Abstract

Investigating the relationship between individual pKa values and the efficacy of aminoglycosides is essential for the development of more effective and targeted therapies. In this work, we measured the pKa values for individual amino groups of the six clinically relevant aminoglycoside antibiotics gentamicin, tobramycin, amikacin, arbekacin, plazomicin, and apramycin using 15N–1H heteronuclear multiple-bond correlation and 1H NMR experiments. For arbekacin and plazomicin, the pKa values are reported for the first time. These pKa values were used to calculate the net charges of the aminoglycosides and the protonation levels of amino groups under various pH conditions. The results were analyzed in relation to the mode of interaction and inhibition to establish pKa relationships for rRNA binding, inhibitory activity, and the pH dependence of the uptake into bacterial cells.
Introduction
Aminoglycosides are a class of natural antibiotics derived from various species of Streptomyces and Micromonospora bacteria and used primarily in the treatment of severe Gram-negative and mycobacterial infections.1,2 They are structurally complex molecules composed of an aminocyclitol moiety and amino sugars connected by glycosidic linkages. Based on the identity of the aminocyclitol, the aminoglycosides can be divided into five groups: (1) derivatives containing streptidine (e.g., streptomycin), (2) those containing streptamine (spectinomycin), (3) those containing a 4,5-disubstituted deoxystreptamine (neomycin), and (4) derivatives containing a 4,6-disubstituted deoxystreptamine moiety (gentamicin, kanamycin, tobramycin, and sisomicin). Apramycin constitutes a fifth group that is structurally unique in that it contains a 4-monosubstituted deoxystreptamine aminocyclitol moiety and a bicyclic sugar moiety. Its unique chemical structure protects apramycin from almost all the bacterial resistance mechanisms that inactivate all other aminoglycoside antibiotics in clinical use, spurring interest in apramycin as a potential drug candidate.4−6 In addition, several semisynthetic aminoglycoside analogues are available bearing a variety of amino and/or hydroxyl substitutions (e.g., amikacin, arbekacin, and plazomicin). These derivatives are used for the treatment of infections caused by multiresistant bacteria as the introduced groups can influence the mechanism of action and susceptibility to various aminoglycoside modifying enzymes.7,8
The aminoglycoside antibiotics act by binding to the 16S ribosomal RNA (rRNA) in the 30S ribosomal subunit, impeding the fidelity of the translation process.9−11 This interference results in the disruption of the bacterial protein synthesis, ultimately leading to cell death. The high-affinity binding to rRNA varies among the distinct classes of aminoglycosides; however, in all cases, it is in part driven by electrostatic interactions between the positively charged aminoglycoside ammonium groups and the negatively charged sugar–phosphate backbone of rRNA.12,13 Additionally, the positive charge facilitates aminoglycoside binding to the negatively charged bacterial cell surface and allows them to penetrate through the outer membrane.14 This implies that the ionization constants (pKas) and locations of the amino groups in the aminoglycoside structure can have a significant impact on the biological activity of these antibiotics.
Understanding structure–activity relationships of aminoglycosides requires knowing the protonation level of individual amino groups at a specific pH found at the site of infection. Traditional 15N NMR titration methods have been used to measure the site-specific pKa values of amino groups in various aminoglycosides, including neomycin B,15 apramycin,16,17 isepamicin,18 ribostamycin,19 and tobramycin.16,20 However, minor differences between the equipment and experimental approaches used in these separate studies complicates a direct comparison of the aminoglycosides in terms of ionization state at a particular pH. Recently, Blagbrough and co-workers published two studies reporting individual pKa values for a total of nine selected aminoglycosides from Streptomyces and Micromonospora (tobramycin, kanamycin B, amikacin, sisomicin, netilmicin, neamine, neomycin, paromomycin, and streptomycin) by multinuclear NMR spectroscopy.21,22 The determined pKa values deviated from previously published data by up to 1.5 units, highlighting the impact of different experimental protocols and calling for more comparative studies. Notably, such differences would preclude the establishment of meaningful structure–activity relationships.
In this work, we determine the pKa values for individual amino groups of apramycin in comparison to the five clinically most relevant aminoglycoside antibiotics using 15N–1H heteronuclear multiple-bond correlation (HMBC) and 1H NMR titration experiments. Based on the pKa values, we calculate the pH dependent net charge of the aminoglycosides and the protonation levels of amino groups. Furthermore, we analyze the structure—pKa relationships and protonation profiles of the aminoglycosides in relation to their reported rRNA-bound structures and uptake from different biological environments. Our findings demonstrate that this approach allows for the identification of the amino groups that contribute most to binding and ribosomal inhibition at specific pH levels.
Results and Discussion
The pKa values of individual amino groups of the studied aminoglycosides were determined using a similar approach as described by Alkhzem et al.21 In short, the aminoglycoside samples were dissolved in D2O and titrated with DCl or NaOD. Changes in the 15N and 1H chemical shifts were monitored by using 15N–1H HMBC and 1H NMR spectroscopy. Assignment of the 1H, 13C, and 15N resonances was performed based on 2D correlation spectra. The pKas were calculated using Microsoft Excel Solver Add-in by fitting the experimental data to a theoretical model (see the Experimental Section for details). Spectroscopic measurements were repeated twice to obtain data points in triplicate for each aminoglycoside.
Gentamicin
Gentamicin is a 4,6-disubstituted deoxystreptamine derivative containing purpurosamine at the 4-O and garosamine (3-methyl amino-3-deoxy-4-C-methyl-β-l-arabinose) at the 6-O positions (Figure 1A). Naturally produced gentamicin is a mixture of five major compounds C1, C1a, C2, C2a, and C2b differing by the substituents at the 6′ position of the purpurosamine ring.23 Gentamicin C1, C2a, and C2b have three primary amine (i.e., monosubstituted ammonia) groups, N-1 and N-3 on the DOS moiety and N-2′ on purpurosamine; as well as two secondary amines, N-6′ on purpurosamine and N-3′′ on garosamine. In gentamicin C1a and C2, N-6′ is a primary amine bringing the balance to four primary and one secondary amine instead. Correlation with N-2′ was not observed by 15N–1H HMBC NMR and the H-1, H-3, and H-2′ chemical shifts could not be assigned in the 1H NMR spectra due to spectral overlap, which probably stems from the fact that the sample is a mixture of congeners. Therefore, the pKas for N-6′, N-3′′ were measured using 15N and 1H chemical shift data, whereas for N-1 and N-3, the pKas were measured using only 15N chemical shift data (Figure 1B,C). The 1H and 15N derived values are in good agreement for N-3′′ but differ by ∼0.6 units for N-6′. The order of the pKa values is N-6′ > N-3′′ > N-1 > N-3, which is consistent with literature data (Figure 1D).24 However, our determined pKa values are about 0.1–1.1 units lower when considering the 15N NMR data and by 0.1–0.3 units lower when considering the 1H NMR data. The reasons for these discrepancies may be incorrect assignments due to the spectral overlap or very different sample concentrations used for the 15N and 1H NMR measurements, respectively, as noted by previous authors.24
Figure 1.
pKa determination for individual amino groups of gentamicin. (A) Chemical structure and atom numbering of gentamicin. (B) 15N NMR chemical shift titration curves. (C) 1H NMR chemical shift titration curves. (D) Determined pKa values in comparison to values published previously.
Tobramycin
Tobramycin is a 4,6-disubstituted deoxystreptamine derivative containing nebrosamine at the 4-O and 3-amino-3-deoxy-d-glucose (kanosamine) at the 6-O positions (Figure 2A). The kanosamine moiety is present in several aminoglycosides (e.g., tobramycin, kanamycin, and amikacin) and is an antibiotic by itself.25 Tobramycin has five primary amine groups, N-1 and N-3 on DOS, N-2′ and N-6′ on nebrosamine, and N-3′′ on kanosamine. Again, the N-2′ atom was not observed by 15N–1H HMBC NMR and its pKa value was determined from the H-2′ chemical shift data (Figure 2B,C). The pKas determined from 15N and 1H chemical shift data agree well, except for N-1 where the difference is 0.05 units taking into account the uncertainty. The order of the pKa values is N-6′ > N2′ > N-3′′ ≈ N-1 > N-3, which is consistent with literature data (Figure 2D).16,20,21 Our determined pKa values differ by about 0.1–0.3 units compared to the latest report in the literature and by 0.3–0.7 units compared to earlier studies.
Figure 2.
pKa determination for the individual amino groups of tobramycin. (A) Chemical structure and atom numbering of tobramycin. (B) 15N NMR chemical shift titration curves. (C) 1H NMR chemical shift titration curves. (D) Determined pKa values in comparison to values published previously.
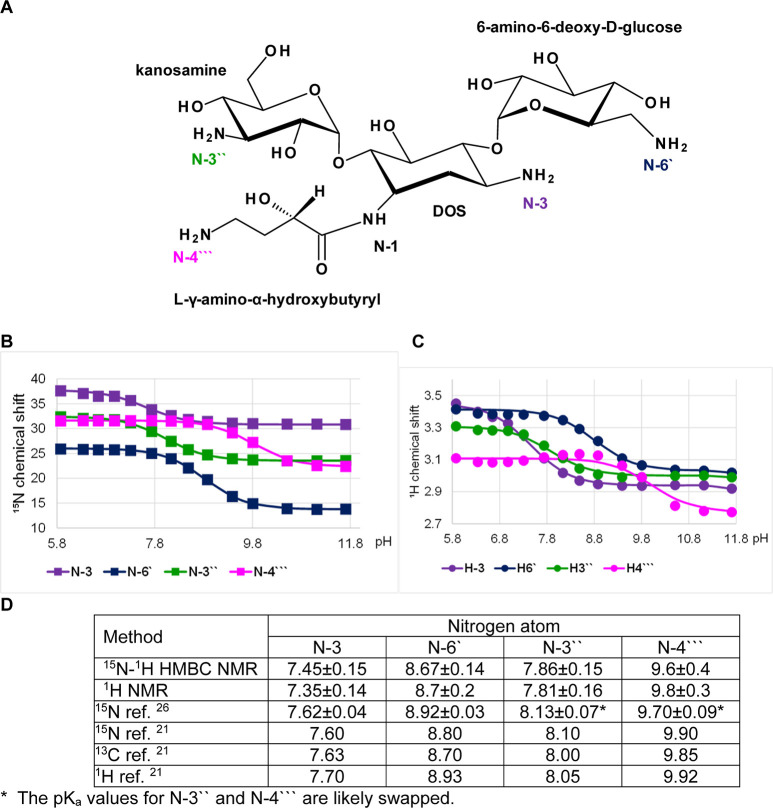
Amikacin
Amikacin is a semisynthetic derivative of kanamycin. It consists of a 4,6-disubstituted deoxystreptamine with 6-amino-6-deoxy-d-glucose at the 4-O and kanosamine at the 6-O positions (Figure 3A). The DOS moiety is acylated with an l-γ-amino-α-hydroxybutyryl side chain at the 1-amino group. Amikacin has four primary amine groups, N-3 and N-4′′′ on DOS, N-6′ on 6-amino-6-deoxy-d-glucose, and N-3′′ on kanosamine. The pKa values were determined from 15N and 1H chemical shift data for all amines and showed very good agreement (Figure 3B,C). The order (N-4′′′ > N-6′ > N-3′′ > N-3) and magnitude of the pKa values is in good agreement with those reported in the literature (Figure 3D).21,26
Figure 3.
pKa determination for the individual amino groups of amikacin. (A) Chemical structure and atom numbering of amikacin. (B) 15N NMR chemical shift titration curves. (C) 1H NMR chemical shift titration curves. (D) Determined pKa values in comparison to values published previously.
Arbekacin
Arbekacin is another semisynthetic derivative of dibekacin (3′,4′-dideoxy-kanamycin). It contains a 4,6-disubstituted deoxystreptamine with purpurosamine C at the 4-O and kanosamine at the 6-O positions (Figure 4A). The DOS moiety is also acylated with an l-γ-amino-α-hydroxybutyryl side chain at the 1-amino group. It has five primary amine groups, N-3 and N-4′′′ on DOS, N-6′ on purpurosamine C, and N-3′′ on kanosamine. The pKa values of N-3, N-3′′, and N-4′′′ amines were determined using both 15N and 1H chemical shift data and showed good agreement, while pKa values of N-2′ and N-6′ were determined only from 1H chemical shifts (Figure 4B–D). The order of the pKa values is N-4′′′ > N-6′ > N-3′′ > N-3 and is reported here for the first time.
Figure 4.
pKa determination for the individual amino groups of arbekacin. (A) Chemical structure and atom numbering of arbekacin. (B) 15N NMR chemical shift titration curves. (C) 1H NMR chemical shift titration curves. (D) Determined pKa values.
Plazomicin
Plazomicin is a semisynthetic aminoglycoside derived from sisomicin. It has a 4,6-disubstituted deoxystreptamine with 6′-N-hydroxyethyl sisosamine at the 4-O and garosamine at the 6-O positions (Figure 5A). Similarly to amikacin and arbekacin, the DOS moiety is acylated with an l-γ-amino-α-hydroxybutyryl side chain at the 1-amino group. Plazomicin has three primary amine groups, N-3 and N-4′′′ on DOS, N-2′ on sisosamine, and two secondary amine groups, N-6′ on sisosamine and N-3′′ on garosamine. The pKa values of N-3, N-2′, N-6′, and N-3′′ amines were determined from 15N and 1H chemical shift data and showed a good match, whereas N-4′′′ amine pKas were determined using 1H and 15N NMR data, respectively (Figure 5B,C). The individual pKa values and their order (N-4′′′ > N-3′′ ≈ N-6′ > N-2′ ≈ N-3) are reported here for the first time.
Figure 5.
pKa determination for the individual amino groups of plazomicin. (A) Chemical structure and atom numbering of plazomicin. (B) 15N NMR chemical shift titration curves. (C) 1H NMR chemical shift titration curves. (D) Determined pKa values.
Apramycin
Apramycin is a structurally unique aminoglycoside that comprises a 4-monosubstituted deoxystreptamine aminocyclitol moiety, an unusual bicyclic eight-carbon dialdose, and 4-amino-4-deoxy-d-glucose (Figure 6A). It has four primary amine groups, N-1 and N-3 on the 2-deoxystreptamine (DOS) fragment, N-2′ on the bicyclic eight-carbon dialdose, and N-4′′ on the terminal amino sugar moiety as well as one secondary amine (N-7′) on the central bicyclic sugar moiety. The N-2′ atom was not observed by 15N–1H HMBC NMR and its pKa value was determined from the H-2′ chemical shift data (Figure 6B–E). For the other amines, the pKa values determined using 15N and 1H chemical shifts are in agreement, taking into account the uncertainty. The order of the pKa values is N-1 > N-2′ > N-7′ > N-3 ≈ N-4′′, which is consistent with literature data (Figure 6F).16,17 However, our determined pKa values are higher by about 0.3 unit, except for N-7′ (the only secondary amine).
Figure 6.
pKa determination for the individual amino groups of apramycin. (A) Chemical structure and atom numbering of apramycin. (B) 15N NMR chemical shift titration curves. (C) 1H NMR chemical shift titration curves. (D) Determined pKa values in comparison to values published previously. (E) 15N–1H HMBC spectra of apramycin in D2O. Arrows indicate the shift of signals upon pH change from 12.05 to 5.14. (F) Stacked 1H spectra of apramycin in D2O in the pH interval between 11.76 and 5.54.
pKa Differences for Particular Amino Groups in Different Aminoglycosides
Our results confirm previous reports that have pointed to a variation in pKa values of homologous amino groups from one aminoglycoside to another depending on their chemical environment. Thus, for example, the primary N-3′′ amines in the kanosamine series are less basic than the secondary N-3′′ amines in the garosamines. The basicity of N-6′ across the compounds studied is gentamicin ≈ arbekacin > tobramycin > amikacin > plazomicin. With the exception of plazomicin, this sequence is readily explained by the absence of electron-withdrawing and basicity-reducing C–O at the 3′ and 4′-positions in gentamicin and arbekacin, and the absence of a 3′–C–O bond in tobramycin, as compared to amikacin with the full complement of C–O bonds at the 3′ and 4′ positions. The absence of electron-withdrawing 3′- and 4′–C–O bonds in plazomicin is offset by the sp2-hybridized nature of C5′ and by the electron-withdrawing β–C–O bond in the hydroxyethyl moiety, which together make its N-6′ the least basic in the series. These trends are in full agreement with well-established patterns of pKa modulation in simple amines.27 Finally, the greater basicity of N-3 in the N-1-γ-amino-α-hydroxybutyryl substituted aminoglycosides amikacin, arbekacin, and plazomicin arises because of the absence of a basic amino group at N-1, whose protonation in apramycin, tobramycin, and gentamicin reduces the basicity of N-3.
Comparison of Protonation Profiles and Implications for Uptake of the Studied Aminoglycosides
As summarized in Table 1, all of the determined pKa values are between 6.4 and 9.8. This means that within a slightly wider pH range of approximately 5–11, the net charge of each aminoglycoside will depend on the exact pKa values of its containing amino groups and the precise pH of the environment. We used the Henderson–Hasselbalch equation to calculate the net positive charges of the studied aminoglycosides in the pH range of 4–12 (Figure 7A). This showed that apramycin has the lowest charge at pH > 7, followed by tobramycin and gentamicin, whereas arbekacin and plazomicin have the highest charges. Amikacin is somewhat different because it contains only four amino groups; therefore, its calculated titration curve crosses the other ones, and it has the lowest positive charge of all tested aminoglycosides at pH < 7.
Table 1. Comparison of the Determined pKa Values of Individual Amino Groups Among Apramycin, Tobramycin, Gentamicin, Amikacin, Arbekacin, and Plazomicina.
| aminoglycoside | method | N-1 | N-3 | N-2′ | N-6′ | N-7′ | N-4′′ | N-3′′ | N-4′′′ |
|---|---|---|---|---|---|---|---|---|---|
| apramycin | 15N–1H HMBC NMR | 8.5 ± 0.3 | 7.0 ± 0.2 | nd | 7.52 ± 0.10 | 7.1 ± 0.3 | |||
| 1H NMR | 8.3 ± 0.2 | 7.17 ± 0.06 | 7.96 ± 0.09 | 7.51 ± 0.08 | 6.92 ± 0.08 | ||||
| tobramycin | 15N–1H HMBC NMR | 7.81 ± 0.06 | 6.93 ± 0.03 | nd | 8.90 ± 0.06 | 7.82 ± 0.08 | |||
| 1H NMR | 7.61 ± 0.09 | 7.03 ± 0.12 | 8.07 ± 0.09 | 9.0 ± 0.3 | 7.75 ± 0.07 | ||||
| gentamicin | 15N–1H HMBC NMR | 7.84 ± 0.10 | 7.15 ± 0.10 | nd | 9.2 ± 0.2 | 8.54 ± 0.07 | |||
| 1H NMR | nd | nd | nd | 9.73 ± 0.16 | 8.53 ± 0.15 | ||||
| amikacin | 15N–1H HMBC NMR | 7.53 ± 0.13 | 8.76 ± 0.13 | 7.96 ± 0.11 | 9.6 ± 0.4 | ||||
| 1H NMR | 7.35 ± 0.14 | 8.7 ± 0.2 | 7.81 ± 0.16 | 9.8 ± 0.3 | |||||
| arbekacin | 15N–1H HMBC NMR | 7.39 ± 0.10 | nd | nd | 7.94 ± 0.07 | 9.71 ± 0.08 | |||
| 1H NMR | 7.26 ± 0.18 | 8.47 ± 0.19 | 9.11 ± 0.04 | 7.7 ± 0.2 | 9.8 ± 0.2 | ||||
| plazomicin | 15N–1H HMBC NMR | 7.84 ± 0.11 | 8.06 ± 0.08 | 8.52 ± 0.11 | 8.57 ± 0.08 | 9.74 ± 0.10 | |||
| 1H NMR | 7.8 ± 0.4 | 7.83 ± 0.14 | 8.43 ± 0.07 | 8.65 ± 0.05 | nd |
nd-not determined.
Figure 7.

Effect of pKa values on the net positive charge, inhibition of ribosomal translation, and bacterial inhibition. (A) Net positive charge of the studied aminoglycosides in the pH range 4–12 calculated using the Henderson–Hasselbalch equation from the determined pKa values of individual amino groups. The dotted line indicates a pH of 7.4. (B) Relationship between the aminoglycoside net positive charge and ribosomal inhibition at physiologic pH. (C) Relationship between the net positive charge and cell-based bacterial inhibition expressed as the minimal inhibitory concentration (MIC), a function of both cellular uptake and ribosomal inhibition. (D) Relationship between the aminoglycoside relative interaction energy with rRNA (in kcal/mol, Table S7) and reported ribosomal inhibition at pH 7.4.
The proton motive force has been established as the driving force for aminoglycoside uptake into bacterial cells,28−30 which in turn correlates with antibacterial activity.31,32 At physiological pH 7.4, the aminoglycoside net charge is between +2.86 e for apramycin and +4.37 e for plazomicin. Under these conditions, the pH difference between the environment (7.4) and bacterial cytoplasm (7.5) does not contribute effectively to the proton motive force and the electrical potential across the membrane provides the main stimulus for aminoglycoside uptake.28 Thus, at physiological pH plazomicin is expected to have a better uptake due to its higher net charge, followed by arbekacin (+4.08 e), gentamicin (+3.72 e), tobramycin (+3.45 e), amikacin (+3.23 e), and apramycin (+2.86 e). This prediction has yet to be tested experimentally by pH-dependent bacterial cell-wall permeability assays. In an acidic environment such as urine with a variable pH of e.g., 6.0, the proton motive force is significantly decreased due to lowered membrane potential.33 However, the net charge of all the aminoglycosides does not differ much from one another at pH 6.0 (+4.79 e for apramycin, +4.86 e for tobramycin, +4.90 e for gentamicin, 4.94 e for arbekacin, and 4.97 e for plazomicin), except for amikacin (+3.95 e), which has only four amino groups. Compared to pH 7.4, the increase in protonation level at pH 6.0 is the highest for apramycin (Δ = 1.93 e), followed by tobramycin (Δ = 1.41 e), gentamicin (Δ = 1.18 e), arbekacin (Δ = 0.86 e), amikacin (Δ = 0.72 e), and lowest for plazomicin (Δ = 0.60 e). A relatively larger increase in the protonation level at pH 6.0 may potentially make up for the reduced proton motive force, at least in part. Thus, apramycin is expected to show a comparable uptake to plazomicin and the other aminoglycosides at slightly acidic sites of infection, which has recently also been observed experimentally.34
Aminoglycoside Net Charge–Activity Relationships
To investigate the relationship between the aminoglycoside pKa values and antibacterial activity, we first compared their net charge at pH 7.4 with the reported cell-free ribosome inhibition levels (Figure 7B). In the series apramycin, amikacin, tobramycin, and gentamicin, the IC50 values clearly decrease (inhibition increases) with increased net positive charge. A higher protonation level as in arbekacin and plazomicin does not further enhance the inhibition, implying either a saturation in effect of positive charge or that the effect of additional protonation in arbekacin and plazomicin is offset by suboptimal fit into the binding pocket. Notably, the higher net positive charge for the latter two aminoglycosides is largely due to protonation of their γ-amino-α-hydroxybutyryl substituent with the high (9.7) pKa of its amino group that is fully protonated at pH 7.4, suggesting that protonation of the N-4′′′ amino group in these compounds might not be important for ribosome inhibitory activity.
Next, we analyzed the correlation with cell-based minimal inhibitory concentration (MIC) values, which measure bacterial inhibition as a combination of both uptake and ribosome inhibition (Figure 7C). In the series apramycin, amikacin, and tobramycin, the MIC values decrease with an increased net positive charge, resembling the effect for ribosome inhibition alone. In this sequence, the net charge effect on ribosomal inhibition alone could be responsible for the lower MICs, although it cannot be ruled out that the effect on uptake is also contributing. In contrast, gentamicin, arbekacin, and plazomicin all show similar MIC values despite differences in their ribosomal IC50 values. This suggests that an increased uptake of plazomicin and arbekacin may potentially compensate for their reduced ribosomal IC50 values in comparison to gentamicin. Along similar lines, we have shown previously that simply increasing the number of amino groups in a given aminoglycoside does not always result in higher activity and may even lead to a reduction in activity. For example, replacement of the C-6′ hydroxy group of apramycin by an amino group resulted in a substantial loss of activity.35 Similarly, exchanging the 5′′-hydroxy group of the 4,5-disubstituted 2-deoxystreptamines for an amino group mostly leads to a reduction in activity because of the proximity of the newly introduced basic residue with the 2′-ammonium ion.36
Structure—pKa—rRNA Binding Relationships
As described above, the aminoglycosides contain primary amine groups either directly attached to the sugar ring or linked through a methylene bridge (aminomethyl group) or through an α-hydroxybutyryl chain as well as secondary amine groups (N-methyl and N-2-hydroxyethyl groups). The lowest pKa values were consistently found for the N-3 and N-1 primary amino groups on the 4,6-disubstituted DOS moiety. The highest pKas were for the primary 1-aminoalkyl groups (γ-amino-α-hydroxybutyryl and aminomethyl groups), followed by the secondary amines. Thereby, the substitution of the N-1 amino group with an l-γ-amino-α-hydroxybutyryl group in amikacin, arbekacin, and plazomicin results in a significant increase in the basicity of the aminoglycoside. Similarly, the addition of a methyl substituent to the primary N-3′′ amino group in gentamicin and plazomicin increases its basicity. In contrast, the introduction of a 2-hydroxyethyl group in the aminomethyl moiety (N-6′) of plazomicin decreases its basicity.
To evaluate the potential impact of the pKa values on the binding strength with rRNA (i.e., binding free energy, ΔG), we analyzed the crystal structures of apramycin, tobramycin, gentamicin, and amikacin bound to the A-site of 16S rRNA available from the Protein Data Bank. As shown in Figure 8A, tobramycin, gentamicin, and amikacin bind rRNA in nearly identical conformation. The DOS moiety of apramycin interacts with rRNA very similarly to and overlays with the other aminoglycosides, except for its additional 4-amino-4-deoxy-d-glucosyl residue (Figure 8B).37
Figure 8.
(A) Superposition of the crystal structures of tobramycin (cyan, PDB ID 1LC4(44)), gentamicin (purple, 2ET3(45)) and amikacin (yellow, 4P20(46)) bound with rRNA. (B) Crystal structure of apramycin (green, PDB ID 4AQY(4)) bound with rRNA. The aminoglycosides are shown in stick representation and the rRNA backbone is shown with thin lines. The N, O, and P atoms are colored in blue, red, and orange, respectively. The distances between amino groups and nearest phosphates are marked with yellow dashed lines and are indicated in Å.
In general, the aminoglycosides interact with the rRNA through specific hydrogen bonding with the nucleotide bases and by nonspecific charge interactions with the rRNA backbone phosphate groups. Since the protonation state of the aminoglycoside amines is primarily expected to modulate the charge interactions, we only consider the latter, i.e., amine–phosphate interactions, in our analysis. The shortest distance from an amine to an rRNA phosphate group in all the complexes is measured from the N-3 amine (about 2.5–2.8 Å), which also has the lowest pKa value (Table 1). Short distance amine–phosphate interactions are also observed with the N-3′′ (about 2.9 Å in gentamicin and 4.6 Å in tobramycin and amikacin), N-6′/N-7′ (4.5–4.6 Å), and N-2′ (4.7–5.3 Å) amino groups. The N-1, N-4′′ (in apramycin), and N-4′′′ (in amikacin) amino groups are >6 Å away from any phosphates. We calculated the relative electrostatic interaction energies for each amino group at pH 7.4 and pH 6.0 by considering interactions with only the closest phosphate group (Table S7). The sum of these energies shows a strong relationship with ribosomal IC50 values, indicating that the electrostatic interactions are primarily responsible for binding strength, whereas the hydrogen bonding with bases determines specificity (Figure 7D). At physiological pH, the N-6′ amino group contributes most to the binding of tobramycin and amikacin, while for gentamicin, the N-3′′ amine shows the highest relative interaction energy (due to a shorter distance and an increased N-methyl pKa value), followed by N-6′. For apramycin, the N-2′ and N-1 amines show comparable relative interaction energies owing to their elevated pKa values. This suggested that combining the central DOS moiety with the N-6′ amine-containing nebrosamine from tobramycin and the N-3′′-containing garosamine from gentamicin (Figure S1) could lead to an aminoglycoside derivative with increased binding affinity. Indeed, the literature reveals that this compound was previously prepared by Mallams and co-workers38 and was found to have excellent activity when tested against Escherichia coli and Pseudomonas aeruginosa.39 At pH 6.0, the N-3 amino group largely determines the binding strength between the aminoglycosides and rRNA in a pKa-dependent manner (highest relative energy for apramycin, then amikacin, gentamicin, and lowest for tobramycin). The protonation levels of the other amines of tobramycin, gentamicin, and amikacin probably have a much smaller effect on the binding strength due to larger distances to phosphates and because their pKas suggest nearly full protonation already at physiological pH. In line with the net charge–ribosomal IC50 relationships, the introduction of the l-γ-amino-α-hydroxybutyryl group in amikacin does not seem to affect the binding strength with rRNA as both, the N-1 and N-4′′′ amino groups, are >6 Å away from any phosphates. Furthermore, it has previously been suggested that amine protonation not only facilitates electrostatic interaction with the negatively charged phosphate backbone but also affects intramolecular charge repulsion and hydrogen bonding that modulate the conformation and flexibility of an aminoglycoside antibiotic, which may in turn determine its specific affinity to the three-dimensional RNA binding pocket, and thus, its biological activity.40,41
Conclusions
We used 15N–1H HMBC and 1H NMR spectroscopy to determine the pKa values of individual amino groups in the aminoglycosides apramycin, tobramycin, gentamicin, amikacin, arbekacin, and plazomicin. The values for arbekacin and plazomicin are reported for the first time. Although 1H NMR is incomparably faster and more sensitive, the signal overlap observed in several cases causes difficulties in following the chemical shift changes, thereby not allowing precise determination of the pKa values. 15N–1H HMBC experiments, on the other hand, may miss signals due to low sensitivity. Thus, a combination of the two experimental approaches is pertinent for the determination of all of the aminoglycoside amine pKa values.
The amine pKa values allow for calculation of the net charge that is important for understanding the antibacterial activity and uptake of the aminoglycosides under various pH conditions. The individual amine pKa values obtained using the same methodology/approach also allow for a direct comparison of the aminoglycoside amino groups in terms of protonation levels at a particular pH, which are important to understand their binding mechanism with rRNA. Thus, we have shown that the N-6′ amino group of tobramycin and amikacin, the N-3′′ and N-6′ amines of gentamicin, and the N-2′ and N-1 amines of apramycin are most important for binding to their drug target. The N-4′′′ amine of the l-γ-amino-α-hydroxybutyryl group does not seem to affect the binding strength with rRNA and does not enhance the ribosomal inhibition of arbekacin and plazomicin. We expect the approach described herein to be valuable for rationalization of the structure–activity relationships of aminoglycosides and for the design of better antibiotics.
Methods
Materials and General Methods
Deuterium oxide, deuterium chloride solution (35 wt % in D2O, ≥99 atom % D), sodium deuteroxide solution (40 wt % in D2O, 99.5 atom % D), tobramycin, gentamicin, amikacin, and apramycin were purchased from Sigma-Aldrich. Plazomicin (Cipla) and arbekacin (Meiji Seika) solutions were obtained from the dispensary.
All NMR spectra, including 1H, 13C, HSQC, HMBC, COSY, and 1H–15N HMBC were recorded on a Bruker Avance Neo 600 MHz (Bruker Biospin Gmbh, Rheinstetten, Germany) with a quadruple resonance CryoProbe (CP QCI 600S3 H/F-C/N-D-05 Z).
Titration Experiments
The initial concentrations of the aminoglycosides were from 0.3 to 0.6 mol/L and during the titration process, the concentration could be decreased up to 2 times.
Each aminoglycoside was titrated three times using the deuterium chloride and sodium deuteroxide solutions to obtain the desired pH value. The pD values were measured using an Oakton pH 11 Portable pH/mV/°C Meter and then converted into pH (pH = pD – 0.5) following the IUPAC Technical Report Guidelines.21,42
Before the pH measurements, the electrode was calibrated using three points (standard buffer solutions with pH values 4, 7, and 10).
The pKa was calculated using MS Excel Solver Add-in to align the experimental titration graph with the predicted values using the formula
The MS Solver can be used for nonlinear calculation of the different values with the same error level as MATLAB or OriginPro.43 The standard deviation was calculated from the three obtained values.
Calculation of Relative Electrostatic Interaction Energies
The relative electrostatic interaction energies
were calculated
using the Coulomb’s equation  , where k is an arbitrary
scaling constant (1000), q1 is the pKa-dependent charge (protonation level) of an
aminoglycoside amino group at a particular pH value calculated using
the Henderson–Hasselbalch equation, q2 is the charge of an rRNA backbone phosphate (fully deprotonated
at pH 6.0–7.4, resulting in −1 e in all cases), D is the dielectric constant of water (80), and r is the distance between the amino group nitrogen and the
phosphate oxygen in the aminoglycoside-bound rRNA crystal structure.
, where k is an arbitrary
scaling constant (1000), q1 is the pKa-dependent charge (protonation level) of an
aminoglycoside amino group at a particular pH value calculated using
the Henderson–Hasselbalch equation, q2 is the charge of an rRNA backbone phosphate (fully deprotonated
at pH 6.0–7.4, resulting in −1 e in all cases), D is the dielectric constant of water (80), and r is the distance between the amino group nitrogen and the
phosphate oxygen in the aminoglycoside-bound rRNA crystal structure.
Calculation of Net Charges
The net positive charge
of all the aminoglycosides in the pH range 4–12 were calculated
using the equation 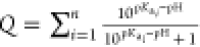 derived from the Henderson–Hasselbalch
equation, where pKai is the
average pKa value of each amino group
measured by 15N–1H HMBC and 1H NMR experiments.
derived from the Henderson–Hasselbalch
equation, where pKai is the
average pKa value of each amino group
measured by 15N–1H HMBC and 1H NMR experiments.
Ribosomal Inhibition Assays
Cell-free in vitro translation inhibition assays were performed as described previously using bacterial S30 extracts and luciferase mRNA.23 In brief, firefly luciferase mRNA was transcribed in vitro using a T7 RNA polymerase. Each reaction comprised bacterial S30 extract, 0.2 mM amino acid mix, 6 μg of tRNA, 0.4 μg of hFluc mRNA, 0.3 μL of protease inhibitor, 12 U of RNase inhibitor, and 6 μL of S30 premix without amino acids, plus a defined concentration of aminoglycoside antibiotic. After 1 h of incubation at 37 °C, the reaction was stopped on ice, 75 μL of luciferase assay reagent was added to each well, and the luminescence recorded.
Bacterial Inhibition Assays
The MICs of aminoglycosides for E. coli strain ATCC 25922, Klebsiella pneumoniae clinical isolate AG215, and P. aeruginosa strain ATCC 27853 were determined by broth microdilution assays according to CLSI reference methodology M07 and as described previously.5 The MIC values plotted represent the geometric mean of n ≥ 3 replicate values for each aminoglycoside.
Acknowledgments
Some of the research leading to these results was conducted as part of the ND4BB European Gram-Negative Antibacterial Engine (ENABLE) Consortium (www.nd4bb-enable.eu) and has received funding from the Innovative Medicines Initiative Joint Undertaking (www.imi.europa.eu) under grant agreement no. 115583, resources of which are composed of financial contribution from the European Union’s Seventh Framework Programme (FP7/2007-2013) and The European Federation of Pharmaceutical Industries and Associations (EFPIA) companies in-kind contribution. The ENABLE project is also financially supported by contributions from Academic and Small and medium-sized enterprise (SME) partners.
Supporting Information Available
The Supporting Information is available free of charge at https://pubs.acs.org/doi/10.1021/acsomega.3c09226.
1H and 13C NMR chemical shift assignments for all compounds and chemical structure of an aminoglycoside derivative combining the central DOS moiety with the N-6′ amine-containing nebrosamine from tobramycin and the N-3′-containing garosamine from gentamicin (PDF)
The authors declare no competing financial interest.
Supplementary Material
References
- Armstrong E. S.; Kostrub C. F.; Cass R. T.; Moser H. E.; Serio A. W.; Miller G. H.. Aminoglycosides. In Antibiotic Discovery and Development; Dougherty T. J., Pucci M. J., Eds.; Springer US: Boston, MA, 2012; pp 229–269. [Google Scholar]
- Thy M.; Timsit J.-F.; De Montmollin E. Aminoglycosides for the Treatment of Severe Infection Due to Resistant Gram-Negative Pathogens. Antibiotics 2023, 12 (5), 860. 10.3390/antibiotics12050860. [DOI] [PMC free article] [PubMed] [Google Scholar]
- Matt T.; Ng C. L.; Lang K.; Sha S.-H.; Akbergenov R.; Shcherbakov D.; Meyer M.; Duscha S.; Xie J.; Dubbaka S. R.; Perez-Fernandez D.; Vasella A.; Ramakrishnan V.; Schacht J.; Böttger E. C. Dissociation of Antibacterial Activity and Aminoglycoside Ototoxicity in the 4-Monosubstituted 2-Deoxystreptamine Apramycin. Proc. Natl. Acad. Sci. U.S.A. 2012, 109 (27), 10984–10989. 10.1073/pnas.1204073109. [DOI] [PMC free article] [PubMed] [Google Scholar]
- Juhas M.; Widlake E.; Teo J.; Huseby D. L.; Tyrrell J. M.; Polikanov Y. S.; Ercan O.; Petersson A.; Cao S.; Aboklaish A. F.; Rominski A.; Crich D.; Böttger E. C.; Walsh T. R.; Hughes D.; Hobbie S. N. In Vitro Activity of Apramycin against Multidrug-Carbapenem- and Aminoglycoside-Resistant Enterobacteriaceae and Acinetobacter Baumannii. J. Antimicrob. Chemother. 2019, 74 (4), 944–952. 10.1093/jac/dky546. [DOI] [PMC free article] [PubMed] [Google Scholar]
- Zhao C.; Chirkova A.; Rosenborg S.; Palma Villar R.; Lindberg J.; Hobbie S. N.; Friberg L. E. Population Pharmacokinetics of Apramycin from First-in-Human Plasma and Urine Data to Support Prediction of Efficacious Dose. J. Antimicrob. Chemother. 2022, 77 (10), 2718–2728. 10.1093/jac/dkac225. [DOI] [PMC free article] [PubMed] [Google Scholar]
- Kondo S.; Hotta K. Semisynthetic Aminoglycoside Antibiotics: Development and Enzymatic Modifications. J. Infect. Chemother. 1999, 5 (1), 1–9. 10.1007/s101560050001. [DOI] [PubMed] [Google Scholar]
- Abdul-Mutakabbir J. C.; Kebriaei R.; Jorgensen S. C. J.; Rybak M. J. Teaching an Old Class New Tricks: A Novel Semi-Synthetic Aminoglycoside, Plazomicin. Infect. Dis. Ther. 2019, 8 (2), 155–170. 10.1007/s40121-019-0239-0. [DOI] [PMC free article] [PubMed] [Google Scholar]
- Carter A. P.; Clemons W. M.; Brodersen D. E.; Morgan-Warren R. J.; Wimberly B. T.; Ramakrishnan V. Functional Insights from the Structure of the 30S Ribosomal Subunit and Its Interactions with Antibiotics. Nature 2000, 407 (6802), 340–348. 10.1038/35030019. [DOI] [PubMed] [Google Scholar]
- Ogle J. M.; Brodersen D. E.; Clemons W. M.; Tarry M. J.; Carter A. P.; Ramakrishnan V. Recognition of Cognate Transfer RNA by the 30 S Ribosomal Subunit. Science 2001, 292 (5518), 897–902. 10.1126/science.1060612. [DOI] [PubMed] [Google Scholar]
- Lin J.; Zhou D.; Steitz T. A.; Polikanov Y. S.; Gagnon M. G. Ribosome-Targeting Antibiotics: Modes of Action, Mechanisms of Resistance, and Implications for Drug Design. Annu. Rev. Biochem. 2018, 87 (1), 451–478. 10.1146/annurev-biochem-062917-011942. [DOI] [PMC free article] [PubMed] [Google Scholar]
- Kaul M.; Barbieri C. M.; Kerrigan J. E.; Pilch D. S. Coupling of Drug Protonation to the Specific Binding of Aminoglycosides to the A Site of 16S rRNA: Elucidation of the Number of Drug Amino Groups Involved and Their Identities. J. Mol. Biol. 2003, 326 (5), 1373–1387. 10.1016/S0022-2836(02)01452-3. [DOI] [PubMed] [Google Scholar]
- Kulik M.; Goral A. M.; Jasiński M.; Dominiak P. M.; Trylska J. Electrostatic Interactions in Aminoglycoside-RNA Complexes. Biophys. J. 2015, 108 (3), 655–665. 10.1016/j.bpj.2014.12.020. [DOI] [PMC free article] [PubMed] [Google Scholar]
- Krause K. M.; Serio A. W.; Kane T. R.; Connolly L. E. Aminoglycosides: An Overview. Cold Spring Harbor Perspect. Med. 2016, 6 (6), a027029. 10.1101/cshperspect.a027029. [DOI] [PMC free article] [PubMed] [Google Scholar]
- Botto R. E.; Coxon B. Nitrogen-15 Nuclear Magnetic Resonance Spectroscopy of Neomycin B and Related Aminoglycosides. J. Am. Chem. Soc. 1983, 105 (4), 1021–1028. 10.1021/ja00342a062. [DOI] [Google Scholar]
- Dorman D. E.; Paschal J. W.; Merkel K. E. Nitrogen-15 Nuclear Magnetic Resonance Spectroscopy. The Nebramycin Aminoglycosides. J. Am. Chem. Soc. 1976, 98 (22), 6885–6888. 10.1021/ja00438a020. [DOI] [PubMed] [Google Scholar]
- Paschal J. W.; Dorman D. E. Determination of pKa Values using15N and13C Nuclear Magnetic Resonance Spectroscopy. The Case of Apramycin. Org. Magn. Reson. 1978, 11 (12), 632–634. 10.1002/mrc.1270111210. [DOI] [Google Scholar]
- DiGiammarino E. L.; Draker K.; Wright G. D.; Serpersu E. H. Solution Studies of Isepamicin and Conformational Comparisons between Isepamicin and Butirosin A When Bound to an Aminoglycoside 6‘- N -Acetyltransferase Determined by NMR Spectroscopy. Biochemistry 1998, 37 (11), 3638–3644. 10.1021/bi972778b. [DOI] [PubMed] [Google Scholar]
- Cox J. R.; Ekman D. R.; DiGiammarino E. L.; Akal-Strader A.; Serpersu E. H. Aminoglycoside Antibiotics Bound to Aminoglycoside-Detoxifying Enzymes and RNA Adopt Similar Conformations. Cell Biochem. Biophys. 2000, 33 (3), 297–308. 10.1385/CBB:33:3:297. [DOI] [PubMed] [Google Scholar]
- Pagano T. G.; Gong Y.; Kong F.; Tsao R.; Fawzi M.; Zhu T. Structural Characterization of the Tobramycin-Piperacillin Reaction Product Formed at pH 6.0. J. Antibiot. 2011, 64 (10), 673–677. 10.1038/ja.2011.72. [DOI] [PubMed] [Google Scholar]
- Alkhzem A. H.; Woodman T. J.; Blagbrough I. S. Individual p K a Values of Tobramycin, Kanamycin B, Amikacin, Sisomicin, and Netilmicin Determined by Multinuclear NMR Spectroscopy. ACS Omega 2020, 5 (33), 21094–21103. 10.1021/acsomega.0c02744. [DOI] [PMC free article] [PubMed] [Google Scholar]
- Alkhzem A. H.; Woodman T. J.; Blagbrough I. S. Multinuclear Nuclear Magnetic Resonance Spectroscopy Is Used to Determine Rapidly and Accurately the Individual p K a Values of 2-Deoxystreptamine, Neamine, Neomycin, Paromomycin, and Streptomycin. ACS Omega 2021, 6 (4), 2824–2835. 10.1021/acsomega.0c05138. [DOI] [PMC free article] [PubMed] [Google Scholar]
- Jana S.; Rajasekaran P.; Haldimann K.; Vasella A.; Böttger E. C.; Hobbie S. N.; Crich D. Synthesis of Gentamicins C1, C2, and C2a and Antiribosomal and Antibacterial Activity of Gentamicins B1, C1, C1a, C2, C2a, C2b, and X2. ACS Infect. Dis. 2023, 9 (8), 1622–1633. 10.1021/acsinfecdis.3c00233. [DOI] [PMC free article] [PubMed] [Google Scholar]
- Lesniak W.; Laren J. M.; Harris W. R.; Pecoraro V. L.; Schacht J. An Isocratic Separation of Underivatized Gentamicin Components, 1H NMR Assignment and Protonation Pattern. Carbohydr. Res. 2003, 338 (24), 2853–2862. 10.1016/j.carres.2003.08.005. [DOI] [PubMed] [Google Scholar]
- Fusetani N.; Ejima D.; Matsunaga S.; Hashimoto K.; Itagaki K.; Akagi Y.; Taga N.; Suzuki K. 3-Amino-3-Deoxy-D-Glucose: An Antibiotic Produced by a Deep-Sea Bacterium. Experientia 1987, 43 (4), 464–465. 10.1007/BF01940457. [DOI] [PubMed] [Google Scholar]
- Cox J. R.; Serpersu E. H. Biologically Important Conformations of Aminoglycoside Antibiotics Bound to an Aminoglycoside 3‘-Phosphotransferase as Determined by Transferred Nuclear Overhauser Effect Spectroscopy. Biochemistry 1997, 36 (9), 2353–2359. 10.1021/bi9626822. [DOI] [PubMed] [Google Scholar]
- Morgenthaler M.; Schweizer E.; Hoffmann-Röder A.; Benini F.; Martin R. E.; Jaeschke G.; Wagner B.; Fischer H.; Bendels S.; Zimmerli D.; Schneider J.; Diederich F.; Kansy M.; Müller K. Predicting and Tuning Physicochemical Properties in Lead Optimization: Amine Basicities. ChemMedChem 2007, 2 (8), 1100–1115. 10.1002/cmdc.200700059. [DOI] [PubMed] [Google Scholar]
- Taber H. W.; Mueller J. P.; Miller P. F.; Arrow A. S. Bacterial Uptake of Aminoglycoside Antibiotics. Microbiol. Rev. 1987, 51 (4), 439–457. 10.1128/mr.51.4.439-457.1987. [DOI] [PMC free article] [PubMed] [Google Scholar]
- Crabbé A.; Ostyn L.; Staelens S.; Rigauts C.; Risseeuw M.; Dhaenens M.; Daled S.; Van Acker H.; Deforce D.; Van Calenbergh S.; Coenye T. Host Metabolites Stimulate the Bacterial Proton Motive Force to Enhance the Activity of Aminoglycoside Antibiotics. PLoS Pathog. 2019, 15 (4), e1007697 10.1371/journal.ppat.1007697. [DOI] [PMC free article] [PubMed] [Google Scholar]
- Webster C. M.; Woody A. M.; Fusseini S.; Holmes L. G.; Robinson G. K.; Shepherd M. Proton Motive Force Underpins Respiration-Mediated Potentiation of Aminoglycoside Lethality in Pathogenic Escherichia Coli. Arch. Microbiol. 2022, 204 (1), 120. 10.1007/s00203-021-02710-y. [DOI] [PMC free article] [PubMed] [Google Scholar]
- Minuth J. N.; Musher D. M.; Thorsteinsson S. B. Inhibition of the Antibacterial Activity of Gentamicin by Urine. J. Infect. Dis. 1976, 133 (1), 14–21. 10.1093/infdis/133.1.14. [DOI] [PubMed] [Google Scholar]
- Yang L.; Wang K.; Li H.; Denstedt J. D.; Cadieux P. A. The Influence of Urinary pH on Antibiotic Efficacy Against Bacterial Uropathogens. Urology 2014, 84 (3), 731.e1–731.e7. 10.1016/j.urology.2014.04.048. [DOI] [PubMed] [Google Scholar]
- Eisenberg E. S.; Mandel L. J.; Kaback H. R.; Miller M. H. Quantitative Association between Electrical Potential across the Cytoplasmic Membrane and Early Gentamicin Uptake and Killing in Staphylococcus Aureus. J. Bacteriol. 1984, 157 (3), 863–867. 10.1128/jb.157.3.863-867.1984. [DOI] [PMC free article] [PubMed] [Google Scholar]
- Becker K.; Cao S.; Nilsson A.; Erlandsson M.; Hotop S.-K.; Kuka J.; Hansen J.; Haldimann K.; Grinberga S.; Berruga-Fernández T.; Huseby D. L.; Shariatgorji R.; Lindmark E.; Platzack B.; Böttger E. C.; Crich D.; Friberg L. E.; Vingsbo Lundberg C.; Hughes D.; Brönstrup M.; Andrén P. E.; Liepinsh E.; Hobbie S. N. Antibacterial Activity of Apramycin at Acidic pH Warrants Wide Therapeutic Window in the Treatment of Complicated Urinary Tract Infections and Acute Pyelonephritis. EBioMedicine 2021, 73, 103652. 10.1016/j.ebiom.2021.103652. [DOI] [PMC free article] [PubMed] [Google Scholar]
- Mandhapati A. R.; Yang G.; Kato T.; Shcherbakov D.; Hobbie S. N.; Vasella A.; Böttger E. C.; Crich D. Structure-Based Design and Synthesis of Apramycin-Paromomycin Analogues: Importance of the Configuration at the 6′-Position and Differences between the 6′-Amino and Hydroxy Series. J. Am. Chem. Soc. 2017, 139 (41), 14611–14619. 10.1021/jacs.7b07754. [DOI] [PMC free article] [PubMed] [Google Scholar]
- Quirke J. C. K.; Sati G. C.; Sonousi A.; Gysin M.; Haldimann K.; Böttger E. C.; Vasella A.; Hobbie S. N.; Crich D. Structure Activity Relationships for 5″ Modifications of 4,5 Aminoglycoside Antibiotics. ChemMedChem 2022, 17 (13), e202200120 10.1002/cmdc.202200120. [DOI] [PMC free article] [PubMed] [Google Scholar]
- Perez-Fernandez D.; Shcherbakov D.; Matt T.; Leong N. C.; Kudyba I.; Duscha S.; Boukari H.; Patak R.; Dubbaka S. R.; Lang K.; Meyer M.; Akbergenov R.; Freihofer P.; Vaddi S.; Thommes P.; Ramakrishnan V.; Vasella A.; Böttger E. C. 4′-O-Substitutions Determine Selectivity of Aminoglycoside Antibiotics. Nat. Commun. 2014, 5 (1), 3112. 10.1038/ncomms4112. [DOI] [PMC free article] [PubMed] [Google Scholar]
- Kugelman M.; Mallams A. K.; Vernay H. F. Semisynthetic Aminoglycoside Antibacterials. Part IV. Synthesis of Antibiotic JI-20A, Gentamicin B, and Related Compounds. J. Chem. Soc., Perkin Trans. 1 1976, (10), 1126–1134. 10.1039/p19760001126. [DOI] [PubMed] [Google Scholar]
- Mallams A. K.Pseudodi- und trisaccharide und Verfahren zu ihrer Herstellung. DE 2349974 A1, 1974.
- Corzana F.; Cuesta I.; Freire F.; Revuelta J.; Torrado M.; Bastida A.; Jiménez-Barbero J.; Asensio J. L. The Pattern of Distribution of Amino Groups Modulates the Structure and Dynamics of Natural Aminoglycosides: Implications for RNA Recognition. J. Am. Chem. Soc. 2007, 129 (10), 2849–2865. 10.1021/ja066348x. [DOI] [PubMed] [Google Scholar]
- Pirrone M. G.; Ande C.; Haldimann K.; Hobbie S. N.; Vasella A.; Böttger E. C.; Crich D. Importance of Co operative Hydrogen Bonding in the Apramycin Ribosomal Decoding A Site Interaction. ChemMedChem 2023, 18 (1), e202200486 10.1002/cmdc.202200486. [DOI] [PMC free article] [PubMed] [Google Scholar]
- Popov K.; Rönkkömäki H.; Lajunen L. H. J. Guidelines for NMR Measurements for Determination of High and Low pKa Values (IUPAC Technical Report). Pure Appl. Chem. 2006, 78 (3), 663–675. 10.1351/pac200678030663. [DOI] [Google Scholar]
- Suwannahong K.; Wongcharee S.; Kreetachart T.; Sirilamduan C.; Rioyo J.; Wongphat A. Evaluation of the Microsoft Excel Solver Spreadsheet-Based Program for Nonlinear Expressions of Adsorption Isotherm Models onto Magnetic Nanosorbent. Appl. Sci. 2021, 11 (16), 7432. 10.3390/app11167432. [DOI] [Google Scholar]
- Vicens Q.; Westhof E. Crystal Structure of a Complex between the Aminoglycoside Tobramycin and an Oligonucleotide Containing the Ribosomal Decoding A Site. Chem. Biol. 2002, 9 (6), 747–755. 10.1016/S1074-5521(02)00153-9. [DOI] [PubMed] [Google Scholar]
- Francois B. Crystal Structures of Complexes between Aminoglycosides and Decoding A Site Oligonucleotides: Role of the Number of Rings and Positive Charges in the Specific Binding Leading to Miscoding. Nucleic Acids Res. 2005, 33 (17), 5677–5690. 10.1093/nar/gki862. [DOI] [PMC free article] [PubMed] [Google Scholar]
- Kondo J.; Francois B.; Russell R.; Murray J.; Westhof E. Crystal Structure of the Bacterial Ribosomal Decoding Site Complexed with Amikacin Containing the γ-Amino-α-Hydroxybutyryl (Haba) Group. Biochimie 2006, 88 (8), 1027–1031. 10.1016/j.biochi.2006.05.017. [DOI] [PubMed] [Google Scholar]
Associated Data
This section collects any data citations, data availability statements, or supplementary materials included in this article.