Summary
Background
Clinical management of Asian BRCA1 and BRCA2 pathogenic variants (PV) carriers remains challenging due to imprecise age-specific breast (BC) and ovarian cancer (OC) risks estimates. We aimed to refine these estimates using six multi-ethnic studies in Asia.
Methods
Data were collected on 271 BRCA1 and 301 BRCA2 families from Malaysia and Singapore, ascertained through population/hospital-based case-series (88%) and genetic clinics (12%). Age-specific cancer risks were estimated using a modified segregation analysis method, adjusted for ascertainment.
Findings
BC and OC relative risks (RRs) varied across age groups for both BRCA1 and BRCA2. The age-specific RR estimates were similar across ethnicities and country of residence. For BRCA1 carriers of Malay, Indian and Chinese ancestry born between 1950 and 1959 in Malaysia, the cumulative risk (95% CI) of BC by age 80 was 40% (36%–44%), 49% (44%–53%) and 55% (51%–60%), respectively. The corresponding estimates for BRCA2 were 29% (26–32%), 36% (33%–40%) and 42% (38%–45%). The corresponding cumulative BC risks for Singapore residents from the same birth cohort, where the underlying population cancer incidences are higher compared to Malaysia, were higher, varying by ancestry group between 57 and 61% for BRCA1, and between 43 and 47% for BRCA2 carriers. The cumulative risk of OC by age 80 was 31% (27–36%) for BRCA1 and 12% (10%–15%) for BRCA2 carriers in Malaysia born between 1950 and 1959; and 42% (34–50%) for BRCA1 and 20% (14–27%) for BRCA2 carriers of the same birth cohort in Singapore. There was evidence of increased BC and OC risks for women from >1960 birth cohorts (p-value = 3.6 × 10−5 for BRCA1 and 0.018 for BRCA2).
Interpretation
The absolute age-specific cancer risks of Asian carriers vary depending on the underlying population-specific cancer incidences, and hence should be customised to allow for more accurate cancer risk management.
Funding
Wellcome Trust [grant no: v203477/Z/16/Z]; CRUK (PPRPGM-Nov20∖100002).
Keywords: Penetrance, Breast cancer risk, Ovarian cancer risk, BRCA1, BRCA2
Research in context.
Evidence before this study
To date, four studies have reported breast cancer risk estimates for BRCA1 and BRCA2 pathogenic variant carriers of Asian ancestry, of which two also reported the risk of ovarian cancer. These studies were based on small numbers of pathogenic variant carriers and there is considerable variability in the relative risk estimates between these studies. Moreover, all the published Asian studies assumed a constant relative risk across all ages, which has been shown in European studies to result in imprecise age specific absolute risks. Taken together, there is a need to provide more accurate lifetime and age-specific risk estimates for Asian BRCA carriers, both for the purposes of management of lifetime risk of cancers, and also for informing at what age risk-reducing are required.
Added value of this study
We have refined age-specific breast and ovarian cancer risks for BRCA1 and BRCA2 carriers of Chinese ancestry and quantified for the first time the cancer risks for carriers of Malay and Indian ancestry. Our findings show that the cancer relative risks in Asian carriers, vary by age, highlighting the importance of using age-specific relative risks for estimating absolute risks of breast and ovarian cancer. Furthermore, the age-specific relative risk estimates were similar across the three ethnic groups and close with those reported for European carriers. Despite similarities in age-specific relative risks, there were marked differences between the absolute risks for Asian carriers of all ancestries residing in Malaysia, a developing Asian country, and the absolute risks for European carriers. However, the absolute risks for Asian carriers residing in Singapore, a developed Asian country, were similar to those for European carriers.
Implications of all the available evidence
The findings provide a framework for estimating age-specific absolute risks for Asian carriers of diverse ancestries and place of residence. Given the comparable relative risk estimates among Asian ethnic subgroups, the absolute age-specific cancer risks for Asian carriers will differ, and will depend on the underlying population-specific incidences. As a result, a clinical management strategy based on European age-specific cancer risks may be suitable for Asian carriers residing in countries with population cancer incidences similar to high income Asian countries like Singapore. However, for Asian carriers residing in countries with markedly lower population cancer incidences, as in Malaysia, it is expected that their age-specific cancer risks would be lower than the risks applicable for European ancestry carriers. The results will optimize the clinical management of Asian BRCA1 and BRCA2 pathogenic variant carriers from diverse ancestries in Asia and beyond.
Introduction
Female carriers of BRCA1 or BRCA2 pathogenic variants (PV) have increased risks of both breast and ovarian cancer that warrant enhanced surveillance or risk-reducing options.1, 2, 3, 4, 5 Large-scale prospective cohort of primarily high-risk European-ancestry carriers have estimated that the breast cancer cumulative risk to age 70 was 66% (95% CI: 61–72%) for BRCA1 and 61% (95% CI: 55–68%) for BRCA2 and the corresponding ovarian cancer cumulative risks were 41% (95% CI: 33–50%) and 15% (95% CI: 10–23%), respectively.6 Notably cancer risk estimates for carriers of European ancestry have been found to vary by age, birth cohort and mutation position.1,4, 5, 6, 7, 8 The estimates from these studies have formed the basis for developing age-specific clinical management guidelines on screening and prophylactic surgery for European-ancestry PV carriers.
By contrast, only four studies (eTable 1) have reported breast cancer risk estimates for Asian carriers of BRCA1 and BRCA2 PV, of which two also estimated ovarian cancer risks.9, 10, 11, 12 These cumulative cancer risk estimates are imprecise and there was substantial variability in the relative risk estimates between studies, likely due to differences in sampling and analytical methods. Moreover, all existing Asian studies, due to the small sizes (125–376 BRCA1 and BRCA2 families), assumed a constant relative risk across all ages and birth cohorts, which is sub-optimal for informing risk-reducing clinical decisions which depend critically on age. Taken together, based on paucity of accurate Asian-specific data, clinical management of Asian PV carriers remains challenging.
Here, we use data from 572 families from Malaysia and Singapore with BRCA1 and BRCA2 PVs to estimate age-specific relative and absolute breast and ovarian cancer risks. We also investigate how these risks vary by ethnicity, birth cohort and PV location.
Methods
Families
Data were obtained on families in Malaysia and Singapore in which a BRCA1 or BRCA2 PV was present.13, 14, 15, 16 Two ascertainment schemes were used: (1) four studies identified PVs by systematic screening of cancer case-series, unselected for family history (235 BRCA1 and 269 BRCA2 families), and (2) two studies screened breast and ovarian cancer patients from multiple-case families recruited through genetics clinics (36 BRCA1 and 32 BRCA2 families). Patients recruited through the case-series and the first family member recruited through multiple-case families was designated as the proband. Genetic testing was offered to family members of probands. Variants were considered pathogenic or likely pathogenic based on ACMG/AMP guidelines.17 The number of eligible families by gene and recruitment scheme are detailed in eTable 2. Details regarding study recruitment, sequencing and PV definitions are provided in Supplementary Methods.
All studies were approved by the relevant institutional ethics committees and review boards, and all participants provided written informed consent for the study and for genetic testing.
Statistical methods
The breast and ovarian cancer-specific relative risks (RRs) were estimated simultaneously, but separately, for BRCA1 and BRCA2, using modified segregation analysis.18 Pedigree likelihoods were constructed using first-degree family members of probands and maximised using pedigree analysis software MENDEL.19 Family members were censored at the age at first cancer diagnosis, age at death, age at last follow-up or age 80 years, whichever occurred first. Since ascertainment criteria varied across studies, ascertainment bias was adjusted for each family separately using the ascertainment assumption free method described and applied previously1,4,20 which has been shown to provide unbiased parameter estimates.21, 22, 23 Briefly, the likelihoods were computed conditional on any data that may be relevant to the ascertainment. For families recruited through population and hospital-based case-series, we maximised the conditional likelihood of the pedigree given the phenotypic and genotypic information of the proband. For families recruited through multiple-case families, we maximised the conditional likelihood of the pedigree given the genotypic information of the proband and phenotypic information of all family members. Mutation status of tested family members were included in the analyses.
We considered models in which: (1) the log RR was assumed to be constant across ages; and (2) the log RRs were assumed to be constant within 10-year age intervals (20–29, 30–39, 40–49, 50–59, 60–69, 70–79). We fitted the above models by constraining the overall cancer incidence over carriers and non-carriers in the model to agree with country-, ethnic- and birth cohort-specific population age-specific incidences.24 We also considered country-, cohort-, self-reported ethnicity and PV location-specific models, where an additional variable for each subgroup (assumed to be constant over all ages) was included in the model to allow for log RRs to differ between subgroups. Nested models were compared against each other using the likelihood ratio test (LRT). Details regarding the statistical analysis are provided in Supplementary Methods.
Role of the funding sources
This study was supported by Wellcome Trust grant [grant no: v203477/Z/16/Z], which was not involved in any aspect of the study, including data collection, analysis, interpretation, trial design, patient recruitment, or any other pertinent aspect related to the writing of the manuscript or the decision to submit it for publication. The authors have not received any payment from pharmaceutical companies or other agencies for writing this article.
Results
Description of families
A total of 271 families in which the proband harboured a BRCA1 and 301 families with BRCA2 PV were eligible for inclusion in the analysis (eTable 2). Majority of the probands were of Chinese ancestry (52–81% depending on the study) except for the ovarian cancer case-series where the majority were Malays (eTable 3). There were 1121 and 1275 female first-degree family members in BRCA1 and BRCA2 families, respectively, of whom 144 and 65 family members in BRCA1 families were diagnosed with breast and ovarian cancer, respectively, and the corresponding number in BRCA2 families were 152 and 19 (eTable 4). The most common recurrent PV in BRCA1 was c.2726dup (7% of BRCA1 families) and in BRCA2 was c.2808_2811del (5% of BRCA2 families) (eTable 5, eFigure 1).
Relative risk estimates
When the RR was assumed to be independent of age, the estimated BRCA1 breast and ovarian cancer RRs (95% CI) were 15.6 (12.6–19.4) and 39.8 (29.6–53.3), respectively, and the estimated BRCA2 breast and ovarian cancer RRs (95% CI) were 10.3 (8.3–12.7) and 6.8 (3.9–11.9), respectively (Table 1). When age-specific RRs were considered, the breast cancer RRs for BRCA1 PV carriers were higher before age 40 (RR (95% CI) was 25.1 (9.1–69.3) for age group 20–29 and 27.0 (18.9–38.9) for age group 30–39) and then decreased with increasing age (RRs (95% CIs) were 16.1 (11.4–22.8), 12.1 (7.9–18.5), 6.4 (2.8–14.8) and 10.1 (3.4–30.0) for age groups 40–49, 50–59, 60–69 and 70–79, respectively, p-value for trend = 0.018). The RRs for ovarian cancer increased from 8.7 (0.9–79.4) in age group 20–29 to 81.7 (47.0–141.9) in age group 60–69, then decreased to 25.6 (5.5–119.5) in age group 70–79, but estimates were associated with wide confidence intervals (p-value for trend = 0.064). For BRCA2, the estimated breast cancer RR (95% CI) increased to a maximum of 14.1 (10.3–19.4) in the 40–49 age group and decreased to 4.9 (1.5–15.7) in the 70–79 age group (p-value for trend = 0.06). The ovarian cancer RRs increased from 2.3 (0.3–17.7) in age group 40–49 to 29.5 (10.1–86.2) in age group 70–79 (p-value for trend <0.001). The age-specific RR models provided a better fit to the data than the constant RR models (LRT: p-value = 7 × 10−5 for BRCA1 and p-value = 0.002 for BRCA2).
Table 1.
Estimated relative risk of breast and ovarian cancer for PV carriers of BRCA1 or BRCA2.
| Model | Breast cancer |
Ovarian cancer |
||||||
|---|---|---|---|---|---|---|---|---|
| BRCA 1 |
BRCA 2 |
BRCA 1 |
BRCA 2 |
|||||
| No. affected relatives | RR (95% CI) | No. affected relatives | RR (95% CI) | No. affected relatives | RR (95% CI) | No. affected relatives | RR (95% CI) | |
| Constant RR across age | ||||||||
| 20–79 | 144 | 15.6 (12.6–19.4) | 152 | 10.3 (8.3–12.7) | 64 | 39.8 (29.6–53.3) | 19 | 6.8 (3.9–11.9) |
| RR varied by age | ||||||||
| 20–29 | 6 | 25.1 (9.1–69.3) | 3 | 9.7 (2–46.1) | 1 | 8.7 (0.9–79.4) | 0 | – |
| 30–39 | 38 | 27 (18.9–38.9) | 21 | 9.5 (5.6–16.1) | 5 | 10.3 (3–35.1) | 0 | – |
| 40–49 | 47 | 16.1 (11.4–22.8) | 60 | 14.1 (10.3–19.4) | 16 | 35.5 (20.5–61.4) | 3 | 2.3 (0.3–17.7) |
| 50–59 | 38 | 12.1 (7.9–18.5) | 47 | 10.9 (7.6–15.6) | 23 | 51.9 (32.0–84.2) | 3 | 6.3 (2.2–18.6) |
| 60–69 | 10 | 6.4 (2.8–14.8) | 16 | 4.8 (2.4–9.8) | 16 | 81.7 (47.0–141.9) | 9 | 15.8 (6.4–38.9) |
| 70–79 | 5 | 10.1 (3.4–30.0) | 5 | 4.9 (1.5–15.7) | 3 | 25.6 (5.5–119.5) | 4 | 29.5 (10.1–86.2) |
| p-value | 0.018a | 0.060b | 0.064b | 0.0056a | ||||
RR, Relative risk (with non-carriers as reference group); CI, Confidence Interval.
Likelihood ratio test comparing logRR as a linear function of age against the model with a constant relative risk, df = 1.
Likelihood ratio test comparing logRR as a piecewise function of age against the model with a constant relative risk, df = 2. See Supplementary methods.
Analyses by country, ethnicity, PV location, ascertainment method
There was no evidence that breast and ovarian cancer RRs were different between countries, between ethnic groups or between ascertainment methods for PV carriers in either gene (eTable 6). Since the gender of unaffected children and the year of birth of unaffected siblings were not collected for Singapore breast cancer case-series families, we repeated the ethnic-specific analyses by using data from Malaysia only. There was no appreciable difference in results compared to the full cohort (eTable 7).
Compared to the region bounded by position c.2282 to c.4071, carriers with BRCA1 PVs outside this region had breast cancer RRs between 1.4 and 1.5 and carriers with PVs in the upstream region (5′ to c.2830) had ovarian cancer RR of 1.3. Compared to carriers of BRCA2 PVs in the OCCR (c.2831 to c.6401), carriers with PVs outside the OCCR had breast and ovarian cancer RRs of 0.4–0.8. However, none of these results were statistically significant (eTable 6).
Age-specific incidences and cumulative risks
Using the ethnic-specific breast cancer incidences experienced by a woman born in Malaysia between 1950 and 1959, the breast cancer incidences for BRCA1 carriers across all three ethnicities increased from age 20 to age 55, then remained similar between ages 55 and 65, and appeared to drop thereafter. A similar pattern was seen for BRCA2 carriers (Table 2). The cumulative risk (95% CI) of breast cancer to age 80 was highest in Chinese-ancestry women followed by Indian-and Malay-ancestry women (Chinese versus Indian versus Malay: 55% (51–60%) versus 49% (44%–53%) versus 40% (36–44%) for BRCA1 (pairwise p-values <0.05), and 42% (38–45%) versus 36% (33%–40%) versus 29% (26–32%) for BRCA2 (pairwise p-values <0.05 except for Chinese versus Indians where p-value was 0.07) (Fig. 1a and b and Table 2).
Table 2.
Breast and ovarian cancer incidences and cumulative risks of carriers of PVs in BRCA1 and BRCA2.
| Ages | Incidence per 1000 person-years (95% CI) |
Cumulative risk, % (95% CI) |
||||||
|---|---|---|---|---|---|---|---|---|
| Breasta |
Ovarianb | Breasta |
Ovarianb | |||||
| Chinese | Malay | Indian | Chinese | Malay | Indian | |||
| BRCA 1 mutation carriers | ||||||||
| 20 | 0 | 0 | 0 | 0 | 0 | 0 | 0 | 0 |
| 25 | 0 | 0 | 0 | 0 | 0 | 0 | 0 | 0 |
| 30 | 3 (2–4) | 2 (1–3) | 2 (1–3) | 0 (0–1) | 1 (1–1) | 1 (0–1) | 1 (0–1) | 0 |
| 35 | 7 (5–11) | 5 (4–7) | 6 (4–8) | 1 (0–3) | 3 (3–4) | 2 (2–3) | 3 (2–3) | 0 (0–1) |
| 40 | 12 (8–17) | 8 (6–11) | 9 (6–13) | 2 (1–4) | 8 (7–9) | 6 (5–6) | 6 (6–7) | 1 (1–2) |
| 45 | 17 (12–24) | 12 (8–17) | 13 (9–18) | 6 (3–10) | 15 (13–16) | 10 (9–12) | 11 (10–13) | 3 (3–4) |
| 50 | 20 (13–31) | 14 (9–22) | 16 (10–24) | 8 (5–13) | 23 (21–25) | 16 (15–18) | 18 (16–19) | 7 (6–8) |
| 55 | 22 (14–33) | 15 (10–23) | 18 (12–27) | 10 (6–16) | 30 (28–33) | 22 (20–24) | 25 (23–27) | 11 (9–12) |
| 60 | 22 (9–50) | 14 (6–32) | 18 (8–42) | 11 (6–19) | 38 (35–41) | 28 (25–30) | 31 (29–34) | 15 (13–17) |
| 65 | 20 (9–46) | 12 (5–27) | 17 (7–39) | 11 (6–19) | 44 (40–47) | 32 (29–35) | 37 (34–40) | 20 (18–22) |
| 70 | 17 (6–51) | 10 (3–29) | 15 (5–45) | 11 (2–51) | 49 (45–53) | 35 (32–39) | 42 (38–45) | 24 (21–27) |
| 75 | 14 (5–42) | 7 (2–22) | 13 (4–39) | 10 (2–47) | 52 (48–57) | 38 (35–42) | 46 (41–50) | 28 (24–32) |
| 80 | 11 (4–32) | 5 (2–14) | 11 (4–31) | 8 (2–40) | 55 (51–60) | 40 (36–44) | 49 (44–53) | 31 (27–36) |
| BRCA 2 mutation carriers | ||||||||
| 20 | 0 | 0 | 0 | 0 | 0 | 0 | 0 | 0 |
| 25 | 0 | 0 | 0 | 0 | 0 | 0 | 0 | 0 |
| 30 | 1 (1–2) | 1 (1–1) | 1 (1–2) | 0 | 0 (0–1) | 0 (0–0) | 0 (0–0) | 0 |
| 35 | 3 (2–6) | 2 (1–4) | 3 (2–4) | 0 | 2 (1–2) | 1 (1–1) | 1 (1–1) | 0 |
| 40 | 6 (5–9) | 4 (3–6) | 5 (4–7) | 0 | 4 (3–5) | 3 (2–3) | 3 (3–4) | 0 |
| 45 | 11 (8–14) | 7 (5–10) | 8 (6–11) | 0 (0–3) | 8 (7–9) | 6 (5–6) | 6 (6–7) | 0 |
| 50 | 15 (10–21) | 10 (7–15) | 12 (8–17) | 1 (0–2) | 14 (13–15) | 10 (9–11) | 11 (10–12) | 0 (0–1) |
| 55 | 18 (13–27) | 13 (9–18) | 15 (10–22) | 1 (0–4) | 21 (19–23) | 15 (14–16) | 17 (15–18) | 1 (1–1) |
| 60 | 17 (8–34) | 11 (5–21) | 14 (7–28) | 2 (1–5) | 28 (25–30) | 20 (18–22) | 23 (21–25) | 2 (1–2) |
| 65 | 14 (7–28) | 8 (4–17) | 12 (6–24) | 4 (1–9) | 33 (30–36) | 23 (21–26) | 27 (25–30) | 3 (3–4) |
| 70 | 11 (3–35) | 6 (2–20) | 10 (3–31) | 5 (2–14) | 37 (34–40) | 26 (24–28) | 31 (28–34) | 5 (4–7) |
| 75 | 8 (3–26) | 4 (1–13) | 7 (2–24) | 7 (3–20) | 40 (36–43) | 28 (25–31) | 34 (31–37) | 8 (7–11) |
| 80 | 6 (2–18) | 3 (1–8) | 6 (2–18) | 10 (4–26) | 42 (38–45) | 29 (26–32) | 36 (33–40) | 12 (10–15) |
Incidences and cumulative risks for breast cancer were calculated based on population calendar, cohort and ethnic-specific breast cancer incidences for a woman born in Malaysia between 1950 and 1959. Disease-specific mortality was not available and hence was not accounted for in cumulative risk estimates.
Incidences and cumulative risks for ovarian cancer were calculated based on overall population calendar and cohort-specific ovarian cancer incidences for a woman born in Malaysia between 1950 and 1959, as ethnic-specific ovarian cancer incidence was not available. Disease-specific mortality was not available and hence was not accounted for in cumulative risk estimat.
Fig. 1.
Estimated cumulative risk of developing breast and ovarian cancer (under a model with no cohort effect) for women with germline BRCA1 and BRCA2 pathogenic variants. Assume that population incidences are applicable to individuals born between 1950 and 1959 from Malaysia. Solid lines represent cumulative risk of BRCA1 or BRCA2 carriers; dashed lines represents cumulative risk of general population; Blue, red and green lines represent Chinese, Indian and Malay women, respectively. The shaded areas show the 95% CIs.
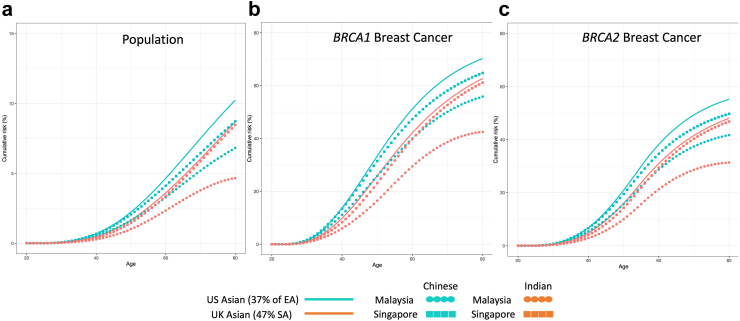
Assuming that age-specific RRs are constant across Asian populations in any country and using the latest available incidences for women of Chinese and Indian-ancestry from Malaysia and Singapore as well as Asians from UK and USA, Fig. 2 aims to demonstrate how cumulative breast cancer risks can vary within the same ethnic groups due to differences in population cancer incidences across different countries. The ethnic-specific population cancer incidences for Asian populations in the USA and UK were not available, thus we made assumption based on the demographic composition of these countries. The cumulative risks of Chinese in Singapore were similar to Asians in USA (with approximately 37% East Asians in the USA25) but higher compared to Chinese in Malaysia. The cumulative risks of Indians in Singapore were similar to Asians in UK (with approximately 47% South Asians in the UK26) but higher compared to Indians from Malaysia.
Fig. 2.
Estimated cumulative risk of breast cancer (under a model with no cohort effect) for Chinese and Indians in Malaysia and Singapore, and Asians in UK and USA. Assume that estimated relative risks are applicable to Asian populations in the UK and US, and women had the incidences as reported between 2016 and 2018 for UK, 2015–2019 for US, and 2014–2016 for Malaysia and Singapore. Blue line, blue circle and blue square represent the cumulative risk of US Asians, Malaysian Chinese and Singaporean Chinese, respectively while orange line, orange circle and orange square represent UK Asians, Malaysian Indians and Singaporean Indians, respectively.
Using the ovarian cancer incidences experienced by a woman born in Malaysia between 1950 and 1959, the ovarian cancer incidences for BRCA1 carriers increased with age up to 60 years old and remained similar between ages 60 and 80. The ovarian cancer incidences for BRCA2 carriers were lower compared to BRCA1 carriers (incidences per 1000 person-years increased from 2 at age 60 to 11 at age 80) (Table 2). The cumulative risk (95% CI) of ovarian cancer to age 80 was 31% (27–36%) for BRCA1 carriers and 12% (10–15%) for BRCA2 carriers (Table 2 and Fig. 1c and d). Ethnic-specific population ovarian cancer incidences were not available to determine ethnicity-specific cumulative risk estimates.
Birth cohort effect
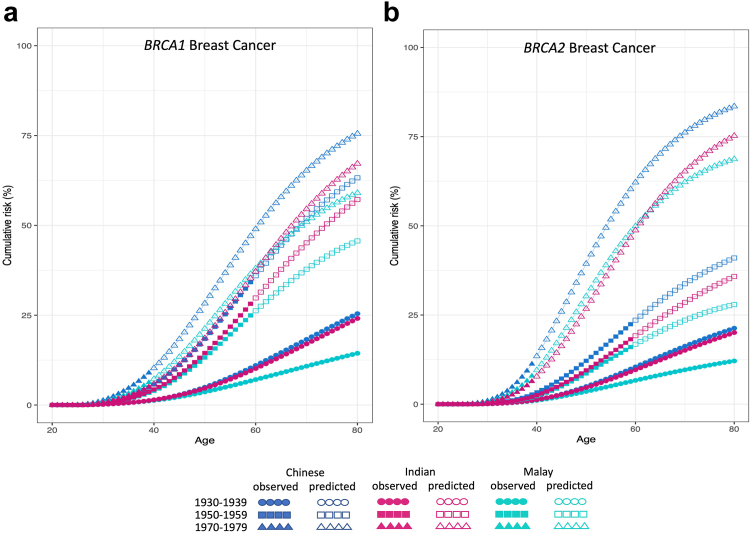
We investigated whether breast and ovarian cancer risks varied by birth cohort: before 1940, 1940–59, 1960–69, and after 1969. For BRCA1, relative to the before-1940 cohort, the breast cancer RRs (95% floating-CI27) were 2.5 (1.8–3.4), 2.9 (1.9–4.6) and 3.3 (1.9–5.7) for the three later birth cohorts, respectively (eTable 6). The corresponding estimates for ovarian cancer were 7.8 (5.3–11.6), 10.7 (5.2–22.1), and 5.7 (1.1–30.5). For BRCA2, the corresponding cohort effect for breast cancer were of 1.4 (1.1–2.2), 2.2 (1.4–3.4) and 4.4 (2.3–8.5). The ovarian cancer RR (95% floating-CI) was 3.3 (1.2–10.2) for BRCA2 carriers born in 1940–1959 compared to those who were born before 1940. The cohort-specific model provided a significantly better fit than the age-only model (LRT: p-value = 3.6 × 10−5 for BRCA1 and 0.018 for BRCA2). Under the birth cohort-specific models, the cumulative risk of breast cancer to age 80 was predicted to be higher in PV carriers born in 1970–79 compared to PV carriers born in 1930–39, across all three ethnicities (Fig. 3a and b).
Fig. 3.
Estimated cumulative risk of developing breast cancer (under a model that allows for cohort-specific relative risks) for women with germline(a)BRCA1 and(b)BRCA2 pathogenic variants. Circle, square and triangle represent birth cohort 1930-1939, 1950-1959, and 1970-1979, respectively, where solid symbol represents observed risk and hollow symbol represents predicted risk; blue, red and green represent Chinese, Indian and Malay ethnicity, respectively.
Discussion
In this study, we have defined breast and ovarian cancer risk estimates for BRCA1 and BRCA2 PV carriers in Malaysia and Singapore. These estimates refine estimates for carriers of Chinese ancestry and quantify for the first time the risks for carriers of Malay and Indian ancestry. We found that the age-specific RRs for BRCA1 and BRCA2 PV carriers were similar across ethnic groups.
Our study showed that breast cancer RRs vary substantially by age, from approximately 25 for BRCA1 and 10 for BRCA2 at age <30 to 10 for BRCA1 and 5 for BRCA2 at age >70. The patterns are largely similar to those reported in women of European ancestry,1 except for age group 40–49 in BRCA1 PV carriers where the breast cancer RRs appeared to be lower compared to European studies (eTable 8). The estimated breast cancer incidences for all ethnicities peak at age 55 then decrease again thereafter for both genes (Table 2 and eTable 9). This pattern is somewhat different from that reported in European populations, where peak incidence occurs earlier (at 45 for BRCA1 carriers and 50 for BRCA2 carriers), plateauing thereafter.1,6 Comparing to the higher breast cancer RRs at younger age for both BRCA1 and BRCA2 carriers, the RRs for ovarian cancer increased with age for both mutations. This pattern is different from that reported in European populations, where RR peaked between age 40–49 for BRCA1 carriers and 50–59 for BRCA2 carriers, though the confidence intervals between the two studies overlapped (eTable8).
The cumulative breast cancer risks of Asian carriers vary depending on underlying population-specific cancer incidences. The corresponding risks to age 70 are substantially lower for Asian carriers in Malaysia than those reported for European women: 40–55% (depending on ethnicity) versus 65% for BRCA1 carriers and 29–42% (depending on ethnicity) versus 45% for BRCA2 carriers).1 These differences in absolute risks reflect the lower population incidences in Malaysia versus Western countries and are consistent with the hypothesis that the risks conferred by PVs combine multiplicatively with the effects of other risk factors (such as lifestyle or genetics factors), and are largely independent of genetic ancestry.13 A similar observation was made for women of African ancestry based on a study from Ghana.28 It is also worth noting that the predicted absolute risks in Singapore, where population incidences are closer to those of European ancestry, are notably higher than those based on Malaysia (57–61% for BRCA1 carriers and 43–47% (depending on ethnicity) for BRCA2 carriers; Fig. 2 and eTable 9). Assuming that RRs are constant across Asian populations in any countries, the absolute risks of Asian carriers in Western countries is close to that of European carriers, whereas the absolute risks of Asian carriers in Asian countries with lower population risk will be lower than that reported here for Malaysians (Fig. 2).
Notably, we found a significant birth cohort effect, likely driven by the substantial increase in breast cancer incidence largely driven by urbanisation and significant changes in reproductive patterns in Asia.29, 30, 31 Even after adjusting for birth-cohort-specific population cancer incidences, breast cancer risk among carriers of PVs has increased over time (Fig. 3 & eTable 6). Although lower life expectancy and under-reporting of cancer cases in earlier decades, and higher uptake of screening for recent generations can at least partly explain this observation,32 the data could also point to the influence of lifestyle, reproductive and hormone-related modifiers on disease risks for PV carriers.33, 34, 35 In this regard, risk assessment tools which integrate genetic modifiers with lifestyle, hormonal or other cancer risk factors could be important for refining personalised breast cancer risks in Asian carriers.
For ovarian cancer, the cumulative risk in carriers of BRCA1 PV is low under the age of 45 years but reaches more than 10% by the age of 55 years, whereas it remains low until the age of 65 years for BRCA2 mutation carriers. The recommended ovarian cancer control guidelines include discussion of risk-reducing salpingo-oophorectomy (RRSO) at ages 35–40 for BRCA1 carriers and 40–45 years for BRCA2 carriers.36 However, given the lower risk in BRCA2 carriers in Asian countries, it may be appropriate to delay this surgical prevention to avoid the potential consequences of premature surgical menopause.37,38 Nevertheless, the confidence intervals for the ovarian cancer RR estimates for BRCA2 carriers are wide, larger studies are needed to improve the precision of these estimates.
Our study has several limitations. While we confirmed cancer family history on all family members who underwent genetic testing, we relied on proband-reported family history on other members and hence may be susceptible to inaccuracies. Although studies have shown high accuracy of proband-reported family history of breast cancer, accuracy has been shown to be lower for less common cancers, including ovarian cancer.39,40 A second limitation would be high proportion (∼33%) of missing information on year of birth, mostly from the Singapore breast cancer case-series where the information on year of birth was not collected for unaffected siblings of proband. An additional limitation is an assumption that unaffected children recruited in the Singapore breast cancer case-series were all females. To access the impact of these limitations, we performed sensitivity analyses to examine how RRs vary across birth cohorts and ethnicities without using the Singapore data and the results remained similar. Another limitation is ethnic-specific ovarian cancer incidence was not available for adjustment. However, additional ethnic-specific parameters included in the models were not significant, suggesting that not adjusting for ethnic-specific population ovarian cancer incidences is unlikely to have resulted in biased RR estimates. It is also important to acknowledge that the RR estimates for the birth cohort >1970 have relatively wide confidence intervals, indicating greater uncertainty in the estimated cumulative risks for the birth cohort 1970–79 based on the birth-cohort model alone. Finally, although information on salpingo-oophorectomy and bilateral mastectomy for relatives were not available, the phenotypic data on relatives were collected at or prior to genetic testing. Hence it is unlikely that these surgeries would have taken place prior to genetic testing results in family members. Moreover, the rate of both of these surgeries have been historically low in Asia and therefore unlikely to impact on the parameter estimates.
In summary, we have shown that the age-specific RR estimates of BRCA1 and BRCA2 carriers of Asian ancestry were similar to those previously observed in European populations, but the corresponding absolute breast and ovarian cancer risks vary depending on the underlying population- and birth-cohort specific cancer incidences. The current European-derived risk management strategies may be appropriate for Asian carriers in developed countries where absolute risks are expected to be similar to Europeans. By contrast, the current European-derived risk management strategies may require refinement in Asian populations with lower risk of these cancers as the current absolute risk estimates are significantly higher than appropriate. It may be critical to develop risk assessment tools which integrate genetics, lifestyle, hormonal and other risk factors for refining population- and age-specific cancer risks, particularly for these Asian carriers in developing countries.
Contributors
Conceptualization: W.K.H., D.F.E., A.C.A., S.H.T.; Resources: J.C., Z.Z., SGBCC investigators, M.H.S., M.K.T., Y.L.W., MaGiC Investigators, A.M.D., M.H., C.H.Y., N.A.M.T., J.Li., J.N., S.H.T.; Data curation: N.T.H., S.Y.Y., J.Lim, N.D.B.I., P.J.H., E.A.W., P.P.S.N., C.L., J.A., M.C.T.; Formal analysis: W.K.H., X.Y., D.F.E., A.C.A., S.H.T.; Validation: W.K.H., A.C.A., S.H.T.; Manuscript writing—original draft: W.K.H., D.F.E., J.N., A.C.A., S.H.T.; Manuscript writing—review and editing: all authors.
Data sharing statement
Request for access to data from the Malaysian studies, Singapore SGBCC study and Singapore NCC study can be made via submission of an inquiry to Prof. Soo-Hwang Teo at genetics@cancerresearch.my, Dr. Mikael Hartman at mikael_hartman@nuhs.edu.sg, and Dr. Joanne Yuen-Yie Ngeow at joanne.ngeow@ntu.edu.sg, respectively.
Declaration of interests
Z.Z received honorarium from AstraZeneca. J.N received research funding from AstraZeneca and MiRXES. A.C.A is listed as creator of the BOADICEA model which has been licensed by Cambridge Enterprise, from which University of Cambridge may receive royalties. N.A.M.T received honoraria for lectures from Zuellig Pharma Sdn Bhd and Astra Zeneca, received support for attending meetings and/or travel from MSD and Astra Zeneca. S.Y.Y received speaker's honoraria from Astra Zeneca, she is the president of Genetic Counselling Society Malaysia.
Acknowledgements
We thank all the participants and their families for taking part in the research studies and all the researchers, clinicians, technicians and administrative staff who have enabled this work to be carried out. MyBrCa, MyOvCa, MyF and MaGiC thank all research staff at Cancer Research Malaysia, University Malaya, participating Ministry of Health Malaysia hospitals, Subang Jaya Medical Centre, Beacon Hospital, Gleneagles Penang, Hospital Universiti Sains Malaysia, KPJ Ampang Puteri Specialist Hospital, KPJ Johor Specialist Hospital, KPJ Sabah Specialist Hospital, Loh Guan Lye Specialist Centre, Mount Miriam Cancer Hospital, Pantai Hospital Kuala Lumpur, Penang Adventist Hospital, Universiti Kebangsaan Malaysia Medical Centre and Sunway Medical Centre who assisted in recruitment and interviews for their contributions and commitment to this study. We want to thank Siti Norhidayu Hasan, Lau Shao Yan and Habibatul Saadiah Isa for assistance with DNA preparation for MyF and MaGiC; Lee Sheau Yee, Daphne SC Lee, Wong Siu Wan and Lee Yong Quan for their assistance in curating family history data for MyBrCa, MyOvCa, MyF and MaGIc.
For SGBCC, we want to thank the program manager Jenny Liu, clinical research coordinators/research assistants Siew-Li Tan, Siok-Hoon Yeo, Amanda Ong, Jin-Yee Lee, Michelle Mo Ying-Jia Chew, Jing-Jing Hong, and Hui-Min Lau for their contributions in recruitment, and Yen-Shing Yeoh, Nur Khaliesah Binte Mohamed Riza, and Ganga Devi d/o Chandrasegran for data preparation. We also want to thank all the participants' and clinicians' who supported SGBCC.
We also want to thank Caroline Baynes and Don Conroy who ran sequencing reactions for MYBRCA and SGBCC studies.
For NCCS study, we thank all our clinical partners and genetic counsellors Shao Tzu Li, Jeanette Yuen, Hui Xuan Goh and laboratory staff Sock Hoai Chan, Ee Ling Chew and Siao Ting Chong for their efforts in patient recruitment and database management. We thank all NCCS patients and families for their support of our research efforts.
Funding: This study was supported by Wellcome Trust grant [grant no: v203477/Z/16/Z]. For the purpose of open access, the author has applied a CC BY public copyright licence to any Author Accepted Manuscript version arising from this submission.
MyBrCa was funded by the Malaysian Ministry of Science, the Malaysian Ministry of Higher Education High Impact Research Grant (Grant No.: UM.C/HIR MOHE/06). MaGiC was funded in part through an AstraZeneca External Investigator Grant. MyBrCa, MyOvCa, MaGiC and MyF were funded by charitable funds from Yayasan Sime Darby, Yayasan PETRONAS, Estee Lauder Group of Companies, Khind Starfish Foundation, Vistage Group of Companies and other donors of Cancer Research Malaysia.
WKH is the recipient of L'Oreal-UNESCO For Women in Science National Fellowship. JL is the recipient of a National Research Foundation Singapore Fellowship (NRF-NRFF2017-02). ACA and XY are supported through Cancer Research—UK (PPRPGM-Nov20∖100002) and the core funding from the NIHR Cambridge Biomedical Research Centre (NIHR203312) [∗]. ∗The views expressed are those of the author(s) and not necessarily those of the NIHR or the Department of Health and Social Care.
SGBCC is funded by NUS Start Up Grant, National University Cancer Institute, Singapore (NCIS) Centre Grant [grant no: MRC/CG/NCIS/2010, NMRC/CG/012/2013, CGAug16M005, CG21APR1005], NMRC Clinical Scientist Award [grant no: NMRC/CSA/0048/2013], NMRC Clinician Scientist Award-Senior Investigator [grant no: NMRC/CSA-SI/0015/2017], Asian Breast Cancer Research Fund, Breast Cancer Prevention Programme under Saw Swee Hock School of Public Health, and Breast Cancer Screening and Prevention Programme under Yong Loo Lin School of Medicine.
J.N. was supported by National Research Foundation Singapore, Clinician Scientist Award (NMRC/CSA-INV/0017/2017, and MOH-000654), Singapore Ministry of Health's National Medical Research Council, National Cancer Centre Research Fund (NCCRF-YR2018-NOV-1) and the Terry Fox Foundation, Canada. NCCS is supported in part by the National Research Foundation, Singapore, through the Singapore Ministry of Health’s National Medical Research Council and the Precision Health Research, Singapore (PRECISE), under PRECISE’s Clinical Implementation Pilot grant scheme.
Footnotes
Supplementary data related to this article can be found at https://doi.org/10.1016/j.lanwpc.2024.101017.
Appendix A. Supplementary data
References
- 1.Antoniou A., Pharoah P.D., Narod S., et al. Average risks of breast and ovarian cancer associated with BRCA1 or BRCA2 mutations detected in case series unselected for family history: a combined analysis of 22 studies. Am J Hum Genet. 2003;72(5):1117–1130. doi: 10.1086/375033. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 2.Anglian Breast Cancer Study Group Prevalence and penetrance of BRCA1 and BRCA2 mutations in a population-based series of breast cancer cases. Br J Cancer. 2000;83(10):1301. doi: 10.1054/bjoc.2000.1407. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 3.Chen S., Iversen E.S., Friebel T., et al. Characterization of BRCA1 and BRCA2 mutations in a large United States sample. J Clin Oncol. 2006;24(6):863. doi: 10.1200/JCO.2005.03.6772. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 4.Milne R.L., Osorio A., Cajal T.R., et al. The average cumulative risks of breast and ovarian cancer for carriers of mutations in BRCA1 and BRCA2 attending genetic counseling units in Spain. Clin Cancer Res. 2008;14(9):2861–2869. doi: 10.1158/1078-0432.CCR-07-4436. [DOI] [PubMed] [Google Scholar]
- 5.Brohet R.M., Velthuizen M.E., Hogervorst F.B., et al. Breast and ovarian cancer risks in a large series of clinically ascertained families with a high proportion of BRCA1 and BRCA2 Dutch founder mutations. J Med Genet. 2014;51(2):98–107. doi: 10.1136/jmedgenet-2013-101974. [DOI] [PubMed] [Google Scholar]
- 6.Kuchenbaecker K.B., Hopper J.L., Barnes D.R., et al. Risks of breast, ovarian, and contralateral breast cancer for BRCA1 and BRCA2 mutation carriers. JAMA. 2017;317(23):2402–2416. doi: 10.1001/jama.2017.7112. [DOI] [PubMed] [Google Scholar]
- 7.Levy-Lahad E., Friedman E. Cancer risks among BRCA1 and BRCA2 mutation carriers. Br J Cancer. 2007;96(1):11–15. doi: 10.1038/sj.bjc.6603535. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 8.Lubinski J., Phelan C.M., Ghadirian P., et al. Cancer variation associated with the position of the mutation in the BRCA2 gene. Fam Cancer. 2004;3(1):1–10. doi: 10.1023/B:FAME.0000026816.32400.45. [DOI] [PubMed] [Google Scholar]
- 9.Park B., Dowty J.G., Ahn C., et al. Breast cancer risk for Korean women with germline mutations in BRCA1 and BRCA2. Breast Cancer Res Treat. 2015;152(3):659–665. doi: 10.1007/s10549-015-3495-z. [DOI] [PubMed] [Google Scholar]
- 10.Yao L., Sun J., Zhang J., et al. Breast cancer risk in Chinese women with BRCA1 or BRCA2 mutations. Breast Cancer Res Treat. 2016;156(3):441–445. doi: 10.1007/s10549-016-3766-3. [DOI] [PubMed] [Google Scholar]
- 11.Zhang L., Shin V.Y., Chai X., et al. Breast and ovarian cancer penetrance of BRCA1/2 mutations among Hong Kong women. Oncotarget. 2018;9(38) doi: 10.18632/oncotarget.24382. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 12.Momozawa Y., Sasai R., Usui Y., et al. Expansion of cancer risk Profile for BRCA1 and BRCA2 pathogenic variants. JAMA Oncol. 2022;8(6):871–878. doi: 10.1001/jamaoncol.2022.0476. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 13.Dorling L., Carvalho S., Allen J. Breast cancer risk genes—association analysis in more than 113,000 women. N Engl J Med. 2021;384(5):428–439. doi: 10.1056/NEJMoa1913948. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 14.Wen W.X., Allen J., Lai K.N., et al. Inherited mutations in BRCA1 and BRCA2 in an unselected multiethnic cohort of Asian patients with breast cancer and healthy controls from Malaysia. J Med Genet. 2018;55(2):97–103. doi: 10.1136/jmedgenet-2017-104947. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 15.Yoon S., Bashah N.A., Wong S., et al. Mainstreaming genetic counselling for genetic testing of BRCA1 and BRCA2 in ovarian cancer patients in Malaysia (MaGiC study) Ann Oncol. 2017;28:x187. [Google Scholar]
- 16.Hasmad H.N., Lai K.N., Wen W.X., et al. Evaluation of germline BRCA1 and BRCA2 mutations in a multi-ethnic Asian cohort of ovarian cancer patients. Gynecol Oncol. 2016;141(2):318–322. doi: 10.1016/j.ygyno.2015.11.001. [DOI] [PubMed] [Google Scholar]
- 17.Richards S., Aziz N., Bale S., et al. Standards and guidelines for the interpretation of sequence variants: a joint consensus recommendation of the American college of medical genetics and genomics and the association for molecular pathology. Genet Med. 2015;17(5):405–423. doi: 10.1038/gim.2015.30. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 18.Antoniou A.C., Casadei S., Heikkinen T., et al. Breast-cancer risk in families with mutations in PALB2. N Engl J Med. 2014;371(6):497–506. doi: 10.1056/NEJMoa1400382. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 19.Lange K., Weeks D., Boehnke M. 1986. Programs for pedigree analysis: MENDEL. Fisher, and dGENE. [DOI] [PubMed] [Google Scholar]
- 20.Yang X., Leslie G., Doroszuk A., et al. Cancer risks associated with germline PALB2 pathogenic variants: an international study of 524 families. J Clin Oncol. 2020;38(7):674. doi: 10.1200/JCO.19.01907. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 21.Cannings C., Thompson E. Ascertainment in the sequential sampling of pedigrees. Clin Genet. 1977;12(4):208–212. doi: 10.1111/j.1399-0004.1977.tb00928.x. [DOI] [PubMed] [Google Scholar]
- 22.Ewens W., Shute N.C. A resolution of the ascertainment sampling problem I. Theory. Theor Popul Biol. 1986;30(3):388–412. doi: 10.1016/0040-5809(86)90042-0. [DOI] [PubMed] [Google Scholar]
- 23.Shute N., Ewens W. A resolution of the ascertainment sampling problem. III. Pedigrees. Am J Hum Genet. 1988;43(4):387. [PMC free article] [PubMed] [Google Scholar]
- 24.Antoniou A.C., Cunningham A., Peto J., et al. The BOADICEA model of genetic susceptibility to breast and ovarian cancers: updates and extensions. Br J Cancer. 2008;98(8):1457–1466. doi: 10.1038/sj.bjc.6604305. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 25.Budiman A., Cilluffo A., Ruiz N.G. Pew Research Center; Washington, DC: 2019. Key facts about Asian origin groups in the US. [Google Scholar]
- 26.(ONS) OfNS . 2018. Population denominators by ethnic group, regions and countries: England and Wales, 2011 to 2018. [Google Scholar]
- 27.Easton D.F., Peto J., Babiker A.G. Floating absolute risk: an alternative to relative risk in survival and case-control analysis avoiding an arbitrary reference group. Stat Med. 1991;10(7):1025–1035. doi: 10.1002/sim.4780100703. [DOI] [PubMed] [Google Scholar]
- 28.Ahearn T.U., Pal Choudhury P., Derkach A., et al. Cancer Epidemiology, Biomarkers & Prevention; 2022. Breast cancer risk in women from Ghana carrying rare germline pathogenic mutations. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 29.Bhoo-Pathy N., Yip C.-H., Hartman M., et al. Breast cancer research in Asia: adopt or adapt Western knowledge? Eur J Cancer. 2013;49(3):703–709. doi: 10.1016/j.ejca.2012.09.014. [DOI] [PubMed] [Google Scholar]
- 30.Heer E., Harper A., Escandor N., Sung H., McCormack V., Fidler-Benaoudia M.M. Global burden and trends in premenopausal and postmenopausal breast cancer: a population-based study. Lancet Glob Health. 2020;8(8):e1027–e1037. doi: 10.1016/S2214-109X(20)30215-1. [DOI] [PubMed] [Google Scholar]
- 31.Sung H., Rosenberg P.S., Chen W.-Q., et al. Female breast cancer incidence among Asian and Western populations: more similar than expected. J Natl Cancer Inst. 2015;107(7):djv107. doi: 10.1093/jnci/djv107. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 32.Hemminki K., Bermejo J.L. Effects of screening for breast cancer on its age-incidence relationships and familial risk. Int J Cancer. 2005;117(1):145–149. doi: 10.1002/ijc.21149. [DOI] [PubMed] [Google Scholar]
- 33.Andrieu N., Goldgar D.E., Easton D.F., et al. Pregnancies, breast-feeding, and breast cancer risk in the International BRCA1/2 Carrier Cohort Study (IBCCS) J Natl Cancer Inst. 2006;98(8):535–544. doi: 10.1093/jnci/djj132. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 34.Chang-Claude J., Andrieu N., Rookus M., et al. Age at menarche and menopause and breast cancer risk in the International BRCA1/2 Carrier Cohort Study. Cancer Epidemiol Biomarkers Prev. 2007;16(4):740–746. doi: 10.1158/1055-9965.EPI-06-0829. [DOI] [PubMed] [Google Scholar]
- 35.Brohet R.M., Goldgar D.E., Easton D.F., et al. Oral contraceptives and breast cancer risk in the international BRCA1/2 carrier cohort study: a report from EMBRACE, GENEPSO, GEO-HEBON, and the IBCCS collaborating group. J Clin Oncol. 2007;25(25):3831–3836. doi: 10.1200/JCO.2007.11.1179. [DOI] [PubMed] [Google Scholar]
- 36.Daly M.B., Pilarski R., Axilbund J.E., et al. Genetic/familial high-risk assessment: breast and ovarian, version 2.2015. J Natl Compr Cancer Netw. 2016;14(2):153–162. doi: 10.6004/jnccn.2016.0018. [DOI] [PubMed] [Google Scholar]
- 37.Walker J.L., Powell C.B., Chen L.M., et al. S ociety of G ynecologic O ncology recommendations for the prevention of ovarian cancer. Cancer. 2015;121(13):2108–2120. doi: 10.1002/cncr.29321. [DOI] [PubMed] [Google Scholar]
- 38.Walker M., Jacobson M., Sobel M. Management of ovarian cancer risk in women with BRCA1/2 pathogenic variants. CMAJ. 2019;191(32):E886–E893. doi: 10.1503/cmaj.190281. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 39.Douglas F.S., O'Dair L.C., Robinson M., Evans D.G.R., Lynch S.A. The accuracy of diagnoses as reported in families with cancer: a retrospective study. J Med Genet. 1999;36(4):309–312. [PMC free article] [PubMed] [Google Scholar]
- 40.John E.M., Canchola A.J., Sangaramoorthy M., Koo J., Whittemore A.S., West D.W. Race/ethnicity and accuracy of self-reported female first-degree family history of breast and other cancers in the Northern California Breast Cancer Family Registry. Cancer Epidemiol Biomarkers Prev. 2019;28(11):1792–1801. doi: 10.1158/1055-9965.EPI-19-0444. [DOI] [PMC free article] [PubMed] [Google Scholar]
Associated Data
This section collects any data citations, data availability statements, or supplementary materials included in this article.