Abstract
Phosphorylcholine (ChoP) is a component of the teichoic acids of Streptococcus pneumoniae and has been recently identified on the lipopolysaccharide of Haemophilus influenzae, also a major pathogen of the human respiratory tract. Other gram-negative pathogens that frequently infect the human respiratory tract were surveyed for the presence of the ChoP epitope as indicated by binding to monoclonal antibodies (MAbs) recognizing this structure. The ChoP epitope was found on a 43-kDa protein on all clinical isolates of Pseudomonas aeruginosa examined and on several class I and II pili of Neisseria meningitidis. The specificity of the anti-ChoP MAb was demonstrated by the inhibition of binding in the presence of ChoP but not structural analogs. As in the case of H. influenzae, the expression of this epitope was phase variable on these species. In P. aeruginosa, this epitope was expressed at detectable levels only at lower growth temperatures. Expression of the ChoP epitope on piliated neisseriae displayed phase variation, both linked to pilus expression and independently of fully piliated bacteria.
Choline, a major constituent of eukaryotic membrane lipids, was previously thought to be an unusual structural feature of prokaryotes. The best-known example is Streptococcus pneumoniae, which accumulates environmental choline and incorporates it in the form of phosphorylcholine (ChoP) into its glycolipid, lipoteichoic acid, as well as its cell wall-associated teichoic acid (8). It has been suggested that ChoP contributes to adherence of the pneumococcus to host cells by binding to the receptor for platelet-activating factor, whose natural ligand also contains ChoP (1). Recently, ChoP has been identified as a unique feature of the lipopolysaccharide (LPS) of Haemophilus influenzae (21, 22). In the case of H. influenzae, choline is also acquired from the growth medium and linked to a glucose residue on the outer core region of the rough LPS (22). The expression of ChoP on the H. influenzae glycolipid undergoes phase variation mediated by a translational switch within the gene licA, a putative choline kinase (20, 22). The only significant homology to licA in protein databases is in a gene found in several species of the genus Mycoplasma, including the common respiratory tract pathogen Mycoplasma pneumoniae. In addition, the ChoP structure has been identified on a polar lipid found in the opportunistic pathogen M. fermentans (2). It appears, therefore, that ChoP is a structure common to several important and distantly related pathogens, including S. pneumoniae, H. influenzae, and various mycoplasma species which reside on the mucosal surface and infect the human respiratory tract. In addition, screening of secretions from the human respiratory tract with a monoclonal antibody (MAb) specific to the ChoP epitope has revealed several other gram-positive species which bind the antibody and may contain the ChoP structure (3).
The focus of this study was the identification of the ChoP epitope on pathogenic gram-negative species other than Haemophilus. Results of this screening show that this epitope is present and displays phase variation on protein structures of pathogenic neisseriae, and Pseudomonas aeruginosa. In the case of the neisseriae, the ChoP epitope was found to be present on pili.
MATERIALS AND METHODS
Bacterial strains and culture conditions.
All strains used in these studies were clinical isolates. Many of the Neisseria meningitidis and N. gonorrhoeae strains used in this study have previously been described (16). The 31 strains of N. meningitidis used comprised 11 blood, 11 cerebrospinal fluid (CSF), and 9 throat isolates representing several serogroups (a, b, c, w, x, y, and 29e) and included nongroupable isolates. Three piliated gonococcal strains were used, and they included several distinct piliated variants of one strain, MS11. Bacteria were grown overnight at 37°C in tryptic soy broth (Branhamella catarrhalis and Klebsiella pneumoniae), Bordet-Gengou agar (Bordetella pertussis), buffered charcoal-yeast extract agar (Legionella pneumonphila), Luria-Bertani broth (Pseudomonas aeruginosa), brain heart infusion broth supplemented with 1% Fildes enrichment (H. influenzae), 5% heated horse blood (N. meningitidis), or GC agar (N. gonorrhoeae) (16). Growth medium was purchased from Difco Laboratories, Detroit, Mich.
Western transfer and immunoblotting.
Bacterial cells were suspended in phosphate-buffered saline (PBS), pH 7.2, to an optical density at 620 nm of 0.5, washed in PBS, concentrated 10-fold, resuspended in gel loading buffer, and treated at 100°C for 5 min. In some experiments, proteinase K was added at a final concentration of 0.5 mg/ml and the sample was incubated at 37°C for 60 min prior to boiling. Following separation by sodium dodecyl sulfate (SDS)–12.5% polyacrylamide gel electrophoresis (PAGE), electrotransfer onto nitrocellulose or Immobilon-P (Millipore Corp., Bedford, Mass.) and Western blotting were carried out on whole-cell lysates or purified pili as described previously (14, 19). Immunoblotting of membranes was carried out in a 1-in-10,000 dilution of MAb TEPC-15 (Sigma Chemical Co., St. Louis, Mo.) or MAb HAS (Statens Serum Institut, Copenhagen, Denmark), and bands were visualized following incubation in alkaline phosphatase-conjugated goat anti-mouse immunoglobulin A (IgA) or IgM, respectively. In some experiments, ChoP or structural analogs were added during incubation with the anti-ChoP MAb. Inhibition of MAb HAS binding was determined in digitalized images by comparison to controls without hapten (positive control) and with a secondary antibody only (negative control). Pili were detected by using SM1, a MAb raised to N. gonorrhoeae pili which reacts with the structural subunit pilin of the class I subgroup of N. meningitidis pili, or AD211 against the class II pilin subunit (15, 16).
Detection of the ChoP epitope in whole-cell lysates and colony immunoblots.
In some experiments, whole-cell lysates or colonies lifted onto nitrocellulose were denatured by urea treatment (3 M final concentration, 100°C for 5 min). Nitrocellulose blots were air dried and immersed in the boiling urea solution, washed in water, blocked with 5% skim milk in PBS with Tween (0.5%), and immunoblotted with antibodies to ChoP as described for Western blots.
Competitive ELISA.
To coat enzyme-linked immunosorbent assay (ELISA) plates, purified, denatured pili from N. meningitidis C311, variant 16, were suspended in 0.5 M bicarbonate buffer, pH 9.5, and added to plates at 2 μg/ml in 96-well polystyrene microtiter plates (Dynatech). After overnight incubation at 37°C, plates were washed and nonspecific sites were blocked in 1% bovine serum albumin in Dulbecco’s PBS containing 0.05% Tween 20. Soluble competitor molecules (ChoP, choline, phosphorylethanolamine, or ethanolamine) were added at a range of concentrations (10 μM to 100 mM) in 1% bovine serum albumin–PBS–0.05% Tween 20 prior to the addition of anti-ChoP antibody HAS. Binding of the antibody to pili was detected by the use of an alkaline phosphatase-conjugated second antibody and a p-nitrophenyl phosphate substrate (Sigma FAST pNPP).
RESULTS
Identification of gram-negative pathogens with the ChoP epitope.
Clinical isolates of various gram-negative pathogens obtained from cultures of the human respiratory tract were screened by Western analysis with MAb TEPC-15, a natural IgA MAb which has been shown to recognize ChoP but not close structural analogs such as phosphorylethanolamine (7). Initial results obtained with whole-cell lysates of B. catarrhalis (6 isolates), K. pneumoniae (3 isolates), B. pertussis (3 isolates), P. aeruginosa (12 isolates), L. pneumonphila (4 isolates), and N. meningitidis (3 isolates) showed no reactivity with MAb TEPC-15 in comparison to the H. influenzae control. Since this epitope is highly variable in H. influenzae, the negative results obtained by screening other pathogens could be explained by lack of expression under the growth conditions used. Further investigation revealed variable expression of the ChoP epitope in two of the above species, P. aeruginosa and N. meningitidis.
Detection of a protein with the ChoP epitope in P. aeruginosa.
A single band of 43 kDa was identified in P. aeruginosa by Western analysis with MAb TEPC-15 and whole-cell lysates from cells grown in Luria-Bertani broth at 20°C instead of 37°C. A separate MAb, HAS, from a mouse IgM myeloma that also binds specifically to ChoP recognized the same 43-kDa band only in cells grown at 20°C, confirming the presence of the ChoP epitope. Controls using irrelevant IgM or IgA MAbs or a secondary antibody against mouse IgM or IgA alone showed no reactivity. The specificity of MAb binding was demonstrated in Western blot experiments in which ChoP or structural analogs were tested for inhibition of MAb HAS reactivity with the 43-kDa protein (Table 1). The binding of MAb HAS was completely inhibited by addition of ChoP at a concentration of only 10 μM. Hapten inhibition by choline required a concentration of 1 mM, whereas ethanolamine and phosphorylethanolamine required a concentration of 100 mM.
TABLE 1.
Inhibition of MAb binding to a 43-kDa protein in P. aeruginosa on Western blots in the presence of ChoP and analogs
| Competitor concn | % Inhibition of MAb binding bya:
|
|||
|---|---|---|---|---|
| ChoP | C | PE | E | |
| 100 mM | 100 | 100 | 100 | 100 |
| 10 mM | 100 | 100 | 15 | 24 |
| 1 mM | 100 | 100 | 20 | 10 |
| 100 μM | 100 | 31 | 0 | 25 |
| 10 μM | 100 | 0 | 0 | 0 |
C, choline; PE, phosphorylethanolamine; E, ethanolamine.
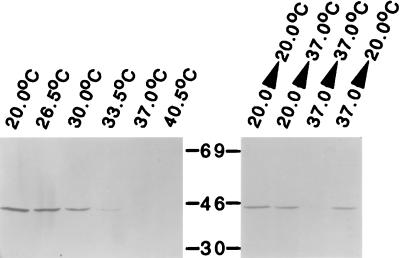
The relationship between growth temperature and expression of the ChoP epitope was further explored. A band of 43 kDa was present in 12 of 12 clinical isolates of P. aeruginosa examined, including strain PAO1 (ATCC 15692) when it was grown at 20°C but not when equivalent numbers of cells grown at 37°C were screened. The inverse correlation between growth temperature and the amount of the 43-kDa ChoP epitope in strain PAO1 is shown in Fig. 1. No detectable expression of the ChoP epitope was apparent in cells grown at 33.5°C and above. The regulation of the expression of this epitope based on temperature was confirmed by analysis of cells grown to stationary phase and then subjected to a shift in temperature. After growth at 20°C, increasing the temperature to 37°C caused partial loss of expression of the 43-kDa epitope. In contrast, shifting the temperature to 20°C for cells grown to stationary phase at 37°C caused increased expression of the ChoP epitope.
FIG. 1.
Relationship between growth temperature and expression of the ChoP epitope. P. aeruginosa PAO1 was grown to stationary phase at the temperature indicated, and equivalent numbers of cells in whole-cell lysates were examined by Western analysis on SDS-PAGE with MAb TEPC-15, which recognizes ChoP. Where indicated, following growth to stationary phase, the growth temperature was shifted for 4 h prior to the preparation of whole-cell lysates. Molecular sizes are in kilodaltons.
Treatment of whole-cell lysates from PAO1 grown at 20°C with proteinase K eliminated all proteins detectable by Coomassie blue staining, as well as the 43-kDa band containing the ChoP epitope, by Western analysis. In controls with whole-cell lysates of H. influenzae, the ChoP epitope on the LPS was not affected by treatment with proteinase K.
No difference associated with growth temperature was detected in Coomassie blue-stained proteins of whole-cell lysates separated by SDS-PAGE. This suggests that the protein with the ChoP epitope is not abundant. An alternative explanation is that the ChoP epitope is present only at lower growth temperatures and the addition of ChoP, which has a molecular mass of only 223 Da, is associated with modification of a protein without an observable shift in its migration on SDS-PAGE.
The possibility that P. aeruginosa incorporates choline obtained from the growth medium was examined. PAO1 was able to grow in a chemically defined medium with 20 mM choline as a sole source of carbon (10). The ability of P. aeruginosa to metabolize choline made it impractical to label specifically the 43-kDa protein by growing cells in the presence of radiolabelled choline as had been demonstrated for H. influenzae LPS (22). In addition, PAO1 grown in a chemically defined medium lacking choline with 0.5% glucose as the sole source of carbon were still able to express the ChoP epitope when grown at 20°C, suggesting that P. aeruginosa may be able to synthesize choline de novo.
Detection of the ChoP epitope on pili of pathogenic neisseriae.
Based on observations of variable expression of the ChoP epitope and the many epitopes shared by Haemophilus and Neisseria, screening of additional N. meningitidis isolates was performed (18). There was no reactivity with purified LPS obtained from several strains of N. meningitidis on Western blots (data not shown). Further evidence that the anti-ChoP MAb was not recognizing LPS was the absence of reactivity in the region of the Western blot below 10 kDa, where the LPS migrated on SDS-PAGE of whole-cell lysates (Fig. 2, lanes 1, 3, and 5 to 8). Western blot analysis of whole-cell lysates of N. meningitidis, however, showed that the anti-ChoP MAb reacted with a single protein whose molecular size corresponded to that of the pilin subunit of that strain (Fig. 2). Only piliated variants reacted with MAbs TEPC-15 and HAS, providing further evidence that the ChoP epitope was present on pili. In addition, the epitope was found on both major structural classes of pili, classes I and II. The identity of the protein with the ChoP epitope was confirmed by the use of purified pili.
FIG. 2.
Western blots showing the reactivity of anti-ChoP antibody with purified pilus preparations and with corresponding protein in whole-cell lysates of piliated meningococcal strains. Lanes: 1 and 3; class I piliated variants 3 and 16 of strain C311; 2 and 4, purified pili from these variants; 5 and 6, whole-cell lysates from nonpiliated variants of strain C311 (class I) or C114 (class II); 7 and 8, class II piliated variants of strains C319 and C114. Molecular sizes are in kilodaltons. Pilin migrations as detected with an anti-ChoP antibody are in agreement with previously known migrations of pilins of these strains and were confirmed by the use of MAbs against the pilin subunit (see Fig. 4). Note that the LPSs of neisseriae migrate farther than the 10-kDa molecular size marker. No reactivity of anti-ChoP antibody was detected in this region of the Western blot, even in the whole-cell lysates used in lanes 1, 3, and 5 to 8.
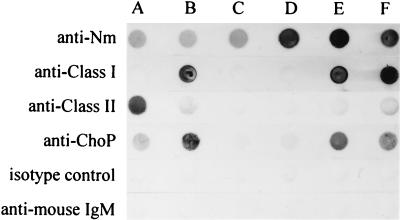
The specificity of anti-ChoP antibodies was confirmed in a dot blot assay (Fig. 3). The anti-ChoP antibody bound only to piliated bacteria. The alkaline phosphatase-conjugated secondary antibody against mouse IgM, alone or together with an isotype-matched control (an irrelevant mouse antibody of the IgM class), did not react with meningococci in parallel experiments. The antibody specificity was further investigated in an ELISA using ChoP, choline, phosphorylethanolamine, and ethanolamine as competitive inhibitors. The binding of HAS to pili was inhibited significantly by ChoP over a wide range of dilutions. Choline caused partial inhibition of binding at a concentration of 100 mM. However, phosphorylethanolamine and ethanolamine were totally ineffective at all dilutions tested (Table 2).
FIG. 3.
Dot blot assay of whole-cell lysates of N. meningitidis (Nm) isolates to demonstrate the specificity of anti-ChoP antibodies. Blots contained whole-cell suspensions of class I piliated (B and E), class II piliated (A), and nonpiliated (C and D) strains of N. meningitidis. Blots in row F contained purified pili of strain C311. One nitrocellulose strip was reacted with polyclonal rabbit antiserum against strain C311 to show the presence of antigen in each dot (top). The stronger but equal reaction in rows D to F is due to the presence of homologous antigen in these dots. Other strips were reacted with MAb SM1 against class I pili, MAb AD211 against class II pili, or anti-ChoP MAb HAS. No reaction in any dots was observed when an irrelevant MAb (IgM class) was used as an isotype-matched control. Also, the alkaline phosphatase-conjugated secondary antibody against mouse IgM used in the experiment gave no reaction when used alone. The bottom three strips were subjected to urea treatment prior to antibody probing.
TABLE 2.
Inhibition of MAb HAS binding to purified pili of N. meningitidis C311 in ELISAs in the presence of ChoP and analogs
| Competitor concn | % Inhibition of MAb binding bya:
|
|||
|---|---|---|---|---|
| ChoP | C | PE | E | |
| 100 mM | 100 | 99 | 0 | 0 |
| 10 mM | 100 | 75 | 0 | 0 |
| 1 mM | 100 | 29 | 0 | 0 |
| 100 μM | 100 | 20 | 0 | 0 |
| 10 μM | 99 | 15 | 0 | 0 |
| 1 μM | 65 | 9 | 0 | 0 |
| 100 nM | 16 | 2 | 0 | 0 |
C, choline; PE, phosphorylethanolamine; E, ethanolamine.
The ChoP epitope is surface exposed on pili of many N. meningitidis isolates.
Whole-cell lysates of 31 strains of N. meningitidis isolated from blood (11), throat (9), or CSF (11) samples were examined by Western analysis using MAb TEPC-15. Twelve (38.7%) of the 31 isolates reacted with the antibody in Western blots. Five of the strains that reacted in Western blots also reacted in a dot blot assay using whole-cell lysates (Fig. 3). The remainder of the strains reacted only when lysates were first treated with urea as described in Materials and Methods. This suggests that the ChoP epitope is surface exposed on some strains but requires denaturation of pili to be available to react with the MAb in a number of strains. Expression of the ChoP epitope was independent of serogroup and the site from which the strain was isolated. However, fewer (18%) of the strains originally isolated from blood expressed the epitope compared with the CSF isolates (45%) or throat isolates (55%) in the present survey.
Piliation-independent phase variation of the ChoP epitope.
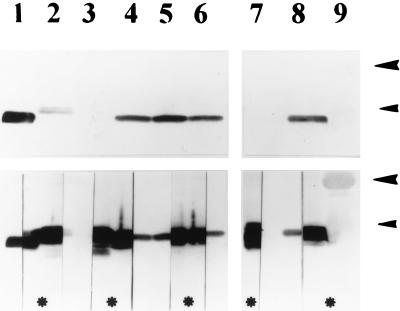
N. gonorrhoeae strains and variants of a single strain, MS11, known to express distinct pilins were also screened for the ability to bind ChoP-reactive MAbs. This investigation showed that the ChoP epitope was present on distinct gonococcal pili and that the epitope was phase variable independently of pilus expression within a strain (Fig. 4).
FIG. 4.
Western blots demonstrating reactivity of pilins of N. gonorrhoeae with MAb TEPC-15 against ChoP. The top and bottom are photographs of the same Western blot developed sequentially. The blot was first developed by using an anti-ChoP antibody (top). After recording the reactivity shown at the top, we cut the lanes in half and reacted the nitrocellulose strips marked with asterisks with MAb SM1 against class I pilins. After development, the strips were combined and rephotographed (bottom composite blot) to show colocation of the ChoP epitope (unmarked strips) and pilins (strips marked with asterisks). Lanes: 1, purified pili of N. meningitidis C311 as a control; 2 to 6, piliated variants of gonococcal strain MS11; 7 and 8, piliated gonococcal strains R10 and SU95; 9, molecular size markers. Size markers of 30 kDa (large arrowheads) and 21.5 kDa (small arrowheads) are shown. Note that the pilins of one variant of strain MS11 (lane 3) do not contain the ChoP epitope.
DISCUSSION
A survey of seven species of gram-negative pathogens of the human mucosa showed that anti-ChoP MAbs bind to at least three species, P. aeruginosa, N. meningitidis, and N. gonorrhoeae, not previously recognized to have this epitope. The bacterial cell surface is generally considered to be antigenically distinct between one species and another. Hence, the presence of a shared epitope, particularly one heretofore found only on cell surface structures in prokaryotes, among so many otherwise diverse pathogens was unexpected. Identification of the ChoP epitope relied on reactivity with two independent MAbs documented to bind to ChoP on the LPS of H. influenzae and teichoic acid of S. pneumoniae, as well as hapten inhibition studies showing specificity for ChoP. Unlike the bacterial structures previously shown to contain ChoP, in both P. aeruginosa and the pathogenic Neisseriae species, this epitope is found on proteins. If the observations based on immunologic methods are confirmed by ongoing structural analysis, these would be the first examples of prokaryotic proteins containing ChoP. This would also demonstrate that ChoP is not uncommon among mucosal pathogens and that these organisms are able to incorporate choline in the form of choline phosphate onto multiple different types of cell surface structures.
A recent report by Kolberg et al. also showed that the presence of an epitope on some strains of pathogenic neisseriae is recognized by antibodies that cross-react with the teichoic acid of the pneumococcus (5). Here we document the presence of the ChoP epitope on meningococcal and gonococcal pili. Class I and II pili of N. meningitidis and N. gonorrhoeae have been shown to be glycosylated and, in addition, contain other modifications such as a phosphodiester-linked glycerol on Ser93 (9, 11, 12, 17). The full range of posttranslational modifications to the pilin glycoprotein of the pathogenic neisseriae has not been defined (12). This study suggests that one such previously unrecognized structure is ChoP. Characterization and identification of a 43-kDa P. aeruginosa protein containing the ChoP epitope is in progress. This protein, whose function is unknown, does not appear to be related to the pilin of this species, based on its size.
The presence of the ChoP epitope was not demonstrated in a number of other gram-negative pathogens. It remains possible, however, that expression of this structure in these species is dependent on specific growth conditions not used in this study. Identification of the ChoP epitope by Western analysis would detect this structure on cell surface components of gram-negative bacteria such as proteins, glycoproteins, and glycolipids. The methods used in this study would not detect the ChoP epitope on other bacterial structures, such as membrane lipids.
As in the case of H. influenzae, expression of the ChoP epitope was subject to phase variation in both P. aeruginosa and the pathogenic neisseriae (22). In addition to phase variation in piliation, there was phase variation in the expression of the ChoP epitope on pilin. The precise function of this epitope on pili is unclear. It is available to bind to antibodies in several strains tested, and therefore it is surface located and exposed in these strains. Its unavailability in some strains suggests that it is partially or totally masked, perhaps by other posttranslational modifications of pili or by folding of pilin itself. However, the fact that the epitope is exposed in some strains means that it is available on these strains to host receptors or antibodies. In addition, the formal possibility remains that it may become exposed in vivo. ChoP appears to contribute to adherence of the pneumococcus to human epithelial cells and to colonization of the nasopharynx by H. influenzae (1, 21). The ability to turn off expression of ChoP may be important in bacterial survival during invasive infection or in the presence of an inflammatory response. A serum protein, C-reactive protein, binds to ChoP and serves as a specific opsonin for organisms expressing this structure (13). Expression of ChoP has been shown to render H. influenzae sensitive to the bactericidal activity of serum by the binding of C-reactive protein and complement (21). The targeting of ChoP by innate humoral immunity in the host is consistent with observations in an animal model of invasive infection where there is a selection for variants of S. pneumoniae with decreased amounts of cell surface ChoP (4). The survey of 31 N. meningitidis isolates in the present study showed that fewer blood isolates expressed the epitope. This is consistent with the notion that expression of the ChoP epitope renders organisms more susceptible to the bactericidal activity of serum. However, a larger number of isolates need to be examined to establish the validity of this observation.
In P. aeruginosa, expression of the ChoP epitope was dependent on the growth temperature. An outer membrane protein of 43 kDa that is expressed in greater quantities in cells of strain PAO1 grown at low temperatures has previously been described (6). P. aeruginosa, unlike the other species displaying the ChoP epitope, exists in a wide variety of environments and at a range of temperatures, including temperatures below 33.5°C, at which this structure is expressed. It is unclear whether this structure contributes to the ability of P. aeruginosa to colonize a mammalian host. The ability of P. aeruginosa to down regulate ChoP expression at 37°C, however, may be a factor in its capacity to cause invasive infection such as that which occurs in immunocompromised hosts.
ACKNOWLEDGMENTS
Clinical isolates were generously provided by Karin McGowan, Paul Edelstein, Staffan Normark, and Anne-Beth Jonsson. We thank Debbie Evans for technical assistance.
J.N.W. is a Lucille P. Markey Charitable Trust Scholar. M.V. is an MRC Senior Fellow. This work was supported by grants from the Lucille P. Markey Charitable Trust (J.N.W.), the Public Health Service (AI38436) (J.N.W.), the Medical Research Council (M.V.), and the National Meningitis Trust (M.V.). Some preliminary work was carried out in the Department of Pediatrics, John Radcliffe Hospital (grant to E. Richard Moxon and M.V. from the Wellcome Trust).
REFERENCES
- 1.Cundell D R, Gerard N P, Gerard C, Idanpaan-Heikkila I, Tuomanen E I. Streptococcus pneumoniae anchor to activated human cells by the receptor for platelet-activating factor. Nature. 1995;377:435–438. doi: 10.1038/377435a0. [DOI] [PubMed] [Google Scholar]
- 2.Deutsch J, Salman M, Rottem S. An unusual polar lipid from the cell membrane of Mycoplasma fermentans. Eur J Biochem. 1995;227:897–902. doi: 10.1111/j.1432-1033.1995.tb20216.x. [DOI] [PubMed] [Google Scholar]
- 3.Gillespie S H, Ainscough S, Dickens A, Lewin J. Phosphorylcholine-containing antigens in bacteria from the mouth and respiratory tract. J Med Microbiol. 1996;44:35–40. doi: 10.1099/00222615-44-1-35. [DOI] [PubMed] [Google Scholar]
- 4.Kim J, Weiser J. Association of intrastrain phase variation in quantity of capsular polysaccharide and teichoic acid with the virulence of Streptococcus pneumoniae. J Infect Dis. 1998;177:368–377. doi: 10.1086/514205. [DOI] [PubMed] [Google Scholar]
- 5.Kolberg J, Holby E A, Jantzen E. Detection of the phosphorylcholine epitope in streptococci, Haemophilus and pathogenic Neisseriae by immunoblotting. Microb Pathog. 1997;22:321–329. doi: 10.1006/mpat.1996.0114. [DOI] [PubMed] [Google Scholar]
- 6.Kropinski A M B, Lewis V, Berry D. Effect of growth temperature on the lipids, outer membrane proteins, and lipopolysaccharides of Pseudomonas aeruginosa PAO1. J Bacteriol. 1987;169:1960–1966. doi: 10.1128/jb.169.5.1960-1966.1987. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 7.Leon M A, Young N M. Specificity for phosphorylcholine of six murine myeloma proteins reactive with pneumococcus C polysaccharide and beta-lipoprotein. Biochemistry. 1971;10:1424–1429. doi: 10.1021/bi00784a024. [DOI] [PubMed] [Google Scholar]
- 8.Mosser J L, Tomasz A. Choline-containing teichoic acid as a structural component of pneumococcal cell wall and its role in sensitivity to lysis by an enzyme. J Biol Chem. 1970;245:287–298. [PubMed] [Google Scholar]
- 9.Parge H, Forest K, Hickey M, Christensen D, Getzoff E, Tainer J. Structure of the fibre-forming protein pilin at 2.6 A resolution. Nature. 1995;378:32–38. doi: 10.1038/378032a0. [DOI] [PubMed] [Google Scholar]
- 10.Salvano M, Lisa T, Domenech C. Choline transport in Pseudomonas aeruginosa. Mol Cell Biochem. 1989;85:81–89. doi: 10.1007/BF00223517. [DOI] [PubMed] [Google Scholar]
- 11.Stimson E, Virji M, Barker S, Panico M, Blench I, Saunders J, Payne G, Moxon E, Dell A, Morris H. Discovery of a novel protein modification: alpha-glycerophosphate is a substituent of meningococcal pilin. Biochem J. 1996;316:29–33. doi: 10.1042/bj3160029. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 12.Stimson E, Virji M, Makepeace K, Dell A, Morris H, Payne G, Saunders J, Jennings M, Barker S, Panico M. Meningococcal pilin: a glycoprotein substituted with digalactosyl 2,4-diacetamido-2,4,6-trideoxyhexose. Mol Microbiol. 1995;17:1201–1214. doi: 10.1111/j.1365-2958.1995.mmi_17061201.x. [DOI] [PubMed] [Google Scholar]
- 13.Szalai A J, Briles D E, Volanakis J E. Role of complement in C-reactive-protein-mediated protection of mice from Streptococcus pneumoniae. Infect Immun. 1996;64:4850–4853. doi: 10.1128/iai.64.11.4850-4853.1996. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 14.Towbin H, Staehelin T, Gordon J. Electrophoretic transfer of proteins from polyacrylamide gels to nitrocellulose sheets: procedure and some applications. Proc Natl Acad Sci USA. 1979;76:4350–4354. doi: 10.1073/pnas.76.9.4350. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 15.Virji M, Heckels J E, Potts W J, Hart C A, Saunders J R. Identification of the epitopes recognised by monoclonal antibodies SM1 and SM2 which react with all pili of Neisseria gonorrhoeae but which differentiate between two structural classes of pili expressed by Neisseria meningitidis and the distribution of their encoding sequences in the genomes of Neisseria spp. J Gen Microbiol. 1989;135:3239–3251. doi: 10.1099/00221287-135-12-3239. [DOI] [PubMed] [Google Scholar]
- 16.Virji M, Kayhty H, Ferguson D J P, Alexandrescu C, Heckels J E, Moxon E R. The role of pili in the interactions of pathogenic Neisseria with cultured human endothelial cells. Mol Microbiol. 1991;5:1831–1841. doi: 10.1111/j.1365-2958.1991.tb00807.x. [DOI] [PubMed] [Google Scholar]
- 17.Virji M, Saunders J R, Sims G, Makepeace K, Maskell D, Ferguson D J P. Pilus-facilitated adherence of Neisseria meningitidis to human epithelial and endothelial cells: modulation of adherence phenotype occurs concurrently with changes in primary amino acid sequence and the glycosylation status of pilin. Mol Microbiol. 1993;10:1013–1028. doi: 10.1111/j.1365-2958.1993.tb00972.x. [DOI] [PubMed] [Google Scholar]
- 18.Virji M, Weiser J N, Lindberg A A, Moxon E R. Antigenic similarities in lipopolysaccharides of Haemophilus and Neisseria and expression of a digalactoside structure also present on human cells. Microb Pathog. 1990;9:441–450. doi: 10.1016/0882-4010(90)90062-u. [DOI] [PubMed] [Google Scholar]
- 19.Wani J, Gilbert J, Plaut A, Weiser J. Identification, cloning, and sequencing of the immunoglobulin A1 protease gene of Streptococcus pneumoniae. Infect Immun. 1996;64:3967–3974. doi: 10.1128/iai.64.10.3967-3974.1996. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 20.Weiser J N, Love J M, Moxon E R. The molecular mechanism of phase variation of H. influenzae lipopolysaccharide. Cell. 1989;59:657–665. doi: 10.1016/0092-8674(89)90011-1. [DOI] [PubMed] [Google Scholar]
- 21.Weiser J N, Pan N, McGowan K L, Musher D, Martin A, Richards J C. Phosphorylcholine on the lipopolysaccharide of Haemophilus influenzae contributes to persistence in the respiratory tract and sensitivity to serum killing mediated by C-reactive protein. J Exp Med. 1998;187:631–640. doi: 10.1084/jem.187.4.631. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 22.Weiser J N, Shchepetov M, Chong S T H. Decoration of lipopolysaccharide with phosphorylcholine: a phase-variable characteristic of Haemophilus influenzae. Infect Immun. 1997;65:943–950. doi: 10.1128/iai.65.3.943-950.1997. [DOI] [PMC free article] [PubMed] [Google Scholar]