Abstract
BACKGROUND.
Chronic opioid exposure leads to hedonic deficits and enhanced vulnerability to addiction, which are observed and even strengthen after a period of abstinence, but the underlying circuit mechanisms are poorly understood. In this study, we test the hypothesis that neurons expressing mu opioid receptors (MORs) in the dorsal raphe nucleus (DRN) are involved in addiction vulnerability associated with morphine abstinence, using both molecular and behavioral approaches.
METHODS.
MOR-Cre mice were exposed to chronic morphine then went through spontaneous withdrawal for 4 weeks, a well-established mouse model of morphine abstinence (ABS). We studied DRN-MOR neurons of ABS mice using (i) viral translating ribosome affinity for transcriptome profiling, (ii) fiber photometry to measure neuronal activity and (iii) opto-intracranial self-stimulation (oICSS) paradigm applied to DRN-MOR neurons to assess responses related to addiction vulnerability: persistence to respond, motivation to obtain the stimulation, self-stimulation despite punishment and cue-induced reinstatement.
RESULTS.
DRN-MOR neurons of ABS animals showed a down-regulation of genes involved in ion conductance and MOR-mediated signaling, as well as altered responding to acute morphine. Opto-ICSS data showed that abstinent animals execute more impulsive-like and persistent responses during acquisition, and score higher for addiction-like criteria.
CONCLUSION.
Our data suggest that protracted abstinence to chronic morphine leads to reduced MOR function in DRN-MOR neurons, and abnormal self-stimulation of these neurons. We propose that DRN-MOR neurons have partially lost their reward-facilitating properties, which in turn may lead to increased propensity to perform addiction-related behaviors.
Keywords: chronic morphine, mu opioid signaling, addiction-related behaviors, opto-intracranial self-stimulation, vTRAP-seq
INTRODUCTION
Opioid use disorder (OUD) is a chronic brain disorder, characterized by intoxication episodes that are followed by phases of withdrawal, craving and relapse (1). Acute opioid withdrawal occurs when the drug clears out of the body and is associated with strong physical and psychological symptoms (2), and contribute to the next intoxication episode (3). Further, after physical withdrawal signs have vanished and abstinence is maintained, hedonic deficits (4-8) develop in the long term, and enhance the risk of relapse (9, 10). Because maintaining abstinence is a true challenge, the neurobiology of this particular condition is being increasingly studied in preclinical research (11).
The dorsal raphe nucleus (DRN), the main source of serotonin (5-HT) in the central nervous system (12), plays a pivotal role in hedonic tone and mood-related psychiatric disorders or substance abuse (13-15). There is strong evidence for interactions between opioid and 5-HT systems in the DRN. For example, systemic morphine (16) and heroin (17) increased 5-HT neuron activity in rodents. Rats pretreated with fluoxetine showed enhanced preference for a morphine-associated environment (18) and DRN dopaminergic neurons were necessary for the expression of morphine conditioned place preference (19). In addition, opioid withdrawal is associated with impaired DRN activity. For example, opioid-dependent rats showed lower 5-HT release in response to morphine compared to naïve controls (20), naltrexone-precipitated withdrawal decreased DRN 5-HT levels (20), and 5-HT2C receptor activation suppressed physical withdrawal symptoms upon naloxone-precipitated withdrawal (21). Also, adaptations in glutamatergic inputs from the lateral habenula to the DRN were shown involved in social deficits associated with naloxone-precipitated withdrawal (22), and expression levels of BDNF, TrkB and CRF-R1 mRNAs were decreased in 5-HT neurons of the DRN during both morphine exposure and after seven days of withdrawal (23). Although non-exhaustive, this set of studies clearly demonstrates implication of the DRN and 5HT transmission in opioid dependence.
We previously developed a mouse model of protracted abstinence to chronic morphine, which revealed the emergence of social interaction deficits and despair-like behavior four weeks after cessation of the chronic morphine exposure (24). Conditional deletion of MORs in the DRN prior to morphine exposure (25) and fluoxetine treatment during abstinence (24, 26) prevented the emergence of these phenotypes in abstinent animals, demonstrating a causal link between chronic receptor activation in the DRN and emotional responses. Here, we used the same mouse model of morphine abstinence to study neurons expressing the receptor (DRN-MOR neurons), as these neurons represent the primary cellular target of opioids in the DRN. We tested the hypothesis that DRN-MOR neurons have adapted to morphine with both molecular and behavioral consequences related to addiction. We found significant downregulation of the Opioid Reactome in DRN-MOR neurons of abstinent animals, and also discovered higher propensity of these animals to opto-self-stimulate these neurons.
METHODS AND MATERIALS
Animals
All experiments were performed in accordance with the Canadian Council of animal Care and by the Animal Care Committees (see Suppl Information for details).
Morphine treatment
Morphine sulfate (NIH, NIDA Drug Supply Program) was prepared in saline (0.98% sodium chloride) and injected twice daily intraperitoneally with escalating doses of morphine (20, 40, 60, 80, 100 mg/kg for 5 days, followed by a single 100mg/kg injection on day 6), according to our previously described protocol (24, 27) and see Suppl Information.
Viral translating ribosome affinity purification and RNA sequencing, fiber photometry and operant optogenetic self-stimulation
See Suppl Information for details.
RESULTS
The transcriptome of DRN-MOR neurons is modified in abstinent mice
We first examined whether the prior history of chronic morphine exposure durably modifies gene expression in DRN-MOR neurons. To specifically analyze gene expression in these neurons, we used the viral translating ribosome affinity purification (vTRAP) methodology (28). We targeted expression of a L10a-mCherry ribosomal transgene to these neurons by injection of a Cre-dependent virus in the DRN of MOR-Cre mice (Figure 1A). Animals were subsequently exposed to chronic morphine (escalating doses 20-100 mg/kg over 6 days, see Methods) or sasuline. Four weeks later, the DRN was micro-dissected and we proceeded to the separation of RNA fractions coming from total DRN tissue (input) or from immuno-precipitated (IP) ribosomes. The two RNA fractions were used for RNA-Seq library preparation and sequenced at similar depth (Suppl Figure S1; two-way ANOVA, Fraction effect, n.s.) with no difference between treatment groups (two-way ANOVA, Treatment effect, n.s.).
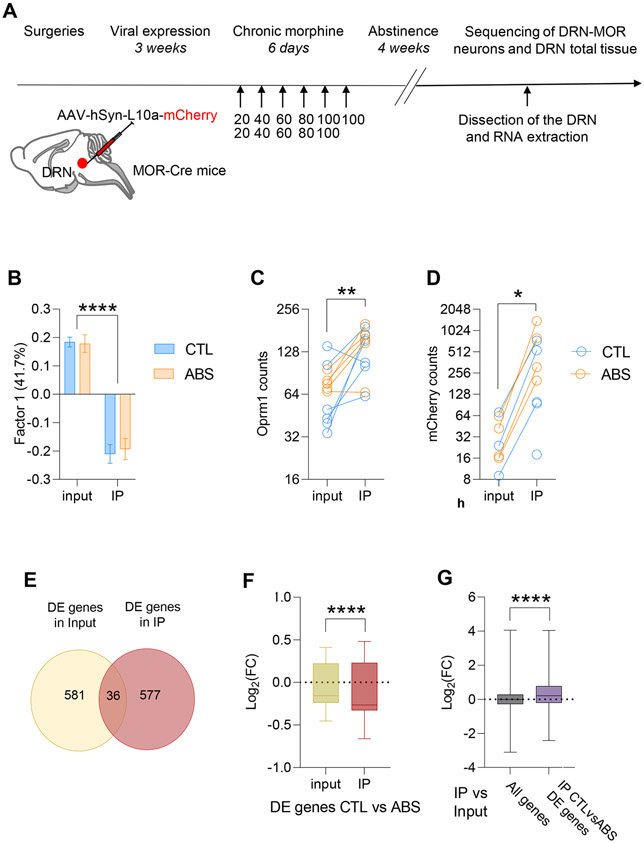
Figure 1. Gene transcription is modified in abstinent mice.
(A-D) Viral translating ribosome affinity purification allows to sequence the transcriptome of DRN-MOR neurons. (A) Schematic of experimental timeline: MOR-Cre mice were injected in the DRN to express L10a-mCherry protein in DRN-MOR neurons, and were subsequently injected chronically either with saline CTL) or morphine (ABS). Four weeks after the last morphine exposure the DRN was micro-dissected. A fraction of total tissue mRNAs was conserved (input samples) and we immuno-precipitated mRNAs bounds to mCherry-L10a (IP samples) (n=6/6). (B) Principal component analysis: Factor 1 explains 41.7% of the variance of the and mostly corresponds to the fraction samples (IP vs input). (C) Quantification of Oprm1 transcripts: IP samples were enriched for Oprm1 transcripts compare to input samples for CTL and ABS. (D) Quantification of mCherry transcripts: IP samples were enriched for mCherry transcripts compare to input samples for CTL and ABS. (E-G) Gene expression is modified in DRN-MOR neurons from abstinent mice. (E) Differentially expressed genes after morphine abstinence (CTL vs ABS) are mainly distinct genes in input vs IP samples. (F) Comparison of the fold change of differentially expressed genes in CTL vs ABS: the fold change of differentially expressed genes is higher in IP versus input samples. (G) Comparison of the fold change of differentially expressed genes in IP vs input for all genes or genes differentially expressed in IP CTL vs IP ABS: the fold change of differentially expressed genes were higher when looking only at genes affected by morphine abstinence (IP CTL vs ABS differentially expressed genes) than at all genes. Data are represented as mean ± SEM. *: p<0.05; **: p<0.01; ****: p<0.0001.
A PCA analysis performed on all samples (IP and input) from control (CTL) and abstinent (ABS) animals revealed that factor 1, explaining 41.71% of the total variance, discriminated IP from input samples (Figure 1B; two-way RM ANOVA, Fraction effect, p<0.0001). In addition, as expected, IP versus input samples showed higher expression counts for MOR (Figure 1C; two-way RM ANOVA, Fraction effect, p<0.01) and mCherry (Figure 1D; two-way RM ANOVA, Fraction effect, p<0.05), suggesting that the procedure successfully enriched MOR-neurons in the IP fraction. This enrichment was similar for CTL and ABS groups (Figure 1C-D; two-way RM ANOVA, Treatment effect, n.s.), enabling unbiased assessment of abstinence-related differences.
We also compared differentially expressed genes in IP vs input samples of CTL mice with recent single-cell expression data generated in the DRN (29) (Suppl Figure S2). We found that IP samples were depleted in markers genes for several non-neuronal cell types (oligodendrocytes, polydendrocytes), and enriched for markers of one type of serotonergic neurons, two types of GABAergic neurons and three types of glutamatergic neurons, which matches our own immunohistochemical characterization indicating that DRN-MOR neurons are mainly glutamatergic and GABAergic, and for a smaller proportion serotonergic (30). Altogether, these results show that the vTRAP procedure enables the analysis of morphine abstinence effects in an enriched DRN-MOR neuron population.
We next analyzed whether gene expression is modified in ABS animals by comparing input or IP RNA-seq data from ABS and CTL mice. While a similar number of genes were differentially expressed at nominal significance (p<0.05) in IP (613 genes) and input RNA fractions (617 genes), there was little overlap between the two (Jaccard index ±3%, 36 differentially expressed genes in common, Figure 1E). Further, morphine abstinence was associated with down-regulation of gene expression (Figure 1F) in both IP (406/613 downregulated genes; One sample t-test, p<0.0001) and input fractions (340/617 downregulated genes; one sample t-test, p<0.01). Downregulation was more pronounced in the IP vs input (Figure 1F, unpaired t-test, p<0.0001), suggesting that the global transcriptional impact of abstinence is stronger in DRN-MOR neurons. This was confirmed by the observation that, compared to the whole genome, genes that were differentially expressed as a function of abstinence in the IP (Figure 1G) showed significantly higher fold changes in the IP/input comparison (unpaired t-test, p<0.0001).
DRN-MOR neurons from abstinent mice show reduced expression of neuronal excitability and opioid signaling genes, as well as altered responsivity to morphine
We then conducted a gene ontology analysis (31) (Figure 2A and Suppl Tables S1-5) of differentially expressed genes in DRN-MOR neurons. The top 10 gene ontology terms that were significantly over-represented in ABS vs CTL IP samples related to the regulation of neuronal excitability and ion conductance (Suppl Tables S1-2). Notably genes regulating potassium (K+) efflux from neuronal cells were downregulated including Kcnj14, Kcnk6, Kcnn4, Kcnmb4 and Kcns2, or genes associated with sodium exchanges (Scn4b), and voltage–dependent regulation of calcium (Ca2+) influx (Cacng3, Cacng5, Cacna1d). Genes such as Gabra3 (GABA A receptor, subunit alpha 3) or Htr3a (5-HT receptor 3A) were also decreased (Suppl Table S1) suggesting that morphine abstinence alters neurotransmission in DRN-MOR neurons. Other sets of differentially expressed genes were linked to axon formation including genes for microtubules functions (Dnah9, Dnah5, Dnah10, Dnah7a or Kif3b), and ciliary functions (Cfap206, Cfap52, Cep162) (Suppl Table S3), indicative of cellular morphology changes.
Figure 2. DRN-MOR neuron function is altered in abstinent mice.
(A-C) DRN-MOR neurons of abstinent mice show decreased gene expression related neuronal excitability and opioid signaling. (A) Over representation-analysis: the gene ontology (GO) terms underrepresented in DRN-MOR neurons of ABS animals included: Molecular function (passive transmembrane transporter activity, channel activity, cation channel activity, ion channel activity, substrate specific channel activity), Cellular component (cilium, ciliary part, axoneme part) and Biological processes (metanephros development, kidney morphogenesis). (B) Gene Set Enrichment Analysis (GSEA) with gene set REACTOME_OPIOID_SIGNALLING (33). (E) GSEA performed on IP ABS vs CTL differentially expressed genes indicated transcriptional depletion of opioid signaling pathway in DRN-MOR neurons of ABS animals. (C) Enrichment profile for differentially expressed genes between IP CTL vs ABS. Data are represented as mean ± SEM. *: p<0.05; ****: p<0.0001. (D). DRN-MOR neurons from ABS mice respond differently to morphine. Left: schematic of viral injection of the GCaMP expressing Cre-dependent virus and optic fiber (OF) implantation in the DRN of MOR-Cre mice. Middle: time courses of average GCaMP6m z-scores following morphine administration (10 mg/kg, i.p) in CTL and ABS animals showing a reduction of DRN-MOR neurons calcium activity with a maximum effect 4min after injection. Right: the average of z-score of DRN-MOR neurons fluorescence levels is reduced from 6 to 12 min after morphine injection in ABS animals compared to the CTL animals. n=5 for CTL and n=6 for ABS. Welch t-test: t(6.45)=2.64; p<0.05.
We next used gene set enrichment analysis (32) to further characterize the impact of morphine abstinence on the Opioid Reactome (33), an annotated gene list that is composed of genes known to be involved in opioid signaling (±90 genes, see Suppl Table S6), including Oprm1 itself, intracellular messengers of GPCR-regulated pathways (such as G protein subunits and adenylate cyclases) and proteins involved in downstream phosphorylation cascades (e.g. calcium/calmodulin kinases or protein kinase C alpha and gamma). We found enrichment of the Opioid Reactome in IP fractions compared to input for CTL and ABS samples (Figure 2B, FDR BH, CTL: q=0.18; ABS: q=0.35), a trend that did not reach significance but still consistent with the enrichment of DRN-MOR neurons in IP samples. Notably, comparison of CTL and ABS IP fractions revealed a significant depletion of the Opioid Reactome in DRN-MOR neurons of ABS mice (Figure 2B, FDR BH, q<0.05), suggesting that morphine abstinence reduces expression levels of genes involved in MOR activity signaling.
RNA-seq data together demonstrate that DRN-MOR neurons of ABS animals show a specific down-regulation of genes related to neuronal excitability and opioid signaling, which could possibly alter their function. To further test this hypothesis, we measured the response of DRN-MOR neurons to acute morphine, using fiber photometry (Figure 2C and Suppl Info). MOR-Cre mice were injected with a Cre-dependent virus expressing the calcium indicator GCaMP6m in the DRN, and implanted with an optic fiber 100μm above the injection site. Animals were then subjected to chronic saline or morphine, as above. After 4 weeks abstinence, modification of the calcium signal upon a single morphine dose (10 mg/kg, i.p.) was recorded. Morphine reduced the activity of DRN-MOR neurons in the SAL group, as expected (30). The morphine effect in ABS mice was different, and notably, a reduced morphine effect was observed in ABS mice in the 6-12 min period post-injection (p<0.05). Transcriptional modifications observed in ABS mice, therefore, are associated with a detectable reduction of DRN-MOR neuron responsivity to morphine, possibly reflecting an impairment of DRN-MOR neuron function.
An oICSS paradigm to study reward-related behaviors controlled by DRN-MOR neurons
Our prior experiments had shown that mice self-administer the opto-stimulation of their DRN-MOR neurons (30), demonstrating that activation of these neurons has rewarding properties, and consistent with previous work (34). We therefore tested whether this behavior, referred as to opto-intracranial self-stimulation (oICSS), is modified in ABS animals.
MOR-Cre mice were injected in the DRN with a Cre-inducible virus expressing ChR2 fused to mCherry (Suppl Fig S3), or mCherry alone, and implanted with an optic fiber targeting the DRN (Figure 3A). Three weeks after surgeries, mice were submitted to the chronic saline (CTL) or morphine (ABS) treatment, and the acquisition of self-stimulation started 28 days after the last morphine injection (24, 25). Animals were trained to self-administer the laser stimulation (LS) during 28 consecutive days under a fixed ratio (FR) that gradually increased from FR1 to FR5. The operant paradigm was also designed as to measure the persistence to respond during time-off periods. Next, we tested i) the motivation to obtain the LS in a progressive ratio procedure, ii) the level of compulsive-like behaviors, evaluated as nosepoking despite receiving a foot-shock, iii) reinstatement of the oICSS behavior, measured as cue-induced nosepoking after extinction training.
Figure 3. Control and abstinent mice learn to self-stimulate DRN-MOR neurons.
(A) Schematic of the experimental timeline: MOR-cre mice were injected in the DRN to express mCherry-ChR2 proteins and implanted with an optic fiber to activate DRN-MOR neurons with laser stimulation. Three weeks after surgeries, mice were injected either with saline (CTL, n=17) or with escalating doses of morphine (ABS, n=17). Four weeks after the last injection, mice were trained to nosepoke for the laser stimulation according to a fixed ratio of 1 (FR1; 4 days), FR3 (9 days) and FR5 (15 days) (acquisition). Mice were also evaluated for different addiction-related behaviors (persistence to respond, motivation, compulsion and reinstatement after extinction training). (B) Left. Total number of nosepokes performed during each 1 hr self-stimulation session during acquisition (28 days). Right. Average nosepokes performed during each reinforcement schedule: CTL and ABS discriminated the active from the inactive and increased the number of active nosepoke according to the schedule. (C) Left. Total number of earned laser stimulation bouts during each self-stimulation session during acquisition. Left. Average laser stimulations earned during each reinforcement schedule: CTL and ABS mice earned similar number laser stimulation across schedules. Data are represented as mean ± SEM.**** / ˚˚˚˚: p<0.0001.
Control and abstinent mice self-stimulate their DRN-MOR neurons
CTL mice discriminated between the active and inactive nosepoke (Figure 1B, left; three-way RM ANOVA, NP effect, p<0.0001) and the number of active responses increased from FR1 (66 ± 9 active nosepokes/session) to FR3 (148 ± 11 active nosepokes/session) and FR5 schedules (196 ± 12 active nosepokes/session) (three-way RM ANOVA, Schedule effect, p<0.0001). ABS animals seemed to self-stimulate at higher levels (FR1: 84 ± 10 active nosepokes/session; FR3: 176 ± 14 active nosepokes/session; FR5: 228 ± 18 active nosepokes/session) during the entire oICSS experiment, but the statistical analysis showed no significant difference between the CTL and ABS groups (Figure 1B, right; three-way RM ANOVA, Treatment effect, n.s.).
The number of LS earned during each 1-hour operant session remained stable across schedules for the two groups (Figure 1C; two-way ANOVA, Schedule effect, n.s.): both CTL and ABS mice adapted their nosepokes to receive 41 ± 1 LS/session (38 ± 1 and 43 ± 1 LS respectively) across the acquisition period. As expected, none of the mCherry-mice achieved the acquisition criteria (Suppl Figure S4A), demonstrating that the self-stimulation behavior was specific to DRN-MOR neuron activation. A post-mortem analysis confirmed that the LS induced a significant increase in the number of c-Fos+ cells in the DRN of ChR2-mice compared to mCherry-mice (Suppl Figure S4B-C; Kruskal-Wallis test, virus effect, p<0.01), and for both CTL (p<0.01) and ABS (p<0.01) groups. The data confirm our prior study showing that DRN-MOR neuron opto-activation has reinforcing properties, and also suggest that acquisition of this behavior does not differ in ABS mice.
Abstinent mice perform more impulsivity-like nose pokes and persistent responses during the acquisition of oICSS
Although ABS mice did not differ from CTL mice in the number of nosepoke and LS earned per session, other parameters indicate differences between the two groups. ABS mice performed on average more impulse-like nosepokes (performed during the first 5 sec of the session with cue-light on and LS off, see (35) and Suppl Figure S5A) than CTL mice (Figure 4A; three-way RM ANOVA, Treatment effect, p<0.05). Nosepokes performed during the following 15s (Two-way RM ANOVA, Treatment effect, p=0.1501, Suppl Figure S5B) was similar in the two groups, further supporting the notion of impulsive-like responses (35, 36). Locomotor activity across all sessions (Two-way RM ANOVA, Treatment effect, p=0.42, Suppl Figure S5C) also was similar, suggesting that no general hyperactivity interferes with these responses.
Figure 4. Abstinent mice perform more impulsivity-like and persistent responses when evaluated for several addiction-related behaviors.
(A) Left. Total number of impulsivity-like responses during each 1 hr self-stimulation session during acquisition (28 days. Right. Average impulsivity-like nose pokes per session for each reinforcement schedule: ABS mice performed in average more impulsivity-like nose pokes during acquisition. (B) Left. Persistence to respond FR3: ABS mice performed more persistent nose pokes than CTL mice. Right. Persistence to respond FR5: ABS did not perform more persistent responses than CTL mice. (C) Motivation: ABS mice did not perform more active nose pokes during the progressive ratio session (D) Compulsivity-like: ABS mice did not earn more laser stimulation during the foot-shock session. (E) Extinction and reinstatement: cue presentation reinstated active nose pokes after extinction in CTL and ABS mice. Data are represented as mean ± SEM. #: p<0.05; **: p<0.01; **** / ˚˚˚˚: p<0.001.
We also measured the persistence to respond by introducing a time-off period (no light cue, no LS), in the middle of the oICSS session during FR3 and FR5 sessions (37, 38). ABS mice performed more active responses during the time-off period in the FR3 schedule (40 ± 3) compared to CTL mice (29 ± 2; Figure 4B; three-way RM ANOVA, effect of Treatment, p<0.05). This result indicates that ABS animals show a more limited ability to inhibit a response once it is initiated.
Abstinent mice display more addiction-related criteria than control mice
Next we recorded motivation to obtain the LS, compulsivity-like behavior and cue-induced reinstatement of the oICSS behavior. During the progressive ratio session, CTL mice performed 396 ± 36 active nosepokes while ABS mice performed 433 ± 38 active nosepokes in total (Figure 4C; unpaired t-test, n.s.). During the foot-shock session, CTL mice earned 11 ± 1 LS in total, while ABS mice earned 13 ± 1 LS (Figure 4D; unpaired t-test, n.s.). After the foot-shock session, mice were trained under a FR5 schedule to restore baseline nosepoking and earned LS (Suppl Figure S6A-B), and the self-stimulation behavior was subsequently extinguished during 10 days. No physical withdrawal sign was observed during extinction.
During the last extinction session, CTL and ABS mice performed 15 ± 2 and 21 ± 3 active nosepokes, respectively (Figure 4E; two-way RM ANOVA, Treatment effect, n.s.). During the cue-induced reinstatement session, both CTL and ABS mice re-established nosepoking (Figure 4E; two-way RM ANOVA, Cue effect, p<0.01) and performed similarly with 28 ± 4 and 34 ± 7 active nosepokes, respectively (Figure 4E; two-way RM ANOVA, Treatment effect, n.s.). None of the group comparison revealed a significant effect of morphine abstinence, suggesting that motivation, compulsive-like behavior and reinstatement were similar for CTL and ABS mice.
Yet ABS mice tended to score slightly higher than CTL for each measured behavior, which prompted us to perform a three-criteria analysis that takes advantage of inter-individual variability (37, 39) (Figure 5). This analysis separates mice into four groups ranging from 0 to 3 criteria of addiction-related behaviors (37). As previously done for cocaine (37) and sucrose self-administration (39), we correlated intensity of responses in the three tests with the intensity of the criterion met by each mouse: mice with the higher criteria of addiction-related behaviors were also mice that scored higher for persistence to respond (Figure 5A; Kruskal-Wallis test, Criteria effect, p<0.001), motivation (Figure 5B; Kruskal-Wallis test, Criteria effect, p<0.01) and compulsivity-like behavior (Figure 5C; Kruskal-Wallis test, Criteria effect, p<0.05).
Figure 5. Abstinent mice score higher for addiction-related criteria in the oICSS behavior.
(A-C) Comparison of addiction-related behaviors in mice presenting 0, 1, 2 or 3 criteria of addiction-related behaviors. The higher the criteria of addiction-related behavior the higher the animal scored for (A) the persistence to respond (B) the motivation (C) the compulsivity-like responses. (D) % of mice in each criterion of addiction-related behaviors for CTL and ABS mice. (E) ABS mice have higher criteria of addiction-related behaviors than CTL mice. (F) Principal component analysis: Factor 1 explains 45.3% of the total variance between individuals and takes into account four behavioral measures (compulsivity, motivation, persistence to respond and reinstatement). (G-H) Representation of individual values in Factor 1 and 2 according to (G) the group-criteria of addiction-related behaviors: Factor 1 ranks the individuals according to their criteria of addiction-related behavior. (H) the treatment: Factor 1 ranks the individuals according to their treatment (morphine or saline). Data are represented as mean ± SEM. *: p<0.05; **: p<0.01; ***: p<0.001; ****: p<0.0001.
The four groups were distributed differently among CTL and ABS animals (Figure 5D), so that 52.9% CTL mice presented 0 criteria vs 11.8% for ABS mice, and only 5.9% CTL mice reached 3 criteria vs 17.6% for ABS mice. In addition, ABS mice were in average attributed more addiction-related criteria that CTL mice (Figure 5E; Chi-square test for trend, p<0.05). This analysis demonstrates that morphine abstinence induces a shift towards a higher score in the three-criteria analysis.
We finally performed a PCA analysis with data collected for persistence, motivation, compulsion and reinstatement tests (Figure 5F: variables’ space, Figure 5G-H: subjects’ space). The first factor aggregated the four behavioral tests and explained 45.3% of the variance. Projection in the subjects’ space revealed that Factor 1 clustered individuals according to their addiction-related criteria (Figure 5G, one-way RM ANOVA on Factor 1, p<0.0001), and subjects were ranked according to their number of criteria along the Factor 1 axis: the higher the values in Factor 1, the higher the criteria of addiction-related behaviors, suggesting that Factor 1 reflects the expression of addiction-related behaviors. In addition, Factor 1 also significantly dissociated CTL from ABS mice (Figure 5H; unpaired t-test, p<0.05), demonstrating distinct patterns of addiction-related behaviors across the two populations. In line with the three-criteria analysis, this result shows that an individual-based analysis allows a significant separation of ABS and CTL animals, and suggests that the prior exposure to morphine enhances the propensity to perform addiction-related behaviors in the DRN-MOR neuron oICSS paradigm.
DISCUSSION
In summary, our data show an alteration of DRN-MOR neuron function in morphine ABS animals at both molecular and behavioral levels. At present, only few neuronal types or microcircuits have been show modified upon protracted abstinence to morphine, and these include kappa opioid receptor-expressing neurons in the DRN projecting to the NAc (40), the VTA-tVTA circuit (41) or the amygdala-NAC pathway (42). Here we show for the first-time major alterations in cells that are the primary target of morphine within a major mood center.
Morphine abstinence impairs the transcriptome of DRN-MOR neurons
Our laboratory reported transcriptional modifications developing during morphine abstinence, notably in the extended amygdala (27, 43), however a cell-specific characterization of morphine abstinence effects has not been done. Using vTRAP in MOR-Cre mice, we were able to isolate and sequence the transcriptome of DRN-MOR neurons.
Differentially expressed genes (CTL vs ABS) largely differed in total DRN tissue versus enriched DRN-MOR neurons. This observation is in line with the significant enrichment of DRN-MOR neurons in IP samples, which results from the high Cre/MOR co-localization demonstrated in MOR-Cre mice (44). Also, the effect size of abstinence was higher in DRN-MOR neurons, suggesting that the chronic morphine regimen has a higher impact on the transcriptome of MOR-neurons compared to whole DRN tissue. This finding is consistent with the notion that MOR-neurons are the primary pharmacological target of opioids in the brain, and encourages future experiments using vTRAP in MOR-Cre mice to better grasp subtle transcriptional modifications in opioid studies across the brain.
The gene ontology analysis shows major alterations in the expression of ion channel-encoding genes, suggesting that protracted morphine abstinence modifies ion conductance in DRN-MOR neurons. Notably, the expression of genes associated with K+ efflux and voltage–dependent regulation of Ca2+ influx in neuronal cells were modified in ABS animals, which could modify the intrinsic excitability of DRN-MOR neurons and disrupt neurotransmitter release (45-47). Therefore, both neuronal function and opioid sensitivity of DRN-MOR neurons are likely impaired by a prior history of morphine exposure.
The gene set enrichment analysis also demonstrates a downregulation of the Opioid Reactome in DRN-MOR neurons of ABS individuals. This is consistent with previous studies revealing modifications in several major GPCR signaling pathways (27, 48, 49) and reduced Penk expression upon long-term morphine withdrawal (43). Even though these results were collected from other brain regions and bulk tissue, these studies together with our data, support the notion that morphine abstinence reduces GPCR signaling, including opioid reactivity, in brain regions associated with the modulation of reward and affective responses.
oICSS of DRN-MOR neurons is a useful approach to study reward seeking behaviors in the context of morphine abstinence
oICSS was used previously to evaluate the reinforcing properties of selected neuronal populations (50-54). More recently, Pascoli et al. proposed that oICSS of dopaminergic neurons (oDASS) located in the ventral tegmental area can also be used as a model of compulsive drug-taking in mice. The authors demonstrated that oDASS was sufficient to recapitulate some behavioral hallmarks of addiction, including compulsive responding for the LS despite a foot-shock (35). Our own paradigm was based on this study: mice trained to self-stimulate their DRN-MOR neurons reached 41 LS in 1 hour, while mice trained with oDASS obtained 80 LS in a 45 min (35). Despite the lower level of self-stimulation for oICSS of DRN-MOR neurons, mice in our study controlled the total number of LS received across sessions just as for oDASS (35), suggesting the existence of a hedonic threshold specific to each reinforcer (drug infusion or opto-stimulation of different neuronal population) (35, 55, 56).
Mice self-stimulating DRN-MOR neurons developed behavioral responses observed in rodent drug self-administration models classically used in rodent addiction research (37, 57-59), including impulsivity-like responses, persistent responses, compulsivity-like responses, and cue-induced reinstatement (60, 61). Therefore, the DRN-MOR neuron oICSS paradigm seems to be an appropriate approach to test whether morphine abstinence has consequences on reward seeking and possibly vulnerability to develop addiction-like behaviors. Morphine self-administration could have been used but re-exposition to the drug would hinder neuro-adaptations specific to the ABS state. Sucrose self-administration also would be inappropriate because opioid abstinence was shown to decrease sucrose preference (25, 62, 63) and self-administration (64).
Protracted morphine abstinence increases vulnerability to perform addiction-related behaviors
Nosepokes and earned LS were similar for ABS and CTL mice during acquisition, suggesting that reinforcing properties of the ChR2-mediated activation of DRN-MOR neurons are intact in ABS mice, despite altered expression of genes related to neuronal excitability and opioid signaling. However, other oICSS responses were different: first, ABS mice performed more non-rewarded active nosepokes between at the onset of the cue-light (first 5s), considered a marker of impulsivity predictive of future vulnerability to addiction (35, 65-69) and consistent with the human literature showing persistent impulsivity in former heroin users (70-73). Second, ABS mice are more prone to perform addiction-related behaviors in the three-criteria analysis. This result is coherent with pre-clinical studies showing that prior opioid exposure increased opioid self-administration (74-76) and clinical work designating prior opioid exposure as a major risk factor for addiction to opioids (77-81) and other drugs (82). This also strengthens the idea that chronic opioids induce neuroadaptations that will later drive addiction (10). An important next step would be to investigate whether this particular behavioral adaptation is detectable through the manipulation of other brain circuits, by testing oICSS of other populations of MOR-neurons. Another important step will be to perform a similar study in female mice, as gender differences are reported in opioid abstinence (74, 83-85).
DRN-mediated mechanisms underlying behavioral alterations in protracted abstinence to chronic morphine: a possible reward deficiency?
The present study demonstrates that a prior history of chronic morphine exposure followed by a period of protracted abstinence leads to (i) a downregulation of gene clusters involved in neuronal excitability and GPCR signaling, including the MOR-regulated pathway, in DRN-MOR neurons, (ii) modified responsivity to an acute morphine challenge of these neurons and (iii) the emergence of increased impulsivity and higher propensity to perform addiction-related behaviors in the DRN-MOR neuron oICSS paradigm. We may speculate that the behavioral phenotype of ABS animals is a consequence of reduced DRN-MOR neuron function, including their ability to respond to endogenous opioids. This deficit may in turn jeopardize reward processing ensured by these neurons (30), and lead to enhanced reward seeking behavior. Likewise, it is possible that this mechanism also underlies the DRN-mediated negative emotional state, which we demonstrated previously in ABS animals (25). Overall, the integrity of DRN neurons responsive to opioids may prove essential to understand the negative affect and high relapse susceptibility of individuals with a history of OUD.
Supplementary Material
ACKNOWLEDGMENTS AND DISCLOSURES
This work was supported by the National Institutes of Health (P50DA005010 and R01048796 to BLK), the Canada Fund for Innovation and the Canada Research Chairs (ED and BLK), Support was also received from the ‘Centre National de la Recherche Scientifique’ (CF and PEL), Strasbourg University (CF and PEL), French National Research Agency (ANR-19-CE37-0010; PEL), ‘Fondation Fyssen’ (‘subvention recherche 2021’; PEL), and ‘Fondation pour la Recherche Médicale’ (FDT202204015236; CF). Sequencing was performed by the GenomEast platform, a member of the ‘France Génomique’ consortium (ANR-10-INBS-0009). We also thank the staff of the animal facility of the Neurophenotyping Center of the Douglas Mental Health University Institute (Montréal, Canada).
LW, PEL and BLK designed the experiments. LW, EC, CF performed the experiments. ED, PEL, SBH and FA helped for experimental design and data analysis. BLK and LW wrote the manuscript. BLK provided resources for the study. All authors read and approved the submitted version.
Footnotes
The authors report no biomedical financial interests or potential conflicts of interest
REFERENCES
- 1.Koob GF, Volkow ND (2016): Neurobiology of addiction: a neurocircuitry analysis. Lancet Psychiatry. 3:760–773. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 2.Kosten TR, Baxter LE (2019): Review article: Effective management of opioid withdrawal symptoms: A gateway to opioid dependence treatment. Am J Addict. 28:55–62. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 3.Hutcheson DM, Everitt BJ, Robbins TW, Dickinson A (2001): The role of withdrawal in heroin addiction: enhances reward or promotes avoidance? Nat Neurosci. 4:943–947. [DOI] [PubMed] [Google Scholar]
- 4.Kenny PJ, Chen SA, Kitamura O, Markou A, Koob GF (2006): Conditioned withdrawal drives heroin consumption and decreases reward sensitivity. J Neurosci. 26:5894–5900. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 5.Swain Y, Muelken P, Skansberg A, Lanzdorf D, Haave Z, LeSage MG, et al. (2020): Higher anhedonia during withdrawal from initial opioid exposure is protective against subsequent opioid self-administration in rats. Psychopharmacology (Berl). 237:2279–2291. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 6.Holtz NA, Radke AK, Zlebnik NE, Harris AC, Carroll ME (2015): Intracranial self-stimulation reward thresholds during morphine withdrawal in rats bred for high (HiS) and low (LoS) saccharin intake. Brain Res. 1602:119–126. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 7.Schaefer GJ, Michael RP (1986): Changes in response rates and reinforcement thresholds for intracranial self-stimulation during morphine withdrawal. Pharmacology Biochemistry and Behavior. 25:1263–1269. [DOI] [PubMed] [Google Scholar]
- 8.Schaefer GJ, Michael RP (1983): Morphine withdrawal produces differential effects on the rate of lever-pressing for brain self-stimulation in the hypothalamus and midbrain in rats. Pharmacology Biochemistry and Behavior. 18:571–577. [DOI] [PubMed] [Google Scholar]
- 9.Baker TB, Piper ME, McCarthy DE, Majeskie MR, Fiore MC (2004): Addiction motivation reformulated: an affective processing model of negative reinforcement. Psychol Rev. 111:33–51. [DOI] [PubMed] [Google Scholar]
- 10.Evans CJ, Cahill CM (2016): Neurobiology of opioid dependence in creating addiction vulnerability. F1000Res. 5. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 11.Welsch L, Bailly J, Darcq E, Kieffer BL (2020): The Negative Affect of Protracted Opioid Abstinence: Progress and Perspectives From Rodent Models. Biol Psychiatry. 87:54–63. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 12.Jacobs BL, Azmitia EC (1992): Structure and function of the brain serotonin system. Physiol Rev. 72:165–229. [DOI] [PubMed] [Google Scholar]
- 13.Baldwin D, Rudge S (1995): The role of serotonin in depression and anxiety. Int Clin Psychopharmacol. 9 Suppl 4:41–45. [DOI] [PubMed] [Google Scholar]
- 14.Valentino RJ, Lucki I, Van Bockstaele E (2010): Corticotropin-releasing factor in the dorsal raphe nucleus: Linking stress coping and addiction. Brain Res. 1314:29–37. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 15.Kirby LG, Zeeb FD, Winstanley CA (2011): Contributions of serotonin in addiction vulnerability. Neuropharmacology. 61:421–432. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 16.Tao R, Auerbach SB (2002): GABAergic and glutamatergic afferents in the dorsal raphe nucleus mediate morphine-induced increases in serotonin efflux in the rat central nervous system. J Pharmacol Exp Ther. 303:704–710. [DOI] [PubMed] [Google Scholar]
- 17.Wei C, Han X, Weng D, Feng Q, Qi X, Li J, et al. (2018): Response dynamics of midbrain dopamine neurons and serotonin neurons to heroin, nicotine, cocaine, and MDMA. Cell Discov. 4:60. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 18.Harris GC, Aston-Jones G (2001): Augmented Accumbal Serotonin Levels Decrease the Preference for a Morphine Associated Environment During Withdrawal. Neuropsychopharmacology. 24:75–85. [DOI] [PubMed] [Google Scholar]
- 19.Lin R, Liang J, Wang R, Yan T, Zhou Y, Liu Y, et al. (2020): The Raphe Dopamine System Controls the Expression of Incentive Memory. Neuron. 106:498–514.e498. [DOI] [PubMed] [Google Scholar]
- 20.Tao R, Ma Z, Auerbach SB (1998): Alteration in regulation of serotonin release in rat dorsal raphe nucleus after prolonged exposure to morphine. J Pharmacol Exp Ther. 286:481–488. [PubMed] [Google Scholar]
- 21.Zhang G, Wu X, Zhang YM, Liu H, Jiang Q, Pang G, et al. (2016): Activation of serotonin 5-HT(2C) receptor suppresses behavioral sensitization and naloxone-precipitated withdrawal symptoms in morphine-dependent mice. Neuropharmacology. 101:246–254. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 22.Valentinova K, Tchenio A, Trusel M, Clerke JA, Lalive AL, Tzanoulinou S, et al. (2019): Morphine withdrawal recruits lateral habenula cytokine signaling to reduce synaptic excitation and sociability. Nature Neuroscience. 22:1053–1056. [DOI] [PubMed] [Google Scholar]
- 23.Lunden JW, Kirby LG (2013): Opiate exposure and withdrawal dynamically regulate mRNA expression in the serotonergic dorsal raphe nucleus. Neuroscience. 254:160–172. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 24.Goeldner C, Lutz P-E, Darcq E, Halter T, Clesse D, Ouagazzal A-M, et al. (2011): Impaired emotional-like behavior and serotonergic function during protracted abstinence from chronic morphine. Biol Psychiatry. 69:236–244. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 25.Lutz P-E, Ayranci G, Chu-Sin-Chung P, Matifas A, Koebel P, Filliol D, et al. (2014): Distinct mu, delta, and kappa opioid receptor mechanisms underlie low sociability and depressive-like behaviors during heroin abstinence. Neuropsychopharmacology. 39:2694–2705. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 26.Lalanne L, Ayranci G, Filliol D, Gavériaux-Ruff C, Befort K, Kieffer BL, et al. (2017): Kappa opioid receptor antagonism and chronic antidepressant treatment have beneficial activities on social interactions and grooming deficits during heroin abstinence. Addict Biol. 22:1010–1021. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 27.Le Merrer J, Befort K, Gardon O, Filliol D, Darcq E, Dembele D, et al. (2012): Protracted abstinence from distinct drugs of abuse shows regulation of a common gene network. Addict Biol. 17:1–12. [DOI] [PubMed] [Google Scholar]
- 28.Nectow AR, Moya MV, Ekstrand MI, Mousa A, McGuire KL, Sferrazza CE, et al. (2017): Rapid Molecular Profiling of Defined Cell Types Using Viral TRAP. Cell Rep. 19:655–667. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 29.Huang KW, Ochandarena NE, Philson AC, Hyun M, Birnbaum JE, Cicconet M, et al. (2019): Molecular and anatomical organization of the dorsal raphe nucleus. eLife. 8:e46464. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 30.Welsch L, Colantonio E, Frison M, Johnson DA, McClain SP, Mathis V, et al. (2023): Mu opioid receptors-expressing neurons in the dorsal raphe nucleus are involved in reward processing and affective behaviors. Biol Psychiatry. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 31.Liao Y, Wang J, Jaehnig EJ, Shi Z, Zhang B (2019): WebGestalt 2019: gene set analysis toolkit with revamped UIs and APIs. Nucleic Acids Res. 47:W199–w205. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 32.Subramanian A, Tamayo P, Mootha VK, Mukherjee S, Ebert BL, Gillette MA, et al. (2005): Gene set enrichment analysis: A knowledge-based approach for interpreting genome-wide expression profiles. Proceedings of the National Academy of Sciences. 102:15545–15550. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 33.Jassal BLN N (2004): Opioid Signalling. Reactome. [Google Scholar]
- 34.Castro DC, Oswell CS, Zhang ET, Pedersen CE, Piantadosi SC, Rossi MA, et al. (2021): An endogenous opioid circuit determines state-dependent reward consumption. Nature. 598:646–651. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 35.Pascoli V, Terrier J, Hiver A, Lüscher C (2015): Sufficiency of Mesolimbic Dopamine Neuron Stimulation for the Progression to Addiction. Neuron. 88:1054–1066. [DOI] [PubMed] [Google Scholar]
- 36.Mancino S, Burokas A, Gutiérrez-Cuesta J, Gutiérrez-Martos M, Martín-García E, Pucci M, et al. (2015): Epigenetic and Proteomic Expression Changes Promoted by Eating Addictive-Like Behavior. Neuropsychopharmacology. 40:2788–2800. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 37.Deroche-Gamonet V, Belin D, Piazza PV (2004): Evidence for Addiction-like Behavior in the Rat. Science. 305:1014–1017. [DOI] [PubMed] [Google Scholar]
- 38.Logan GD, Schachar RJ, Tannock R (1997): Impulsivity and Inhibitory Control. Psychological Science. 8:60–64. [Google Scholar]
- 39.Domingo-Rodriguez L, Ruiz de Azua I, Dominguez E, Senabre E, Serra I, Kummer S, et al. (2020): A specific prelimbic-nucleus accumbens pathway controls resilience versus vulnerability to food addiction. Nature Communications. 11:782. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 40.Pomrenze MB, Cardozo Pinto DF, Neumann PA, Llorach P, Tucciarone JM, Morishita W, et al. (2022): Modulation of 5-HT release by dynorphin mediates social deficits during opioid withdrawal. Neuron. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 41.Kaufling J, Aston-Jones G (2015): Persistent Adaptations in Afferents to Ventral Tegmental Dopamine Neurons after Opiate Withdrawal. J Neurosci. 35:10290–10303. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 42.Zan GY, Wang YJ, Li XP, Fang JF, Yao SY, Du JY, et al. (2021): Amygdalar κ-opioid receptor-dependent upregulating glutamate transporter 1 mediates depressive-like behaviors of opioid abstinence. Cell Rep. 37:109913. [DOI] [PubMed] [Google Scholar]
- 43.Becker JAJ, Kieffer BL, Le Merrer J (2017): Differential behavioral and molecular alterations upon protracted abstinence from cocaine versus morphine, nicotine, THC and alcohol. Addiction Biology. 22:1205–1217. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 44.Bailly J, Del Rossi N, Runtz L, Li J-J, Park D, Scherrer G, et al. (2020): Targeting Morphine-Responsive Neurons: Generation of a Knock-In Mouse Line Expressing Cre Recombinase from the Mu-Opioid Receptor Gene Locus. eneuro. 7:ENEURO.0433–0419.2020. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 45.Fornasari D (2014): Pain pharmacology: focus on opioids. Clin Cases Miner Bone Metab. 11:165–168. [PMC free article] [PubMed] [Google Scholar]
- 46.Cohen GA, Doze VA, Madison DV (1992): Opioid inhibition of GABA release from presynaptic terminals of rat hippocampal interneurons. Neuron. 9:325–335. [DOI] [PubMed] [Google Scholar]
- 47.Johnson SW, North RA (1992): Opioids excite dopamine neurons by hyperpolarization of local interneurons. J Neurosci. 12:483–488. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 48.Terwilliger RZ, Beitner-Johnson D, Sevarino KA, Crain SM, Nestler EJ (1991): A general role for adaptations in G-proteins and the cyclic AMP system in mediating the chronic actions of morphine and cocaine on neuronal function. Brain Res. 548:100–110. [DOI] [PubMed] [Google Scholar]
- 49.Jolas T, Nestler EJ, Aghajanian GK (2000): Chronic morphine increases GABA tone on serotonergic neurons of the dorsal raphe nucleus: association with an up-regulation of the cyclic AMP pathway. Neuroscience. 95:433–443. [DOI] [PubMed] [Google Scholar]
- 50.Faget L, Zell V, Souter E, McPherson A, Ressler R, Gutierrez-Reed N, et al. (2018): Opponent control of behavioral reinforcement by inhibitory and excitatory projections from the ventral pallidum. Nature Communications. 9:849. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 51.Zell V, Steinkellner T, Hollon NG, Warlow SM, Souter E, Faget L, et al. (2020): VTA Glutamate Neuron Activity Drives Positive Reinforcement Absent Dopamine Co-release. Neuron. 107:864–873.e864. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 52.Tooley J, Marconi L, Alipio JB, Matikainen-Ankney B, Georgiou P, Kravitz AV, et al. (2018): Glutamatergic Ventral Pallidal Neurons Modulate Activity of the Habenula-Tegmental Circuitry and Constrain Reward Seeking. Biol Psychiatry. 83:1012–1023. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 53.Nieh EH, Vander Weele CM, Matthews GA, Presbrey KN, Wichmann R, Leppla CA, et al. (2016): Inhibitory Input from the Lateral Hypothalamus to the Ventral Tegmental Area Disinhibits Dopamine Neurons and Promotes Behavioral Activation. Neuron. 90:1286–1298. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 54.Urstadt KR, Berridge KC (2020): Optogenetic mapping of feeding and self-stimulation within the lateral hypothalamus of the rat. PLoS One. 15:e0224301. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 55.Kornetsky C, Esposito RU, McLean S, Jacobson JO (1979): Intracranial Self-stimulation Thresholds: A Model for the Hedonic Effects of Drugs of Abuse. Archives of General Psychiatry. 36:289–292. [DOI] [PubMed] [Google Scholar]
- 56.Ausubel DP (1965): Note on a Threshold Concept of Reinforcement. The Journal of General Psychology. 72:239–240. [DOI] [PubMed] [Google Scholar]
- 57.Augier E, Barbier E, Dulman RS, Licheri V, Augier G, Domi E, et al. (2018): A molecular mechanism for choosing alcohol over an alternative reward. Science. 360:1321–1326. [DOI] [PubMed] [Google Scholar]
- 58.Berger AC, Whistler JL (2011): Morphine-induced mu opioid receptor trafficking enhances reward yet prevents compulsive drug use. EMBO Mol Med. 3:385–397. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 59.O’Neal TJ, Nooney MN, Thien K, Ferguson SM (2020): Chemogenetic modulation of accumbens direct or indirect pathways bidirectionally alters reinstatement of heroin-seeking in high- but not low-risk rats. Neuropsychopharmacology. 45:1251–1262. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 60.Vanderschuren L, Ahmed SH (2021): Animal Models of the Behavioral Symptoms of Substance Use Disorders. Cold Spring Harb Perspect Med. 11. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 61.Lüscher C, Janak PH (2021): Consolidating the Circuit Model for Addiction. Annu Rev Neurosci. 44:173–195. [DOI] [PubMed] [Google Scholar]
- 62.Harris GC, Aston-Jones G (2003): Altered Motivation and Learning Following Opiate Withdrawal: Evidence for Prolonged Dysregulation of Reward Processing. Neuropsychopharmacology. 28:865–871. [DOI] [PubMed] [Google Scholar]
- 63.Harris GC, Aston-Jones G (2007): Activation in extended amygdala corresponds to altered hedonic processing during protracted morphine withdrawal. Behav Brain Res. 176:251–258. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 64.Zhang D, Zhou X, Wang X, Xiang X, Chen H, Hao W (2007): Morphine withdrawal decreases responding reinforced by sucrose self-administration in progressive ratio. Addict Biol. 12:152–157. [DOI] [PubMed] [Google Scholar]
- 65.Dalley JW, Everitt BJ, Robbins TW (2011): Impulsivity, compulsivity, and top-down cognitive control. Neuron. 69:680–694. [DOI] [PubMed] [Google Scholar]
- 66.Leyton M, Vezina P (2014): Dopamine ups and downs in vulnerability to addictions: a neurodevelopmental model. Trends Pharmacol Sci. 35:268–276. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 67.Swain Y, Gewirtz JC, Harris AC (2021): Behavioral predictors of individual differences in opioid addiction vulnerability as measured using i.v. self-administration in rats. Drug Alcohol Depend. 221:108561. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 68.Vest N, Reynolds CJ, Tragesser SL (2016): Impulsivity and risk for prescription opioid misuse in a chronic pain patient sample. Addict Behav. 60:184–190. [DOI] [PubMed] [Google Scholar]
- 69.Belin D, Mar AC, Dalley JW, Robbins TW, Everitt BJ (2008): High impulsivity predicts the switch to compulsive cocaine-taking. Science. 320:1352–1355. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 70.Tolomeo S, Gray S, Matthews K, Steele JD, Baldacchino A (2016): Multifaceted impairments in impulsivity and brain structural abnormalities in opioid dependence and abstinence. Psychological Medicine. 46:2841–2853. [DOI] [PubMed] [Google Scholar]
- 71.Zhai T, Shao Y, Chen G, Ye E, Ma L, Wang L, et al. (2015): Nature of functional links in valuation networks differentiates impulsive behaviors between abstinent heroin-dependent subjects and nondrug-using subjects. Neuroimage. 115:76–84. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 72.Lee TM, Pau CW (2002): Impulse control differences between abstinent heroin users and matched controls. Brain Inj. 16:885–889. [DOI] [PubMed] [Google Scholar]
- 73.Xie C, Shao Y, Fu L, Goveas J, Ye E, Li W, et al. (2011): Identification of hyperactive intrinsic amygdala network connectivity associated with impulsivity in abstinent heroin addicts. Behav Brain Res. 216:639–646. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 74.Mavrikaki M, Lintz T, Constantino N, Page S, Chartoff E (2021): Chronic opioid exposure differentially modulates oxycodone self-administration in male and female rats. Addict Biol. 26:e12973. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 75.Cooper ZD, Truong YN, Shi YG, Woods JH (2008): Morphine deprivation increases self-administration of the fast- and short-acting mu-opioid receptor agonist remifentanil in the rat. J Pharmacol Exp Ther. 326:920–929. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 76.Townsend EA, Kim RK, Robinson HL, Marsh SA, Banks ML, Hamilton PJ (2021): Opioid withdrawal produces sex-specific effects on fentanyl-vs.-food choice and mesolimbic transcription. Biol Psychiatry Glob Open Sci. 1:112–122. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 77.Chaudhary MA, Bhulani N, de Jager EC, Lipsitz S, Kwon NK, Sturgeon DJ, et al. (2019): Development and Validation of a Bedside Risk Assessment for Sustained Prescription Opioid Use After Surgery. JAMA Network Open. 2:e196673–e196673. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 78.Lanzillotta JA, Clark A, Starbuck E, Kean EB, Kalarchian M (2018): The Impact of Patient Characteristics and Postoperative Opioid Exposure on Prolonged Postoperative Opioid Use: An Integrative Review. Pain Management Nursing. 19:535–548. [DOI] [PubMed] [Google Scholar]
- 79.McAuliffe PF, Gold MS, Bajpai L, Merves ML, Frost-Pineda K, Pomm RM, et al. (2006): Second-hand exposure to aerosolized intravenous anesthetics propofol and fentanyl may cause sensitization and subsequent opiate addiction among anesthesiologists and surgeons. Medical Hypotheses. 66:874–882. [DOI] [PubMed] [Google Scholar]
- 80.Gold MS, Byars JA, Frost-Pineda K (2004): Occupational exposure and addictions for physicians: case studies and theoretical implications. Psychiatric Clinics. 27:745–753. [DOI] [PubMed] [Google Scholar]
- 81.Juurlink DN, Dhalla IA (2012): Dependence and Addiction During Chronic Opioid Therapy. Journal of Medical Toxicology. 8:393–399. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 82.Fishbain DA, Cole B, Lewis J, Rosomoff HL, Rosomoff RS (2008): What percentage of chronic nonmalignant pain patients exposed to chronic opioid analgesic therapy develop abuse/addiction and/or aberrant drug-related behaviors? A structured evidence-based review. Pain Med. 9:444–459. [DOI] [PubMed] [Google Scholar]
- 83.Bravo IM, Luster BR, Flanigan ME, Perez PJ, Cogan ES, Schmidt KT, et al. (2020): Divergent behavioral responses in protracted opioid withdrawal in male and female C57BL/6J mice. Eur J Neurosci. 51:742–754. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 84.Kosten TR, Rounsaville BJ, Kleber HD (1985): Ethnic and gender differences among opiate addicts. Int J Addict. 20:1143–1162. [DOI] [PubMed] [Google Scholar]
- 85.Yu J, Zhang S, Epstein DH, Fang Y, Shi J, Qin H, et al. (2007): Gender and stimulus difference in cue-induced responses in abstinent heroin users. Pharmacol Biochem Behav. 86:485–492. [DOI] [PubMed] [Google Scholar]
Associated Data
This section collects any data citations, data availability statements, or supplementary materials included in this article.