Abstract
The improved survival outcomes of patients with non-small-cell lung cancer (NSCLC), largely owing to improved control of systemic disease from immunotherapy and novel targeted therapies, have highlighted the challenges posed by CNS metastases as a devastating yet common complication, with up to 50% of patients developing such lesions during the course of the disease. Early generation tyrosine-kinase inhibitors (TKIs) often provide robust systemic disease control in patients with oncogene-driven NSCLC, although these agents are usually unable to accumulate to therapeutically relevant concentrations in the CNS owing to an inability to cross the blood–brain barrier (BBB). However, the past few years have seen a paradigm shift with the emergence of several novel or later-generation TKIs with improved CNS penetrance. Such agents have promising levels of activity against brain metastases, as demonstrated by data from preclinical and clinical studies. In this Review, we describe current preclinical and clinical evidence of the intracranial activity of TKIs targeting various oncogenic drivers in patients with NSCLC, with a focus on newer agents with enhanced CNS penetration, leptomeningeal disease (LMD), and the need for intrathecal treatment options. We also discuss evolving assessment criteria and regulatory considerations for future clinical investigations.
Introduction
Non-small-cell lung cancer (NSCLC) remains the leading cause of cancer-related mortality in the USA and worldwide. CNS metastases are common in patients with NSCLC, with 10–20% having such lesions at initial diagnosis1,2 and up to 50% developing brain metastases over the course of the disease3–5. CNS metastases substantially reduce patients’ quality of life, often owing to seizures, cognitive impairment and/or other forms of neurological dysfunction6. The prognosis of patients with brain-metastatic NSCLC is often poor, with median overall survival (OS) durations estimated to be around 17 months for patients with lung adenocarcinomas and 8 months for those with other subtypes of NSCLC7,8. However, as treatment strategies evolve, the cumulative incidence of brain metastases will probably also change based on the extent of intracranial penetrance of systemic therapies.
The treatment of patients with intracranial metastases from NSCLC is highly multidisciplinary. Radiotherapy, including stereotactic radiosurgery (SRS) and whole-brain radiotherapy (WBRT), as well as surgical resection when feasible, have been the main treatment modalities for the management of brain metastases. Traditionally, systemic chemotherapy is less effective in patients with CNS metastases relative to those with metastases in other organs owing to the inability of most agents to cross the blood–brain barrier (BBB), typically resulting in intracranial response rates ranging from 15–50%9,10. Immune-checkpoint inhibitors such as anti-PD-(L)1 agents have shown promising initial intracranial activity in patients with other advanced-stage solid tumours that are often associated with CNS metastases11. For example, in melanoma, nivolumab in combination with ipilimumab have demonstrated 57% intracranial response among patients with untreated CNS metastases11. Investigations designed to understand the extent of any CNS benefits of ICIs both with and without radiotherapy in patients with NSCLC are ongoing12–14.
The number of systemic treatment options [to editor: the systemic options expanded, but CNS penetrating options are still limited] has expanded substantially with the emergence of therapies targeting specific oncogenes that drive different subtypes of NSCLC. Targeted therapies for nine such driver oncogenes have received regulatory approval for patients with oncogene-driven NSCLCs, most of which are small-molecule tyrosine-kinase inhibitors (TKIs)15. First-generation TKIs such as erlotinib and crizotinib demonstrated poor BBB permeability and limited activity against CNS metastases, although these agents were nonetheless associated with lower rates of CNS progression compared with chemotherapy16–19. When used in combination with radiotherapy, early generation TKIs are well-tolerated and moderately effective20, although the contribution of many of these TKIs to the overall efficacy of the combination can be difficult to determine. Later-generation TKIs such as osimertinib and lorlatinib have demonstrated marked improvements in CNS penetrance and have now entered clinical practice21,22.
In this Review, we summarize the available preclinical evidence on the BBB penetrance of targeted therapies approved for use in patients with NSCLC harbouring specific driver alterations, as well as the clinical intracranial response rates of patients receiving these various agents. The evolving assessment criteria and regulatory considerations are also discussed, given that these criteria are increasingly used in trials and susbsequently in clinical practice. These criteria might also be applicable to CNS metastases from other solid tumours.
The blood-brain and blood-tumour barriers
The BBB consists of endothelial cells lining cerebral capillaries that are connected by tight junctions and are able to interact with astrocytes, pericytes and microganglia (FIG. 1a). Tight junctions generally restrict the paracellular diffusion of most molecules into the CNS. Polarized transport proteins expressed on the endothelial cells enable the selective transport of a range of substrates, including signalling proteins, carbohydrates, fatty acids, amino acids and ions, across the BBB, thus protecting the brain from pathogens and toxins while also ensuring access to essential nutrients. Active transport takes place by efflux via various transporters, such as the ATP-binding cassette (ABC), or by movement against the concentration gradients of other molecules23. Small molecules that lack a specific transport protein must cross the BBB passively via paracellular or transcellular diffusion along their concentration gradient, which is limited by tight junctions for most molecules. Certain molecules are able undergo passive transcellular diffusion through endothelial cells to cross the BBB. Macromolecules (>400 Da) require carrier-mediated transport to cross the BBB. After internalization, molecules are excreted from the brain through active transporters (such as P-glycoproteins) with efflux pumps along the endothelium, entrance into the CSF or lymphatic system, or by enzymatic metabolism24.
Figure 1. Schematic illustration of the (A) blood–brain barrier and (B) the blood–tumour barrier.

(A) The BBB consists of endothelial cells lining cerebral capillaries that are connected by tight junctions and interact with astrocytes, pericytes and microganglia. Transport proteins on the endothelial cells enable the selective transport of various substrates. Active transport takes place by efflux via transporters such as P-glycoprotein.
(B) In the case of brain metastases, the protective BBB becomes disrupted and transforms into a blood–tumour barrier (BTB) with increased permeability due to a modified basement membrane and loss of tight junctions (as a result of tumor-secreted VEGF and angiopoietin-2). Pericytes and astrocytes are often also lost, and transcytosis components are downregulated
In patients with brain metastases, the protective BBB becomes disrupted and transforms into a blood–tumour barrier (BTB) with modified and more-variable permeability25. Loss of BBB tight junctions is typically seen in the endothelium of the BTB owing to increased vascular endothelial growth factor (VEGF) secretion by tumour cells and hypoxia-induced angiogenesis, causing increased fenestration and vascular permeability. Pericytes and astrocytes are often also lost, and transcytosis components are downregulated25 (FIG. 1b). This loss of barrier function enables greater endocytic penetration of certain macromolecules compared to that seen with an intact BBB25,26, and these macromolecules can include HER2-targeted antibody–drug conjugates (ADCs) and chemotherapies in mouse xenograft models26,27. Data from such models indicate varying drug concentrations within the brain, across different metastases and even among different areas of the same lesion, which is likely to reflect variations in paracellular permeability25.
The newer, rationally designed, CNS-penetrating drugs are optimized to facilitate uptake and minimize efflux across the BBB with the aim of maximizing intracranial retention. Most early generation TKIs are substrates of P-glycoprotein efflux transporters. Indeed, certain older TKIs such as crizotinib are very readily excreted via P-glycoprotein efflux pumps, resulting in subtherapeutic28 intracranial concentrations and thus limited efficacy. Hence, later-generation TKIs such as osimertinib, alectinib and lorlatinib were specificially designed to bypass recognition by P-glycoproteins in order to limit their elimination from the CNS29. Further understanding of the heterogenous permeability of the BTB is necessary to develop more-effective therapeutic strategies.
Preclinical quantification of CNS drug concentrations
Preclinical quantification of the CNS concentration of a drug is the first step in predicting BBB penetrance. Therefore, pharmacokinetic models specifically for this purpose have been developed in small animals (FIG. 1). Kpuu, the unbound partition coefficient, is increasingly used to quantify drug accumulation in such studies30. Kpuu,brain, which indicates the unbound brain-to-plasma partition ratio, is defined as the ratio between the free drug concentration in the brain parenchyma and the free concentration of the same drug in plasma at a steady state30,31. Similarly, Kpuu,CSF, the cerebrospinal fluid (CSF)-to-plasma ratio, indicates the ratio of the unbound drug concentration in CSF versus plasma at equilibrium. CSF drug concentrations provide a fairly good estimate of brain extracellular fluid concentrations but are not an exact measure of parenchymal drug accumulation, as Kpuu,CSFvalues generally tend to be slightly higher than Kpuu,brain32. This difference is thought to be attributed to enhanced drug elimination from the brain parenchymal through active efflux transporters at the BBB32. While CSF is the most easily sampled CNS specimen in humans, it is worth noting that Kpuu,CSF is not entirely representative of the intraparenchymal drug concentration. Values >0.3 reflect high levels of diffusion across the BBB31,33. Transporter proteins expressed at the BBB and blood–CSF barrier, such as P-glycoprotein, which excrete drugs from the CSF via active efflux, have a major role in limiting CNS penetrance31. P-glycoprotein seems to have opposing functions when expressed at the BBB versus the blood-CSF barrier34. The available Kpuu,brain and Kpuu,CSF data for various CNS-penetrant TKIs discussed throughout this review can be found in Table 1.
Table 1 │.
CNS accumulation and activity of targeted therapies in patients with brain-metastatic NSCLC
| Drug | Kpuu brain | Kpuu, CSF | CNS ORR (%) | Median CNS DoR | CNS PFS |
|---|---|---|---|---|---|
| EGFR classical mutations | |||||
| Gefitinib51,197 | 0.0021a | 0.088a | 65% | – | – |
| Erlotinib51,198 | 0.11a | 0.29a | 50% | – | – |
| Icotinib46,199 | 0.12a | 0.61a | 67.9% | – | Median 10.0 months |
| Afatinib51,200 | 0.0066a | 0.27a | 67% | – | – |
| Osimertinib21,48 | 0.21–0.39b | 0.29a | 91% | 15.2 months | – |
| Lazertinib46,201 | 0.087a | – | 85.7% in patients with EGFRT790M | 15.1 months | Median 26.0 months |
| Zorifertinib52 | 0.65b | 0.42b | 75% | 12.4 months | 15.2 months |
| BLU-70157 | 0.56–0.98a | – | – | – | – |
| BDTX-153560 | 0.55–0.58a | – | – | – | – |
| EGFR exon 20 | |||||
| BLU-45169 | 0.66a | – | – | – | – |
| ALK | |||||
| Crizotinib28 | – | 0.0026c | 23–50% | 3.7–10.2 months | – |
| Ceritinib81 | 0.15a | – | 72.7% | 16.6 months | – |
| Alectinib84,85 | 0.63–0.94a | – | 81% | – | – |
| Brigatinib78,202 | – | – | 78% | – | 2-year PFS 48% |
| Ensartinib87 | – | – | 63.7% | – | – |
| Lorlatinib92,203 | – | 0.75c | 82% (first-line); 59% (third-line or later) |
– | Median 24.6 months (third line or later) |
| NVL-65594 | 0.16a | – | – | – | – |
| ROS1 | |||||
| Entrectinib101 | 0.43b | – | 80% | 12.9 months | Median 8.8 months |
| Repotrectinib104 | – | – | 100% (TKI-naive); 75% (TKI-pretreated) | – | – |
| Taletrectinib204 | – | – | 91.6% | 100% | – |
| Lorlatinib205,206 | – | 0.75c | 66.7% | – | Median 38.8 months |
| RET | |||||
| Selpercatinib110,207 | 0.20b | – | 85% | 9.4 months | Median 19.4 months |
| Pralsetinib208 | – | – | 70% | 10.5 months | – |
| METex14 | |||||
| Capmatinib128 | – | 0.34a | 54% | – | – |
| Tepotinib129,131 | 0.25a | – | 55–86.7% | – | – |
| KRAS G12C | |||||
| Sotorasib139 | – | – | DCR 88% | – | – |
| Adagrasib141–142 |
– | 0.47c | 42% | 12.7 months | Median 5.4 months |
| NTRK | |||||
| Larotrectinib99 | – | 0.03a | 63% | – | – |
| BRAF V600E | |||||
| Dabrafenib plus trametinib | – | – | – | – | – |
| HER2 | |||||
| Trastuzumab deruxtecan | – | – | – | – | – |
CSF, cerebrospinal fluid; DCR, disease control rate; DoR, duration of response; NR, not reported; ORR, objective response rate; PFS, progression-free survival; TKI, tyrosine-kinase inhibitor.
As measured in rats.
As measured in mice.
As measured in humans.
CNS activity of targeted therapies
The range of targeted therapies available for patients with NSCLC has expanded substantially over the past 5 years. Targeted therapies are now available for nine different driver oncogenes with 10 actionable alterations, including EGFR aberrations (classical activating mutations and exon 20 insertions), ERBB2 (HER2) mutations, ALK, ROS1, RET or NTRK rearrangements, MET exon 14 skipping mutations, BRAFV600E, or KRASG12C. Mostly small-molecule inhibitors are indicated for all of these alterations, except for HER2 mutations. For HER2-mutant NSCLC, antibody drug conjugate (ADC) trastuzumab deruxtecan was approved, which has recently demonstrated promising CNS activity in patients with both untreated and previously treated baseline brain metastases35. The intracranial efficacy of certain small-molecule TKIs was initially attributed to their low molecular weight; however, larger molecules such as ADCs have also been demonstrated to penetrate the brain parenchyma, most likely owing to disruptions of the BBB when brain metastases occur36,37. With the newer TKIs having unprecedented levels of intracranial efficacy, very good systemic treatment options are now available for patients with CNS metastasis from certain subtypes of NSCLC. These drugs, which include those targeting classical EGFR mutations (osimertinib), ALK rearrangements (alectinib, brigatinib and lorlatinib), RET rearrangements (selpercatinib), and ROS1 or NTRK rearrangements (entrecitinib), can produce CNS objective response rates (ORRs) ≥80% in each patient population (TABLE 1). However, an unmet need exists to develop small molecules with intracranial activity against other driver alterations and to establish intracranial efficacy of newer next-generation TKIs, such as EGFR exon 20 insertions, HER2 mutations, MET exon 14 skipping, BRAFV600E and KRASG12C.
Classical EGFR mutations
The ability to inhibit EGFR not only set in motion the TKI renaissance that would later define precision medicine for NSCLC, but also provided the first demonstration of the importance of CNS activity. Brain metastases are found in about a quarter (24.4%) of patients with EGFR-mutant NSCLC at the time of a diagnosis of advanced-stage disease38, with a 5-year cumulative incidence as high as 52.9%38,39. First-generation and second-generation TKIs such as gefitinib, erlotinib and afatinib have poor BBB permeability (Kpuu,brain <0.11) and thus limited activity in patients with CNS metastases40,41. Early generation EGFR TKIs in combination with SRS or WBRT have demonstrated improved systemic control and time to CNS progression in patients with brain metastases, possibly as a result of the disrupted BTB42. One approach to augment BBB penetrance involves the ‘pulsed’ oral administration of high-dose (1,500 mg, which is 10 times the recommended daily dose) erlotinib once or twice per week, with or without daily continuous dosing. This approach is reportedly well-tolerated and enables improved CNS activity (ORR 67%; median CNS progression-free survival (PFS) 2.7 months)43 44. These pulsed-dose studies illustrate the strategy of generating a higher peak concentration over a shorter duration in an effort to enhance the CNS drug concentration and thus promote intracranial disease control, as supported by clinical evidence and mathematical PK modeling45.
Osimertinib is a third-generation EGFR TKI with activity against the T790M variant, as well as classical activating mutations, with a much improved BBB penetrance compared to that of earlier-generation TKIs. Osimertinib is currently the standard-of-care first-line therapy for patients with metastatic EGFR-mutant NSCLC. Osimertinib is a weak substrate for human BBB efflux transporters and, therefore, has a low BBB influx:efflux ratio and thus high CNS penetrance46,47. Data from preclinical studies support this observation, with a Kpuu,brain of 0.21–0.39 compared to <0.01 for afatinib and gefitinib (TABLE 1)48. The superiority of osimertinib over first-generation TKIs has been established in the first-line setting through the phase III FLAURA trial, with an impressive intracranial ORR of 91% among patients with measurable asymptomatic or stable CNS metastases (included both untreated and pretreated)21,49. FLAURA included patients with LMD (seen in 7 of 128 patients with CNS metastases) who historically have been excluded from most clinical trials.
Novel BBB-penetrant EGFR TKIs with even higher Kpuu (brain and CSF) values are in clinical development (TABLE 1). For example, zorifertinib is an EGFR TKI designed to fully penetrate the BBB by evading efflux transporters. Impressive early pharmacokinetic results were achieved with this agent, which demonstrated Kpuu,brain of 0.65 and Kpuu,CSF of 0.42 in mice51,52. Profound suppression of intracranial tumours and improved survival relative to that achieved with erlotinib have also been demonstrated in mouse models51,53. Combining zorifertinib with radiation has been found to have more-potent antitumour effects in mouse models, by suppressing both cellular proliferation and DNA damage repair54. Intracranial tumour regression and CSF tumour cell reductions >50% were observed in 4 of 5 patients with LMD receiving zorifertinib as part of a larger phase I study55. Phase III data on the efficacy of this agent recently confirmed improved intracranial PFS of 15.2 months with zorifertinib versus 8.3 months in control group treated with gefitinib or erlotinib (HR 0.47, p<0.0001) (NCT03653546)56. Another novel drug, BLU-701, is designed to target EGFR TKI-resistant tumours harbouring the C797S resistance mutation in EGFR and has demonstrated high levels of BBB penetration in rat models (Kpuu,brain 0.56–0.98)57,58. BDTX-1535, another developmental highly CNS-penetrant irreversible EGFR TKI, has also been shown to induce CNS tumour regression in preclinical models59,60. A phase I study of BDTX-1535 in patients with advanced-stage EGFR-mutated glioblastoma and NSCLC is currently recruiting patients (NCT05256290)61.
EGFR exon 20 insertions
EGFR exon 20 insertions can be found in approximately 2–3% of all NSCLCs62 and are resistant to conventional EGFR TKIs63. Two targeted agents, amivantamab and mobocertinib, are currently approved for this patient population64,65. Amivantamab is a novel bispecific antibody targeting EGFR and MET that has shown good levels of systemic efficacy and tolerability in the phase I CHRYSALIS trial66,67. However, intracranial activity is unknown owing to the exclusion of patients with untreated and/or active brain metastases67. Mobocertinib is a TKI targeting EGFR exon 20 insertions that demonstrated an inferior ORR among patients with baseline brain metastases (25%) compared to those without (56%) in the phase II EXCLAIM study, suggesting limited CNS activity65,68. Similar to CHRYSALIS, EXCLAIM only allowed the enrolment of patients with stable and treated brain metastases and excluded those with untreated or active lesions65,67, thus making the CNS efficacy of mobocertinib very difficult to evaluate. On the basis of current data, both amivantimab and mobocertinib are considered to have minimal CNS activity.
Several novel TKIs targeting EGFR exon 20 insertions are currently at various stages of clinical investigation in an attempt to address this unmet need. BLU-451 is a highly CNS-penetrant inhibitor of exon 20 insertion-mutant EGFR (Kpuu,brain 0.66)69 that is currently being tested in a phase I/II study involving patients with EGFR exon 20 insertion-driven NSCLC and untreated brain metastases (NCT05241873)70. Sunvozertinib is another new TKI that has received FDA breakthrough therapy designation for patients with NSCLC harbouring EGFR exon 20 insertions. This agent demonstrated an ORR of 48.5% among 33 patients with pretreated stable and asymptomatic brain metastases at study enrolment in a phase II study71. However, additional evaluations in patients with untreated active brain metastases are needed to better elucidate the intracranial activity of sunvozertinib. Finally, ORIC-114, a CNS-penetrant irreversible TKI with activity against both EGFR and HER2 exon 20 insertions, has shown promising preclinical intracranial activity in mouse models, with a phase I trial underway (NCT05315700)72.
HER2 mutations
HER2 mutations occur in about 3% of all NSCLCs, and are newest addition to the list of targetable driver alterations following the approval of T-DXd based on results from the DESTINY-lung trials35. Because T-DXd is an ADC and a macromolecule, the trial allowed patients with irradiated and non-irradiated CNS metastases71. Nonetheless, T-DXd has demonstrated a CNS ORR of 73% in 15 patients with HER2-positive breast cancer with new or progressing CNS metastases73. Data in patients with HER2-mutant NSCLC and CNS metastases in DESTINY-Lung01 will mature over time.
Various non-selective HER2 TKIs, such as afatinib, dacomitinib and pyrotinib, have demonstrated some systemic activity in patients with HER2-mutant NSCLC, although CNS-specific data are again unavailable. The pan-ErbB TKI poziotinib was evaluated in a cohort of pretreated patients with stable CNS metastases, demonstrating an ORR of 28.6% with a median PFS duration of 7.4 months. However, interpretation of these data is limited by the small sample size, the use of prior CNS radiation in 13 of the 14 patients (the majority within 3 months of study initiation), and the lack of a mandatory baseline brain MRI74. BI-1810631 is a promising new HER2-selective TKI that that does not inhibit EGFR, showing a systemic ORR of 42% and disease control rate (DCR) of 95% in a phase I trial involving 19 patients. Further evaluations of the efficacy of this agent, including intracranial activity are ongoing (NCT04886804). Novel HER2-targeting ADCs are also in early clinical development for the treatment of HER2-driven lung cancers, including disitamab vedotin, SBT-6050, GQ-1001 and ZW-49.
ALK rearrangements
ALK rearrangements occur in approximately 3–7% of patients with NSCLC75. Around 20–30% of patients with ALK-rearranged NSCLC will have CNS metastases at the time of diagnosis, and up to 50% will develop such lesions throughout the course of the disease38,76, although this incidence will probably decline following the availability of CNS-penetrant TKIs. Crizotinib, the first FDA approved TKI for patients with ALK-rearranged NSCLC, has limited CNS penetrance (Kpuu,CSF 0.0026) and, as expected, more than a third of treatment failures among patients receiving crizotinib failures involve CNS progression, thus limiting the extent of clinical benefit77. Newer and more-specific ALK TKIs such as alectinib, brigatinib and lorlatinib all have improved levels of CNS penetrance, with 82% of patients having an objective intracranial response to lorlatinib78–80.
Indeed, the second-generation ALK TKIs ceritinib, alectinib and brigatinib seem to have improved CNS penetration relative to that of crizotinib or systemic chemotherapy. Ceritinib had far superior intracranial efficacy compared to that of chemotherapy in the phase III ASCEND-4 trial (TABLE 1)81,82. The CNS penetrance of alectinib is additionally enhanced relative to ceritinib (Kpuu,brain 0.63–0.94), reflecting the fact that this agent is not a P-glycoprotein substrate83,84. Data from the phase III ALEX trial confirmed the impressive intracranial activity of alectinib in patients with asymptomatic measurable brain metastases or LMD, with an intracranial ORR of 81%79,85. Brigatinib has similar levels of systemic and intracranial activity to alectinib, among patients with baseline measurable brain metastases and no LMD (TABLE 1). Analyses of long term CNS DoR and CNS PFS of both alectinib and brigatinib remain under investigation78,86. Ensartinib has also demonstrated superiority over crizotinib; data from the phase III eXalt3 trial demonstrated that fewer patients without baseline brain metastases develop brain metastases at 12 months when treated with ensartinib compared with crizotinib (4.2% versus 23.9%; HR 0.32, 95% CI 0.16–0.63), demonstrating not only regression of pre-exisiting brain metastases, but also prevention of CNS metastases87.
Lorlatinib is a third-generation ALK and ROS1 TKI with inhibitory activity against all known acquired crizotinib resistance mutations88. The effective BBB penetrance of this agent (Kpuu,CSF of 0.75–0.77) has been confirmed in both preclinical animal models and in patients22,89,90. Clinically, lorlatinib has shown the most impressive CNS efficacy of all ALK TKIs, with a 71% intracranial complete response rate and HR of 0.10 (95% CI 0.04–0.27) for time to intracranial progression among treatment-naive patients with baseline CNS metastases in the phase III CROWN study80,91. Furthermore, lorlatinib is the only TKI that has demonstrated both intracranial and systemic disease control in patients who previously received ≥1 earlier-generation ALK TKI, making it an excellent second-line option. An impressive median CNS PFS duration of 24.6 months was reported in patients who had previously received at least one ALK TKI. However, the inclusion of patients with both parenchymal disease and LMD, the limited sample size of ths study (15 patients), and the history of prior intracranial radiotherapy in 65% of patients make these results more difficult to interpret92. Nevertheless, in light of the impressive intracranial data from the CROWN study, lorlatinib should be used in ALK-TKI pre-treated patients with CNS metastases, or as first-line therapy in those with CNS-predominant metastases.
Several 4th-generation ALK TKIs are currently being tested in clinical trials. NVL-655 is an ALK TKI currently in development that has activity against both single and compound ALK resistance mutations, while also sparing NTRK and thus minimizing the risk of adverse events related to TRK inhibition seen in other ALK TKIs, as well as a Kpuu,brain of 0.1694.
ROS1 rearrangements
ROS1 rearrangements occur in 1–2% of all NSCLCs and are associated with a similar incidence of CNS metastasis to that of patients with ALK-rearranged NSCLC95,96. Also similar to ALK-rearranged NSCLC, crizotinib confers limited CNS benefit in patients with ROS1-rearranged NSCLC97. Newer ROS1-targeting TKIs have more promising levels of CNS activity98. Entrectinib is an inhibitor of ALK, ROS1 and NTRK and is approved by the FDA as both a ROS1 and NTRK inhibitor96. As a weak P-glycoprotein substrate, entrectinib has good CNS penetrance, as indicated by its low apical efflux ratio and Kpuu,brain of 0.4399–101. An updated integrated analysis of data from the ALKA-372–001, STARTRK-1 and STARTRK-2 trials confirmed durable intracranial activity in patients with measurable CNS disease at enrolment (intracranial ORR 55%; median intracranial DoR 12.9 months102,103. Repotrectinib and taletrectinib are both next-generation ROS1 TKIs with clinical data demonstrating impressive intracranial activity, including CNS ORRs of 100% (specifically in TKI-naive patients) and 91.6%, respectively, albeit in small cohorts of patients (TABLE 1)104,105. Both drugs have received FDA breakthrough therapy designation for ROS1-rearranged NSCLC. Similar to its excellent intracranial activity in ALK-rearranged NSCLC, lorlatinib has been shown to induce durable responses with a median intracranial PFS duration of 38.8 months in patients with CNS-only progression on crizotinib106. Overall, data on the intracranial activity of TKIs in patients with ROS1-rearranged NSCLC remains limited owing to the small sample sizes of many of the cohorts and a lack of head-to-head comparison between different TKIs. Nonetheless, a phase III trial comparing the efficacy of entrectinib versus that of crizotinib in patients with advanced-stage NSCLC, including those with brain metastases (NCT04603807), is underway.
RET rearrangements
RET rearrangements are oncogenic drivers in 1–2% of NSCLCs107. The highest prevalence of brain metastases at diagnosis in NSCLC was seen in RET-rearranged NSCLC (32.2%), along with ALK-translocated NSCLC (34.9%)108. While older, broad-spectrum kinase inhibitors (such as cabozantinib, vandetanib and lenvatinib) have enabled confirmed systemic responses107, the limited available data suggest very poor intracranial response rates in patients with RET-rearranged NSCLC109. However, the highly selective RET TKIs selpercatinib and pralsetinib, both of which have received FDA approval for RET-rearranged NSCLC, have excellent intracranial efficacy.
In an updated analysis of data from the phase I/II LIBRETTO-001 study, selpercatinib yielded impressive and durable intracranial activity, including a median CNS PFS of 19.4 months among patients with baseline measurable CNS metastases independent of previous systemic therapy or radiotherapy. The phase I part of this study did not require brain imaging at baseline for all study participants. However, phase II did mandate both baseline and subsequent brain imaging, regardless of the presence of pre-existing brain metastases. None of the 178 patients without baseline CNS metastases developed intracranial disease progression (the estimated probability of intracranial disease progression at 2 years was 0.7%), suggesting that selpercatinib either prevents or delays the development of CNS metastases110. In a combined analysis of data from LIBRETTO-001 and LIBRETTO-201, all patients with disease progression who lacked baseline CNS metastases had extracranial-only progression111. Pralsetinib is similarly efficacious in terms of overall intracranial response (all 9 patients with baseline brain metastases had an objective response, and one response detected on post-baseline assessments, although the duration of CNS response is unknown at this time, and sample size was limited to 10 patients in the phase I/II ARROW cohort112. In a recent update of ARROW in the Chinese cohort, pralsetinib demonstrated CNS ORR of 73.7% (14/19) in chemotherapy-pretreated patients and 82.6% (19/23) in treatment-naïve patients113.
NTRK rearrangements
NTRK rearrangements occur in <1% of NSCLCs, and the NTRK inhibitors entrectinib and larotrectinib are now FDA-approved for this rare population, based on histology- and tumor-agnostic trials of NTRK-rearranged solid tumors. For entrectinib, data from patients with NTRK-rearranged NSCLC with CNS metastases indicate similar levels of activity to that seen in patients with ROS1-rearranged NSCLC in the previously mentioned integrated analysis of data from ALKA-372–001, STARTRK-1 and STARTRK-2, with 66.7% (4 of 6 patients) having an intracranial response102,103,114. However, neurological adverse events can occur with TRK inhibition, as TRK receptors and pathway activation have been found to be closely involved in regulating several aspects of CNS function, including appetite, balance and pain sensitivity115. For example, dysgeusia, or disturbed taste sensations, is the most frequently reported grade 1–2 adverse event in patients receiving entrectinib, with most clinically serious events also being neurological in nature (including one incidence of cognitive disorder, cerebellar ataxia and dizziness)114. Larotrectinib is a pan-TRK inhibitor designed to have poor CNS penetration and thus avoid CNS toxicities. In contrast to entrectinib, larotrectinib has a relatively high apical efflux ratio, which translates to a lower Kpuu,CSF of 0.0399. Despite low BBB penetration, larotrectinib has demonstrated activity among 8 patients with baseline CNS metastases (ORR 63%) in an integrated analysis of data from a phase I trial (NCT02122913) and the phase II NAVIGATE trial, although CNS-specific ORR is unknown116. No clinically serious neurological adverse events have been observed in patients receiving larotrectinib116. Several newer ROS1 and NTRK inhibitors, such as taletrectinib, repotrectinib and selirectinib, are under investigation and some have shown good BBB penetrance and promising overall and intracranial activity117–119.
BRAFV600E
Oncogenic alterations in BRAF can be identified in approximately 4% of patients with NSCLC, and around half of these (1–2%) are BRAFV600E 120,121. The BRAF–MEK inhibitor combination of dabrafenib plus trametinib has been demonstrated to induce durable responses in patients with metastatic BRAFV600E-mutant NSCLC, although intracranial efficacy is less well known owing to the exclusion of patients with symptomatic brain metastases from this phase II trial122. An intracranial ORR of 58% has been reported among 125 patients with metastatic BRAFV600E-mutant melanoma123, suggesting that this combination has a high level of CNS activity. Nonetheless, investigations involving patients with BRAF-mutant NSCLC with CNS metastases are needed.
MET exon 14 skipping mutations
MET exon 14 skipping mutations are found in 3–4% of all NSCLCs124–127. The incidence of CNS metastases at diagnosis in this group is around 17%, with a lifetime incidence of 36%126. The TKIs capmatinib and tepotinib are the both approved by the FDA for patients with NSCLC harbouring METexon 14 skipping mutations124. Capmatinib is a highly selective and potent inhibitor of exon-14 altered MET127, and has a Kpuu,CSF of 0.34128. In the phase II GEOMETRY mono-1 study, capmatinib demonstrated an intracranial ORR of 54%, with 30.8% of patients having a CNS complete response128. Patients receiving tepotinib, another agent targeting exon-14 altered MET, had a similar intracranial ORR in the phase II VISION study129,130, with an updated analysis indicating that 71.4% (5 of 7 patients) had a partial intracranial response with 86.7% (13 of 15) having intracranial disease control according to response assessment in neuro-oncology brain metastases (RANO-BM) criteria131. The addition of the highly selective MET inhibitor savolitinib to osimertinib has demonstrated efficacy in overcoming resistance owing to acquired secondary MET amplification and/or overexpression in patients with EGFR-mutant NSCLC, though CNS efficacy was not evaluated132. Other MET TKIs currently in early clinical trial development include ABN-401 (NCT04052971)133 and APL-101 (NCT03175224), although no CNS data are currently available for these agents.
KRASG12C
Despite being the most common oncogenic alteration, including in up to 30% of all patients with NSCLC134,135, oncogenic KRAS alterations have been deemed ‘undruggable’ for several decades — until the FDA accelerated approval of the KRASG12C inhibitor sotorasib in 2021 for patients with advanced-stage KRASG12C-mutant NSCLC, an alteration found in 13% of all NSCLCs. Data from a retrospective analysis indicate that up to 40% of patients with KRAS-mutant NSCLC will develop brain metastases at any time during the course of disease, with a 6-month cumulative incidence of CNS metastases since initial diagnosis of 42%136.
Sotorasib is a specific and irreversible KRASG12C inhibitor that locks the mutated protein in the inactive GDP-bound state, thus inhibiting subsequent phosphorylation of downstream signalling proteins137,138. In a post-hoc analysis of data from the phase II CodeBreaK100 trial, 14 of 16 patients (88%) with evaluable brain metastases had intracranial disease control per the RANO-BM criteria and 2 (13%) had a complete response139. However, only patients with resected CNS lesions or who received brain radiotherapy >4 weeks prior to enrollment were included in this analysis, whereas those with untreated brain metastases were excluded. Thus, the intracranial activity of sotorasib in patients with active, untreated brain metastases remains unknown and will be difficult to evaluate.
Adagrasib is another KRASG12C inhibitor that received accelerated approval for patients with advanced-stage KRASG12C-mutant NSCLC in December 2022. This agent has demonstrated BBB penetrance and intracranial activity in both preclinical and clinical studies. Preclinically, adagrasib accumulates to adequate concentrations in the CNS partially through the saturation of P-glycoprotein-mediated efflux140. Adagrasib is the first KRASG12C inhibitor with data available on intracranial activity in patients with active, untreated brain metastases, with a CNS ORR of 42%, intracranial PFS of 5.4 months, and median IC DOR of 12.7 months reported in the phase Ib KRYSTAL-1 trial141. The average Kpuu,CSF was 0.47 among 2 patients enrolled in this study, thus further confirming the CNS penetration of this drug142.
An unmet need remains for intracranially penetrant treatments for patients with NSCLC harbouring KRASG12C, as well as other KRAS alterations without an approved targeted therapy. Various KRASG12C inhibitors are in early clinical development for solid tumors including NSCLC, including JDQ-443 (NCT04699188), GDC-6036 (NCT04449874), JNJ-74699157 (NCT04006301), MK-1084 (NCT05067283) and BI-1823911(NCT04973163), with many more agents expected to follow in the next few years. Furthermore, there are also ongoing phase 1/2 studies evaluating therapies targeting KRAS non-G12C alterions, such as MRTX1133 (NCT05737706) and RMC6236 (NCT05379985).
LMD and intrathecal therapies
LMD describes the secondary spread of malignant cells into the CSF and leptomeninges of the brain and spinal cord, a complication occurring in 3–5% of patients with advanced-stage NSCLC. This pattern of dissemination is viewed as the worst type of CNS metastasis, owing to the limited treatment options available and an extremely poor prognosis50 (Figure 2).
Figure 2. MRI brain images (axial view, T1 sequence, post-contrast) demonstrating.

(A) diffuse LMD with enhancement within the cerebral sulci (B) normal brain without metastases or LMD
Osimertinib has a Kpuu,CSF of 0.29 and was therefore evaluated in the phase II BLOOM study as a systemic therapy for patients with EGFR-mutant NSCLC and LMD. In this study, 41 patients of Asian ethnicity with cytologically confirmed LMD and disease progression on an EGFR TKI received 160 mg daily doses of osimertinib, which is double the standard dose46. Measurable CNS lesions were assessed using RECIST 1.1, whereas LMDs were evaluated using blinded central independent review according to RANO leptomeningeal metastases (RANO-LM) criteria. Patients receivivng osimertinib had a confirmed leptomeningeal ORR of 41% and a median DoR of 8.3 months143. Almost half (49%) of the cohort had received prior radiotherapy, most of whom completed treatment ≤6 months prior to enrolment. Hence, ambiguity exists regarding the extent to which radiotherapy might have contributed to the responses of these patients. Furthermore, 160 mg daily dose is not the standard dose of osimertinib used in patients with metastatic EGFR-mutant NSCLC without LMD, and carries additional toxicities compared to the 80 mg daily dose, including 66% grade ≥ 3 adverse events leading to drug discontinuation in 22% of patients, much higher than that observed in the 80mg trials. Nevertheless, as one of very few clinical studies capturing an LMD-only trial that is often excluded from clinical trials, BLOOM is a major stride forward in the highly understudied field of LMD. The efficacy of osimertinib 80 mg once daily has also been evaluated in patients with LMD through retrospective analyses of the AURA and FLAURA trials, with LM ORRs of 55% and 80% reported, respectively, supporting the efficacy of standard-dose osimertinib in this setting but with fewer serious toxicities21,144.
A phase II study in which patients with ALK-rearranged NSCLC and intracranial disease progression on ≥1 ALK TKI, including 4 of 23 with LMD, received lorlatinib provides some evidence of the activity of this agent in this setting. The intracranial ORR for the entire cohort was 59%, although the best response among patients with LMD was stable disease92.
Intrathecal injections, via either the lumbar or intraventricular routes, in which drugs are delivered directly to the CNS either via the intrathecal spaces in the spinal cord or the subarachnoid space near the base of the brain, are an avenue that requires further exploration especially for patients with LMD (Figure 3). This approach has the advantages of bypassing the BBB and also avoiding drug metabolism within any part of the systemic circulation while also minimizing the risk of systemic toxicities24. However, the increase risk of neurological complications associated with IT chemotherapy such as paresthesia, cranial nerve palsy or paralysis, as well as the expertise required for administration, must also be acknowledged145. The intrathecal approach is particularly appealing for the administration of cytotoxic chemotherapy and/or macromolecules. A phase I/II trial evaluating the safety and efficacy of intrathecally administered pemetrexed plus dexamethasone in patients with EGFR-mutant NSCLC and disease progression on a TKI yielded an impressive ORR of 84.6% (22 of 26 patients) and a median OS duration of 9.0 months. The intervention was also well tolerated, with most patients having low-grade adverse events only146.
Figure 3. Intrathecal chemotherapy can be delivered through two different methods.
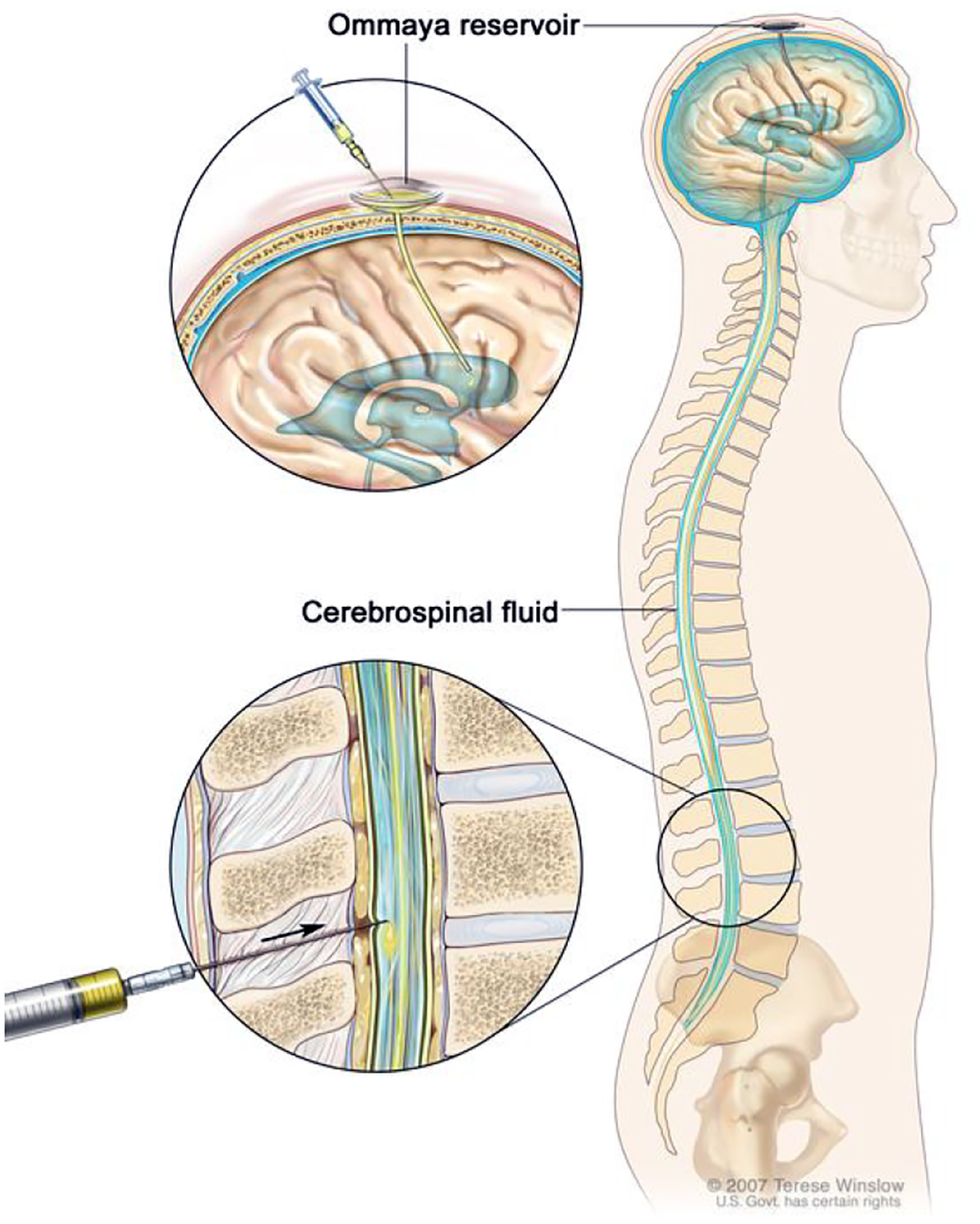
A) Direct injection into the CSF in the lower lumbar spinal column or B) Injection into an Ommaya reservoir (intraventricular implant that is surgically placed beneath the scalp)
Intrathecal topotecan did not yield impressive survival outcomes (6-month PFS of 19%) among 62 patients with LMDs from advanced-stage solid tumours, including 13 with NSCLC, in a phase II trial conducted in the early 2000s. A larger-cohort single-centre study evaluating a similar approach in a similar setting and including 29 patients with lung cancer demonstrated a median OS duration of 6.5 months150,151.
Rapid clearance of the administered drug is a common challenge associated with intrathecal therapy153–155. Intrathecal formulations with encapsulated chambers designed to preserve bioactivity while slowly releasing the active drug provide one method of overcoming this problem. Liposomal cytarabine, which has been withdrawn from the market due to product-specific manufacturing problems, is a slow-release form of cytarabine with the drug remaining within the patient’s CSF for >14 days156. This formulation improves the response rates of patients with lymphomatous meningitis and offers some benefit for patients with LMD from certain solid tumours156–158. This example suggests that innovative chemical engineering approaches could be leveraged to develop formulations that are able to overcome rapid drug clearance from the CSF. Various technologies, such as polymer-protein bio-conjugation (PEGylation), polymer complexation, polymer encapsulation, and microparticle-mediated intrathecal protein or gene delivery155, are expected to greatly accelerate the speed of development of such intrathecal formulations.
Assessment criteria and trial design
The measurement of intracranial response across clinical trials involving patients with solid tumours has been inconsistent owing to the use of different evaluation criteria (TABLE 2). The RANO-BM criteria originate from a multidisciplinary effort to standardize the evaluation of response and progression of brain metastases in clinical trials. Prior to the development of RANO-BM, most trials used RECIST, which has various shortcomings when used to evaluate brain metastases (TABLE 2). For example, under RECIST, the CNS and systemic lesions are considered to be a single compartment, whereas RANO-BM stipulates that intracranial disease status is assessed separately from systemic disease146. Unlike RECIST, RANO-BM accounts for steroid use, clinical neurological deterioration (related to the tumor) and neurological symptoms, and also allows for the consideration of pseudoprogression from immunotherapy and/or radiosurgery (SRS)159. Furthermore, RANO-BM establishes separate guidelines and end points for assessing the activity of local and systemic therapies160,161. Evaluations of intracranial tumour volume need to be more commonly incorporated into response assessments in clinical trials. Despite the lack of a standardized mandate for volumetric response assessment in trials assessing CNS metastases due to insufficient preexisting data, RANO-BM encourages inclusion of volumetric response evaluation (using gadolinium-enhanced MRI) in effort to expand the current knowledge base and potentially substantiate its use in future trial design159.
Table 2 │.
| Domain | RECIST 1.1 | RANO-BM | RANO-LM |
|---|---|---|---|
| Imaging modality | CT or MRI | CT or MRI | MRI brain and spine |
| Measurement technique | Unidimensional | Bidimensional | Bidimensional |
| Target lesion | Longest diameter 310 mm | Contrast-enhancing lesions with two perpendicular diameters 310 mm | Nodular lesions, leptomeningeal enhancement, cranial nerve enhancement, hydrocephalus or nerve root enhancement |
| Maximum CNS target lesions | ≤2 | ≤5 | ≤5 |
| Definition of progressive disease | ≥20% increase in sum of lesion diameter, any new lesion | ≥25% increase in sum of enhancing lesion diameter, any new lesions | Worsening neurological function owing to LM, worsening neuroradiographical assessments (≥25% increase in sum of lesion diameters), positive CSF cytology (lack consensus) |
| Shrinkage needed for partial response | ≥30% | ≥50% | ≥50% |
| Durability of response | Confirmatory scans only needed in non-randomized trials in which response is the primary end point | Confirmatory scans required at ≥4 weeks apart | Confirmatory scans at baseline and follow-up; CSF analysis not needed |
| T2/FLAIR | Not required | Required | Not required |
| Corticosteroids use considered | No | Yes | Only in patients with haematological malignancies |
| Neurological symptoms considered | No | Yes | Yes |
BM, brain metastases; CSF, cerebrospinal fluid; LM, leptomeningeal metastases; RECIST, Response Evaluation Criteria in Solid Tumors; RANO, Response Assessment in Neuro-Oncology
Evaluation of response in patients with LMD is even more challenging and lacks standardization. The RANO-LM group recommends that all patients with LMD enrolled in trials undergo a standardized neurological examination, CSF analysis including cytology, complete brain and spinal MRI, as well as radioisotope CSF flow studies in those receiving intrathecal therapy (at baseline and after treatment to assess response)162. No single disease characteristic is specific or definitive of LMD; therefore, the RANO-LM criteria incorporate three core components to quantify response and/or disease progression. On the basis of a radiological LM response score designed to standardize subjectivity, imaging results are graded as either stable, progressive or improved162. Progressive disease is characterized by worsening neurological findings and/or worsening neuroradiographic assessments, with the current criteria defining progression as conversion of negative to positive CSF cytology or failure to convert positive cytology to negative cytology after therapy. However, persistently positive CSF cytology with otherwise unchanged clinical and radiographical assessments is insufficient to qualify as disease progression; how best to assess such responses remains unclear163.
Regulatory considerations
In 2021, the FDA provided new guidelines for the design of trials evaluating cancer drugs in patients with CNS metastases from solid tumours, recognizing “that treatment of CNS metastases presents several challenges and unique considerations (e.g., circumventing the blood-brain barrier)”164.This guidance stipulates that either of the standard response criteria used to evaluate CNS disease in prospective studies, such as the modified version of RECIST 1.1 or RANO-BM, can be used in clinical trials. The FDA also recommends concurrent imaging for patients with both intracranial and extracranial disease and provides criteria for including previously irradiated lesions as target lesions. Notably, the FDA guidance also recommends for case report forms to include response assessments, including changes in neurological symptoms, as well as concurrent steroid use and any use of antiseizure medications164. The guidance also favours the use of the RANO-BM or RANO-LM criteria over RECIST 1.1 owing to the inclusion of neurological symptoms and steroid use only in the former but not the latter159. These considerations are not limited to trials testing targeted therapies in patients with brain-metastatic NSCLC, but are also generally applicable to any trials involving patients with CNS metastases from solid tumours.
Clinical considerations
When evaluating the intracranial activity of targeted therapies for CNS metastases, several practical and clinical considerations should be taken into account besides FDA guidelines. First, any measurements of clinical benefit need to take into account both clinical and radiological evidence. Neurological function is a crucial outcome for patients with CNS metastases that should be an integral part of any assessment. Furthermore, the duration of CNS benefit, which can be measured using various different parameters, such as CNS PFS or CNS intervention-free survival, is an important clinical parameter, in addition to response rate. Independent of response, the duration of clinical benefit, defined as the period of time in which a change in systemic therapy is not needed while allowing SRS as required, could also be considered as a parameter of clinical benefit given the minimal adverse effects on patients’ quality of life.
Second, receipt of previous CNS radiotherapy and the types of CNS radiotherapy received need to be specified in order to understand the contributions of any systemic therapy to CNS end points. Currently, many trials, including some of the pivotal trials described herein, allow both patients with untreated and previously irradiated CNS lesions to be evaluated. For trials or cohorts dedicated to assessing the intracranial activity of systemic therapies, receipt of previous radiotherapy needs to be distinguished from no previous radiotherapy, with the types of CNS radiotherapy received in relation to the time of initiation of trial drug considered separately. Assessing the contribution of any targeted therapy is particularly challenging in patients who previously received WBRT, as this intervention can provide oncological benefit across the entire brain parenchyma that might last for several months165. In patients who previously received SRS, responses to systemic therapy among lesions located in the untreated brain parenchyma are likely to reflect control by systemic therapy regardless of the relative timing of SRS. However, any previously SRS-irradiated lesions would not be ideal for clinical response assessments166.
Finally, adequately powered dedicated trials involving cohorts of patients with LMD or untreated brain metastases with CNS-specific primary end points are needed. Other than the BLOOM trial, which exclusively enrolled patients with LMD, such patients are typically included alongside other patients with CNS metastases, when allowed to enrol143. The LMD subgroups from such trials provide preliminary data and a certain level of clinical confidence in the intracranial efficacy of systemic therapies for patients with LMD, although the sizes of such subgroups are generally very small. For example, the large-cohort AURA, FLAURA and CROWN trials allowed patients with LMD to enrol, although these subgroups all contained <10 patients21,49,80. Going forward, dedicated trials or trial cohorts for patients with LMD or those with untreated brain metastases will enable a more-homogenous population to be evaluated and provide the optimal level of evidence to support the development of new CNS-active therapies in the most appropriate populations. A big challenge with this approach, however, would be the accrual of a sufficiently powered LMD cohort, given its rarity, especially in trials focused on specific molecular alterations.
Future directions
Many areas of active research, beyond the development of novel CNS-penetrant targeted therapies and/or formulations for intrathecal administration, might support translational and clinical research efforts aiming to improve the diagnosis and treatment of patients with brain metastases from solid tumours.
Advanced imaging techniques
Both the detection of CNS metastasis and assessments of their response to therapy primarily relies on MRI. T1-weighted acquisition post-gadolinium contrast imaging is typically used to assess the extent of BBB disruption and delineate the tumour, whereas T2-weighted fluid-attenuated inversion recovery (T2-FLAIR) enables the detection of vasogenic oedema located around the tumour167. CNS MRI is the preferred imaging method in both the RANO and RECIST criteria. These criteria stipulate the evaluation of treatment activity based on changes in the diameter of CNS metastases, which can be confounded by several other factors when assessed using standard MRI, including peritumoural oedema, treatment-related inflammation (from immunotherapy or radiotherapy) and delayed complications such as radiation necrosis. Therefore, an unmet need exists for advanced imaging techniques capable of delineating a genuine tumour response from other treatment-related changes.
Perfusion-weighted imaging (PWI) and diffusion-weighted imaging (DWI) are both MRI methods that are already used in neuro-oncology clinics. PWI has shown to detect viable tumour specifically in brain metastases168,169. Dynamic contrast-enhanced (DCE)-MRI is a subtype of PWI that enables modelling of the distribution of contrast between the vascular and interstitial space, enabling the detection of changes in vascular permeability (for example, owing to BBB breakdown at the tumour interface). Dynamic susceptibility contrast (DSC)-MRI, which measures signal loss on a T2-weighted sequence as contrast passes through is another widely used PWI technique170. These methods enable qualitative assessments of whether changes in tumour diameter and/or the tumour microenvironment reflect the presence of viable tumour material or inflammation and are being increasingly used clinically for this purpose. DWI also provides information on tumour cellularity through measurements of the apparent diffusion coefficient (ADC), which is expected to inversely correlate with cellularity for a progressing metastatic lesion171,172. In addition to PWI and DWI, magnetic resonance spectroscopy (MRS) is another technique currently in clinical use. MRS enables the measurement of biochemical alterations in tumour tissue and can be used to deduce treatment response based on metabolic alterations in brain metastases. Specifically in brain metastases, MRS has been shown to effectively distinguish benign from malignant tissues and treatment-induced changes in tumour metabolites. These metabolic alterations (involving choline, creatine and N-acetyl-aspartate) have been found to correlate with prognosis and disease recurrence after SRS in patients with NSCLC, and can therefore be used to predict clinical outcomes173,174.
Several novel imaging techniques have also been developed but are currently not widely implemented clinically, including those involving PET175, single-photon emission computed tomography (SPECT) and chemical exchange saturation transfer (CEST). 18F-Fluorodeoxyglucose (FDG)–PET is not routinely used to evaluate brain metastases owing to high levels of background brain metabolic activity; however, delayed FDG-PET has demonstrated some potential in differentiating between disease progression and treatment effects176. Furthermore, various PET imaging modalities involving alternative tracers, such as radiolabelled nucleotides or amino acids, are being developed specifically for brain tumour imaging. For example, 18F-flourothymidine (FLT) provides a moderately specific method of detecting cellular proliferation as thymidine does not typically accumulate to high levels in brain cells or in regions of inflammation; however, FLT requires BBB breakdown in order to penetrate the CNS, thus limiting its use in assessing treatment responses in patients with brain metastases. Another PET amino acid tracer, O-(2-[18F] fluoroethyl)-L-tyrosine (FET), enables the identification of treatment-related metabolic alterations earlier than changes in tumour diameter on MRI in patients with glioblastoma receiving bevacizumab177. Several natural or synthetic amino acid-based PET probes (including 18F-FET as well as 11C-MET, 8F-Fluciclovine and 18F-FDOPA) are gradually entering clinical use178,179 following recommendations from the RANO working group for use in patients with gliomas180.
Adapting these advanced imaging techniques into routine clinical practice comes with several challenges. Perfusion-weighted and diffusion-weighted MRI can be performed readily without the need for additional equipment at most comprehensive medical centres, although image processing sometimes requires specialized expertise and/or software. Non-FDG-PET is currently not available for clinical use and might require access to a cyclotron if using radioactive tracers that are not commercially available. Delayed PET can be time-intensive and costly without insurance approval. However, the potential advantages of using one imaging method (non-FDG-PET) to assess tumour status in both the intracranial and extracranial compartments are appealing to both patients and clinicians. Tumour-type or treatment-specific radiotracers, such as PET involving a radiolabelled DLL3 tracer in patients with small-cell lung cancer181 and a radiolabelled PD-L1 tracer for patients who might be eligible for anti-PD-1 or anti-PD-L1 antibodies182, are currently under preclinical investigation.
Molecular profiling
A deeper understanding of the molecular underpinnings of CNS metastases and the extent of genomic concordance between primary tumours and their associated CNS metastases will enabled improved guidance of treatment selection, both at initial diagnosis and at the time of acquired resistance. This area of research will be greatly facilitated by the profiling of cell-free DNA (cfDNA) from CSF samples, which is a much more sensitive method than CSF cytology183. Furthermore, CSF-derived cfDNA has demonstrated higher sensitivity compared to plasma-derive cfDNA, particularly in brain metastases and primary brain tumors, presumably owing to the relative volumes of these compartments184. Studies involving patients with LMD from EGFR-mutant NSCLC have revealed a high level of concordance of EGFR mutations, as well as TP53, CDKN2A and PIK3CA aberrations, between the primary tumour and cfDNA from the CSF, albeit with notable differences in alterations in genes associated with cell cycle regulation and the DNA damage response185. After disease progression on osimertinib, EGFRC797S, MET dysregulation and the co-ocurrence of alterations in TP53 plus RB1 were reported as potential mechanisms of resistance detectable in the CSF cfDNA185,186. Similarly high levels of concordance between ALK rearrangements and TP53 mutations have been reported, although different secondary ALK mutations were observed in CSF samples in a patient who received brigatinib, suggesting the occurrence of parallel clonal evolution in the CNS and systemic compartments187. However, data on shared and distinct mutations between CNS metastases and the primary tumour are limited and will require validation in larger-cohort studies.
In addition to genetic profiling, analysis of cfDNA in CSF samples can also enable more accurate disease monitoring188. A prospective study involving 28 patients with EGFR-mutant NSCLC and LMD demonstrated a 100% sensitivity of cfDNA profiling in detecting driver genes in CSF samples, as well as the ability to capture multiple copy number variations (CNVs), concomitant resistance mutations and loss of heterozygosity more frequently than the equivalent analysis of plasma samples188. Another study demonstrated an association between >50% reduction in CSF cfDNA after treatment and longer median intracranial PFS, while shorter intracranial PFS was associated with a higher residual burden of clonal mutations in CSF cfDNA189. These studies have laid the foundations for future use of dynamic assessments of CSF cfDNA to monitor intracranial responses and CNS clonal evolution during treatment188,189.
Obtaining CSF samples for cfDNA analysis is relatively non-invasive compared with tumour biopsy sampling and thus enables longitudinal assessments. Nonetheless, these methods should not replace tissue profiling, especially for characterization of the tumour immune microenvironment (TIME). In one study, tumour PD-L1 levels were comparable between the primary tumour and brain metastases, although the latter had substantially lower levels of all T cell subsets relative to the matched primary tumour190, highlighting the need to evaluate tissue samples from brain metastases to gain insights into the TIME. Window-of-opportunity trials have the potential to add value in this area, as these trials typically involve short-term treatment exposures followed by surgical resection. The resected post-treatment specimens can provide an extremely valuable resource that can advances our knowledge of treatment-induced histological and molecular alterations, especially when paired with initial pretreatment biopsy samples. In one such trial in which patients with recurrent glioblastoma received pembrolizumab followed by surgical resection, comparisons of pretreatment and post-treatment specimens indicated a lack of response, characterized by a macrophage-dominated TIME with limited effector T cell infiltration, in most patients191. Patients enrolled in this trial had limited levels of clinical benefit (3 partial responses in 15 patients), although molecular profiling of the TIME of those without a response provides clear directions for future research efforts towards overcoming macrophage-related immunosuppression in this setting.
A small subset of patients with brain-metastatic NSCLC will receive upfront palliative surgical resection of one or more dominant CNS lesions. For this population, offering a highly CNS-penetrant TKI in the context of a well-designed window-of-opportunity trial as systemic maintenance therapy should be considered. An ongoing window-of-opportunity trial (NCT05120960) is evaluating tepotinib in patients with MET-altered brain tumours, including patients with brain metastases from EGFR-mutant NSCLCs with acquired MET amplifications after disease progression on EGFR TKIs.
Targeted therapy and radiotherapy
An increasing number of novel targeted therapies have demonstrated robust intracranial activity; however, an emerging question exists regarding how best to incorporate targeted therapies into the current standard-of-care (comprising radiotherapy and surgery) for patients with brain metastases. This consideration is particularly important given that the approach to radiotherapy has moved away from WBRT owing to the substantial incidence of cognitive toxicities and limited OS benefit, as well as advances in more-focussed radiotherapy techniques. SRS has been increasingly used for patients with up to 15 lesions192, while multi-fraction stereotactic body radiation therapy (SBRT) enables the targeted irradiation of larger brain metastases or lesions located close to crucial structures such as the brainstem or optic chiasm. In patients receiving first-generation EGFR or ALK TKIs (gefitinib, erlotinib or crizotinib), upfront radiotherapy in combination with a TKI demonstrated survival advantages compared to a TKI only193. However, the improved CNS activity of newer-generation TKIs, with intracranial ORRs >70–80% observed with certain agents, raises questions as to whether upfront radiotherapy can be delayed or even omitted in this new era194. At least two randomized trials are recruiting patients in an attempt to examine whether upfront SRS (NCT03497767) or early SRS (NCT05033691) is superior to osimertinib without radiotherapy in patients with newly diagnosed brain metastatic EGFR-mutant NSCLC. Evidence in these studies will be derived from patients with EGFR-mutant NSCLC, although the principal findings will likely inform the treatment approach for other molecular subtypes with highly CNS-penetrant TKIs available.
Before data from such trials become available, we recommend that the treatment approach for each patient is discussed in a multidisciplinary fashion including clinicians specializing in medical oncology, CNS radiation oncology and neurosurgery. In this regard, ASCO/SNO/ASTRO jointly published guidelines for the treatment of patients with brain metastases in 2022195, and these recommendations should provide a solid multidisciplinary foundation for the individualized treatment of each patient. Currently, early intervention with local therapy (radiotherapy and/or surgery) is generally recommended for patients with symptomatic or rapidly progressing CNS metastases. For those with asymptomatic CNS metastases from NSCLC, the overall recommendation is that local therapy should not be deferred, with a few exceptions when a highly effective CNS-penetrant TKI is available, such as osimertinib for EGFR-mutant NSCLC and lorlatinib for ALK-rearranged NSCLC. Consensus was not reached on the role of immunotherapy.
Treatment decisions become even more complex upon disease progression. For patients with CNS-only disease progression on a CNS-penetrant TKI, local therapy with radiotherapy and continuation of the TKI is often recommended. For those with extracranial-only progression in the context of continued control of CNS disease, many medical oncologists would continue the TKI to ensure CNS protection, while adding chemotherapy for systemic disease control. This strategy has demonstrated a favorable safety profile (with most frequent G3 adverse event being neutropenia, 11%), while delaying intracranial disease progression in 28 of 37 (75.7%) patients with brain metastases196. For progression in both the CNS and extracranial compartments, a change of systemic therapy while leveraging radiotherapy for CNS control is a reasonable approach given that the tumour cells will most likely have acquired one or more new mechanisms of resistance. Owing to the rapid pace of development of CNS-penetrant TKIs, evidence informing the optimal sequences and combinations of multimodality treatment is still accumulating; therefore, treatment-related decisions should be made in a multidisciplinary fashion that also takes into account other clinical characteristics and the availability of CNS-penetrant TKIs.
Conclusions
In summary, CNS metastases have always been recognized as a devastating complication of advanced-stage solid tumours. Over the past decade, and despite the rapid development of numerous novel therapeutics that provide improved extracranial tumour control, CNS metastases, including LMD, are emerging as an increasing unmet need that can limit long-term survival. CNS metastases are considered a distinct challenge because the tumour cells reside within a physiologically unique compartment protected by the BBB, and thus demand a different treatment strategy. For patients with oncogene-driven NSCLC, a wave of novel TKIs with excellent brain penetrance has already delivered clinical successes in subsets of patients with tumours harbouring certain driver oncogenes. At the same time, advances in radiotherapy technology and surgical techniques are continuing to improve outcomes, while making the management of CNS metastases highly complex and multidisciplinary in nature, requiring close collaboration. Novel diagnostic and intrathecal therapeutic technologies are also expanding the potential to treat patients with CNS metastases and LMD. With the development of improved comprehensive outcome measurement criteria and the availability of regulatory recommendations guiding drug development towards the unmet needs of patients with CNS metastases, we anticipate strong growth in clinical investigations over the next few years in this area, ultimately leading to improved clinical outcomes for patients.
Supplementary Material
Key points.
CNS metastasis and leptomeningeal disease are clinical challenges in the treatment of patients with advanced-stage NSCLC that are often associated with inferior outcomes [Au: Details added, OK?]
Targeted therapies have improved the outcomes of several molecularly defined subgroups of patients with oncogene-driven NSCLC, although certain small-molecule inhibitors confer only limited levels of CNS benefit, often owing to an inability to cross the blood–brain barrier [Au: Details added here also, OK?].
Preclinical evaluations of Kpuu are commonly used to assess the CNS penetrance of novel agents during drug development
Several newer TKIs having improved CNS efficacy, including osimertinib in EGFR-mutant NSCLC and alectinib and lorlatinib in ALK-rearranged NSCLC, with many others in clinical development
Response Assessment in Neuro-Oncology (RANO) criteria for response assessment in patients with brain or leptomeningeal metastases are different to RECIST; and future clinical trials should incorporate those criteria into the study design
BOX 1. Summary of FDA recommendations for evaluating cancer drugs in patients with CNS metastases.
Patient population
CNS response rates should be evaluated in the context of the entire disease burden, regardless of the incidence of CNS metastases in the trial population.
CNS-specific approvals might not be appropriate.
Available therapy
Therapies with existing approvals for patients with metastatic disease from a given primary tumour type (such as ALK inhibitors in ALK-rearranged NSCLC) will be prioritized.
Prior therapies
Attempt to collect information on previous interventions, such as any CNS-directed surgery, SRS, and WBRT, including timing and response rates
Specify the permitted interval length between completion of CNS-directed therapy and study enrolment (typically at least 12 weeks).
Include one additional stratification factor to minimize bias (such as treated versus untreated CNS metastases).
Assessments of CNS metastases
Use RECIST or RANO-BM assesment criteria with gadolinium MRI as the preferred imaging modality, including a baseline scan.
CNS and extra-CNS disease evaluation should occur simultaneously.
Pre-specify which previously irradiated lesions are included as target lesions across all trial sites, and their time from irradiation.
Attempt to capture data at baseline and during the study on variables potentially affecting the interpretation of radiographic images including neurological symptoms, steroid use and use of anti-seizure medications.
Study end points
Time-to-event and all-cause mortality are preferred end points.
Evaluate the entire burden of disease as end points (including ORR or PFS, and not only CNS-specific forms of these end points), with assessments verified by independent, blinded review by experts in CNS radiology.
Specify CNS PFS versus systemic PFS progression to prevent censoring.
Leptomeningeal disease
LMD should be confirmed and monitored using MRI and/or CSF analysis for response evaluation.
Additional Funding information:
Xiuning Le is supported by Damon Runyon Foundation and V Foundation for Cancer Research. John Heymach is supported by NIH R50CA265307, 5R01CA247975, R01CA234183
Kyle Concannon is supported by NIH T32 CA009666.
Jianjun Zhang is supported by Cancer Prevention and Research Institute of Texas Multi-Investigator Research Award grant (RP160668), the National Cancer Institute of the National Institute of Health Research Project Grant (R01CA234629-01 and 5U01CA256780).
Competing interests
JZ declares personal fees from BMS, AZ, Novartis, Johnson and Johnson, GenePlus, Innovent, Research grants from Merck, Novartis, Johnson and Johnson. JVH declares stock and Other Ownership Interests: Cardinal Spine, Bio-Tree Consulting or Advisory Role: AstraZeneca, Bristol Myers Squibb, Spectrum Pharmaceuticals, Guardant Health, Hengrui Pharmaceutical, GlaxoSmithKline, EMD Serono, Lilly, Takeda, Sanofi/Aventis, Genentech/Roche, Boehringer Ingelheim, Catalyst Biotech, Foundation medicine, Novartis, Mirati Therapeutics, BrightPath Biotheraputics, Janssen, Nexus Health Systems, Pneuma Respiratory, Kairos Ventures, Roche, Leads Biolabs Research Funding: AstraZeneca (Inst), Spectrum Pharmaceuticals, GlaxoSmithKline Patents, Royalties, Other Intellectual Property: Licensing agreement between Spectrum and MD Anderson (including myself) regarding intellectual property for treatment of EGFR and HER2 exon 20 mutations. XL declares consulting/advisory fees from EMD Serono (Merck KGaA), AstraZeneca, Spectrum Pharmaceutics, Novartis, Eli Lilly, Boehringer Ingelheim, Hengrui Therapeutics, Janssen, Blueprint Medicines, Sensei Biotherapeutics, and Abbvie Research Funding from Eli Lilly, EMD Serono, Regeneron, and Boehringer Ingelheim.
Glossary
- CSF
cerebrospinal fluid
- LMD
leptomeningeal disease
- NSCLC
non-small-cell lung cancer
- ORR
objective response rate
- PFS
progression-free survival
- RANO-BM
Response Assessment in Neuro-Oncology – Brain Metastases
- RECIST
Response Evaluation Criteria in Solid Tumors
- SRS
stereotactic radiosurgery
- WBRT
whole-brain radiotherapy
References
- 1.Barnholtz-Sloan JS et al. Incidence proportions of brain metastases in patients diagnosed (1973 to 2001) in the Metropolitan Detroit Cancer Surveillance System. J Clin Oncol 22, 2865–72 (2004). [DOI] [PubMed] [Google Scholar]
- 2.Siegel RL, Miller KD & Jemal A Cancer statistics, 2019. CA Cancer J Clin 69, 7–34 (2019). [DOI] [PubMed] [Google Scholar]
- 3.D’Antonio C et al. Bone and brain metastasis in lung cancer: recent advances in therapeutic strategies. Ther Adv Med Oncol 6, 101–114 (2014). [DOI] [PMC free article] [PubMed] [Google Scholar]
- 4.Sorensen JB, Hansen HH, Hansen M & Dombernowsky P Brain metastases in adenocarcinoma of the lung: frequency, risk groups, and prognosis. 10.1200/JCO.1988.6.9.1474 6, 1474–1480 (2016). [DOI] [PubMed] [Google Scholar]
- 5.Rangachari D et al. Brain metastases in patients with EGFR-mutated or ALK-rearranged non-small-cell lung cancers. Lung Cancer 88, 108–11 (2015). [DOI] [PMC free article] [PubMed] [Google Scholar]
- 6.Coelho JC et al. Non–Small-Cell Lung Cancer With CNS Metastasis: Disparities From a Real-World Analysis (GBOT-LACOG 0417). JCO Glob Oncol (2022) doi: 10.1200/GO.21.00333. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 7.Sperduto PW et al. Estimating Survival in Patients With Lung Cancer and Brain Metastases: An Update of the Graded Prognostic Assessment for Lung Cancer Using Molecular Markers (Lung-molGPA). JAMA Oncol 3, 827–831 (2017). [DOI] [PMC free article] [PubMed] [Google Scholar]
- 8.Sperduto PW et al. Graded Prognostic Assessment (GPA) for Patients With Lung Cancer and Brain Metastases: Initial Report of the Small Cell Lung Cancer GPA and Update of the Non-Small Cell Lung Cancer GPA Including the Effect of Programmed Death Ligand 1 and Other Prognostic Factors. International Journal of Radiation Oncology*Biology*Physics 114, 60–74 (2022). [DOI] [PMC free article] [PubMed] [Google Scholar]
- 9.Zimmermann S, Dziadziuszko R & Peters S Indications and limitations of chemotherapy and targeted agents in non-small cell lung cancer brain metastases. Cancer Treat Rev 40, 716–722 (2014). [DOI] [PubMed] [Google Scholar]
- 10.Besse B et al. Bevacizumab in Patients with Nonsquamous Non–Small Cell Lung Cancer and Asymptomatic, Untreated Brain Metastases (BRAIN): A Nonrandomized, Phase II Study. Clinical Cancer Research 21, 1896–1903 (2015). [DOI] [PubMed] [Google Scholar]
- 11.Tawbi HA et al. Combined Nivolumab and Ipilimumab in Melanoma Metastatic to the Brain. New England Journal of Medicine 379, 722–730 (2018). [DOI] [PMC free article] [PubMed] [Google Scholar]
- 12.Li J et al. Concurrent nivolumab and ipilimumab with brain stereotactic radiosurgery for brain metastases from non-small cell lung cancer: A phase I trial. Journal of Clinical Oncology 38, 2531–2531 (2020). [Google Scholar]
- 13.Goldberg SB et al. Pembrolizumab for management of patients with NSCLC and brain metastases: long-term results and biomarker analysis from a non-randomised, open-label, phase 2 trial. Lancet Oncol 21, 655–663 (2020). [DOI] [PMC free article] [PubMed] [Google Scholar]
- 14.Hou X et al. Efficacy, Safety, and Health-Related Quality of Life With Camrelizumab Plus Pemetrexed and Carboplatin as First-Line Treatment for Advanced Nonsquamous NSCLC With Brain Metastases (CAP-BRAIN): A Multicenter, Open-Label, Single-Arm, Phase 2 Study. Journal of Thoracic Oncology (2023) doi: 10.1016/j.jtho.2023.01.083. [DOI] [PubMed] [Google Scholar]
- 15.Non-Small Cell Lung Cancer. National Comprehensive Cancer Network (NCCN) (2023).
- 16.Heon S et al. The impact of initial gefitinib or erlotinib versus chemotherapy on central nervous system progression in advanced non-small cell lung cancer with EGFR mutations. Clin Cancer Res 18, 4406–14 (2012). [DOI] [PMC free article] [PubMed] [Google Scholar]
- 17.Johung KL et al. Extended Survival and Prognostic Factors for Patients With ALK-Rearranged Non-Small-Cell Lung Cancer and Brain Metastasis. J Clin Oncol 34, 123–9 (2016). [DOI] [PMC free article] [PubMed] [Google Scholar]
- 18.Sperduto PW et al. The Effect of Gene Alterations and Tyrosine Kinase Inhibition on Survival and Cause of Death in Patients With Adenocarcinoma of the Lung and Brain Metastases. Int J Radiat Oncol Biol Phys 96, 406–413 (2016). [DOI] [PMC free article] [PubMed] [Google Scholar]
- 19.Bohn J-P, Pall G, Stockhammer G & Steurer M Targeted Therapies for the Treatment of Brain Metastases in Solid Tumors. Target Oncol 11, 263–75 (2016). [DOI] [PubMed] [Google Scholar]
- 20.Welsh JW et al. Phase II Trial of Erlotinib Plus Concurrent Whole-Brain Radiation Therapy for Patients With Brain Metastases From Non–Small-Cell Lung Cancer. Journal of Clinical Oncology 31, 895–902 (2013). [DOI] [PMC free article] [PubMed] [Google Scholar]
- 21.Reungwetwattana T et al. CNS Response to Osimertinib Versus Standard Epidermal Growth Factor Receptor Tyrosine Kinase Inhibitors in Patients With Untreated EGFR-Mutated Advanced Non–Small-Cell Lung Cancer. 10.1200/JCO.2018.78.3118 36, 3290–3297 (2018). [DOI] [PubMed] [Google Scholar]
- 22.Shaw AT et al. Lorlatinib in non-small-cell lung cancer with ALK or ROS1 rearrangement: an international, multicentre, open-label, single-arm first-in-man phase 1 trial. Lancet Oncol 18, 1590–1599 (2017). [DOI] [PMC free article] [PubMed] [Google Scholar]
- 23.Sweeney MD, Zhao Z, Montagne A, Nelson AR & Zlokovic BV Blood-Brain Barrier: From Physiology to Disease and Back. Physiol Rev 99, 21–78 (2019). [DOI] [PMC free article] [PubMed] [Google Scholar]
- 24.Mo F, Pellerino A, Soffietti R & Rudà R Blood-Brain Barrier in Brain Tumors: Biology and Clinical Relevance. Int J Mol Sci 22, (2021). [DOI] [PMC free article] [PubMed] [Google Scholar]
- 25.Steeg PS The blood–tumour barrier in cancer biology and therapy. Nat Rev Clin Oncol 18, 696–714 (2021). [DOI] [PubMed] [Google Scholar]
- 26.Lockman PR et al. Heterogeneous Blood–Tumor Barrier Permeability Determines Drug Efficacy in Experimental Brain Metastases of Breast Cancer. Clinical Cancer Research 16, 5664–5678 (2010). [DOI] [PMC free article] [PubMed] [Google Scholar]
- 27.Gril B et al. HER2 antibody-drug conjugate controls growth of breast cancer brain metastases in hematogenous xenograft models, with heterogeneous blood–tumor barrier penetration unlinked to a passive marker. Neuro Oncol 22, 1625–1636 (2020). [DOI] [PMC free article] [PubMed] [Google Scholar]
- 28.Ahn HK et al. ALK inhibitor crizotinib combined with intrathecal methotrexate treatment for non-small cell lung cancer with leptomeningeal carcinomatosis. Lung Cancer 76, 253–254 (2012). [DOI] [PubMed] [Google Scholar]
- 29.Angeli E & Bousquet G Brain Metastasis Treatment: The Place of Tyrosine Kinase Inhibitors and How to Facilitate Their Diffusion across the Blood-Brain Barrier. Pharmaceutics 13, (2021). [DOI] [PMC free article] [PubMed] [Google Scholar]
- 30.Sánchez-dengra B, Gonzalez-alvarez I, Bermejo M & Gonzalez-alvarez M Physiologically based pharmacokinetic (Pbpk) modeling for predicting brain levels of drug in rat. Pharmaceutics 13, (2021). [DOI] [PMC free article] [PubMed] [Google Scholar]
- 31.Singh Badhan RK, Chenel M & Penny JI Development of a Physiologically-Based Pharmacokinetic Model of the Rat Central Nervous System. Pharmaceutics 6, 97 (2014). [DOI] [PMC free article] [PubMed] [Google Scholar]
- 32.De Lange ECM Utility of CSF in translational neuroscience. J Pharmacokinet Pharmacodyn 40, 315 (2013). [DOI] [PMC free article] [PubMed] [Google Scholar]
- 33.Varadharajan S et al. Exploring In Silico Prediction of the Unbound Brain-to-Plasma Drug Concentration Ratio: Model Validation, Renewal, and Interpretation. J Pharm Sci 104, 1197–1206 (2015). [DOI] [PubMed] [Google Scholar]
- 34.Wilcox JA & Boire AA Leveraging Molecular and Immune-Based Therapies in Leptomeningeal Metastases. CNS Drugs 37, 45–67 (2023). [DOI] [PMC free article] [PubMed] [Google Scholar]
- 35.Li BT et al. Trastuzumab Deruxtecan in HER2 -Mutant Non–Small-Cell Lung Cancer. New England Journal of Medicine 386, 241–251 (2022). [DOI] [PMC free article] [PubMed] [Google Scholar]
- 36.Bartsch R et al. Trastuzumab deruxtecan in HER2-positive breast cancer with brain metastases: a single-arm, phase 2 trial. Nat Med 28, 1840–1847 (2022). [DOI] [PMC free article] [PubMed] [Google Scholar]
- 37.Kabraji S et al. Preclinical and Clinical Efficacy of Trastuzumab Deruxtecan in Breast Cancer Brain Metastases. Clinical Cancer Research 29, 174–182 (2023). [DOI] [PMC free article] [PubMed] [Google Scholar]
- 38.Rangachari D et al. Brain metastases in patients with EGFR-mutated or ALK-rearranged non-small-cell lung cancers. Lung Cancer 88, 108–11 (2015). [DOI] [PMC free article] [PubMed] [Google Scholar]
- 39.Doebele RC et al. Oncogene status predicts patterns of metastatic spread in treatment-naive nonsmall cell lung cancer. Cancer 118, 4502–11 (2012). [DOI] [PMC free article] [PubMed] [Google Scholar]
- 40.Owen S & Souhami L The management of brain metastases in non-small cell lung cancer. Front Oncol 4, 248 (2014). [DOI] [PMC free article] [PubMed] [Google Scholar]
- 41.de Vries NA et al. Restricted brain penetration of the tyrosine kinase inhibitor erlotinib due to the drug transporters P-gp and BCRP. Invest New Drugs 30, 443–9 (2012). [DOI] [PubMed] [Google Scholar]
- 42.Luo S, Chen L, Chen X & Xie X Evaluation on efficacy and safety of tyrosine kinase inhibitors plus radiotherapy in NSCLC patients with brain metastases. Oncotarget 6, 16725–16734 (2015). [DOI] [PMC free article] [PubMed] [Google Scholar]
- 43.Grommes C et al. ‘Pulsatile’ high-dose weekly erlotinib for CNS metastases from EGFR mutant non-small cell lung cancer. Neuro Oncol 13, 1364–1369 (2011). [DOI] [PMC free article] [PubMed] [Google Scholar]
- 44.Arbour KC et al. Twice weekly pulse and daily continuous-dose erlotinib as initial treatment for patients with epidermal growth factor receptor-mutant lung cancers and brain metastases. Cancer 124, 105–109 (2018). [DOI] [PMC free article] [PubMed] [Google Scholar]
- 45.Yu HA et al. Phase 1 study of twice weekly pulse dose and daily low-dose erlotinib as initial treatment for patients with EGFR-mutant lung cancers. Annals of Oncology 28, 278–284 (2017). [DOI] [PMC free article] [PubMed] [Google Scholar]
- 46.Ballard P et al. Preclinical Comparison of Osimertinib with Other EGFR-TKIs in EGFR-Mutant NSCLC Brain Metastases Models, and Early Evidence of Clinical Brain Metastases Activity. Clin Cancer Res 22, 5130–5140 (2016). [DOI] [PubMed] [Google Scholar]
- 47.Varadharajan S et al. Exploring in silico prediction of the unbound brain-to-plasma drug concentration ratio: model validation, renewal, and interpretation. J Pharm Sci 104, 1197–206 (2015). [DOI] [PubMed] [Google Scholar]
- 48.Colclough N et al. Preclinical Comparison of the Blood-brain barrier Permeability of Osimertinib with Other EGFR TKIs. Clin Cancer Res 27, 189–201 (2021). [DOI] [PubMed] [Google Scholar]
- 49.Wu Y-L et al. CNS Efficacy of Osimertinib in Patients With T790M-Positive Advanced Non-Small-Cell Lung Cancer: Data From a Randomized Phase III Trial (AURA3). J Clin Oncol 36, 2702–2709 (2018). [DOI] [PubMed] [Google Scholar]
- 50.Yang JCH et al. Osimertinib in Patients With Epidermal Growth Factor Receptor Mutation-Positive Non-Small-Cell Lung Cancer and Leptomeningeal Metastases: The BLOOM Study. J Clin Oncol 38, 538–547 (2020). [DOI] [PMC free article] [PubMed] [Google Scholar]
- 51.Zeng Q et al. Discovery and Evaluation of Clinical Candidate AZD3759, a Potent, Oral Active, Central Nervous System-Penetrant, Epidermal Growth Factor Receptor Tyrosine Kinase Inhibitor. (2015) doi: 10.1021/acs.jmedchem.5b01073. [DOI] [PubMed] [Google Scholar]
- 52.Yang Z et al. AZD3759, a BBB-penetrating EGFR inhibitor for the treatment of EGFR mutant NSCLC with CNS metastases. Sci Transl Med 8, (2016). [DOI] [PubMed] [Google Scholar]
- 53.Yang Z et al. AZD3759, a BBB-penetrating EGFR inhibitor for the treatment of EGFR mutant NSCLC with CNS metastases. Sci Transl Med 8, 368ra172 (2016). [DOI] [PubMed] [Google Scholar]
- 54.Li X et al. Enhanced efficacy of AZD3759 and radiation on brain metastasis from EGFR mutant non-small cell lung cancer. Int J Cancer 143, 212–224 (2018). [DOI] [PubMed] [Google Scholar]
- 55.Ahn M-J et al. Phase I study of AZD3759, a CNS penetrable EGFR inhibitor, for the treatment of non-small-cell lung cancer (NSCLC) with brain metastasis (BM) and leptomeningeal metastasis (LM). Journal of Clinical Oncology 34, 9003 (2016). [Google Scholar]
- 56.Wu Y-L et al. Randomized phase 3 study of first-line AZD3759 (zorifertinib) versus gefitinib or erlotinib in EGFR-mutant (EGFR m +) non–small-cell lung cancer (NSCLC) with central nervous system (CNS) metastasis. Journal of Clinical Oncology 41, 9001–9001 (2023). [Google Scholar]
- 57.Conti C et al. BLU-701 is a highly potent, brain-penetrant and WT-sparing next-generation EGFR TKI for the treatment of sensitizing (ex19del, L858R) and C797S resistance mutations. in (Blueprint Medicines Corporation, 2021). [Google Scholar]
- 58.Zapata A, Chefer VI & Shippenberg TS Microdialysis in rodents. Curr Protoc Neurosci Chapter 7, Unit7.2 (2009). [DOI] [PMC free article] [PubMed] [Google Scholar]
- 59.BDTX-1535 Goes after Osimertinib Resistance. Cancer Discov 11, 2952–2953 (2021). [DOI] [PubMed] [Google Scholar]
- 60.Lucas MC et al. 27MO BDTX-1535, a CNS penetrant, irreversible inhibitor of intrinsic and acquired resistance EGFR mutations, demonstrates preclinical efficacy in NSCLC and GBM PDX models. Annals of Oncology 33, S14 (2022). [Google Scholar]
- 61.Study of BDTX-1535, in Participants With Glioblastoma or Non-Small Cell Lung Cancer - Full Text View - ClinicalTrials.gov. https://clinicaltrials.gov/ct2/show/NCT05256290.
- 62.Remon J, Hendriks LEL, Cardona AF & Besse B EGFR exon 20 insertions in advanced non-small cell lung cancer: A new history begins. Cancer Treat Rev 90, 102105 (2020). [DOI] [PubMed] [Google Scholar]
- 63.Leal JL et al. EGFR Exon 20 Insertion Mutations: Clinicopathological Characteristics and Treatment Outcomes in Advanced Non–Small Cell Lung Cancer. Clin Lung Cancer 22, e859–e869 (2021). [DOI] [PubMed] [Google Scholar]
- 64.Syed YY Amivantamab: First Approval. Drugs 81, 1349–1353 (2021). [DOI] [PubMed] [Google Scholar]
- 65.Zhou C et al. Treatment Outcomes and Safety of Mobocertinib in Platinum-Pretreated Patients With EGFR Exon 20 Insertion–Positive Metastatic Non–Small Cell Lung Cancer. JAMA Oncol 7, e214761 (2021). [DOI] [PMC free article] [PubMed] [Google Scholar]
- 66.Yun J et al. Antitumor activity of amivantamab (Jnj-61186372), an egfr–met bispecific antibody, in diverse models of egfr exon 20 insertion–driven nsclc. Cancer Discov 10, 1194–1209 (2020). [DOI] [PubMed] [Google Scholar]
- 67.Park K et al. Amivantamab in egfr exon 20 insertion- mutated non-small-cell lung cancer progressing on platinum chemotherapy: Initial results from the chrysalis phase i study. Journal of Clinical Oncology 39, 3391–3402 (2021). [DOI] [PMC free article] [PubMed] [Google Scholar]
- 68.Riely GJ et al. Activity and Safety of Mobocertinib (TAK-788) in Previously Treated Non-Small Cell Lung Cancer with EGFR Exon 20 Insertion Mutations from a Phase I/II Trial. Cancer Discov 11, 1688–1699 (2021). [DOI] [PMC free article] [PubMed] [Google Scholar]
- 69.Pearson PG et al. Abstract 3261: LNG-451, a potent inhibitor of EGFR exon 20 insertion mutations with high CNS exposure. Cancer Res 82, 3261–3261 (2022). [Google Scholar]
- 70.Study of BLU-451 in Advanced Cancers With EGFR Exon 20 Insertion Mutations - Full Text View - ClinicalTrials.gov. https://clinicaltrials.gov/ct2/show/NCT05241873.
- 71.Wang M, hu YFMSYWJFYZBJY, Z. H. X. S. A. L. K. T. C. D. L. L. L. W. J. G. J. W. J. C. Y. P. A. J. L. Z. 987P - Sunvozertinib for NSCLC patients with EGFR exon 20 insertion mutations: Preliminary analysis of WU-KONG6, the first pivotal study. in ESMO Congress S448–S554 (Annals of Oncology, 2022). [Google Scholar]
- 72.Junttila MR et al. Abstract 1466: ORIC-114, a brain penetrant, orally bioavailable, irreversible inhibitor selectively targets EGFR and HER2 exon20 insertion mutants and regresses intracranial NSCLC xenograft tumors. Cancer Res 81, 1466–1466 (2021). [Google Scholar]
- 73.Bartsch R et al. Trastuzumab deruxtecan in HER2-positive breast cancer with brain metastases: a single-arm, phase 2 trial. Nat Med 28, 1840–1847 (2022). [DOI] [PMC free article] [PubMed] [Google Scholar]
- 74.Cornelissen R et al. Poziotinib in Treatment-Naive NSCLC Harboring HER2 Exon 20 Mutations: ZENITH20–4, A Multicenter, Multicohort, Open-Label, Phase 2 Trial (Cohort 4). J Thorac Oncol (2023) doi: 10.1016/j.jtho.2023.03.016. [DOI] [PubMed] [Google Scholar]
- 75.Koivunen JP et al. EML4-ALK fusion gene and efficacy of an ALK kinase inhibitor in lung cancer. Clin Cancer Res 14, 4275–83 (2008). [DOI] [PMC free article] [PubMed] [Google Scholar]
- 76.Costa DB et al. Clinical Experience With Crizotinib in Patients With Advanced ALK-Rearranged Non-Small-Cell Lung Cancer and Brain Metastases. J Clin Oncol 33, 1881–8 (2015). [DOI] [PMC free article] [PubMed] [Google Scholar]
- 77.Costa DB et al. CSF Concentration of the Anaplastic Lymphoma Kinase Inhibitor Crizotinib. Journal of Clinical Oncology 29, e443–e445 (2011). [DOI] [PubMed] [Google Scholar]
- 78.Camidge DR et al. Brigatinib versus Crizotinib in ALK -Positive Non–Small-Cell Lung Cancer. New England Journal of Medicine 379, 2027–2039 (2018). [DOI] [PubMed] [Google Scholar]
- 79.Peters S et al. Alectinib versus Crizotinib in Untreated ALK -Positive Non–Small-Cell Lung Cancer. New England Journal of Medicine 377, 829–838 (2017). [DOI] [PubMed] [Google Scholar]
- 80.Shaw AT et al. First-Line Lorlatinib or Crizotinib in Advanced ALK -Positive Lung Cancer. New England Journal of Medicine 383, 2018–2029 (2020). [DOI] [PubMed] [Google Scholar]
- 81.Chow LQM et al. ASCEND-7: Efficacy and Safety of Ceritinib Treatment in Patients With ALK -Positive Non-Small Cell Lung Cancer Metastatic to the Brain and/or Leptomeninges. Clinical Cancer Research clincanres.1838.2021 (2022) doi: 10.1158/1078-0432.CCR-21-1838. [DOI] [PubMed] [Google Scholar]
- 82.Soria J-C et al. First-line ceritinib versus platinum-based chemotherapy in advanced ALK -rearranged non-small-cell lung cancer (ASCEND-4): a randomised, open-label, phase 3 study. The Lancet 389, 917–929 (2017). [DOI] [PubMed] [Google Scholar]
- 83.Kodama T, Tsukaguchi T, Yoshida M, Kondoh O & Sakamoto H Selective ALK inhibitor alectinib with potent antitumor activity in models of crizotinib resistance. Cancer Lett 351, 215–221 (2014). [DOI] [PubMed] [Google Scholar]
- 84.Kodama T et al. Antitumor activity of the selective ALK inhibitor alectinib in models of intracranial metastases. Cancer Chemother Pharmacol 74, 1023–1028 (2014). [DOI] [PubMed] [Google Scholar]
- 85.Gadgeel S et al. Alectinib versus crizotinib in treatment-naive anaplastic lymphoma kinase-positive (ALK+) non-small-cell lung cancer: CNS efficacy results from the ALEX study. Annals of Oncology 29, 2214–2222 (2018). [DOI] [PMC free article] [PubMed] [Google Scholar]
- 86.Mok T et al. Updated overall survival and final progression-free survival data for patients with treatment-naive advanced ALK-positive non-small-cell lung cancer in the ALEX study. Annals of Oncology 31, 1056–1064 (2020). [DOI] [PubMed] [Google Scholar]
- 87.Horn L et al. Ensartinib vs Crizotinib for Patients With Anaplastic Lymphoma Kinase−Positive Non–Small Cell Lung Cancer. JAMA Oncol 7, 1617 (2021). [DOI] [PMC free article] [PubMed] [Google Scholar]
- 88.Landi L & Cappuzzo F Achievements and future developments of ALK-TKIs in the management of CNS metastases from ALK-positive NSCLC. Transl Lung Cancer Res 5, 579–587 (2016). [DOI] [PMC free article] [PubMed] [Google Scholar]
- 89.Zou HY et al. PF-06463922 is a potent and selective next-generation ROS1/ALK inhibitor capable of blocking crizotinib-resistant ROS1 mutations. Proc Natl Acad Sci U S A 112, 3493–8 (2015). [DOI] [PMC free article] [PubMed] [Google Scholar]
- 90.Sun S, Pithavala YK, Martini J-F & Chen J Evaluation of Lorlatinib Cerebrospinal Fluid Concentrations in Relation to Target Concentrations for ALK Inhibition. J Clin Pharmacol (2022) doi: 10.1002/jcph.2056. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 91.Solomon BJ et al. Efficacy and safety of first-line lorlatinib versus crizotinib in patients with advanced, ALK-positive non-small-cell lung cancer: updated analysis of data from the phase 3, randomised, open-label CROWN study. Lancet Respir Med 11, 354–366 (2023). [DOI] [PubMed] [Google Scholar]
- 92.Dagogo-Jack I et al. Phase II Study of Lorlatinib in Patients With Anaplastic Lymphoma Kinase–Positive Lung Cancer and CNS-Specific Relapse. JCO Precis Oncol (2022) doi: 10.1200/PO.21.00522. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 93.Murray BW et al. TPX-0131, a Potent CNS-penetrant, Next-generation Inhibitor of Wild-type ALK and ALK-resistant Mutations. Mol Cancer Ther 20, 1499–1507 (2021). [DOI] [PMC free article] [PubMed] [Google Scholar]
- 94.Pelish HE et al. Abstract 1468: NUV-655 (NVL-655) is a selective, brain-penetrant ALK inhibitor with antitumor activity against the lorlatinib-resistant G1202R/L1196M compound mutation. Cancer Res 81, 1468–1468 (2021). [Google Scholar]
- 95.Ernani V & Stinchcombe TE Management of Brain Metastases in Non–Small-Cell Lung Cancer. J Oncol Pract 15, 563–570 (2019). [DOI] [PMC free article] [PubMed] [Google Scholar]
- 96.Ou S-HI & Zhu VW CNS metastasis in ROS1+ NSCLC: An urgent call to action, to understand, and to overcome. Lung Cancer 130, 201–207 (2019). [DOI] [PubMed] [Google Scholar]
- 97.Wu Y-L et al. Phase II Study of Crizotinib in East Asian Patients With ROS1-Positive Advanced Non–Small-Cell Lung Cancer. Journal of Clinical Oncology 36, 1405–1411 (2018). [DOI] [PubMed] [Google Scholar]
- 98.Azelby CM, Sakamoto MR & Bowles DW ROS1 Targeted Therapies: Current Status. Curr Oncol Rep 23, 94 (2021). [DOI] [PubMed] [Google Scholar]
- 99.Fischer H et al. Entrectinib, a TRK/ROS1 inhibitor with anti-CNS tumor activity: differentiation from other inhibitors in its class due to weak interaction with P-glycoprotein. Neuro Oncol 22, 819–829 (2020). [DOI] [PMC free article] [PubMed] [Google Scholar]
- 100.Frampton JE Entrectinib: A Review in NTRK+ Solid Tumours and ROS1+ NSCLC. Drugs 81, 697–708 (2021). [DOI] [PMC free article] [PubMed] [Google Scholar]
- 101.Menichincheri M et al. Discovery of Entrectinib: A New 3-Aminoindazole As a Potent Anaplastic Lymphoma Kinase (ALK), c-ros Oncogene 1 Kinase (ROS1), and Pan-Tropomyosin Receptor Kinases (Pan-TRKs) inhibitor. J Med Chem 59, 3392–3408 (2016). [DOI] [PubMed] [Google Scholar]
- 102.Frampton JE Entrectinib: A Review in NTRK+ Solid Tumours and ROS1+ NSCLC. Drugs 81, 697 (2021). [DOI] [PMC free article] [PubMed] [Google Scholar]
- 103.De Braud FGM et al. Entrectinib in locally advanced/metastatic ROS1 and NTRK fusion-positive non-small cell lung cancer (NSCLC): Updated integrated analysis of STARTRK-2, STARTRK-1 and ALKA-372–001. Annals of Oncology 30, v609 (2019). [Google Scholar]
- 104.Doebele RC et al. TRIDENT-1: A global, multicenter, open-label Phase II study investigating the activity of repotrectinib in advanced solid tumors harboring ROS1 or NTRK1–3 rearrangements. 10.1200/JCO.2020.38.15_suppl.TPS9637 38, TPS9637–TPS9637 (2020). [DOI] [Google Scholar]
- 105.Ou SHI et al. Efficacy of Taletrectinib (AB-106/DS-6051b) in ROS1+ NSCLC: An Updated Pooled Analysis of U.S. and Japan Phase 1 Studies. JTO Clin Res Rep 2, (2021). [DOI] [PMC free article] [PubMed] [Google Scholar]
- 106.Schneider JL et al. A Phase 2 Study of Lorlatinib in Patients With ROS1-Rearranged Lung Cancer With Brain-Only Progression on Crizotinib. JTO Clin Res Rep 3, 100347 (2022). [DOI] [PMC free article] [PubMed] [Google Scholar]
- 107.Cascetta P et al. RET Inhibitors in Non-Small-Cell Lung Cancer. Cancers (Basel) 13, 4415 (2021). [DOI] [PMC free article] [PubMed] [Google Scholar]
- 108.Gillespie CS et al. Genomic alterations and the incidence of brain metastases in advanced and metastatic non-small cell lung cancer: a systematic review and meta-analysis. Journal of Thoracic Oncology (2023) doi: 10.1016/j.jtho.2023.06.017. [DOI] [PubMed] [Google Scholar]
- 109.Drilon A et al. Frequency of Brain Metastases and Multikinase Inhibitor Outcomes in Patients With RET–Rearranged Lung Cancers. Journal of Thoracic Oncology 13, 1595–1601 (2018). [DOI] [PMC free article] [PubMed] [Google Scholar]
- 110.Drilon A et al. Selpercatinib in Patients With RET Fusion–Positive Non–Small-Cell Lung Cancer: Updated Safety and Efficacy From the Registrational LIBRETTO-001 Phase I/II Trial. Journal of Clinical Oncology 41, 385–394 (2023). [DOI] [PMC free article] [PubMed] [Google Scholar]
- 111.Murciano-Goroff YR et al. Central Nervous System Disease in Patients With RET Fusion-Positive NSCLC Treated With Selpercatinib. Journal of Thoracic Oncology 18, 620–627 (2023). [DOI] [PMC free article] [PubMed] [Google Scholar]
- 112.Gainor JF et al. Pralsetinib for RET fusion-positive non-small-cell lung cancer (ARROW): a multi-cohort, open-label, phase 1/2 study. Lancet Oncol 22, 959–969 (2021). [DOI] [PubMed] [Google Scholar]
- 113.Zhou Q et al. Efficacy and safety of pralsetinib in patients with advanced RET fusion-positive non–small cell lung cancer. Cancer (2023) doi: 10.1002/cncr.34897. [DOI] [PubMed] [Google Scholar]
- 114.Doebele RC et al. Entrectinib in patients with advanced or metastatic NTRK fusion-positive solid tumours: integrated analysis of three phase 1–2 trials. Lancet Oncol 21, 271–282 (2020). [DOI] [PMC free article] [PubMed] [Google Scholar]
- 115.Liu D et al. Characterization of on-target adverse events caused by TRK inhibitor therapy. Annals of Oncology 31, 1207–1215 (2020). [DOI] [PMC free article] [PubMed] [Google Scholar]
- 116.Drilon A et al. Efficacy and Safety of Larotrectinib in Patients With Tropomyosin Receptor Kinase Fusion–Positive Lung Cancers. JCO Precis Oncol 6, (2022). [DOI] [PMC free article] [PubMed] [Google Scholar]
- 117.Liu F et al. NTRK Fusion in Non-Small Cell Lung Cancer: Diagnosis, Therapy, and TRK Inhibitor Resistance. Front Oncol 12, (2022). [DOI] [PMC free article] [PubMed] [Google Scholar]
- 118.Nagasaka M et al. TRUST-II: A global phase II study for taletrectinib in ROS1 fusion–positive lung cancer and other solid tumors. Journal of Clinical Oncology 40, TPS8601–TPS8601 (2022). [Google Scholar]
- 119.Besse B et al. Abstract P02–01: Repotrectinib in patients with NTRK fusion-positive advanced solid tumors: update from the registrational phase 2 TRIDENT-1 trial. Mol Cancer Ther 20, P02–01–P02–01 (2021). [Google Scholar]
- 120.Negrao M. v. et al. Molecular landscape of BRAF-mutant NSCLC reveals an association between clonality and driver mutations and identifies targetable non-V600 driver mutations. J Thorac Oncol 15, 1611 (2020). [DOI] [PMC free article] [PubMed] [Google Scholar]
- 121.Leonetti A et al. BRAF in non-small cell lung cancer (NSCLC): Pickaxing another brick in the wall. Cancer Treat Rev 66, 82–94 (2018). [DOI] [PubMed] [Google Scholar]
- 122.Planchard D et al. Dabrafenib plus trametinib in patients with previously untreated BRAFV600E-mutant metastatic non-small-cell lung cancer: an open-label, phase 2 trial. Lancet Oncol 18, 1307–1316 (2017). [DOI] [PubMed] [Google Scholar]
- 123.Davies MA et al. Dabrafenib plus trametinib in patients with BRAFV600-mutant melanoma brain metastases (COMBI-MB): a multicentre, multicohort, open-label, phase 2 trial. Lancet Oncol 18, 863–873 (2017). [DOI] [PMC free article] [PubMed] [Google Scholar]
- 124.Mathieu LN et al. FDA Approval Summary: Capmatinib and Tepotinib for the Treatment of Metastatic NSCLC Harboring MET Exon 14 Skipping Mutations or Alterations. Clinical Cancer Research 28, 249–254 (2022). [DOI] [PubMed] [Google Scholar]
- 125.Paik PK et al. Tepotinib in Non-Small-Cell Lung Cancer with MET Exon 14 Skipping Mutations. N Engl J Med 383, 931 (2020). [DOI] [PMC free article] [PubMed] [Google Scholar]
- 126.Offin M et al. CNS Metastases in Patients With MET Exon 14–Altered Lung Cancers and Outcomes With Crizotinib. JCO Precis Oncol 4, 871–876 (2020). [DOI] [PMC free article] [PubMed] [Google Scholar]
- 127.Drusbosky LM, Dawar R, Rodriguez E & Ikpeazu C v. Therapeutic strategies in METex14 skipping mutated non-small cell lung cancer. J Hematol Oncol 14, 129 (2021). [DOI] [PMC free article] [PubMed] [Google Scholar]
- 128.Garon EB et al. Abstract CT082: Capmatinib in METex14-mutated (mut) advanced non-small cell lung cancer (NSCLC): Results from the phase II GEOMETRY mono-1 study, including efficacy in patients (pts) with brain metastases (BM). Cancer Res 80, CT082–CT082 (2020). [Google Scholar]
- 129.Viteri S et al. 1286P Activity of tepotinib in brain metastases (BM): Preclinical models and clinical data from patients (pts) with MET exon 14 (METex14) skipping NSCLC. Annals of Oncology 31, S831 (2020). [Google Scholar]
- 130.Paik PK et al. Tepotinib in Non-Small-Cell Lung Cancer with MET Exon 14 Skipping Mutations. N Engl J Med 383, 931 (2020). [DOI] [PMC free article] [PubMed] [Google Scholar]
- 131.Le X et al. Tepotinib Efficacy and Safety in Patients with MET Exon 14 Skipping NSCLC: Outcomes in Patient Subgroups from the VISION Study with Relevance for Clinical Practice. Clin Cancer Res 28, 1117–1126 (2022). [DOI] [PMC free article] [PubMed] [Google Scholar]
- 132.Hartmaier RJ et al. Osimertinib + Savolitinib to Overcome Acquired MET-Mediated Resistance in Epidermal Growth Factor Receptor–Mutated, MET -Amplified Non–Small Cell Lung Cancer: TATTON. Cancer Discov 13, 98–113 (2023). [DOI] [PMC free article] [PubMed] [Google Scholar]
- 133.Lee DH et al. ABN401 in patients with NSCLC with MET exon 14 (MET ex14) skipping: Result from the pilot expansion study. Journal of Clinical Oncology 41, e21148–e21148 (2023). [Google Scholar]
- 134.Judd J et al. Characterization of KRAS Mutation Subtypes in Non–small Cell Lung Cancer. Mol Cancer Ther 20, 2577–2584 (2021). [DOI] [PMC free article] [PubMed] [Google Scholar]
- 135.Reita D et al. Direct Targeting KRAS Mutation in Non-Small Cell Lung Cancer: Focus on Resistance. Cancers (Basel) 14, 1321 (2022). [DOI] [PMC free article] [PubMed] [Google Scholar]
- 136.Sabari JK et al. Activity of Adagrasib (MRTX849) in Brain Metastases: Preclinical Models and Clinical Data From Patients With KRASG12C-Mutant Non-Small Cell Lung CancerPreliminary Activity of Adagrasib in Brain Metastases. Clinical Cancer Research (2022) doi: 10.1158/1078-0432.CCR-22-0383. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 137.Canon J et al. The clinical KRAS(G12C) inhibitor AMG 510 drives anti-tumour immunity. Nature | 575, (2019). [DOI] [PubMed] [Google Scholar]
- 138.Skoulidis F et al. Sotorasib for Lung Cancers with KRAS p.G12C Mutation. New England Journal of Medicine 384, 2371–2381 (2021). [DOI] [PMC free article] [PubMed] [Google Scholar]
- 139.Ramalingam SS et al. Efficacy of sotorasib in KRAS p.G12C-mutated NSCLC with stable brain metastases: a post-hoc analysis of CodeBreaK100. doi: 10.1378/chest.12-2366. [DOI] [Google Scholar]
- 140.Sabari JK et al. Activity of Adagrasib (MRTX849) in Brain Metastases: Preclinical Models and Clinical Data from Patients with KRASG12C-Mutant Non–Small Cell Lung Cancer. Clinical Cancer Research 28, 3318–3328 (2022). [DOI] [PMC free article] [PubMed] [Google Scholar]
- 141.Negrao MV et al. Intracranial Efficacy of Adagrasib in Patients From the KRYSTAL-1 Trial With KRAS G12C –Mutated Non–Small-Cell Lung Cancer Who Have Untreated CNS Metastases. Journal of Clinical Oncology (2023) doi: 10.1200/JCO.23.00046. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 142.Sabari JK et al. Activity of adagrasib (MRTX849) in patients with KRAS G12C -mutated NSCLC and active, untreated CNS metastases in the KRYSTAL-1 trial. Journal of Clinical Oncology 40, LBA9009–LBA9009 (2022). [Google Scholar]
- 143.Yang JCH et al. Osimertinib in Patients With Epidermal Growth Factor Receptor Mutation–Positive Non–Small-Cell Lung Cancer and Leptomeningeal Metastases: The BLOOM Study. Journal of Clinical Oncology 38, 538 (2020). [DOI] [PMC free article] [PubMed] [Google Scholar]
- 144.Ahn M-J et al. Osimertinib for patients (pts) with leptomeningeal metastases (LM) associated with EGFRm advanced NSCLC: The AURA LM study. Annals of Oncology 30, ii48 (2019). [DOI] [PubMed] [Google Scholar]
- 145.Byrnes DM et al. Incidence of Neurological Complications Secondary to Intrathecal Chemotherapy Used As Either Prophylaxis or Treatment of Leptomeningeal Carcinomatosis. Blood 128, 5973–5973 (2016). [Google Scholar]
- 146.Fan C et al. Efficacy and Safety of Intrathecal Pemetrexed Combined With Dexamethasone for Treating Tyrosine Kinase Inhibitor-Failed Leptomeningeal Metastases From EGFR-Mutant NSCLC-a Prospective, Open-Label, Single-Arm Phase 1/2 Clinical Trial (Unique Identifier: ChiCTR1800016615). J Thorac Oncol 16, 1359–1368 (2021). [DOI] [PubMed] [Google Scholar]
- 147.Zagouri F et al. Intrathecal administration of trastuzumab for the treatment of meningeal carcinomatosis in HER2-positive metastatic breast cancer: a systematic review and pooled analysis. Breast Cancer Res Treat 139, 13–22 (2013). [DOI] [PubMed] [Google Scholar]
- 148.Figura NB et al. Intrathecal trastuzumab in the management of HER2+ breast leptomeningeal disease: a single institution experience. Breast Cancer Res Treat 169, 391–396 (2018). [DOI] [PubMed] [Google Scholar]
- 149.Figura NB et al. Clinical outcomes of breast leptomeningeal disease treated with intrathecal trastuzumab, intrathecal chemotherapy, or whole brain radiation therapy. Breast Cancer Res Treat 175, 781–788 (2019). [DOI] [PubMed] [Google Scholar]
- 150.Groves MD et al. A multicenter phase II trial of intrathecal topotecan in patients with meningeal malignancies. Neuro Oncol 10, 208–215 (2008). [DOI] [PMC free article] [PubMed] [Google Scholar]
- 151.Sinicrope KD et al. LPTO-09. INTRATHECAL TOPOTECAN FOR LEPTOMENINGEAL METASTASIS IN SOLID TUMORS: THE MD ANDERSON EXPERIENCE. Neurooncol Adv 1, i8–i8 (2019). [Google Scholar]
- 152.Jaeckle KA et al. Intra-CSF topotecan in treatment of breast cancer patients with leptomeningeal metastases. Cancer Med 9, 7935–7942 (2020). [DOI] [PMC free article] [PubMed] [Google Scholar]
- 153.Abbott NJ Evidence for bulk flow of brain interstitial fluid: significance for physiology and pathology. Neurochem Int 45, 545–52 (2004). [DOI] [PubMed] [Google Scholar]
- 154.Lin JH CSF as a surrogate for assessing CNS exposure: an industrial perspective. Curr Drug Metab 9, 46–59 (2008). [DOI] [PubMed] [Google Scholar]
- 155.Soderquist RG & Mahoney MJ Central nervous system delivery of large molecules: challenges and new frontiers for intrathecally administered therapeutics. Expert Opin Drug Deliv 7, 285–93 (2010). [DOI] [PMC free article] [PubMed] [Google Scholar]
- 156.Chamberlain MC Treatment of Leptomeningeal Metastasis With Intraventricular Administration of Depot Cytarabine (DTC 101). Arch Neurol 50, 261 (1993). [DOI] [PubMed] [Google Scholar]
- 157.Glantz MJ et al. Randomized Trial of a Slow-Release Versus a Standard Formulation of Cytarabine for the Intrathecal Treatment of Lymphomatous Meningitis. Journal of Clinical Oncology 17, 3110–3116 (1999). [DOI] [PubMed] [Google Scholar]
- 158.Ohyashiki K, Ohyashiki JH, Iwabuchi A, Ito H & Toyama K Central nervous system involvement in acute nonlymphocytic leukemia with inv(16)(p13q22). Leukemia 2, 398–9 (1988). [PubMed] [Google Scholar]
- 159.Lin NU et al. Response assessment criteria for brain metastases: proposal from the RANO group. Lancet Oncol 16, e270–8 (2015). [DOI] [PubMed] [Google Scholar]
- 160.Lin NU et al. Challenges relating to solid tumour brain metastases in clinical trials, part 1: patient population, response, and progression. A report from the RANO group. Lancet Oncol 14, e396–406 (2013). [DOI] [PubMed] [Google Scholar]
- 161.Chukwueke UN & Wen PY Use of the Response Assessment in Neuro-Oncology (RANO) criteria in clinical trials and clinical practice. CNS Oncol 8, CNS28 (2019). [DOI] [PMC free article] [PubMed] [Google Scholar]
- 162.Chamberlain M et al. Leptomeningeal metastases: a RANO proposal for response criteria. Neuro Oncol 19, 484–492 (2017). [DOI] [PMC free article] [PubMed] [Google Scholar]
- 163.Wen PY et al. Response Assessment in Neuro-Oncology Clinical Trials. Journal of Clinical Oncology 35, 2439–2449 (2017). [DOI] [PMC free article] [PubMed] [Google Scholar]
- 164.Food and Drug Administration. Evaluating Cancer Drugs in Patients with Central Nervous System Metastases. (2021). [DOI] [PubMed]
- 165.Zhou K, Cai X, Wang X, Lan X & Zhang X Efficacy and safety of WBRT+EGFR-TKI versus WBRT only in the treatment of NSCLC patients with brain metastasis: An updated meta-analysis. Thorac Cancer 13, 563–570 (2022). [DOI] [PMC free article] [PubMed] [Google Scholar]
- 166.Brown PD et al. Postoperative stereotactic radiosurgery compared with whole brain radiotherapy for resected metastatic brain disease (NCCTG N107C/CEC·3): a multicentre, randomised, controlled, phase 3 trial. Lancet Oncol 18, 1049–1060 (2017). [DOI] [PMC free article] [PubMed] [Google Scholar]
- 167.Mehrabian H, Detsky J, Soliman H, Sahgal A & Stanisz GJ Advanced Magnetic Resonance Imaging Techniques in Management of Brain Metastases. Front Oncol 9, 440 (2019). [DOI] [PMC free article] [PubMed] [Google Scholar]
- 168.Zakaria R et al. The Role of the Immune Response in Brain Metastases: Novel Imaging Biomarkers for Immunotherapy. Front Oncol 11, 711405 (2021). [DOI] [PMC free article] [PubMed] [Google Scholar]
- 169.Daqqaq TS & Alhasan AS Positron emission tomography and perfusion weighted imaging in the detection of brain tumors recurrence. Neurosciences 27, 131–142 (2022). [Google Scholar]
- 170.Kwee RM & Kwee TC Dynamic susceptibility MR perfusion in diagnosing recurrent brain metastases after radiotherapy: A systematic review and meta-analysis. J Magn Reson Imaging 51, 524–534 (2020). [DOI] [PMC free article] [PubMed] [Google Scholar]
- 171.Zakaria R et al. The Role of the Immune Response in Brain Metastases: Novel Imaging Biomarkers for Immunotherapy. Front Oncol 11, 711405 (2021). [DOI] [PMC free article] [PubMed] [Google Scholar]
- 172.Mehrabian H, Detsky J, Soliman H, Sahgal A & Stanisz GJ Advanced Magnetic Resonance Imaging Techniques in Management of Brain Metastases. Front Oncol 9, 440 (2019). [DOI] [PMC free article] [PubMed] [Google Scholar]
- 173.Jia C et al. Brain Metastases of Non-Small Cell Lung Cancer: Magnetic Resonance Spectroscopy for Clinical Outcome Assessment in Patients with Stereotactic Radiotherapy. Onco Targets Ther Volume 13, 13087–13096 (2020). [DOI] [PMC free article] [PubMed] [Google Scholar]
- 174.Guo D et al. A Novel Score Combining Magnetic Resonance Spectroscopy Parameters and Systemic Immune-Inflammation Index Improves Prognosis Prediction in Non-Small Cell Lung Cancer Patients With Brain Metastases After Stereotactic Radiotherapy. Front Oncol 12, (2022). [DOI] [PMC free article] [PubMed] [Google Scholar]
- 175.Najjar AM, Johnson JM & Schellingerhout D The Emerging Role of Amino Acid PET in Neuro-Oncology. Bioengineering (Basel) 5, (2018). [DOI] [PMC free article] [PubMed] [Google Scholar]
- 176.Otman H et al. Delayed [18 F]-FDG PET Imaging Increases Diagnostic Performance and Reproducibility to Differentiate Recurrence of Brain Metastases From Radionecrosis. Clin Nucl Med 47, 800–806 (2022). [DOI] [PubMed] [Google Scholar]
- 177.Najjar AM, Johnson JM & Schellingerhout D The Emerging Role of Amino Acid PET in Neuro-Oncology. Bioengineering (Basel) 5, (2018). [DOI] [PMC free article] [PubMed] [Google Scholar]
- 178.Pauleit D et al. PET with O-(2–18F-Fluoroethyl)-L-Tyrosine in peripheral tumors: first clinical results. J Nucl Med 46, 411–6 (2005). [PubMed] [Google Scholar]
- 179.Celli M et al. Diagnostic and Prognostic Potential of 18F-FET PET in the Differential Diagnosis of Glioma Recurrence and Treatment-Induced Changes After Chemoradiation Therapy. Front Oncol 11, 721821 (2021). [DOI] [PMC free article] [PubMed] [Google Scholar]
- 180.Albert NL et al. Response Assessment in Neuro-Oncology working group and European Association for Neuro-Oncology recommendations for the clinical use of PET imaging in gliomas. Neuro Oncol 18, 1199–208 (2016). [DOI] [PMC free article] [PubMed] [Google Scholar]
- 181.Chou J et al. Immunotherapeutic Targeting and PET Imaging of DLL3 in Small-Cell Neuroendocrine Prostate Cancer. Cancer Res 83, 301–315 (2023). [DOI] [PMC free article] [PubMed] [Google Scholar]
- 182.Lv G et al. PET Imaging of Tumor PD-L1 Expression with a Highly Specific Nonblocking Single-Domain Antibody. J Nucl Med 61, 117–122 (2020). [DOI] [PMC free article] [PubMed] [Google Scholar]
- 183.Boire A et al. Liquid biopsy in central nervous system metastases: a RANO review and proposals for clinical applications. Neuro Oncol 21, 571–584 (2019). [DOI] [PMC free article] [PubMed] [Google Scholar]
- 184.Wu X et al. Cerebrospinal Fluid Cell-Free DNA-Based Detection of High Level of Genomic Instability Is Associated With Poor Prognosis in NSCLC Patients With Leptomeningeal Metastases. Front Oncol 12, (2022). [DOI] [PMC free article] [PubMed] [Google Scholar]
- 185.Fan Y et al. Cell-Cycle and DNA-Damage Response Pathway Is Involved in Leptomeningeal Metastasis of Non–Small Cell Lung Cancer. Clinical Cancer Research 24, 209–216 (2018). [DOI] [PubMed] [Google Scholar]
- 186.Zheng M-M et al. Genotyping of Cerebrospinal Fluid Associated With Osimertinib Response and Resistance for Leptomeningeal Metastases in EGFR-Mutated NSCLC. Journal of Thoracic Oncology 16, 250–258 (2021). [DOI] [PubMed] [Google Scholar]
- 187.Zheng M-M et al. Clinical Utility of Cerebrospinal Fluid Cell-Free DNA as Liquid Biopsy for Leptomeningeal Metastases in ALK-Rearranged NSCLC. Journal of Thoracic Oncology 14, 924–932 (2019). [DOI] [PubMed] [Google Scholar]
- 188.Li YS et al. Unique genetic profiles from cerebrospinal fluid cell-free DNA in leptomeningeal metastases of EGFR-mutant non-small-cell lung cancer: a new medium of liquid biopsy. Annals of Oncology 29, 945–952 (2018). [DOI] [PubMed] [Google Scholar]
- 189.Li M et al. Dynamic monitoring of cerebrospinal fluid circulating tumor DNA to identify unique genetic profiles of brain metastatic tumors and better predict intracranial tumor responses in non-small cell lung cancer patients with brain metastases: a prospective cohort study (GASTO 1028). BMC Med 20, 398 (2022). [DOI] [PMC free article] [PubMed] [Google Scholar]
- 190.Lu BY et al. Spatially resolved analysis of the T cell immune contexture in lung cancer-associated brain metastases. J Immunother Cancer 9, (2021). [DOI] [PMC free article] [PubMed] [Google Scholar]
- 191.de Groot J et al. Window-of-opportunity clinical trial of pembrolizumab in patients with recurrent glioblastoma reveals predominance of immune-suppressive macrophages. Neuro Oncol 22, 539–549 (2020). [DOI] [PMC free article] [PubMed] [Google Scholar]
- 192.Jing Li MP Stereotactic Radiosurgery Versus Whole-brain Radiation Therapy For Patients With 4–15 Brain Metastases: A Phase III Randomized Controlled Trial. in (American Society for Radiation Oncology Annual Meeting, 2020). [Google Scholar]
- 193.Magnuson WJ et al. Management of Brain Metastases in Tyrosine Kinase Inhibitor-Naïve Epidermal Growth Factor Receptor-Mutant Non-Small-Cell Lung Cancer: A Retrospective Multi-Institutional Analysis. J Clin Oncol 35, 1070–1077 (2017). [DOI] [PubMed] [Google Scholar]
- 194.Soffietti R, Ahluwalia M, Lin N & Rudà R Management of brain metastases according to molecular subtypes. Nat Rev Neurol 16, 557–574 (2020). [DOI] [PubMed] [Google Scholar]
- 195.Vogelbaum MA et al. Treatment for Brain Metastases: ASCO-SNO-ASTRO Guideline. J Clin Oncol 40, 492–516 (2022). [DOI] [PubMed] [Google Scholar]
- 196.White MN et al. Combining Osimertinib With Chemotherapy in EGFR-Mutant NSCLC at Progression. Clin Lung Cancer 22, 201–209 (2021). [DOI] [PMC free article] [PubMed] [Google Scholar]
- 197.Chen G et al. Central nervous system efficacy of furmonertinib versus gefitinib in patients with non–small cell lung cancer with epidermal growth factor receptor mutations: Results from FURLONG study. Journal of Clinical Oncology 40, 9101–9101 (2022). [Google Scholar]
- 198.Deng Y et al. The concentration of erlotinib in the cerebrospinal fluid of patients with brain metastasis from non-small-cell lung cancer. Mol Clin Oncol 2, 116–120 (2014). [DOI] [PMC free article] [PubMed] [Google Scholar]
- 199.Yang J-J et al. Icotinib versus whole-brain irradiation in patients with EGFR -mutant non-small-cell lung cancer and multiple brain metastases (BRAIN): a multicentre, phase 3, open-label, parallel, randomised controlled trial. Lancet Respir Med 5, 707–716 (2017). [DOI] [PubMed] [Google Scholar]
- 200.Jung HA et al. Totality outcome of afatinib sequential treatment in patients with EGFR mutation-positive non-small cell lung cancer in South Korea (TOAST): Korean Cancer Study Group (KCSG) LU-19–22. Transl Lung Cancer Res 11, 1369–1379 (2022). [DOI] [PMC free article] [PubMed] [Google Scholar]
- 201.Cho BC et al. A Phase 1/2 Study of Lazertinib 240 mg in Patients With Advanced EGFR T790M-Positive NSCLC After Previous EGFR Tyrosine Kinase Inhibitors. Journal of Thoracic Oncology 17, 558–567 (2022). [DOI] [PubMed] [Google Scholar]
- 202.Camidge DR et al. Brigatinib Versus Crizotinib in Advanced ALK Inhibitor–Naive ALK-Positive Non–Small Cell Lung Cancer: Second Interim Analysis of the Phase III ALTA-1L Trial. Journal of Clinical Oncology 38, 3592–3603 (2020). [DOI] [PMC free article] [PubMed] [Google Scholar]
- 203.Solomon BJ et al. Lorlatinib in patients with ALK-positive non-small-cell lung cancer: results from a global phase 2 study. Lancet Oncol 19, 1654–1667 (2018). [DOI] [PubMed] [Google Scholar]
- 204.Li W, L. K., F. H. . . Z. C. TRUST - Updated Efficacy and Safety of Taletrectinib in Patients (pts) with ROS1+ Non-Small Cell Lung Cancer (NSCLC). in (European Lung Cancer Congress 2023, 2023). [Google Scholar]
- 205.Ou S et al. OA02. 03 clinical activity of lorlatinib in patients with ROS1+ advanced non-small cell lung cancer: phase 2 study cohort EXP-6. Journal of Thoracic Oncology 13, S322–S323 (2018). [Google Scholar]
- 206.Frost N et al. Lorlatinib in pretreated ALK- or ROS1-positive lung cancer and impact of TP53 co-mutations: results from the German early access program. Ther Adv Med Oncol 13, 175883592098055 (2021). [DOI] [PMC free article] [PubMed] [Google Scholar]
- 207.Odintsov I et al. Comparison of TAS0953/HM06 and selpercatinib in RET fusion-driven preclinical disease models of intracranial metastases. Journal of Clinical Oncology 40, 2024–2024 (2022). [Google Scholar]
- 208.Griesinger F et al. Safety and efficacy of pralsetinib in RET fusion–positive non-small-cell lung cancer including as first-line therapy: update from the ARROW trial. Annals of Oncology 33, 1168–1178 (2022). [DOI] [PubMed] [Google Scholar]
Associated Data
This section collects any data citations, data availability statements, or supplementary materials included in this article.


