Abstract
Synthesizing polymeric materials that are both sustainable and practical has become a priority. Polyurethanes (PUs) are becoming more popular because of their countless applications and exclusive properties in many sectors. While considering the current issue of environmental problems and the excessive use of petroleum products, nonisocyanate PU (NIPU) are favored due to their sustainability and low toxicity compared to conventional PU. In this work, flexible NIPU films were made using a green and facile method. For that, soybean oil (SBO) was used as the starting material and converted into epoxide SBO, followed by its chemical conversion into carbonated SBO (CSBO) using carbon dioxide gas. Following that, the CSBO reacted with three different aliphatic amines, namely, 1,2-ethylenediamine, 1,4-butylenediamine, and 1,6-hexamethylenediamine, in a solventless and catalyst-free system. The films were cast and cured at 85 °C for different curing times. The effects of the aliphatic diamines and curing times on the NIPU films were evaluated. The individual materials were confirmed with Fourier transform infrared, 1H nuclear magnetic resonance, and gel permeation chromatography. To analyze the thermal and mechanical properties, thermogravimetric analysis, dynamic mechanical analysis, and differential scanning calorimetry were performed. Furthermore, mechanical tests such as hardness and tensile strength were also performed along with the degree of swelling, gel content, and contact angle by using several solvents. This study elucidated the structure–property relationship based on the effect of curing time and aliphatic chain size of diamines in the properties of a NIPU film. The satisfactory thermal and mechanical properties, accompanied by a green and facile approach, displayed the potential scalability of the NIPU films.
1. Introduction
The versatility in the fabrication of polyurethanes (PUs) has allowed several changes in its manufacturing processes over the years. One of the first cases was the reduction of the chlorofluorocarbon gases that were used as blowing agents, which, aside from being highly flammable, were also attributed as a cause of the depletion of the ozone layer in the atmosphere.1,2 Such a situation led to the introduction of other blowing agents, such as hydrocarbons and water, which were less harmful to the environment and considerably cheaper. Also, there was a push to diminish the large use of organic solvents in the production of PU dispersions, which prompted the development of waterborne PUs.3−5 These dispersions found applications as coatings for protection against corrosion, adhesives, inks, and paints, among other products.3,6,7
Nowadays, there is an effort from the scientific community to find alternatives to the use of isocyanates, which, even though they are a core component for the fabrication of PU, are also harmful compounds as they can lead to respiratory diseases and severe skin irritation.8−10 Alongside that, one of the reagents used for its synthesis is phosgene, which is extremely toxic.11 Other factors, such as its nonrenewable origin and the limited number of commercially available products when compared to polyols, also instigate the research to find alternatives for this monomer.12−14 This situation pushed research for the development of nonisocyanate PUs (NIPUs). Based on that, among the available synthetic routes, the reaction through a cyclic carbonated compound that reacts with a primary amine became one of the most notorious.15−17 One of the reasons is that the cyclic carbonate monomer can be synthesized through the reaction between epoxy and carbon dioxide (CO2). In this sense, the epoxy groups can be chemically introduced into unsaturated vegetable oil. Along with that, CO2 is used as a reagent to convert the epoxy into a cyclic carbonate, which makes the process highly sustainable. Then, a primary amine can react with the cyclic carbonated monomer to yield the NIPU.18 This considerable change in the synthetic route opened several possibilities for the fabrication of a wide set of NIPUs with different properties, which can be obtained by varying the catalysts,19−22 utilizing multifunctional carbonates, compositing with inorganic materials, or other polymers.22−25 Such a variety allows for the design of polymeric structures that can potentially cover a broad range of applications.
This work presents the synthesis and characterization of NIPU derived from soybean oil (SBO). The approach consisted of converting the double bonds in the SBO into epoxy groups, epoxide SBO (ESBO), followed by its reaction with CO2 to obtain carbonated SBO (CSBO). Then, three diamines, i.e., 1,2-ethylenediamine (EDA), 1,4-butylenediamine (BDA), and 1,6-hexamethylenediamine (HDA), reacted with the CSBO to obtain NIPU films. The schematic for the synthetic process and the proposed chemical structures are presented in Figure 1. The structure–property relation along with the thermo-mechanical properties of the NIPUs were elucidated, which displayed a material with potential use in the industry due to its appreciable thermal, mechanical, and hydrophobic properties alongside its eco-friendly and sustainable credentials. The novelty of this work resides in the high content of eco-friendly and renewable materials that can be used to synthesize a flexible NIPU with appreciable mechanical properties. The synthesis of the CSBO utilizes CO2, which can function to capture carbon from the environment while using an abundant source to synthesize the monomers for the NIPU. Lastly, the synthesis of the NIPU itself is a solvent- and catalyst-free method consisting of the direct addition of different aliphatic diamines followed by mixing and curing to obtain the polymeric films with comparable properties to previous reports in terms of thermal and mechanical properties.26−28 Such aspects make this type of approach highly desired for scaling to make processing more sustainable and potentially cheaper.
Figure 1.
Schematic for the synthesis of NIPU films using different diamines.
2. Experimental Details
2.1. Materials
SBO and distilled water were purchased from a local Walmart (Pittsburg, KS, USA). Glacial acetic acid (99.7%), toluene (99.5%), Amberlite IR 120H, sodium chloride, sodium sulfate, and tetrabutylammonium bromide (TBAB) (99%) were purchased from Acros Organics (NJ, USA). Hydrogen peroxide 30% (w/w), EDA, BDA, and HDA were purchased from Sigma-Aldrich (St. Louis, MO, USA). CO2 was supplied by Matheson Trigas (Basking Ridge, NJ, USA).
2.2. Test Methods and Instruments
The spectroscopic characterizations of the starting materials and NIPU films were performed using a PerkinElmer Spectrum Two Fourier transform infrared (FT-IR) spectrometer. Gel permeation chromatography (GPC) was performed through an Acquity instrument from Waters system using tetrahydrofuran as solvent, a 7.8 × 300 mm Styrogel HR 0.5, and an OMNISEC REVEAL detector system from Malvern. The viscosity was measured through the use of an AR 2000 ex rheometer from TA Instruments. A 2° angle cone plate with a 12.5 mm radius was used in the measurements. The 1H nuclear magnetic resonance (NMR) was performed using a Spinsolve 80 Multi X from Magitek. Thermogravimetric analysis (TGA) was performed using a TGA 550 with a heating rate of 10 °C/min from 25 to 600 °C. Differential scanning calorimetry (DSC) was performed using a DSC Q100 by performing three cycles of cooling and heating with a temperature rate of 10 °C/min from −60 to 110 °C while keeping the system isothermal at −60 and 110 °C for 5 min. Dynamic mechanical analysis (DMA) was performed using a DMA Q800. The test was performed in the tension mode at a frequency of 1 Hz with a heating rate of 3 °C/min from −60 to 110 °C. The specimen obtained from the NIPU films was cut in a rectangular prism shape with the dimensions of 10 × 15 × 2.45 mm (W × L × T). The TGA, DSC, and DMA were obtained from TA Instruments. The tensile strength test was performed using an Instron 3367 instrument following the ASTM D882-97 standard. The specimens were films with dimensions of 50 mm of length, 10 mm of uniform width, and a thickness of around 2.45 mm. The test was performed at a rate of 10 mm/min.
The hardness test was performed using a Type D durometer from PTC Instruments under the ASTM D2240 procedure. The water contact angle was measured using an Ossila Contact Angle Goniometer. Every analysis was performed three times.
2.3. Synthesis
2.3.1. Synthesis of ESBO
A commercially available SBO (100 g) was charged in a four-neck round-bottom flask equipped with a mechanical stirrer. Then, SBO was diluted with 50 mL of toluene. After that, 25 g of Amberlite IR 120H resin was added to the solution and cooled to 0 °C with vigorous stirring. After cooling, glacial acetic acid (14.9 g, 0.248 mol, 0.5 equiv) was added dropwise, and the reaction mixture was stirred for 30 min at 0 °C. Then 30% hydrogen peroxide (84.33 g, 0.744 mol, 1.5 equiv) was added to the reaction mixture. After complete addition, the system was heated to 70 °C for 7 h. After completion, the reaction mixture was allowed to cool to room temperature. After that, the resin was filtered out with the help of a 50 μm filter with a nylon mesh. The remaining oil was washed with brine several times. Later, the organic layer was dried over sodium sulfate and concentrated under reduced pressure to collect ESBO. The obtained product was confirmed through FT-IR and GPC. After that, ESBO was used in the next step.
2.3.2. Synthesis of CSBO
In a 500 mL autoclave reactor, ESBO (100 g) and TBAB (3.7 g, 0.011 mol, 0.27 equiv) catalysts were mixed and stirred at 1100 rpm. Then, CO2 gas was purged through low pressure in the reaction mixture three times. After a uniform environment was established, the system was gradually heated to 110 °C and initially set at a pressure of 300 psi. After that, CO2 pressure rose to 800 psi for 36 h. The reaction progress was monitored through the oxirane oxygen percentage (EOC %) and viscosity analysis. For the EOC % wet chemistry analysis, the sample was dissolved in glacial acetic acid in the presence of tetraethylammonium bromide. Then, crystal violet was used as an indicator, and the solution was titrated with 0.1 N HClO4 until it transitioned from blue to the green end point. The EOC % was calculated based on eq 1
| 1 |
where V and N are the volume for titration and the normality of HClO4, respectively. During the reaction, small aliquots were taken from the reaction system to monitor its progress. That step was done by gently opening a valve connected to a tube inserted into the reaction mixture. Through that, small aliquots could be collected without interfering with the system or causing variations in the internal pressure. Figure 2 displays the decrease in epoxy concentration over time for the carbonation reaction, which showed that after around 40 h, the epoxy groups were completely consumed from the system. After the reaction was completed, it was allowed to cool to 50 °C. After that, the pressure was slowly released from the system. Finally, a light brown, viscous, waxy liquid was obtained.
Figure 2.

Decrease of epoxy concentration as a function of reaction time.
2.3.3. Synthesis of NIPU
To fabricate the NIPU films, diamine (20.5 wt % of CSBO) and CSBO were added at room temperature and gently mixed with a glass rod to avoid air bubbles, until the mixture became viscous. Simultaneously, a Teflon mold was prepared by spraying a mold releaser, followed by heating it at 85 °C. After that, the viscous mixture was poured into a mold and cured at 85 °C at different curing times of 1.5, 3, 6, 12, and 24 h.
3. Results and Discussion
3.1. Viscosity
The analysis of the rheological properties of a material can provide important information regarding the success of a reaction, changes in the viscoelastic properties, and molecular weight, among many other factors. In this regard, the viscosity of the starting materials, i.e., SBO, ESBO, and CSBO, was measured and observed to be 0.04, 2.9, and 89.38 Pa s measured at 25 °C. Such a variation in viscosity was expected and can be explained mostly through intermolecular interactions. SBO presented mostly van der Waals weak interactions, which made it easier to flow, therefore displaying the lowest viscosity. Upon epoxidation, ESBO was obtained, which presented oxirane groups that increased the polarity of the ESBO chemical structure, leading to the formation of stronger intermolecular interactions. Such a factor was reflected in the increase in viscosity. CSBO presented the highest viscosity as it displayed highly polarized groups of the cyclic carbonate, which could promote stronger hydrogen bonding interactions with other CSBO molecules. Despite the high viscosity, the CSBO still presented good processability as it could be easily mixed with the other components by hand mixing. Thus, CSBO could be easily incorporated into a mixing process when considering larger-scale systems.
3.2. Fourier Transform Infrared Spectroscopy
Figure 3a shows the FT-IR spectra for the SBO, ESBO, and CSBO. For the case of SBO, a small peak at 3009 cm–1 could be observed assigned to the H–C=C stretch. In the ESBO spectra, the H–C=C peak at 3009 cm–1 disappeared, whereas a new peak related to the C–O–C bending around 844 cm–1 appeared, which suggested the conversion of the unsaturation from the vegetable oil into epoxy rings.29 In the CSBO spectra, there was the appearance of a peak around 1800 cm–1 assigned to the C=O stretch from the cyclic carbonated group, along with the disappearance of the peak at 844 cm–1. Also, the peak at around 1700 cm–1 has been attributed to the C=O stretch of the ester group.17Figure 3b presents the FT-IR for NIPUs containing increasing concentrations of EDA that were measured after 6 h of reaction. It could be observed that when concentrations above 20.5 wt % of EDA concerning CSBO were utilized to react with the CSBO, there was a disappearance of the sharp peak around 1800 cm–1 assigned to the C=O stretch related to the carbonated groups. Simultaneously, there was also the appearance of the N–H stretch at 3300 cm–1 that suggested the presence of amine groups. Also, there were two peaks at 1632 and 1540 cm–1 that were assigned to CONH and C–N stretch, respectively, which suggested the presence of amides. Lastly, the peak from around 1710 to 1733 cm–1 could be attributed to the presence of urethane groups.17,30 Based on that, it could be observed that the increasing wt % of EDA led to a continuous decrease in the sharp peak around 1800 cm–1 assigned to C=O stretch from cyclic carbonated groups, suggesting their reaction with the diamine. From there, the addition of 20.5 wt % of EDA in relation to CSBO led to the consumption of the cyclic carbonates, given their disappearance in the FT-IR spectra after a curing process of 6 h. It is worth noting that the curing process occurred at 85 °C, which could function as enough driving force for the reactions to occur. Yet, it could be likely that several reactions could take place considering that the diamines could react with cyclic carbonates, carbonyls from the triglycerides, and leftover epoxies.30,31 Given that situation, 20.5 wt % of CSBO was the selected weight ratio for the diamines to analyze their properties. It could be observed that this amount could promote the consumption of cyclic carbonates, as seen from the FT-IR spectra (Figure 3c–e), along with yielding flexible NIPU films used for testing. Based on that, the curing process over time for the EDA-, BDA-, and HDA-based NIPU is presented in Figure 3c–e, respectively. Through that, some characteristic peaks could be observed even after short curing times, such as the amide bond stretch (–CONH) at 1630 cm–1, –C=O ester stretch at 1700 cm–1, C–N stretch at 1540 cm–1, and the N–H and O–H stretches in the range of 3300–3440 cm–1.30 The O–H stretches around 3500 cm–1 could also be observed simultaneously with the N–H as the reaction between the cyclic carbonates and primary amines leads to the formation of OH groups forming hydroxy-urethane moieties.20,32,33 Also, C–O stretch vibrations at around 1170 cm–1 could be observed. The presence of OH groups after the reaction between cyclic carbonates and primary amines is one of the aspects that differs the NIPUs from the isocyanate-based PU. It has been reported that when higher ratios of isocyanate are used to react with a polyol, the FT-IR spectra of those PUs present peaks around 3320 cm–1 assigned to N–H stretches as well as peaks around 2260–2270 cm–1 assigned to unreacted isocyanate groups.34,35
Figure 3.
(a) FT-IR spectra of SBO, ESBO, and CSBO. (b) Chromatogram of ESBO and CSBO from GPC. (c) Different EDA to CSBO weight ratio at 6 h. FT-IR spectra were obtained from the increasing curing time for the NIPUs obtained from (d) EDA, (e) BDA, and (f) HDA.
3.3. Gel Permeation Chromatography
The GPC is one of the most utilized techniques, as it is a relative method used to determine important aspects of a material, such as its molecular weight and polydispersity index, among others. GPC was performed to confirm the conversion of epoxy groups into cyclic carbonates, as presented by the chromatograms of ESBO and CSBO in Figure 3f. It could be observed that CSBO presented a shorter elution time when compared to ESBO, which indicated that it presented a higher molecular weight as it could go faster through the column.
3.4. Nuclear Magnetic Resonance
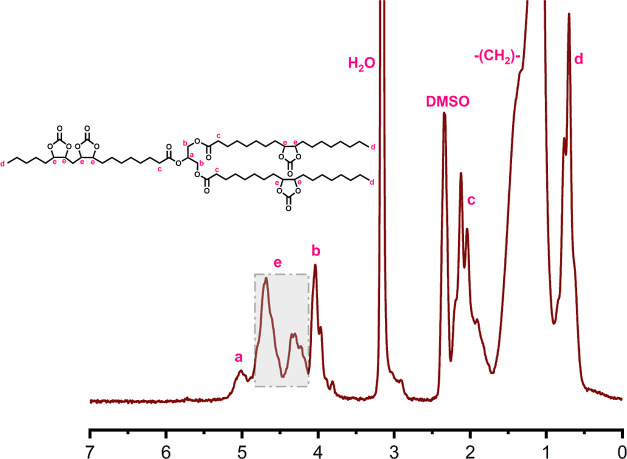
NMR is one of the most important techniques to elucidate the chemical structure of a compound, serving as one of the most important tools in chemistry and material science. The 1H NMR spectra for SBO, ESBO, and CSBO are presented in Figure 4–6, respectively. The spectra of SBO (Figure 4) had the peaks assigned to protons adjacent to the bonded to the C=C and the highly polarized proton bonded to the secondary C–O from the triglyceride, which were within the range of 4.9 to 5.3 ppm and marked as “a”.36 The spectra of ESBO (Figure 5) showed a peak at around 2.5 to 3.0 ppm marked as “e” that were assigned to the C–H in the α-position of the epoxy group.30 The spectra of CSBO (Figure 6) showed new peaks around 4.2 to 5.0 ppm that were assigned to the C–H in the α-position of the cyclic carbonate groups, along with the disappearance of the peaks at around 2.5 to 3.0 ppm assigned to the epoxy groups.37 Based on that, the conversion of epoxy into cyclic carbonate groups could be confirmed based on the shift of these peaks. Based on that, the proposed structures for NIPU are presented in Figure 7.
Figure 4.
1H NMR spectra of SBO.
Figure 6.
1H NMR spectra for CSBO.
Figure 5.
1H NMR spectra of ESBO.
Figure 7.
Proposed chemical structures after the reaction between CSBO and a diamine to form NIPU films.
3.5. Thermogravimetric Analysis
The TGA is an important test to understand the thermal properties of a polymer, such as its working temperature, degradation behavior, and overall thermal stability. In this sense, Figure 8 shows the TGA and derivative TGA (DTGA) for the NIPU obtained from EDA, BDA, and HDA at increasing curing times. In general, the TGA plots displayed similar degradation profiles. However, some slight differences were observed, as presented in Table 1. Generally, there was an increase in degradation temperature with a longer curing time. Such results were expected since a shorter curing time would be more likely to present unreacted diamines in the system, which degrade at lower temperatures. From that, as presented in Table 1, considering the NIPUs cured for 24 h, the decomposition temperature at 5% wt. loss (Td5%), decomposition temperature at 50% wt. loss (Td50%), and maximum decomposition temperature (Tmax), determined as the highest peak at the DTGA plot, were progressively higher for BDA-, EDA-, and HDA-based NIPUs. Also, longer aliphatic chains can introduce some improvement in thermal stability; hence, the relatively larger aliphatic chain of HDA could have been a factor for higher thermal degradation temperatures when compared to EDA and BDA since there was a larger population of C–C bonds that present a dissociation energy of 359.2 kJ/mol, which is higher than C–N 337.7 kJ/mol.38,39 It could also be observed that on the DTGA, the NIPU films obtained from EDA presented three distinct peaks. The first peak could be associated with the decomposition of the diamine segment from the structure. Correlated to that, the first decomposition of the BDA- and HDA-containing NIPUs occurred at higher temperatures, which could be due to the higher dissociation energy of C–C bonds that are present in a larger population in HDA and BDA in comparison to EDA. The second decomposition temperature could be attributed to the polymer’s backbone chain. Yet, in the case of BDA- and HDA-containing NIPUs, the first and second thermal decompositions could have occurred simultaneously due to the higher temperatures required for the degradation of BDA and HDA. This thermal behavior was similar to a previous report from He et al.38
Figure 8.
TGA and DTGA plots for the NIPU films cured at different times were obtained from the diamines (a,b) EDA, (c,d) BDA, and (e,f) HDA.
Table 1. Decomposition Temperatures for NIPU Containing EDA, BDA, and HAD.
| diamine | curing time (h) | Td5% (°C) | Td50% (°C) | Tmax (°C) | residue at 600 °C (%) |
|---|---|---|---|---|---|
| EDA | 1.5 | 197 | 342.9 | 378.1 | 0.86 |
| 3 | 192 | 342.4 | 381.7 | 0.58 | |
| 6 | 198 | 345.9 | 379.7 | 0.62 | |
| 12 | 214 | 349.8 | 384.5 | 0.61 | |
| 24 | 219 | 353.4 | 383.6 | 0.71 | |
| BDA | 1.5 | 154 | 346.6 | 372.9 | 0.99 |
| 3 | 166 | 346.9 | 377.8 | 0.56 | |
| 6 | 171 | 345.1 | 387.1 | 0.44 | |
| 12 | 179 | 349.9 | 376.9 | 0.38 | |
| 24 | 192 | 352.3 | 375.3 | 0.40 | |
| HDA | 1.5 | 199 | 374.3 | 431.3 | 0.51 |
| 3 | 211 | 372.8 | 423.5 | 0.30 | |
| 6 | 209 | 373.5 | 416.9 | 0.33 | |
| 12 | 214 | 374.3 | 415.0 | 0.32 | |
| 24 | 211 | 370.7 | 412.5 | 0.29 |
3.6. Differential Scanning Calorimetry
The DSC is a valuable tool to elucidate the thermal transitions of a polymer in a controlled environment. Under this line, Figure 9 displays the DSC plots for EDA, BDA, and HDA-based NIPUs, respectively. Generally, it could be observed that the glass transition temperature (Tg) tended to increase with curing time. Such behavior was expected since the amine groups would have more favorable conditions to react with the CSBO, likely leading to longer polymeric chains that could be more entangled and, therefore, promote an increase in the Tg. However, both EDA and BDA displayed at least two thermal transitions, regardless of the curing time. These thermal transitions could be, perhaps, associated with the different mobilities of urethane and amide linkages, as it could be possible that both moieties are present in the polymeric structure of the film as backed by FTIR. In addition, EDA and BDA are generally more reactive than HDA, which could suggest that the former two were less selective to react with either the carbonyl from the ester bond inherently present in the vegetable oil that would lead to an amide or the carbonyl from the cyclic carbonate group that would lead to a urethane group.30 Also, as the aliphatic chain size of the diamines increased, there was a slight increase in the Tg range as EDA-, BDA-, and HDA-based NIPUs presented values from −25 to −20, −10 to 0, and 5 to 10 °C, respectively. This range of values was similar to previously reported studies on NIPUs.17,38
Figure 9.
DSC for the NIPU films based on (a) EDA, (b) BDA, and (c) HDA.
Further on, the comparison of EDA-, BDA-, and HDA-based NIPU at the same curing time of 1.5, 3, 6, 12, and 24 h is shown in Figure 10a–e, respectively. It could be observed that both BDA- and EDA-based NIPUs presented multiple thermal transitions at shorter curing times, whereas HDA-based NIPU presented two that, as previously mentioned, could be associated with the transition temperatures associated with the amide and the urethane segments.
Figure 10.
DSC thermograms for the EDA-, BDA-, and HDA-based NIPU films at increasing curing times of (a) 1.5, (b) 3, (c) 6, (d) 12, and (e) 24 h.
3.7. Dynamic Mechanical Analysis
The DMA is a versatile technique that can be used to elucidate some of the thermal and mechanical properties of a material under specific conditions, such as varying dynamic forces as a function of temperature. From that, valuable information can be obtained regarding the viscoelastic behavior of the material. The DMA of the NIPU containing EDA, BDA, and HDA is presented in Figure 11. The compiled information for DSC and DMA is presented in Table 2. It was notable from the DMA plots for EDA-, BDA-, and HDA-based NIPUs that at short curing times, there were two distinct peaks for the tan delta for the NIPU films, whereas at longer curing times, the two peaks tended to merge. Based on that, it could be proposed that the short curing time could have prevented some of the reactions of the diamines with the cyclic carbonates, which was more likely to result in a shorter polymeric chain with a lower degree of cross-linking.17 Taking the information from FT-IR, it was observed that regardless of the curing times, moieties such as carbonyl and amines were observed, which could suggest the presence of both urethanes and amides in the NIPUs. Yet, at shorter curing times, the NIPUs presented more separated tan delta peaks, which suggested the occurrence of two Tg processes. Perhaps, one could be attributed to the Tg of chains formed from the reaction of diamines with cyclic carbonates that resulted in urethanes, whereas the other could be related to the reaction of diamines with the carbonyl esters derived from the triglycerides in the vegetable oil’s structure that led to the formation of amides. At longer curing times, it could be observed as an approximation of the peaks. Such an effect could be due to the longer time that allowed the diamines or end-reactive groups to react, leading to polymeric chains containing both urethanes and amide groups.
Figure 11.
DMA plots for EDA-, BDA-, and HDA-based NIPU films, respectively, for (a–c) tan delta, (d–f) storage modulus, and (g–i) loss modulus.
Table 2. Transition Temperatures for NIPU Containing EDA, BDA, and HDA for DSC and DMA.
| diamine | curing time (h) | Tg DSC (°C) | Tg DMA (°C) |
|---|---|---|---|
| EDA | 1.5 | 15.27 | 55.28 |
| 3 | 16.07 | 52.75 | |
| 6 | 14.04 | 52.22 | |
| 12 | 26.92 | 44.90 | |
| 24 | 24.79 | 47.08 | |
| BDA | 1.5 | 12.74 | 52.77 |
| 3 | 12.97 | 48.95 | |
| 6 | 14.23 | 48.69 | |
| 12 | 13.42 | 45.93 | |
| 24 | 20.25 | 39.80 | |
| HDA | 1.5 | 27.80 | 35.72 |
| 3 | 15.18 | 37.74 | |
| 6 | 21.58 | 35.18 | |
| 12 | 24.62 | 37.64 | |
| 24 | 25.90 |
Further on, it could be observed that there was a decreasing value of Tg from the order EDA-, BDA-, and HDA-based NIPUs, as shown in Table 2. It could be proposed that the shorter aliphatic chain of EDA led to the formation of less spaced, rigid, and polarized segments that could present more hydrogen-bonding interactions when compared with BDA- and HDA-based NIPUs that presented slightly longer aliphatic chains that displayed weaker intermolecular interactions. Because of that, the EDA-based NIPU was more likely to present higher temperatures to disrupt these intermolecular interactions, to allow the polymeric chains to move. Hence, higher Tg values. In addition to that, the decrease in the trend in the decrease of Tg could be attributed to the reduction of the content of amine groups at a fixed concentration of carbonate moieties, which could have led to a decrease in the cross-linking density of the NIPU. Such a condition facilitates the movement of polymeric chains, causing the Tg to decrease.
3.8. Mechanical Properties
The mechanical properties of the NIPU films obtained through the curing process with different diamines were evaluated. Figure 12a displays the Shore D hardness of the EDA-, BDA-, and HDA-based NIPUs at different curing times. It could be observed that all the NIPU films presented an increase in hardness with an increasing curing time. Such an effect was expected as longer curing times promoted the reaction of the diamines with the cyclic carbonate moieties, which could lead to an increase in molecular weight, cross-linking density, and hydrogen bonding in the NIPU backbone. Such factors are likely to increase the hardness of a polymeric film. After 24 h of curing process, the EDA-, BDA-, and HDA-based NIPUs presented a Shore D hardness of around 20, 18, and 22, respectively. The highest hardness for the HDA-based NIPU could perhaps be attributed to a larger polymeric chain entanglement, given that the slightly larger aliphatic chain of HDA could promote an increase in the hardness of the NIPU.
Figure 12.
(a) Hardness shore D and (b) tensile strength tests for the NIPU films. (c) Stretchability of the NIPU films during tensile testing. (d) Various aspects of the NIPU film’s flexibility. (e) Bone-shaped specimen used for the tensile test.
The tensile strengths of the NIPU films are presented in Figure 12b. It could be observed that longer curing times led to an increase in tensile strength. From that, the highest values were obtained for the NIPU films cured at 24 h, which were around 2.7, 0.7, and 0.5 MPa for EDA-, BDA-, and HDA-based NIPU films, respectively. The higher tensile strength observed for the EDA-based NIPU film could be attributed to the smaller aliphatic chain of the EDA, which led to a higher degree of cross-linking that was likely to make the NIPU structure more densely packed. Because of that, the polymeric structure of EDA-based NIPU required a larger amount of force to enable its movement as it presented slightly smaller aliphatic chains that are less flexible than the ones of BDA- and HDA-based NIPU, which resulted in higher tensile strength. The NIPU film’s flexibility during the tensile test is shown in Figure 12c. The general flexibility of the NIPU-based films in terms of stretching, twisting, folding, and wrapping is presented in Figure 12d as well, and the NIPU film specimen that was cast in a mold to attain the bone shape to be used for tensile testing is presented in Figure 12e. The plots of stress vs strain demonstrated that increasing curing times led to an overall increase in tensile stress accompanied by a decrease in strain. Based on that, it could be proposed that there was an increase in cross-linking degree at longer curing times, which led to a more brittle film resulting in higher tensile strengths with lower elongations. Yet, HDA-based NIPU film cured for 24 h displayed appreciable mechanical properties by presenting an elongation of around 2% and a stress of around 0.5 MPa. Based on that, it could be proposed that the curing time promoted some degree of cross-linking that influenced the tensile stress, whereas the longer aliphatic chain of HDA introduced some degree of flexibility on the chains. Further on that, the Young’s modulus (MPa) is the slope of the gradient of stress/gradient of strain in a given range of elongation within the elastic region, such as from 0.02 to 0.3%, which is displayed in Table 3. It could be observed that the EDA-based NIPU displayed the highest Young’s modulus at the elongation for the elongation strain from 0.02 to 0.3% with a value of 3.64 MPa, whereas the BDA- and HDA-based NIPU presented 0.9925 and 0.4878 MPa, respectively. This data suggested that EDA-based NIPU was stiffer than the other NIPU films, likely due to the smaller aliphatic chain that hindered the mobility of the polymeric chains, making it more brittle in comparison to the others.
Table 3. Young’s Modulus (E) for the NIPU Films Obtained from Different Diamines with 24 h Curing Time.
| diamine-based NIPU | Young’s modulus(E) (MPa) | tensile stress (σ) (MPa) | tensile strain (ε) (%) |
|---|---|---|---|
| EDA | 3.64 | 1.97 | 0.30 |
| 0.95 | 0.02 | ||
| BDA | 0.99 | 0.59 | 0.30 |
| 0.31 | 0.02 | ||
| HDA | 0.49 | 0.22 | 0.30 |
| 0.08 | 0.02 |
Some of the mechanical properties of the NIPU films were comparable to or higher than the results reported in previous studies on NIPU films.40−42
3.9. Degree of Swelling and Gel Content
The degree of swelling (DS) provides some information regarding a polymer’s capability to absorb and retain a solvent, whereas the gel content (GC) is the insoluble fraction of a cross-linked polymer. Hence, the higher the DS, the higher the affinity of the polymer with the solvent, and the higher the GC, the higher the degree of cross-linking. To perform the test, the NIPU films were immersed in dimethyl sulfoxide (DMSO), N-methyl-2-pyrrolidone (NMP), dimethylformamide (DMF), and toluene for 24 h. After that, the samples were dried in a vacuum oven at 75 °C for 48 h. The swelling capability of the polymer is intrinsically related to its cross-linking degree and its interaction with a given solvent. In this sense, attractive interactions between a cross-linked polymer with solvent lead to a higher DS. The DS can be calculated through eq 2
| 2 |
where m0 is the initial weight and m1 is the weight after swelling. Yet, a fraction of the polymer itself is dissolved, which is removed from the polymer structure upon drying. That condition leads to a reduction of the polymer’s weight in comparison to the initial weight of the polymer before swelling, which allows the calculation of GC through eq 3
| 3 |
where m2 is the weight after drying. Figure 13a–d displays the DS for the NIPU films at different curing times after their immersion in solvents, such as DMSO, NMP, DMF, and toluene, respectively. In general, it could be observed that EDA-based NIPU films presented the highest swelling degree in every solvent when compared to both BDA- and HDA-based NIPU films. The order of solvents that promoted the highest DS was NMP, DMF, DMSO, and toluene. Also, generally, with the increase in curing time, there was a decrease in the swelling degree. Alongside these general aspects that were observed, the HDA-based NIPU film presented the overall lowest DS. Such phenomena could be attributed to a slightly larger chain entanglement of the polymeric chains from the NIPU obtained from HDA that could limit the gradient of solvent that was absorbed by the polymeric matrix.43
Figure 13.
DS for the NIPU films at increasing curing times at (a) DMSO, (b) NMP, (c) DMF, and (d) toluene.
After the swelling test, the NIPU films were dried for 48 h at 75 °C. The final weight of the NIPU films after drying can provide some information regarding the GC of the polymers, as it can be defined as the amount of insoluble fraction of the cross-linked polymer. The GC for the NIPU films after drying from different solvents DMSO, NMP, DMF, and toluene is shown in Figure 14a–d, respectively. It could be observed that DMSO, NMP, and DMF could dissolve a larger fraction of the polymeric chain within the network structure due to the general decrease in the GC of the NIPU films. On the other hand, toluene presented a considerable solubility of the NIPU polymeric chains. Also, similarly to the DS, the GC tended to increase with the increase in curing time for all of the NIPU films. Based on that, taking into consideration the NIPU films cured at 24 h, it could be observed that the lowest GC was around 85%, which suggested a satisfactory cross-linking degree for the NIPU films. Further on that, the HDA-based NIPU presented the highest GC, suggesting that most of its structure was cross-linked. On the other hand, the EDA-based NIPU presented the overall lowest GC, which suggested that it presented a considerable amount of soluble polymeric chains that could be extracted from the network upon drying. The physical aspects of the NIPU films after drying are presented in Figure 15.
Figure 14.
GC for the NIPU films at increasing curing times in (a) DMSO, (b) NMP, (c) DMF, and (d) toluene.
Figure 15.

Physical aspect of the swollen NIPU films after drying.
3.10. Water Contact Angle
The contact angles for the NIPU films were performed in several solvents such as water, glycerol, ethylene glycol, DMSO, and DMF, which are shown in Figure 16a–e, respectively. For the case of water (Figure 16a), the average contact angles for EDA-, BDA-, and HDA-based NIPU films were 111.83, 103.12, and 104.11°, respectively. It was observed that the films presented relatively similar contact angles with values above 90°, which can characterize them as hydrophobic. Yet, upon decreasing the polarity of the solvents in the order of glycerol (Figure 16b), ethylene glycol (Figure 16c), DMSO (Figure 16d), and DMF (Figure 16e), there was an overall decrease in the contact angle, which reinforced the hydrophobic behavior of the NIPU films. Yet, generally, the HDA-based NIPU film presented the highest contact angles in comparison to EDA- and BDA-based NIPU films, except for the case of DMF. Based on that, it could be proposed that the longer aliphatic chains of HDA led to a general increase in contact angle, suggesting that it tended to repel the solvents in comparison to EDA- and BDA-based NIPU films. Yet, HDA-based NIPU film had a stronger interaction with a nonpolar solvent such as DMF as compared to the other solvents, which led to a lower contact angle. Based on that, in general, the films presented a hydrophobic nature.
Figure 16.
Contact angles for the 24 h cured EDA-, BDA-, and HDA-based NIPU films for (a) water, (b) glycerol, (c) ethylene glycol, (d) DMSO, and (e) DMF.
4. Conclusions
In conclusion, this paper describes a feasible approach to obtaining NIPU films using CSBO that were polymerized with the use of different diamines with increasing sizes of their aliphatic chains. The solvent- and catalyst-free procedures, along with a straightforward film casting technique to obtain the NIPU film, can make the scalability of these polymeric materials more feasible. On top of that, the isocyanate-free and use of bio-based starting materials such as SBO account for an eco-friendly credential for the NIPU. This study revealed that through the control of simple variables such as type of diamine and curing time, the films can cover a wide range of mechanical properties as they went from relatively brittle and rigid, as for the case of EDA-based NIPU, to gradually more elastic and flexible, as for the case of BDA- and HDA-based NIPUs. Following that, it was observed that curing time held the most detrimental effect on overall properties, leading to a general improvement in thermal decomposition, hardness, tensile strength, and reduction in the DS. On the other hand, an increase in the size of the aliphatic chains of the diamines, i.e., EDA, BDA, and HDA, led to an increase in thermal decomposition and a decrease of hardness, tensile stress, and DS. In terms of mechanical properties, it was notable that with the increase of the aliphatic chain size of the diamines, there was a shift toward tougher NIPU films, which could be relatively more elastic while withstanding higher stress values, as for the case of HDA-based NIPU. Based on these findings, the NIPU films obtained in this work help in the elucidation of the structure–property relationship, along with providing a facile approach for the fabrication of polymeric materials with sustainable and eco-friendly aspects.
Acknowledgments
The authors acknowledge Pittsburg State University for providing research and financial support.
The authors declare no competing financial interest.
References
- Protocol M.Montreal protocol on substances that deplete the ozone layer; DC US Gov Print Off: WA, 1987; Vol. 26; pp 128–136. [Google Scholar]
- Molina M. J.; Rowland F. S. Stratospheric sink for chlorofluoromethanes: chlorine atom-catalysed destruction of ozone. Nature 1974, 249, 810–812. 10.1038/249810a0. [DOI] [Google Scholar]
- de Souza F. M.; Gupta R. K.. Waterborne Polyurethanes for Corrosion Protection BT. In Sustainable Production and Applications of Waterborne Polyurethanes; Inamuddin, Boddula R., Khan A., Eds.; Springer International Publishing: Cham, 2021; pp 1–27. [Google Scholar]
- Zhou X.; Li Y.; Fang C.; Li S.; Cheng Y.; Lei W.; Meng X. Recent Advances in Synthesis of Waterborne Polyurethane and Their Application in Water-based Ink: A Review. J. Mater. Sci. Technol. 2015, 31, 708–722. 10.1016/j.jmst.2015.03.002. [DOI] [Google Scholar]
- Ahmadi Y.; Ahmad S. Recent progress in the synthesis and property enhancement of waterborne polyurethane nanocomposites: promising and versatile macromolecules for advanced applications. Polym. Rev. 2020, 60, 226–266. 10.1080/15583724.2019.1673403. [DOI] [Google Scholar]
- Liu X.; Hong W.; Chen X. Continuous Production of Water-Borne Polyurethanes: A Review. Polymers 2020, 12, 2875. 10.3390/polym12122875. [DOI] [PMC free article] [PubMed] [Google Scholar]
- Honarkar H. Waterborne polyurethanes: A review. J. Dispers Sci. Technol. 2018, 39, 507–516. 10.1080/01932691.2017.1327818. [DOI] [Google Scholar]
- Karol M. H.; Kramarik J. A. Phenyl isocyanate is a potent chemical sensitizer. Toxicol. Lett. 1996, 89, 139–146. 10.1016/S0378-4274(96)03798-8. [DOI] [PubMed] [Google Scholar]
- Barbhuiya M.; Bhunia S.; Kakkar M.; Shrivastava B.; Tiwari P. K.; Gupta S. Fine needle aspiration cytology of lesions of liver and gallbladder: An analysis of 400 consecutive aspirations. J. Cytol Acad. Cytol 2014, 31, 20. 10.4103/0970-9371.130634. [DOI] [PMC free article] [PubMed] [Google Scholar]
- Bello D.; Herrick C. A.; Smith T. J.; Woskie S. R.; Streicher R. P.; Cullen M. R.; Liu Y.; Redlich C. A. Skin Exposure to Isocyanates: Reasons for Concern. Environ. Health Perspect. 2007, 115, 328–335. 10.1289/ehp.9557. [DOI] [PMC free article] [PubMed] [Google Scholar]
- Slocombe R. J.; Hardy E. E.; Saunders J. H.; Jenkins R. L. Phosgene derivatives. The preparation of isocyanates, carbamyl chlorides and cyanuric acid1. J. Am. Chem. Soc. 1950, 72, 1888–1891. 10.1021/ja01161a009. [DOI] [Google Scholar]
- Akindoyo J. O.; Beg M. D. H.; Ghazali S.; Islam M. R.; Jeyaratnam N.; Yuvaraj A. R. Polyurethane types, synthesis and applications-a review. RSC Adv. 2016, 6, 114453–114482. 10.1039/C6RA14525F. [DOI] [Google Scholar]
- de Souza F. M.; Kahol P. K.; Gupta R. K.. Polyols from Sustainable Resources. In Polyurethane Chemistry: Renewable Polyols and Isocyanates; Gupta R. K., Kahol P. K., Eds.; American Chemical Society: Washington, D.C., 2021; pp 2–25. [Google Scholar]
- Asare M. A.; de Souza F. M.; Gupta R. K. Waste to Resource: Synthesis of Polyurethanes from Waste Cooking Oil. Ind. Eng. Chem. Res. 2022, 61, 18400–18411. 10.1021/acs.iecr.2c03718. [DOI] [Google Scholar]
- Cornille A.; Auvergne R.; Figovsky O.; Boutevin B.; Caillol S. A perspective approach to sustainable routes for non-isocyanate polyurethanes. Eur. Polym. J. 2017, 87, 535–552. 10.1016/j.eurpolymj.2016.11.027. [DOI] [Google Scholar]
- Sardon H.; Pascual A.; Mecerreyes D.; Taton D.; Cramail H.; Hedrick J. L. Synthesis of Polyurethanes Using Organocatalysis: A Perspective. Macromolecules 2015, 48, 3153–3165. 10.1021/acs.macromol.5b00384. [DOI] [Google Scholar]
- Javni I.; Hong D. P.; Petrović Z. S. Soy-based polyurethanes by nonisocyanate route. J. Appl. Polym. Sci. 2008, 108, 3867–3875. 10.1002/app.27995. [DOI] [Google Scholar]
- Magliozzi F.; Chollet G.; Grau E.; Cramail H. Benefit of the Reactive Extrusion in the Course of Polyhydroxyurethanes Synthesis by Aminolysis of Cyclic Carbonates. ACS Sustain. Chem. Eng. 2019, 7, 17282–17292. 10.1021/acssuschemeng.9b04098. [DOI] [Google Scholar]
- Panchireddy S.; Grignard B.; Thomassin J.-M.; Jerome C.; Detrembleur C. Catechol Containing Polyhydroxyurethanes as High-Performance Coatings and Adhesives. ACS Sustain. Chem. Eng. 2018, 6, 14936–14944. 10.1021/acssuschemeng.8b03429. [DOI] [Google Scholar]
- Panchireddy S.; Grignard B.; Thomassin J.-M.; Jerome C.; Detrembleur C. Bio-based poly(hydroxyurethane) glues for metal substrates. Polym. Chem. 2018, 9, 2650–2659. 10.1039/C8PY00281A. [DOI] [Google Scholar]
- Fleischer M.; Blattmann H.; Mülhaupt R. Glycerol-pentaerythritol- and trimethylolpropane-based polyurethanes and their cellulose carbonate composites prepared via the non-isocyanate route with catalytic carbon dioxide fixation. Green Chem. 2013, 15, 934–942. 10.1039/c3gc00078h. [DOI] [Google Scholar]
- Cornille A.; Ecochard Y.; Blain M.; Boutevin B.; Caillol S. Synthesis of hybrid polyhydroxyurethanes by Michael addition. Eur. Polym. J. 2017, 96, 370–382. 10.1016/j.eurpolymj.2017.09.028. [DOI] [Google Scholar]
- Türünç O.; Kayaman-Apohan N.; Kahraman M. V.; Menceloğlu Y.; Güngör A. Nonisocyanate based polyurethane/silica nanocomposites and their coating performance. J. Sol-Gel Sci. Technol. 2008, 47, 290–299. 10.1007/s10971-008-1786-0. [DOI] [Google Scholar]
- Assumption H. J.; Mathias L. J. Photopolymerization of urethane dimethacrylates synthesized via a non-isocyanate route. Polymer 2003, 44, 5131–5136. 10.1016/S0032-3861(03)00530-5. [DOI] [Google Scholar]
- Figovsky O. L.; Shapovalov L. D. Features of reaction amino-cyclocarbonate for production of new type nonisocyanate polyurethane coatings. Macromol. Symp. 2002, 187, 325–332. . [DOI] [Google Scholar]
- Wang C.; Wu Z.; Tang L.; Qu J. Synthesis and properties of cyclic carbonates and non-isocyanate polyurethanes under atmospheric pressure. Prog. Org. Coat. 2019, 127, 359–365. 10.1016/j.porgcoat.2018.11.040. [DOI] [Google Scholar]
- Steblyanko A.; Choi W.; Sanda F.; Endo T. Addition of five-membered cyclic carbonate with amine and its application to polymer synthesis. J. Polym. Sci., Part A: Polym. Chem. 2000, 38, 2375–2380. . [DOI] [Google Scholar]
- Mao H.-I.; Chen C.-W.; Yan H.-C.; Rwei S.-P. Synthesis and characteristics of nonisocyanate polyurethane composed of bio-based dimer diamine for supercritical CO2 foaming applications. J. Appl. Polym. Sci. 2022, 139, e52841 10.1002/app.52841. [DOI] [Google Scholar]
- Dhore N.; Prasad E.; Narayan R.; Rao C. R. K.; Palanisamy A. Studies on Biobased Non-Isocyanate Polyurethane Coatings with Potential Corrosion Resistance. Sustain. Chem. 2023, 4, 95–109. 10.3390/suschem4010008. [DOI] [Google Scholar]
- Doley S.; Dolui S. K. Solvent and catalyst-free synthesis of sunflower oil based polyurethane through non-isocyanate route and its coatings properties. Eur. Polym. J. 2018, 102, 161–168. 10.1016/j.eurpolymj.2018.03.030. [DOI] [Google Scholar]
- Mora A.-S.; Tayouo R.; Boutevin B.; David G.; Caillol S. A perspective approach on the amine reactivity and the hydrogen bonds effect on epoxy-amine systems. Eur. Polym. J. 2020, 123, 109460. 10.1016/j.eurpolymj.2019.109460. [DOI] [Google Scholar]
- Bähr M.; Bitto A.; Mülhaupt R. Cyclic limonene dicarbonate as a new monomer for non-isocyanate oligo- and polyurethanes (NIPU) based upon terpenes. Green Chem. 2012, 14, 1447–1454. 10.1039/c2gc35099h. [DOI] [Google Scholar]
- Tamami B.; Sohn S.; Wilkes G. L. Incorporation of carbon dioxide into soybean oil and subsequent preparation and studies of nonisocyanate polyurethane networks. J. Appl. Polym. Sci. 2004, 92, 883–891. 10.1002/app.20049. [DOI] [Google Scholar]
- Asefnejad A.; Khorasani M. T.; Behnamghader A.; Farsadzadeh B.; Bonakdar S. Manufacturing of biodegradable polyurethane scaffolds based on polycaprolactone using a phase separation method: physical properties and in vitro assay. Int. J. Nanomedicine 2011, 6, 2375–2384. 10.2147/IJN.S15586. [DOI] [PMC free article] [PubMed] [Google Scholar]
- Dias R. C. M.; Góes A. M.; Serakides R.; Ayres E.; Oréfice R. L. Porous biodegradable polyurethane nanocomposites: preparation, characterization, and biocompatibility tests. Mater. Res. 2010, 13, 211–218. 10.1590/S1516-14392010000200015. [DOI] [Google Scholar]
- Mendes A. N. F.; Gregório J. R.; Rosa R. G. d. Studies on the experimental variables effects on rhodium catalyzed hydroformylation of unsaturated fatty esters and comparison of [RhH (CO)(PPh3) 3] and [RhCl3. 3H2O] as starting catalytic precursors. J. Braz. Chem. Soc. 2005, 16, 1124–1129. 10.1590/s0103-50532005000700006. [DOI] [Google Scholar]
- Liu W.; Lu G.; Xiao B.; Xie C. Potassium iodide-polyethylene glycol catalyzed cycloaddition reaction of epoxidized soybean oil fatty acid methyl esters with CO2. RSC Adv. 2018, 8, 30860–30867. 10.1039/C8RA05947K. [DOI] [PMC free article] [PubMed] [Google Scholar]
- He X.; Xu X.; Wan Q.; Bo G.; Yan Y. Solvent- and Catalyst-free Synthesis, Hybridization and Characterization of Biobased Nonisocyanate Polyurethane (NIPU). Polymers 2019, 11, 1026. 10.3390/polym11061026. [DOI] [PMC free article] [PubMed] [Google Scholar]
- Mó O.; Yáñez M.; Eckert-Maksić M.; Maksić Z. B.; Alkorta I.; Elguero J. Periodic Trends in Bond Dissociation Energies. A Theoretical Study. J. Phys. Chem. A 2005, 109, 4359–4365. 10.1021/jp050857o. [DOI] [PubMed] [Google Scholar]
- Wu H.; Jin B.; Wang H.; Wu W.; Cao Z.; Wu J.; Huang G. A Degradable and Self-Healable Vitrimer Based on Non-isocyanate Polyurethane. Front. Chem. 2020, 8, 585569. 10.3389/fchem.2020.585569. [DOI] [PMC free article] [PubMed] [Google Scholar]
- Lee A.; Deng Y. Green polyurethane from lignin and soybean oil through non-isocyanate reactions. Eur. Polym. J. 2015, 63, 67–73. 10.1016/j.eurpolymj.2014.11.023. [DOI] [Google Scholar]
- Beniah G.; Fortman D. J.; Heath W. H.; Dichtel W. R.; Torkelson J. M. Non-Isocyanate Polyurethane Thermoplastic Elastomer: Amide-Based Chain Extender Yields Enhanced Nanophase Separation and Properties in Polyhydroxyurethane. Macromolecules 2017, 50, 4425–4434. 10.1021/acs.macromol.7b00765. [DOI] [Google Scholar]
- Ke J.; Li X.; Jiang S.; Liang C.; Wang J.; Kang M.; Li Q.; Zhao Y. Promising approaches to improve the performances of hybrid non-isocyanate polyurethane. Polym. Int. 2019, 68, 651–660. 10.1002/pi.5746. [DOI] [Google Scholar]