Abstract

Ischemic stroke is a neurological deficit caused by a lack of blood supply to the brain. Evidence indicates that ischemic stroke leads to dementia of the Alzheimer’s disease (AD) phenotype; however, the underlying molecular mechanism remains unclear. Tau hyperphosphorylation is a common pathological feature of both ischemic stroke and AD. In human AD, the pThr231 residue preceding a pro residue is the primary phosphorylation site that emerges in the phosphorylation cascade before tau tangles, and its levels in cerebrospinal fluid can track AD progression. The pThr231-Pro motif in phosphorylated tau has two distinct cis or trans conformations. Unlike trans P-tau, cis P-tau is the neurotoxic tau conformer, acting as an early precursor of tau pathology in several neurodegenerative disorders, including AD. In a similar pattern, ischemic stroke triggers tau hyperphosphorylation, leading to the formation of tau tangles and promoting neuronal apoptosis. However, it is still unknown whether ischemic stroke induces pathogenic cis P-tau. In this study, we employed both in vitro and in vivo stroke models to investigate cis P-tau formation at different time points by performing immunoblotting and immunofluorescence analyses. We found that cellular stress due to a lack of oxygen and nutrients stimulates cis P-tau formation and accumulation, leading to cistauosis and ultimately neuronal cell death. Therefore, our results suggest a novel molecular mechanism for ischemic stroke and a therapeutic target to fight tau-mediated neurodegeneration after ischemic stroke.
1. Introduction
Ischemic stroke, the most common stroke type, is a neurological deficit caused by a lack of blood supply to the brain.1 At present, the only available medical treatment for acute ischemic strokes is intravenous thrombolysis. However, it is time-dependent and may cause a reperfusion injury. Moreover, stroke survivors are at a high risk of developing cognitive deficits. Thus, there is a great need to develop new therapeutic approaches. Alzheimer’s disease (AD) is the leading cause of dementia worldwide. Growing evidence indicates that ischemic stroke results in neurodegeneration of the AD phenotype, and it provides new insight into a similar mechanism that might be involved in both diseases; however, the underlying mechanism has remained unknown.2,3
Ischemic stroke shares multiple pathological similarities with AD, among which tau hyperphosphorylation is of crucial importance.3 Tau is a phosphoprotein that stabilizes the microtubule structure. However, tau hyperphosphorylation loses the normal function of tau. It causes tau mislocalization4−6 and microtubule destabilization that forms neurofibrillary tangles (NFTs), which are associated with neuronal loss and cognitive deficits in AD and related neurodegenerative disorders, collectively known as tauopathies.7 In a similar pattern, tau hyperphosphorylation induced by ischemic stroke is also prone to tangle formation in humans and animal models.8−11 Additionally, it contributes to neuronal apoptosis in mice after brain ischemia,3,10,12,13 whereas its deletion reduces the infarct volume.14 Therefore, the abnormal phosphorylation of tau in ischemic stroke seems to share a similar pattern of tau pathology with AD. However, it is still unknown whether it leads to neurodegeneration after ischemic stroke with the AD phenotype, and if so, what is the underlying mechanism?
For decades, the importance of specific tau phosphorylation sites has been controversial. In AD, tau hyperphosphorylation occurs on diverse residues.4 However, the Thr231 residue preceding a pro residue (Thr231-Pro) is the primary phosphorylation site that appears in the cascade of phosphorylation before the formation of tau tangles, and its levels can track AD progression.15,16 Notably, the pThr231-Pro motif in phosphorylated tau exists in two distinct cis and trans conformations.16 It has been revealed that Pin1, a peptidyl–prolyl isomerase, hinders tau pathology and neurodegeneration by converting the pThr231-Pro motif from cis to trans in human AD cells and animal models.16 Given the development of antibodies capable of recognizing these conformations, it has been disclosed that trans pThr231-tau (trans P-tau) is the physiological tau conformation, whereas cis pThr231-tau (cis P-tau) is the pathogenic tau conformer.17 Indeed, trans P-tau is associated with microtubule assembly, but cis P-tau, which is resistant to dephosphorylation, promotes dysfunctional tau and acts as an early precursor of tau pathology in acute and chronic neurodegenerative disorders, such as traumatic brain injury (TBI), chronic traumatic encephalopathy (CTE), and AD.16,17 According to studies, in mouse models of TBI or neuronal stress in vitro, neurons generate toxic cis P-tau long before tau aggregation, which leads to axonal pathology, spreads to other neurons, and ultimately results in apoptosis, a process termed cistauosis.17,18 Strikingly, immunotherapy against cis P-tau acts efficiently in preclinical models of TBI and cultured neurons by preventing cistauosis and eventually neuronal cell death.17,19 However, despite extensive research, it is still unknown whether ischemic stroke induces pathogenic cis P-tau. Therefore, we aimed to evaluate cis P-tau induction, an early driver of tau-mediated neurodegeneration in AD,19 in both in vitro and in vivo stroke models.
2. Results
2.1. Hypoxia Stress and/or Nutrient Deprivation Induced cis P-tau Accumulation in Cultured Neurons
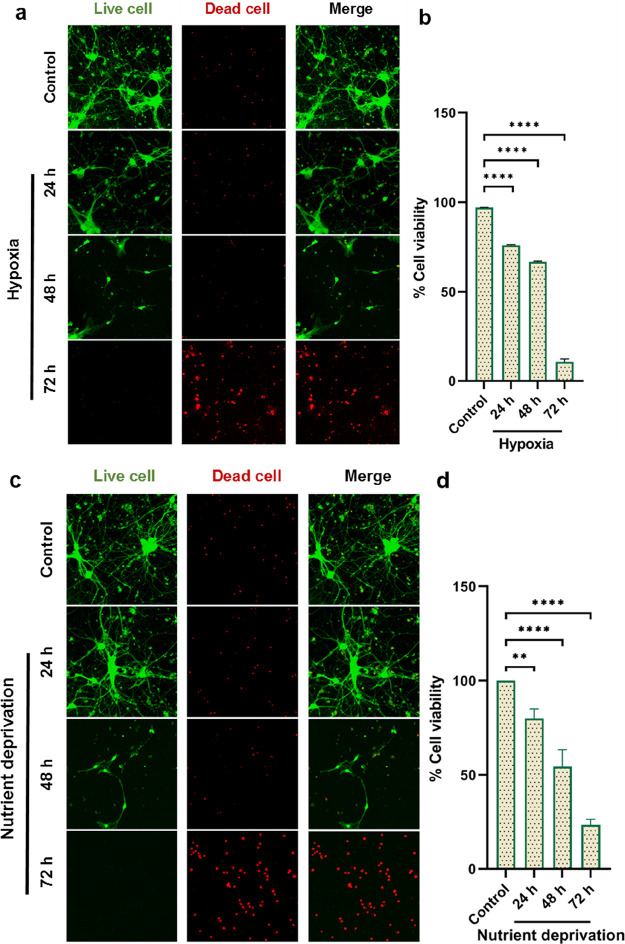
As described above, reduced blood flow to the brain reflects a disrupted oxygen and glucose supply to neuronal cells.1 We herein considered hypoxia or nutrient deprivation in primary cortical neurons as the same stress as an ischemic stroke. Thus, we examined whether toxic cis P-tau might be induced under the same stress conditions in the in vitro cultured neurons. Here, we used cis-pT231-tau mouse monoclonal antibodies to indicate the toxic cis P-tau in primary cultured neurons via immunofluorescence staining and immunoblotting. Immunofluorescence analyses showed that 24 h after hypoxia or nutrient deprivation, cis P-tau was induced and gradually increased over time (Figure 1a). These findings are in line with prior reports, which have shown that neuronal stress caused by serum starvation or hypoxia induces cis P-tau.17 We also showed that both stress conditions not only induced cis P-tau but also resulted in neurodegeneration, confirmed by the live/dead cell assay. According to the live/dead cell assay, hypoxia or nutrient deprivation promotes neuronal death at 24 and 48 h, which is increased significantly to a peak at 72 h (Figure 2a,c). Indeed, based on the prior studies, following these stress conditions, cis P-tau caused the death of cultured neurons, as cis mAbs have been shown to inhibit neuronal cell death induced by serum starvation or hypoxia in SH-SY5Y cells and primary neurons by blocking cis P-tau.17 These results are consistent with studies that have shown that tau hyperphosphorylation induced by ischemic stroke leads to apoptosis in cortical neurons.11,13 Therefore, our results suggest that toxic cis P-tau would be induced and lead to neurodegeneration under the same stress conditions as in ischemic stroke. Based on our findings, there was a dramatic neuronal loss at 72 h, and because of that, we were unable to measure immunofluorescence data to detect cis P-tau.
Figure 1.
Hypoxia and/or nutrient deprivation induce cis P-tau formation in the primary cultured neurons. (a) Primary neurons were cultured and underwent hypoxia followed by immunofluorescence with cis mAb to detect cis P-tau formation at different time points. Primary neurons were cultured without serum at different times followed by immunofluorescence with cis mAb to detect cis P-tau induction at the indicated times. Scale bars, 50 μm. (b) Data were evaluated by one-way analysis of variance (ANOVA), with Tukey’s multiple comparison test (**p < 0.01, ****p < 0.0001). (c) Mean fluorescence intensity was determined by Image J software.
Figure 2.
Hypoxia and/or nutrient deprivation induce neuronal cell death in primary cultured neurons. (a, c) Primary neurons were cultured and after hypoxia and/or serum deprivation were followed by the live and dead cell kit at different time points. The percentage of cell viability was measured after hypoxia and nutrient deprivation at 24, 48, and 72 h. Hypoxia reduced the viabilities of cells to 76, 66.80, and 10.58%, while nutrient deprivation reduced the viabilities to 80, 54.33, and 23.33%, respectively. (b, d) Data were evaluated by one-way analysis of variance (ANOVA), with Tukey’s multiple comparison test (**p < 0.01, ****p < 0.0001).
We also showed cis P-tau formation by immunoblot analysis at 24, 48, and 72 h of hypoxia treatment in a primary neuron culture (Figure 3). We found that hypoxia stress induced neurotoxic cis P-tau in a time-dependent manner. Thus, we demonstrated that hypoxia or nutrient deprivation induced prominent pathogenic cis P-tau in the in vitro cultured neurons, resulting in neurodegeneration (Figures 1 and 2), consistent with the findings that have shown that oxygen and glucose deficit promote tau hyperphosphorylation and then neurodegeneration.20,21
Figure 3.

Hypoxia induces cis P-tau formation in the primary cultured neurons. (a) Primary neurons were cultured and underwent hypoxia followed by immunoblotting with cis mAb to detect cis P-tau formation and accumulation at different time points. (b) Data were evaluated by one-way analysis of variance (ANOVA), with Tukey’s multiple comparison test (***p < 0.001, ****p < 0.0001).
2.2. Ischemia/Reperfusion Induced cis P-tau Accumulation in the Mouse Models
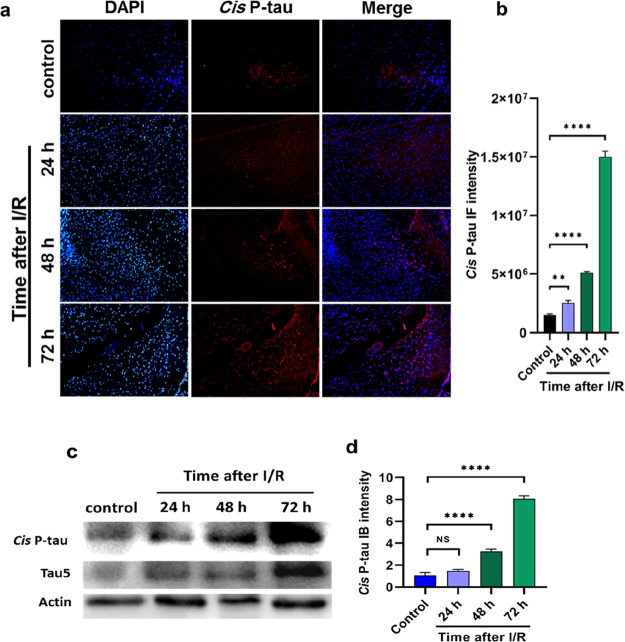
It has been shown that mouse models of ischemia/reperfusion (I/R) induce pathogenic tau hyperphosphorylation associated with apoptosis.12,13,22 Here, we investigated whether mouse models of I/R would induce neurotoxic cis P-tau. Using immunofluorescence analyses, we revealed that 24 h after I/R, cis P-tau was induced and gradually enhanced over time, which was in high levels at 72 h (Figure 4a). Moreover, western blot analysis of brain ischemia indicated cis P-tau formation after 24 h, which increased significantly at 48 and 72 h after injury (Figure 4c). Therefore, our analysis showed that toxic cis P-tau was induced and accumulated in the brains of mouse models after I/R injury. Along with our results, cis P-tau induction has been previously observed in TBI, CTE, and AD, which provides a direct link between these diseases.17 Therefore, we suggest that cistauosis may be a common pathological feature of ischemic stroke, TBI, CTE, and AD.
Figure 4.
I/R induces cis P-tau formation and accumulation in the brain of mouse models. (a) I/R brain was followed by the immunostaining assay with cis mAb to indicate cis P-tau formation at different times. (c) I/R brain was also followed by immunoblotting with cis mAb to reveal cis P-tau formation and accumulation at different times. (b, d) Data were evaluated by one-way analysis of variance (ANOVA), with Tukey’s multiple comparison test. NS, not significant, (**p <0.01, ****p < 0.0001).
3. Discussion
Although growing evidence has shown that ischemic stroke results in neurodegeneration of the AD phenotype,3 the underlying molecular mechanism remains unknown. cis P-tau has been recognized as an early driver of tau pathology and neurodegeneration in AD.17,19 Here, we evaluated the presence of cis P-tau following an ischemic stroke. We showed that cellular stress due to a lack of oxygen and nutrients associated with ischemic stroke induces cis P-tau formation and accumulation, which can lead to cistauosis and eventually neurodegeneration (Figure 5). We used nutrient deprivation or hypoxic stress in primary cortical neurons as in vitro stroke conditions. Then, we examined cis P-tau formation by performing immunoblotting and immunostaining analyses using cis pT231-tau mouse monoclonal antibodies. Our research revealed that following nutrient deprivation and hypoxia stress, there is a gradual accumulation of cis P-tau over time, increasing with poststress. We also showed that both stress conditions result in neuronal cell death in a time-dependent manner. Indeed, cis P-tau is responsible for neuronal death in such a condition, as was shown previously by immunotherapy against cis P-tau.17 Furthermore, we examined cis P-tau induction in ischemic stroke mouse models and showed that cis P-tau is induced, enhancing over time (in a pattern similar to the in vitro models). Our findings are consistent with investigations that have suggested that progressive degeneration after ischemic stroke correlates with tau protein dysfunction.23−25
Figure 5.
Mechanism for the role of cis P-tau in the progression of neurodegeneration after an ischemic stroke. Following ischemic stroke, cellular stress due to a lack of oxygen and nutrients induces the aberrant phosphorylation of tau, leading to the generation of the cis conformation of phosphorylated Thr231-tau (cis P-tau). The cis P-tau promotes cistauosis, leading to neurodegeneration, and also spreads to other neurons and causes neuronal death.
Tau hyperphosphorylation is one of the main pathological hallmarks of AD, and it is also known to be a pathogenic occurrence after reversible brain ischemia.11,13,22,25 Studies have shown that tau hyperphosphorylation seems to have pathological effects on various molecular mechanisms of stroke, including excitotoxicity, oxidative stress, apoptosis, autophagy, inflammation, changes in the blood-brain barrier, and mitochondrial dysfunction. Thus, it may play a crucial role in stroke pathogenesis.24,26 Furthermore, oxidative stress, blood-brain barrier dysfunction, and excitotoxicity, which are negatively influenced by tau hyperphosphorylation, contribute to tau phosphorylation,24,27,28 which, in turn, causes further tau phosphorylation after ischemic stroke. Studies have demonstrated that tau hyperphosphorylation caused by ischemic stroke leads to the formation of paired helical filaments, neurofibrillary tangle-like tauopathy, and neurofibrillary tangles.8−10 Therefore, it can indicate the main pathological hallmarks of AD. In addition, studies have shown that tau depletion plays a neuroprotective role against neurological deficits caused by excitotoxicity in both AD and ischemic stroke mouse models.29,30 As a result, these studies not only demonstrate the importance of tau in ischemic stroke but also suggest a similar tau pathology in both ischemic stroke and AD. Thus, they may explain a mechanism of neurodegeneration after ischemic stroke with the AD phenotype.
In both ischemic stroke and AD, tau is phosphorylated at many phosphorylation sites.4,13 In human AD, pThr231 is the initial event that emerges in the phosphorylation cascade before tangle formation.15 In cerebrospinal fluid (CSF), it can be an early biomarker for detecting the initial pathology in the preamyloid phase of AD.31 Increased levels of the pThr231 in the CSF link with cognitive decrease, neocortical tangle aggregation, and hippocampal atrophy rates in mild cognitive impairment and AD and act as an indicator of progression from mild cognitive impairment to AD.17,19 The pThr231 is also potentially induced following ischemic stroke.13 According to studies, in pathogenic conditions like AD and TBI, the pThr231-pro motif tends to undergo trans-to-cis isomerization, leading to increased toxic cis P-tau.16,17 Likewise, we revealed that the pThr231-tau induced by ischemic stroke13 exhibits pathogenic cis P-tau conformation. Therefore, cis P-tau is induced not only in AD but also after an ischemic stroke. Hyperphosphorylation of tau can be due to the activation of specific kinases.10 Investigations indicate that aberrant cdk5-p25, a kinase activated by p25, plays a crucial role in the development of neurodegeneration and NFTs.32 Notably, ischemic stroke induces potential cdk5-p25, leading to the initial tau phosphorylation.10 Studies have shown that tau neurotoxicity caused by the coexpression of cdk5-p25 and GFP-tau in neurons depends on the cis P-tau induction.17 Therefore, this suggests that cis P-tau may play a critical role in tau neurotoxicity after ischemic stroke. Furthermore, AD, TBI, and ischemic stroke induce activated death-associated protein (DAPK1), a calcium-/calmodulin-regulated serine/threonine–protein kinase. In such a condition, activated DAPK1 phosphorylates tau and results in neuronal degeneration.33−36 According to studies, DAPK1-dependent regulation of tau pathology is mediated by its phosphorylation on Pin1 at Ser71, which inactivates Pin1 function, leading to increased toxic cis P-tau.33,35 Therefore, our results align with the studies indicating that ischemic stroke triggers activated DAPK134 because we suggest that it may contribute to increased cis P-tau after ischemic stroke. As a result, these studies support the idea that cis P-tau mediates stroke pathogenesis and may be one of the earliest events involved in neuronal degeneration after ischemic stroke.
Taken together, our data disclose that ischemic stroke induces cis P-tau, an early driver of tau pathology and neurodegeneration in AD and TBI. Therefore, it may be a causative agent of neurodegeneration after brain ischemia. TBI mouse models have shown that cis P-tau not only is limited to the cortex but also, within 6 months after injury, spreads to other areas, such as the hippocampus,17 which has been confirmed in CTE human patients.18 Hence, we propose that cis P-tau may contribute to the progressive neurodegeneration in remote regions of the primary infarction within days or months after ischemic stroke onset.37 Importantly, TBI mouse models have shown that immunotherapy with cis mAbs is highly effective17,18 as cis P-tau mAbs efficiently block cistauosis by suppressing both intracellular and extracellular cis P-tau and preventing their spread and neuronal death.17 Thus, our results not only suggest a novel molecular mechanism for ischemic stroke but also, given the prior research on immunotherapy against cis P-tau in preclinical TBI,17,18 propose an effective therapeutic agent to suppress cistauosis and neurodegeneration after ischemic stroke.
4. Materials and Methods
4.1. Primary Culture Neuron
Primary neuronal cells were isolated from a 16 day old embryonic mouse brain cerebral cortex. Neuronal cells were seeded on precoated culture dishes with poly-l-ornithine and laminin (Gibco). The medium was then exchanged for a neurobasal medium (Gibco) supplemented with B-27 (Gibco) and 1 mM l-glutamax (Gibco).17 For hypoxia stress, the primary cultured neurons at day 7 were treated with CoCl238 as a hypoxia-mimetic agent. Also, to induce nutrient deprivation stress, the cells were cultured in the absence of serum.
4.2. Animal Model
We performed bilateral common carotid artery occlusion (BCCAO) as an animal model of ischemic stroke.39 In brief, mice were anaesthetized through a mixture of ketamine (10%) and xylazine (2%). Then, both carotid arteries were isolated from the vagus nerve and clamped for 5 min with vascular clamps. Perfusion was restored by removing the vascular clamps for 10 min. Afterward, a subsequent occlusion was performed for 5 min again, and finally, the blood circulation was allowed to return in both carotid arteries. After surgery, all mice were transported to an individual cage, kept at the standard temperature, and allowed to recover normal body temperature.
4.3. Immunostaining Analysis
Cultured neurons were fixed in 4% paraformaldehyde (PFA) for 20 min at 37 °C. The cells were permeabilized with 0.5% Triton-X 100 and blocked in 10% goat serum for 1 h. Then, neuronal cells were stained with a primary antibody overnight. The cells were incubated with secondary antibody Alex flour 488 and/or 594 for 1 h at 37 °C and then washed three times with PBS for 5 min. Next, the samples were incubated with DAPI, and by the use of a fluorescent microscope, the images were recorded. For brain tissue sections, we first deparaffinized units with xylene (Merk, 108633) and dehydrated them with serial dilutions of ethanol followed by incubation in 5% ammonium chloride overnight.
4.4. Immunoblotting Analysis
Brain tissues or cultured neurons were lysed in a RIPA buffer (Sigma-Aldrich, MFCD02100484) containing proteinase and phosphatase inhibitors. Protein concentrations were measured by a Pierce BCA protein assay kit (Thermo Fisher Scientific). Then, samples were mixed with the SDS sample buffer and loaded onto a gel after boiling. The proteins were resolved by polyacrylamide gel and electrophoretically transferred to a nitrocellulose PVDF membrane. Membranes were blocked by staining with 5% milk in TBST (10 mM Tris-HCl pH 7.6, 150 mM NaCl, 0.1% Tween 20) for 1 h followed by incubation with the primary antibody (cis mAb) in 5% milk in TBST overnight at 4 °C. Then, the membranes were incubated with the HRP-conjugated secondary antibody in 5% milk in TBST. The signals were indicated using a chemiluminescence reagent (PerkinElmer, San Jose, CA). The membranes were washed six times with TBST after each step.17
4.5. Live and Dead Cell Assay
Cell viability was examined using a live and dead cell assay kit (Abcam) according to the manufacturer. Cultured neurons were stained with the live and dead dye diluted in PBS and then incubated for 5 min at room temperature in the dark. After the induction of hypoxia and nutrient deprivation in primary cortical neurons, the percentages of cell viability were calculated at 24 h: 76%, 48 h: 66.80%, 72 h: 10.58% and 24 h: 80%, 48 h: 54.33%, 72 h: 23.33% respectively.
4.6. Statistical Analysis
Statistical analysis was carried out with GraphPad Prism version 9.0. Results were presented as the means ± SD, and data were evaluated by one-way analysis of variance (ANOVA), with Tukey’s multiple comparison test. A p value less than 0.05 (p < 0.05) was considered statistically significant. NS, not significant. Mean fluorescence intensity and standard deviation were determined by Image J software.
Acknowledgments
This research did not receive any specific grant from funding agencies in the public, commercial, or not-for-profit sectors.
Author Contributions
⊥ N.S. and D.T. contributed equally to the manuscript. N.S. and K.S. designed the methods, performed the experiments, and analyzed the data; N.S. wrote the manuscript; K.S. edited the manuscript; D.T. designed the methods and performed the experiments; M.D. performed the experiments.
The authors declare no competing financial interest.
References
- Kuriakose D.; Xiao Z. Pathophysiology and treatment of stroke: present status and future perspectives. International Journal of Molecular Sciences. 2020, 21, 7609. 10.3390/ijms21207609. [DOI] [PMC free article] [PubMed] [Google Scholar]
- Wei C.-J. J.; Cui P.; Li H.; Lang W.-J. J.; Liu G.-Y. Y.; Ma X.-F. F. Shared genes between Alzheimer’s disease and ischemic stroke. CNS Neuroscience and Therapeutics. 2019, 25, 855–864. 10.1111/cns.13117. [DOI] [PMC free article] [PubMed] [Google Scholar]
- Pluta R.; Ułamek-Kozioł M.; Januszewski S.; Czuczwar S. J. Tau protein dysfunction after brain ischemia. J. Alzheimer’s Disease. 2018, 66, 429–437. 10.3233/JAD-180772. [DOI] [PMC free article] [PubMed] [Google Scholar]
- Wang Y.; Mandelkow E. Tau in physiology and pathology. Nature Reviews Neuroscience. 2016, 17, 22–35. 10.1038/nrn.2015.1. [DOI] [PubMed] [Google Scholar]
- Ballatore C.; Lee V.M.-Y.; Trojanowski J. Q. Tau-mediated neurodegeneration in Alzheimer’s disease and related disorders. Nature Reviews Neuroscience. 2007, 8, 663–672. 10.1038/nrn2194. [DOI] [PubMed] [Google Scholar]
- Barbier P.; Zejneli O.; Martinho M.; Lasorsa A.; Belle V.; Smet-Nocca C.; Tsvetkov P. O.; Devred F.; Landrieu I. Role of tau as a microtubule-associated protein: structural and functional aspects. Front. Aging Neurosci. 2019, 11, 204. 10.3389/fnagi.2019.00204. [DOI] [PMC free article] [PubMed] [Google Scholar]
- Kaur P.; Khera A.; Alajangi H. K.; Sharma A.; Jaiswal P. K.; Singh G.; Barnwal R. P. Role of tau in various tauopathies, treatment approaches, and emerging role of nanotechnology in neurodegenerative disorders. Molecular Neurobiology. 2023, 60, 1690–1720. 10.1007/s12035-022-03164-z. [DOI] [PubMed] [Google Scholar]
- Khan S.; Yuldasheva N. Y.; Batten T. F. C.; Pickles A. R.; Kellett K. A. B.; Saha S. Tau pathology and neurochemical changes associated with memory dysfunction in an optimized murine model of global cerebral ischaemia-A potential model for vascular dementia?. Neurochem. Int. 2018, 118, 134–144. 10.1016/j.neuint.2018.04.004. [DOI] [PubMed] [Google Scholar]
- Kato T.; Hirano A.; Katagiri T.; Sasaki H.; Yamada S. Neurofibrillary tangle formation in the nucleus basalis of Meynert ipsilateral to a massive cerebral infarct. Ann. Neurol. 1988, 23, 620–623. 10.1002/ana.410230617. [DOI] [PubMed] [Google Scholar]
- Wen Y.; Yang S.-H.; Liu R.; Perez E.J.; Brun-zinkernagel A. M.; Koulen P.; Simpkins J.W. Cdk5 is involved in NFT-like tauopathy induced by transient cerebral ischemia in female rats. Biochim. Biophys. Acta, Mol. Basis Dis. 2007, 1772, 473–483. 10.1016/j.bbadis.2006.10.011. [DOI] [PubMed] [Google Scholar]
- Fujii H.; Takahashi T.; Mukai T.; Tanaka S.; Hosomi N.; Maruyama H.; Sakai N.; Matsumoto M. Modifications of tau protein after cerebral ischemia and reperfusion in rats are similar to those occurring in Alzheimer’s disease--Hyperphosphorylation and cleavage of 4-and 3-repeat tau. J. Cerebral Blood Flow Metab. 2017, 37, 2441–2457. 10.1177/0271678X16668889. [DOI] [PMC free article] [PubMed] [Google Scholar]
- Basurto-Islas G.; Gu J. h.; Tung Y. C.; Liu F.; Iqbal K. Mechanism of tau hyperphosphorylation involving lysosomal enzyme asparagine endopeptidase in a mouse model of brain ischemia. J. Alzheimer’s Disease. 2018, 63, 821–833. 10.3233/JAD-170715. [DOI] [PubMed] [Google Scholar]
- Wen Y.; Yang S.; Liu R.; Simpkins J. W. Transient cerebral ischemia induces site-specific hyperphosphorylation of tau protein. Brain Res. 2004, 1022, 30–38. 10.1016/j.brainres.2004.05.106. [DOI] [PubMed] [Google Scholar]
- Mehta S. L.; Kim T.; Chelluboina B.; Vemuganti R. Tau and GSK-3$β$ are critical contributors to $α$-synuclein-mediated post-stroke brain damage. Neuromolecular Medicine. 2023, 25, 94–101. 10.1007/s12017-022-08731-0. [DOI] [PMC free article] [PubMed] [Google Scholar]
- Luna-Mun̈oz J.; Chávez-Macías L.; García-Sierra F.; Mena R. Earliest stages of tau conformational changes are related to the appearance of a sequence of specific phospho-dependent tau epitopes in Alzheimer’s disease. J. Alzheimer’s Disease. 2007, 12, 365–375. 10.3233/JAD-2007-12410. [DOI] [PubMed] [Google Scholar]
- Nakamura K.; Greenwood A.; Binder L.; Bigio E. H.; Denial S.; Nicholson L.; Zhou X. Z.; Lu K. P. Proline isomer-specific antibodies reveal the early pathogenic tau conformation in Alzheimer’s disease. Cell. 2012, 149, 232–244. 10.1016/j.cell.2012.02.016. [DOI] [PMC free article] [PubMed] [Google Scholar]
- Kondo A.; Shahpasand K.; Mannix R.; Qiu J.; Moncaster J.; Chen C. H.; Yao Y.; Lin Y. M.; Driver J. A.; Sun Y.; Wei S.; Luo M. L.; Albayram O.; Huang P.; Rotenberg A.; Ryo A.; Goldstein L. E.; Pascual-Leone A.; McKee A. C.; Meehan W.; Zhou X. Z.; Lu K. P. Antibody against early driver of neurodegeneration cis P-tau blocks brain injury and tauopathy. Nature. 2015, 523, 431–436. 10.1038/nature14658. [DOI] [PMC free article] [PubMed] [Google Scholar]
- Albayram O.; Kondo A.; Mannix R.; Smith C.; Tsai C. Y.; Li C.; Herbert M. K.; Qiu J.; Monuteaux M.; Driver J.; Yan S.; Gormley W.; Puccio A. M.; Okonkwo D. O.; Lucke-Wold B.; Bailes J.; Meehan W.; Zeidel M.; Lu K. P.; Zhou X. Z. others, Cis P-tau is induced in clinical and preclinical brain injury and contributes to post-injury sequelae. Nat. Commun. 2017, 8, 1–17. 10.1038/s41467-017-01068-4. [DOI] [PMC free article] [PubMed] [Google Scholar]
- Lu K. P.; Kondo A.; Albayram O.; Herbert M. K.; Liu H.; Zhou X. Z. Potential of the antibody against cis-phosphorylated tau in the early diagnosis, treatment, and prevention of Alzheimer disease and brain injury. JAMA Neurology. 2016, 73, 1356–1362. 10.1001/jamaneurol.2016.2027. [DOI] [PubMed] [Google Scholar]
- Lauretti E.; Li J. G.; Di Meco A.; Praticò D. Glucose deficit triggers tau pathology and synaptic dysfunction in a tauopathy mouse model. Transl. Psych. 2017, 7, e1020–e1020. 10.1038/tp.2016.296. [DOI] [PMC free article] [PubMed] [Google Scholar]
- Raz L.; Bhaskar K.; Weaver J.; Marini S.; Zhang Q.; Thompson J. F.; Espinoza C.; Iqbal S.; Maphis N. M.; Weston L.; Sillerud L. O.; Caprihan A.; Pesko J. C.; Erhardt E. B.; Rosenberg G. A. Hypoxia promotes tau hyperphosphorylation with associated neuropathology in vascular dysfunction. Neurobiol. Disease 2019, 126, 124–136. 10.1016/j.nbd.2018.07.009. [DOI] [PMC free article] [PubMed] [Google Scholar]
- Wen Y.; Yang S.; Liu R.; Brun-Zinkernagel A. M.; Koulen P.; Simpkins J. W. Transient cerebral ischemia induces aberrant neuronal cell cycle re-entry and Alzheimer’s disease-like tauopathy in female rats. J. Biol. Chem. 2004, 279, 22684–22692. 10.1074/jbc.M311768200. [DOI] [PubMed] [Google Scholar]
- Pluta R.; Kiś J.; Januszewski S.; Jabłoński M.; Czuczwar S. J. Cross-Talk between Amyloid, Tau Protein and Free Radicals in Post-Ischemic Brain Neurodegeneration in the Form of Alzheimer’s Disease Proteinopathy. Antioxidants. 2022, 11, 146. 10.3390/antiox11010146. [DOI] [PMC free article] [PubMed] [Google Scholar]
- Pluta R.; Czuczwar S. J.; Januszewski S.; Jabłoński M. The many faces of post-ischemic tau protein in brain neurodegeneration of the alzheimer’s disease type. Cells. 2021, 10, 2213. 10.3390/cells10092213. [DOI] [PMC free article] [PubMed] [Google Scholar]
- Zhang Q.; Gao T.; Luo Y.; Chen X.; Gao G.; Gao X.; Zhou Y.; Dai J. Transient focal cerebral ischemia/reperfusion induces early and chronic axonal changes in rats: its importance for the risk of Alzheimer’s disease. PloS One. 2012, 7, e33722 10.1371/journal.pone.0033722. [DOI] [PMC free article] [PubMed] [Google Scholar]
- Chen X.; Jiang H. Tau as a potential therapeutic tar[1] X. Chen, H. Jiang, Tau as a potential therapeutic target for ischemic stroke, Aging (Albany NY). 11 (2019) 12827.get for ischemic stroke, Aging (Albany NY). Aging 2019, 11, 12827. 10.18632/aging.102547. [DOI] [PMC free article] [PubMed] [Google Scholar]
- Zhou S.; Yu G.; Chi L.; Zhu J.; Zhang W.; Zhang Y.; Zhang L. Neuroprotective effects of edaravone on cognitive deficit, oxidative stress and tau hyperphosphorylation induced by intracerebroventricular streptozotocin in rats. Neurotoxicology. 2013, 38, 136–145. 10.1016/j.neuro.2013.07.007. [DOI] [PubMed] [Google Scholar]
- Ho P. I.; Ortiz D.; Rogers E.; Shea T. B. Multiple aspects of homocysteine neurotoxicity: glutamate excitotoxicity, kinase hyperactivation and DNA damage. Journal of Neuroscience Research. 2002, 70, 694–702. 10.1002/jnr.10416. [DOI] [PubMed] [Google Scholar]
- Bi M.; Gladbach A.; van Eersel J.; Ittner A.; Przybyla M.; van Hummel A.; Chua S. W.; van der Hoven J.; Lee W. S.; Müller J.; Parmar J.; Jonquieres G. v.; Stefen H.; Guccione E.; Fath T.; Housley G. D.; Klugmann M.; Ke Y. D.; Ittner L. M. others, Tau exacerbates excitotoxic brain damage in an animal model of stroke. Nat. Commun. 2017, 8, 1–15. 10.1038/s41467-017-00618-0. [DOI] [PMC free article] [PubMed] [Google Scholar]
- Tuo Q. z.; Lei P.; Jackman K. A.; Li X. l.; Xiong H.; Li X. l.; Liuyang Z. y.; Roisman L.; Zhang S. t.; Ayton S.; Wang Q.; Crouch P. J.; Ganio K.; Wang X. c.; Pei L.; Adlard P. A.; Lu Y. m.; Cappai R.; Wang J. z.; Liu R.; Bush A. I. others, Tau-mediated iron export prevents ferroptotic damage after ischemic stroke. Mol. Psych. 2017, 22, 1520–1530. 10.1038/mp.2017.171. [DOI] [PubMed] [Google Scholar]
- Ashton N. J.; Benedet A. L.; Pascoal T. A.; Karikari T. K.; Lantero-Rodriguez J.; Brum W. S.; Mathotaarachchi S.; Therriault J.; Savard M.; Chamoun M.; Stoops E.; Francois C.; Vanmechelen E.; Gauthier S.; Zimmer E. R.; Zetterberg H.; Blennow K.; Rosa-Neto P. others, Cerebrospinal fluid p-tau231 as an early indicator of emerging pathology in Alzheimer’s disease. EBioMedicine 2022, 76, 103836 10.1016/j.ebiom.2022.103836. [DOI] [PMC free article] [PubMed] [Google Scholar]
- Cruz J. C.; Tseng H.-C.; Goldman J. A.; Shih H.; Tsai L.-H. Aberrant Cdk5 activation by p25 triggers pathological events leading to neurodegeneration and neurofibrillary tangles. Neuron. 2003, 40, 471–483. 10.1016/S0896-6273(03)00627-5. [DOI] [PubMed] [Google Scholar]
- Kim N.; Wang B.; Koikawa K.; Nezu Y.; Qiu C.; Lee T. H.; Zhou X. Z. Inhibition of death-associated protein kinase 1 attenuates cis P-tau and neurodegeneration in traumatic brain injury. Progress in Neurobiology. 2021, 203, 102072 10.1016/j.pneurobio.2021.102072. [DOI] [PMC free article] [PubMed] [Google Scholar]
- Kim N.; Chen D.; Zhou X. Z.; Lee T. H. Death-associated protein kinase 1 phosphorylation in neuronal cell death and neurodegenerative disease. International Journal of Molecular Sciences. 2019, 20, 3131. 10.3390/ijms20133131. [DOI] [PMC free article] [PubMed] [Google Scholar]
- Kim B. M.; You M. H.; Chen C. H.; Lee S.; Hong Y.; Hong Y.; Kimchi A.; Zhou X. Z.; Lee T. H. Death-associated protein kinase 1 has a critical role in aberrant tau protein regulation and function. Cell Death Disease 2014, 5, e1237–e1237. 10.1038/cddis.2014.216. [DOI] [PMC free article] [PubMed] [Google Scholar]
- Pei L.; Wang S.; Jin H.; Bi L.; Wei N.; Yan H.; Yang X.; Yao C.; Xu M.; Shu S.. others, A novel mechanism of spine damages in stroke via DAPK1 and tau, Cerebral Cortex. (2015) 254559–4571. [DOI] [PMC free article] [PubMed]
- Zhang J.; Zhang Y.; Xing S.; Liang Z.; Zeng J. Secondary neurodegeneration in remote regions after focal cerebral infarction: a new target for stroke management?. Stroke. 2012, 43, 1700–1705. 10.1161/STROKEAHA.111.632448. [DOI] [PubMed] [Google Scholar]
- Guo M.; Song L.-P.; Jiang Y.; Liu W.; Yu Y.; Chen G.-Q. Hypoxia-mimetic agents desferrioxamine and cobalt chloride induce leukemic cell apoptosis through different hypoxia-inducible factor-1$α$ independent mechanisms. Apoptosis. 2006, 11, 67–77. 10.1007/s10495-005-3085-3. [DOI] [PubMed] [Google Scholar]
- Nabavi S. F.; Habtemariam S.; Di Lorenzo A.; Sureda A.; Khanjani S.; Nabavi S. M.; Daglia M. Post-stroke depression modulation and in vivo antioxidant activity of gallic acid and its synthetic derivatives in a murine model system. Nutrients. 2016, 8, 248. 10.3390/nu8050248. [DOI] [PMC free article] [PubMed] [Google Scholar]