Abstract
Climate change is one of the most significant challenges facing the sustainability of global poultry production. Stress resulting from extreme temperature swings, including cold snaps, is a major concern for food production birds. Despite being well-documented in mammals, the effect of environmental stress on enteric neurophysiology and concomitant impact on host-microbiome interactions remains poorly understood in birds. As early life stressors may imprint long-term adaptive changes in the host, the present study sought to determine whether cold temperature stress, a prominent form of early life stress in chickens, elicits changes in enteric stress-related neurochemical concentrations that coincide with compositional and functional changes in the microbiome that persist into the later life of the bird. Chicks were, or were not, subjected to cold ambient temperature stress during the first week post-hatch and then remained at normal temperature for the remainder of the study. 16S rRNA gene and shallow shotgun metagenomic analyses demonstrated taxonomic and functional divergence between the cecal microbiomes of control and cold stressed chickens that persisted for weeks following cessation of the stressor. Enteric concentrations of serotonin, norepinephrine, and other monoamine neurochemicals were elevated (P < 0.05) in both cecal tissue and luminal content of cold stressed chickens. Significant (P < 0.05) associations were identified between cecal neurochemical concentrations and microbial taxa, suggesting host enteric neurochemical responses to environmental stress may shape the cecal microbiome. These findings demonstrate for the first time that early life exposure to environmental temperature stress can change the developmental trajectory of both the chicken cecal microbiome and host neuroendocrine enteric physiology. As many neurochemicals serve as interkingdom signaling molecules, the relationships identified here could be exploited to control the impact of climate change-driven stress on avian enteric host-microbe interactions.
Key words: stress, microbiome, avian, neurochemical, gut
INTRODUCTION
Inclement weather as a result of climate change is a major threat to the sustainability of the global production of poultry (Mottet and Tempio, 2017; Cole and Desphande, 2019), which is now the most consumed animal protein in the world (Whitton et al., 2021). Environmental stressors, including extreme temperatures, encountered in food animal production settings increase animal susceptibility to enteric infection, yet the mechanisms underpinning this observation are not fully understood. Stress-related neurochemicals that are part of the neurophysiological stress response common in both mammals and birds have been demonstrated to encourage colonization and augment the pathogenicity of a wide variety of bacterial species commonly found in the intestinal tract of food production animals (Vlisidou et al., 2004; Pullinger et al., 2010; Aroori et al., 2014). Environmental sources of stress have therefore been proposed to increase intestinal foodborne pathogen carriage in food production animals, in part, through neurochemical mediated host-microbe interactions (Humphrey, 2006; Truccollo et al., 2020). Indeed, the chicken intestinal tract is regarded as a major reservoir of the human foodborne disease-causing organisms Campylobacter jejuni and Salmonella spp. (Scharff, 2020). No study to date has investigated in chickens whether host physiological stress can affect in vivo the production of enteric neurochemicals. Likewise, while the use of germ-free chickens has established a clear role of the microbiome in shaping the enteric neurochemical environment (Phillips et al., 1961), and the production environment, in turn, shapes the chicken gut microbiome (McKenna et al., 2020), it is unknown whether avian host stress may affect related microbial metabolic pathways. Establishing that environmental temperature-based stress increases local concentrations of monoamine neurochemicals in the gut that are related with shifts in microbial taxonomic and functional profiles, will likely have major implications in the design of next-generation poultry husbandry practices that prevent climate change-driven acquisition and transmission of foodborne pathogens.
The study of neurochemicals as interkingdom signaling molecules has been termed Microbial Endocrinology (Neuman et al., 2015). Like in mammals, the avian intestinal tract is a major source of many monoamine neurochemicals (Lyte et al., 2022) as it is highly innervated by the enteric nervous system and contains non-neuronal endocrine cell populations (Komori et al., 1979; Rawdon, 1984). Another similarity between the mammalian and avian intestinal microbiota are several resident bacterial taxa that synthesize and respond to structurally identical neurochemicals of the host neuroendocrine system (Villageliu and Lyte, 2017). Any condition that would induce stress in the avian host to cause the local enteric release of monoamine neurochemicals would therefore likely impact functional aspects of the intestinal microbiome as has been reported in Japanese quail (Lyte et al., 2021) and in mammals (Strandwitz, 2018). Indeed, in vitro exposure of C. jejuni to norepinephrine increased its ability to colonize the ceca of orally inoculated chickens (Cogan et al., 2007; Methner et al., 2008). Such findings warrant investigation of the plasticity of chicken intestinal neurochemistry to environmental stressors and its resultant interaction with the intestinal microbiome, an area of study in birds that has so far received only sparse attention.
Low environmental temperatures, often termed cold stress, are one of the most important environmental stressors in the rearing of chickens during early life. Recognition of the significance of thermoregulation in preventing bacterial infection in birds dates back to the mid-1800s with Pasteur's work relating avian body temperature and susceptibility to Bacillus anthracis infection (Pasteur et al., 1878). Since that time, multiple investigations have reported that chicks, as poor thermoregulators of body temperature (Shinder et al., 2007), are uniquely susceptible during early life to cold stress. Not surprisingly, cold stress has been widely demonstrated to increase avian mortality and susceptibility to enteric infection (Mallmann, 1934; Thaxton et al., 1974; Soerjadi et al., 1979; Matsumoto and Huang, 2000; Borsoi et al., 2015). Indeed, the frequent and intense weather events ushered in by climate change, including rapid ambient temperature swings such as cold snaps, are of particular concern for avian species (Garrett et al., 2022). Whether the stress-induced modulation of enteric neurochemical concentrations constitutes, in part, the mechanism driving avian susceptibility to infection following cold stress is unknown. Most critically, it has not yet been demonstrated that cold stress, or any non-infectious environmental stressor, can cause changes in the concentrations of neurochemicals in the chicken gut as well as concomitantly alter community structure or metabolic pathways of the chicken intestinal microbiome.
As such, we hypothesized that cold stress exposure during the first week post-hatch (i.e., early life) would elicit increases in stress-related neurochemical concentrations in the chicken gut as well as alter the taxonomic and functional trajectory of the intestinal microbiome into the chicken's later life. This study provides the first evidence that an ambient temperature environmental stressor, specifically cold stress exposure, during the early life of a chicken can cause short and long-term changes in the concentration of neurochemicals in the chicken gut as well as shift compositional and functional aspects of the intestinal microbiome. These results provide novel insight into the potential importance of early life stress management in poultry husbandry practices in determining changes in the concentration of neurochemicals in the chicken gut that have recognized roles in mediating bacterial pathogen colonization.
MATERIALS AND METHODS
Animals
All procedures were approved by the University of Arkansas Division of Agriculture Institutional Animal Care and Use Committee before the study was initiated. Male by-product breeder chicks were obtained on d of hatch from a local hatchery (Cobb, Siloam Springs, AR) and transported to the United States Department of Agriculture (USDA) poultry facility which is separate from but located on the grounds of the University of Arkansas Poultry Research Facility (Fayetteville, AR). This USDA poultry facility consists of 2 identical fully contained environmentally controllable housing structures that are separated by approximately 5 m distance. Upon arrival, chicks were weighed, individually tagged, and randomly allocated into 2 groups (control or cold stress). Due to the ability to maintain a single temperature at any one time within each housing structure, the control and cold stress groups were housed separately but maintained under identical conditions for the entirety of the study except during the cold stress challenge periods. Within each house, 1 large floor pen lined with fresh pine shavings was subdivided into 4 pens (each of the 4 pens measured (L x W; 6 ft x 7 ft). Each of the 4 pens were separated by metal fencing that permitted social interaction but prevented movement of chickens between pens. The number of chickens in each experimental group was 40 (i.e., 40 chickens in the control group and 40 chickens in the cold stress group), so that 10 chickens per group for each sampling timepoint (i.e., at 3, 7, 9, and 21 d of age) were included in analyses. An n of 10 birds per sampling timepoint was selected as this number was previously demonstrated as sufficient to detect a statistically significant (P < 0.05) change in chicken intestinal neurochemical concentrations (Redweik et al., 2019). Chicken was the experimental unit. To account for possible early life mortality, the stocking density of each pen was 20 chickens per pen so that the total number of birds used in this study was 160 chickens. A standard temperature curve in each house was automatically maintained at 27°C during the first and second week of age (except on d of age 4, 5, and 6 in the cold stress group house), and 25°C during the third week of age. A standard corn/soy-based diet was produced at the University of Arkansas feed mill and provided to the chickens in floor feeders. All chickens were provided ad libitum access to feed and water throughout the entire study.
Cold Stress
At 4, 5, and 6 d of age the temperature in the cold stress group house was maintained at 20°C for 6 h per d. For the remaining 18 h of each d, the cold stress group house temperature was matched to that of the control group house. Broiler houses were equipped with NIST-traceable thermometers (VWR) that were placed at the level of the chicks’ heads. Readings were manually recorded at regular intervals throughout the 24 h period. Cold stress temperatures were maintained and verified during the 6 h cold stress periods. Immediately on d 7 of age and for the entire remaining duration of the study, the cold stress group house was returned to the temperature curve of the control group house. This temperature (20°C) was selected as a cold stress as it was previously reported that a range of cold stress temperatures (from 15°C to 20°C) cause a temporary stress response without causing mortality in broiler chickens during the first week post-hatch (Borsoi et al., 2015; Shinder et al., 2002; Shinder et al., 2007; Shahir et al., 2012). In addition, as the focus of the present study was to investigate the impact of cold stress on gut neurochemical and microbial dynamics that are known to influence enteric infection, this cold stress temperature was chosen as it was demonstrated to influence broiler chicken susceptibility to infection of the gastrointestinal tract (Borsoi et al., 2015).
Tissue Collection
Chickens were euthanized via decapitation (n = 10 chickens/group/timepoint) on 3, 7, 9, and 21 d of age. At each timepoint, 10 birds from each group were randomly selected, euthanized, and tissues collected. The purpose of the 3-day-old group was to assess baseline differences between control and cold stress groups the day immediately preceding the start of the cold stress challenge considering these groups were housed in separate but identical facilities. Due to the young age of this baseline group of birds, there was not enough cecal content for both microbiome profiling and neurochemical quantification. In order to address the potential confounding factor of housing environment in the interpretation of the effect of cold stress on the microbiome, we chose to assess baseline differences in the cecal microbiome. In addition, only within the 3-day-old group, due to the limited amount of cecal tissue per bird, this tissue was used exclusively for the determination of neurochemical concentrations.
Immediately following euthanasia, the intestinal tract was manually removed and dissected on Petri dishes packed with ice. A new petri dish was used for each chicken in order to avoid cross-contamination between birds. As the ceca in chickens is bifurcated, ceca were removed at the site of bifurcation. In each 3-day-old chicken, the content of both ceca was pooled for microbiome sample collection. For all other age groups, cecal content and tissue were collected from a single ceca pouch. For microbiome sample collection, cecal content was carefully collected using sterile technique into sterile 2 mL microcentrifuge tubes (Catalog # 10018-754, VWR Life Science). Following cecal content sample collection, each ceca was opened longitudinally using ball-tipped scissors and gently washed using sterile PBS to remove any remaining content. In the 3-day-old chickens, both emptied ceca were collected for neurochemical analysis. In all other age groups, full thickness pieces of the same cecal pouch from which microbiome and other samples were obtained, were collected for neurochemical and qPCR analyses. The other empty ceca was Swiss-rolled for histological analysis.
Intestinal tissue and content samples collected for later ultra-high performance liquid chromatography (UHPLC) analysis were processed as previously described (Lyte et al., 2022). In brief, tissues were weighed and then immediately submerged in 2 mL reinforced tubes containing 6 ceramic beads (Catalog #s: 19-648 and 19-646, Omni International, Kennesaw, GA) and 1 mL of 0.2 N perchloric acid (0.2 N perchloric acid consisted of HPLC grade water (Catalog # 7732-18-5, VWR Life Science, Radnor, PA) and perchloric acid (Catalog #:AAA44464-AP, VWR Life Science)), then snap frozen on dry ice and stored at −80°C until analysis. All samples collected for histological analysis were first Swiss-rolled and then promptly placed into 10% neutral-buffered formalin (Catalog #: 16004-128, VWR Life Science) and stored at room temperature until sectioning and staining. Cecal content samples collected for microbiome analyses were immediately snap frozen on dry ice and stored at −80°C until DNA extraction. All content and tissue samples for short chain fatty acids (SCFA) and qPCR analysis were collected into DNAase/RNAase free microcentrifuge tubes (Catalog #: 10025-738, VWR Life Science), snap frozen on dry ice, then stored at −80°C until analysis.
Ultra-High Performance Liquid Chromatography
Tissues were thawed in the tubes in which they were placed following dissection and immediately placed into a Bead Ruptor (Catalog #: 19-040E, Omni International). The tissues were homogenized twice for 30 s at 5 m/s, with samples allowed to rest for 10 s in between each 30 s cycle. Homogenized samples were promptly centrifuged at 3000 × g at 4°C for 15 min, and the supernatant was then transferred to 2–3 kDa molecular weight cut-off spin filters (Catalog #: 89132-006, VWR Life Science) for further purification. Flow-through was collected and stored at −80°C until analysis by UHPLC as previously described (Lyte et al., 2022) with minor modification. In brief, the UHPLC consisted of a Dionex Ultimate 3,000 autosampler and a Dionex Ultimate 3,000 pump, equipped with a Dionex Ultimate 3,000 RS electrochemical detector and VWD-3400 UV/Vis detector (Thermo Fisher Scientific, Sunnyvale, CA). Mobile phase was a buffered 10% acetonitrile mobile phase (Catalog #: NC9777698, Thermo Fisher Scientific), and the flow rate was 0.6 mL/min on a 150-mm (length), 3 mm (internal diameter), and 3 μm (particle size) Hypersil BDS C18 column (Catalog #: 28103-153030, Thermo Fisher Scientific). Samples were maintained at 4°C on the autosampler before injection, and electrochemical detection was achieved using a 6041RS glassy carbon electrode set at 400 mV. The UV detection was set to 210 nm. Data was analyzed using the Chromeleon software package (version 7.2, Thermo Fisher Scientific), and neurochemical identification was confirmed using retention times of corresponding analytical standards from Millipore-Sigma (norepinephrine, Catalog #: 636-88-4; serotonin, Catalog #: 61-47-2; 5-hydroxyindoleacetic acid (5-HIAA), Catalog #: 59709-57-8; dopamine, Catalog #: 59-92-7; histamine, Catalog # 56-92-8).
RNA Isolation and Quantitative Reverse Transcription Polymerase Chain Reaction
Total RNA was extracted from full thickness cecal tissue samples using RNAzol RT (Fisher Scientific, Catalog # NC0477546) according to the manufacturer's protocol. RNA quality and quantity were determined using a NanoDrop One Microvolume UV-Vis spectrophotometer (Nanodrop Products, Wilmington, DE). The iScript cDNA Synthesis Kit (BioRad, Hercules, CA, Catalog # 1708891) was used to reverse transcribe 0.3 µg of RNA per sample in a 4 µL total volume reaction. The cDNA was diluted 4 times with RNase-free water prior to real-time PCR analysis of intestinal genes. PCR reactions consisted of 1 µL diluted cDNA, 5 µL iTaq Universal SYBR Green Supermix (BioRad, Catalog # 1725121), 0.5 µL of chicken specific PrimePCR SYBR Green Assay Primers (BioRad, Catalog # 10042976), and 3.5 µL of molecular grade nuclease free water (Fisher Scientific, Catalog # 10-977-023). PCR reaction was carried out with an initial activation at 95°C for 30 s, followed by 40 cycles of 94°C for 5 s and 60°C for 30 s. Melting curve analysis was performed to confirm the specificity of PCR amplification and relative fold change in gene expression was determined using the 2− ΔΔCt method (Livak and Schmittgen, 2001) normalized against the housekeeping gene GAPDH.
Gas Chromatography-Mass Spectrometry
Cecal SCFA quantification was carried out as previously described (van de Wouw et al., 2018) with minor modification. Cecal content (∼100 mg) was weighed and then immediately placed into individual 2 mL reinforced tubes that each contained 5 ceramic beads (Catalog #s: 19-648 and 19-646, Omni International). To each tube was added 800 µL of 0.003M hydrochloric acid (Catalog #: H1758, Millipore-Sigma). Samples were homogenized using an Omni Bead Ruptor at 5 m/s for 30 sec and then promptly placed onto ice for 1 min prior to centrifugation. Samples were then centrifuged at 15000 x g at 4°C at for 5 min. Following centrifugation, 800 µL of supernatant was collected and filtered through a 0.2 µm syringe filter (VWR, Catalog # 76479-044). Filtrate was transferred to a new 2 mL tube and a solvent mixture (1:3 v/v chloroform: methanol (Millipore-Sigma) was added at a ratio of 1:4 (v/v supernatant: solvent) to establish a single phase solution to precipitate out protein. Tubes were briefly vortexed, then immediately centrifuged at 4°C at 15000 x g for 10 min. Supernatant was collected and transferred to an HPLC vial (VWR, Catalog # 89523-484) and 6 µL internal standard (decanoic acid, Catalog #: C1875, Sigma Aldrich) was added at a volume of 2 μL to each sample. Vials were stored at −20°C until injection into the gas chromatograph (GC).
Samples were analyzed using an Agilent 6890N gas chromatograph with 5973 single quadrupole mass spectrometer (GC/MS) equipped with an Agilent 7683B autosampler. An Agilent 30m G3903-63011 DB-FastFAME column (internal diameter 0.25 mm and 0.25 µm film liquid layer) was used for the analysis. A sample volume of 1 μL was injected in spitless mode to the injector port where the temperature was maintained at 280°C. Temperature of the oven was changed linearly from 37 to 150°C at a rate of 10°C/min followed by a 5 min hold. The carrier gas was helium, the flow rate was variable throughout the run as method used constant pressure, and the mass spectrometer was scanned in the m/z range of 30–500 in fast scanning mode. SCFA identification was performed using both retention time and 70 eV electron impact ionization fragment ion spectra of corresponding analytical standards (Millipore-Sigma, butyric acid, Catalog #: 19215; propionic acid, Catalog #: 94425; acetic acid, Catalog #: 695092) in NIST search 2.0 library (v2.0). Each fatty acid was quantified by using a calibration curve generated in the concentration range of 0.02 mg/ml (20 ppm) to 0.0006 mg/ml (0.6 ppm) for 6 calibration points. Relative intensities (Intensity for fatty acid/intensity of internal standard) instead of absolute intensities for each concentration of fatty acid were used for the calibration curves to correct for injection volume during the sample injection into the GC-MS.
Genomic DNA Isolation and Quantification, Library Preparation, and Sequencing
Cecal content samples were sent to the company Corebiome (now Diversigen, New Brighton, MN) for DNA extraction as well as subsequent 16S rRNA gene and shallow shotgun sequencing. Shallow shotgun sequencing (sWGS) achieves species-level taxonomic identification and functional resolution that closely approximates deep shotgun sequencing (Hillmann et al., 2018; Johnson et al., 2019; La Reau et al., 2023). All samples were included in 16S rRNA gene analysis. The 21 d of age group samples were additionally processed for sWGS. Financial cost limitations disallowed all samples to be sent for metagenomic sequencing. All samples were extracted using the MO Bio PowerFecal (Qiagen) automated for high throughput on QiaCube (Qiagen), with bead beating in 0.1 mm glass bead plates. For samples designated for 16S analysis, samples were quantified via qPCR using primers for the variable region 4 (515f/806r) of the 16S rRNA gene. For samples used in sWGS analysis, samples were quantified with the Qiant-iT Picogreen dsDNA Assay (Invitrogen).
To identify any contribution of potential kit contamination, isolation of genomic DNA was also performed on molecular grade water (Catalog #: 60-2450, ATCC, Manassas, VA) using the same kit and following the same protocol used for cecal content samples. The molecular grade water sample was carried through for the entirety of the 16S rRNA gene and shallow shotgun processes.
For 16S rRNA gene analysis, samples were prepared with a protocol derived from (Gohl et al., 2016), using KAPA HiFi Polymerase to amplify variable region 4 (515f/806r) of the 16S rRNA gene. Libraries were sequenced on an Illumina MiSeq using paired-end 2 × 250 reads and the MiSeq Reagent Kit v3. For metagenomic analysis, libraries were prepared with a procedure adapted from the Nextera Library Prep kit (Illumina). Libraries were sequenced on an Illumina NextSeq using single-end 1 × 150 reads with a NextSeq 500/550 High Output v2 kit (Illumina). Microbial community standards (Catalog #’s: D6311 and D6305 ZymoBIOMICS, Irvine, CA) were purchased to determine any bias or error in library preparation and subsequent sequencing, respectively. These standards were processed along with the DNA extracted from chicken cecal content and included in the same sequencing runs. 16S rRNA gene and metagenomic datasets were deposited on the NCBI Sequence Read Archive database under the BioProject accession number PRJNA977671.
Histology
Ceca specimens from 8 chickens/group were subjected to histological analysis. Tissues were placed in 10% neutral buffered formalin, transferred to Monosette IV cassettes (Catalog #: 15154-273 VWR Life Science), and fixed overnight in an automated processor (Model 2500, Shandon Lipshaw, Pittsburgh, PA). The following morning, tissue samples were removed from the processor and embedded in paraffin. Tissue blocks were sectioned at a thickness of 5 μm using a microtome (Shandon Lipshaw, Pittsburgh, PA), transferred onto Superfrost Plus microscope slides (Catalog #: 48311-703 VWR Life Science), and placed in a slide dryer (Shandon Lipshaw, Pittsburgh, PA) overnight. In the morning, the paraffin was removed. For Hematoxylin/Eosin (H/E) staining, slides were stained using Harris Hematoxylin solution (Catalog #: 95057-858, VWR Life Science), Harleco blueing reagent (Catalog #:34172-018, VWR Life Science), and Eosin Y solution (Catalog # 34172-002, VWR Life Science). Cover slips were fixed using Xylene substitute mountant (Catalog # 1900231: Thermo Scientific, Waltham, MA). Slides were dried overnight before imaging.
Slides were digitally imaged using a Cytation 5 imaging multi-mode plate reader (Biotek) equipped with a × 4 magnification (Catalog #: 1220519, Biotek) brightfield objective. Measurements of intestinal parameters were conducted blinded to control or cold stress sample identity in the cecum using the NIH Fiji ImageJ software (version 1.53q). The thickness of the muscularis externa (including the circular and longitudinal muscle layers) was measured in 8 images/bird (8 birds/group = 64 images per group). Villus height (VH; measured from the villus-crypt junction to the villus tip) and crypt depth (CD; measured from the base of the crypt to the villus-crypt junction in crypts with open lumens and a continuous cell column on each side) were determined in at least 4 well-oriented, villus-crypt units per images (15 images/bird × 8 birds/group = 120 images per group; Supplemental Figure 1). These values were averaged to obtain the mean height of intestinal structures and the ratio of VH to CD was calculated.
Statistics
UHPLC, GC-MS, RT-qPCR, and histological data were analyzed via 2-way ANOVA with Sidak`s multiple comparison test (GraphPad Prism, La Jolla, CA (v. 9.4.1)). Data are presented as mean ± SEM. The threshold of statistical significance was set at P < 0.05.
16S rRNA Gene Sequencing Data Processing and Analysis
Fastq files were explored using FastQC (v. 0.11.3) (Andrews, 2010) before being quality filtered and trimmed using Trimmomatic (v. 0.36) (Bolger et al., 2014) to ensure an average quality score of 30. The remaining reads were then imported into the R environment (v.3.5.1) (R Core Team, 2021) for further quality filtering, error correction and chimera removal using the DADA2 pipeline (v. 1.10.1) (Callahan et al., 2016) before collapsing the reads into amplicon sequence variants (ASVs). A second chimera filtering step was performed, utilizing the “uchime_ref” command in USEARCH against the ChimeraSlayer GOLD database (v. 20110519) (Edgar et al., 2011). Taxonomic classification of the filtered ASVs up to genus level was performed with the “classify.seqs” command in the mothur software (v 1.39.5) (Schloss et al., 2009) against the SILVA ribosomal RNA reference database (release 132) (Quast et al., 2013) with a bootstrapping cut-off of 80%. In order to identify taxa that are likely over- or underrepresented in samples, the compositions of several mock microbial community DNA standards (Catalog #’s: D6311 and D6305 ZymoBIOMICS, Irvine, CA) were compared against their theoretical composition at the family level. The results of the mock microbial community DNA standards are presented in (Supplemental Figure 2).
Statistical analysis of microbiota 16S rRNA gene data was performed in an R software environment (v. 4.1.0) (R Core Team, 2021). Beta diversity was evaluated with the vegan package (v.2.6.4) by performing principal component analysis (PCA) on Aitchison distances, which were calculated with the ALDEx2 package (v.1.26.0) (Oksanen et al., 2018; Fernandes et al., 2013). ALDEx2 was also used to calculate pairwise differential abundances. The package uses the ratio of the median between-group differences and the larger value of the median within-group variation as effect size measure. An effect size larger than absolute one is considered a reproducible difference between groups as outlined by authors of the package (Fernandes et al., 2019). Differences between groups were assessed using permutational multivariate analysis of variance (PERMANOVA) which was implemented using the “adonis2” function. Alpha diversity was estimated as Chao1 richness, Shannon diversity, and Simpson index within the iNEXT package (v.3.0.0). iNEXT computes asymptotic diversity profiles based on the statistical estimation of the true Hill number of any order q> = 0. Kruskal-Wallis signed rank test (for more than 2 groups) or a Wilcox signed rank test (when comparing 2 groups) were used to evaluate the significance between the groups (Chao et al., 2014).
Canonical Correspondence Analysis (CCA) was also carried out using the vegan package (Oksanen et al., 2018). Neurochemical concentrations were used to constrain the ordination of each group's microbial composition with a separate model produced for cecal content and tissue. An additional model, constrained by SCFA concentrations was also produced for cecal content only. The resulting eigenvalues display only the variance that is explainable by the chemical used to constrain the model. Correspondence Analysis (CA) was also conducted so that the variance of each constrained model could be compared against the total variance. ANOVA-like permutation tests were performed to evaluate the significance of each CCA model using the “anova.cca” function from the vegan package.
Metagenomic Sequencing Data Processing and Analysis
Raw sequence reads were pre-processed using the Kneaddata (version 0.12.0) pipeline (http://huttenhower.sph.harvard.edu/kneaddata). This involved the removal of overrepresented and low complexity sequences by FastQC (Andrews, 2010) and TRF (Benson, 1999) respectively. Subsequently, low quality bases, truncated reads and adapter sequences were removed using Trimmomatic (Bolger et al., 2014), before depletion of host derived sequences by mapping to the broiler chicken reference genome assembly (bGalGal1 GRCg7b) using bowtie2 (Langmead and Salzberg, 2012). Microbial gene content and metabolic potential were subsequently estimated using the HUMAnN3.0 (version 3.6) pipeline (Beghini et al., 2021) against the UniProt Reference database (uniref50_201901b) with reduced nucleotide and translated subject coverage thresholds of zero.
Statistical analysis of metagenomic sequencing data was performed in an R software environment (v 4.1.0). Differences in microbial gene content was evaluated with the vegan package (v.2.6.4) by performing PCA on Aitchison distances, which were calculated with the ALDEX2 package (v.1.26.0) (Oksanen et al., 2018; Fernandes et al., 2013). ALDEX2 was also used to calculate pairwise differential abundance. Differences between groups were assessed using permutational multivariate analysis of variance (PERMANOVA) which was implemented using the “adonis” function.
Enzyme annotations corresponding to outputted KEGG Orthology (KO) identifiers were downloaded and collated using the KEGGREST package (v 1.38) (Tenenbaum, 2022) in R. Functional enrichment analysis of differentially abundant gene families was subsequently carried out using the R/shiny package MicrobiomeProfiler (v 1.4.0) (Chen and Yu, 2022). Heat trees displaying differences in abundances between stress and control groups in KEGG gene families corresponding to enzyme annotations were generated using metacoder (v 0.3.5) (Foster et al., 2017).
RESULTS
Cecal Neurochemical Concentrations Are Affected by Cold Stress
Although the microbiota displays compositional and functional differences along the length of the gastrointestinal tract, it is important to appreciate that in each gut region there is transverse microbial distribution from the mucosa to the lumen. As interkingdom signaling molecules, neurochemicals, in part, mediate interaction between the microbiota and host, tissue and luminal concentrations were measured to provide a transverse perspective to the neurochemical distribution in the ceca. The measurement of luminal neurochemical concentrations can be interpreted as directly relevant for the bacterial microbiota, and tissue concentrations as applicable to bacteria that colonize the ceca.
Cecal tissue neurochemical concentrations (μg per g of tissue) were not significantly different (P > 0.05) between the control and cold stress groups at 3 d of age (Table 1). Cecal luminal content neurochemical concentrations at 3 d of age were not measured due to the limited amount of content in the ceca at this age, as described in Methods. Luminal content concentration of norepinephrine was significantly (P < 0.0001) increased in the cold stress group compared to the controls at 21 d of age. At 9 and 21 d of age, luminal content dopamine was lesser (P = 0.0012) and greater (P = 0.0037), respectively, in the cold stress group compared to controls. At 9 d of age, serotonin in cecal tissue was significantly increased (P < 0.0001) in the cold stress group compared to control. Cecal luminal content serotonin at 21 d of age was significantly lower (P = 0.0086) in the cold stress group compared to control. 5-HIAA was increased in the cecal tissue (P = 0.0039) of the cold stress group compared to control only at 9 d of age. Histamine concentrations in cecal tissue were elevated (P = 0.0014) in the cold stress group compared to control at 9 d of age.
Table 1.
Neurochemical concentrations in chicken cecal luminal content and tissue
| Neurochemical | Timepoint (d of age) |
||||
|---|---|---|---|---|---|
| 3 d | 7 d | 9 d | 21 d | ||
| Norepinephrine | |||||
| Tissue | Control | 1.17 ± 0.08 | 1.46 ± 0.10 | 2.10 ± 0.13 | 1.74 ± 0.15 |
| Cold stress | 1.26 ± 0.14 | 1.68 ± 0.20 | 2.58 ± 0.14 | 2.19 ± 0.32 | |
| Luminal content | Control | NM | 1.08 ± 0.37 | 2.19 ± 0.92 | 0.75 ± 0.18* |
| Cold stress | NM | 1.35 ± 0.48 | 0.85 ± 0.15 | 12.98 ± 4.26 | |
| Dopamine | |||||
| Tissue | Control | 0.19 ± 0.01 | 0.30 ± 0.04 | 0.31 ± 0.01 | 0.17 ± 0.01 |
| Cold stress | 0.18 ± 0.02 | 0.29 ± 0.04 | 0.29 ± 0.01 | 0.15 ± 0.01 | |
| Luminal content | Control | NM | 0.32 ± 0.02 | 0.33 ± 0.03* | 0.06 ± 0.04* |
| Cold stress | NM | 0.23 ± 0.02 | 0.15 ± 0.03 | 0.23 ± 0.03 | |
| Serotonin | |||||
| Tissue | Control | 8.34 ± 0.53 | 12.28 ± 1.34 | 10.16 ± 1.02* | 6.69 ± 0.70 |
| Cold stress | 8.28 ± 0.65 | 11.49 ± 0.77 | 37.02 ± 3.17 | 5.61 ± 0.71 | |
| Luminal content | Control | NM | 0.54 ± 0.11 | 0.57 ± 0.07 | 1.87 ± 0.57* |
| Cold stress | NM | 0.39 ± 0.05 | 0.23 ± 0.01 | 0.79 ± 0.07 | |
| 5-HIAA | |||||
| Tissue | Control | 0.25 ± 0.01 | 0.37 ± 0.02 | 0.52 ± 0.03* | 0.29 ± 0.01 |
| Cold stress | 0.28 ± 0.02 | 0.43 ± 0.07 | 0.71 ± 0.06 | 0.25 ± 0.02 | |
| Luminal content | Control | NM | 0.03 ± 0.03 | 0.64 ± 0.18 | 1.85 ± 1.15 |
| Cold stress | NM | 0.32 ± 0.26 | 0.28 ± 0.08 | 0.16 ± 0.16 | |
| Histamine | |||||
| Tissue | Control | 5.37 ± 0.25 | 7.47 ± 0.61 | 7.77 ± 0.59* | 8.13 ± 1.07 |
| Cold stress | 5.26 ± 0.26 | 6.41 ± 0.49 | 11.07 ± 0.83 | 7.88 ± 0.36 | |
| Luminal content | Control | NM | 6.01 ± 1.14 | 6.75 ± 1.72 | ND |
| Cold stress | NM | 6.86 ± 1.28 | 8.09 ± 0.32 | 1.07 ± 0.73 | |
Abbreviations: NM, not measured; ND, not detected; 5-HIAA, 5-hydroxyindoleacetic acid.
Values are μg of neurochemical per g of tissue or luminal content. All values are expressed as mean ± SEM (n = 10 chickens/group/timepoint). Chickens were (cold stress) or were not (control) subjected to ambient environmental cold stress on 4, 5, and 6 d of age and then sacrificed on either 7, 9, or 21 d of age as described in the Methods section. Data was analyzed using 2-way ANOVA followed by Sidak's post-hoc test.
Significant difference (P < 0.05) of control tissue or luminal content compared to respective cold stress group tissue or luminal content in same column (e.g. control tissue norepinephrine vs cold stress tissue norepinephrine, or control luminal content norepinephrine vs cold stress luminal content norepinephrine).
Microbial Diversity and Differential Abundance Analysis
The 16S rRNA V4 region of all samples (n = 80) were amplified, sequenced and subjected to quality and chimaera filtering, resulting in a mean of 174283 +/− 8125 usable reads per sample which were collapsed into 1203 ASVs for further analyses.
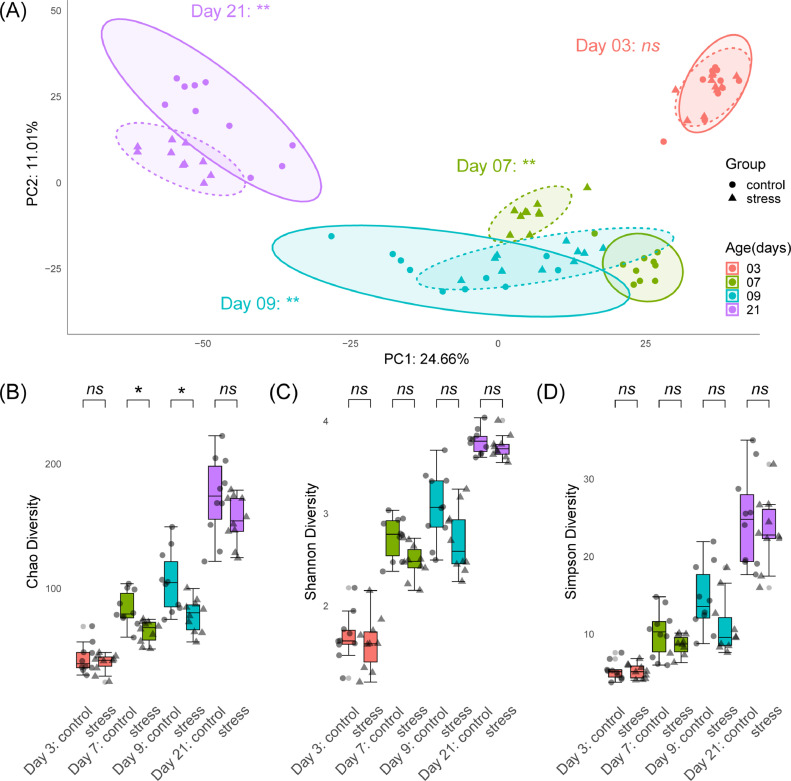
Principal component analysis based on Aitchison distances, the primary and secondary axes of which explained 35.67% of variation in the data, revealed a clear separation in cecal microbial communities based on both age (P = 0.0001) and stress (P = 0.0001) (Figure 1A). No significant separation between cold stress and control chickens presented at d 3 (PERMANOVA adjusted P = 0.708). The largest divergence between stress groups presented at d 7 (PERMANOVA adjusted P = 0.0028), with significant separations also being evident at d 9 (PERMANOVA adjusted P = 0.0028) and d 21 (PERMANOVA adjusted P = 0.0056) (Supplemental Table 1).
Figure 1.
Microbial diversity in control and cold stress group chickens across time-points. Chickens were cold stressed at 4, 5, and 6 d of age as described in Methods: (A) Principal coordinate analysis plot based on Aitchison distances exhibits significant separation (**P < 0.01) between the gut microbiomes of control and cold stress chickens at 7, 9 and 21 d. (B) Chao diversity significantly decreases (*P < 0.05) in cold stress chickens at 7 and 9 d before recovering at 21 d. (C) Shannon and (D) Simpson diversity do not significantly differ between groups at any time-point.
As expected, alpha diversity indices Chao (Figure 1B), Shannon (Figure 1C) and Simpson (Figure 1D) all show age-dependent increases in the diversity of chicken cecal microbial communities (Supplemental Table 2). While no significant difference between cold stress and control chickens presented in Chao richness at d 3 (Wilcoxon Rank Sum test adjusted P = 0.704), significantly lower diversity was observed in the cold stress group at d 7 and d 9 (Wilcoxon Rank Sum test adjusted, P = 0.0127 and 0.031, respectively), before increasing again and displaying no significant difference compared to control chickens at d 21 (Wilcoxon Rank Sum test adjusted P = 0.231). Similar trends were also observed for Shannon and Simpson diversity metrics, although no significant differences were observed between control and cold stress groups at any time-point (Supplemental Table 2).
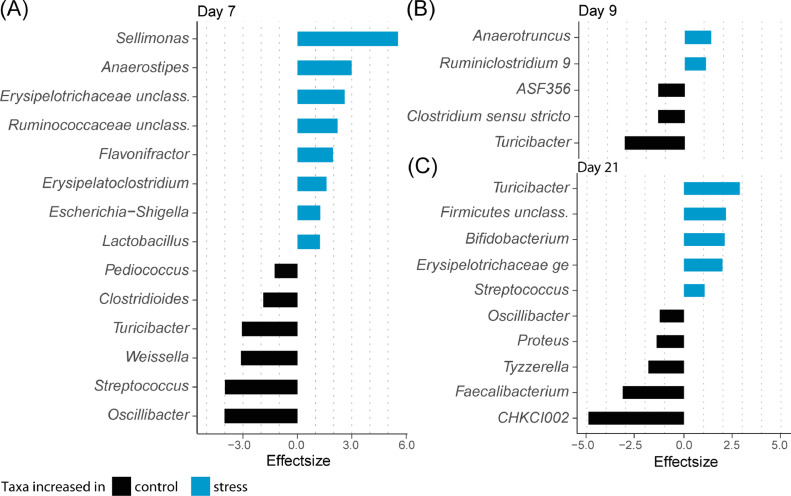
Differential analysis of taxonomic abundances at the genus level with ALDEx2 (Supplemental Table 3) identified 18 significantly differential taxa between cold stress and control groups at d 7 (Figure 2A), 14 of which had an effect size of larger than 1. Eight of these, including Sellimonas, Anaerostipes and Unclassified Erysipelotrichaceae were significantly increased in the cold stress group compared to control, while the remaining 7, including Oscillibacter, Streptococcus and Weissella exhibited significantly higher abundances in the control group. At d 9 (Figure 2B), 6 genera were observed to be significantly differentially abundant, 5 of which had an effect size of larger than 1. Anaerotruncus and Ruminoclostridium were significantly increased in the cold stress group compared to control, while Turicibacter, Clostridium Sensu Stricto and ASF356 (member of the Anaerotignaceae family) exhibited significantly higher abundances in the control group. At d 21 (Figure 2C), 10 genera were observed to be significantly differentially abundant with an effect size of larger than 1. Turicibacter and Bifidobacterium were among the taxa significantly increased in the cold stress group compared to control, while CHKCI002 (member of the Eggerthellaceae family), Faecalibacterium and Tyzzerella were among those who exhibited significantly higher abundances in the control group. Notably, Turicibacter was among the taxa found to be significantly higher in the control group both at d 7 and d 9 but was found to be more highly abundant in the cold stress group at d 21. Similarly, while Streptococcus exhibited significantly higher abundances in the control group at d 7, it was found to be more highly abundant in the cold stress group at d 21.
Figure 2.
Differentially abundant taxa between control and cold stress group chickens at (A) 7 d, (B) 9 d and (C) 21 d. Chickens were cold stressed at 4, 5, and 6 d of age as described in Methods. Effect size is defined as the between group differences divided by the within group differences, an effect size cut-off of absolute 1 is suggested for reproducible results.
Canonical Correspondence Analysis
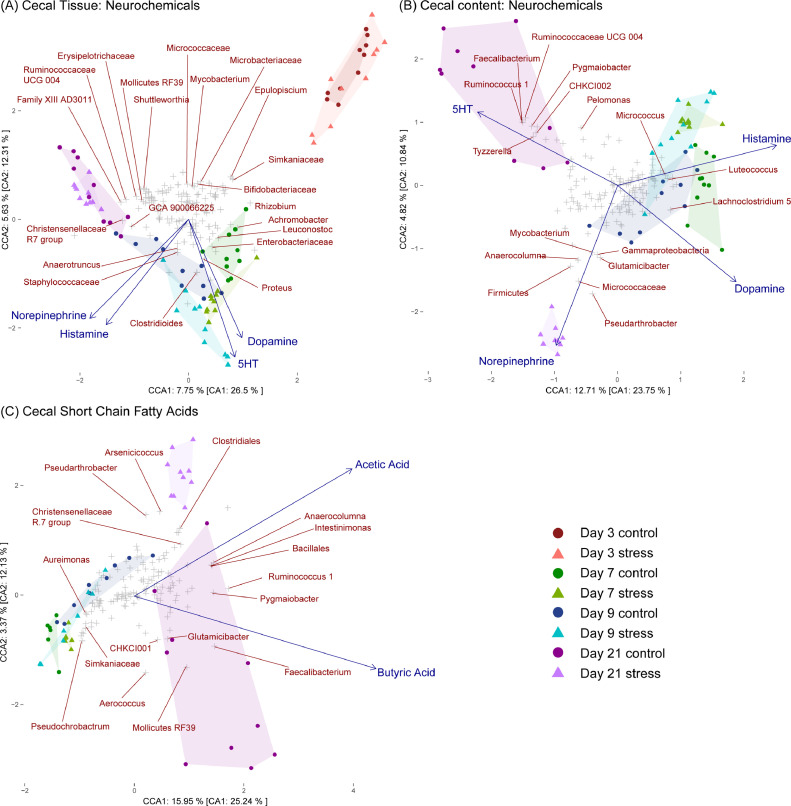
CCA results are visualized in the form of triplots (as shown in Figure 3), where 3 types of data are represented; individual samples by filled circles, microbial species by crosses and neurochemical/SCFA data by arrow vectors from the center-point of each axis. The distances between sample or species sites within the plot indicate their similarity (sites near each other are more closely associated than more distant ones). The positioning of sample or species points relative to a neurochemical/SCFA vector indicates the strength of association with that vector (for example, the abundance of a taxa whose site appears in close proximity to a vector is positively associated with the concentration of the represented neurochemical or SCFA; the abundance of a taxa whose site appears orthogonal and distant to a vector is not influenced by the represented neurochemical or SCFA; the abundance of a taxa whose site appears in the opposing direction to a vector is negatively associated with the concentration of the represented neurochemical or SCFA). The direction of a neurochemical/SCFA vector points to which sample or species it associates the strongest with, while the vector length indicates the magnitude of its importance in explaining the bacterial dispersion observed (longer vectors contribute more to the explained variance). Finally, the angles between neurochemical/SCFA vectors represent the strength of correlation between them (for example, a 5° angle between vectors indicates they are strongly correlated; a 90° angle between vectors indicates they are not correlated; a 180° angle between vectors indicates they are strongly inversely correlated).
Figure 3.
Associations between cecal bacterial relative abundances and neurochemical or short chain fatty acid concentrations in the ceca. (A) CCA of microbiota composition regressed on neurochemical concentrations in cecal tissue. The canonical variates explain 26.5% and 12.31% of the total explainable variance in the first and second axis respectively. (B) CCA of microbiota composition regressed on neurochemical concentrations within cecal content. The canonical variates explain 23.75% and 10.84% of the total explainable variance in the first and second axis respectively. (C) CCA of microbiota composition regressed on short chain fatty acid concentrations within cecal content. The canonical variates explain 25.24% and 12.13% of the total explainable variance in the first and second axis respectively. Filled circles represent samples while grey crosses represent bacterial taxa (genus level) with the top 5% of taxa which best fit the axes being indicated by name. Neurochemical or SCFA vectors point to the direction of taxa with which they exhibit the strongest association with while their magnitude indicates the strength of the variable in explaining the bacterial dispersion observed (only significant vectors, P < 0.05, are displayed). 5-HT: 5-hydroxytryptamine.
The proportion of unconstrained inertia resolved by neurochemical data from cecal tissue (Figure 3A) and cecal content (Figure 3B) were 29.2% and 53.5% in axis 1, and 45.7% and 44.5% in axis 2, respectively, while the proportion resolved by SCFA data from cecal content (Figure 3C) was 63.2% in axis 1 and 27.8% in axis 2. Four of the 5 neurochemicals assessed in cecal tissue were found to exhibit significant relationships with microbial communities and stress condition groups (Supplemental Table 4). The serotonin (5-hydroxytryptamine; 5HT) (F7,72 = 2.921, P = 0.004) and dopamine (F7,72 = 3.727, P = 0.003) vectors are both directed towards the d 7 and d 9 cold stress groups and in close proximity to genera including Clostridioides, Proteus and Enterobacteriaceae. The norepinephrine (F7,72 = 4.526, P = 0.001) and histamine (F7,72 = 2.477, P = 0.013) vectors are directed equidistant from these 2 groups and the d 21 cold stress group, indicating that they may impact the microbial composition of the cold stress groups at all 3 time-points. Genera located close to the norepinephrine and histamine vectors include Staphylococcaceae and Anaerotruncus.
Four of the 5 neurochemicals assessed in cecal content were also found to exhibit significant relationships with microbial communities and stress condition groups (Supplemental Table 4). The 5HT (F5,54 = 3.628, P = 0.001) vector is directed towards the d 21 cold stress group and near genera including CHKCI002 (member of the Eggerthellaceae family), Faecalibacterium and Tyzzerella (all of which also exhibited the most significantly higher abundances in the control group compared to the cold stress group at d 21—see Figure 2C). In contrast, the norepinephrine vector (F5,54 = 3.357, P = 0.001) is directed towards the d 21 control groups and close to genera including Firmicutes, Micrococcaceae and Pseudarthrobacter. The dopamine (F5,54 = 5.264, P = 0.001) vector is directed towards the d 7 and d 9 control groups and near Lachnoclostridium 5, while the histamine (F5,54 = 2.205, P = 0.016) vector is directed closer to d 7 and d 9 cold stress groups and in close proximity to Micrococcus and Luteococcus.
Two of the 3 short chain fatty acids assessed in cecal content were also found to exhibit significant relationships with microbial communities and stress condition groups (Supplemental Table 4). Both acetic acid (F5,43 = 7.267, P = 0.001) and butyric acid (F5,43 = 3.747, P = 0.002) vectors are directed in the opposite direction from d 7 and d 9 groups. The butyric acid vector is directed towards the d 21 control groups and close to genera including Faecalibacterium, Pygmaiobacter and Ruminococcus 1, while the acetic acid vector is directed closer to the d 21 cold stress group and near genera including Bacillales, Intestinimonas, and Anaerocolumna.
Metagenomic Diversity and Functional Enrichment Analysis
Cecal content samples for the 21 d of age group (10 stress and 10 control) were subjected to sWGS resulting in an average of 4,237,392 ± 1,016,870 reads after removal of host sequences per sample.
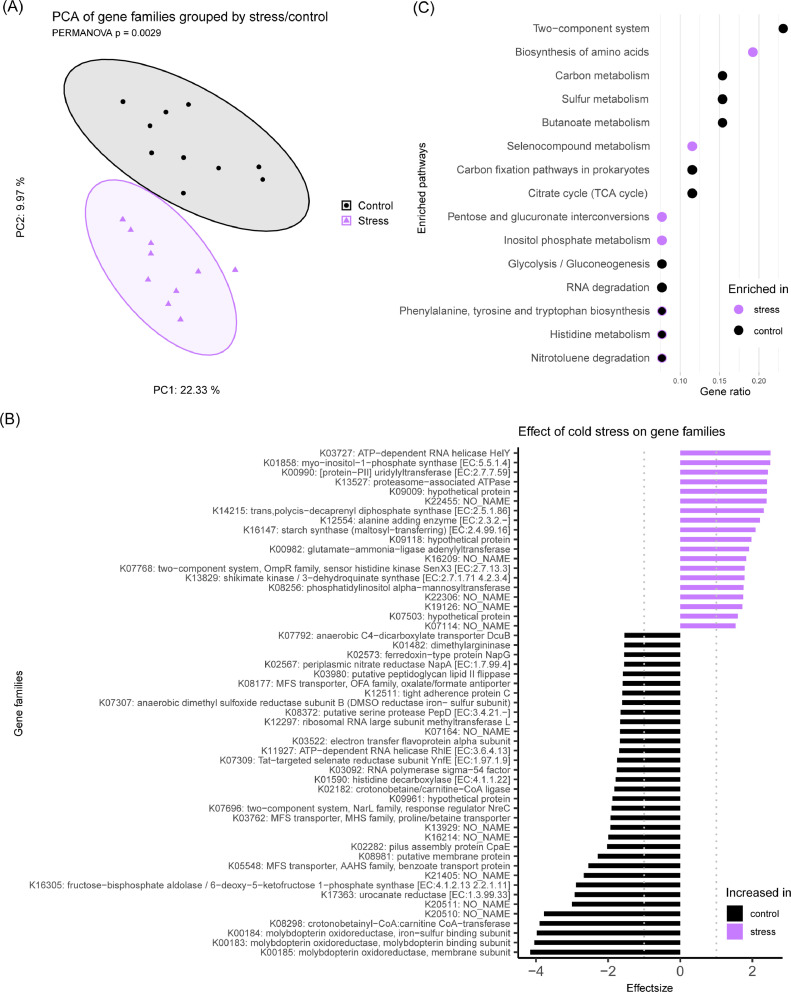
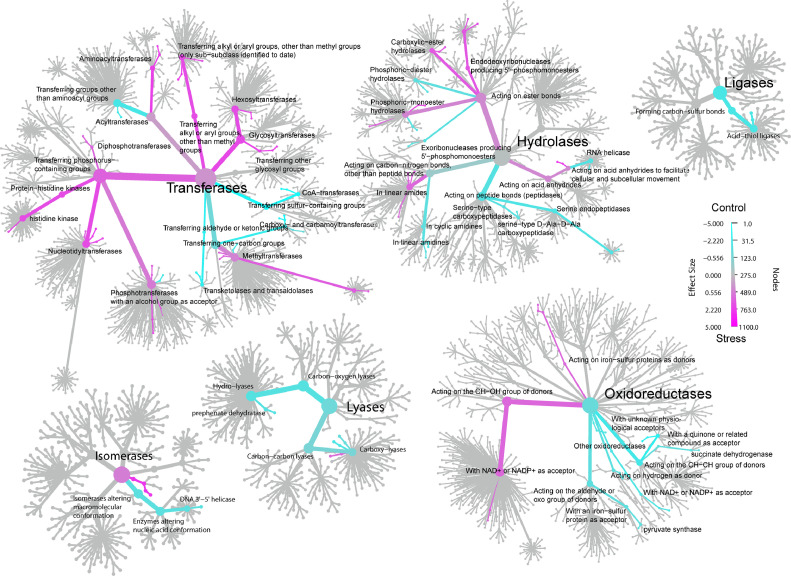
Principal component analysis of gene families based on Aitchison distances, the primary and secondary axes of which explained 32.3% of variation in the data, revealed a clear separation of gene family abundances based on stress condition (P = 0.0029) (Figure 4A). Differential analysis of gene families with ALDEx2 (Supplemental Table 5) identified 98 significantly differential families with an effect size of larger than 1 between cold stress and control groups (Figure 4B). Of the 43 gene families which were significantly increased in the cold stress group compared to control group, ATP-dependent RNA helicase HelY, myo-inositol-1-phosphate synthase and [protein-PII] uridylyltransferase exhibited the highest effect sizes. Of the 55 gene families which were significantly increased in the control group compared to cold stress group, genes with the highest effect sizes belonged to molybdopterin oxidoreductase subunits. Functional enrichment analysis of 98 differentially abundant gene families, identified 11 enriched pathways, including those involved in 2-component regulatory systems as well as carbon, sulfur, and butanoate metabolism, in the control group compared to the stress group (Figure 4C). Conversely, only 4 enriched pathways, including ones involved in biosynthesis of amino acids and selenocompound metabolism, were identified in the stress group compared to the control group.
Figure 4.
Metagenomic diversity in control and cold stressed chickens at 21 d. Chickens were cold stressed at 4, 5, and 6 d of age as described in Methods. (A) Principal coordinate analysis plot based on Aitchison distances exhibits significant separation between gene family abundances of control and cold stress chickens at 21 d. (B) Differentially abundant gene families between control and cold stress chickens at 21 d. Effect size is defined as the between group differences divided by the within group differences, an effect size cut-off of absolute 1 is suggested for reproducible results. (C) Functional enrichment analysis of pathways based on differentially abundant gene families.
Differential abundances of gene families corresponding to enzyme annotations between cold stress and control groups are presented as heat trees in (Figure 5). Visually it is apparent that a higher proportion of transferase enzymes are enriched for in the cold stress group while ligase, lyase and oxidoreductase enzymes are primarily enriched for in the control group.
Figure 5.
Heat trees of differentially abundant genes. Heat trees displaying differentially abundant genes encoding for enzymes between cold stress and control groups across the BRITE functional hierarchy for enzymes.
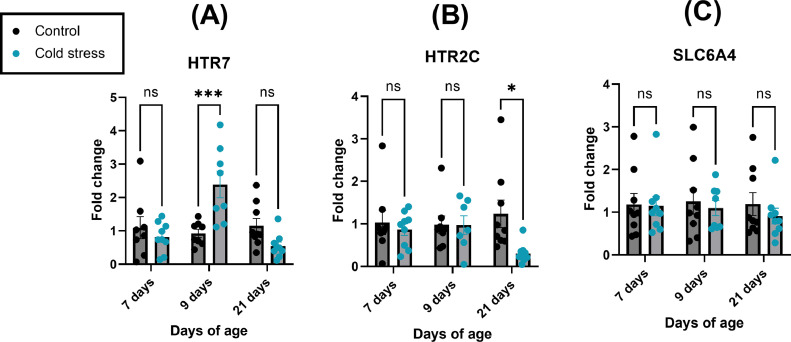
Cecal Serotonergic Gene Expression Is Altered Following Cold Stress
Serotonergic signaling is an important part of the enteric neuroimmune system. As studies have demonstrated a role for microbial influence of enteric serotonin in chickens, and the present study identified stress to alter both tissue and luminal concentrations of serotonin, we also sought to understand how these changes may be related to host serotonin receptor expression in the ceca. Serotonin receptor 7 (HTR7) gene expression was increased in the cold stress group at 9 d of age compared to the control group (P = 0.0004; Figure 6A). HTR7 expression at 7 and 21 d of age was not significantly different (P > 0.05) between cold stress and control groups. Serotonin receptor 2C (HTR2C) expression was decreased in the cold stress group at 21 d of age compared to the control group (P = 0.0113; Figure 6B). HTR2C expression at 7 and 9 d of age was not significantly different (P > 0.05) between cold stress and control groups. Serotonin transporter (sodium dependent serotonin transporter; SLC6A4) was not significantly affected (P > 0.05; Figure 6C) by cold stress at any timepoint. Primers are listed in Supplemental Table 6.
Figure 6.
Serotonergic gene expression is altered in the chicken ceca following cold stress. Chickens were cold stressed at 4, 5, and 6 d of age as described in Methods. mRNA fold change of (A) 5-Hydroxytryptamine Receptor 7 (HTR7), (B) 5-Hydroxytryptamine Receptor 2C (HTR2C), and (C) Sodium dependent serotonin transporter (SLC6A4) were determined in full thickness chicken ceca tissue (n = 8-10 chickens per group) using RT-qPCR as described in Methods. Outliers were assessed using ROUT (q = 1%), and data analyzed using 2-way ANOVA with outliers removed followed by Sidak post-hoc test. Data are presented as mean ± SEM. Statistical significance was set at P < 0.05; * = P < 0.05; *** = P < 0.001.
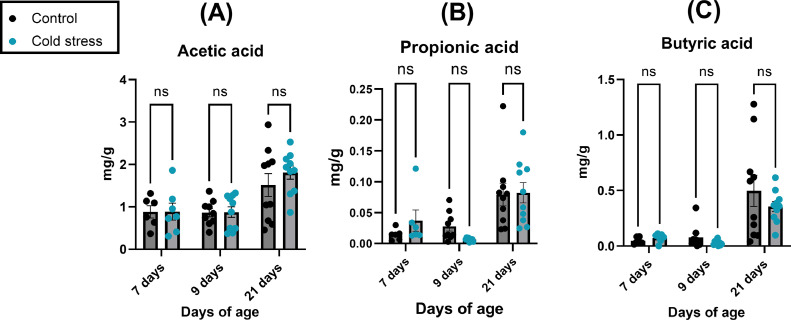
Cecal SCFA Concentrations Are Not Significantly Altered Following Cold Stress
Cecal acetic, propionic, and butyric acid concentrations (mg of SCFA per g of cecal content; Figure 7A–C) were not significantly different (P > 0.05) between cold stress and normal temperature groups at 7, 9, or 21 d of age.
Figure 7.
Short chain fatty acids concentrations are not significantly affected in chicken cecal content following cold stress. Chickens were cold stressed at 4, 5, and 6 d of age as described in Methods. Short chain fatty acids (SCFA) (A) Acetic, (B) Propionic, and (C) Butyric acids were determined in chicken ceca content (n=8-10 chickens per group) using GC-MS as described in Methods. SCFA concentrations are reported in mg of SCFA per g of cecal content. Data are presented as mean ± SEM. Data was analyzed using 2-way ANOVA followed by Sidak post-hoc test. Statistical significance was set at P < 0.05. * = P < 0.05.
Histology
Stress and the enteric microbiota are well known to impact intestinal architecture, including aspects of the mucosa and muscularis externa (Ducatelle et al., 2018). Thickening of the muscularis externa can be associated with enteric neuronal loss and motility dysfunction. Likewise, alterations in mucosal epithelial populations can impact villi height and CD, which contribute to changes in nutrient absorption, intestinal health and overall performance. Histological examination of cecal cross-sections stained with H&E showed a significant increase (P < 0.05) in the thickness of the muscularis externa and CD in the cold stress and in the control groups at 21 d of age compared to both groups at 9 and 7 d of age (Table 2). In the ceca of the cold stress group chickens, villus height (VH) was significantly higher (P < 0.05) in birds at 21 d of age compared to those at 9 and 7 d of age, however no differences were observed in the control groups between any timepoint. At 21 d of age, cold stress chickens displayed higher VH compared to the control group (P < 0.05, Table 2). No differences in VH-CD ratio were observed between the control and the cold stress groups at any timepoints (P > 0.05, Table 2).
Table 2.
Cecal structural morphologies of control and cold stressed chickens.
| Timepoint (d of age) |
|||
|---|---|---|---|
| Criterion (μm) | 7 d | 9 d | 21 d |
| Muscularis externa | |||
| Control | 328.28 ± 16.64# | 343.97 ± 15.61# | 452.33 ± 26.23 |
| Cold stress | 307.46 ± 18.61# | 354.62 ± 14.36# | 499.51 ± 34.04 |
| Villus height | |||
| Control | 200.67 ± 7.55 | 189.77 ± 7.99 | 206.13 ± 9.15 |
| Cold stress | 185.55 ± 5.8# | 192.21 ± 6.12# | 239.45 ± 9.26* |
| Crypt depth | |||
| Control | 72.02 ± 1.06# | 70.95 ± 2.57# | 82.62 ± 3.22 |
| Cold stress | 71.51 ± 1.30# | 67.45 ± 4.26# | 88.14 ± 2.04 |
| VH/CD ratio | |||
| Control | 2.79 ± 0.18 | 2.69 ± 0.14 | 2.51 ± 0.12 |
| Cold stress | 2.60 ± 0.08 | 2.85 ± 0.08 | 2.71 ± 0.09 |
Abbreviations: VH, villus height; CD, crypt depth.
Data are expressed as mean ± SEM and were analyzed using 2-way ANOVA with Sidak's multiple comparison test. N = 8 chicken/group.
P < 0.05 vs respective group at 21 d (i.e. control 7 d or 9 d vs control 21 d; or cold stress 7 d or 9 d vs cold stress 21 d).
P < 0.05 vs control at 21 d.
DISCUSSION
Conditions that induce stress in the host are well recognized to alter host-microbe dynamics. Neurochemicals that are a component of the host neuroendocrine response to stress serve as interkingdom signaling molecules that, in part, drive bacterial response to host stress. Although environmental stressors have been demonstrated to increase norepinephrine and other stress-related neurochemicals in the gut lumen of mammals, it is virtually unknown if, in avian species, environmental stressors similarly affect intestinal neurochemical concentrations. The results presented herein demonstrate that early life exposure to an environmental stressor, namely cold stress, in the production environment can elicit local neurochemical responses in the ceca of the chicken that correlate with compositional and functional changes in the cecal microbiome. Cold stress exposure within the first week post-hatch caused prolonged changes in cecal stress-related neurochemical concentrations and microbial community that were found to persist for weeks following cessation of the stressor. Together, these findings suggest early life stress in chickens may have profound consequences in shaping host-microbe interactions that are dependent on the gut luminal neuroendocrine environment.
Evidence of monoamine neurochemicals in the chicken intestinal tract dates back to at least the 1960s (Phillips et al., 1961; Beaver and Wostmann, 1962). Perhaps unsurprisingly, neurochemical concentrations along the intestinal tract exhibit extensive spatial variation between gross anatomical sections as well as between tissue and luminal content (Lyte et al., 2022). The present study focused on the ceca as this part of the avian intestine harbors the greatest microbial density (Oakley et al., 2014) and functional diversity (Sergeant et al., 2014; Pandit et al., 2018). Critically, it is also where host stress, including ambient temperature stressor (Campos et al., 2023), has been demonstrated to alter local microbial populations (Line et al., 1997) and modulate susceptibility to bacterial colonization (Burkholder et al., 2008). The cecal tissue and luminal concentrations of norepinephrine, dopamine, serotonin, 5-HIAA, and histamine reported herein were demonstrated to be in a similar range to those previously described in the chicken ceca (Redweik et al., 2019; Lyte et al., 2022).
In the present report, cold stress was found to alter neurochemical concentrations. It should be recognized that chickens employed in the production of meat have been bred over decades to undergo a rapid period of growth to maximize the greatest amount of weight gain in the shortest period of time, typically 5–7 wk from hatch until slaughter (Siegel, 2014). In mammals an early life stress has been widely demonstrated to effect long-term negative consequences for the organism, and while the impact of early life stress, including cold stress (Shahir et al., 2012) in chickens has received attention in effecting changes in avian physiology and microbiota (Elfwing et al., 2015; Ramirez et al., 2020), the present study is the first to report the role of an early life stressor in determining avian enteric neurochemical concentrations. As the neurochemicals reported in the present study play a variety of roles in driving avian gastrointestinal function and development as well as mediating host-microbe communication (Dennis, 2016), we sought to examine both short- and longer-term changes outside of the immediate stress response. Hence, it is important to highlight that while monoaminergic changes were observed at 3 d following the stressor, changes in norepinephrine concentrations were shown in chickens at 21 d of age. Our results are in agreement with a previous study (Borsoi et al., 2015) in which broiler chicks that were subjected to cold stress (19oC) for 6h per day during the first week post-hatch had increased plasma norepinephrine concentrations on 21 d of age, but not at 7 d of age. An enteric neurochemical response was not observed at 1-d post-stressor, which was the earliest sampled time-point following stress. This suggests that an early life cold stressor may elicit a combination of short-term enteric stress response and longer-term changes in enteric neuroendocrine plasticity. Indeed, a delayed enteric response to stress, including in chronic stress models that involved cold stress (Choudhury et al., 2009), has been previously demonstrated in mammals (Demaude et al., 2006).
As the variety of neuroendocrine pathways that govern stress-related neurochemical production are largely conserved (Denver, 2009), albeit with some functional differences (Romero and Gormally, 2019), between avians and mammals it is reasonable to suggest that the measured neurochemical concentrations are a product of both host and microbial metabolism (Villageliu and Lyte, 2017), and not solely the product of the host stress response. Indeed, the use of germ-free animals has demonstrated the role of the microbiota in shaping intestinal serotonin and histamine concentrations in both the chicken (Beaver and Wostmann, 1962) and the mouse (Hata et al., 2017). Likewise in mammals, the microbial contribution to catecholamine concentrations in the gut is well-documented (Asano et al., 2012). In the present study, canonical correspondence analyses identified cecal concentrations of serotonin, norepinephrine, dopamine, and histamine to affect microbial gradients between control and stressed chickens. High confidence associations were identified between neurochemical concentrations and microbial relative abundances. As enteric norepinephrine, serotonin, and other monoamines play a variety of roles in birds ranging from modulating gut health and inflammation to the regulation of bacterial pathogenesis, the results presented here may serve as a launching point for the dissection of distinct host-microbe dynamics that underpin chicken enteric neurochemical plasticity following stress. Indeed, Bifidobacterium spp. is a known probiotic genus in poultry (Mountzouris et al., 2007; Igbafe et al., 2020), and in the present study was observed to be increased in the chicken ceca due to cold stress. These findings are in agreement with past studies which also found host stress to increase Bifidobacterium spp. abundances in the lower gastrointestinal tract (Yang et al., 2017; Tian et al., 2020). As Bifidobacterium spp. have been shown to increase central serotonin in mammals (Tian et al., 2019), that we found cold stress to increase abundance of this genus as well as serotonin concentrations in the chicken ceca provides a potential intersection to understand how early life stress may drive related changes in the avian microbiota and enteric neurochemical concentrations.
Several studies have reported on the ability of cold stress to enhance the pathogenesis of cecal bacterial infection in chickens and other poultry (Matsumoto and Huang, 2000; Shahir et al., 2012; Borsoi et al., 2015; Tsiouris et al., 2015), yet surprisingly this has not involved investigation into an effect of cold stress on the cecal microbiome. Cecal microbial community structure has been previously shown to play a role in preventing enteric infection in young chickens (La Ragione and Woodward, 2003) as well as at later ages (Chica Cardenas et al., 2021; Borrelli et al., 2023). Therefore, perturbation of the cecal microbiome in an early life stage may be reasonably suggested as a contributing factor in avian susceptibility to enteric infection. Indeed, it was previously reported that chickens infected with C. jejuni at 6 d of age versus infection at 20 d of age exhibited distinct changes in the cecal microbiome (Connerton et al., 2018). Herein we observed that cold stress exposure resulted in sustained changes in the developmental trajectory of cecal microbial diversity in chickens up to and including at 3 wk of age.
As discussed above, it is important to keep in mind the short production lifespan of the broiler chicken insofar that bacterial infections in chickens that result in avian mortality and economic loss, such as avian pathogenic E. coli (Kemmett et al., 2013) or pose food safety risks for consumers of poultry products (Evans and Sayers, 2000), typically occur within the first few weeks post-hatch. As would therefore be expected, early life interventions within the first week post-hatch that target the cecal microbiome have been associated with reduced bacterial pathogen carriage (Ramirez et al., 2020). Similarly, early life stress events have been recognized for decades to enhance chicken susceptibility to enteric bacterial pathogens in terms of increasing either mortality (Leitner and Heller, 1992) or pathogen load (Borsoi et al., 2015). The cold stress driven changes in cecal microbial taxonomic and metagenomic diversities observed in the present report provide further insight into how an early life stressor may initiate persistent alterations in the microbiome that future studies may leverage as targetable points for intervention.
As we identified microbial taxonomic shifts concomitant with serotonergic as well as noradrenergic alterations in the ceca of cold stressed chickens, we also sought to quantify concentrations of short chain fatty acids as these are largely, but not exclusively, microbially-derived products that are abundantly found in the ceca (González-Ortiz et al., 2020) and known to both affect host synthesis of serotonin (Reigstad et al., 2015) and catecholamines (Parab et al., 2007; LaGamma et al., 2021). While concentrations of SCFA in chicken cecal content reported herein were similar to previous reports (Wang et al., 2021; Dai et al., 2022), we did not observe an effect of cold stress on SCFA concentrations. Changes in SCFA concentrations may depend on type of stress as, for example, cecal acetate in chickens was increased by heat stress (Wang et al., 2021) but reduced by stocking density stress (Dai et al., 2022).
Likewise, histological examination of the chicken ceca did not find a significant effect of cold stress on gross ceca morphology. Interestingly, to date we are not aware of any peer-reviewed study that has investigated the impact of cold stress on avian ceca morphology. As chronic forms of severe heat stress have been previously shown to alter ceca morphology in chickens (Mazzoni et al., 2022), future studies may be warranted to examine if different durations and intensities of cold stress induce changes in ceca histological structures.
Early life stress in chickens, like in mammals, can imprint long-lasting changes in both host and microbiome. Climate change-related extreme temperature swings, including cold snaps, are expected to increase the frequency of early life environmental stress events in poultry. As interkingdom signaling molecules, enteric neurochemical concentrations could be used to inform novel poultry husbandry stress management strategies that leverage host-microbe interaction. The results presented herein demonstrate that an early life stressor, namely cold temperature stress, can initiate long-term changes in the neuroendocrine environment of the chicken gut lumen that coincide with taxonomic and functional shifts in the microbiome. That these changes were identified in the chicken ceca, which plays major roles in chicken health and is a primary intestinal site of foodborne pathogen carriage, suggests wide-ranging utility for the benefit of both poultry producers and consumers. Together, the present study highlights chicken enteric neuroendocrine plasticity as a focal point of host-microbe interaction that is susceptible to stress.
SUPPLEMENTAL DATA
Description of data (Supplemental table 1: Differences in microbial composition calculated via PERMANOVA; Supplemental table 2: Differences in microbial diversity calculated via Wilcoxon test; Supplemental table 3: Differential abundant genera at d 7, 9 and 21 calculated via ALDEx2; Supplemental table 4: Canonical correspondence analysis; Supplemental table 5: Differential abundant gene families at d 21 calculated via ALDEx2; Supplemental Table 6. List of primers used in RT-qPCR).
Supplemental Figure 1. Intestinal architecture of control and cold stressed chickens. Representative microphotographs showing H&E stained cecum from control (A-C) and cold stressed chickens (D-F). In the muscularis externa the thickness of the muscle layer was measured (dashed green lines, B, E). In the mucosa the villus height was measured (VH, measured from villus-crypt junction to villus tip in the mucosa; dashed green lines, panels C, F), and crypt depth (CD, measured from crypt base to villus-crypt junction in the mucosa; solid green lines, panels C, F). Scale bars = 500 μm (panels a and d) and 200 μm (panels B, C, E, F).
Supplemental Figure 2. Mock Microbial Community DNA standards. Theoretical (manufacturer reported taxa distribution) distribution of each standard was compared against composition obtained in the present study (Sample) as described in Methods. (A) ZymoBIOMICS Microbial Community Standard # D6305; and (B) ZymoBIOMICS Microbial Community Standard # D6311).
ACKNOWLEDGMENTS
The authors would like to thank Mr. Phil Matsler (Department of Poultry Science, University of Arkansas) for assistance with tissue fixation and processing. The USDA is an equal opportunity provider and employer. Mention of trade names or commercial products in this publication is solely for the purpose of providing specific information and does not imply recommendation or endorsement by the USDA. This research was supported, in part, by the USDA, Agricultural Research Service. This study was, in part, supported by internal Iowa State University funds provided by the W. Eugene Lloyd Chair in Toxicology to Mark Lyte. Rohana Liyanage would like to acknowledge the statewide mass spectrometry facility and the COBRE grant NIH P30 GM103450.
DISCLOSURES
The authors declare no conflicts of interest.
Footnotes
Supplementary material associated with this article can be found in the online version at doi:10.1016/j.psj.2023.103393.
Appendix. Supplementary materials
Supplemental Figure 1. Intestinal architecture of control and cold stressed chickens. Representative microphotographs showing H&E stained cecum from control (A–C) and cold stressed chickens (D–F). In the muscularis externa the thickness of the muscle layer was measured (dashed green lines, B, E). In the mucosa the villus height was measured (VH, measured from villus-crypt junction to villus tip in the mucosa; dashed green lines, panels C, F), and CD (measured from crypt base to villus-crypt junction in the mucosa; solid green lines, panels C, F). Scale bars = 500 μm (panels a and d) and 200 μm (panels B, C, E, F).
Supplemental Figure 2. Mock Microbial Community DNA standards. Theoretical (manufacturer reported taxa distribution) distribution of each standard was compared against composition obtained in the present study (Sample) as described in methods: (A) ZymoBIOMICS Microbial Community Standard # D6305; and (B) ZymoBIOMICS Microbial Community Standard # D6311.
REFERENCES
- Andrews S. FastQC: a quality control tool for high throughput sequence data. http://www.bioinformatics.babraham.ac.uk/projects/fastqc/
- Aroori S.V., Cogan T.A., Humphrey T.J. Effect of noradrenaline on the virulence properties of Campylobacter species. Int. J. Microbiol. 2014 doi: 10.1155/2014/279075. [DOI] [PMC free article] [PubMed] [Google Scholar]
- Asano Y., Hiramoto T., Nishino R., Aiba Y., Kimura T., Yoshihara K., Koga Y., Sudo N. Critical role of gut microbiota in the production of biologically active, free catecholamines in the gut lumen of mice. Am. J. Physiol. Gastrointest. Liver Physiol. 2012;303:G1288–G1295. doi: 10.1152/ajpgi.00341.2012. [DOI] [PubMed] [Google Scholar]
- Beaver M.H., Wostmann B.S. Histamine and 5-hydroxytryptamine in the intestinal tract of germ-free animals, animals harbouring one microbial species and conventional animals. Br. J. Pharmacol. Chemother. 1962;19:385–393. doi: 10.1111/j.1476-5381.1962.tb01443.x. [DOI] [PMC free article] [PubMed] [Google Scholar]
- Beghini, F., L. J. McIver, A. Blanco-Miguez, L. Dubois, F. Asnicar, S. Maharjan, A. Mailyan, P. Manghi, M. Scholz, A. M. Thomas, M. Valles-Colomer, G. Weingart, Y. Zhang, M. Zolfo, C. Huttenhower, E. A. Franzosa, and N. Segata. 2021. Integrating taxonomic, functional, and strain-level profiling of diverse microbial communities with bioBakery 3. Elife 10. [DOI] [PMC free article] [PubMed]
- Benson G. Tandem repeats finder: a program to analyze DNA sequences. Nucleic Acids Res. 1999;27:573–580. doi: 10.1093/nar/27.2.573. [DOI] [PMC free article] [PubMed] [Google Scholar]
- Bolger A.M., Lohse M., Usadel B. Trimmomatic: a flexible trimmer for Illumina sequence data. Bioinformatics. 2014;30:2114–2120. doi: 10.1093/bioinformatics/btu170. [DOI] [PMC free article] [PubMed] [Google Scholar]
- Borrelli L., Varriale L., Coretti L., Pace A., Russo T.P., Santaniello A., Gavazzi L., Fioretti A., Dipineto L. Research note: cecal microbiota harbored by free-range chickens may influence the reduction of Helicobacter pullorum relative abundance. Poult. Sci. 2023;102 doi: 10.1016/j.psj.2022.102222. [DOI] [PMC free article] [PubMed] [Google Scholar]
- Borsoi A., Quinteiro-Filho W.M., Calefi A.S., Ferreira A.J., Astolfi-Ferreira C.S., Florio J.C., Palermo-Neto J. Effects of cold stress and Salmonella Heidelberg infection on bacterial load and immunity of chickens. Avian Pathol. 2015;44:490–497. doi: 10.1080/03079457.2015.1086976. [DOI] [PubMed] [Google Scholar]
- Burkholder K.M., Thompson K.L., Einstein M.E., Applegate T.J., Patterson J.A. Influence of stressors on normal intestinal microbiota, intestinal morphology, and susceptibility to Salmonella enteritidis colonization in broilers. Poult. Sci. 2008;87:1734–1741. doi: 10.3382/ps.2008-00107. [DOI] [PubMed] [Google Scholar]
- Callahan B.J., McMurdie P.J., Rosen M.J., Han A.W., Johnson A.J., Holmes S.P. DADA2: high-resolution sample inference from Illumina amplicon data. Nat. Methods. 2016;13:581–583. doi: 10.1038/nmeth.3869. [DOI] [PMC free article] [PubMed] [Google Scholar]
- Campos P.M., Schreier L.L., Proszkowiec-Weglarz M., Dridi S. Cecal microbiota composition differs under normal and high ambient temperatures in genetically distinct chicken lines. Sci. Rep. 2023;13:16037. doi: 10.1038/s41598-023-43123-9. [DOI] [PMC free article] [PubMed] [Google Scholar]
- Chao A., Gotelli N.J., Hsieh T.C., Sander E.L., Ma K.H., Colwell R.K., Ellison A.M. Rarefaction and extrapolation with Hill numbers: a framework for sampling and estimation in species diversity studies. Ecolog. Mono. 2014;84:45–67. [Google Scholar]
- Chen, M., G. Yu. 2022. MicrobiomeProfiler: an R/shiny package for microbiome functional enrichment analysis. R package version 1.4.0.
- Chica Cardenas L.A, Clavijo V., Vives M., Reyes A. Bacterial meta-analysis of chicken cecal microbiota. PeerJ. 2021;9:e10571. doi: 10.7717/peerj.10571. [DOI] [PMC free article] [PubMed] [Google Scholar]
- Choudhury B.K., Shi X.Z., Sarna S.K. Norepinephrine mediates the transcriptional effects of heterotypic chronic stress on colonic motor function. Am. J. Physiol. Gastrointest. Liver Physiol. 2009;296:G1238–G1247. doi: 10.1152/ajpgi.90712.2008. [DOI] [PMC free article] [PubMed] [Google Scholar]
- Cogan T.A., Thomas A.O., Rees L.E., Taylor A.H., Jepson M.A., Williams P.H., Ketley J., Humphrey T.J. Norepinephrine increases the pathogenic potential of Campylobacter jejuni. Gut. 2007;56:1060–1065. doi: 10.1136/gut.2006.114926. [DOI] [PMC free article] [PubMed] [Google Scholar]
- Cole J., Desphande J. Poultry farming, climate change, and drivers of antimicrobial resistance in India. Lancet Planet. Health. 2019;3:e494–e495. doi: 10.1016/S2542-5196(19)30236-0. [DOI] [PubMed] [Google Scholar]
- Connerton P.L., Richards P.J., Lafontaine G.M., O'Kane P.M., Ghaffar N., Cummings N.J., Smith D.L., Fish N.M., Connerton I.F. The effect of the timing of exposure to Campylobacter jejuni on the gut microbiome and inflammatory responses of broiler chickens. Microbiome. 2018;6:88. doi: 10.1186/s40168-018-0477-5. [DOI] [PMC free article] [PubMed] [Google Scholar]
- Dai D., Qi G., Wang J., Zhang H., Qiu K., Han Y., Wu Y., Wu S. Dietary organic acids ameliorate high stocking density stress-induced intestinal inflammation through the restoration of intestinal microbiota in broilers. J. Anim. Sci. and Biotech. 2022;13:124. doi: 10.1186/s40104-022-00776-2. [DOI] [PMC free article] [PubMed] [Google Scholar]
- Demaude J., Salvador-Cartier C., Fioramonti J., Ferrier L., Bueno L. Phenotypic changes in colonocytes following acute stress or activation of mast cells in mice: implications for delayed epithelial barrier dysfunction. Gut. 2006;55:655–661. doi: 10.1136/gut.2005.078675. [DOI] [PMC free article] [PubMed] [Google Scholar]
- Dennis R.L. Adrenergic and noradrenergic regulation of poultry behavior and production. Domest. Anim. Endocrinol. 2016;56(Suppl):S94–S100. doi: 10.1016/j.domaniend.2016.02.007. [DOI] [PubMed] [Google Scholar]
- Denver R.J. Structural and functional evolution of vertebrate neuroendocrine stress systems. Ann. N Y Acad. Sci. 2009;1163:1–16. doi: 10.1111/j.1749-6632.2009.04433.x. [DOI] [PubMed] [Google Scholar]
- Ducatelle R., Goossens E., De Meyer F., Eeckhaut V., Antonissen G., Haesebrouck F., Van Immerseel F. Biomarkers for monitoring intestinal health in poultry: present status and future perspectives. Vet. Res. 2018;49:43. doi: 10.1186/s13567-018-0538-6. [DOI] [PMC free article] [PubMed] [Google Scholar]
- Edgar R.C., Haas B.J., Clemente J.C., Quince C., Knight R. UCHIME improves sensitivity and speed of chimera detection. Bioinformatics. 2011;27:2194–2200. doi: 10.1093/bioinformatics/btr381. [DOI] [PMC free article] [PubMed] [Google Scholar]
- Elfwing M., Natt D., Goerlich-Jansson V.C., Persson M., Hjelm J., Jensen P. Early stress causes sex-specific, life-long changes in behaviour, levels of gonadal hormones, and gene expression in chickens. PLoS One. 2015;10 doi: 10.1371/journal.pone.0125808. [DOI] [PMC free article] [PubMed] [Google Scholar]
- Evans S.J., Sayers A.R. A longitudinal study of Campylobacter infection of broiler flocks in Great Britain. Prevent. Vet. Med. 2000;46:209–223. doi: 10.1016/s0167-5877(00)00143-4. [DOI] [PubMed] [Google Scholar]
- Fernandes A.D., Macklaim J.M., Linn T.G., Reid G., Gloor G.B. ANOVA-like differential expression (ALDEx) analysis for mixed population RNA-Seq. PLoS One. 2013;8:e67019. doi: 10.1371/journal.pone.0067019. [DOI] [PMC free article] [PubMed] [Google Scholar]
- Fernandes A.D., Vu M., Edward L.M., Macklaim J.M., Gloor G.B. A reproducible effect size is more useful than an irreproducible hypothesis test to analyze high throughput sequencing datasets. Arxiv Genom. 2019 [Google Scholar]
- Foster Z.S., Sharpton T.J., Grunwald N.J. Metacoder: an R package for visualization and manipulation of community taxonomic diversity data. PLoS Comput. Biol. 2017;13 doi: 10.1371/journal.pcbi.1005404. [DOI] [PMC free article] [PubMed] [Google Scholar]
- Garrett D.R., Pelletier F., Garant D., Bélisle M. Interacting effects of cold snaps, rain, and agriculture on the fledging success of a declining aerial insectivore. Ecolog. App. 2022;32:e2645. doi: 10.1002/eap.2645. [DOI] [PubMed] [Google Scholar]
- Gohl D.M., Vangay P., Garbe J., MacLean A., Hauge A., Becker A., Gould T.J., Clayton J.B., Johnson T.J., Hunter R., Knights D., Beckman K.B. Systematic improvement of amplicon marker gene methods for increased accuracy in microbiome studies. Nat. Biotechnol. 2016;34:942–949. doi: 10.1038/nbt.3601. [DOI] [PubMed] [Google Scholar]
- González-Ortiz G., Olukosi O.A., Jurgens G., Apajalahti J., Bedford M.R. Short-chain fatty acids and ceca microbiota profiles in broilers and turkeys in response to diets supplemented with phytase at varying concentrations, with or without xylanase. Poult. Sci. 2020;99:2068–2077. doi: 10.1016/j.psj.2019.11.051. [DOI] [PMC free article] [PubMed] [Google Scholar]
- Hata T., Asano Y., Yoshihara K., Kimura-Todani T., Miyata N., Zhang X.T., Takakura S., Aiba Y., Koga Y., Sudo N. Regulation of gut luminal serotonin by commensal microbiota in mice. PLoS One. 2017;12 doi: 10.1371/journal.pone.0180745. [DOI] [PMC free article] [PubMed] [Google Scholar]
- Hillmann B., Al-Ghalith G.A., Shields-Cutler R.R., Zhu Q., Gohl D.M., Beckman K.B., Knight R., Knights D. Evaluating the information content of shallow shotgun metagenomics. mSystems. 2018:3. doi: 10.1128/mSystems.00069-18. [DOI] [PMC free article] [PubMed] [Google Scholar]
- Humphrey T. Are happy chickens safer chickens? Poultry welfare and disease susceptibility. Br. Poult. Sci. 2006;47:379–391. doi: 10.1080/00071660600829084. [DOI] [PubMed] [Google Scholar]
- Igbafe J., Kilonzo-Nthenge A., Nahashon S.N., Mafiz A.I., Nzomo M. Probiotics and antimicrobial effect of Lactiplantibacillus plantarum, Saccharomyces cerevisiae, and Bifidobacterium longum against common foodborne pathogens in poultry. Agriculture. 2020;10:368. [Google Scholar]
- Johnson A.J., Vangay P., Al-Ghalith G.A., Hillmann B.M., Ward T.L., Shields-Cutler R.R., Kim A.D., Shmagel A.K., Syed A.N., Personalized Microbiome Class S., Walter J., Menon R., Koecher K., Knights D. Daily sampling reveals personalized diet-microbiome associations in humans. Cell Host Micro. 2019;25:789–802.e785. doi: 10.1016/j.chom.2019.05.005. [DOI] [PubMed] [Google Scholar]
- Kemmett K., Humphrey T., Rushton S., Close A., Wigley P., Williams N.J. A longitudinal study simultaneously exploring the carriage of APEC virulence associated genes and the molecular epidemiology of faecal and systemic E. coli in commercial broiler chickens. PLoS One. 2013;8:e67749. doi: 10.1371/journal.pone.0067749. [DOI] [PMC free article] [PubMed] [Google Scholar]
- Komori S., Ohashi H., Okada T., Takewaki T. Evidence that adrenaline is released from adrenergic neurones in the rectum of the fowl. Br. J. Pharmacol. 1979;65:261–269. doi: 10.1111/j.1476-5381.1979.tb07827.x. [DOI] [PMC free article] [PubMed] [Google Scholar]
- La Ragione R.M., Woodward M.J. Competitive exclusion by Bacillus subtilis spores of Salmonella enterica serotype Enteritidis and Clostridium perfringens in young chickens. Vet. Microbiol. 2003;94:245–256. doi: 10.1016/s0378-1135(03)00077-4. [DOI] [PubMed] [Google Scholar]
- La Reau A.J., Strom N.B., Filvaroff E., Mavrommatis K., Ward T.L., Knights D. Shallow shotgun sequencing reduces technical variation in microbiome analysis. Sci. Rep. 2023;13:7668. doi: 10.1038/s41598-023-33489-1. [DOI] [PMC free article] [PubMed] [Google Scholar]
- LaGamma E.F., Hu F., Pena Cruz F., Bouchev P., Nankova B.B. Bacteria - derived short chain fatty acids restore sympathoadrenal responsiveness to hypoglycemia after antibiotic-induced gut microbiota depletion. Neurobio. Stress. 2021;15 doi: 10.1016/j.ynstr.2021.100376. [DOI] [PMC free article] [PubMed] [Google Scholar]
- Langmead B., Salzberg S.L. Fast gapped-read alignment with Bowtie 2. Nat. Methods. 2012;9:357–359. doi: 10.1038/nmeth.1923. [DOI] [PMC free article] [PubMed] [Google Scholar]
- Leitner G., Heller E.D. Colonization of Escherichia coli in young turkeys and chickens. Avian Dis. 1992;36:211–220. [PubMed] [Google Scholar]
- Line J.E., Bailey J.S., Cox N.A., Stern N.J. Yeast treatment to reduce Salmonella and Campylobacter populations associated with broiler chickens subjected to transport stress. Poult. Sci. 1997;76:1227–1231. doi: 10.1093/ps/76.9.1227. [DOI] [PubMed] [Google Scholar]
- Livak K.J., Schmittgen T.D. Analysis of relative gene expression data using real-time quantitative PCR and the 2−ΔΔCT method. Methods. 2001;25:402–408. doi: 10.1006/meth.2001.1262. [DOI] [PubMed] [Google Scholar]
- Lyte J.M., Martinez D.A., Robinson K., Donoghue A.M., Daniels K.M., Lyte M. A neurochemical biogeography of the broiler chicken intestinal tract. Poult. Sci. 2022;101 doi: 10.1016/j.psj.2021.101671. [DOI] [PMC free article] [PubMed] [Google Scholar]
- Lyte J.M., Keane J., Eckenberger J., Anthony N., Shrestha S., Marasini D., Daniels K.M., Caputi V., Donoghue A.M., Lyte M. Japanese quail (Coturnix japonica) as a novel model to study the relationship between the avian microbiome and microbial endocrinology-based host-microbe interactions. Microbiome. 2021;9:38. doi: 10.1186/s40168-020-00962-2. [DOI] [PMC free article] [PubMed] [Google Scholar]
- Mallmann W.L. Effects of different brooding temperatures on pullorum disease. Vet. Med. 1934;29:254. [Google Scholar]
- Matsumoto M., Huang H.J. Induction of short-term, nonspecific immunity against Escherichia coli infection in chickens is suppressed by cold stress or corticosterone treatment. Avian Pathol. 2000;29:227–232. doi: 10.1080/03079450050045486. [DOI] [PubMed] [Google Scholar]
- Mazzoni M., Zampiga M., Clavenzani P., Lattanzio G., Tagliavia C., Sirri F. Effect of chronic heat stress on gastrointestinal histology and expression of feed intake-regulatory hormones in broiler chickens. Animal. 2022;16 doi: 10.1016/j.animal.2022.100600. [DOI] [PubMed] [Google Scholar]
- McKenna, A., U. Z. Ijaz, C. Kelly, M. Linton, W. T. Sloan, B. D. Green, U. Lavery, N. Dorrell, B. W. Wren, A. Richmond, N. Corcionivoschi, and O. Gundogdu. 2020. Impact of industrial production system parameters on chicken microbiomes: mechanisms to improve performance and reduce Campylobacter. Microbiome 8:128. [DOI] [PMC free article] [PubMed]
- Methner U., Rabsch W., Reissbrodt R., Williams P.H. Effect of norepinephrine on colonisation and systemic spread of Salmonella enterica in infected animals: role of catecholate siderophore precursors and degradation products. Int. J. Med. Microbiol. 2008;298:429–439. doi: 10.1016/j.ijmm.2007.07.013. [DOI] [PubMed] [Google Scholar]
- Mottet A., Tempio G. Global poultry production: current state and future outlook and challenges. W. Poult. Sci. J. 2017;73:245–256. [Google Scholar]
- Mountzouris K.C., Tsirtsikos P., Kalamara E., Nitsch S., Schatzmayr G., Fegeros K. Evaluation of the efficacy of a probiotic containing Lactobacillus, Bifidobacterium, Enterococcus, and Pediococcus strains in promoting broiler performance and modulating cecal microflora composition and metabolic activities. Poult. Sci. 2007;86:309–317. doi: 10.1093/ps/86.2.309. [DOI] [PubMed] [Google Scholar]
- Neuman H., Debelius J.W., Knight R., Koren O. Microbial endocrinology: the interplay between the microbiota and the endocrine system. FEMS Microbiol. Rev. 2015;39:509–521. doi: 10.1093/femsre/fuu010. [DOI] [PubMed] [Google Scholar]
- Oakley B.B., Lillehoj H.S., Kogut M.H., Kim W.K., Maurer J.J., Pedroso A., Lee M.D., Collett S.R., Johnson T.J., Cox N.A. The chicken gastrointestinal microbiome. FEMS Microbiol. Lett. 2014;360:100–112. doi: 10.1111/1574-6968.12608. [DOI] [PubMed] [Google Scholar]
- Oksanen J, B. F., M. Friendly, R. Kindt, P. Legendre, D. McGlinn, P. R. Minchin, R. B. O'Hara, G. L. Simpson, P. Solymos, M. H. H. Stevens, E. Szoecs, H. Wagner. 2018. Vegan: community ecology package. R package version 2.5-6.
- Pandit R.J., Hinsu A.T., Patel N.V., Koringa P.G., Jakhesara S.J., Thakkar J.R., Shah T.M., Limon G., Psifidi A., Guitian J., Hume D.A., Tomley F.M., Rank D.N., Raman M., Tirumurugaan K.G., Blake D.P., Joshi C.G. Microbial diversity and community composition of caecal microbiota in commercial and indigenous Indian chickens determined using 16s rDNA amplicon sequencing. Microbiome. 2018;6:115. doi: 10.1186/s40168-018-0501-9. [DOI] [PMC free article] [PubMed] [Google Scholar]
- Parab S., Nankova B.B., La Gamma E.F. Differential regulation of the tyrosine hydroxylase and enkephalin neuropeptide transmitter genes in rat PC12 cells by short chain fatty acids: concentration-dependent effects on transcription and RNA stability. Brain Res. 2007;1132:42–50. doi: 10.1016/j.brainres.2006.11.013. [DOI] [PubMed] [Google Scholar]
- Pasteur L., Joubert J., Chamberland C. Sur le charbon des poules. Comptes Rendus des seances De L'Academie Des Sciences. 1878;87:47–48. [Google Scholar]
- Phillips A.W., Newcomb H.R., Smith J.E., Lachapeller Serotonin in the small intestine of conventional and germfree chicks. Nature. 1961;192:380. doi: 10.1038/192380a0. [DOI] [PubMed] [Google Scholar]
- Pullinger G.D., van Diemen P.M., Carnell S.C., Davies H., Lyte M., Stevens M.P. 6-hydroxydopamine-mediated release of norepinephrine increases faecal excretion of Salmonella enterica serovar Typhimurium in pigs. Vet. Res. 2010;41:68. doi: 10.1051/vetres/2010040. [DOI] [PMC free article] [PubMed] [Google Scholar]
- Quast C., Pruesse E., Yilmaz P., Gerken J., Schweer T., Yarza P., Peplies J., Glockner F.O. The SILVA ribosomal RNA gene database project: improved data processing and web-based tools. Nucleic Acids Res. 2013;41:D590–D596. doi: 10.1093/nar/gks1219. [DOI] [PMC free article] [PubMed] [Google Scholar]
- Ramirez G.A., Richardson E., Clark J., Keshri J., Drechsler Y., Berrang M.E., Meinersmann R.J., Cox N.A., Oakley B.B. Broiler chickens and early life programming: microbiome transplant-induced cecal community dynamics and phenotypic effects. PLoS One. 2020;15 doi: 10.1371/journal.pone.0242108. [DOI] [PMC free article] [PubMed] [Google Scholar]
- R Core Team. 2021. R: a language and environment for statistical computing. https://www.r-project.org/.
- Rawdon B.B. Gastrointestinal hormones in birds: morphological, chemical, and developmental aspects. J. Exp. Zool. 1984;232:659–670. doi: 10.1002/jez.1402320335. [DOI] [PubMed] [Google Scholar]
- Redweik G.A.J., Daniels K., Severin A.J., Lyte M., Mellata M. Oral treatments with probiotics and live Salmonella Vaccine induce unique changes in gut neurochemicals and microbiome in chickens. Front. Microbiol. 2019;10:3064. doi: 10.3389/fmicb.2019.03064. [DOI] [PMC free article] [PubMed] [Google Scholar]
- Reigstad C.S., Salmonson C.E., Rainey J.F., 3rd, Szurszewski J.H., Linden D.R., Sonnenburg J.L., Farrugia G., Kashyap P.C. Gut microbes promote colonic serotonin production through an effect of short-chain fatty acids on enterochromaffin cells. FASEB J. 2015;29:1395–1403. doi: 10.1096/fj.14-259598. [DOI] [PMC free article] [PubMed] [Google Scholar]
- Romero L.M., Gormally B.M.G. How truly conserved is the "well-conserved" vertebrate stress response? Integr. Comp. Biol. 2019;59:273–281. doi: 10.1093/icb/icz011. [DOI] [PubMed] [Google Scholar]
- Scharff R.L. Food attribution and economic cost estimates for meat- and poultry-related illnesses. J. Food Prot. 2020;83:959–967. doi: 10.4315/JFP-19-548. [DOI] [PubMed] [Google Scholar]
- Schloss P.D., Westcott S.L., Ryabin T., Hall J.R., Hartmann M., Hollister E.B., Lesniewski R.A., Oakley B.B., Parks D.H., Robinson C.J., Sahl J.W., Stres B., Thallinger G.G., Van Horn D.J., Weber C.F. Introducing mothur: open-source, platform-independent, community-supported software for describing and comparing microbial communities. Appl. Environ. Microbiol. 2009;75:7537–7541. doi: 10.1128/AEM.01541-09. [DOI] [PMC free article] [PubMed] [Google Scholar]
- Sergeant M.J., Constantinidou C., Cogan T.A., Bedford M.R., Penn C.W., Pallen M.J. Extensive microbial and functional diversity within the chicken cecal microbiome. PLoS One. 2014;9:e91941. doi: 10.1371/journal.pone.0091941. [DOI] [PMC free article] [PubMed] [Google Scholar]
- Shahir M.H., Dilmagani S., Tzschentke B. Early-age cold conditioning of broilers: effects of timing and temperature. Br. Poult. Sci. 2012;53:538–544. doi: 10.1080/00071668.2012.719604. [DOI] [PubMed] [Google Scholar]
- Shinder D., Luger D., Rusal M., Rzepakovsky V., Bresler V., Yahav S. Early age cold conditioning in broiler chickens (Gallus domesticus): thermotolerance and growth responses. J. Therm. Bio. 2002;27:517–523. [Google Scholar]
- Shinder D., Rusal M., Tanny J., Druyan S., Yahav S. Thermoregulatory responses of chicks (Gallus domesticus) to low ambient temperatures at an early age. Poult. Sci. 2007;86:2200–2209. doi: 10.1093/ps/86.10.2200. [DOI] [PubMed] [Google Scholar]
- Siegel P.B. Evolution of the modern broiler and feed efficiency. Annu. Rev. Anim. Biosci. 2014;2:375–385. doi: 10.1146/annurev-animal-022513-114132. [DOI] [PubMed] [Google Scholar]
- Soerjadi A.S., Druitt J.H., Lloyd A.B., Cumming R.B. Effect of environmental temperature on susceptibility of young chickens to Salmonella typhimurium. Aust. Vet. J. 1979;55:413–417. doi: 10.1111/j.1751-0813.1979.tb05591.x. [DOI] [PubMed] [Google Scholar]
- Strandwitz P. Neurotransmitter modulation by the gut microbiota. Brain Res. 2018;1693:128–133. doi: 10.1016/j.brainres.2018.03.015. [DOI] [PMC free article] [PubMed] [Google Scholar]
- Tenenbaum D., M. B. 2022. KEGGREST: client-side REST access to the Kyoto Encyclopedia of Genes and Genomes (KEGG). R package version 1.38.0.
- Thaxton P., Wyatt R.D., Hamilton P.B. The effect of environmental temperature on parathyphoid infection in the neonatal chicken. Poult. Sci. 1974;53:88–94. doi: 10.3382/ps.0530088. [DOI] [PubMed] [Google Scholar]
- Tian P., Wang G., Zhao J., Zhang H., Chen W. Bifidobacterium with the role of 5-hydroxytryptophan synthesis regulation alleviates the symptom of depression and related microbiota dysbiosis. J. Nutr. Biochem. 2019;66:43–51. doi: 10.1016/j.jnutbio.2019.01.007. [DOI] [PubMed] [Google Scholar]
- Tian P., O'Riordan K.J., Lee Y.K., Wang G., Zhao J., Zhang H., Cryan J.F., Chen W. Towards a psychobiotic therapy for depression: bifidobacterium breve CCFM1025 reverses chronic stress-induced depressive symptoms and gut microbial abnormalities in mice. Neurobiol. Stress. 2020;12 doi: 10.1016/j.ynstr.2020.100216. [DOI] [PMC free article] [PubMed] [Google Scholar]
- Truccollo, B., P. Whyte, and D. J. Bolton. 2020. An investigation of the effect of catecholamines and glucocorticoids on the growth and pathogenicity of Campylobacter jejuni. Pathogens 9 [DOI] [PMC free article] [PubMed]
- Tsiouris V., Georgopoulou I., Batzios C., Pappaioannou N., Ducatelle R., Fortomaris P. The effect of cold stress on the pathogenesis of necrotic enteritis in broiler chicks. Avian Pathol. 2015;44:430–435. doi: 10.1080/03079457.2015.1083094. [DOI] [PubMed] [Google Scholar]
- van de Wouw M., Boehme M., Lyte J.M., Wiley N., Strain C., O'Sullivan O., Clarke G., Stanton C., Dinan T.G., Cryan J.F. Short-chain fatty acids: microbial metabolites that alleviate stress-induced brain–gut axis alterations. J. Phys. 2018;596:4923–4944. doi: 10.1113/JP276431. [DOI] [PMC free article] [PubMed] [Google Scholar]
- Villageliu D.N., Lyte M. Microbial endocrinology: why the intersection of microbiology and neurobiology matters to poultry health. Poult. Sci. 2017;96:2501–2508. doi: 10.3382/ps/pex148. [DOI] [PubMed] [Google Scholar]
- Vlisidou I., Lyte M., van Diemen P.M., Hawes P., Monaghan P., Wallis T.S., Stevens M.P. The neuroendocrine stress hormone norepinephrine augments Escherichia coli O157:H7-induced enteritis and adherence in a bovine ligated ileal loop model of infection. Infect. Immun. 2004;72:5446–5451. doi: 10.1128/IAI.72.9.5446-5451.2004. [DOI] [PMC free article] [PubMed] [Google Scholar]
- Wang G., Li X., Zhou Y., Feng J., Zhang M. Effects of heat stress on gut-microbial metabolites, gastrointestinal peptides, glycolipid metabolism, and performance of broilers. Animals. 2021;11 doi: 10.3390/ani11051286. [DOI] [PMC free article] [PubMed] [Google Scholar]
- Whitton C., Bogueva D., Marinova D., Phillips C.J.C. Are we approaching peak meat consumption? Analysis of meat consumption from 2000 to 2019 in 35 countries and its relationship to gross domestic product. Animals. 2021:11. doi: 10.3390/ani11123466. [DOI] [PMC free article] [PubMed] [Google Scholar]
- Yang C., Fujita Y., Ren Q., Ma M., Dong C., Hashimoto K. Bifidobacterium in the gut microbiota confer resilience to chronic social defeat stress in mice. Sci. Rep. 2017;7:45942. doi: 10.1038/srep45942. [DOI] [PMC free article] [PubMed] [Google Scholar]
Associated Data
This section collects any data citations, data availability statements, or supplementary materials included in this article.
Supplementary Materials
Supplemental Figure 1. Intestinal architecture of control and cold stressed chickens. Representative microphotographs showing H&E stained cecum from control (A–C) and cold stressed chickens (D–F). In the muscularis externa the thickness of the muscle layer was measured (dashed green lines, B, E). In the mucosa the villus height was measured (VH, measured from villus-crypt junction to villus tip in the mucosa; dashed green lines, panels C, F), and CD (measured from crypt base to villus-crypt junction in the mucosa; solid green lines, panels C, F). Scale bars = 500 μm (panels a and d) and 200 μm (panels B, C, E, F).
Supplemental Figure 2. Mock Microbial Community DNA standards. Theoretical (manufacturer reported taxa distribution) distribution of each standard was compared against composition obtained in the present study (Sample) as described in methods: (A) ZymoBIOMICS Microbial Community Standard # D6305; and (B) ZymoBIOMICS Microbial Community Standard # D6311.