Abstract
Liver fat storage, also called hepatic steatosis, is increasingly common and represents a very frequent diagnosis in the medical field. Excess fat is not without consequences. In fact, hepatic steatosis contributes to the progression toward liver fibrosis. There are two main types of fatty liver disease, alcoholic fatty liver disease (AFLD) and nonalcoholic fatty liver disease (NAFLD). Although AFLD and NAFLD are similar in their initial morphological features, both conditions involve the same evolutive forms. Moreover, there are various common mechanisms underlying both diseases, including alcoholic liver disease and NAFLD, which are commonalities. In this Review, the authors explore similar downstream signaling events involved in the onset and progression of the two entities but not completely different entities, predominantly focusing on the gut microbiome. Downstream molecular events, such as the roles of sirtuins, cytokeratins, adipokines and others, should be considered. Finally, to complete the feature, some new tendencies in the therapeutic approach are presented.
Keywords: Hepatic steatosis, Alcoholic liver disease, Nonalcoholic fatty liver disease, Microbiome, Adipokines, Cytokeratins, Sirtuins, Spleen, Patatin-like phospholipase domain-containing proteins, miRs
Introduction
Fatty liver or hepatic steatosis is increasingly diagnosed worldwide. In addition to histology, noninvasive techniques, such as ultrasound, computerized tomography magnetic resonance imaging and proton magnetic resonance spectroscopy, can be used to detect this disease [1]. A Japanese study showed that in 1989, the prevalence of fatty liver was 12.6%, which reached 30.3% in 1998, corresponding to a 2.4-fold increase over the previous rate; this figure was twice as high in males (26.0%) as in females (12.7%) [2]. Its main characteristic is the accumulation of triacylglycerol-rich macrovesicular and/or microvesicular lipid droplets within hepatocytes. This fat storage is not without consequences. In fact, hepatic steatosis contributes to the progression toward liver fibrosis [3]. There are two main types of fatty liver disease, alcoholic fatty liver disease (AFLD) and nonalcoholic fatty liver disease (NAFLD).
In recent years, there have been many discussions about the nomenclature of fatty liver disease among experts. In 2020, an international expert consensus recommended a new definition, MAFLD, which is metabolic dysfunction-associated fatty liver disease and represents the central concept of metabolic dysfunction [4]. The fatty liver disease terminology was also changed as of June 2023. MASLD has replaced NAFLD. To clarify the suggested nomenclature, MASLD can be diagnosed based on the presence of one of five cardiovascular risk factors, unlike MAFLD, which requires patients to have two of seven parameters of metabolic dysfunction [5]. The MASLD score is a very useful definition for lean patients with NAFLD [6]. Interestingly, patients who meet both the MASLD and alcohol-related fatty liver disease (ALD) criteria are categorized as having MetALD to include those with MASLD who consume greater amounts of alcohol per week (140 g/week and 210 g/week for females and males, respectively) [7]. Another necessary change in terminology was proposed, i.e., metabolic dysfunction-associated steatohepatitis (MASH), as a replacement for NASH to avoid the use of exclusionary confounder terms and potentially stigmatizing language [8]. A recent study revealed that the new nomenclature MASLD almost always confirmed NAFLD, while there were significant differences among MASLD, MetALD and MAFLD [9]. With respect to the various diagnostic terms used by patients, there were no substantial distinctions between “NAFLD”, “fatty liver disease”, “NASH”, or “MAFLD”. Furthermore, regarding the new nomenclature MASLD, the percentage of providers reporting “steatotic liver disease” as stigmatizing was low (14%) [10].
Finally, it has been confirmed that the discrepancies between NAFLD and MASLD criteria are negligible [11]. Based on such considerations and because most of the discussed studies, mainly preclinical studies, still illustrate the disease as NAFLD, we will employ such a definition throughout the manuscript, except when the pieces of research address MAFLD or MASLD.
AFLD and NAFLD exhibit the same progressive histological manifestations, i.e., steatohepatitis and cirrhosis, but the natural history of alcoholic liver disease (ALD) is often dissimilar from that of NAFLD in terms of clinical aspects [12]. In a registry-based study from 2003, the mortality of patients with a diagnosis of fatty liver increased 5.4-fold (95% CI 5.2–5.6) in patients with ALD and 2.6-fold (95% CI 2.4–2.9) in patients with NAFLD or unspecified fatty liver [13]. Currently, the scenarios are completely different. In fact, with high rates of obesity (likely one of the most important drivers) and type 2 diabetes mellitus (T2DM) among adult patients, which are associated with an aging population, NAFLD mortality is reported to steadily increase. Specifically, in the United States, the estimated incidence of hepatocarcinoma, the most severe sequela of NAFLD, will increase by 137% in 2030 [14]. According to 2017–2018 data from the National Health and Nutrition Examination Survey, more than 2 in 5 adults (42.4%) were obese, and approximately 1 in 11 adults (9.2%) had severe obesity [15]. The interaction of the changing food context combined with other environmental factors and genetic predispositions has created the current obesity pandemic, but the underlying mechanism has not been fully elucidated, resulting in many unanswered questions [16]. The fact that NAFLD and metabolic syndrome, with their main components, i.e., central obesity, abnormal glycemic values, dyslipidemia and hypertension, are intertwined is a finding dating back many years [17, 18]. However, the reciprocal effects of alcohol consumption and several metabolic syndrome criteria merit great attention in the risk stratification of severe liver complications and mortality [19, 20].
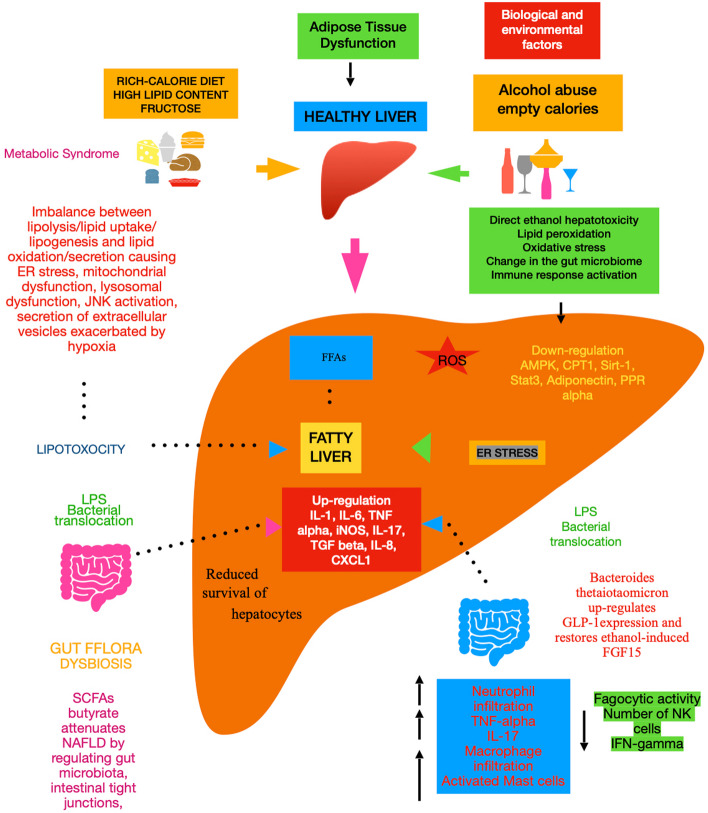
The default position in categorizing the two initially discussed forms of hepatic steatosis and the successive spectra is that there is no relationship between the two entities. In this review, we try to test this null (i.e., zero) hypothesis by examining the biological mechanisms that explain these diseases, drawing some conclusions from our current approach to elicit the potential of new interventions (Fig. 1).
Fig. 1.
Common and different mechanisms of ALD and NAFLD/MASLD. Abbreviations: ER, endoplasmic reticulum; JNK, c-Jun N-terminal kinase; FFAs, free fatty acids; AMPK, AMP-activated protein kinase; ROS, reactive oxygen species; CPT1, carnitine palmitoyltransferase I; Sirt, sirtuin; Stat3, signal transducer and activator of transcription 3; PPR alpha, peroxisome proliferator-activated receptor alpha; IL, interleukin; TNF, tumor necrosis factor; iNOS, inducible nitric oxide synthase; TGF, transforming growth factor; CXCL1, C-X-C motif chemokine ligand 1; GLP, glucagon-like peptide; FGF15, fibroblast growth factor 15; IFN, interferon; NK, natural killer
Characteristics of NAFLD and ALD
Zeroing in on the main factors able to clinically differentiate NAFLD from ALD is essential. Alcohol consumption can be evaluated as alcohol consumption (e.g., drinks per week) or as a lifetime measure (e.g., alcohol dependence). One of the most common criteria for evaluating excess alcohol consumption is drinking more than 21 drinks per week for men and more than 14 drinks per week for women [21]. Indeed, the two liver diseases often coexist. Among 1,216 patients with alcohol-associated acute-on-chronic liver failure (ACFL), overweight/obese status and dyslipidemia increase the severity of alcohol-associated ACLF, and a high BMI augments short-term mortality [22]. Paradoxically, a meta-analysis of 43,175 subjects showed that modest alcohol consumption decreases the risk of NAFLD [23].
With respect to risk factors, it is necessary to stress the differences between the two main entities. The relative risk of ALD is greater in women for any given amount of alcohol intake [24]. Conversely, NAFLD has a greater prevalence in men. Compared with men, women of fertile age are at reduced risk of NAFLD, whereas after menopause, women have a comparable prevalence of NAFLD to men [25]. The proportion of Hispanic and White/Caucasian patients with severe alcoholic hepatitis was similar but was lower for African/American subjects [26]. These racial differences are likely due to genetic differences in the enzymes involved in alcohol metabolism. Accordingly, genome-wide association studies.
GWASs of alcohol consumption in European Americans and African Americans have identified significant associations with the well-known functional locus alcohol dehydrogenase 1B (ADH1B) [27]. Very recently, authors, carrying out a GWAS meta-analysis of imaging and diagnostic code-measured NAFLD across diverse ancestries, identified NAFLD-associated variants at torsin family 1 member B, fat mass and obesity associated, cordon-bleu WH2 repeat protein like 1/growth factor receptor-bound protein 14, insulin receptor, sterol regulatory element-binding transcription factor 1 and patatin-like phospholipase domain-containing protein 2, as well as validated NAFLD-associated variants at patatin-like phospholipase domain-containing protein 3, transmembrane 6 superfamily 2, apolipoprotein E, glucokinase regulator, tribbles homolog 1, glycerol-3-phosphate acyltransferase, mitochondrial amidoxime-reducing component 1 (MARC1), microsomal triglyceride transfer protein large subunit, transmembrane channel like 4/membrane-bound O-acyltransferase domain containing 7 and receptor-type tyrosine-protein phosphatase δ, and, surprisingly, ADH1B [28]. It should be stressed that the aforementioned variants in MARC1 are also novel genetic risk factors for ALD [29].
Now, we attempt to determine the similarities but also differences in histopathology features. Macrovesicular hepatic steatosis is the main feature of NAFLD and is the initial and most common consequence of excessive alcohol consumption due to the impaired intracellular redox potential that leads to the accumulation of lipids within liver cells. In addition, small fat droplets collect around hepatocytes in areas filled with venules, proceeding toward the portal tracts. The first form of ALD, i.e., pure AFLD, may not be a benign condition every time. In fact, when retrospectively analyzing patients with a histologic diagnosis of AFLD without alcoholic steatohepatitis, as many as 18% progressed to fibrosis or cirrhosis when followed for a median of ten years. However, it is necessary to stress that the severe forms were linked to continuing alcohol consumption of more than 40 units per week at follow-up, while in the other patients, consumption was unknown [30], leaving doubts whether the progression could be due to initial liver fat storage. Thus, additional evidence is needed to determine whether hepatic steatosis plays a role in the onset and progression of advanced liver disease.
Eosinophilic fibrillar material (Mallory hyaline or Mallory-Denk bodies that are “damaged intermediate filaments” within liver cells) produces swollen hepatocytes. Ballooned cells are typically two to three times the size of adjacent hepatocytes. This histological feature distinguishes steatohepatitis from simple steatosis. Severe lobular infiltration of polymorphonuclear leukocytes (neutrophils) is abundant in alcoholic steatohepatitis, unlike in other types of hepatitis, such as viral hepatitis, where mononuclear cells are restricted around portal triads. The final stage of ALD is alcoholic cirrhosis, characterized by the deposition of collagen that typically starts around the terminal hepatic vein (perivenular fibrosis) and along the sinusoids, leading to a peculiar “chickenwire” pattern of fibrosis [31]. Some authors insist that individual cases of ALD can be differentiated from NAFLD by the presence of sclerosing hyaline necrosis, fibro-obliterative and inflammatory lesions of the outflow veins, alcoholic foamy degeneration, and acute cholestasis [32]. According to other pathologists, hepatic steatosis is more common in NAFLD patients than in ALD patients. Conversely, inflammatory cell infiltration is more pronounced in ALD than in NAFLD. Furthermore, venous or perivenular fibrosis, phlebosclerosis, and (less commonly) lymphocytic phlebitis are more common in ALD patients than in NAFLD patients [33]. Indeed, upon careful examination of silver impregnation histology, the progressive form of ALD, i.e., steatohepatitis, could be detected as lattice fibrosis in NASH and solid fibrosis in ALD. In addition to previous findings, megamitochondria, bile stasis, hemosiderin deposition, vacuolic nuclei, and lipogranuromas were less common in ALDs [34]. Overall, there are subtle differences between NAFLD and ALD, even though they are more advanced (Table 1). For this reason, distinguishing one from the other is difficult, according to the classic study by Ludwig [35].
Table 1.
Main features of alcoholic and nonalcoholic fatty liver disease at histology
| Alcoholic Liver Disease | Non Alcoholic Fatty Liver Disease |
|---|---|
| Macrovescicular steatosis is largely represented | Macrovescicular steatosis is less represented |
| Mallory hyaline is recurrent | Mallory hyaline is not occurring often |
| Swollen hepatocytes/Ballooned cells are more frequent | Swollen hepatocytes/Ballooned cells are less present |
| Lobular infiltration of polymorphonuclear leukocytes (neutrophils) is severe | Usually, there is mild lobular infiltration with foci of mononuclear cell clusters, and occasional eosinophils or neutrophils. |
| Inflammatory cell infiltration is more pronounced | Inflammatory cell infiltration is less marked |
| Perivenular fibrosis with the chicken wire” pattern of fibrosis is common | Fibrosis typically begins in zone 3 with the characteristic pericellular “chicken wire” pattern |
| Fibro-obliterative/inflammatory lesions of the outflow veins, alcoholic foamy degeneration are present | Fibro-obliterative lesions are not constant and foamy degeneration is rare |
| Acute cholestasis is often present | Intrahepatic cholestasis is associated with more advanced histological impairment |
| Phlebosclerosis, and (less commonly) lymphocytic phlebitis are present | Phlebosclerosis is rare |
| There is solid fibrosis | There is lattice fibrosis |
| Megamitochondria, bile stasis, hemosiderin deposition, vacuolic nuclei, and lipogranuroma are scarcely represented | Megamitochondria, bile stasis, hemosiderin deposition, vacuolic nuclei, and lipogranuroma are more often represented |
| Bridging necrosis is frequent | Bridging necrosis is rare |
| Fibrosis/cirrhosis is more frequent | Fibrosis/cirrhosis is less frequent |
The features at histology overlap, and it is not easy to clearly separate the two entities
Common molecular events
Lipid metabolism alterations
As previously described, the initial process characterizing hepatic steatosis consists of lipid droplets that are enriched in saturated triglycerides and result in the typical histological pattern of steatosis common to both NAFLD and AFLD [36]. Liver fat storage originates from a mismatch of fatty acid uptake (either by nutrients or from adipose tissue), fatty acid synthesis, fatty acid oxidation, or the export of lipids from the liver through effects on apolipoprotein B and microsomal transport protein [37]. Zeroing on lipid synthesis, sterol regulatory element-binding protein (SREBP) 1 plays a central role in NAFLD but not only [38]. In fact, activation of SREBP-1 by ethanol feeding was associated with increased expression of hepatic lipogenic genes as well as the accumulation of triglycerides in the liver in both the rat H4IIEC3 and McA-RH7777 hepatoma cell lines [39]. However, downstream signaling events involving transcription factor-regulating genes involved in lipid synthesis and the endoplasmic reticulum (ER) stress response are present in both NAFLD and ALD in the context of fatty acid oxidation mediated by AMP-activated protein kinase (AMPK), adiponectin, and peroxisome proliferator-activated receptors (PPARs) [40]. Interestingly, findings suggest that activation of this latter pathway may improve NAFLD due to the intrinsic link between NAFLD and mitochondrial metabolism and the mechanisms by which mitochondrial dysfunctions contribute to NAFLD progression [41].
Redox imbalance
Importantly, another central effect of ethanol on lipids is to activate (e.g., via ER stress, tumor necrosis factor (TNF)-alpha and/or hepatic PPAR-gamma) de novo lipogenesis (DNL) while concomitantly inhibiting processes that block this response, such as AMP-activated protein kinase (AMPK) and sirtuin (SIRT) 1 [42]. As a significant event, ROS production and oxidative stress in liver cells play pivotal roles in the development of ALD [43]. Confirming the importance of oxidative stress in ALD, the authors showed that Humulus japonicus extract decreases ethanol-induced lipid accumulation, lipogenic protein expression, and cellular oxidative stress in hepatocytes [44]. A further epiphenomenon induced by triglyceride excess and the accumulation of other lipids is the generation of insulin resistance in both NAFLD [45] and AFLD [46].
The key molecular event represented by DNL is also central to NAFLD. DNL was measured isotopically and was significantly associated with liver fatty acid synthase protein content, total steatosis assessed by histology, and the fraction of DNL fatty acids in plasma very low-density lipoprotein-triacylglycerol in 49 patients scheduled for bariatric surgery [47].
The main role of adipokines
It is well known that adiponectin lowers insulin resistance by decreasing triglyceride content in the muscle and liver in obese mice [48]. In this context, the role of adiponectin, among other mechanisms, needs to be clarified (Table 2). In fact, previous findings have shown that altered adiponectin production in adipose tissue and impaired expression of hepatic adiponectin receptors are associated with the development of AFLD in several rodent models [49]. Similarly, adiponectin generally predicts steatosis grade and the severity of NAFLD, although to what extent this is a direct effect or related to the presence of more severe insulin resistance or obesity remains to be addressed [50]. In vitro and in vivo studies have shown that another crucial adipokine, gremlin-1, promotes hepatic ER stress through the impairment of autophagy, which we briefly focus on, ultimately causing hepatic steatosis in the obese state [51].
Table 2.
Signaling events of alcoholic liver disease and nonalcoholic fatty liver disease
| Alcoholic Liver Disease | Non Alcoholic Liver Disease |
|---|---|
| SCFAs are decreased | Fecal SCFAs are increased |
| Secondary BAs are high acting on farnesoid X receptor | BAs binds to the farnesoid X receptor increasing inflammation and fibrosis |
| Lactobacillaceae correlates with postprandial peripheral ethanol | It is evident a reduction in the abundance of Ruminococcus gnavus and Bifidobacterium longum subsp infantis |
| Klebsiella pneumoniae strain W14 bears the higher ethanol production | Some forms of NAFLD are due to endogenous production of ethanol |
| Chronic intake of alcohol beyond provoking ROS formation raises the multiplication of intestinal bacteria and changes the gut permeability | High-alcohol-producing Klebsiella pneumoniae is associated with up to 60% of individuals with NAFLD in a Chinese cohort. |
| Pathogenic species of bacteria results in a fast replenishment of the marginal zone of the spleen | The spleen has been recognized to take a great part in lipid metabolism via the mononuclear phagocytic system |
| Functional hyposplenism is present | There is a trend in increased spleen volume |
| K18 is the main component of Mallory-Denk bodies, which are a hallmark of alcoholic steatohepatitis | K18 fragments have been proven to be associated with NASH |
| Serum TPS, mirroring K18, is frequently increased in alcoholics and may be a marker of alcoholic hepatitis | TPS is a better marker for differentiating NASH from simple fatty liver |
| Ethanol-induced oxidative stress directly downregulates NAD + levels, increases SIRT1 nucleocytoplasmic shuttling, and ultimately inhibits SIRT1 gene and protein expression levels in the liver | SIRT1 activator E1231, alleviated NAFLD induced in C57BL/6J mice fed a high-fat and high-cholesterol diet and improved liver injury by regulating the SIRT1-AMPK alpha pathway |
| SIRT 4 is strongly associated with heavy drinker | Low circulating levels of SIRT4 are found in obese patients with NAFLD |
| There is a close relationship between SIRT7 and age-related processes | SIRT7 is linked to age-related processes |
| Impaired expression of hepatic adiponectin receptors are associated with AFLD | Adiponectin predicts steatosis grade and the severity of NAFLD |
There are more common than dissimilar mechanisms involved
Abbreviations: BA biliary acid, TPS polypeptide-specific antigen, K18 karykeratin 18, ROS reactive oxygen species, SCFAs short-chain fatty acids, SIRT sirtuin, AFLD alcoholic fatty liver disease, NAFLD nonalcoholic fatty liver disease, NASH nonalcoholic steatohepatitis, NAD nicotinamide adenine dinucleotide, AMPK adenosine monophosphate-activated protein kinase
Cellular senescence
It is necessary to stress which molecular processes could be involved in the pathogenesis of ALD and NAFLD, in addition to canonical insulin resistance, mitochondrial dysfunction, and adipokine imbalance. Cellular senescence, better defined as a stress-responsive program, limits the proliferation of damaged cells by inducing telomere shortening, enlargement of the nuclear area and genomic and mitochondrial DNA damage, ultimately leading to irreversible cell cycle arrest and the secretion of proinflammatory cytokines. Accordingly, dysfunctional lysosomes, a hallmark of cellular senescence, contribute to lipid droplet accumulation in fatty liver disease [52]. Recent results highlight that plasminogen activator inhibitor-1 and early growth response protein 1 are important regulators of lipopolysaccharide-induced cellular senescence and alcoholic hepatitis [53]. Another fascinating study showed that a novel factor, zinc‐finger protein 281, is a driver of hepatocyte senescence during ALD by reducing hexokinase II-stabilized PTEN-induced kinase 1/Parkin-mediated mitophagy [54]. Returning to lysosomes, chronic ethanol consumption disrupts hepatic biogenesis and inhibits the ubiquitin‒proteasome system by impeding hepatic proteasome activity [55]. In line with previous results, several human and animal studies have shown the involvement of senescence in determining NAFLD [56]. Very recently, restoration of lysosomal acidification was shown to rescue autophagy, which shares the same characteristics as senescence and metabolic dysfunction in NAFLD [57]. This field of research could help discover novel therapeutic targets for halting NAFLD progression.
Main pathogenetic mechanisms of ALD
In addition to the downstream molecular events of ALD, ethanol oxidative metabolism influences intracellular signaling pathways and disrupts the transcriptional control of several genes, leading to fat accumulation and fibrogenesis [58]. Specifically, the role of microsomal ethanol oxidizing system, i.e., P4502E1 is central [59]. In parallel with NAFLD, there was also a marked increase in hepatic CYP2E1 activity in obese T2DM patients, one of the most common risk factors for NAFLD, as assessed by chlorzoxazone administration [60]. Furthermore, alcohol and its metabolites initiate and aggravate inflammatory conditions by sensitizing immune cells to stimulation and by activating innate immune pathways [61]. By decreasing the production of proinflammatory cytokines, chronic exposure to ethanol leads to the translocation of LPS from the gut, activating Kupffer cells through Toll-like receptor 4 (TLR4) and overexpressing IL-1 and TNF-alpha. These cytokines contribute to hepatocyte dysfunction and programmed cell death, as well as to the activation of hepatic stellate cells (HSCs), which generate extracellular matrix proteins leading to fibrosis development [62].
IL-6 plays dual roles in the pathogenesis of ALD by not only favoring inflammation and injury but also promoting liver regeneration [63]. In fact, IL-6 deficiency in mice has been shown to protect against ethanol-induced oxidative stress and mitochondrial dysfunction in hepatocytes via the induction of metallothionein protein expression [64]. This occurs at least in the early phase of ALD. Conversely, in alcoholic liver cirrhosis, increased serum levels and spontaneous or induced production of IL-6 by peripheral blood monoclonal cells have been found [65]. IL-8, also known as CXCL8, and CXCL1 promote liver inflammation and injury by stimulating neutrophil infiltration [66]. After the progression of ALD, mechanistically, acetaldehyde, a major toxic metabolite of ethanol, causes adduct formation, representing one of the principal causes of the fibrogenic and mutagenic effects of alcohol in the liver [67]. Malondialdehyde and acetaldehyde react with proteins in a synergistic manner and form hybrid protein adducts, playing an important role in the pathogenesis of alcoholic liver injury [68]. Finally, ALD is associated with the generation of IgAs and IgGs against acetaldehyde-derived antigens [69]. These last molecular initiation events fundamentally differ from those of NAFLD.
Inflammation and NAFLD, recently renamed MASLD
The mechanisms underlying MAFLD involve intertwined processes involving lipotoxicity and infiltration of proinflammatory cells with cytokine/chemokine overexpression, resulting in HSC activation and fibrogenesis [70]. However, the interaction between mitochondrial dysfunction and insulin resistance has also been typically described in the context of NAFLD, and this mechanism is being increasingly regarded as pivotal for leading to hepatic steatosis [71]. Dysregulated mitochondrial oxidative metabolism mediates inflammation and liver fibrosis [72]. Coming back to inflammation/immune system involvement as a link between excess fat storage in the liver and NAFLD progression, authors used an animal model of NASH and found increased serum concentrations of TNF-alpha, TLR4 and CD14 in male F344 rats that were fed a choline-deficient L-amino-acid-defined diet [73]. Similarly, in 52 obese patients, a remarkable increase in the expression of TNF-alpha mRNA was found in hepatic tissue and peripheral fat in patients with NASH [74]. The expression of IL-6, the main proinflammatory cytokine, was markedly increased in the livers of patients with NASH compared to patients with simple steatosis or normal biopsies, confirming that hepatic IL-6 expression plays an important role in NAFLD progression, as well as in systemic insulin resistance, correlating plasma IL-6 levels with the degree of systemic insulin resistance [75]. IL-10(-/-) mice are resistant to steatosis and hepatocellular damage induced by ethanol or HFD feeding. This is due to the increase in inflammation-associated hepatic IL-6/STAT3 activation, which subsequently downregulates lipogenic genes but upregulates fatty acid oxidation-associated genes in the liver [76].
Similarly, in an animal model of HFD-induced NAFLD, the inhibition of IL-10 results in increased expression of inflammatory cytokines, worsening of insulin signaling and activation of gluconeogenic and lipidogenic pathways [77]. As previously mentioned, activated IL-8 mediates neutrophil infiltration, a pivotal process in the pathogenesis of ALD. Furthermore, high IL-8 levels might reflect the severity of ALD [78]. Surprisingly, increased concentrations of IL-8, a consequence of hepatic gene expression, were found in 97 patients with biopsy-proven NAFLD, lending credence to such chemokines as ideal surrogate serum biomarkers for the severity of hepatic fibrosis [79]. Another attractive cytokine involved in this very common liver disease, MASLD, is IL-17. Clinical trials have shown that inhibiting IL-17A could contribute to the improvement of MAFLD patients with psoriasis [80]. Interestingly, palmitic acid-induced cellular stress in liver epithelial cells enhances the expression of IL-32 and CCL20, contributing to increased expression of these fibrosis-driving molecules in MASLD [81].
High-fat diet/T2DM induces NAFLD/MASLD
Canonically, fatty liver is generally connected with a HFD, insulin resistance and obesity.
Various downstream signaling events, including mitochondrial dysfunction, endoplasmic reticulum stress, derangement of autophagy, cellular apoptosis, gut flora dysbiosis, dysregulation of microRNAs, and genetic/epigenetic risk factors, and an increase in inflammatory responses, have been found to account for this liver damage [82]. Progress over the last decade has been substantial in that the role of adipose tissue inflammation and the gut microbiome (gastrointestinal tract emerging as critical drivers of inflammation) have evolved as crucial players in the pathogenesis of NAFLD, suggesting multiple parallel hits based on mechanistic insights gained from animal models and descriptive clinical trials [83]. First, there is a complex two-way relationship between the progression of NAFLD and the development of T2DM [84]. A central pathogenic mechanism of NAFLD is insulin resistance in the liver and extrahepatic tissues such as skeletal muscle, beyond the already emphasized adipose tissue. Specifically, the molecular initiator of hepatic insulin resistance is protein kinase C translocated to the membrane compartment from the cytosol, causing impaired activation of the insulin receptor substrate phosphatidylinositol-3 kinase [85]. Recent data suggest that decreased mitochondrial content in the muscle of insulin-resistant offspring may be due in part to reductions in lipoprotein lipase expression in skeletal muscle resulting in decreased PPAR-delta activation [86].
Therapeutic approaches for ALD and NAFLD/MAFLD
Long-term abstinence is currently the main treatment for ALD [87]. As a further approach, nutritional support is recommended for patients by providing them with high-protein, low-fat diets and by balancing the levels of vitamin B, C, K, and folic acid [88].
The authors found that an association between silymarin and S-adenosyl-L-methionine, recently introduced to the market, seems to be promising in such cases of ALD [89]. An interesting study of Gclm KO mice revealed a novel mechanism that protects against liver steatosis via an oxidative stress adaptive response that activates the AMPK pathway. Accordingly, the authors suggested that redox activation of AMPK may represent a new therapeutic strategy for preventing ALD. Oxidative stress and associated decreases in glutathione levels are known to play central roles in ALD [90]. Lipin 1 is a key regulator of hepatic lipid metabolism and a downstream target of miR-203. Recent results suggested that overexpression of miR-203 inhibits liver lipid accumulation and the progression of AFLD by targeting lipin1 [91]. IL-22 has antiapoptotic, antifibrotic, antioxidative, antibacterial and regenerative effects on protecting against liver injury in many preclinical models, including several recently developed models, such as chronic-plus-binge ethanol feeding [92].
Returning to the gut flora, the colonic microbiome is impaired in alcoholism patients, in which the median abundance of Bacteroidetes is lower and that of Proteobacteria is greater [93]. Thus, both intestinal dysbiosis and disturbed integrity of the intestinal barrier play a role in the promotion of inflammatory liver damage in chronic excessive use of alcohol. Prebiotics, probiotics, postbiotics, and symbiotics could be useful therapeutic interventions for the treatment of ALD [94]. Oral ethanol supplementation diminishes the intestinal abundance of A. muciniphila in both mice and humans, but this effect can be reversed in mice with experimental ALD. In fact, A. muciniphila promotes intestinal barrier integrity and ameliorates experimental ALD [95]. Oral supplementation with living Bacteroides thetaiotaomicron, which upregulates glucagon-like peptide expression and restores ethanol-induced fibroblast growth factor 15 downregulation, was associated with restored intestinal homeostasis and ameliorated experimental ALD [96]. As various cytokines are upregulated in the liver and increased in the serum of patients with mild/moderate and severe forms of ALD, they may be valid targets for curing this disease [97]. Authors have shown that mice deficient in interferon regulator factor 3 or TLR4 expression are protected from alcohol-induced liver steatosis, inflammation and hepatocyte injury, suggesting that inflammasome and caspase-1 activation occur in ALD and that IL-1 significantly contributes to both steatosis and inflammation in the liver in ALD [98]. The finding that the inflammatory cascade plays a central role in the pathophysiology of ALD was further confirmed by a classical clinical investigation that showed that the anti-TNF monoclonal antibody infliximab resulted in an early, though not significant, decrease in the plasma levels of the proinflammatory cytokines IL-1beta, IL-6, IL-8, and interferon-gamma, whereas the plasma levels of TNF-alpha remained near the sensitivity limit of the assay throughout the treatment course in ten out of the 12 patients [99].
The mainstream NAFLD therapy consists of intensive lifestyle modifications, in the sense of a proper diet and exercise [100], beyond weight control [101]. Indeed, there are many drugs in the pipeline that are considered good candidates for curing NAFLD/NASH, as reviewed previously [102]. In addition to the key role of cytokines, IL-22 activates the JAK1/signal transducer and activator of transcription 3, c-Jun N-terminal kinase and extracellular-signal regulated kinase pathways, leading to subsequent regulation of the expression of genes involved in inflammation, metabolism, tissue repair, and regeneration, thus creating therapeutic opportunities for alleviating NAFLD [103]. Another protective effect on liver injury is provided by the noncoding gene miR-29a due to its ability to regulate epigenetic activity, mitochondrial homeostasis and immune modulation, which could have therapeutic applications in NAFLD [104]. Finally, the findings of these studies suggest that sodium butyrate and Clostridium butyricum are potential adjuvant treatment strategies for T2DM-induced NAFLD [105].
The gut microbiome: a key regulator of host physiology
The gut microbiome plays a central role in modifying blood lipid levels, independent of age, sex, and host genetics [106]. On the other hand, lipid metabolism is instrumental to the onset of hepatic steatosis, as previously emphasized. The gut microbiota transforms and synthesizes lipids and breaks down dietary lipids to generate secondary metabolites with host modulatory properties. Previously, lipids have largely been considered to play structural roles, such as in cell membranes, or viewed as metabolic fuel, but recent research has led to an increased appreciation of their signaling activities [107]. In keeping with this emerging role, some endogenous bioactive lipids produced from arachidonic acid and other polyunsaturated fatty acids exert their biological activity via specific receptors by triggering distinct signal transduction pathways [108].
An increasing body of evidence points to a bidirectional interaction between the gut and the liver mediated by the microbiome. On this basis, chronic intake of alcohol beyond provoking ROS formation increases the multiplication of intestinal bacteria and changes the gut permeability to food antigens, commensal or pathogenic bacteria and bacterial components, ultimately increasing endotoxins in the portal circulation and activating Kupffer cells through Toll-like receptor pathways [109]. In contrast, there is a major group of microbial metabolites, i.e., short-chain fatty acids (SCFAs), with favorable effects. They promote the growth of intestinal epithelial cells and strengthen intestinal tight junctions, ultimately maintaining the integrity of the gut barrier. Moreover, these products regulate peripheral innate immune responses [110]. There is wide consensus on the finding that SCFA levels decrease with alcohol consumption [111]. Accordingly, linolenic acid, by shaping the microbial metabolome, could be used for the treatment of ALD [112].
Conversely, data on SCFAs in NAFLD patients are controversial. However, for some authors, higher fecal SCFA levels and higher abundance levels of SCFA-producing bacteria were found in NAFLD patients [113]; moreover, fresh evidence shows that circulating SCFA levels are lower in NAFLD patients and negatively correlated with the severity of T2DM-associated NAFLD [114]. The likely mechanism linking SCFAs to the progression of NAFLD is based on the finding that these SCFAs are negatively related to TNF-alpha [115].
In addition to the previously mentioned events, intestinal bacteria transform primary bile acids (BAs) to secondary BAs, which can modify the gut barrier. In this context, alcohol abuse is associated with a significant increase in the secondary formation of BAs, which are implicated in the pathogenesis of ALD [116, 117]. Notably, BA binds to the farnesoid X receptor, which is critically involved in many molecular processes responsible for maintaining glucose and lipid homeostasis. Alterations to these pathways can also lead to dysregulation of energy balance and increased inflammation and fibrosis in NAFLD patients [118].
Considering the phylogenetic profiles of the gut microbiota of NAFLD patients, we should note that studies partially converge on the class, family and genus levels of the involved bacteria. Specifically, low abundances of Faecalibacterium, Bacteroides and Prevotella and high abundances of Gemmiger were associated specifically with the degree of inflammation, ballooning, and stage of fibrosis, even after taking other cofactors into account, in 57 NAFLD patients [119]. Alternatively, the intestinal bacterial microbiota in NAFLD patients with coronary artery disease showed an increase in the abundance of Copococcus and Veillonella and a reduction in the abundance of Ruminococcus gnavus and Bifidobacterium longum subsp. infantis, in addition to Parabacteroides, Bacteroides fragilis and Bacteroides dorei [120].
Interestingly, in this field of research, alcohol produced by some intestinal bacteria is associated with liver damage. The endogenous production of ethanol was confirmed by an interesting preclinical study in which two mutants with different alcohol-producing abilities, W14-adh and W14Δadh, were constructed using the clinical high alcohol-producing Klebsiella pneumoniae strain W14 as a parent. Mice in the W14-adh group, which had the highest ethanol production, exhibited the most severe liver damage, characterized by ROS accumulation, likely as a consequence of mitochondrial dysfunction [121]. Data from an intervention study of ten NAFLD patients in which ethanol was measured in the portal vein blood of patients infused with a selective alcohol dehydrogenase inhibitor before a mixed meal test was performed showed that the first-pass effect obscures the level of endogenous ethanol production, suggesting that microbial ethanol could be considered to be involved in the pathogenesis of NAFLD. Furthermore, Lactobacillaceae correlated with postprandial peripheral ethanol concentrations, findings that were prospectively obtained [122]. These results suggest that, at least in some cases of NAFLD, an alteration in the gut microbiome drives this pathological condition due to excess endogenous alcohol production. Moreover, in a Chinese cohort, investigators discovered that high-alcohol-producing Klebsiella pneumoniae (HiAlc Kpn) is associated with up to 60% of individuals with NAFLD. Nevertheless, the same group found that the oral gavage of clinical isolates of HiAlc Kpn into mice induced NAFLD. Finally, selective elimination of the HiAlc Kpn strain before focal microbiome transplantation prevents NAFLD in recipient mice [123]. Other data suggest that phage therapy targeting the gut microbiota is an alternative to antibiotics, with potential efficacy and safety, at least in HiAlc Kpn-induced NAFLD [124]. In NASH patients, an increased abundance of alcohol-producing bacteria corresponds to an elevated blood ethanol concentration, suggesting a role for alcohol-producing microbiota in the pathogenesis of NASH [125]. However, the main role of the microbiome in NAFLD has not been fully elucidated.
Spleen and microbiome/cytokeratins
The spleen has been recognized to play a great role in lipid metabolism, likely due to its natural immune function, i.e., via the mononuclear phagocytic system [126]. An increase in the levels of total cholesterol and low-density lipoprotein was observed in splenectomized patients [127]. At this point, we should consider that there is a reciprocal link in the spleen-gut-microbiota axis. In fact, data suggest that splenectomy leads to an abnormal composition of the gut microbiota, impacting SCFA levels [128]. On the other hand, the presence of a greater representation of pathogenic species of bacteria results in rapid replenishment of the marginal zone of the spleen, thus impacting the maturation of the spleen and its complete function [129]. Notably, the authors demonstrated that after 4 days of antibiotic treatment, which eliminated specific families of the Bacteroidetes, Firmicutes, Tenericutes and Actinobacteria phyla in the intestine, there was a consequent decrease in the levels of multiple pattern recognition receptor (PRR) ligands. A reduction in the serum concentration of PRR ligands led to a decrease in the number of splenic Ly6Chigh monocytes, which acquired an immature phenotype characterized by decreased inflammatory cytokine levels and increased phagocytic ability [130]. It has long been known that functional hyposplenism occurs in ALD [131], but the spleen-liver axis has gained much attention in recent years concerning NAFLD, as this phenomenon has not been detected in diagnostic versants [132].
An increasingly important topic is the role of cytokeratins in ALD and NAFLD. First, by immunoelectron microscopy, several authors have identified a direct linkage between lipid droplet-binding proteins and intermediate-sized filament cytokeratins (K) 8 and 18 [133]. K18 represents the major intermediate filament protein in liver cells [134] but is also the main component of Mallory-Denk bodies, which are a hallmark of alcoholic steatohepatitis [135]. In NAFLD, K18 fragments have been proven to be associated with NASH, although with reduced sensitivity but good specificity (66% and 82%, respectively) [136]. A previous study showed that polypeptide-specific antigen (TPS), a serological marker of K18 that is widely used as a marker for various cancers, is a better marker for differentiating NASH from simple fatty liver [137]. The serum TPS is frequently increased in alcoholic patients and may be a marker of alcoholic hepatitis [138].
Sirtuins: as therapeutic targets
SIRTs are effective at modulating energy metabolism by intervening in oxidative stress and mitochondrial homeostasis, regulating the inflammatory response, activating autophagy and necroptosis and acting on lipid metabolism in ALD. Its actions include mediating multiple signal transduction pathways, such as the mTOR, AMPK, PPAR alpha and gamma, LKB1, PGC-1 alpha, FoxO1/3a, Nrf2/p62, TFEB, RIPK1/3, HMGB1, NFATc4, NF-κB, TLR4, NLRP3, P2X7R, MAPK, TGF1β/Smads, Wnt/β-catenin, Lipin1, and SREBP1 pathways [139].
One of the most common SIRTs involved is SIRT1 because ethanol-induced oxidative stress directly downregulates NAD + levels, increases SIRT1 nucleocytoplasmic shuttling, and ultimately inhibits SIRT1 gene and protein expression in the liver [140].
A recent study illustrated that the SIRT1 activator E1231, a piperazine 1,4-diamide compound, alleviated NAFLD induced in C57BL/6 J mice fed a high-fat and high-cholesterol diet and improved liver injury by regulating the SIRT1-AMPK alpha pathway, suggesting that this compound is a promising candidate compound for NAFLD treatment [141].
Treatment of C2C12 myocytes and HepG2 cells with patchouli alcohol augmented AMPK phosphorylation and SIRT1 expression in a dose-dependent manner, attenuating skeletal muscle insulin resistance and hepatic steatosis in high-fat diet-fed mice [142].
Interestingly, SIRT4 is an important regulator of lipid homeostasis, and malonyl CoA decarboxylase has been identified as a SIRT4 target [143].
There are conflicting results related to the role of SIRT4 in the development of atherosclerosis, which is strongly associated with NAFLD [144] and heavy alcohol consumption [145]. SIRT4 overexpression suppressed the PI3K/Akt/NF‑κB pathway by inhibiting PI3K phosphorylation and phosphorylated (p)‑Akt and inhibiting the expression of inflammatory cytokines in oxidized low-density lipoprotein‑induced human umbilical vein endothelial cells [146]. In contrast with the findings of previous studies, other authors have shown that SIRT4 deficiency exacerbates inflammation and promotes the development of atherosclerosis, activating the phosphorylation of NF-κB [147]. Consistent with these data, low circulating levels of SIRT4 were found in obese patients with NAFLD and early atherosclerosis, likely mirroring its reduced mitochondrial expression in an attempt to increase fat oxidative capacity and mitochondrial function in the liver and muscle [148].
Nevertheless, researchers revealed that treating hepatocytes with valdecoxib increases AMPK phosphorylation and SIRT6 expression in human primary hepatocytes and ameliorates hepatic lipid accumulation and lipogenic protein expression in HFD-fed mice, thus attenuating hepatic steatosis under hyperlipidemic conditions [149]. Several reports have substantiated the close relationship between SIRT7 and age-related processes [150]. Earlier findings suggest that musclin can suppress palmitate-induced ER stress by upregulating SIRT7 and autophagy signaling, thereby alleviating lipid accumulation in primary hepatocytes [151].
Other common mechanisms
Close examination of the oxidative damage associated with NAFLD revealed that ablation of catalase promoted NAFLD via oxidative stress and mitochondrial dysfunction in diet-induced obese mice. Furthermore, knockdown of catalase expedited cellular lipid accumulation and weakened mitochondrial biogenesis in fatty acid-treated HepG2 cells [152]. Catalase knockout mice were not effective at metabolizing alcohol or inducing hydrogen peroxide clearance and were more prone to alcohol-induced liver injury. Catalase-mediated hydrogen peroxide removal has been found to be an inherent mechanism through which PPAR-alpha maintains the nicotinamide adenine dinucleotide pool [153]. However, preclinical studies concerning catalase favor its role in addition to preclinical studies. Recent in vivo observations have shown that decreased catalase levels, leading to an insufficient antioxidant defense system, are a characteristic of 139 NAFLD patients [154]. In addition, methyl donors, such as S-adenosylmethionine (SAMe) and betaine, may act via the attenuation of alcohol-induced oxidative stress in male C57BL/6 J mice [155]. Moreover, interesting findings highlight the beneficial effect of SAMe as an antisteatotic agent in two in vitro models of fatty liver [156]. Now, we try to explain which mechanisms NAFLD and AFL share on the genetic side. Patatin-like phospholipase domain-containing proteins (PNPLA1-9) are a family of proteins that have diverse lipolytic activities toward diverse substrates, such as triacylglycerols, phospholipids, and retinol esters [157]. Previous data have shown that the PNPLA3 I148M mutation promotes triglyceride accumulation by limiting triglyceride hydrolysis [158]. It has recently been suggested that the rs738409 G allele in PNPLA3, which encodes adiponutrin, is strongly associated with increased liver fat content in different ethnic groups [159]. Genetic variation in PNPLA3 confers susceptibility to NAFLD [160, 161] and is associated with ALD [162, 163].
Substitution of lysine for glutamic acid at residue 167 in transmembrane 6 superfamily member 2 (TM6SF2), located on chromosome 19, is associated with fatty liver disease. The authors found that TM6SF2 acts in the smooth endoplasmic reticulum to promote bulk lipidation of apolipoprotein B-containing lipoproteins, thus preventing fatty liver disease in a study comparing Tm6sf2-/- mice with their wild-type littermates [164].
Because carriers of the TM6SF2 variant have favorable cardiovascular outcomes, identification of downstream pathways altered by the TM6SF2 E167K variant could help unravel its noxious role in NAFLD development. Furthermore, this study contributes to the development of new therapeutic approaches due to its cardioprotective effect [165].
In addition, miR-34a, miR-122 and miR-155 are the most involved in the pathogenesis of NAFLD. These three miRNAs have also been implicated in ALD, reinforcing a common disease mechanism between these two entities and the pleiotropic effects of specific miRNAs [166].
Future directions
First, in patients with T2DM, the new definition of MAFLD increased the prevalence of fatty liver disease by 68.89%, resulting in an overdiagnosis of fatty liver disease, exaggerated mortality, and morbidity [167]. Thus, rigorous surveillance is needed.
Considering the n-3 polyunsaturated fatty acid (PUFA) mechanism, i.e., a reduction in de novo lipogenesis and lipid mobilization from adipose tissue, an increase in mitochondrial fatty acid β-oxidation, a decrease in hepatic inflammation and oxidative stress and a positive impact on the intestinal flora, n-3 PUFAs could represent a promising management tool for ALD [168]. However, twelve months of n-3-PUFA treatment in patients with NAFLD was also associated with a significant decrease in liver fat and beneficial changes in the plasma lipid profile [169].
Interestingly, taking into account the mechanisms by which mechanistic target of rapamycin (mTOR) influences insulin resistance by acting on Foxo1 and lipin1; regulating lipid metabolism via SREBPs; interfering with the intestinal microbiota via TLRs; moderating oxidative stress through PIG3, p53, and JNK; and counteracting autophagy, inflammation, genetic polymorphisms, and epigenetics in NAFLD, the authors have stressed the importance of mTOR as an influencing factor of NAFLD [170]. Nevertheless, targeting the signaling of adipose mTOR, nutritional sensors that regulate lipid metabolism, cell proliferation and autophagy, and adipocyte lipolysis could be a potential approach for improving ALD [171] based on the finding that alcohol consumption changes mTORC1 and mTORC2 activity [172]. Importantly, mTOR inhibitors could play a protective role in coronary artery disease [173], a common comorbidity of NAFLD. Furthermore, a recent study provided evidence of how mitochondrial dysfunction, which plays a pivotal role in the alteration of lipid metabolism beyond inflammation and oxidative stress, could be counteracted by natural and bioactive compounds in the context of MASLD [174]. Nevertheless, it would be interesting to further explore why, in heavy drinkers, ethanol oxidation shortens hepatic lipid metabolism, converting the liver from a lipid burning to a lipid-storing organ. In fact, studies involving rodents chronically fed alcohol have shown that ethanol consumption reduces adipose tissue mass by increasing lipolysis in adipose tissue. In this sense, the FFAs released from adipose tissue are taken up by the liver and esterified into triglycerides, thereby exacerbating fat accumulation in the liver [175]. On the other hand, attractive data indicate that the total amount of triglycerides stored in hepatocytes is not the major determinant of lipotoxicity, having the role of ceramides recently emerged in NASH [176]. Capturing, concerning the similar prevention of ALD and NAFLD, is the following. Cyclic guanosine monophosphate–adenosine monophosphate (GMP-AMP) synthase (cGAS) triggers innate immune responses by producing the secondary messenger cyclic 2′,3′-cGAMP, which binds to and triggers STING, resulting in IRF3 activation. RNA sequence analysis of liver cells from ALD patients revealed that activation of the cGAS-IRF3 pathway was related to the severity of ALD. Therefore, cGAS is central to the pathogenesis of ALD and can be used as a potential therapeutic target for liver protection [177]. Accordingly, a study of liver samples from 98 patients with NAFLD also showed that STING expression in Kupffer cells and monocyte-derived macrophages was closely related to inflammation and fibrosis [178]. Failure of the factors that regulate the cGAS-STING pathway is responsible for ALD/NAFLD. Therefore, the cGAS–STING signaling pathway could be a novel and valuable therapeutic target for NAFLD in the future.
Finally, a recent study suggested that genetic variations in neuregulin (NRG4) may give rise to mutant proteins with altered functions and that impaired or enhanced Nrg4 function could be either a risk factor or a protective factor for NAFLD and associated metabolic disorders [179].
Limitations
Currently, many issues persist, and there are still unknown factors involved in the differential diagnosis of NAFLD and ALD. Although these two diseases share similar mechanistic biochemical events, their epidemiological and clinical characteristics are distinct, and there are significant dissimilarities in the prognoses of both illnesses, with alcoholic fatty liver burdened by a much greater risk of developing liver cirrhosis and liver cancer.
Conclusions
We have tried to answer the main question of whether NAFLD is a completely different disease from ALD or if NAFLD is a two-stage disease caused by the same treatment. The more we learn, the more we realize how similar NAFLD and ALD truly are, at least from pathogenesis. Indeed, there are some other intriguing aspects that must be reported in interpreting the molecular processes displayed during the onset and progression of NAFLD and ALD that are still obscure, as autophagy is a protective or dangerous mechanism and still others. Scientists should prompt new questions to be asked about the molecular events and up-to-date interpretations, challenging and scrutinizing the old ones.
Authors’ contributions
GT conceived the idea and drafted the manuscript; VC contributed to searching the literature data.
Funding
No funding was received.
Availability of data and materials
Not applicable.
Declarations
Ethics approval and consent to participate
Not applicable.
Competing interests
The authors declare no competing interests.
Footnotes
Publisher’s Note
Springer Nature remains neutral with regard to jurisdictional claims in published maps and institutional affiliations.
References
- 1.Mehta SR, Thomas EL, Bell JD, Johnston DG, Taylor-Robinson SD. Non-invasive means of measuring hepatic fat content. World J Gastroenterol. 2008;14(22):3476–3483. doi: 10.3748/wjg.14.3476. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 2.Kojima S, Watanabe N, Numata M, Ogawa T, Matsuzaki S. Increase in the prevalence of fatty liver in Japan over the past 12 years: analysis of clinical background. J Gastroenterol. 2003;38(10):954–961. doi: 10.1007/s00535-003-1178-8. [DOI] [PubMed] [Google Scholar]
- 3.El-Zayadi AR. Hepatic steatosis: a benign disease or a silent killer. World J Gastroenterol. 2008;14(26):4120–4126. doi: 10.3748/wjg.14.4120. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 4.Eslam M, Newsome PN, Sarin SK, Anstee QM, Targher G, Romero-Gomez M, et al. A new definition for metabolic dysfunction-associated fatty liver disease: an international expert consensus statement. J Hepatol. 2020;73(1):202–209. doi: 10.1016/j.jhep.2020.03.039. [DOI] [PubMed] [Google Scholar]
- 5.Rinella ME, Lazarus JV, Ratziu V, Francque SM, Sanyal AJ, Kanwal F, NAFLD Nomenclature consensus group A multisociety Delphi consensus statement on new fatty liver disease nomenclature. Hepatology. 2023;78(6):1966–1986. doi: 10.1097/HEP.0000000000000520. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 6.De A, Bhagat N, Mehta M, Taneja S, Duseja A. Metabolic dysfunction-associated steatotic liver disease (MASLD) definition is better than MAFLD criteria for lean patients with NAFLD. J Hepatol. 2023:S0168–8278(23)05044–4. 10.1016/j.jhep.2023.07.031. Epub ahead of print. PMID: 37558135. [DOI] [PubMed]
- 7.New MASLD nomenclature. https://www.aasld.org/new-masld-nomenclature. Last retrieved December 2023.
- 8.Lichtenstein GR. Shifting our focus from NASH to MASH. Gastroenterol Hepatol (N Y) 2023;19(9):511. [PMC free article] [PubMed] [Google Scholar]
- 9.Chen L, Tao X, Zeng M, Mi Y, Xu L. Clinical and histological features under different nomenclatures of fatty liver disease: NAFLD, MAFLD, MASLD and MetALD. J Hepatol. 2023:S0168–8278(23)05075–4. 10.1016/j.jhep.2023.08.021. Epub ahead of print. PMID: 37714381. [DOI] [PubMed]
- 10.Younossi MZ et al. Global survey of stigma among physicians and patients with nonalcoholic fatty liver disease. J Hepatol. 2023. 10.1016/j.jhep.2023.11.004. [DOI] [PubMed]
- 11.Song SJ, Che-To Lai J, Lai-Hung Wong G, Wai-Sun Wong V, Cheuk-Fung YT. Can we use old NAFLD data under the new MASLD definition? J Hepatol. 2023 doi: 10.1016/j.jhep.2023.07.021. [DOI] [PubMed] [Google Scholar]
- 12.Mills SJ, Harrison SA. Comparison of the natural history of alcoholic and nonalcoholic fatty liver disease. Curr Gastroenterol Rep. 2005;7(1):32–36. doi: 10.1007/s11894-005-0063-4. [DOI] [PubMed] [Google Scholar]
- 13.Jepsen P, Vilstrup H, Mellemkjaer L, Thulstrup AM, Olsen JH, Baron JA, Sørensen HT. Prognosis of patients with a diagnosis of fatty liver–a registry-based cohort study. Hepatogastroenterology. 2003;50(54):2101–4. [PubMed] [Google Scholar]
- 14.Estes C, Razavi H, Loomba R, Younossi Z, Sanyal AJ. Modeling the epidemic of nonalcoholic fatty liver disease demonstrates an exponential increase in burden of disease. Hepatology. 2018;67(1):123–133. doi: 10.1002/hep.29466. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 15.Fryar CD, Carroll MD, Afful J. Prevalence of overweight, obesity, and severe obesity among adults aged 20 and over: United States, 1960–1962 through 2017–2018. NCHS Health E-Stats, Centers for Disease Control and Prevention. 2020. Updated February 8, 2021. www.cdc.gov/nchs/data/hestat/obesity-adult-17-18/obesity-adult.htm. Last retrieved on September 2023.
- 16.Speakman JR, Sørensen TIA, Hall KD, Allison DB. Unanswered questions about the causes of obesity. Science. 2023;381(6661):944–946. doi: 10.1126/science.adg2718. [DOI] [PubMed] [Google Scholar]
- 17.Marchesini G, Brizi M, Bianchi G, Tomassetti S, Bugianesi E, Lenzi M, McCullough AJ, Natale S, Forlani G, Melchionda N. Nonalcoholic fatty liver disease: a feature of the metabolic syndrome. Diabetes. 2001;50(8):1844–1850. doi: 10.2337/diabetes.50.8.1844. [DOI] [PubMed] [Google Scholar]
- 18.Tarantino G, Saldalamacchia G, Conca P, Arena A. Non-alcoholic fatty liver disease: further expression of the metabolic syndrome. J Gastroenterol Hepatol. 2007;22(3):293–303. doi: 10.1111/j.1440-1746.2007.04824.x. [DOI] [PubMed] [Google Scholar]
- 19.Åberg F, Färkkilä M, Männistö V. Interaction between alcohol use and metabolic risk factors for liver disease: a critical review of epidemiological studies. Alcohol Clin Exp Res. 2020;44(2):384–403. doi: 10.1111/acer.14271. [DOI] [PubMed] [Google Scholar]
- 20.Younossi ZM, Stepanova M, Ong J, Yilmaz Y, Duseja A, Eguchi Y, El Kassas M, Castellanos-Fernandez M, George J, Jacobson IM, Bugianesi E, Wong VW, Arrese M, de Ledinghen V, Romero-Gomez M, Mendez-Sanchez N, Ahmed A, Wong R, Papatheodoridis G, Serfaty L, Younossi I, Nader F, Ziayee M, Afendy A, Global NASH Council Effects of alcohol consumption and metabolic syndrome on mortality in patients with nonalcoholic and alcohol-related fatty liver disease. Clin Gastroenterol Hepatol. 2019;17(8):1625–1633.e1. doi: 10.1016/j.cgh.2018.11.033. [DOI] [PubMed] [Google Scholar]
- 21.Alcoholic liver disease. https://www.ncbi.nlm.nih.gov/books/NBK546632/. Last retrieved on September 2023.
- 22.Duseja A, De A, Taneja S, Choudhury AK, Devarbhavi H, Hu J, Hamid SS, Butt AS, Jafri SMW, Ghazinian H, Chawla YK, Dhiman RK, Duan Z, Chen Y, Tan SS, Lee GH, Lim SG, Kim DJ, Sahu M, Sollano JD, Carpio G, Mohan Prasad VG, Abbas Z, Lesmana LA, Lesmana CR, Eapen CE, Goel A, Sood A, Midha V, Goyal O, Dokmeci AK, Ning Q, Chen T, Ma K, Payawal DA, Lau GKK, Al Mahtab M, Rahman S, Alam MS, Shukla A, Shrestha A, Shah S, Kalal CR, Kumar G, Jain P, Paulson I, Sarin SK, APASL ACLF Working Party, APASL ACLF Research Consortium (AARC) Impact of metabolic risk factors on the severity and outcome of patients with alcohol-associated acute-on-chronic liver failure. Liver Int. 2021;41(1):150–157. doi: 10.1111/liv.14671. [DOI] [PubMed] [Google Scholar]
- 23.Sookoian S, Castaño GO, Pirola CJ. Modest alcohol consumption decreases the risk of non-alcoholic fatty liver disease: a meta-analysis of 43 175 individuals. Gut. 2014;63(3):530–532. doi: 10.1136/gutjnl-2013-305718. [DOI] [PubMed] [Google Scholar]
- 24.Ceylan-Isik AF, McBride SM, Ren J. Sex difference in alcoholism: who is at a greater risk for development of alcoholic complication? Life Sci. 2010;87(5–6):133–8. doi: 10.1016/j.lfs.2010.06.002. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 25.Burra P, Bizzaro D, Gonta A, Shalaby S, Gambato M, Morelli MC, Trapani S, Floreani A, Marra F, Brunetto MR, Taliani G, Villa E, Special Interest Group Gender in Hepatology of the Italian Association for the Study of the Liver (AISF) Clinical impact of sexual dimorphism in non-alcoholic fatty liver disease (NAFLD) and non-alcoholic steatohepatitis (NASH) Liver Int. 2021;41(8):1713–1733. doi: 10.1111/liv.14943. [DOI] [PubMed] [Google Scholar]
- 26.Levy R, Catana AM, Durbin-Johnson B, Halsted CH, Medici V. Ethnic differences in presentation and severity of alcoholic liver disease. Alcohol Clin Exp Res. 2015;39(3):566–574. doi: 10.1111/acer.12660. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 27.Xu K, Kranzler HR, Sherva R, Sartor CE, Almasy L, Koesterer R, Zhao H, Farrer LA, Gelernter J. Genomewide Association study for maximum number of alcoholic drinks in European Americans and African Americans. Alcohol Clin Exp Res. 2015;39(7):1137–47. doi: 10.1111/acer.12751. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 28.Chen Y, Du X, Kuppa A, Feitosa MF, Bielak LF, O’Connell JR, Musani SK, Guo X, Kahali B, Chen VL, Smith AV, Ryan KA, Eirksdottir G, Allison MA, Bowden DW, Budoff MJ, Carr JJ, Chen YI, Taylor KD, Oliveri A, Correa A, Crudup BF, Kardia SLR, Mosley TH, Jr, Norris JM, Terry JG, Rotter JI, Wagenknecht LE, Halligan BD, Young KA, Hokanson JE, Washko GR, Gudnason V, Province MA, Peyser PA, Palmer ND, Speliotes EK. Genome-wide association meta-analysis identifies 17 loci associated with nonalcoholic fatty liver disease. Nat Genet. 2023;55:1640–1650. doi: 10.1038/s41588-023-01497-6. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 29.Innes H, Buch S, Hutchinson S, Guha IN, Morling JR, Barnes E, Irving W, Forrest E, Pedergnana V, Goldberg D, Aspinall E, Barclay S, Hayes PC, Dillon J, Nischalke HD, Lutz P, Spengler U, Fischer J, Berg T, Brosch M, Eyer F, Datz C, Mueller S, Peccerella T, Deltenre P, Marot A, Soyka M, McQuillin A, Morgan MY, Hampe J, Stickel F. Genome-wide Association study for alcohol-related cirrhosis identifies risk Loci in MARC1 and HNRNPUL1. Gastroenterology. 2020;159(4):1276–1289.e7. doi: 10.1053/j.gastro.2020.06.014. [DOI] [PubMed] [Google Scholar]
- 30.Teli MR, Day CP, Burt AD, Bennett MK, James OF. Determinants of progression to cirrhosis or fibrosis in pure alcoholic fatty liver. Lancet. 1995;346(8981):987–990. doi: 10.1016/s0140-6736(95)91685-7. [DOI] [PubMed] [Google Scholar]
- 31.Webpathology. Alcoholic steatohepatitis. https://www.webpathology.com/image.asp?case=715&n=12#:~:text=Pericellular%20(%22chicken%2Dwire%22,is%20highlighted%20by%20Trichrome%20stains. Last retrieved September 2023.
- 32.Lackner C, Tiniakos D. Fibrosis and alcohol-related liver disease. J Hepatol. 2019;70(2):294–304. doi: 10.1016/j.jhep.2018.12.003. [DOI] [PubMed] [Google Scholar]
- 33.Toshikuni N, Tsutsumi M, Arisawa T. Clinical differences between alcoholic liver disease and nonalcoholic fatty liver disease. World J Gastroenterol. 2014;20(26):8393–8406. doi: 10.3748/wjg.v20.i26.8393. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 34.Nakano M, Fukusato T. Histological study on comparison between NASH and ALD. Hepatol Res. 2005;33(2):110–115. doi: 10.1016/j.hepres.2005.09.016. [DOI] [PubMed] [Google Scholar]
- 35.Ludwig J, Viggiano TR, McGill DB, Oh BJ. Nonalcoholic steatohepatitis: Mayo Clinic experiences with a hitherto unnamed disease. Mayo Clin Proc. 1980;55(7):434–438. [PubMed] [Google Scholar]
- 36.Bedossa P. Pathology of non-alcoholic fatty liver disease. Liver Int. 2017;37 Suppl 1:85–89. doi: 10.1111/liv.13301. [DOI] [PubMed] [Google Scholar]
- 37.Sirwi A, Hussain MM. Lipid transfer proteins in the assembly of apoB-containing lipoproteins. J Lipid Res. 2018;59(7):1094–1102. doi: 10.1194/jlr.R083451. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 38.Higuchi N, Kato M, Shundo Y, Tajiri H, Tanaka M, Yamashita N, Kohjima M, Kotoh K, Nakamuta M, Takayanagi R, Enjoji M. Liver X receptor in cooperation with SREBP-1c is a major lipid synthesis regulator in nonalcoholic fatty liver disease. Hepatol Res. 2008;38(11):1122–1129. doi: 10.1111/j.1872-034X.2008.00382.x. [DOI] [PubMed] [Google Scholar]
- 39.You M, Fischer M, Deeg MA, Crabb DW. Ethanol induces fatty acid synthesis pathways by activation of sterol regulatory element-binding protein (SREBP) J Biol Chem. 2002;277(32):29342–29347. doi: 10.1074/jbc.M202411200. [DOI] [PubMed] [Google Scholar]
- 40.Sozio MS, Liangpunsakul S, Crabb D. The role of lipid metabolism in the pathogenesis of alcoholic and nonalcoholic hepatic steatosis. Semin Liver Dis. 2010;30(4):378–390. doi: 10.1055/s-0030-1267538. [DOI] [PubMed] [Google Scholar]
- 41.Zheng Y, Wang S, Wu J, Wang Y. Mitochondrial metabolic dysfunction and non-alcoholic fatty liver disease: new insights from pathogenic mechanisms to clinically targeted therapy. J Transl Med. 2023;21(1):510. doi: 10.1186/s12967-023-04367-1. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 42.You M, Arteel GE. Effect of ethanol on lipid metabolism. J Hepatol. 2019;70(2):237–248. doi: 10.1016/j.jhep.2018.10.037. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 43.Wu D, Cederbaum AI. Alcohol, oxidative stress, and free radical damage. Alcohol Res Health. 2003;27(4):277–84. [PMC free article] [PubMed] [Google Scholar]
- 44.Cho W, Park SY, Oh H, Abd El-Aty AM, Hacimüftüoğlu A, Kim DS, Jung TW, Jeong JH. Humulus japonicus extract ameliorates hepatic steatosis through the PPARα-mediated suppression of alcohol-induced oxidative stress. J Med Food. 2023;26(3):193–200. doi: 10.1089/jmf.2022.K.0093. [DOI] [PubMed] [Google Scholar]
- 45.Samuel VT, Petersen KF, Shulman GI. Lipid-induced insulin resistance: unravelling the mechanism. Lancet. 2010;375(9733):2267–2277. doi: 10.1016/S0140-6736(10)60408-4. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 46.Lakshman MR. Some novel insights into the pathogenesis of alcoholic steatosis. Alcohol. 2004;34(1):45–48. doi: 10.1016/j.alcohol.2004.08.004. [DOI] [PubMed] [Google Scholar]
- 47.Syed-Abdul MM, Moore MP, Wheeler AA, Ganga RR, Diaz-Arias A, Petroski GF, Rector RS, Ibdah JA, Parks EJ. Isotope labeling and biochemical assessment of liver-triacylglycerol in patients with different levels of histologically-graded liver disease. J Nutr. 2023:S0022–3166(23)72613–8. 10.1016/j.tjnut.2023.09.018. Epub ahead of print. PMID: 37774841. [DOI] [PMC free article] [PubMed]
- 48.Yamauchi T, Kamon J, Waki H, Terauchi Y, Kubota N, Hara K, Mori Y, Ide T, Murakami K, Tsuboyama-Kasaoka N, Ezaki O, Akanuma Y, Gavrilova O, Vinson C, Reitman ML, Kagechika H, Shudo K, Yoda M, Nakano Y, Tobe K, Nagai R, Kimura S, Tomita M, Froguel P, Kadowaki T. The fat-derived hormone adiponectin reverses insulin resistance associated with both lipoatrophy and obesity. Nat Med. 2001;7(8):941–946. doi: 10.1038/90984. [DOI] [PubMed] [Google Scholar]
- 49.Rogers CQ, Ajmo JM, You M. Adiponectin and alcoholic fatty liver disease. IUBMB Life. 2008;60(12):790–797. doi: 10.1002/iub.124. [DOI] [PubMed] [Google Scholar]
- 50.Finelli C, Tarantino G. What is the role of adiponectin in obesity related non-alcoholic fatty liver disease? World J Gastroenterol. 2013;19(6):802–812. doi: 10.3748/wjg.v19.i6.802. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 51.Choi SW, Oh H, Park SY, Cho W, Abd El-Aty AM, Hacimuftuoglu A, Jeong JH, Jung TW. Adipokine gremlin-1 promotes hepatic steatosis via upregulation of ER stress by suppressing autophagy-mediated signaling. J Cell Physiol. 2023;238(5):966–975. doi: 10.1002/jcp.30982. [DOI] [PubMed] [Google Scholar]
- 52.Larsen LE, van den Boogert MAW, Rios-Ocampo WA, Jansen JC, Conlon D, Chong PLE, Levels JHM, Eilers RE, Sachdev VV, Zelcer N, Raabe T, He M, Hand NJ, Drenth JPH, Rader DJ, Stroes ESG, Lefeber DJ, Jonker JW, Holleboom AG. Defective lipid droplet-lysosome interaction causes fatty liver disease as evidenced by human mutations in TMEM199 and CCDC115. Cell Mol Gastroenterol Hepatol. 2022;13(2):583–597. doi: 10.1016/j.jcmgh.2021.09.013. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 53.Meng F, Lorenzo SR, Francis H, Glaser S, Alpini G. Characterization of cellular senescence mechanisms in alcoholic liver injury. FASEB J. 2017;31(S1):8043. doi: 10.1096/fasebj.31.1_supplement.804.3. [DOI] [Google Scholar]
- 54.Lu C, Ge T, Shao Y, Cui W, Li Z, Xu W, Bao X. ZNF281 drives hepatocyte senescence in alcoholic liver disease by reducing HK2-stabilized PINK1/Parkin-mediated mitophagy. Cell Prolif. 2023;56(3):e13378. doi: 10.1111/cpr.13378. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 55.Donohue MT, Osna AN, Kharbanda KK, Thomes GP. Lysosome and proteasome dysfunction in alcohol-induced liver injury. Liver Res. 2019;3(3–4):191–205. doi: 10.1016/j.livres.2019.11.001. [DOI] [Google Scholar]
- 56.Engelmann C, Tacke F. The potential role of cellular senescence in non-alcoholic fatty liver disease. Int J Mol Sci. 2022;23(2):652. doi: 10.3390/ijms23020652. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 57.Zeng J, Acin-Perez R, Assali EA, Martin A, Brownstein AJ, Petcherski A, Fernández-Del-Rio L, Xiao R, Lo CH, Shum M, Liesa M, Han X, Shirihai OS, Grinstaff MW. Restoration of lysosomal acidification rescues autophagy and metabolic dysfunction in non-alcoholic fatty liver disease. Nat Commun. 2023;14(1):2573. doi: 10.1038/s41467-023-38165-6. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 58.Ceni E, Mello T, Galli A. Pathogenesis of alcoholic liver disease: role of oxidative metabolism. World J Gastroenterol. 2014;20(47):17756–17772. doi: 10.3748/wjg.v20.i47.17756. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 59.Seitz HK. The role of cytochrome P4502E1 in the pathogenesis of alcoholic liver disease and carcinogenesis. Chem Biol Interact. 2020;316:108918. doi: 10.1016/j.cbi.2019.108918. [DOI] [PubMed] [Google Scholar]
- 60.Wang Z, Hall SD, Maya JF, Li L, Asghar A, Gorski JC. Diabetes mellitus increases the in vivo activity of cytochrome P450 2E1 in humans. Br J Clin Pharmacol. 2003;55(1):77–85. doi: 10.1046/j.1365-2125.2003.01731.x. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 61.Wang HJ, Gao B, Zakhari S, Nagy LE. Inflammation in alcoholic liver disease. Annu Rev Nutr. 2012;21(32):343–68. doi: 10.1146/annurev-nutr-072610-145138. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 62.Tilg H, Moschen AR, Szabo G. Interleukin-1 and inflammasomes in alcoholic liver disease/acute alcoholic hepatitis and nonalcoholic fatty liver disease/nonalcoholic steatohepatitis. Hepatology. 2016;64(3):955–965. doi: 10.1002/hep.28456. [DOI] [PubMed] [Google Scholar]
- 63.Gao B, Ahmad MF, Nagy LE, Tsukamoto H. Inflammatory pathways in alcoholic steatohepatitis. J Hepatol. 2019;70(2):249–259. doi: 10.1016/j.jhep.2018.10.023. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 64.El-Assal O, Hong F, Kim WH, Radaeva S, Gao B. IL-6-deficient mice are susceptible to ethanol-induced hepatic steatosis: IL-6 protects against ethanol-induced oxidative stress and mitochondrial permeability transition in the liver. Cell Mol Immunol. 2004;1(3):205–211. [PubMed] [Google Scholar]
- 65.Deviere J, Content J, Denys C, Vandenbussche P, Schandene L, Wybran J, Dupont E. High interleukin-6 serum levels and increased production by leucocytes in alcoholic liver cirrhosis. Correlation with IgA serum levels and lymphokines production. Clin Exp Immunol. 1989;77(2):221–5. [PMC free article] [PubMed] [Google Scholar]
- 66.Dominguez M, Miquel R, Colmenero J, Moreno M, García-Pagán JC, Bosch J, Arroyo V, Ginès P, Caballería J, Bataller R. Hepatic expression of CXC chemokines predicts portal hypertension and survival in patients with alcoholic hepatitis. Gastroenterology. 2009;136(5):1639–1650. doi: 10.1053/j.gastro.2009.01.056. [DOI] [PubMed] [Google Scholar]
- 67.Setshedi M, Wands JR, Monte SM. Acetaldehyde adducts in alcoholic liver disease. Oxid Med Cell Longev. 2010;3(3):178–85. doi: 10.4161/oxim.3.3.12288. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 68.Tuma DJ. Role of malondialdehyde-acetaldehyde adducts in liver injury. Free Radic Biol Med. 2002;32(4):303–308. doi: 10.1016/s0891-5849(01)00742-0. [DOI] [PubMed] [Google Scholar]
- 69.Latvala J, Hietala J, Koivisto H, Järvi K, Anttila P, Niemelä O. Immune responses to ethanol metabolites and cytokine profiles differentiate alcoholics with or without liver disease. Am J Gastroenterol. 2005;100(6):1303–1310. doi: 10.1111/j.1572-0241.2005.41509.x. [DOI] [PubMed] [Google Scholar]
- 70.Kuchay MS, Choudhary NS, Mishra SK. Pathophysiological mechanisms underlying MAFLD. Diabetes Metab Syndr. 2020;14(6):1875–1887. doi: 10.1016/j.dsx.2020.09.026. [DOI] [PubMed] [Google Scholar]
- 71.Sanyal AJ, Campbell-Sargent C, Mirshahi F, Rizzo WB, Contos MJ, Sterling RK, Luketic VA, Shiffman ML, Clore JN. Nonalcoholic steatohepatitis: association of insulin resistance and mitochondrial abnormalities. Gastroenterology. 2001;120(5):1183–1192. doi: 10.1053/gast.2001.23256. [DOI] [PubMed] [Google Scholar]
- 72.Hughey CC, Puchalska P, Crawford PA. Integrating the contributions of mitochondrial oxidative metabolism to lipotoxicity and inflammation in NAFLD pathogenesis. Biochim Biophys Acta Mol Cell Biol Lipids. 2022;1867(11):159209. doi: 10.1016/j.bbalip.2022.159209. [DOI] [PubMed] [Google Scholar]
- 73.Kawaratani H, Tsujimoto T, Kitazawa T, Kitade M, Yoshiji H, Uemura M, Fukui H. Innate immune reactivity of the liver in rats fed a choline-deficient L-amino-acid-defined diet. World J Gastroenterol. 2008;14(43):6655–6661. doi: 10.3748/wjg.14.6655. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 74.Crespo J, Cayón A, Fernández-Gil P, Hernández-Guerra M, Mayorga M, Domínguez-Díez A, Fernández-Escalante JC, Pons-Romero F. Gene expression of tumor necrosis factor alpha and TNF-receptors, p55 and p75, in nonalcoholic steatohepatitis patients. Hepatology. 2001;34(6):1158–1163. doi: 10.1053/jhep.2001.29628. [DOI] [PubMed] [Google Scholar]
- 75.Wieckowska A, Papouchado BG, Li Z, Lopez R, Zein NN, Feldstein AE. Increased hepatic and circulating interleukin-6 levels in human nonalcoholic steatohepatitis. Am J Gastroenterol. 2008;103(6):1372–1379. doi: 10.1111/j.1572-0241.2007.01774.x. [DOI] [PubMed] [Google Scholar]
- 76.Miller AM, Wang H, Bertola A, Park O, Horiguchi N, Ki SH, Yin S, Lafdil F, Gao B. Inflammation-associated interleukin-6/signal transducer and activator of transcription 3 activation ameliorates alcoholic and nonalcoholic fatty liver diseases in interleukin-10-deficient mice. Hepatology. 2011;54(3):846–856. doi: 10.1002/hep.24517. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 77.Cintra DE, Pauli JR, Araújo EP, Moraes JC, de Souza CT, Milanski M, Morari J, Gambero A, Saad MJ, Velloso LA. Interleukin-10 is a protective factor against diet-induced insulin resistance in liver. J Hepatol. 2008;48(4):628–637. doi: 10.1016/j.jhep.2007.12.017. [DOI] [PubMed] [Google Scholar]
- 78.Huang YS, Wu JC, Chang FY, Lee SD. Interleukin-8 and alcoholic liver disease. Zhonghua Yi Xue Za Zhi (Taipei) 1999;62(7):395–401. [PubMed] [Google Scholar]
- 79.Glass O, Henao R, Patel K, Guy CD, Gruss HJ, Syn WK, Moylan CA, Streilein R, Hall R, Mae Diehl A, Abdelmalek MF. Serum interleukin-8, osteopontin, and monocyte chemoattractant protein 1 are associated with hepatic fibrosis in patients with nonalcoholic fatty liver disease. Hepatol Commun. 2018;2(11):1344–1355. doi: 10.1002/hep4.1237. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 80.Olveira A, Augustin S, Benlloch S, Ampuero J, Suárez-Pérez JA, Armesto S, Vilarrasa E, Belinchón-Romero I, Herranz P, Crespo J, Guimerá F, Gómez-Labrador L, Martín V, Carrascosa JM. The essential role of IL-17 as the pathogenetic link between psoriasis and metabolic-associated fatty liver disease. Life (Basel) 2023;13(2):419. doi: 10.3390/life13020419. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 81.Schilcher K, Dayoub R, Kubitza M, Riepl J, Klein K, Buechler C, Melter M, Weiss TS. Saturated fat-mediated upregulation of IL-32 and CCL20 in hepatocytes contributes to higher expression of these fibrosis-driving molecules in MASLD. Int J Mol Sci. 2023;24(17):13222. doi: 10.3390/ijms241713222. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 82.Lian CY, Zhai ZZ, Li ZF, Wang L. High fat diet-triggered non-alcoholic fatty liver disease: a review of proposed mechanisms. Chem Biol Interact. 2020;330:109199. doi: 10.1016/j.cbi.2020.109199. [DOI] [PubMed] [Google Scholar]
- 83.Tilg H, Adolph TE, Moschen AR. Multiple parallel hits hypothesis in nonalcoholic fatty liver disease: revisited after a decade. Hepatology. 2021;73(2):833–842. doi: 10.1002/hep.31518. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 84.Xia MF, Bian H, Gao X. NAFLD and diabetes: two sides of the same coin? Rationale for gene-based personalized NAFLD treatment. Front Pharmacol. 2019;6(10):877. doi: 10.3389/fphar.2019.00877. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 85.Lipina C, Hundal HS. Sphingolipids: agents provocateurs in the pathogenesis of insulin resistance. Diabetologia. 2011;54(7):1596–607. doi: 10.1007/s00125-011-2127-3. [DOI] [PubMed] [Google Scholar]
- 86.Morino K, Petersen KF, Sono S, Choi CS, Samuel VT, Lin A, Gallo A, Zhao H, Kashiwagi A, Goldberg IJ, Wang H, Eckel RH, Maegawa H, Shulman GI. Regulation of mitochondrial biogenesis by lipoprotein lipase in muscle of insulin-resistant offspring of parents with type 2 diabetes. Diabetes. 2012;61(4):877–87. doi: 10.2337/db11-1391. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 87.Kim MS, Ong M, Qu X. Optimal management for alcoholic liver disease: conventional medications, natural therapy or combination? World J Gastroenterol. 2016;22(1):8–23. doi: 10.3748/wjg.v22.i1.8. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 88.Suk KT, Kim MY, Baik SK. Alcoholic liver disease: treatment. World J Gastroenterol. 2014;20(36):12934–12944. doi: 10.3748/wjg.v20.i36.12934. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 89.Testino G, Leone S, Ansaldi F, Borro P. Silymarin and S-adenosyl-L-methionine (SAMe): two promising pharmacological agents in case of chronic alcoholic hepathopathy. A review and a point of view. Minerva Gastroenterol Dietol. 2013;59(4):341–56. [PubMed] [Google Scholar]
- 90.Chen Y, Singh S, Matsumoto A, Manna SK, Abdelmegeed MA, Golla S, Murphy RC, Dong H, Song BJ, Gonzalez FJ, Thompson DC, Vasiliou V. Chronic glutathione depletion confers protection against alcohol-induced steatosis: implication for redox activation of AMP-activated protein kinase pathway. Sci Rep. 2016;12(6):29743. doi: 10.1038/srep29743. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 91.Cheng XY, Liu JD, Lu XY, Yan X, Huang C, Meng XM, Li J. miR-203 inhibits alcohol-induced hepatic steatosis by targeting Lipin1. Front Pharmacol. 2018;4(9):275. doi: 10.3389/fphar.2018.00275. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 92.Xiang X, Hwang S, Feng D, Shah VH, Gao B. Interleukin-22 in alcoholic hepatitis and beyond. Hepatol Int. 2020;14(5):667–676. doi: 10.1007/s12072-020-10082-6. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 93.Mutlu EA, Gillevet PM, Rangwala H, Sikaroodi M, Naqvi A, Engen PA, Kwasny M, Lau CK, Keshavarzian A. Colonic microbiome is altered in alcoholism. Am J Physiol Gastrointest Liver Physiol. 2012;302(9):G966–78. doi: 10.1152/ajpgi.00380.2011. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 94.Dukić M, Radonjić T, Jovanović I, Zdravković M, Todorović Z, Kraišnik N, Aranđelović B, Mandić O, Popadić V, Nikolić N, Klašnja S, Manojlović A, Divac A, Gačić J, Brajković M, Oprić S, Popović M, Branković M. Alcohol, inflammation, and microbiota in alcoholic liver disease. Int J Mol Sci. 2023;24(4):3735. doi: 10.3390/ijms24043735. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 95.Grander C, Adolph TE, Wieser V, Lowe P, Wrzosek L, Gyongyosi B, Ward DV, Grabherr F, Gerner RR, Pfister A, Enrich B, Ciocan D, Macheiner S, Mayr L, Drach M, Moser P, Moschen AR, Perlemuter G, Szabo G, Cassard AM, Tilg H. Recovery of ethanol-induced Akkermansia muciniphila depletion ameliorates alcoholic liver disease. Gut. 2018;67(5):891–901. doi: 10.1136/gutjnl-2016-313432. [DOI] [PubMed] [Google Scholar]
- 96.Sangineto M, Grander C, Grabherr F, Mayr L, Enrich B, Schwärzler J, Dallio M, Bukke VN, Moola A, Moschetta A, Adolph TE, Sabbà C, Serviddio G, Tilg H. Recovery of Bacteroides thetaiotaomicron ameliorates hepatic steatosis in experimental alcohol-related liver disease. Gut Microbes. 2022;14(1):2089006. doi: 10.1080/19490976.2022.2089006. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 97.Xu MJ, Zhou Z, Parker R, Gao B. Targeting inflammation for the treatment of alcoholic liver disease. Pharmacol Ther. 2017;180:77–89. doi: 10.1016/j.pharmthera.2017.06.007. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 98.Szabo G, Petrasek J, Bala S. Innate immunity and alcoholic liver disease. Dig Dis. 2012;30 Suppl 1(Suppl 1):55–60. doi: 10.1159/000341126. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 99.Tilg H, Jalan R, Kaser A, Davies NA, Offner FA, Hodges SJ, Ludwiczek O, Shawcross D, Zoller H, Alisa A, Mookerjee RP, Graziadei I, Datz C, Trauner M, Schuppan D, Obrist P, Vogel W, Williams R. Anti-tumor necrosis factor-alpha monoclonal antibody therapy in severe alcoholic hepatitis. J Hepatol. 2003;38(4):419–25. 10.1016/s0168-8278(02)00442-7. [DOI] [PubMed]
- 100.Semmler G, Datz C, Reiberger T, Trauner M. Diet and exercise in NAFLD/NASH: beyond the obvious. Liver Int. 2021;41(10):2249–2268. doi: 10.1111/liv.15024. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 101.Finer N. Weight loss interventions and nonalcoholic fatty liver disease: optimizing liver outcomes. Diabetes Obes Metab. 2022;24 Suppl 2:44–54. doi: 10.1111/dom.14569. [DOI] [PubMed] [Google Scholar]
- 102.Negi CK, Babica P, Bajard L, Bienertova-Vasku J, Tarantino G. Insights into the molecular targets and emerging pharmacotherapeutic interventions for nonalcoholic fatty liver disease. Metabolism. 2022;126:154925. doi: 10.1016/j.metabol.2021.154925. [DOI] [PubMed] [Google Scholar]
- 103.Zai W, Chen W, Liu H, Ju D. Therapeutic opportunities of IL-22 in Non-alcoholic fatty liver disease: from molecular mechanisms to clinical applications. Biomedicines. 2021;9(12):1912. doi: 10.3390/biomedicines9121912. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 104.Lin HY, Yang YL, Wang PW, Wang FS, Huang YH. The emerging role of MicroRNAs in NAFLD: highlight of MicroRNA-29a in modulating oxidative stress, inflammation, and beyond. Cells. 2020;9(4):1041. doi: 10.3390/cells9041041. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 105.Yang T, Yang H, Heng C, Wang H, Chen S, Hu Y, Jiang Z, Yu Q, Wang Z, Qian S, Wang J, Wang T, Du L, Lu Q, Yin X. Amelioration of non-alcoholic fatty liver disease by sodium butyrate is linked to the modulation of intestinal tight junctions in db/db mice. Food Funct. 2020;11(12):10675–10689. doi: 10.1039/d0fo01954b. [DOI] [PubMed] [Google Scholar]
- 106.Fu J, Bonder MJ, Cenit MC, Tigchelaar EF, Maatman A, Dekens JA, Brandsma E, Marczynska J, Imhann F, Weersma RK, Franke L, Poon TW, Xavier RJ, Gevers D, Hofker MH, Wijmenga C, Zhernakova A. The gut microbiome contributes to a substantial proportion of the variation in blood lipids. Circ Res. 2015;117(9):817–24. doi: 10.1161/CIRCRESAHA.115.306807. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 107.Brown EM, Clardy J, Xavier RJ. Gut microbiome lipid metabolism and its impact on host physiology. Cell Host Microbe. 2023;31(2):173–186. doi: 10.1016/j.chom.2023.01.009. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 108.Maccarrone M. Deciphering complex interactions in bioactive lipid signaling. Molecules. 2023;28(6):2622. doi: 10.3390/molecules28062622. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 109.Kawaratani H, Moriya K, Namisaki T, Uejima M, Kitade M, Takeda K, Okura Y, Kaji K, Takaya H, Nishimura N, Sato S, Sawada Y, Seki K, Kubo T, Mitoro A, Yamao J, Yoshiji H. Therapeutic strategies for alcoholic liver disease: focusing on inflammation and fibrosis (Review) Int J Mol Med. 2017;40(2):263–270. doi: 10.3892/ijmm.2017.3015. [DOI] [PubMed] [Google Scholar]
- 110.Kim CH. Immune regulation by microbiome metabolites. Immunology. 2018;154(2):220–229. doi: 10.1111/imm.12930. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 111.Lin R, Zhang Y, Chen L, Qi Y, He J, Hu M, Zhang Y, Fan L, Yang T, Wang L, Si M, Chen S. The effects of cigarettes and alcohol on intestinal microbiota in healthy men. J Microbiol. 2020;58(11):926–937. doi: 10.1007/s12275-020-0006-7. [DOI] [PubMed] [Google Scholar]
- 112.Huang RY, Raymond Herr D, Moochhala S. Manipulation of alcohol and short-chain fatty acids in the metabolome of Commensal and Virulent Klebsiella pneumoniae by linolenic acid. Microorganisms. 2020;8(5):773. doi: 10.3390/microorganisms8050773. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 113.Rau M, Rehman A, Dittrich M, Groen AK, Hermanns HM, Seyfried F, Beyersdorf N, Dandekar T, Rosenstiel P, Geier A. Fecal SCFAs and SCFA-producing bacteria in gut microbiome of human NAFLD as a putative link to systemic T-cell activation and advanced disease. United Eur Gastroenterol J. 2018;6(10):1496–1507. doi: 10.1177/2050640618804444. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 114.Tsai HJ, Hung WC, Hung WW, Lee YJ, Chen YC, Lee CY, Tsai YC, Dai CY. Circulating short-chain fatty acids and non-alcoholic fatty liver disease severity in patients with type 2 diabetes mellitus. Nutrients. 2023;15(7):1712. doi: 10.3390/nu15071712. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 115.Xiong J, Chen X, Zhao Z, Liao Y, Zhou T, Xiang Q. A potential link between plasma short-chain fatty acids, TNF-α level and disease progression in non-alcoholic fatty liver disease: a retrospective study. Exp Ther Med. 2022;24(3):598. doi: 10.3892/etm.2022.11536. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 116.Kakiyama G, Hylemon PB, Zhou H, Pandak WM, Heuman DM, Kang DJ, Takei H, Nittono H, Ridlon JM, Fuchs M, Gurley EC, Wang Y, Liu R, Sanyal AJ, Gillevet PM, Bajaj JS. Colonic inflammation and secondary bile acids in alcoholic cirrhosis. Am J Physiol Gastrointest Liver Physiol. 2014;306(11):G929–37. doi: 10.1152/ajpgi.00315.2013. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 117.Way GW, Jackson KG, Muscu SR, Zhou H. Key signaling in alcohol-associated liver disease: the role of bile acids. Cells. 2022;11(8):1374. doi: 10.3390/cells11081374. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 118.Chow MD, Lee YH, Guo GL. The role of bile acids in nonalcoholic fatty liver disease and nonalcoholic steatohepatitis. Mol Aspects Med. 2017;56:34–44. doi: 10.1016/j.mam.2017.04.004. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 119.Lang S, Martin A, Zhang X, Farowski F, Wisplinghoff H, J G T Vehreschild M, Krawczyk M, Nowag A, Kretzschmar A, Scholz C, Kasper P, Roderburg C, Mohr R, Lammert F, Tacke F, Schnabl B, Goeser T, Steffen HM, Demir M. Combined analysis of gut microbiota, diet and PNPLA3 polymorphism in biopsy-proven non-alcoholic fatty liver disease. Liver Int. 2021;41(7):1576–1591. doi: 10.1111/liv.14899. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 120.Zhang Y, Xu J, Wang X, Ren X, Liu Y. Changes of intestinal bacterial microbiota in coronary heart disease complicated with nonalcoholic fatty liver disease. BMC Genomics. 2019;20(1):862. doi: 10.1186/s12864-019-6251-7. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 121.Chen X, Zhang Z, Li H, Zhao J, Wei X, Lin W, Zhao X, Jiang A, Yuan J. Endogenous ethanol produced by intestinal bacteria induces mitochondrial dysfunction in non-alcoholic fatty liver disease. J Gastroenterol Hepatol. 2020;35(11):2009–2019. doi: 10.1111/jgh.15027. [DOI] [PubMed] [Google Scholar]
- 122.Meijnikman AS, Davids M, Herrema H, Aydin O, Tremaroli V, Rios-Morales M, Levels H, Bruin S, de Brauw M, Verheij J, Kemper M, Holleboom AG, Tushuizen ME, Schwartz TW, Nielsen J, Brandjes D, Dirinck E, Weyler J, Verrijken A, De Block CEM, Vonghia L, Francque S, Beuers U, Gerdes VEA, Bäckhed F, Groen AK, Nieuwdorp M. Microbiome-derived ethanol in nonalcoholic fatty liver disease. Nat Med. 2022;28(10):2100–2106. doi: 10.1038/s41591-022-02016-6. [DOI] [PubMed] [Google Scholar]
- 123.Yuan J, Chen C, Cui J, Lu J, Yan C, Wei X, Zhao X, Li N, Li S, Xue G, Cheng W, Li B, Li H, Lin W, Tian C, Zhao J, Han J, An D, Zhang Q, Wei H, Zheng M, Ma X, Li W, Chen X, Zhang Z, Zeng H, Ying S, Wu J, Yang R, Liu D. Fatty liver disease caused by high-alcohol-producing Klebsiella pneumoniae. Cell Metab. 2019;30(4):675–688.e7. doi: 10.1016/j.cmet.2019.08.018. [DOI] [PubMed] [Google Scholar]
- 124.Gan L, Feng Y, Du B, et al. Bacteriophage targeting microbiota alleviates non-alcoholic fatty liver disease induced by high alcohol-producing Klebsiella pneumoniae. Nat Commun. 2023;14:3215. doi: 10.1038/s41467-023-39028-w. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 125.Zhu L, Baker SS, Gill C, Liu W, Alkhouri R, Baker RD, Gill SR. Characterization of gut microbiomes in nonalcoholic steatohepatitis (NASH) patients: a connection between endogenous alcohol and NASH. Hepatology. 2013;57(2):601–609. doi: 10.1002/hep.26093. [DOI] [PubMed] [Google Scholar]
- 126.Ai XM, Ho LC, Han LL, Lu JJ, Yue X, Yang NY. The role of splenectomy in lipid metabolism and atherosclerosis (AS) Lipids Health Dis. 2018;17(1):186. doi: 10.1186/s12944-018-0841-2. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 127.Gunes O, Turgut E, Bag YM, Gundoğan E, Gunes A, Sumer F. The impact of splenectomy on human lipid metabolism. Ups J Med Sci. 2022;127. 10.48101/ujms.v127.8500. PMID: 35756571; PMCID: PMC9199581.Furthermore is there a link between microbiome and spleen?. [DOI] [PMC free article] [PubMed]
- 128.Wei Y, Chang L, Ishima T, Wan X, Ma L, Wuyun G, Pu Y, Hashimoto K. Abnormalities of the composition of the gut microbiota and short-chain fatty acids in mice after splenectomy. Brain Behav Immun Health. 2021;11:100198. doi: 10.1016/j.bbih.2021.100198. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 129.Rosado MM, Aranburu A, Scarsella M, Cascioli S, Giorda E, Del Chierico F, Mortera SL, Mortari EP, Petrini S, Putignani L, Carsetti R. Spleen development is modulated by neonatal gut microbiota. Immunol Lett. 2018;199:1–15. doi: 10.1016/j.imlet.2018.04.010. [DOI] [PubMed] [Google Scholar]
- 130.Kolypetri P, Liu S, Cox LM, Fujiwara M, Raheja R, Ghitza D, Song A, Daatselaar D, Willocq V, Weiner HL. Regulation of splenic monocyte homeostasis and function by gut microbial products. iScience. 2021;24(4):102356. doi: 10.1016/j.isci.2021.102356. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 131.Muller AF, Toghill PJ. Splenic function in alcoholic liver disease. Gut. 1992;33(10):1386–1389. doi: 10.1136/gut.33.10.1386. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 132.Tarantino G, Citro V, Balsano C. Liver-spleen axis in nonalcoholic fatty liver disease. Expert Rev Gastroenterol Hepatol. 2021;15(7):759–769. doi: 10.1080/17474124.2021.1914587. [DOI] [PubMed] [Google Scholar]
- 133.Heid H, Rickelt S, Zimbelmann R, Winter S, Schumacher H, Dörflinger Y. Lipid droplets, perilipins and cytokeratins–unravelled liaisons in epithelium-derived cells. PLoS One. 2013;8(5):e63061. doi: 10.1371/journal.pone.0063061. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 134.Romano V, Hatzfeld M, Magin TM, Zimbelmann R, Franke WW, Maier G, Ponstingl H. Cytokeratin expression in simple epithelia. I. Identification of mRNA coding for human cytokeratin no. 18 by a cDNA clone. Differentiation. 1986;30(3):244–53. doi: 10.1111/j.1432-0436.1986.tb00787.x. [DOI] [PubMed] [Google Scholar]
- 135.Stumptner C, Omary MB, Fickert P, Denk H, Zatloukal K. Hepatocyte cytokeratins are hyperphosphorylated at multiple sites in human alcoholic hepatitis and in a mallory body mouse model. Am J Pathol. 2000;156(1):77–90. doi: 10.1016/S0002-9440(10)64708-6. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 136.Wong VW, Adams LA, de Lédinghen V, Wong GL, Sookoian S. Noninvasive biomarkers in NAFLD and NASH - current progress and future promise. Nat Rev Gastroenterol Hepatol. 2018;15(8):461–478. doi: 10.1038/s41575-018-0014-9. [DOI] [PubMed] [Google Scholar]
- 137.Tarantino G, Conca P, Coppola A, Vecchione R, Di Minno G. Serum concentrations of the tissue polypeptide specific antigen in patients suffering from non-alcoholic steatohepatitis. Eur J Clin Invest. 2007;37(1):48–53. doi: 10.1111/j.1365-2362.2007.01745.x. [DOI] [PubMed] [Google Scholar]
- 138.González-Quintela A, Mella C, Pérez LF, Abdulkader I, Caparrini AM, Lojo S. Increased serum tissue polypeptide specific antigen (TPS) in alcoholics: a possible marker of alcoholic hepatitis. Alcohol Clin Exp Res. 2000;24(8):1222–1226. doi: 10.1111/j.1530-0277.2000.tb02087.x. [DOI] [PubMed] [Google Scholar]
- 139.Zhao X, Xue X, Wang C, Wang J, Peng C, Li Y. Emerging roles of Sirtuins in alleviating alcoholic liver disease: a comprehensive review. Int Immunopharmacol. 2022;108:108712. doi: 10.1016/j.intimp.2022.108712. [DOI] [PubMed] [Google Scholar]
- 140.Ren R, Wang Z, Wu M, Wang H. Emerging roles of SIRT1 in alcoholic liver disease. Int J Biol Sci. 2020;16(16):3174–3183. doi: 10.7150/ijbs.49535. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 141.Han J, Li S, Wang W, Jiang X, Liu C, Lei L, Li Y, Sheng R, Zhang Y, Wu Y, Zhang J, Zhang Y, Xu Y, Si S. SIRT1 activator E1231 alleviates nonalcoholic fatty liver disease by regulating lipid metabolism. Curr Issues Mol Biol. 2023;45(6):5052–5070. doi: 10.3390/cimb45060321. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 142.Pyun DH, Kim TJ, Park SY, Lee HJ, Abd El-Aty AM, Jeong JH, Jung TW. Patchouli alcohol ameliorates skeletal muscle insulin resistance and NAFLD via AMPK/SIRT1-mediated suppression of inflammation. Mol Cell Endocrinol. 2021;538:111464. doi: 10.1016/j.mce.2021.111464. [DOI] [PubMed] [Google Scholar]
- 143.Laurent G, German NJ, Saha AK, de Boer VC, Davies M, Koves TR, Dephoure N, Fischer F, Boanca G, Vaitheesvaran B, Lovitch SB, Sharpe AH, Kurland IJ, Steegborn C, Gygi SP, Muoio DM, Ruderman NB, Haigis MC. SIRT4 coordinates the balance between lipid synthesis and catabolism by repressing malonyl CoA decarboxylase. Mol Cell. 2013;50(5):686–698. doi: 10.1016/j.molcel.2013.05.012. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 144.Finney AC, Das S, Kumar D, McKinney MP, Cai B, Yurdagul A, Jr, Rom O. The interplay between nonalcoholic fatty liver disease and atherosclerotic cardiovascular disease. Front Cardiovasc Med. 2023;2(10):1116861. doi: 10.3389/fcvm.2023.1116861. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 145.Kiechl S, Willeit J, Rungger G, Egger G, Oberhollenzer F, Bonora E. Alcohol consumption and atherosclerosis: what is the relation? Prospective results from the Bruneck Study. Stroke. 1998;29(5):900–907. doi: 10.1161/01.str.29.5.900. [DOI] [PubMed] [Google Scholar]
- 146.Tao Y, Yu S, Chao M, Wang Y, Xiong J, Lai H. SIRT4 suppresses the PI3K/Akt/NF-κB signaling pathway and attenuates HUVEC injury induced by oxLDL. Mol Med Rep. 2019;19(6):4973–4979. doi: 10.3892/mmr.2019.10161. [DOI] [PubMed] [Google Scholar]
- 147.Chang S, Zhang G, Li L, Li H, Jin X, Wang Y, Li B. Sirt4 deficiency promotes the development of atherosclerosis by activating the NF-κB/IκB/CXCL2/3 pathway. Atherosclerosis. 2023;373:29–37. doi: 10.1016/j.atherosclerosis.2023.04.006. [DOI] [PubMed] [Google Scholar]
- 148.Tarantino G, Finelli C, Scopacasa F, Pasanisi F, Contaldo F, Capone D, Savastano S. Circulating levels of sirtuin 4, a potential marker of oxidative metabolism, related to coronary artery disease in obese patients suffering from NAFLD, with normal or slightly increased liver enzymes. Oxid Med Cell Longev. 2014;2014:920676. doi: 10.1155/2014/920676. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 149.Park SY, Cho W, Abd El-Aty AM, Hacimuftuoglu A, Jeong JH, Jung TW. Valdecoxib attenuates lipid-induced hepatic steatosis through autophagy-mediated suppression of endoplasmic reticulum stress. Biochem Pharmacol. 2022;199:115022. doi: 10.1016/j.bcp.2022.115022. [DOI] [PubMed] [Google Scholar]
- 150.Lagunas-Rangel FA. SIRT7 in the aging process. Cell Mol Life Sci. 2022;79(6):297. doi: 10.1007/s00018-022-04342-x. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 151.Cho W, Choi SW, Oh H, Baygutalp F, Abd El-Aty AM, Jeong JH, Song JH, Shin YK, Jung TW. Musclin attenuates lipid deposition in hepatocytes through SIRT7/autophagy-mediated suppression of ER stress. Biochem Biophys Res Commun. 2023;28(658):62–68. doi: 10.1016/j.bbrc.2023.03.065. [DOI] [PubMed] [Google Scholar]
- 152.Shin SK, Cho HW, Song SE, Bae JH, Im SS, Hwang I, Ha H, Song DK. Ablation of catalase promotes non-alcoholic fatty liver via oxidative stress and mitochondrial dysfunction in diet-induced obese mice. Pflugers Arch. 2019;471(6):829–843. doi: 10.1007/s00424-018-02250-3. [DOI] [PubMed] [Google Scholar]
- 153.Yue R, Chen GY, Xie G, Hao L, Guo W, Sun X, Jia W, Zhang Q, Zhou Z, Zhong W. Activation of PPARα-catalase pathway reverses alcoholic liver injury via upregulating NAD synthesis and accelerating alcohol clearance. Free Radic Biol Med. 2021;174:249–263. doi: 10.1016/j.freeradbiomed.2021.08.005. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 154.Kosmalski M, Szymczak-Pajor I, Drzewoski J, Śliwińska A. Non-alcoholic fatty liver disease is associated with a decreased Catalase (CAT) Level, CT genotypes and the T Allele of the -262 C/T CAT Polymorphism. Cells. 2023;12(18):2228. doi: 10.3390/cells12182228. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 155.Powell CL, Bradford BU, Craig CP, Tsuchiya M, Uehara T, O’Connell TM, Pogribny IP, Melnyk S, Koop DR, Bleyle L, Threadgill DW, Rusyn I. Mechanism for prevention of alcohol-induced liver injury by dietary methyl donors. Toxicol Sci. 2010;115(1):131–9. doi: 10.1093/toxsci/kfq031. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 156.Vergani L, Baldini F, Khalil M, Voci A, Putignano P, Miraglia N. New perspectives of S-Adenosylmethionine (SAMe) applications to attenuate fatty acid-induced steatosis and oxidative stress in hepatic and endothelial cells. Molecules. 2020;25(18):4237. doi: 10.3390/molecules25184237. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 157.Kienesberger PC, Oberer M, Lass A, Zechner R. Mammalian patatin domain containing proteins: a family with diverse lipolytic activities involved in multiple biological functions. J Lipid Res. 2009;50 Suppl(Suppl):S63–8. doi: 10.1194/jlr.R800082-JLR200. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 158.He S, McPhaul C, Li JZ, Garuti R, Kinch L, Grishin NV, Cohen JC, Hobbs HH. A sequence variation (I148M) in PNPLA3 associated with nonalcoholic fatty liver disease disrupts triglyceride hydrolysis. J Biol Chem. 2010;285(9):6706–15. doi: 10.1074/jbc.M109.064501. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 159.Kotronen A, Johansson LE, Johansson LM, Roos C, Westerbacka J, Hamsten A, Bergholm R, Arkkila P, Arola J, Kiviluoto T, Fisher RM, Ehrenborg E, Orho-Melander M, Ridderstråle M, Groop L, Yki-Järvinen H. A common variant in PNPLA3, which encodes adiponutrin, is associated with liver fat content in humans. Diabetologia. 2009;52(6):1056–1060. doi: 10.1007/s00125-009-1285-z. [DOI] [PubMed] [Google Scholar]
- 160.Romeo S, Kozlitina J, Xing C, Pertsemlidis A, Cox D, Pennacchio LA, Boerwinkle E, Cohen JC, Hobbs HH. Genetic variation in PNPLA3 confers susceptibility to nonalcoholic fatty liver disease. Nat Genet. 2008;40(12):1461–5. doi: 10.1038/ng.257. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 161.Sookoian S, Pirola CJ. Meta-analysis of the influence of I148M variant of patatin-like phospholipase domain containing 3 gene (PNPLA3) on the susceptibility and histological severity of nonalcoholic fatty liver disease. Hepatology. 2011;53(6):1883–1894. doi: 10.1002/hep.24283. [DOI] [PubMed] [Google Scholar]
- 162.Tian C, Stokowski RP, Kershenobich D, Ballinger DG, Hinds DA. Variant in PNPLA3 is associated with alcoholic liver disease. Nat Genet. 2010;42(1):21–23. doi: 10.1038/ng.488. [DOI] [PubMed] [Google Scholar]
- 163.Stickel F, Buch S, Lau K, Meyer zu Schwabedissen H, Berg T, Ridinger M, Rietschel M, Schafmayer C, Braun F, Hinrichsen H, Günther R, Arlt A, Seeger M, Müller S, Seitz HK, Soyka M, Lerch M, Lammert F, Sarrazin C, Kubitz R, Häussinger D, Hellerbrand C, Bröring D, Schreiber S, Kiefer F, Spanagel R, Mann K, Datz C, Krawczak M, Wodarz N, Völzke H, Hampe J. Genetic variation in the PNPLA3 gene is associated with alcoholic liver injury in caucasians. Hepatology. 2011;53(1):86–95. doi: 10.1002/hep.24017. [DOI] [PubMed] [Google Scholar]
- 164.Luo F, Smagris E, Martin SA, Vale G, McDonald JG, Fletcher JA, Burgess SC, Hobbs HH, Cohen JC. Hepatic TM6SF2 is required for lipidation of VLDL in a Pre-Golgi compartment in mice and rats. Cell Mol Gastroenterol Hepatol. 2022;13(3):879–899. doi: 10.1016/j.jcmgh.2021.12.008. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 165.Luo F, Oldoni F, Das A. TM6SF2: a novel genetic player in nonalcoholic fatty liver and cardiovascular disease. Hepatol Commun. 2022;6(3):448–460. doi: 10.1002/hep4.1822. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 166.Torres JL, Novo-Veleiro I, Manzanedo L, Alvela-Suárez L, Macías R, Laso FJ, Marcos M. Role of microRNAs in alcohol-induced liver disorders and non-alcoholic fatty liver disease. World J Gastroenterol. 2018;24(36):4104–4118. doi: 10.3748/wjg.v24.i36.4104. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 167.Muthiah M, Ng CH, Chan KE, Fu CE, Lim WH, Tan DJH, Nah B, Kong G, Xiao J, Yong JN, Tan B, Syn N, Wang JW, Sayed N, Tan E, Chew NW, Dan YY, Siddiqui MS, Sanyal AJ, Noureddin M. Type 2 diabetes mellitus in metabolic-associated fatty liver disease vs. type 2 diabetes mellitus non-alcoholic fatty liver disease: a longitudinal cohort analysis. Ann Hepatol. 2023;28(1):100762. doi: 10.1016/j.aohep.2022.100762. [DOI] [PubMed] [Google Scholar]
- 168.Wang M, Ma LJ, Yang Y, Xiao Z, Wan JB. n-3 Polyunsaturated fatty acids for the management of alcoholic liver disease: a critical review. Crit Rev Food Sci Nutr. 2019;59(sup1):S116–S129. doi: 10.1080/10408398.2018.1544542. [DOI] [PubMed] [Google Scholar]
- 169.Šmíd V, Dvořák K, Šedivý P, Kosek V, Leníček M, Dezortová M, Hajšlová J, Hájek M, Vítek L, Bechyňská K, Brůha R. Effect of Omega-3 polyunsaturated fatty acids on lipid metabolism in patients with metabolic syndrome and NAFLD. Hepatol Commun. 2022;6(6):1336–1349. doi: 10.1002/hep4.1906. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 170.Feng J, Qiu S, Zhou S, Tan Y, Bai Y, Cao H, Guo J, Su Z. mTOR: a potential new target in nonalcoholic fatty liver disease. Int J Mol Sci. 2022;23(16):9196. doi: 10.3390/ijms23169196. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 171.Rodriguez Y, Dunfield J, Roderique T, Ni HM. Liver-adipose tissue crosstalk in alcohol-associated liver disease: the role of mTOR. Liver Res. 2022;6(4):227–237. doi: 10.1016/j.livres.2022.11.006. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 172.Chao X, Williams SN, Ding WX. Role of mechanistic target of rapamycin in autophagy and alcohol-associated liver disease. Am J Physiol Cell Physiol. 2022;323(4):C1100–C1111. doi: 10.1152/ajpcell.00281.2022. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 173.Tarantino G, Capone D. Inhibition of the mTOR pathway: a possible protective role in coronary artery disease. Ann Med. 2013;45(4):348–356. doi: 10.3109/07853890.2013.770333. [DOI] [PubMed] [Google Scholar]
- 174.Caputo V, Tarantino G, Santini SJ, Fracassi G, Balsano C. The role of epigenetic control of mitochondrial (Dys)Function in MASLD onset and progression. Nutrients. 2023;15(22):4757. doi: 10.3390/nu15224757. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 175.Osna NA, Donohue TM, Jr, Kharbanda KK. Alcoholic liver disease: pathogenesis and current management. Alcohol Res. 2017;38(2):147–161. [PMC free article] [PubMed] [Google Scholar]
- 176.Marra F, Svegliati-Baroni G. Lipotoxicity and the gut-liver axis in NASH pathogenesis. J Hepatol. 2018;68(2):280–295. doi: 10.1016/j.jhep.2017.11.014. [DOI] [PubMed] [Google Scholar]
- 177.Chen B, Rao X, Wang X, Luo Z, Wang J, Sheng S, Liu Y, Zhang N, Jin S, Chen H, Sun C, Xu T, Du Y. cGAS-STING signaling pathway and liver disease: from basic research to clinical practice. Front Pharmacol. 2021;18(12):719644. doi: 10.3389/fphar.2021.719644. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 178.Wang X, Rao H, Zhao J, Wee A, Li X, Fei R, Huang R, Wu C, Liu F, Wei L. STING expression in monocyte-derived macrophages is associated with the progression of liver inflammation and fibrosis in patients with nonalcoholic fatty liver disease. Lab Invest. 2020;100(4):542–552. doi: 10.1038/s41374-019-0342-6. [DOI] [PubMed] [Google Scholar]
- 179.Li Y, Jin L, Jiang F, Yan J, Lu Y, Yang Q, Zhang Y, Zhang H, Yu H, Zhang Y, He Z, Zhang R, Yang J, Hu C. Mutations of NRG4 contribute to the pathogenesis of nonalcoholic fatty liver disease and related metabolic disorders. Diabetes. 2021;70(10):2213–2224. doi: 10.2337/db21-0064. [DOI] [PubMed] [Google Scholar]
Associated Data
This section collects any data citations, data availability statements, or supplementary materials included in this article.
Data Availability Statement
Not applicable.