Highlights
-
•
HNSCC TIL functionality remains rescuable even in exhausted recurrent disease.
-
•
A subset of TILs demonstrate tumor-specificity across HNSCCs.
-
•
Individual neoantigen-specific TILs can be identified by single-cell co-culture.
Keywords: Head and neck cancer, T cell, Tumor-infiltrating lymphocyte, Immunotherapy
Abstract
Background
Human papillomavirus (HPV)-negative head and neck squamous cell carcinoma (HNSCC) remains a treatment-resistance disease with limited response to immunotherapy. While T cells in HNSCC are known to display phenotypic dysfunction, whether they retain rescuable functional capacity and tumor-killing capability remains unclear.
Methods
To investigate the functionality and tumor-specificity of tumor-infiltrating lymphocytes (TILs) across HNSCCs, malignant cell lines and TILs were derived from 31 HPV-negative HNSCCs at the time of standard surgical resection. T cell functional capacity was evaluated through ex vivo expansion, immunophenotyping, and IsoLight single-cell proteomics. Tumor-specificity was investigated through both bulk and single-cell tumor-TIL co-culture.
Results
TILs could be successfully generated from 24 patients (77%), including both previously untreated and radiation recurrent HNSCCs. We demonstrate that across HNSCCs, TILs express multiple exhaustion markers but maintain a predominantly effector memory phenotype. After ex vivo expansion, TILs retain immunogenic functionality even from radiation-resistant, exhausted, and T cell-depleted disease. We further demonstrate tumor-specificity of T cells across HNSCC patients through patient-matched malignant cell-T cell co-culture. Finally, we use optofluidic technology to establish an autologous single tumor cell-single T cell co-culture platform for HNSCC. Cells derived from three HNSCC patients underwent single-cell co-culture which enabled identification and visualization of individual tumor-killing TILs in real-time in all patients.
Conclusions
These studies show that cancer-specific T cells exist across HNSCC patients with rescuable immunogenicity and can be identified on a single-cell level. These data lay the foundation for development of patient-specific T cell immunotherapies in HNSCC.
Graphical abstract
Introduction
Head and neck squamous cell carcinoma (HNSCC), including cancers of the oral cavity, pharynx, and larynx, has an estimated incidence of more than 66,000 cases and 14,000 deaths per year in the United States [1]. Unlike Human Papillomavirus (HPV)-associated HNSCC which maintains favorable treatment outcomes, HPV-negative disease is highly treatment-resistant. For locoregionally advanced HPV-negative HNSCC, the mainstays of current therapy include surgery followed by radiation or definitive chemoradiation [2]. Multi-modality and multi-disciplinary management is essential to optimize treatment outcomes [3,4]. Patients often undergo extensive oral and pharyngeal resections only have to endure 6 and 7 weeks of post-operative radiotherapy or chemoradiotherapy after surgery. However, despite these aggressive treatments, 5-year survival rates remain under 50% [5]. Given the high morbidity and mortality associated with available treatment options for HNSCC, there is a need to develop improved management strategies. However, modifications of traditional therapies for HNSCC, such as surgery and chemoradiation, are unlikely to lead to major advances due to the inevitable development of resistant tumor clones [6].
T cell therapies, including immune checkpoint inhibition and adoptive cell transfer, are promising strategies to improve outcomes due to their polyclonal immune-based approach. By harnessing the adaptive immune response, these techniques have potential to deliver critical improvements in disease control. Current FDA approved immunotherapeutic agents for HNSCC target the PD-1/PD-L1 axis to reverse T cell exhaustion within the tumor microenvironment (TME). Although this treatment strategy has shown recent promise in HNSCC, including substantial early response rates, durable disease control is rare [7], [8], [9], [10].
Several barriers remain to improving cure rates in HNSCC using immunotherapies. Design of rational treatments is limited by gaps in knowledge regarding tumor-infiltrating lymphocyte (TIL) functionality and specificity in HNSCC. While PD-1/PD-L1-based therapies are well-founded, the efficacy of these agents may be constrained by other immune checkpoint pathways [11]. Further, these agents are limited by the functional capacity, absolute number, and tumor-specificity of effector T cells which they release from checkpoint inhibition [12,13].
Given the limited data characterizing the functionality of T cells within and across HNSCCs [14], [15], [16], we developed a prospective workflow to study HNSCC TILs, including autologous tumor-immune investigations. We established malignant cell lines directly from 31 HPV-negative HNSCC patient tumor samples. From separate pieces of matched tumor specimens, we analyzed TILs through multiplex flow cytometry and used orthogonal models to identify immunogenic tumor-specific T cells across HNSCC patients. We show upregulation of multiple immune checkpoint markers in TILs in a patient-specific manner. Despite this, we demonstrate that TIL functionality remains rescuable, even in treatment-resistant T cell-depleted disease. We then show that tumor-specific immunogenic T cells exist across HNSCCs, both within the TME and among the circulating T cell pool. Finally, we adapt a chip-based optofluidics system to perform single tumor cell-single T cell co-culture to directly identify individual tumor-killing TILs from HNSCC patients.
To improve immunotherapy for HNSCC, we must understand not only the phenotype but the objective functionality of TILs. Emerging single-cell datasets in HNSCC demonstrate a wide spectrum of TIL immunophenotypes, predicting a range of TIL dysfunctional states [11,[17], [18], [19]]. These studies, however, do not objectively determine if TIL functionality is rescuable or whether TILs retain capability to induce patient-matched tumor cell death. Through these observations, our data will inform the design of future immunotherapies and provide a platform to isolate individual tumor-killing T cells in HNSCC. These techniques may enable mechanistic identification of tumor-specific T cell receptors (TCRs) and patient-specific neoantigens.
Materials and methods
HNSCC specimen acquisition
Thirty-one patients were included in this cross-sectional report, including 17 female and 14 male patients. Patients were eligible for inclusion who had HPV-negative HNSCC undergoing either surgical resection or a clinically indicated biopsy of the primary tumor at the Medical College of Wisconsin. Patients consented for additional research-specific biopsies in conjunction with these indicated procedures. Study size was planned as a fixed available sample of HNSCCs from which a primary malignant cell line was successfully obtained over approximately a one-year period. Patients were consented consecutively. Demographic and tumor characteristics are listed in Supplementary Table 1. All data and specimen acquisition were approved by the Medical College of Wisconsin Institutional Review Board and Institutional Biosafety Committee. The STROBE cross sectional reporting guidelines were used to ensure rigor in reporting.
HNSCC specimen processing
Biopsy specimens were obtained fresh at the time of surgical resection or as part of a research-specific biopsy between February 27, 2022 and March 15, 2023. Samples were washed in PBS with 4% antibiotic-antimycotic (Gibco). The specimen was then split for multiple parallel analytic pathways. A piece was preserved in 10% buffered formalin for subsequent histologic analysis. The remaining tumor was diced into segments and separated for (1) immediate flow cytometry, (2) malignant cell culture, (3) TIL culture. The tumor piece for flow cytometry was then digested according to the Miltenyi Tumor dissociation kit protocol in a Miltenyi gentleMACS C tube using the Miltenyi gentleMACS tissue dissociator. Cellular debris was removed from the dissociated cell suspension using Debris Removal Solution (Miltenyi). Live cells were enumerated using trypan blue dye on Countess Automated Cell Counter Hemocytometer C10227 (Invitrogen).
HNSCC cell lines
Cell lines were directly derived from fresh patient HNSCC biopsies. Tumor specimens were minced and cultured such that a broad surface of tumor was directly adherent to the culture plate. Culture media included DMEM/F12 with 1.2 g/L sodium bicarbonate, 2.5 mML-glutamine, 15mMHEPES, and 0.5 mM sodium pyruvate supplemented with 400 ng/mL hydrocortisone with 10% FBS. 2% antibiotic-antimycotic (Gibco) was used to limit contamination. Bulk tumor pieces were removed from the plate once adherent cells were established. All cell lines were cultured in an incubator at 37 °C with 5% CO2. Malignant populations were separated from cancer-associated fibroblasts (CAFs) through serial differential trypsinization given the absence of fully reliable surface markers for malignant cells in HNSCC. Mixed cell lines were incubated for approximately 2 min in the presence of TrypLE (Gibco) which preferentially harvests the less adherent fibroblastic population. Since malignant HNSCC cells can lose epithelial surface markers and express fibroblastic and mesenchymal signatures [20,21], serial differential trypsinization remains the most reliable method to enrich for malignant lines while maximally preserving tumor clonal heterogeneity.
TIL isolation
TILs were extracted from separate pieces of the same biopsy specimens used to generated malignant lines. For TIL generation, fresh HNSCC specimens were finely minced (1 mm sections) and cultured. TIL culture media included ImmunoCult XF Expansion media (StemCell Technologies), 6000 U/mL IL2 (Peprotech), and 2% antibiotic-antimycotic (Gibco). Cultures were inoculated on Day 0 with soluble CD3/CD28 activator (StemCell Technologies). Once expanding T cell colonies were established, T cells were enriched with CD45 magnetic column selection (Miltenyi). The early TILs were then cryopreserved (90% FBS/10% DMSO) until patient-matched malignant lines had matured. As required, TILs were later column selected for CD4 or CD8 (Miltenyi) for each experiment.
Circulating T cell isolation
For HNSCC patients who underwent peripheral blood sampling concurrent with biopsy, approximately 45 mL of blood was taken. Ficoll-gradient separation was used to isolate the buffy coat which was aspirated. For flow cytometry, circulating T cells were immediately processed fresh. The remaining T cells were then cryopreserved (90% FBS/10% DMSO). After thaw, circulating T cells were stimulated with CD3/CD28 activation to obtain sufficient numbers for ex vivo study and to remain comparable to extracted TILs in downstream analyses.
Flow cytometry
106 cells per sample were used for immunostaining with conjugated monoclonal antibodies. 7-AAD (420404, BioLegend) was used to exclude dead cells. Single color tubes were employed to set up a compensation matrix, and a fluorescence minus one control tube was included to ensure specific staining. For immunophenotyping of digested HNSCC specimens, the following antibodies were used: VioBlue anti-human CD223 (Miltenyi, clone REA351), VioGreen anti-human CD4 (Miltenyi, clone M-T466), FITC anti-human CD45 (Miltenyi, clone 5B1), PE anti-human TIGIT (Miltenyi, clone REA 1202), PE-Vio770 anti-human CD279 (Miltenyi, clone REA1165), APC anti-human TIM3 (Miltenyi, clone F38–2E2), APC-Vio770 anti-human (Miltenyi, clone REA734), VioBlue anti-human CD45RA (Miltenyi, clone REA562), FITC anti-human CD3 (Miltenyi, clone REA613), PE-Vio770 anti-human CD62L (Miltenyi, clone REA615), APC anti-human CD45RO (Miltenyi, clone REA611). Memory T cell subsets were defined as previously described [22,23], including naïve (CD45RA+/CD45RO-/CD62L+), central memory (CD45RA-/CD45RO+/CD62L+), effector memory (CD45RA-/CD45RO+/CD62L-), and terminally differentiated effector re-expressing CD45RA (CD45RA+/CD45RO-/CD62L-). Antibody staining was done at 4 °C for 30 min. Cells were washed and suspended in 0.2 mL staining buffer (PBS with 2% FBS). The samples were then acquired on a MACSQuant 10 Analyzer Flow Cytometer (Miltenyi). Data was analyzed using FlowJo software version 10.9 (BD Life Sciences). To verify gating and purity, all populations were routinely backgated.
Immunofluorescence
Tumor-immune spatial heterogeneity was quantified through histologic analysis. Multiplex immunostaining was performed using an Opal Polaris 7-Color Automation IHC Kit (Akoya Biosciences, Marlborough, MA; Cat#NEL871001KT), an optimized multiplex for CD8 (Cell Marque, Cat#108M-95), CD4 (Cell Marque, Cat#104R-25), FoxP3 (Cell Signaling, Cat#12653S), CD68 (Cell Marque, Cat#168M-95), CD20 (Cell Marque, Cat#120M-85), panCK (Dako/Agilent, Cat#M3515) and DAPI (Akoya, Cat#210713036). Analysis was conducted on a Leica Bond Rx fully automated autostainer (Leica Biosystems, Deer Park, IL). Biomarkers that could co-localize in the same cellular compartment were paired with a spectrally separated Opal fluorophore to avoid potential spectral interference, as recommended by the manufacturer. All multiplex immunofluorescence slides were scanned on an Akoya Vectra Polaris (Akoya Biosciences, Marlborough, MA) at 20X using MOTiF™ protocol, which generates a single unmixed whole slide scan of up to 7 colors. This single image facilitates a rapid application of digital image analysis across the entire slide in a streamlined workflow, without the requirement of stitching many spectrally unmixed image tiles. Thereafter, whole slide images were loaded into InForm image analysis software for automated cell type density quantification.
xCELLigence cytolysis assay
Autologous adherent tumor cells derived from HNSCC patients were plated in a 96-well xCELLigence E-Plate at 10,000 cells/well. The plate was incubated at room temperature for 30 min to facilitate uniform immobilization of the target cells on plate bottom and then placed in the xCELLigence instrument. Data acquisition was initiated and the plate was incubated at 37 °C. After 24 h, effector cells (circulating T cells or TILs) were plated in triplicate at several effector to target ratios. Appropriate negative (effector or target cells only) and positive (addition of Agilent cytolysis reagent-Cat#006641920) controls were performed. After addition of the effector cells, the plate was incubated at room temperature for 30 min to facilitate uniform distribution of the effectors. The plate was placed back onto the xCELLigence instrument and incubated for 24 h at 37 °C with continuous data acquisition. Data were analyzed using RTCA Software Pro-v2.3.2), Microsoft Excel Mac v16.77.1, and Graphpad Prism v10.0.3.
IsoLight single-cell proteomics
Circulating T cells and TILs were separated into CD4 and CD8 T-cell subsets by magnetic bead separation using the human CD4 and CD8 MicroBeads (Miltenyi) per manufacturer's instructions. Cells were then stimulated by incubation with T cell TransACT at a titer of 1:100 for 24 h in ImmunoCultTM-XF T Cell Expansion Medium (Stemcell Technologies) supplemented with 6000 U/ml human IL-2 (Peprotech). After stimulation, cells were stained with either CD4 or CD8 AF647 and loaded onto human adaptive immune IsoCode chips (IsoPlexis). The chips and detection reagents were loaded into the IsoLight instrument (IsoPlexis) for automated incubation and data acquisition. Data analysis was performed using IsoSpeak software v2.9.0.
Single tumor cell-single T cell co-culture
For each Phenomenex Lightning Optofluidics run, individual CD8 T cells and tumor cells were loaded into nanopen chambers on the optofluidics chip for single-cell functional assessment. Each chip contains 1500 nanopen chambers for analysis. For successful nanopen loading, at least 4 × 105 viable CD8 TILs and 4 × 105 tumor cells are required. Early experience with fresh HNSCC digestions demonstrated that immediate sorting of primary cells into malignant and TIL populations by flow cytometry or magnetic bead separation leads to insufficient cellular viability for successful single tumor cell-single T cell co-culture. The ischemic injury consequent to surgical biopsy and immediate manipulation by tumor digestion and sorting yields cell populations that cannot adequately survive in single-cell suspension for the requisite 48 h. To overcome this barrier, primary tumor and TIL cultures were first established from tumor specimens. Malignant and TIL populations can then be isolated from culture with excellent viability, sufficient for optofluidic analyses. Immediately prior to chip loading, tumor cells were labeled with CFSE for cytotoxicity profiling. Chip loading was performed through optofluidic technology to include an individual CD8 T cell and an individual tumor cell within each of the 1500 nanopen chambers. Due to the stochastic nature of nanopen loading, a minority of nanopens are loaded with tumor cells only, which serve as negative controls. Loaded cells were cultured in growth media supplemented with IL-7 and IL-15. T cell-mediated tumor cell killing was measured during 48 h of culture through fluorescent reporting via Caspase-3 detection.
Quantification and statistical analysis
All comparisons were performed using IBM SPSS Statistics v28. All data were checked for normal distribution and similar variance between groups. Differences between groups were determine by Mann-Whitney U test, independent sample t-test, or one-way ANOVA, depending on data normality and number of involved variables. Data are derived from multiple independent experiments from distinct HNSCC patients.
Study approval
Study activities were approved by the Medical College of Wisconsin Institutional Review Board (approval PRO00040992) and the Institutional Biosafety Committee (approval IBC20210026). All included patients provided written informed consent.
Results
Study population and tumor samples
Over a period of 12 months, tumor samples from 31 patients with HPV-negative HNSCC underwent ex vivo culture with successful generation of malignant cell lines. Twenty-four (77%) HNSCC samples were obtained in treatment naïve patients, while 7 (23%) were taken from locoregionally recurrent disease after prior radiotherapy. Patient tumor and treatment characteristics are shown in Table 1. For 29 (93%) HNSCC samples, separate cultures were also set up to isolate patient-matched autologous TILs. Twenty-eight (90%) patients underwent concurrent peripheral blood sampling with isolation and processing of circulating T cells.
Table 1.
Study population tumor and treatment characteristics.
| Characteristic | N = 31 tumor samples |
|---|---|
| Age: mean (standard deviation) | 66.1 years (2.4 years) |
| Gender Male Female |
17 (53%) 14 (47%) |
| Smoking history Never smoker or <10 pack-years ≥ 10 pack-years |
5 (16%) 26 (84%) |
Alcohol
|
21 (67%) 10 (33%) |
| Tumor subsite Oral cavity Larynx/hypopharynx |
29 (95%) 2 (5%) |
| T-stage T2 T3 T4a |
8 (26%) 7 (23%) 16 (51%) |
| N-stage N0 N+ |
17 (55%) 14 (45%) |
| Prior treatment None: de novo HNSCC Radiotherapy: recurrent HNSCC |
24 (77%) 7 (23%) |
| Histologic grade* Well differentiated Moderately differentiated Poorly differentiated |
2 (6%) 25 (81%) 4 (13%) |
| Perineural invasion | 22 (71%) |
| Depth of invasion: mean (standard deviation)⁎⁎ | 11.7 mm (1.2 mm) |
| High-risk pathology⁎⁎ | 9 (32%) |
| Cell subset density (cells/mm2)⁎⁎⁎ CD8 T cells: median (range) CD4 T cells: median (range) Macrophages: median (range) Regulatory T cells: median (range) B cells: median (range) Malignant cells: median (range) |
81 (2–630) 568 (17–2180) 216 (35–1227) 104 (10–626) 61 (0–584) 1389 (44–4363) |
Histologic grade not reported in 6 samples.
3 patients underwent research biopsy only without definitive resection.
7-color immunophenotyping performed for 29 patients.
Autologous malignant cell lines with matched TILs can be isolated across HNSCCs
Tumor samples generated malignant cell lines at a median of 6 weeks (range 2–32 weeks). After serial differential trypsinization, malignant cultures were predominantly epithelial with a smaller subpopulation expressing non-epithelial markers (Supplementary Fig. 1). Malignant lines could be successfully generated from both early stage and advanced tumors, de novo and radioresistant tumors, and from tumors with low and high stromal composition (Table 1). Of 29 TIL cultures set up, 24 generated sufficient early TILs for column enrichment and cryopreservation at a median of 10 days (range 4–14 days). A median of 2.8 × 106 (range 0.2–3.6 × 106) early TILs were recovered per patient. Success of early TIL isolation was not significantly associated with baseline intra-tumoral T cell density and TILs could be successfully expanded even from patients with very low T cell infiltrate (Supplementary Fig. 2).
HNSCC TILs express multiple exhaustion markers in a patient-specific manner but maintain a predominantly memory phenotype with rescuable functional capacity
In addition to malignant and TIL cultures, HNSCC tumor pieces were also digested at the time of biopsy for immediate multiplex flow cytometry to evaluate phenotypic exhaustion and memory marker expression. Blood samples taken at the time of HNSCC biopsy were used to evaluate circulating T cells with the same immunophenotyping panels. TILs expressed higher levels of several exhaustion markers, including PD-1, TIM-3, and LAG-3 across HNSCCs (Fig. 1, top panels). However, TILs maintained a memory phenotype similar to circulating T cells. In subset analysis, TILs were found to be predominantly effector memory while circulating T cells had larger central memory populations (Fig. 1, bottom panels).
Fig. 1.
Comparison of exhaustion marker expression (top panels) and memory marker expression (bottom panels) from flow cytometry of freshly digested tumor biopsies or patient-matched circulating T cells for CD4 and CD8 subsets. ***p<0.001, **p<0.01. Exhaustion markers assessed in n = 57 samples (n = 29 TIL and n = 28 circulating T cell). Memory markers assessed in n = 52 samples (n = 24 TIL and n = 28 circulating T cell).
These immunophenotyping data suggested that despite exhaustion marker expression, TILs may have replicative capacity with rescuable functionality. To investigate the effects of TCR stimulation and expansion on TIL immunophenotype we performed ex vivo CD3/CD28 activation and compared TIL phenotypic change to patient-matched circulating T cells. Although activation induced early upregulation of multiple exhaustion markers, an effector memory phenotype was preserved in both TIL and circulating T cell populations. Continued culture in the presence of pro-immunogenic cytokine maintained a memory phenotype with a shift towards central memory populations (Fig. 2, bottom panels). Expression of exhaustion markers subsequently demonstrated downregulation over time (Fig. 2, top panel; Supplementary Fig. 3). These data suggested that TILs in HNSCC retain phenotypic plasticity and may have rescuable functional capacity.
Fig. 2.
Comparison of exhaustion marker expression (top panels) and memory marker expression (bottom panels) from flow cytometry of expanded TILs or patient-matched circulating T cells at early (day 4–5 post-activation) and late (day 10–11 post-activation) for CD4 and CD8 subsets. ***p<0.0001, +p = 0.038, *p = 0.050. Exhaustion markers assessed in n = 6 samples (n = 3 TIL and n = 3 circulating T cell). Memory markers assessed in n = 4 samples (n = 2 TIL and n = 2 circulating T cell).
We then evaluated whether this observed phenotypic plasticity in HNSCC TILs could translate to objective functionality. Specifically, we asked whether TILs in a tumor with adverse clinical characteristics, such as recurrent treatment-resistant disease and low baseline TIL infiltration, retained rescuable pro-immunogenic capacity. To examine this possibility, we identified a patient with locally recurrent HNSCC after extensive prior treatment (surgery and chemoradiotherapy) with very low T cell infiltrate and extensive baseline phenotypic exhaustion of viable TILs (Fig. 3, top panel). We then isolated TILs and circulating T cells for IsoLight single-cell proteomics (Fig. 3, bottom panel). Despite these adverse tumor characteristics, TILs isolated from this recurrent HNSCC expanded (2.8 × 106 early TILs, falling at the median of all our clinical samples), retained a central memory phenotype, and demonstrated potent effector and chemotactic immunogenicity upon T cell receptor stimulation ex vivo (Fig. 4). Surprisingly, this patient subsequently failed anti-PD1 therapy with pembrolizumab. Taken together, the above data suggest that HNSCC TILs in treatment-resistant disease are not senescent, but instead have recoverable functional capacity, even in an end-stage T cell-depleted tumor. However, a combinatorial approach is likely necessary, as anti-PD1 treatment alone was insufficient to reinvigorate TILs clinically despite their objective functional potential.
Fig. 3.
MCW Biobank patient 12 (P012) underwent biopsy of the primary tumor (top panel, top section) at the time of upfront surgical resection and then a subsequent biopsy from the locally recurrent tumor (top panel, bottom section) after radiotherapy. The recurrent tumor was significantly T cell-depleted as demonstrated by immunohistochemistry (top panel, left section) with upregulation of exhaustion markers (top panel, right section). (Bottom panel) Schematic for the methodology to evaluate TIL and circulating T cell single-cell functionality from the radiorecurrent T cell-depleted tumor of patient P012.
Fig. 4.
After TIL and circulating T cell isolation, T cells from P012 were rested overnight and underwent evaluation with IsoLight single-cell proteomics immediately after CD3/CD28 activation. CD8 and CD4 TILs secreted effector proteins to a greater extent than patient-matched CD8 and CD4 circulating T cells. In particular, CD8 TILs demonstrated polyfunctional secretion of both effector and chemoattractive proteins.
Both HNSCC TILs and circulating T cells demonstrate concentration-dependent autologous tumor killing
While our phenotypic and functionality data suggest TILs in HNSCC retain tumor-killing potential, the extent of TIL tumor-specificity has not been established. To address this, we first asked whether HNSCC TILs can induce an objective immunogenic response against patient-matched malignant cells. We, therefore, performed bulk co-culture of autologous tumor cells and matched TILs derived from five HNSCC samples. These studies demonstrated concentration-dependent TIL-mediated killing of autologous tumor cells across HNSCC patients (Fig. 5). Surprisingly, we also identified robust anti-tumor functionality of circulating T cells across HNSCC samples, again, in a concentration-dependent manner (Fig. 5). To confirm that these effects were tumor-specific, we tested autologous co-culture with patient-matched T cells and cancer-associated fibroblasts (CAF). Indeed, autologous co-culture of both circulating T cells and TILs with patient-matched CAFs demonstrated less cytolysis than co-culture with patient-matched enriched malignant cells (Supplementary Fig. 4). Some level of target cell cytolysis in the CAF population is not surprising given that CAFs isolated from HNSCCs are heterogenous, growth promoting, and commonly include a subpopulation of malignant cells [24].
Fig. 5.
(Left panel) Both TILs and circulating T cells demonstrate concentration-dependent killing of patient-matched malignant cells (n = 5 patients, ***p<0.001). Representative kill curves using tumor cells and T cells from patient P080 demonstrate concentration-dependent killing by circulating (middle panel) circulating T cells and (right panel) TILs.
Immunogenic tumor-killing T cells exist across HNSCCs and can be identified through autologous single tumor cell-single T cell co-culture
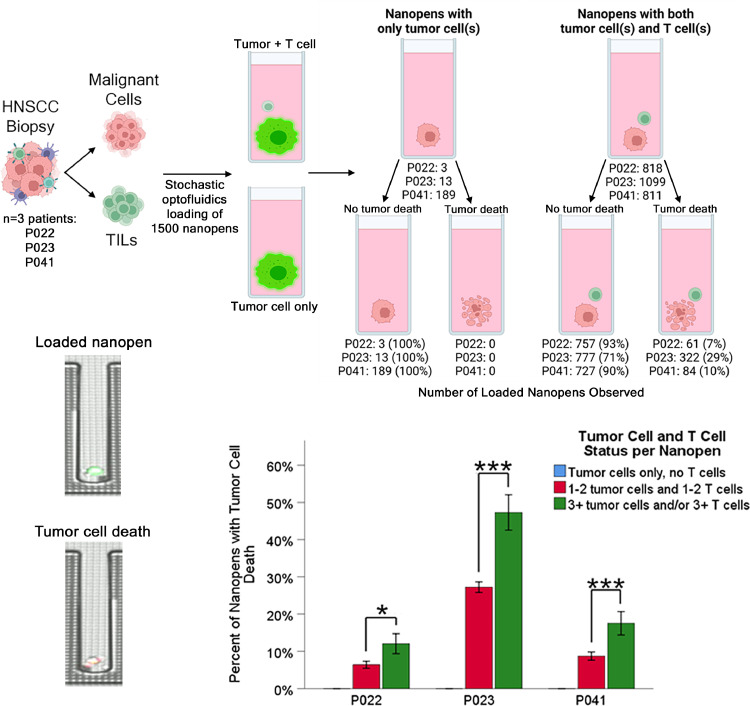
The concentration-dependent tumor-killing observed among TILs and circulating T cells suggested that only a low percentage of tumor-specific T cells existed within these immune populations. In that way, large numbers of bulk T cells were required for the smaller population of tumor-specific T cells to reach a threshold concentration for observable tumor cell killing. To further examine this hypothesis, and visually identify individual tumor-killing T cells from HNSCC patients, we performed single tumor cell-single T cell co-culture using patient-derived malignant cells and patient-matched TILs from three separate HNSCC patients (Fig. 6). These studies identified a subpopulation of TILs on a single-cell level across HNSCCs that demonstrated objective tumor cell killing (Fig. 6, top panel, Supplementary Video 1). Negative control wells, which included tumor only without T cells, demonstrated full viability, supporting the immune-mediated nature of observed tumor cell death.
Fig. 6.
(Top panels) Results from three HNSCC patients who under tumor biopsy, creation of malignant cell lines and TIL cultures, followed by autologous single tumor cell-single TIL co-culture. Observed tumor cell death is shown from both control nanopens without T cells and from experimental nanopens with both tumor cells and T cells. (Bottom left panels) Representative micrographs from a loaded nanopen with a viable tumor cell (CFSE green stain) and a nanopen with T cell-mediated tumor cell death (Caspase-3 red stain) are shown. (Bottom right panel) Due to the stochastic nature of nanopen loading, some nanopens will contain more than one T cell and or tumor cell. The occurrence of T cell-mediated tumor cell death is significantly greater in multiply-loaded nanopens compared with the majority single tumor cell-single T cell loaded nanopens. ***p<0.001, *p = 0.018. Data represent mean ± SEM.
Even in this single-cell assay format, we again identified concentration-dependent tumor cell killing. When performing single tumor cell-single T cell co-culture, optofluidics are used to load individual nanopens with tumor cells and T cells. While this mechanism achieves single tumor cell-single T cell resolution in the majority of nanopens, a minority include doublets or triplets of tumor cells and/or T cells. Unsurprisingly, the inclusion of multiple tumor cells (more antigenic presentation) or multiple T cells (more TCR variation) led to greater tumor cell killing (Fig. 6, bottom panel).
Discussion
For effective immunotherapy in solid malignancies, cancer-specific T cells must traffic to the tumor in sufficient numbers, have access to suitable nutrients, and overcome soluble and cellular mechanisms of intra-tumoral immunosuppression [25]. The foundation of this cascade is T cell tumor-specificity. Without TCRs that can be activated by tumor neoantigen and lead to a cytolytic response, T cell-based immunotherapies will be ineffective, including immune checkpoint blockade and adoptive cell transfer. For this reason, efforts to enhance in vivo expansion of tumor-specific TILs or ex vivo engineering and transfer of tumor-specific T cells are integral to improved immunotherapy development and design.
Our data suggest that such tumor-specific T cells exist in low percentages among both the TIL and circulating T cell pools across HNSCC patients. These data are consistent with recent reports in HNSCC demonstrating tumor antigen-specific TCR identification both within the TME and circulating in peripheral blood [18,19,26]. These previous studies, however, rely on indirect assessments of tumor-specificity through IFNγ secretion in response to putative neoantigen identified in silico and manufactured ex vivo. Although elegant efforts, these engineered designs using predicted peptides and T cell cytokine secretion do not evaluate tumor killing in the setting of native antigen presentation [27], [28], [29], [30], [31], [32], [33], [34], [35]. This methodology has several potential limitations. First, IFNγ-negative T cells can induce antigen-specific cytotoxicity, thereby overlooking relevant TCRs [36]. Second, there is no guarantee that neoantigens identified in silico will be expressed unmodified at the protein level and presented by tumor cells in their native state. Further, reliance on exomic mutational profiling omits immunogenic neoantigens derived from other tumor metabolic derangements, such as altered RNA editing, proteomic processing, or non-canonical translation [37], [38], [39]. This is supported by studies demonstrating TCR recognition of patient-derived xenograft tissue in the absence of reactivity against predicted peptide neoantigens [32]. Select recent reports in solid malignancies that have used tumor cell-T cell co-culture to identify tumor-reactive T cells have similarly relied on cytokine or surface marker expression as surrogates for tumor cell killing or employ an engineered non-native target cell system, and none have achieved single tumor cell-single T cell resolution [39], [40], [41], [42], [43].
To more precisely identify T cells which lead to observable tumor cell death, we developed a workflow for extraction of patient-matched tumor cells and TILs to identify T cell-mediated autologous tumor cell killing in HNSCC. By performing this workflow for over 30 patients, we demonstrate feasibility of acquiring these critical cells at a large scale. By extracting patient TILs and optimizing their growth conditions, we can mechanistically study tumor-TIL interactions. Even in exhausted TILs from recurrent heavily treated tumors, we show robust immunogenic functionality ex vivo upon TCR binding. Therefore, our system will allow for isolation of TCR:neoantigen interactions in patient-matched tumor cells and TILs with positive identification of those T cells which objectively kill cancer.
Better characterizing these key immunogenic cancer-specific T cells may improve both the in vivo study of immunotherapeutics and aid in polyclonal engineered T cell design. Using this workflow, we first demonstrate in bulk co-culture that tumor-killing T cells exist across HNSCC patients. We found this effect to be concentration-dependent, implying that most TILs and circulating T cells are non-reactive. Many bulk T cells are required for the small subset of cancer-specific immunogenic T cells to achieve threshold concentration and induce observable tumor cytolysis. This is supported by recent reports which suggest that most TILs are likely bystanders, without tumor-killing capability [26,41]. This finding also emphasizes the potential importance of T cell concentration within the tumor microenvironment. If tumor killing can be improved in vitro through stoichiometric manipulation, it suggests that tumor-directed T cell chemotaxis and trafficking may be valuable synergistic components to the immunotherapeutic approach.
Our bulk co-culture findings also suggested that if tumor cells can be targeted for T cell-mediated death, immunogenic neoantigens must also exist across HNSCCs. To improve resolution of these interactions, we adapted a chip-based optofluidics technology to perform co-culture at the single cell-level, including individual autologous tumor cell-T cell interactions. This platform enables direct identification of individual cancer-specific T cells which induce objective pro-immunogenic responses that lead to patient-matched tumor cell death. This platform may be leveraged in future studies for cancer-specific TCR and neoantigen discovery. Current approaches to identify neoantigens use in silico prediction from exomics, transcriptomics, or immunopeptidomics but cannot determine clinical immunogenicity, relying instead on T cell cytokine secretion or surface marker expression, as described above [44]. Our approach addresses this central limitation by directly identifying individual T cells that induce tumor cell death.
In addition to demonstrating tumor-specificity of TILs across HNSCCs, we also show that TILs retain rescuable immunogenic functionality despite broad phenotypic exhaustion. This evidence is supported by other recent reports in HNSCC showing that neoadjuvant immunotherapy leads to expansion of exhausted T cells in vivo [18,19,45]. These studies, however, evaluated previously untreated HNSCC. We demonstrate that even in T cell-depleted, radiorecurrent, heavily exhausted disease, TCR activation with cytokine supplementation leads to pro-immunogenic effector activity including secretion of granzyme and perforin. Although current approaches in recurrent/metastatic HNSCC with immune checkpoint blockade induce very limited durable control [8], our results suggest further development of combinatorial agents which can better reinvigorate TILs in the recurrent setting. In particular, we identify upregulation of multiple concurrent exhaustion pathways in HNSCC TILs both within the TME and during TCR activation, including LAG3 and TIM3.
There are several limitations to this study which will require further investigation in future work. The maintenance of malignant cell neoantigen expression and heterogeneity from the in vivo tumor microenvironment to our in vitro system is not certain. Recent studies in other solid malignancies, however, demonstrate the close overlap between exomic and RNA sequencing profiles of patient-derived malignant lines and the initial tumor sample [42,46]. Additionally, given malignant cell heterogeneity, single tumor cell-single T cell co-culture may miss tumor-specific TCRs based on the stochastic nature of nanopen loading. However, across 1500 nanopens, it is likely that many relevant tumor clone-TIL clone pairs are represented. Additionally, although many TILs in HNSCC appear to retain rescuable functionality based on our IsoLight proteomics data, individual irreversibly exhausted TILs carrying high avidity tumor-specific TCRs may not react at the single-cell level, resulting in a false negative result [32].
Finally, while T cell tumor-specificity is the foundation from which all T cell-based immunotherapeutics must be built, tumor-specificity alone will be insufficient for cure in most cases. Once expansion and transfer of tumor-specific T cells can be more readily achieved, we must address T cell tumor infiltration, intra-tumoral nutrient supply, and overcome the myriad of TME immunosuppressive factors. Nonetheless, our autologous tumor cell-T cell platform delivers the most rigorous methodology to-date to identify immunogenic tumor-specific T cells in HNSCC. This work lays the foundation for personalized tumor-specific TCR and neoantigen discovery.
Funding
This work was supported by funding from the MCW Department of Radiation Oncology, the OTO Clinomics pilot grant from the Advancing a Healthier Wisconsin Endowment at the Medical College of Wisconsin with support by the National Center for Advancing Translational Sciences, Award Number UL1TR001436, NCI R21CA279935, the Benjamin Garmer Gift Fund, and the Don and Sharyn Blatnik Endowment at the Medical College of Wisconsin. The content is solely the responsibility of the authors and does not necessarily represent the official views of the NIH and Cancer Center as well as from Advancing a Healthier Wisconsin.
Supplementary Fig. 1.(Right) Flow cytometry from a representative patient-derived cell line enriched for malignancy using differential trypsinization. (Left) Representative EpCAM and FAP immunocytochemical staining.
CRediT authorship contribution statement
Joseph Zenga: Conceptualization, Formal analysis, Funding acquisition, Writing – original draft, Writing – review & editing. Musaddiq Awan: Conceptualization, Formal analysis, Funding acquisition, Writing – original draft, Writing – review & editing. Anne Frei: Data curation, Methodology. Jamie Foeckler: Data curation. Rachel Kuehn: Data curation. Oscar Villareal Espinosa: Data curation. Jennifer Bruening: Writing – original draft, Writing – review & editing. Becky Massey: Writing – original draft, Writing – review & editing. Stuart Wong: Writing – original draft, Writing – review & editing. Aditya Shreenivas: Writing – original draft, Writing – review & editing. Monica Shukla: Writing – original draft, Writing – review & editing. Julia Kasprzak: Writing – original draft, Writing – review & editing. Yunguang Sun: Data curation, Formal analysis. Md Shaheduzzaman: Data curation, Formal analysis. Fanghong Chen: Data curation, Formal analysis. Tyce Kearl: Formal analysis, Methodology, Writing – original draft, Writing – review & editing. Heather A. Himburg: Conceptualization, Formal analysis, Funding acquisition, Project administration, Writing – original draft, Writing – review & editing.
Declaration of competing interest
The authors have no competing interests.
Footnotes
Supplementary material associated with this article can be found, in the online version, at doi:10.1016/j.tranon.2024.101899.
Appendix. Supplementary materials
Supplementary Fig. 2. Success of TIL expansion and isolation from patient tumor biopsies based on T cell infiltrate as determined by digital image analysis from immunohistochemical staining. N = 29 total samples.
Supplementary Fig. 3. Flow cytometric characterization of exhaustion markers on TILs from freshly digested tumor at the time of surgery resection. TILs were subsequently extracted from additional biopsy specimens from the same tumor, stimulated with CD3/CD28 activation, and incubated in T cell media. The same exhaustion marker panel was examined by flow cytometry on these active TILs on post-activation day 4, day 7, and day 11.
Supplementary Fig. 4.(Left) Circulating T cells and (right) TILs demonstrate less target cell cytolysis of a patient-matched cell line enriched for cancer-associated fibroblasts when compared with the patient-matched enriched malignant cell line.
Supplementary Video 1. Time-lapse video demonstrating autologous single tumor cell-single TIL co-culture from a HNSCC patient resulting in T cell-mediated tumor cell death (Caspase-3 red fluorescence).
Data availability
Information and requests for resources and reagents should be directed to and will be fulfilled by the lead contact, Dr Heather Himburg (hhimburg@mcw.edu). Any additional information required to reanalyze the data reported in this paper is available from the lead contact upon request.
References
- 1.American Cancer Society. Cancer statistics: analysis tool. 2023 Accessed September 26, 2023. https://cancerstatisticscenter.cancer.org.
- 2.NCCN clinical practice guidelines in oncology: head and neck cancer. Updated February 2023. Accessed October 12, 2023. https://www.nccn.org/professionals/physician_gls/pdf/head-and-neck.pdf.
- 3.De Felice F., Tombolini V., de Vincentiis M., et al. Multidisciplinary team in head and neck cancer: a management model. Med. Oncol. 2018;36(1):2. doi: 10.1007/s12032-018-1227-z. Nov 13. [DOI] [PubMed] [Google Scholar]
- 4.Machiels J.P., René Leemans C., Golusinski W., et al. Squamous cell carcinoma of the oral cavity, larynx, oropharynx and hypopharynx: EHNS-ESMO-ESTRO clinical practice guidelines for diagnosis, treatment and follow-up. Ann. Oncol. 2020;31(11):1462–1475. doi: 10.1016/j.annonc.2020.07.011. Nov. [DOI] [PubMed] [Google Scholar]
- 5.Lee N.Y., Ferris R.L., Psyrri A., et al. Avelumab plus standard-of-care chemoradiotherapy versus chemoradiotherapy alone in patients with locally advanced squamous cell carcinoma of the head and neck: a randomised, double-blind, placebo-controlled, multicentre, phase 3 trial. Lancet Oncol. 2021;22(4):450–462. doi: 10.1016/S1470-2045(20)30737-3. 04. [DOI] [PubMed] [Google Scholar]
- 6.Jiang A.M., Ren M.D., Liu N., et al. Tumor mutation burden, immune cell infiltration, and construction of immune-related genes prognostic model in head and neck cancer. Int. J. Med. Sci. 2021;18(1):226–238. doi: 10.7150/ijms.51064. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 7.Cohen E.E.W., Bell R.B., Bifulco C.B., et al. The Society for Immunotherapy of Cancer consensus statement on immunotherapy for the treatment of squamous cell carcinoma of the head and neck (HNSCC) J. Immunother. Cancer. 2019;7(1):184. doi: 10.1186/s40425-019-0662-5. Jul. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 8.Burtness B., Harrington K.J., Greil R., et al. Pembrolizumab alone or with chemotherapy versus cetuximab with chemotherapy for recurrent or metastatic squamous cell carcinoma of the head and neck (KEYNOTE-048): a randomised, open-label, phase 3 study. Lancet. 2019;394(10212):1915–1928. doi: 10.1016/S0140-6736(19)32591-7. 11. [DOI] [PubMed] [Google Scholar]
- 9.Schoenfeld J.D., Hanna G.J., Jo V.Y., et al. Neoadjuvant nivolumab or nivolumab plus ipilimumab in untreated oral cavity squamous cell carcinoma: a phase 2 open-label randomized clinical trial. JAMA Oncol. 2020;6(10):1563–1570. doi: 10.1001/jamaoncol.2020.2955. Oct 01. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 10.Uppaluri R., Campbell K.M., Egloff A.M., et al. Neoadjuvant and adjuvant pembrolizumab in resectable locally advanced, human papillomavirus-unrelated head and neck cancer: a multicenter, phase II trial. Clin. Cancer Res. 2020;26(19):5140–5152. doi: 10.1158/1078-0432.CCR-20-1695. Oct. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 11.Cillo A.R., Kürten C.H.L., Tabib T., et al. Immune landscape of viral- and carcinogen-driven head and neck cancer. Immunity. 2020;52(1):183–199. doi: 10.1016/j.immuni.2019.11.014. Jan 14e9. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 12.Qin H., Hu H., Liao X., et al. Antitumor effect of neoantigen-reactive T cells combined with PD1 inhibitor therapy in mouse lung cancer. J. Cancer Res. Clin. Oncol. 2023:1–16. doi: 10.1007/s00432-023-04683-5. Mar 18. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 13.Sun J., Zhang J., Hu H., et al. Anti-tumour effect of neo-antigen-reactive T cells induced by RNA mutanome vaccine in mouse lung cancer. J. Cancer Res. Clin. Oncol. 2021;147(11):3255–3268. doi: 10.1007/s00432-021-03735-y. Nov. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 14.De Meulenaere A., Vermassen T., Aspeslagh S., Vandecasteele K., Rottey S., Ferdinande L. TILs in Head and Neck Cancer: ready for Clinical Implementation and Why (Not)? Head Neck Pathol. 2017;11(3):354–363. doi: 10.1007/s12105-016-0776-8. Sep. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 15.Spector M.E., Bellile E., Amlani L., et al. Prognostic value of tumor-infiltrating lymphocytes in head and neck squamous cell carcinoma. JAMA Otolaryngol. Head Neck Surg. 2019;145(11):1012–1019. doi: 10.1001/jamaoto.2019.2427. Nov 01. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 16.Lechner A., Schlößer H., Rothschild S.I., et al. Characterization of tumor-associated T-lymphocyte subsets and immune checkpoint molecules in head and neck squamous cell carcinoma. Oncotarget. 2017;8(27):44418–44433. doi: 10.18632/oncotarget.17901. Jul 04. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 17.Kürten C.H.L., Kulkarni A., Cillo A.R., et al. Investigating immune and non-immune cell interactions in head and neck tumors by single-cell RNA sequencing. Nat Commun. 2021;12(1):7338. doi: 10.1038/s41467-021-27619-4. Dec 17. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 18.Redman J.M., Friedman J., Robbins Y., et al. Enhanced neoepitope-specific immunity following neoadjuvant PD-L1 and TGF-β blockade in HPV-unrelated head and neck cancer. J. Clin. Invest. 2022;132(18) doi: 10.1172/JCI161400. Sep 15. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 19.Sievers C., Craveiro M., Friedman J., et al. Phenotypic plasticity and reduced tissue retention of exhausted tumor-infiltrating T cells following neoadjuvant immunotherapy in head and neck cancer. Cancer Cell. 2023;41(5):887–902. doi: 10.1016/j.ccell.2023.03.014. May 08,e5. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 20.Puram S.V., Parikh A.S., Tirosh I. Single cell RNA-seq highlights a role for a partial EMT in head and neck cancer. Mol. Cell Oncol. 2018;5(3) doi: 10.1080/23723556.2018.1448244. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 21.Puram S.V., Tirosh I., Parikh A.S., et al. Single-cell transcriptomic analysis of primary and metastatic tumor ecosystems in head and neck cancer. Cell. 2017;171(7):1611–1624. doi: 10.1016/j.cell.2017.10.044. Dec 14e24. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 22.Verfuerth S., Sousa P.S., Beloki L., et al. Generation of memory T cells for adoptive transfer using clinical-grade anti-CD62L magnetic beads. Bone Marrow Transplant. 2016;51(4):620. doi: 10.1038/bmt.2016.30. Apr. [DOI] [PubMed] [Google Scholar]
- 23.Mangare C., Tischer-Zimmermann S., Riese S.B., et al. Robust identification of suitable T-cell subsets for personalized CMV-specific T-cell immunotherapy using CD45RA and CD62L microbeads. Int. J. Mol. Sci. 2019;20(6) doi: 10.3390/ijms20061415. Mar 20. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 24.Zhou J., Schwenk-Zieger S., Kranz G., et al. Isolation and characterization of head and neck cancer-derived peritumoral and cancer-associated fibroblasts. Front. Oncol. 2022;12 doi: 10.3389/fonc.2022.984138. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 25.Ruffin A.T., Li H., Vujanovic L., Zandberg D.P., Ferris R.L., Bruno T.C. Improving head and neck cancer therapies by immunomodulation of the tumour microenvironment. Nat. Rev. Cancer. 2023;23(3):173–188. doi: 10.1038/s41568-022-00531-9. Mar. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 26.Eberhardt C.S., Kissick H.T., Patel M.R., et al. Functional HPV-specific PD-1. Nature. 2021;597(7875):279–284. doi: 10.1038/s41586-021-03862-z. Sep. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 27.Li H., van der Leun A.M., Yofe I., et al. Dysfunctional CD8 T cells form a proliferative, dynamically regulated compartment within human melanoma. Cell. 2019;176(4):775–789. doi: 10.1016/j.cell.2018.11.043. Feb 07e18. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 28.Lowery F.J., Krishna S., Yossef R., et al. Molecular signatures of antitumor neoantigen-reactive T cells from metastatic human cancers. Science. 2022;375(6583):877–884. doi: 10.1126/science.abl5447. Feb 25. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 29.Lu Y.C., Zheng Z., Lowery F.J., et al. Direct identification of neoantigen-specific TCRs from tumor specimens by high-throughput single-cell sequencing. J. Immunother. Cancer. 2021;9(7) doi: 10.1136/jitc-2021-002595. Jul. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 30.Lu Y.C., Zheng Z., Robbins P.F., et al. An efficient single-cell RNA-Seq approach to identify neoantigen-specific T cell receptors. Mol. Ther. 2018;26(2):379–389. doi: 10.1016/j.ymthe.2017.10.018. Feb 07. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 31.Yossef R., Tran E., Deniger D.C., et al. Enhanced detection of neoantigen-reactive T cells targeting unique and shared oncogenes for personalized cancer immunotherapy. JCI Insight. 2018;3(19) doi: 10.1172/jci.insight.122467. Oct 04. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 32.Chatani P.D., Lowery F.J., Parikh N.B., et al. Cell surface marker-based capture of neoantigen-reactive CD8. J. Immunother. Cancer. 2023;11(5) doi: 10.1136/jitc-2022-006264. May. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 33.Tran E., Ahmadzadeh M., Lu Y.C., et al. Immunogenicity of somatic mutations in human gastrointestinal cancers. Science. 2015;350(6266):1387–1390. doi: 10.1126/science.aad1253. Dec 11. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 34.Komuro H., Shinohara S., Fukushima Y., et al. Single-cell sequencing on CD8. J. Immunother. Cancer. 2023;11(8) doi: 10.1136/jitc-2023-007180. Aug. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 35.Hanada K.I., Zhao C., Gil-Hoyos R., et al. A phenotypic signature that identifies neoantigen-reactive T cells in fresh human lung cancers. Cancer Cell. 2022;40(5):479–493. doi: 10.1016/j.ccell.2022.03.012. May 09e6. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 36.Nakiboneka R., Mugaba S., Auma B.O., et al. Interferon gamma (IFN-γ) negative CD4+ and CD8+ T-cells can produce immune mediators in response to viral antigens. Vaccine. 2019;37(1):113–122. doi: 10.1016/j.vaccine.2018.11.024. Jan 03. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 37.Li J., Xiao Z., Wang D., et al. The screening, identification, design and clinical application of tumor-specific neoantigens for TCR-T cells. Mol. Cancer. 2023;22(1):141. doi: 10.1186/s12943-023-01844-5. Aug 30. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 38.Schwarz S., Schmitz J., Löffler M.W., et al. T cells of colorectal cancer patients' stimulated by neoantigenic and cryptic peptides better recognize autologous tumor cells. J. Immunother. Cancer. 2022;10(12) doi: 10.1136/jitc-2022-005651. Dec. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 39.Yamaguchi S., Hamana H., Shitaoka K., et al. TCR function analysis using a novel system reveals the multiple unconventional tumor-reactive T cells in human breast cancer-infiltrating lymphocytes. Eur. J. Immunol. 2021;51(9):2306–2316. doi: 10.1002/eji.202049070. Sep. [DOI] [PubMed] [Google Scholar]
- 40.Tan Q., Zhang C., Yang W., et al. Isolation of T cell receptor specifically reactive with autologous tumour cells from tumour-infiltrating lymphocytes and construction of T cell receptor engineered T cells for esophageal squamous cell carcinoma. J. Immunother. Cancer. 2019;7(1):232. doi: 10.1186/s40425-019-0709-7. Aug 28. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 41.Scheper W., Kelderman S., Fanchi L.F., et al. Low and variable tumor reactivity of the intratumoral TCR repertoire in human cancers. Nat. Med. 2019;25(1):89–94. doi: 10.1038/s41591-018-0266-5. Jan. [DOI] [PubMed] [Google Scholar]
- 42.Oliveira G., Stromhaug K., Klaeger S., et al. Phenotype, specificity and avidity of antitumour CD8. Nature. 2021;596(7870):119–125. doi: 10.1038/s41586-021-03704-y. Aug. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 43.Pasetto A., Gros A., Robbins P.F., et al. Tumor- and neoantigen-reactive T-cell receptors can be identified based on their frequency in fresh tumor. Cancer Immunol. Res. 2016;4(9):734–743. doi: 10.1158/2326-6066.CIR-16-0001. Sep 02. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 44.Leko V., Rosenberg S.A. Identifying and targeting human tumor antigens for T cell-based immunotherapy of solid tumors. Cancer Cell. 2020;38(4):454–472. doi: 10.1016/j.ccell.2020.07.013. Oct 12. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 45.Friedman J., Moore E.C., Zolkind P., et al. Neoadjuvant PD-1 immune checkpoint blockade reverses functional immunodominance among tumor antigen-specific T cells. Clin. Cancer Res. 2020;26(3):679–689. doi: 10.1158/1078-0432.CCR-19-2209. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 46.Quah H.S., Cao E.Y., Suteja L., et al. Single cell analysis in head and neck cancer reveals potential immune evasion mechanisms during early metastasis. Nat. Commun. 2023;14(1):1680. doi: 10.1038/s41467-023-37379-y. Mar 27. [DOI] [PMC free article] [PubMed] [Google Scholar]
Associated Data
This section collects any data citations, data availability statements, or supplementary materials included in this article.
Supplementary Materials
Supplementary Fig. 2. Success of TIL expansion and isolation from patient tumor biopsies based on T cell infiltrate as determined by digital image analysis from immunohistochemical staining. N = 29 total samples.
Supplementary Fig. 3. Flow cytometric characterization of exhaustion markers on TILs from freshly digested tumor at the time of surgery resection. TILs were subsequently extracted from additional biopsy specimens from the same tumor, stimulated with CD3/CD28 activation, and incubated in T cell media. The same exhaustion marker panel was examined by flow cytometry on these active TILs on post-activation day 4, day 7, and day 11.
Supplementary Fig. 4.(Left) Circulating T cells and (right) TILs demonstrate less target cell cytolysis of a patient-matched cell line enriched for cancer-associated fibroblasts when compared with the patient-matched enriched malignant cell line.
Supplementary Video 1. Time-lapse video demonstrating autologous single tumor cell-single TIL co-culture from a HNSCC patient resulting in T cell-mediated tumor cell death (Caspase-3 red fluorescence).
Data Availability Statement
Information and requests for resources and reagents should be directed to and will be fulfilled by the lead contact, Dr Heather Himburg (hhimburg@mcw.edu). Any additional information required to reanalyze the data reported in this paper is available from the lead contact upon request.