Abstract
l-carnitine is an essential dietary supplement of physiological importance. Handling and manufacture of l-carnitine is difficult due to its hygroscopic nature, resulting in impairing its flow properties, as well as solid dosage form stability. The study aimed at reducing l-carnitine hygroscopicity through its encapsulation within a hydrophobic, pH-insensitive polymer. A solid in oil in oil (s/o/o) emulsion solvent evaporation technique for microencapsulation was adopted to exclude the possibility of water uptake. The polymers used were two ethyl cellulose (EC) grades with different viscosities. The chosen solvent for the polymer was acetone, and liquid paraffin was the dispersion medium in which both the drug and polymer were insoluble. Sixteen formulations were developed, and evaluated to study the formulation parameters as anti-coalescent type, mixing speed, surfactant type and polymer ratio, and viscosity grade. A “One Factor at A Time” (OFAT) design of experiment, and a factorial design were utilized. Study results revealed that successful microencapsulation occurred by using Aerosil 200 (0.1 %) as anti-coalescent, a mixing speed of 1000 rpm, and Ethocel Std 20 at a 3:1 drug-to-polymer ratio. Microcapsule formulation containing l-carnitine base, successfully compressed into tablets, showed acceptable water content, disintegration time, hardness, and dissolution. Moreover, it showed acceptable stability upon storage at 40 °C at 75 % RH for six months compared to l-carnitine tablets prepared by wet granulation.
Keywords: l-carnitine base, Solid/oil/oil emulsion solvent evaporation, Microencaspsulation, One Factor at A time (OFAT), Hygroscopic, Ethocel
Graphical abstract
1. Introduction
Carnitine, an amino acid derivative, is an essential cofactor of fatty acid metabolism in the heart, liver, and skeletal muscle [1,2]. The natural sources of carnitine include meat, fish, and poultry [3]. The plants represent a limited source of carnitine [4]. The body can synthesize it [5]. The physiological importance of carnitine lies in its role in transferring long-chain fatty acids as acylcarnitine esters across the mitochondrial membranes [6].
Levocarnitine (l-carnitine) is (R)-3-Carboxy-2-hydroxy-N, N, N-trimethyl-1-propanaminium. It is hygroscopic, freely water soluble, and practically insoluble in organic solvents such as acetone, and dichloromethane [7,8]. l-carnitine represents the biologically active enantiomer [9].
The hygroscopicity of l-carnitine rendered its formulation as solid dosage forms extremely difficult, especially during scale-up [10]. The hygroscopic powder shows poor flowability and sticks to machine parts during its processing during manufacture [11,12]. Among the solutions suggested was using a less hygroscopic l-carnitine salt as l-carnitine tartrate [13]. Also, the use of adsorbents to adsorb moisture, thus stabilizing l-carnitine [14]. Drug encapsulation can represent a barrier separating the hygroscopic drug from the moisture. Usually, a water-repellent polymer is used [15]. l-carnitine was previously formulated as liposomes, and nanoparticles to control its release for 12 h [16]. No trials were reported on the encapsulation of l-carnitine to reduce its hygroscopic properties.
Many studies used the micro-encapsulation technique in surrounding and protecting various drugs and natural bioactive compounds [17]. Micro-encapsulation allowed the sparing of volatile oil marjoram [18], encapsulation of Bacillus bacteria which stimulated plant growth [19], encapsulation of Streptomyces fulvissimus as a biocontrol agent [20] using alginate as biopolymer [21], chitosan [22], and starch [23].
Hydrophilic water-soluble materials could be successfully micro-encapsulated using a few techniques. The techniques must avoid water inclusion, as with the s/o/o (solid in oil in oil) emulsion solvent evaporation method. This method is suitable for highly hydrophilic water-soluble drugs such as amino acids and proteins. The method involves dispersing the drug into an organic volatile solvent containing the dissolved polymer. Oil is the continuous phase, in which neither the drug nor the polymer are soluble. A low-HLB surfactant is involved. Using other emulsion solvent evaporation techniques, such as o/w, or w/o, carries the risk of loss of water-soluble drugs like l-carnitine to the external phase of o/w emulsions. Accordingly, a hydrophilic drug in an oil phase has no chance to dissolve into the external organic solvent, thus increasing its encapsulation efficiency [24,25].
The major problem facing the l-carnitine solid dosage forms manufacture is its hygroscopic nature. Atmospheric moisture adsorption by the drug impairs solid dosage form stability. Complete sealing of the drug away from moisture is an acceptable solution. Hence, the process managed the microencapsulation of l-carnitine using s/o/o emulsion solvent evaporation to ensure maximum drug encapsulation efficiency. A water-insoluble, pH-insensitive polymer was employed to achieve isolation from the surrounding moisture. The intended result was to reduce the hygroscopicity of l-carnitine and facilitate its large-scale production as a solid dosage form, as well as ensure its physical stability.
2. Materials and methods
l-Carnitine Base (Kaiyuan Hengtai Chemical Co. Ltd., China); Croscarmellose sodium “Ac-Di-Sol” and microcrystalline cellulose “Avicel PH 101 and Avicel PH 112” (FMC biopolymer, Ireland); Magnesium stearate (Peter Greven, Malaysia); Polyvinylpyrrolidone K30 “Povidone K30” (Fluka, USA); Colloidal silicon dioxide “Aerosil 200” (Evonik Degussa, Germany); Aqualon ® Ethylcellulose N 100 (Ashland, USA); Ethocel ® Standard 20 Premium (Dow Chemicals, USA); Span 80 (Sorbitan monooleate) (Loba Chemie, India); Acetonitrile HPLC grade (Merck, Germany); Potassium dihydrogen phosphate, Phosphoric acid, Liquid Paraffin, Acetone and Hexane (El-Nasr Chemicals Co., Egypt).
2.1. Preparation of l-Carnitine base microcapsules
The emulsion solvent evaporation method was used in the preparation of l-carnitine microcapsules [26]. The choice of parameter involved, namely, solvent, surfactant, polymer solvent ratio, volume of continuous phase, and temperature was based on preliminary trials.
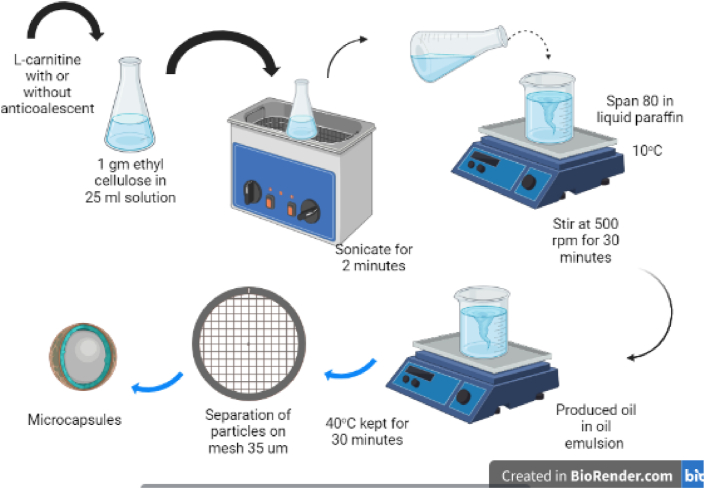
The method involved dissolving 1 g of ethylcellulose (polymer) in 25 ml of acetone. After obtaining a clear solution, the corresponding weights of the l-Carnitine base with (or without) a suitable anti-coalescent (colloidal silicon dioxide or magnesium stearate) were prepared. Then, l-carnitine (10 % w/w) was dispersed in the polymer solution. The beaker containing the polymer-drug dispersion was tightly covered with Parafilm® M and then sonicated in the ultrasonic bath at room temperature for 2 min until it attained homogeneity. The mixture was poured into 100 ml of liquid paraffin containing a predetermined concentration of sorbitan monooleate (Span 80); previously cooled to 10 ± 0.5 °C while being stirred by a mechanical stirrer at 500 rpm for 30 min. That was followed by the gradual heating of the oil in oil emulsion to a temperature of 40 ± 2 °C, while stirring for another 30 min to remove the volatile solvent. The solidified microcapsules were filtered using a mesh of size 35 μm, washed with 50 ml of n-hexane, and then filtered. That was followed by washing five consecutive times with 50 ml of n-hexane to remove the remaining liquid paraffin and drying at 50 °C for 1 h in an oven. The dried microcapsules were collected in tightly closed glass bottles and stored in a desiccator for further investigation. Fig. 1 illustrates the method of encapsulation.
Fig. 1.
Formulation of l-carnitine microcapsules by S/O/O solvent evaporation method.
2.2. Optimization of formulation and process parameters
The formulation and process parameters involved both the usage of OFAT (one factor at a time) design of experiments-which was suitable for studying variables involved in microencapsulation- and factorial design of experiments (Minitab® 19 Statistical Software, version 19.2020.1, Minitab, LLC, USA). Sixteen formulations were prepared in four separate stages. The best formulation promoted to the next stage. Table 1 describes the details of the study, and Table 2 shows the composition of formulations prepared by the emulsion solvent evaporation method.
Table 1.
Stages of microencapsulation of l-Carnitine base using emulsion solvent evaporation method.
| Stage | No. of factors | No. of levels | Factor(s) studied |
|---|---|---|---|
| 1st (OFAT) | 1 | 3 | Anti-coalescent type |
| 2nd (OFAT) | 1 | 3 | Mixing speed |
| 3rd (OFAT) | 1 | 5 | Emulsifier conc. |
| 4th (Factorial) | 2 | 2 4 |
Polymer viscosity grade drug/polymer ratio |
Table 2.
Microencapsulation formulations of l-Carnitine base using emulsion solvent evaporation method.
| Trial No. | Polymer Viscosity grade | Drug/Polymer Ratio | Anti-coalescent | mixing speed | Span 80 (%) |
|---|---|---|---|---|---|
| F1 | EC N100 | 3:1 | – | 500 | 0.1 |
| F2 | EC N100 | 3:1 | Aerosil 200 | 500 | 0.1 |
| F3 | EC N100 | 3:1 | Mg stearate | 500 | 0.1 |
| F4 | EC N100 | 3:1 | Aerosil 200 | 1000 | 0.1 |
| F5 | EC N100 | 3:1 | Aerosil 200 | 1500 | 0.1 |
| F6 | EC N100 | 3:1 | Aerosil 200 | 1000 | – |
| F7 | EC N100 | 3:1 | Aerosil 200 | 1000 | 0.25 |
| F8 | EC N100 | 3:1 | Aerosil 200 | 1000 | 0.5 |
| F9 | EC N100 | 3:1 | Aerosil 200 | 1000 | 1 |
| F10 | EC N100 | 4:1 | Aerosil 200 | 1000 | 0.1 |
| F11 | EC N100 | 2:1 | Aerosil 200 | 1000 | 0.1 |
| F12 | EC N100 | 1:1 | Aerosil 200 | 1000 | 0.1 |
| F13 | Ethocel Std. 20 | 4:1 | Aerosil 200 | 1000 | 0.1 |
| F14 | Ethocel Std. 20 | 3:1 | Aerosil 200 | 1000 | 0.1 |
| F15 | Ethocel Std. 20 | 2:1 | Aerosil 200 | 1000 | 0.1 |
| F16 | Ethocel Std. 20 | 1:1 | Aerosil 200 | 1000 | 0.1 |
F1, F2, and F3 represented the study of the Anti-coalescent type factor. From that stage, F2 succeeded to continue. F2, F4, and F5 demonstrated the mixing speed variable in the second-stage, from which F4 showed superiority. The study of the effect of emulsifier concentration (on F4, F6, F7, F8, and F9) in the third stage resulted in the choice of F4.
2.3. The effects of polymer viscosity grade and drug/polymer ratio
A 2 × 4 Factorial design for microencapsulation of l-Carnitine base, using the emulsion solvent evaporation method, was adopted. The selected independent variables were the effects of polymer viscosity grade (X1) and the drug-polymer ratio (X2). Two different polymer viscosity grades of EC were used, namely Ethylcellulose N 100 (80–105 mPa s), and Ethocel Std. 20P Premium (18–22 mPa s). Four drug-polymer ratios were studied, namely 4:1, 3:1, 2:1, and 1:1. The summary of the factorial design of experiments is given in Table 3. The material quantities were doubled to increase the yield of microcapsules obtained.
Table 3.
2 × 4 Factorial design of microencapsulation of l-Carnitine base studying the effects of polymer viscosity grade and drug/polymer ratio.
| X2 (drug/polymer ratio) |
|||||
|---|---|---|---|---|---|
| 4:1 | 3:1 | 2:1 | 1:1 | ||
| X1 (polymer viscosity grade) | EC N 100 | F10 | F4 | F11 | F12 |
| Ethocel Std. 20 | F13 | F14 | F15 | F16 | |
2.4. Evaluation of microcapsules
2.4.1. Water content
A Karl Fischer titration instrument (905 Titrando, Metrohm, Switzerland) determined the water content of the tested samples. After equilibration of the equipment, 1 g of microcapsules was accurately weighed and placed inside the titration chamber. Samples were dispersed at 100 rpm. The moisture content was recorded from the instrument [27]. All measurements were repeated three times.
2.4.2. Micromeritics
Microcapsules of l-carnitine micromeritics were determined, including flow rate [28], angle of repose [29], bulk and tapped densities [30], Carr's index, and Hausner ratio [31]. The experiments were run in triplicates.
Analysis of particle size by Laser Diffraction: The particle size of l-carnitine microcapsules chosen from the first stage and l-carnitine base was examined by a Laser Diffraction Particle Size Analyzer (Shimadzu SALD-2201, Japan), which covered a range of 0.03–1000 μm. The particle size was compared to that of l-carnitine base powder. One hundred milligrams of the powder were dispersed in hexane and loaded in the quartz cell of the device. Hexane was used as a blank. The test was repeated three times. A semiconductor laser was used as a light source at a wavelength of 680 μm.
2.4.3. Encapsulation efficiency
An accurately weighed amount of the microcapsules, equivalent to 330 mg l-carnitine base, was transferred into a 50-ml glass beaker containing 25 ml of methylene chloride. The mixture was sonicated for 5 min to dissolve the ethylcellulose shell. The broken microcapsules were geometrically transferred and filtered using filter paper to discard methylene chloride and then washed using deionized water into a 100-ml volumetric flask. The volume was adjusted to 100 ml. An aliquot of 10 ml was diluted to 100 ml with deionized water and passed through a millipore membrane of 0.45 μm pore size. The assay was carried out based on an HPLC-based method according to the USP drug monograph [32], and the drug concentration was calculated on the basis of the previously constructed calibration curve.
The encapsulation efficiency of different microcapsule formulations was calculated according to the following equation Eq. 1 [33]:
Encapsulation efficiency (EE) =
| 1 |
The theoretical amount of drug loaded in microcapsules was 330 mg of l-carnitine base.
2.4.4. Production yield
The percentage production yield for each microcapsule formulation was calculated. The actual weight of microcapsules was divided by the sum of the theoretical weights of microcapsule components [34]. The following equation Eq. 2 was used:
| 2 |
2.5. Preparation of l-carnitine base microcapsules as tablets
Microcapsules prepared during the fourth stage were compressed into tablets using the direct compression method of multiunit particulate system (MUPS). The eight suggested tablet formulations contained a dose of l-carnitine microcapsules equivalent to 330 mg l-carnitine base. Microcrystalline cellulose MCC (Avicel PH 112) represented the filler, 5 % croscarmellose sodium (Ac-Di-Sol), a disintegrant, and 2 % magnesium stearate, a lubricant. The final tablet weight was adjusted to 1000 mg using Avicel PH 112, according to the mass of used microcapsules. All formulations were then compressed into tablets. Each batch consisted of 100 tablets. The compositions of suggested directly compressible tablet formulations are summarized in the following Table 4.
Table 4.
Compositions of directly compressible tablet formulations containing l-carnitine base microcapsules.
| Trial | Formulation | quantity (mg) | MCC PH 112 (mg) | AcDiSol (mg) | Mg stearate (mg) | Tablet weight (mg) |
|---|---|---|---|---|---|---|
| C1 | F10 | 445.5 | 484.5 | 50 | 20 | 1000 |
| C2 | F4 | 473 | 457 | 50 | 20 | 1000 |
| C3 | F11 | 528 | 402 | 50 | 20 | 1000 |
| C4 | F12 | 693 | 237 | 50 | 20 | 1000 |
| C5 | F13 | 445.5 | 484.5 | 50 | 20 | 1000 |
| C6 | F14 | 473 | 457 | 50 | 20 | 1000 |
| C7 | F15 | 528 | 402 | 50 | 20 | 1000 |
| C8 | F16 | 693 | 237 | 50 | 20 | 1000 |
2.6. Evaluation of l-carnitine tablet formulations
Tablets were evaluated for thickness, hardness [35], friability [36], and disintegration time [37].
Drug Content: Ten randomly selected tablets from each formulation were accurately weighed and then transferred into a 500-ml volumetric flask, then water was added. Each flask was shaken till the tablet disintegrated, and the volume was completed to 500 ml with water. The flasks were sonicated for 5 min. Three 10-ml aliquots of each tablet formulation were diluted to 20 ml with deionized water, sonicated, and passed through a Millipore membrane of 0.45 μm pore size. The assay was carried out using HPLC [32].
Dissolution rate: The dissolution of each tablet formulation was determined using the USP apparatus II (paddle method) in six vessels (n = 6). The dissolution medium was 900 ml of deionized water maintained at 37 ± 0.5 °C. The paddle rotation speed was set to 75 rpm. After 30 min, samples of dissolution medium were withdrawn and filtered through a 0.45 μm Millipore filter and assayed by HPLC [32].
The formulation results were compared to those of a control tablet formulation containing an l-carnitine base prepared using a wet granulation technique. It consisted of Avicel PH-101 as filler, 1.0 % of Aerosil 200 as adsorbent, 5 % povidone K30 as a binder, 5 % of Ac-Di-Sol as a disintegrant, and 2 % magnesium stearate as lubricant.
2.7. Accelerated stability studies on l-carnitine base microcapsule tablets
Samples: Accelerated stability studies were conducted on l-carnitine base microcapsules formulation C6, which was chosen based on tablet evaluation results. Formulation C6 was compared to the l-carnitine base control tablet (mentioned in the above section). The sample size allowed the study for six months.
Accelerated stability study conditions: The accelerated stability cabinet controls were adjusted to be a temperature of 40 °C ± 2 °C and a relative humidity (RH) of 75 % ± 5 %. The study duration was six months with a testing frequency after 0, 1, 3, and 6 months [29]. The container closure system used was carton boxes containing Aluminum/Triplex (PVC/PE/PVdC) blisters, each of the ten tablets.
The collected tablet samples were evaluated for moisture content, tablet hardness, uniformity of weight, assay, and dissolution test.
2.8. Statistical analysis
The results of the formulations evaluation tests were compared based on ANOVA (one-way) test and factorial design analysis using Minitab® 19 Statistical Software (version 19.2020.1, Minitab, LLC, USA). Statistical analysis was based on the null hypothesis that all means were equal and the alternative hypothesis that all means were different at significance level α = 0.05.
3. Results and discussion
3.1. Choice of microcapsule additives
EC was chosen as a polymer for microencapsulation of the l-carnitine base because it was practically insoluble in water and other polar solvents. It had almost no tendency to adsorb water from humid air [38,39]. Hence, it suited l-carnitine, which was very hygroscopic and required a barrier against moisture.
The solvent for microencapsulation could dissolve the polymer but not l-carnitine. Acetone was the solvent of choice despite its medium dielectric constant (ε = 20.01). It slowly diffused from nascent microcapsules, leading to the gradual solidification of microcapsules [40].
The emulsifier chosen, Sorbitan monooleate (Span 80), was characterized by a low HLB value of 4.3. It had the advantage of being liquid at room temperature and of better miscibility with light liquid paraffin [38,41].
3.2. Optimization outcomes
The results of the effect of an anti-coalescent type, mixing speed, and emulsifier concentration on the properties of microcapsules (F1– F9) are represented in Table 5.
Table 5.
Results of evaluation of Formulations F1– F9.
| Evaluation test | F1 | F2 | F3 | F4 | F5 | F6 | F7 | F8 | F9 |
|---|---|---|---|---|---|---|---|---|---|
| Water content (%) | 3.30 ± 0.22 | 1.06 ± 0.056 | 1.647 ± 0.175 | 1.11 ± 0.046 | 1.66 ± 0.11 | 2.940 ± 0.184 | 1.180 ± 0.062 | 1.250 ± 0.075 | 1.350 ± 0.053 |
| Flowability | Poor | Excellent | good | Excellent | good | poor | excellent | good | good |
| Bulk density (g/cm3) | 0.384 ± 0.010 | 0.299 ± 0.004 | 0.353 ± 0.004 | 0.295 ± 0.003 | 0.292 ± 0.004 | 0.327 ± 0.003 | 0.300 ± 0.002 | 0.303 ± 0.005 | 32.953 ± 1.517 |
| Tapped density (g/cm3) | 0.430 ± 0.005 | 0.314 ± 0.003 | 0.411 ± 0.005 | 0.319 ± 0.002 | 0.328 ± 0.007 | 0.397 ± 0.010 | 0.318 ± 0.003 | 0.328 ± 0.007 | 0.343 ± 0.008 |
| Angle of repose | 45.637 ± 1.737 | 31.607 ± 1.266 | 36.670 ± 1.650 | 29.163 ± 1.053 | 33.580 ± 1.396 | 41.533 ± 1.201 | 29.903 ± 00.658 | 30.707 ± 0.445 | 32.953 ± 1.517 |
| Carr's index | 10.644 ± 2.004 | 4.775 ± 0.990 | 14.167 ± 1.781 | 5.225 ± 0.583 | 11.073 ± 2.448 | 17.734 ± 1.384 | 5.751 ± 1.607 | 7.476 ± 0.939 | 10.081 ± 0.601 |
| Hausner ratio | 1.119 ± 0.025 | 1.050 ± 0.011 | 1.165 ± 0.024 | 1.055 ± 0.006 | 1.125 ± 0.031 | 1.216 ± 0.020 | 1.061 ± 0.018 | 1.081 ± 0.011 | 1.112 ± 0.007 |
| Encapsulation Efficiency (%) | 81.703 ± 2.297 | 96.250 ± 0.789 | 88.610 ± 1.127 | 96.893 ± 0.883 | 89.237 ± 3.373 | 84.667 ± 3.607 | 95.430 ± 0.598 | 93.050 ± 0.861 | 92.003 ± 2.317 |
| Production yield (%) | 81.78 | 91.22 | 87.34 | 94.31 | 83.12 | 88.16 | 93.25 | 94.33 | 91.75 |
*Indicates a significantly different value at p‹0.05. Results represent the average of 3 recorded reading for each test.
The use of OFAT was suitable for studying the variables associated with microencapsulation [42]. The first study stage revealed that the absence of anti-coalescent in F1 resulted in high water content and poor flowability. The inclusion of magnesium stearate, a lubricant in F3, caused droplet stabilization and prevention of coalescence [40]. The role of Aerosil 200 in F2 was as an adsorbent, emulsion stabilizer, glidant, and suspending agent. Its presence led to the powder's excellent flow properties. ANOVA of the above results showed a significant difference between the three formulations (p-value‹ 0.05), where F2 showed a superiority in its properties. Thus, the outcome of the first stage of the study was the choice of F2, which contained Aerosil 200 as an adsorbent [43] and anti-coalescent as well.
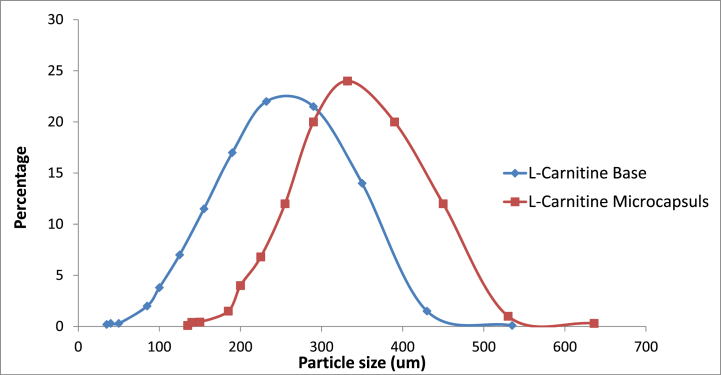
Results of particle size analysis for this formulation revealed that about 66 % of the prepared microcapsules ranged in size between 290 and 390 μm. The size distribution of formulated microcapsules compared to that of the l-carnitine base is demonstrated in Fig (2).
Fig. 2.
Particle size distribution of F2 compared to l-Carnitine base.
The second stage-aiming to choose the optimal mixing speed during microencapsulation-utilized the chosen anti-coalescent (Aerosil 200). Table 5 revealed that by increasing the mixing speed, the water content was increased significantly (p = 0.000). Entrapment of excess air bubbles during formulation was associated with rapid mixing. Hence, the absorption of higher amounts of moisture from the entrapped air bubbles took place [44]. High mixing speed (1500 rpm) resulted in the accumulation of a polymer viscous layer on the mixer shaft and its loss. Also, some l-carnitine stuck to the walls of the beaker and adsorbed water and was out of the encapsulation process. That caused a low yield for F5, which omitting it from further study. Briefly, high mixing speeds caused an increase in water content and low microcapsule yield. According to these results, F4 was chosen to continue to the next stage.
Results of the third stage showed the importance of the presence of Span 80 (emulsifier) in producing uniform, non-aggregated microcapsules within a size range of 250–450 μm. The emulsifier reduced the interfacial tension of the emulsion [45,46]. Higher concentrations of Span 80 worsened the flow properties, encapsulation efficiency, and water content. That was due to the increased capacity to emulsify water entrapped by the hygroscopic l-carnitine during the mixing process [47]. Accordingly, F4 (with a Span concentration of 0.1 %), which showed the best evaluation results, was promoted to the next stage.
The fourth stage involved eight formulations based on a 2 x 4 factorial design to study the effect of polymer viscosity grade on l-carnitine microencapsulation. The results of evaluation tests of the eight formulations are summarized in Table 6.
Table 6.
Results of evaluation tests of microcapsule formulations studied in stage 4.
|
Evaluation test |
Formulation |
|||||||
|---|---|---|---|---|---|---|---|---|
| F10 | F4 | F11 | F12 | F13 | F14 | F15 | F16 | |
| Polymer viscosity grade | Ethylcellulose N 100 | Ethocel St 20 | ||||||
| Drug Polymer ratio | 4:1 | 3:1 | 2:1 | 1:1 | 4:1 | 3:1 | 2:1 | 1:1 |
| Water content (%) | 1.727 ± 0.211 | 1.120 ± 0.110 | 1.093 ± 0.131 | 1.113 ± 0.154 |
1.650 ± 0.131 | 1.110 ± 0.082 | 1.190 ± 0.111 | 1.173 ± 0.121 |
| Flowability | Excellent | Excellent | Excellent | Excellent | Excellent | Excellent | Excellent | Excellent |
| Bulk density (g/cm3 | 0.277 ± 0.001 | 0.293 ± 0.003 | 0.332 ± 0.003 | 0.399 ± 0.003 |
0.274 ± 0.001 | 0.298 ± 0.004 | 0.333 ± 0.005 | 0.398 ± 0.004 |
| Tapped density (g/cm3) | 0.294 ± 0.002 | 0.311 ± 0.002 | 0.363 ± 0.003 | 0.439 ± 0.003 |
0.299 ± 0.003 | 0.313 ± 0.003 | 0.359 ± 0.004 | 0.441 ± 0.004 |
| Angle of repose | 28.423 ± 0.855 | 27.447 ± 0.604 | 27.713 ± 0.596 | 27.036 ± 1.027 |
28.313 ± 0.908 | 26.323 ± 0.947 | 26.337 ± 0.530 | 27.370 ± 0.771 |
| Carr's index | 5.911 ± 0.340 | 5.632 ± 0.298 | 8.465 ± 0.349 | 9.269 ± 1.066 |
8.223 ± 0.975 | 4.863 ± 0.356 | 7.177 ± 0.297 | 9.729 ± 0.214 |
| Hausner ratio | 1.063 ± 0.004 | 1.060 ± 0.003 | 1.092 ± 0.004 | 1.102 ±0.013 |
1.090 ± 0.012 | 1.051 ± 0.004 | 1.077 ± 0.003 | 1.108 ± 0.003 |
| Encapsulation Efficiency (%) | 96.327 ± 0.763 | 97.107 ± 0.994 | 98.717 ± 0.880 | 101.060 ± 1.091 | 97.647 ± 0.869 | 98.323 ± 0.854 | 99.240 ± 0.404 | 101.220 ± 1.484 |
| Production Yield (%) (pooled) | 96.34 | 97.27 | 95.66 | 93.15 | 97.03 | 96.89 | 96.93 | 94.76 |
Results represent the average of 3 recorded reading for each test.
The interaction plots and main effects of water content, angle of repose, Carr's index, Hausner's ratio vs. Polymer viscosity, and Drug: Polymer ratio of formulations F4 and F10– F16 are illustrated in Fig. 3(a–d).
Fig. 3.
Interaction Plots and main effect plots of water content (a), angle of repose (b), Carr's index (c) and Hausner's ratio (d) Vs Polymer Viscosity and Drug: Polymer ratio of formulation F4 and F10– F16.
Results for water content were acceptable (less than 1.2 %). In formulations containing the highest drug percentage, the water content increased due to the hygroscopic nature of the drug.
The observed increase in bulk and tapped densities was associated with the increase in polymer content, which was associated with a decrease in the percentage of Aerosil 200, characterized by low density [48].
As the evaluation results for all microcapsule formulations were acceptable, the eight formulations (C1– C8) were compressed into tablets and evaluated. The evaluation results are summed up in the following Table 7.
Table 7.
Evaluation results of formulations C1– C8.
| Formula No. | Hardness (kp) | Disintegration time (min) | Dissolution (%) |
|---|---|---|---|
| C1 (F10) | 19.72 ± 0.36 | 1.5 | 90.53 ± 3.73 |
| C2 (F4) | 20.44 ± 0.27 | 2.5 | 87.40 ± 5.12 |
| C3 (F11) | 20.27 ± 0.52 | 5 | 73.67 ± 8.19 |
| C4 (F12) | 19.92 ± 0.44 | 7.5 | 39.27 ± 9.66 |
| C5 (F13) | 19.82 ± 0.61 | 1 | 96.57 ± 4.90 |
| C6 (F14) | 19.63 ± 0.37 | 2 | 95.24 ± 2.84 |
| C7 (F15) | 20.37 ± 0.49 | 4.5 | 87.50 ± 5.83 |
| C8 (F16) | 19.47 ± 0.32 | 8.25 | 63.03 ± 6.76 |
Results represent the average of 3 recorded reading for each test.
As it is clear from the results, the higher the polymer percentage, the slower the disintegration [49,50]. In tablets with a high percentage of l-carnitine, rapid drug dissolution happened due to its hygroscopic nature. Following dissolution, channels appeared within tablets that allowed water entry, which aided the disintegrant in its role.
Concerning the dissolution test results, formulations C1, C5, and C6 were the only formulations to conform to the official requirements. The formulations released not less than 75 % of labeled content after 30 min (which meant that individual results of dissolution must be ≥ 80 % of the labeled amount). The other formulations showed that some or all of the six tested tablets released less than 80 % of labeled potency after 30 min. Thus formulations C1, C5, and C6 contained 4:1 EC N100, 4:1 EC ST 20 and 3:1 EC ST20, respectively. Although C2 and C7 containing 3:1 EC N100 and 2:1 EC ST20 showed only one tablet that failed the test, they did not conform to the dissolution test. That resulted from the high polymer percentage in these failing formulations. The ANOVA analysis of dissolution results revealed a significant difference (p = 0.000) between formulations due to EC viscosity grade, as well as the drug-to-polymer ratio. C6 showed acceptable dissolution results at a higher polymer ratio when compared to C1 and C5. Hence, the stability of formulation C6 was assessed.
3.3. Stability study
The study involved storing formulation C6 under accelerated stability study conditions with l-carnitine base tablets prepared by wet granulation as a control. Both were tested every month for six months for water content, weight uniformity, drug assay, hardness, and dissolution. The results are listed in Table 8.
Table 8.
Results of tablet evaluation tests during the period of accelerated stability storage conditions.
|
l-Carnitine base tablets prepared by wet granulation |
Formulation C6 containing l-Carnitine base microcapsules |
|||||||
|---|---|---|---|---|---|---|---|---|
| Zero months | One month | Three months | Six months | Zero months | One month | Three months | Six months | |
| Moisture content (%w/w) | 1.623 ±0.02 |
1.843 ±0.1 |
2.353 ±0.18 |
3.250 ±0.12 |
1.323 ±0.09 |
1.393 ±0.02 |
1.520 ±0.06 |
1.843 ±0.04 |
| Weight uniformity (mg) | 998.80 ±0.14 |
999.50 ±0.35 |
1004.60 ±1.13 |
1012.20 ±0.85 |
1002.00 ±0.71 |
1002.10 ±0.10 |
1003.70 ±0.60 |
1006.00 ±0.40 |
| Tablet hardness (kp) | 20.033 ±0.020 |
18.833 ±0.590 |
16.783 ±0.480 |
13.667 ±0.470 |
20.017 ±0.060 |
19.317 ±0.340 |
18.167 ±0.240 |
17.150 ±0.180 |
| Percentage dissolution (%) | 97.905 ±0.770 |
96.21 ±0.90 |
92.773 ±0.870 |
87.55 ±1.80 |
96.33 ±1.18 |
95.683 ±0.220 |
94.125 ±1.060 |
91.87 ±2.03 |
| Drug assay (%) | 99.33 ±0.94 |
98.20 ±1.60 |
96.24 ±0.81 |
93.21 ±0.85 |
99.32 ±1.01 |
98.57 ±1.11 |
97.07 ±0.4 |
95.07 ±0.66 |
Results represent the average of 3 recorded reading for each test.
Compared to l-Carnitine base tablets, C6 showed acceptable results with an overall increase in moisture content by 0.5 % within six months. Consequently, there was only a slight increase in average tablet weight over the storage period due to moisture gain. A high moisture uptake by the l-carnitine base tablets resulted in a reduction in tablet hardness, unlike C6, where hardness was not affected. The variation in drug assay and dissolution results within the storage period was acceptable for C6 [51,52].
4. Conclusion
It was possible to reduce the hygroscopicity of the l-Carnitine base by changing it to microcapsules. Microencapsulation of l-carnitine improved the stability of formulated tablets. Ethocel ST 20, used at a ratio of 3:1 (drug: polymer), provided a protective drug coat in the presence of 10 % colloidal silicon dioxide as anti-coalescent and 0.1 % Span 80 as surfactant. The formulated microcapsules into tablets had minimal moisture uptake, acceptable hardness, assay, and dissolution results under accelerated stability storage conditions. Thus, microencapsulation minimized manufacturing problems arising from the handling of the hygroscopic l-carnitine base.
Funding statement
This research did not receive any specific grant from funding agencies in the public, commercial, or not-for-profit sectors.
Data availability statement
The authors declare that data associated with our study has not been deposited into a publicly available repository. Data included in article/supp. material is referenced in article.
CRediT authorship contribution statement
Mahmoud M. Hegazy: Writing – original draft, Validation, Software, Resources, Methodology, Formal analysis, Data curation, Conceptualization. Alia A. Badawi: Visualization, Conceptualization. Mohamed A. El-Nabarawi: Conceptualization. Mohammed A. Eldegwy: Software, Resources, Formal analysis, Data curation, Conceptualization. Dina Louis: Writing – review & editing, Writing – original draft, Visualization, Methodology, Formal analysis, Conceptualization.
Declaration of competing interest
The authors declare that they have no known competing financial interests or personal relationships that could have appeared to influence the work reported in this paper.
Footnotes
Supplementary data to this article can be found online at https://doi.org/10.1016/j.heliyon.2023.e23637.
Contributor Information
Mahmoud M. Hegazy, Email: dr.mahmoud_hegazy_2007@hotmail.com.
Alia A. Badawi, Email: alia.badawi@pharma.cu.edu.eg.
Mohamed A. El-Nabarawi, Email: mohamed.elnabarawi@pharma.cu.edu.eg.
Mohammed A. Eldegwy, Email: Degweys78@hotmail.com.
Dina Louis, Email: dina.nassif@pharma.cu.edu.eg.
Appendix A. Supplementary data
The following is the Supplementary data to this article:
References
- 1.Buckingham R., editor. Martindale - the Complete Drug Reference. 40th Editi. The Pharmaceutical Press; UK: 2020. Carnitine derivatives. 2053–5. [Google Scholar]
- 2.Gvozdanovi′ Kristina, Kralik Zlata, Radiši′ Žarko, Manuela Koševi′ G.K. Kušec and ID. Chicken Genome. Animals. 2023;13:1831. doi: 10.3390/ani13111831. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 3.Mateus F.G., Moreira S., Martins A.D., Oliveira P.F., Alves M.G., Pereira M.d. L-carnitine and male fertility: is supplementation beneficial? J. Clin. Med. 2023;12:5796. doi: 10.3390/jcm12185796. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 4.Mason P. Pharmaceutical Press; 2009. Dietary Supplements Pocket Companion. [Google Scholar]
- 5.Sharma B., Yadav D.K. L-carnitine and chronic kidney disease: a comprehensive review on nutrition and health perspectives. J Pers Med. 2023;13(2):289. doi: 10.3390/jpm13020298. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 6.Martins L.F., Silva W.V., Nascimento N.F., Melo M.P., Crispim B.A., Barufatti Cch A. Zootechnical performance, degree of steatosis and the genotoxic potential in yellowtail tetra Astyanax lacustris fed with different levels of L-carnitine. Arq. Bras. Med. Vet. Zootec. 2023;75(4):753–758. [Google Scholar]
- 7.Levocarnitine. in: European Pharmacopoeia. 2019. p. 1339. [Google Scholar]
- 8.The United States Pharmacopoeia USP42-NF37. 2020. Description and relative solubility. [Google Scholar]
- 9.Xue Q. Proc SPIE 12789, International Conference on Modern Medicine and Global Health. 2023. The mechanism of action, side effects and clinical application of L-carnitine. [Google Scholar]
- 10.Uner B., Ergin A.D., Ansari I.A., Macit-Celebi M.S., Ansari S.A., Kahtani H.M.A. Assessing the in vitro and in vivo performance of L-carnitine-loaded nanoparticles in combating obesity. Molecules. 2023;28:7115. doi: 10.3390/molecules28207115. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 11.Chattoraj S., et al. Sticking and picking in pharmaceutical tablet compression: an IQ consortium review. J Pharm Sci. 2018;107(9):29. doi: 10.1016/j.xphs.2018.04.029. [DOI] [PubMed] [Google Scholar]
- 12.Dhondale M.R., Thakor P., Nambiar A.G., Singh M., Agrawal A.K., Shastri N.R., et al. Co-crystallization approach to enhance the stability of moisture-sensitive drugs. Pharmaceutics. 2023;15(1):189. doi: 10.3390/pharmaceutics15010189. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 13.Group L. Carnipure - purity you can trust. [Internet] 2013. http://bio.lonza.com/uploads/tx_mwaxmarketingmaterial/Lonza_Brochures_Carnipure_Purity_You_Can_Trust.pdf Available from:
- 14.Badawi A.A., Hegazy M.M., D Louis M.E. Solving manufacturing problems for L-carnitine-L-tartrate to improve likelihood of successful product scale-up. Acta Pharm. 2017;67(4):511–525. doi: 10.1515/acph-2017-0033. [DOI] [PubMed] [Google Scholar]
- 15.Suganya V., Anuradha V. Microencapsulation and nanoencapsulation : a review. Int J Pharm Clin Res. 2017;9(3):233–239. [Google Scholar]
- 16.Suganya V., Microencapsulation Anuradha V., Nanoencapsulation A review. Int J Pharm Clin Res. 2017;9(3):233–239. [Google Scholar]
- 17.Halahlah Abedalghani, Vieno Piironen Ksm TMH. Polysaccharides as wall materials in spray-dried microencapsulation of bioactive compounds: physicochemical properties and characterization. Crit. Rev. Food Sci. Nutr. 2023;63(24):6983–7015. doi: 10.1080/10408398.2022.2038080. [DOI] [PubMed] [Google Scholar]
- 18.Karen Elbert Leal Mazza, André Mesquita Magalhães Costa, Janine Passos Lima da Silva, Daniela Sales Alviano, Humberto Ribeiro Bizzo RVT. Microencapsulation of marjoram essential oil as a food additive using sodium alginate and whey protein isolate. Int. J. Biol. Macromol. 2023:233. doi: 10.1016/j.ijbiomac.2023.123478. [DOI] [PubMed] [Google Scholar]
- 19.Pour M.M., Riseh R.S., Ranjbar-Karimi R., Hassanisaadi M., Rahdar A.B.F. Microencapsulation of Bacillus velezensis using alginate-gum polymers enriched with TiO2 and SiO2 nanoparticles. Micromachines. 2022;13(9):1423. doi: 10.3390/mi13091423. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 20.Saberi Riseh R., Moradi Pour M., Ait Barka E. A novel route for double-layered encapsulation of Streptomyces fulvissimus uts22 by alginate–Arabic gum for controlling of pythium aphanidermatum in cucumber. Agronomy. 2022;12:655. [Google Scholar]
- 21.Saberi Riseh R., Skorik Y.A., Thakur V.K., Moradi Pour M., Tamanadar E., Noghabi S.S. Encapsulation of plant biocontrol bacteria with alginate as a main polymer material. Int. J. Mol. Sci. 2021;22 doi: 10.3390/ijms222011165. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 22.Roohallah Saberi Riseh, Elahe Tamanadar, Najmeh Hajabdollahi, Masoumeh Vatankhah, Vijay Kumar Thakur YAS. Chitosan microencapsulation of rhizobacteria for biological control of plant pests and diseases: recent advances and applications. Rhizosphere. 23:100565..
- 23.Saberi Riseh R., Ebrahimi-Zarandi M., Gholizadeh Vazvani M.S.Y. Reducing drought stress in plants by encapsulating plant growth-promoting bacteria with polysaccharides. Int. J. Mol. Sci. 2021;22(23) doi: 10.3390/ijms222312979. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 24.Jelvehgari M., Shm Comparison of microencapsulation by emulsion-solvent extraction/evaporation technique using derivatives cellulose and acrylate-methacrylate copolymer as carriers. Jundishapur J. Nat. Pharm. Prod. 2012;7(4):144–152. [PMC free article] [PubMed] [Google Scholar]
- 25.Lu Xiaocun, Katz Joshua S., Schmitt Adam K., Jsm A robust oil-in-oil emulsion for the nonaqueous encapsulation of hydrophilic payloads. J. Am. Chem. Soc. 2018;140(10):3619–3625. doi: 10.1021/jacs.7b11847. [DOI] [PubMed] [Google Scholar]
- 26.Patil Jayesh S., Pawar Y.D. Preparation of metformin biodegradable polymeric microparticles by O/O emulsion solvent evaporation: a 32 full factorial design approach. Lett. Drug Des. Discov. 2023;20(11):1775–1783. [Google Scholar]
- 27.Gillet C., Splawski W., Aguirre F., Hassoune-Rhabbour B., Tchalla T.N.V. Use of Karl Fischer titration for the localized measurement of water content in FRP composite aircraft parts removed from service. J. Compos. Mater. 2023;57(8):1495–1510. [Google Scholar]
- 28.Appendix XVII E. British Pharmacopoeia. Flowability (Ph. Eur. method; 2020. 16) [Google Scholar]
- 29.Appendix X.V.I.I.N. British Pharmacopoeia. 2020. Powder flow (ph. Eur. Method 2.9.36) [Google Scholar]
- 30.British Pharmacopoeia. 2020. Appendix V Q, (ph. Eur. Method 2.9.15) [Google Scholar]
- 31.Aulton M.E. In: Aulton’s Pharmaceutics - the Design and Manufacture of Medicines. Aulton K.E., editor. Elsevier; 2018. Flow, powder; pp. 189–200. [Google Scholar]
- 32.The United States Pharmacopoeia USP43-NF38. 2020. Levocarnitine tablets; p. 2601. [Google Scholar]
- 33.Yang Guangze, Liu Yun, Jin Song, Yue Hui, Wang Xing, Xu Letao, Chen Dong, David Weitz C.-X.Z. Phase separation-induced nanoprecipitation for making polymer nanoparticles with high drug loading. Aggregate. 2023;4(2):314. [Google Scholar]
- 34.Aguiar A., Mariquito A., Gonçalves D., Pinho I., Marques A.C. Biodegradable microcapsules of poly(butylene adipate-co-terephthalate) (PBAT) as isocyanate carriers and the effect of the process parameters. Polymers. 2023;15:665. doi: 10.3390/polym15030665. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 35.Lachman L., Lieberman H.A., Jlk . Varghese Publishing House; 1987. The Theory and Practice of Industrial Pharmacy. [Google Scholar]
- 36.Appendix XVII G. British Pharmacopoeia. Friability (Ph. Eur. method; 2020. 7) [Google Scholar]
- 37.Appendix X.I.I.A. 2020. 1), British Pharmacopoeia. Disintegration (Ph. Eur. method. [Google Scholar]
- 38.Rowe R.C., Sheskey P.J., MEQ, editors. Handbook of Pharmaceutical Excipients. ninth ed. Pharmaceutical Press and American Pharmacists Association.; 2020. [Google Scholar]
- 39.Li Shu-Fang, Wu Jia-Hui, Teng-Gen Hu H.W. Encapsulation of quercetin into zein-ethyl cellulose coaxial nanofibers: preparation, characterization and its anticancer activity. Int. J. Biol. Macromol. 2023;248 doi: 10.1016/j.ijbiomac.2023.125797. [DOI] [PubMed] [Google Scholar]
- 40.Zaki Rizkalla C.M., latif Aziz R. S. In vitro and in vivo evaluation of hydroxyzine hydrochloride microsponges for topical delivery. AAPS PharmSciTech. 2011;12(2):989–1001. doi: 10.1208/s12249-011-9663-5. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 41.Akshay M., Akotkar, Ashwini A., Zanke Kvg Formulation and evaluation of oral microspheres containing antihypertensive drugs by emulsion solvent evaporation method. J Popul Ther Clin Pharmacol. 2023;30(5):635–645. [Google Scholar]
- 42.Paulo F., Santos L. vol. 77. 2017. pp. 1327–1340. (Design of Experiments for Microencapsulation Applications: A Review. Mater Sci Eng C [Internet]). Available from: [DOI] [PubMed] [Google Scholar]
- 43.Hassan Doaa H., Ammar Aar& Mky Amal A. Enhanced bioavailability and pharmacokinetics parameters of Enalapril solid self nanoemulsifying oral dispersible tablet: formulation, in vitro and in vivo evaluation. Pharmaceut. Dev. Technol. 2023;28(3–4):371–382. doi: 10.1080/10837450.2023.2198005. [DOI] [PubMed] [Google Scholar]
- 44.Li M., Rouaud O. DP. Microencapsulation by solvent evaporation: state of the art for process engineering approaches. Int J Pharm. 2008;363(1–2):26–39. doi: 10.1016/j.ijpharm.2008.07.018. [DOI] [PubMed] [Google Scholar]
- 45.Vanichtanunkul D., Vayumhasuwan P., UN The effect of core-to-wall ratio and Span 80 concentration on the properties of ascorbic acid microcapsules. J. Microencapsul. 1998;15(6):53–759. doi: 10.3109/02652049809008258. [DOI] [PubMed] [Google Scholar]
- 46.ElSabagh M.R.N.E. AM. Preparation of water-in-hexane nanoemulsions using low energy emulsification method. J Dispers Sci Technol. 2012;33(1):68–74. [Google Scholar]
- 47.Sawant A., et al. Solid-in-Oil-in-Water emulsion: an innovative paradigm to improve drug stability and biological activity. AAPS PharmSciTech. 2021;22(5):199. doi: 10.1208/s12249-021-02074-y. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 48.Khalid Waleed Khalid S.N.A.A. Preparation and characterization of ticagrelor solid self nano-emulsion. J. Pharm. Negat. Results. 2022;13(3):284–290. [Google Scholar]
- 49.Yang Y., Wang H., Li H., Ou Z.Y.G. 3D printed tablets with internal scaffold structure using ethyl cellulose to achieve sustained ibuprofen release. Eur J Pharm Sci. 2018;115(11):5. doi: 10.1016/j.ejps.2018.01.005. [DOI] [PubMed] [Google Scholar]
- 50.Tonk Megha, Shabnam Ain B.K. QA. Study the effect of polymers on the release rate of propranolol hydrochloride from controlled release matrix tablets. Ann Phytomedicine. 2023;12(1):1–8. [Google Scholar]
- 51.Nokhodchi A. YJ. The effect of storage conditions on the physical stability of tablets. Pharm Technol Eur. 2007;19:20–26. [Google Scholar]
- 52.Avbunudiogba J. Effects of humidity on the dissolution profiles of controlled release theophylline matrix tablets containing release enhancers prepared by melt granulation and coacervation techniques. J. Appl. Sci. Environ. Manag. 2020;24(9):1563–1567. [Google Scholar]
Associated Data
This section collects any data citations, data availability statements, or supplementary materials included in this article.
Supplementary Materials
Data Availability Statement
The authors declare that data associated with our study has not been deposited into a publicly available repository. Data included in article/supp. material is referenced in article.