Abstract
Background:
Multiple sclerosis (MS) frequently affects women of childbearing age and pregnant women.
Objective:
To assess the use of MS disease-modifying therapies (DMTs) during pregnancy in France over the last decade, marked by an increasing DMTs availability.
Methods:
All pregnancies ended from April 2010 to December 2021 in women with MS were identified based on the nationwide Mother–Child Register EPI-MERES, built from the French National Health Data System (Système National des Données de Santé (SNDS)).
Results:
Of a total of 20,567 pregnancies in women with MS, 7587 were exposed to DMT. The number of DMT-exposed pregnancies markedly increased from 1079 in 2010–2012 to 2413 in 2019–2021 (+124%), especially those exposed to glatiramer acetate, natalizumab, dimethyl fumarate, and anti-CD20. Among pregnancies of women on DMT 6 months before pregnancy, 78.0% underwent DMT discontinuation and 7.6% switched DMT, generally before (33.0% and 77.0%, respectively) or during the first trimester of pregnancy (58.3% and 17.8%, respectively). DMT discontinuation decreased from 84.0% in 2010–2012 to 72.4% in 2019–2021 and was less frequent among women aged ⩾35 years and those socioeconomically disadvantaged.
Conclusion:
Despite MS therapeutic management adaptations to pregnancy, exposure during pregnancy to treatments whose safety profile has not yet been clearly established has increased sharply over the last decade.
Keywords: Multiple sclerosis, pregnancy, disease-modifying therapy, drug utilization study, French National Health Data System
Introduction
Multiple sclerosis (MS) is an autoimmune neurodegenerative chronic disease of the central nervous system, most commonly diagnosed in young adults and more frequent in women.1–3 MS cannot be cured, but disease-modifying therapies (DMTs) reduce relapses, short-term and long-term disability. Over the past decade, the number of available DMTs has increased and changes toward earlier initiation of DMT after MS onset have occurred.4–6
Based on limited available safety data, DMT-treated women considering pregnancy are generally recommended to stop DMT before conception, although some DMTs including beta-interferon, glatiramer acetate, natalizumab, and azathioprine may be maintained during pregnancy if disease activity requires it. Teriflunomide, fingolimod, cladribine, mitoxantrone, cyclophosphamide, mycophenolate mofetil, methotrexate, and biotin are contraindicated during pregnancy, whereas dimethyl fumarate and anti-CD20 are not recommended. Change or discontinuation of DMT before or during pregnancy should be individually adapted to women’s age, severity of disability, and benefits and risks of continuing or discontinuing DMT.7–9
Since the 2010s, the number of pregnancies exposed to MS DMT has increased over time in various countries.10–12 However, most reports on the use of DMTs during pregnancy focused on specific sub-populations of pregnant women with MS or on specific DMTs, and only few large-scale studies are available, especially on the use of the most recent DMTs.11,13,14 In addition, to date only few studies have assessed changes in DMT use around the time of pregnancy.11,12,15
This study aimed to assess the level and characteristics of all available MS DMTs use during pregnancy in France and their evolution over the period 2010–2021, and to describe changes in DMT use before, during, and after pregnancy in a large-scale, population-based, nationwide study.
Material and methods
Data source and information available
This comprehensive population-based study was performed using the National Mother–Child Register EPI-MERES, built from the French National Health Data System (Système National des Données de Santé, SNDS) covering 99% of the population. Data of the EPI-MERES Register were previously used to conduct pharmacoepidemiological studies.16–23
Study population
The study included all pregnancies ended between 1 April 2010 and 31 December 2021 in women aged 15–49 years. Pregnancies were considered to occur among MS-affected women if MS was identified before or during pregnancy. MS cases were identified based either on a diagnosis of MS (International Classification of Disease, 10th Revision (ICD-10) code G35) coded as the reason of hospitalization, long-term disease (LTD), disability or long-term sickness absence, or on a reimbursement for MS-specific DMT including beta-interferon, glatiramer acetate, teriflunomide, dimethyl fumarate, fingolimod, natalizumab, ocrelizumab, cladribine, and ofatumumab. The date of MS identification corresponded to the earliest date of MS diagnosis or DMT reimbursement.
Use of MS DMTs before, during, and after pregnancy
All MS-specific DMTs commercialized in France before 31 December 2021, listed above, were considered, as well as biotin which obtained a temporary use authorization for progressive MS between 2016 and 2020. Additional treatments prescribed off-label for MS (i.e. azathioprine, mycophenolate mofetil, methotrexate, and rituximab), while not used to identify MS-population, were considered as MS DMTs in women without any other comorbidity potentially motivating such treatments. Ocrelizumab and rituximab were grouped in a single “anti-CD20” category.
Exposure to MS DMTs was assessed, based on reimbursement dates, during each trimester (i.e. 91-day periods) between 6 months before and 6 months after pregnancy: the two trimesters before pregnancy (Trim-2 and Trim-1), the three trimesters of pregnancy (Trim1, Trim2, and Trim3), and the two trimesters after the end of pregnancy (Trim+1 and Trim+2). Pre-pregnancy anti-CD20 exposure was assessed by considering the two semesters (i.e. 182-day periods) before pregnancy (Sem-2 and Sem-1) as they are administered every 6 months. A trimester or semester was considered exposed if at least one dispensation of MS DMT occurred during the period. A pregnancy was considered exposed if at least one reimbursement of MS DMT occurred within the 30 days before the beginning of pregnancy and/or during pregnancy.
Characteristics of MS DMT use during pregnancy included timing of exposure (until the first trimester only; first trimester and after; second and/or third trimester only), number of DMT dispensations, and prescribers’ type of practice and specialty (for private practitioners). Among pregnancies of women who were on DMT during Trim-2, DMT discontinuation was defined as the absence of any DMT dispensation during at least 70 days (i.e. twice the period covered by a dispensation plus a grace period of 5 days per month) for DMTs dispensed monthly, and during at least 212 days (i.e. the period covered by a dispensation plus a 30-day grace period) for anti-CD20; DMT switch was defined as the dispensation of a new DMT within 70 days (212 days, respectively) of the last dispensation of a previously used DMT.
Maternal and pregnancy characteristics
Maternal characteristics at pregnancy start included age and level of resources categorized according to affiliation to complementary universal health insurance (CMUc, which provides access to free healthcare for people with low income in France) and, for those not affiliated to CMUc, the amount of their salary. Pregnancy characteristics included the use of any assisted reproductive technology (ART), folic acid reimbursement, pregnancy-related hospitalizations, pregnancy outcome, mode and clinical setting of delivery, if any, and pregnancy term. For pregnancies of women with MS, additional information included MS-related hospitalizations during pregnancy and MS severity at the time of pregnancy start, assessed by the existence of a functional impairment and the use of a second-line DMT.
Statistical analysis
Annual numbers of pregnancies in women with MS, whether exposed to DMT or not, and of those in women without MS were reported over the study period. Maternal and pregnancy characteristics and DMT use during pregnancy were described, overall and by sub-periods (2010–2012, 2013–2015, 2016–2018, and 2019–2021). Among pregnancies of women who were on DMT during Trim-2, DMT use was assessed during each trimester between Trim-2 and Trim+2, and DMT use in Trim+2 was described as compared to that in Trim-2. DMT switches and discontinuations before and during pregnancy were described overall and by sub-period, and their correlates were identified using a multinomial logistic regression model including period of pregnancy end, maternal age and level of resources, use of ART, line of DMT used during Trim-2, and functional impairment.
All analyses were performed using SAS Enterprise Guide software, version 7.15. The Sankey diagram was built using SankeyMatic.
Results
Study population
Among a total of 11,891,167 pregnancies ended between 1 April 2010 and 31 December 2021, 20,567 occurred in 13,747 women with MS. Of these 20,567 pregnancies, 8664 (42.1%) occurred in women who were on MS DMT during Trim-2 and 7587 (36.9%) were exposed to at least one DMT. The annual number of DMT-exposed pregnancies increased over time from 247 (accounting for 21.4% of pregnancies in women with MS overall) between April and December 2010 to 833 (43.8%) in 2021 (Supplemental Figure S1).
Among the 13,747 women with MS and at least one pregnancy over the period, 53.4% had one pregnancy, 30.7% had two, and 15.9% had three or more. Median age at MS identification was 27 years. At the time of pregnancy start, median time since MS identification was 4 years overall, with an increasing trend over time from 3 years in 2010–2012 to 5 years in 2019–2021. The frequencies of functional impairment and use of a second-line DMT before pregnancy reached 19.6% and 15.9% overall, respectively, and showed increases over time (Supplemental Table S1).
MS DMT exposure during pregnancy
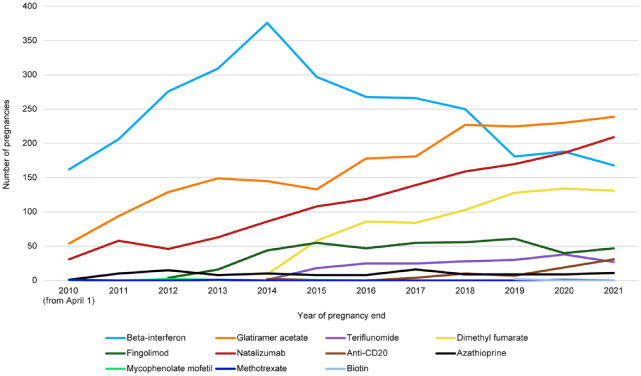
Overall, among the 7587 pregnancies exposed to at least one DMT between 2010 and 2021, 2947 (38.8%) were exposed to beta-interferon, 1984 (26.1%) to glatiramer acetate, 1374 (18.1%) to natalizumab, 733 (9.7%) to dimethyl fumarate, 425 (5.6%) to fingolimod, 192 (2.5%) to teriflunomide, 74 (1.0%) to an anti-CD20, 114 (1.5%) to azathioprine, and 10 (0.1%) to other DMTs (mycophenolate mofetil, methotrexate, or biotin). The numbers of pregnancies exposed to glatiramer acetate, natalizumab, dimethyl fumarate, and anti-CD20 steadily increased over time to 694, 565, 393, and 57, respectively, in 2019–2021, whereas those exposed to beta-interferon peaked at 982 in 2013–2015 before decreasing to 537 in 2019–2021. The number of pregnancies exposed to fingolimod and teriflunomide remained steady over the recent years, reaching 148 and 95, respectively, in 2019–2021 (Figure 1 and Supplemental Table S2).
Figure 1.
Annual number of MS DMT-exposed pregnancies ended between 1 April 2010 and 31 December 2021, by type of DMT.
Maternal and pregnancy characteristics in women with and without MS
Compared to pregnancies of women without MS, those of women with MS were characterized by an older maternal age (32 years vs 29 years in median) and a higher socioeconomic status (CMUc beneficiaries: 7.3% vs 13.2%; salary of 2000€/month or more: 22.8% vs 19.0%) (Table 1). ART was used in 3.4% of pregnancies of women with MS (vs 2.2% in those without MS) and folic acid supplementation in 42.6% (vs 33.0%). These proportions increased over time, especially among DMT-exposed pregnancies (Supplemental Table S3). Compared to pregnancies of women without MS, DMT-exposed pregnancies were more frequently early terminated (19.8%)—especially those exposed to fingolimod (50.1%), teriflunomide (57.3%), anti-CD20 (48.6%), and dimethyl fumarate (28.8%)—but the rate of elective/therapeutic termination decreased over time to a level close to that of DMT-unexposed pregnancies in 2019–2021 (18.8% vs 18.5%). Among pregnancies ended in a delivery, cesarean section and premature birth were more frequent in pregnancies of women with MS compared to those without (21.7% vs 20.8% and 8.4% vs 6.7%, respectively). The rate of prematurity decreased over time, especially for DMT-exposed pregnancies.
Table 1.
Maternal and pregnancy characteristics of pregnancies ended between 2010 and 2021 in women with MS, overall and according to DMT exposure during pregnancy, and in women without MS.
| Non-MS (N = 11,870,600) | MS | |||
|---|---|---|---|---|
| Overall (N = 20,567) | MS DMT-unexposed (N = 12,980) | MS DMT-exposed (N = 7587) | ||
| Age (years) | ||||
| Median [IQR] | 29 [25–33] | 32 [28–35] | 32 [28–35] | 31 [28–35] |
| Level of resources | ||||
| CMUc | 1,564,922 (13.2) | 1496 (7.3) | 1029 (7.9) | 467 (6.2) |
| No CMUc, salary < 2000€/month | 2,258,597 (19.0) | 3976 (19.3) | 2531 (19.5) | 1445 (19.0) |
| No CMUc, salary ⩾ 2000€/month | 2,259,850 (19.0) | 4681 (22.8) | 2934 (22.6) | 1747 (23.0) |
| No CMUc, unknown salary | 5,787,231 (48.7) | 10,414 (50.6) | 6486 (49.9) | 3928 (51.8) |
| Assisted reproduction | 262,789 (2.2) | 692 (3.4) | 471 (3.6) | 221 (2.9) |
| Folic acid dispensation a | 3,916,317 (33.0) | 8769 (42.6) | 5352 (41.2) | 3417 (45.0) |
| Pregnancy-related hospitalization | ||||
| 0 | 10,242,462 (86.3) | 17,370 (84.5) | 10,942 (84.3) | 6428 (84.7) |
| ⩾1 | 1,628,138 (13.7) | 3197 (15.5) | 2038 (15.7) | 1159 (15.3) |
| MS-related hospitalization during pregnancy | ||||
| 0 | – | 19,100 (92.9) | 12,285 (94.6) | 6815 (89.8) |
| ⩾1 | – | 1467 (7.1) | 695 (5.4) | 772 (10.2) |
| Pregnancy outcome | ||||
| Live birth | 8,777,791 (73.9) | 15,320 (74.5) | 9794 (75.5) | 5526 (72.8) |
| Stillbirth | 46,300 (0.4) | 80 (0.4) | 57 (0.4) | 23 (0.3) |
| Therapeutic abortion ⩾ 22 weeks | 37,206 (0.3) | 67 (0.3) | 45 (0.3) | 22 (0.3) |
| Elective/therapeutic abortion < 22 weeks | 2,280,496 (19.2) | 3571 (17.4) | 2066 (15.9) | 1505 (19.8) |
| Spontaneous abortion | 448,429 (3.8) | 924 (4.5) | 620 (4.8) | 304 (4.0) |
| Others | 280,378 (2.3) | 605 (3.0) | 398 (3.0) | 207 (2.8) |
| Cesarean section b | 1,833,650 (20.8) | 3336 (21.7) | 2208 (22.4) | 1128 (20.3) |
| Clinical setting of delivery b | ||||
| University hospital | 1,712,131 (19.4) | 3843 (25.0) | 2332 (23.7) | 1511 (27.2) |
| General hospital | 4,927,575 (55.8) | 8097 (52.6) | 5183 (52.6) | 2914 (52.5) |
| Private hospital | 2,171,400 (24.6) | 3445 (22.4) | 2322 (23.6) | 1123 (20.2) |
| Outpatient | 12,985 (0.1) | 15 (0.1) | 14 (0.1) | 1 (0.0) |
| Pregnancy term at delivery b | ||||
| Full-term (⩾37 weeks) | 8,239,498 (93.4) | 14,118 (91.7) | 9046 (91.8) | 5072 (91.4) |
| Preterm (<37 weeks) | 481,076 (5.5) | 1058 (6.9) | 656 (6.7) | 402 (7.2) |
| Very preterm (<32 weeks) | 103,517 (1.2) | 224 (1.5) | 149 (1.5) | 75 (1.4) |
MS: multiple sclerosis; DMT: disease-modifying therapy; IQR: interquartile range; CMUc: complementary universal health insurance allowing access to free health care for people with low income.
Between 2 months before and 3 months after the beginning of pregnancy.
Among pregnancies ended in live birth or stillbirth.
Characteristics of MS DMT use during pregnancy
Exposed pregnancies were mostly exposed to a single type of DMT (96.6%) and until the first trimester of pregnancy at most (80.7%, of which 23.2% were exposed during the 30 days before pregnancy only). The mean number of DMTs dispensations during pregnancy reached 2.7 overall, increasing from 2.1 in 2010–2012 to 3.3 in 2019–2021. MS DMTs used during pregnancy were mostly prescribed by hospital practitioners (81.2%) (Table 2).
Table 2.
Characteristics of MS DMT use during pregnancy among pregnancies ended between 2010 and 2021 in women with MS, overall and by sub-periods.
| Overall (N = 7587) | By sub-periods | ||||
|---|---|---|---|---|---|
| 2010–2012 (N = 1079) | 2013–2015 (N = 1854) | 2016–2018 (N = 2241) | 2019–2021 (N = 2413) | ||
| Number of DMT types reimbursed a | |||||
| 1 | 7327 (96.6) | 1069 (99.1) | 1811 (97.7) | 2142 (95.6) | 2305 (95.5) |
| ⩾2 | 260 (3.4) | 10 (0.9) | 43 (2.3) | 99 (4.4) | 108 (4.4) |
| Period of exposure to DMTs | |||||
| Until the first trimester only | 6119 (80.7) | 955 (88.5) | 1629 (87.9) | 1821 (81.3) | 1714 (71.0) |
| First trimester and after | 1277 (16.8) | 76 (7.0) | 193 (10.4) | 367 (16.4) | 641 (26.6) |
| Second and/or third trimester only | 191 (2.5) | 48 (4.4) | 32 (1.7) | 53 (2.4) | 58 (2.4) |
| Number of DMTs dispensations a | |||||
| Mean ± SD | 2.7 ± 2.3 | 2.1 ± 1.5 | 2.3 ± 1.8 | 2.6 ± 2.3 | 3.3 ± 2.9 |
| Prescribers | |||||
| Hospital practitioner | 6162 (81.2) | 763 (70.7) | 1321 (71.3) | 1875 (83.7) | 2194 (90.9) |
| Private practitioner | |||||
| General practitioner | 282 (3.7) | 33 (3.1) | 65 (3.5) | 90 (4.0) | 94 (3.9) |
| Neurologist | 1912 (25.2) | 317 (29.4) | 529 (28.5) | 555 (24.8) | 511 (21.2) |
| Other specialties | 209 (2.8) | 22 (2.0) | 41 (2.2) | 63 (2.8) | 83 (3.4) |
MS: multiple sclerosis; DMT: disease-modifying therapy; SD: standard deviation.
Between 30 days before pregnancy start and the end of pregnancy.
Changes in MS DMT before, during, and after pregnancy
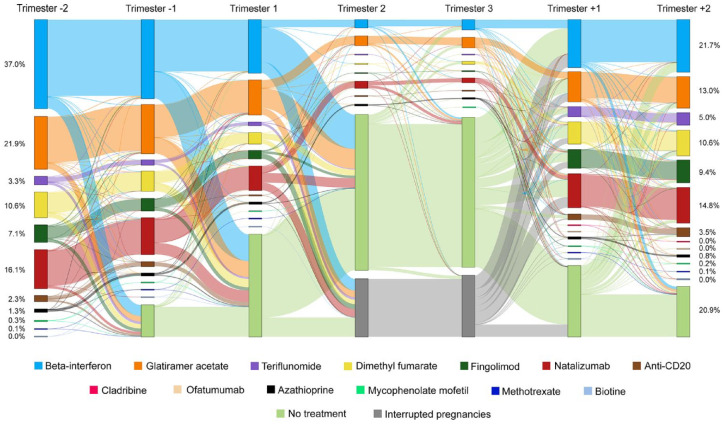
Figure 2 shows the changes in MS DMT during each trimester between Trim-2 and Trim+2 for the 8664 pregnancies of women treated in Trim-2. Between Trim-2 and the end of pregnancy, a large majority of these pregnancies (78.0%) underwent at least one period of DMT discontinuation, and in 7.6%, the DMT used during Trim-2 was switched to another, while it was continuously maintained in 19.5% (Table 3).
Figure 2.
DMT use before, during, and after pregnancy among pregnancies ended between 2010 and 2021 in women who were on DMT during trimester-2.
Table 3.
DMT switches and discontinuations before and during pregnancy among pregnancies ended between 2010 and 2021 in women who were on DMT during trimester-2, overall and by sub-periods.
| Overall (N = 8664) | By sub-periods | ||||
|---|---|---|---|---|---|
| 2010–2012 (N = 1392) | 2013–2015 (N = 2083) | 2016–2018 (N = 2487) | 2019–2021 (N = 2702) | ||
| Pregnancies with at least one DMT switch, n (%) | 661 (7.6) | 48 (3.4) | 114 (5.5) | 219 (8.8) | 280 (10.4) |
| Total number of DMT switches | 762 | 49 | 139 | 250 | 324 |
| Trimester of DMT switch, n (%) | |||||
| Trimester-2 a | 245 (32.2) | 15 (30.6) | 44 (31.7) | 73 (29.2) | 113 (34.9) |
| Trimester-1 b | 341 (44.8) | 28 (57.1) | 70 (50.4) | 102 (40.8) | 141 (43.5) |
| First trimester | 136 (17.8) | 6 (12.2) | 21 (15.1) | 64 (25.6) | 45 (13.9) |
| Second trimester | 28 (3.7) | 0 (0.0) | 3 (2.2) | 6 (2.4) | 19 (5.9) |
| Third trimester | 12 (1.6) | 0 (0.0) | 1 (0.7) | 5 (2.0) | 6 (1.9) |
| Pregnancies with at least one DMT discontinuation, n (%) | 6754 (78.0) | 1169 (84.0) | 1692 (81.2) | 1937 (77.9) | 1956 (72.4) |
| Total number of DMT discontinuations | 7385 | 1286 | 1816 | 2138 | 2145 |
| Trimester of DMT discontinuation, n (%) | |||||
| Trimester-2 a | 911 (12.3) | 227 (17.6) | 207 (11.4) | 248 (11.6) | 229 (10.7) |
| Trimester-1 b | 1525 (20.7) | 366 (28.5) | 333 (18.3) | 414 (19.4) | 412 (19.2) |
| First trimester | 4309 (58.3) | 653 (50.7) | 1192 (65.6) | 1313 (61.4) | 1151 (53.7) |
| Second trimester | 385 (5.2) | 29 (2.3) | 63 (3.5) | 104 (4.9) | 189 (8.8) |
| Third trimester | 255 (3.5) | 11 (0.9) | 21 (1.2) | 59 (2.8) | 164 (7.6) |
| Pregnancies without any DMT switch or discontinuation, n (%) | 1689 (19.5) | 213 (15.3) | 365 (17.5) | 472 (19.0) | 639 (23.6) |
DMT: disease-modifying therapy.
Semester-2 for ocrelizumab and rituximab.
Semester-1 for ocrelizumab and rituximab.
Discontinuations were particularly frequent for azathioprine (89.4%) and beta-interferon (82.2%) (Supplemental Table S4); they mainly occurred during the first trimester of pregnancy (58.3%) or before pregnancy start (33.0%), and their frequency decreased over time from 84.0% in 2010–2012 to 72.4% in 2019–2021. Switches were particularly frequent for fingolimod (20.4%), teriflunomide (14.8%), dimethyl fumarate (12.8%), natalizumab (11.0%), and anti-CD20 (9.7%); they generally occurred before pregnancy start (77.0%) or during the first trimester of pregnancy (17.8%), mainly toward glatiramer acetate (38.3%), beta-interferon (24.7%), and natalizumab (15.2%). Switches occurred in an increasing proportion of pregnancies over time from 3.4% in 2010–2012 to 10.4% in 2019–2021. Accounting for the period of pregnancy end, maternal age ⩾ 35 years, and low level of resources were associated with decreased odds of discontinuation (adjusted odds ratio (aOR) = 0.57 (0.48–0.67) and 0.26 (0.20-0.35), respectively) and switch (aOR = 0.46 (0.35–0.61) and 0.33 (0.22–0.51), respectively), while the use of a second-line DMT during Trim-2 was associated with a decreased odds of discontinuation (aOR = 0.71 (0.62–0.80)) but increased odds of switch (aOR = 1.77 (1.45–2.16)) (Supplemental Table S5).
After the end of pregnancy, DMT was mostly resumed by the time of Trim+2 (79.1%). The DMT used in Trim+2 was generally the same as that used in Trim-2 (60.6%), particularly for fingolimod (70.6%), natalizumab (73.9%), and anti-CD20 (73.6%), whereas women treated with beta-interferon, glatiramer acetate, teriflunomide, or dimethyl fumarate in Trim-2 were more likely to use a different DMT or no DMT in Trim+2 (Table 4).
Table 4.
DMT use in Trim+2 as compared to that in Trim-2 among pregnancies ended between 2010 and 2021 in women who were on DMT during trimester-2.
| DMTs used during trimester+2 a | Total | |||
|---|---|---|---|---|
| Same DMT | Different DMT | No DMT | ||
| DMTs used during trimester-2 b | ||||
| Beta-interferon | 1796 (56.5) | 632 (19.9) | 749 (23.6) | 3177 (100.0) |
| Glatiramer acetate | 983 (53.0) | 444 (24.0) | 426 (23.0) | 1853 (100.0) |
| Teriflunomide | 155 (53.3) | 53 (18.2) | 83 (28.5) | 291 (100.0) |
| Dimethyl fumarate | 587 (62.4) | 143 (15.2) | 210 (22.3) | 940 (100.0) |
| Fingolimod | 463 (70.6) | 104 (15.9) | 89 (13.6) | 656 (100.0) |
| Natalizumab | 1031 (73.9) | 204 (14.6) | 161 (11.5) | 1396 (100.0) |
| Anti-CD20 c | 159 (73.6) | 7 (3.2) | 50 (23.1) | 216 (100.0) |
| Azathioprine | 62 (59.6) | 11 (10.6) | 31 (29.8) | 104 (100.0) |
| Other DMTs d | 15 (48.3) | 2 (6.2) | 14 (45.2) | 31 (100.0) |
| Total | 5251 (60.6) | 1600 (18.5) | 1813 (20.9) | 8664 (100.0) |
DMT: disease-modifying therapy.
During trimester+1 and trimester+2 for anti-CD20.
Semester-2 for anti-CD20.
Ocrelizumab and rituximab.
Mycophenolate mofetil, methotrexate, and biotin.
Discussion
This study provides a comprehensive, detailed characterization of MS DMTs use during pregnancy and its evolution over the last decade at the national scale in France. Given the particularly evolving context of MS management and new DMTs marketing, these results provide useful information for health authorities, health professionals, and people with MS.
Our findings highlight major changes in pregnancy opportunities and management among women with MS over the past decade in France. First, the number of pregnancies in women with MS has markedly increased (+24% between 2010–2012 and 2019–2021) as a result of a dramatic rise in pregnancies in women using DMT during pregnancy (+124%), while those in women untreated throughout pregnancy have slightly decreased (−8%). In a context of stable MS incidence and increasing DMTs availability over the period, these trends likely reflect changes in treatment strategy toward an earlier initiation of DMT after MS diagnosis to reduce long-term disability. 24 In addition, our results suggest that the advent of more and more powerful DMTs has also benefited to pregnancy plans of women with a long-standing and/or advanced MS, as reflected by increasing MS duration and frequencies of functional impairment and history of a second-line DMT over time among women with MS initiating a pregnancy. However, the marked increase in use of second-line DMTs (+16%) as compared to trend in functional impairment (+1.2%) suggests that these DMTs, especially natalizumab, may actually also be used for less active forms of MS.
Moreover, our results highlight significant changes in the management of DMT-exposed pregnancies over the last decade. Indeed, whereas in the early 2010s, early pregnancy termination in women with MS was almost twice as frequent in DMT-exposed pregnancies as in those unexposed; more recently, it has become less so, as suggested by similar rates of elective/therapeutic abortions in 2019–2021 among DMT-exposed and unexposed pregnancies of women with MS. This evolution probably reflects a decrease in prescribers’ and patients’ concerns about DMT safety during pregnancy, as data on their use in pregnant women have accumulated. Meanwhile, folic acid supplementation markedly increased and became more frequent for DMT-exposed than unexposed pregnancies, suggesting particularly close monitoring of these exposed pregnancies (especially as figures based on reimbursement data may be underestimated due to over-the-counter folic acid dispensations).
As reported in other settings and time periods,11,12,25,26 in France, between 2010 and 2021, MS treatments used during pregnancy were mostly limited to DMTs for which available safety data do not support use restrictions during pregnancy (i.e. beta-interferon, glatiramer acetate, natalizumab, and azathioprine, which altogether accounted for almost 85% of all exposed pregnancies over the study period) and to the first trimester of pregnancy only (>80%). Our results show that this results from the widespread discontinuation of DMT—particularly first-line DMTs—and, to a lesser extent, the switch from DMTs whose use is restricted during pregnancy toward others with a less concerning safety profile regarding pregnancy. In addition, by showing that such changes among women treated prior to pregnancy generally occur in the few months preceding pregnancy or during the first trimester of pregnancy, our findings provide evidence of actual adaptations of the therapeutic management of MS to a project of pregnancy or to pregnancy onset in real life. Our finding that older and socioeconomically disadvantaged women are less likely than the others to undergo DMT switch or discontinuation during pregnancy deserves further investigations.
Although these findings apply to the whole period between 2010 and 2021, we found that the proportion of pregnancies with DMT discontinuation substantially decreased over time. Considering the concomitant decrease in early pregnancy termination rates mentioned above, such a trend may not be attributed to a shortening of pregnancies duration, but rather results from changes in DMT use guidelines during pregnancy occurred in the 2010s (i.e. removal of the warnings regarding the use during pregnancy of glatiramer acetate in 2016 and of beta-interferon in 2019, as well as recommendations to lessen restrictions on the use of natalizumab during the first two trimesters of pregnancy in case of active MS7–9) and from the evolving profile of pregnant women with MS toward patients with a more active and/or advanced disease. In contrast, among women previously using contraindicated (i.e. fingolimod and teriflunomide) or non-recommended (i.e. dimethyl fumarate and anti-CD20) DMTs during pregnancy, switching to avoid pregnancy exposure increased over time. Even so, a total of 617 and 807 pregnancies were identified as exposed to a contraindicated or a non-recommended DMT, respectively, between 2010 and 2021 in France, probably as a result of accidental treatment continuation during unplanned or undiagnosed pregnancy27,28 or the lack of information on safety concerns.29,30 Among these pregnancies, the termination rate was high (around one in two for those exposed to fingolimod, teriflunomide, or anti-CD20 and one in three to four for those exposed to dimethyl fumarate).
This study was based on the National Mother–Child EPI-MERES Register, which contains comprehensive information on medication use and patients and pregnancies characteristics at the scale of over 12 million pregnancies, that is, almost all pregnancies occurred in France between 2010 and 2021, regardless of their outcome. Thus, the study findings provide a detailed picture of the therapeutic management of pregnant women with MS at the national level and its evolution over the past decade, a period marked by dramatic changes in MS therapeutic management. As such, they provide new insights into the use of MS DMTs in real life, including the most recent ones for which data have remained limited until now.
Despite these strengths, this study has some limitations. First, in the absence of clinical data available, women with MS could be identified if MS was the cause of hospitalization, LTD, disability or long-term sick leave, or if an MS-specific DMT was implemented, suggesting that some cases, especially the most benign forms, could have been missed. However, the number of cases identified is consistent with figures previously reported in France.25,31,32 Second, information was lacking on key neurological parameters such as MS relapses or Expanded Disability Status Scale (EDSS) score, which constitute strong determinants of the choices of DMT type and of switching or discontinuing treatment during pregnancy. However, information on disease duration was available, and functional impairment and history of second-line DMT were used as proxies of disease severity. Third, for DMTs not administered in hospital, information on the actual consumption of reimbursed drugs is lacking, which may have led to overestimate the number of exposed pregnancies and/or the duration of exposure. However, DMT dispensations were generally repeated during exposed pregnancies (2.7 dispensations on average), suggesting that the treatments dispensed were probably actually used. Fourth, since information on the use of alemtuzumab, mitoxantrone, and cyclophosphamide was not available, the overall number of pregnancies DMTs-exposed may have been slightly underestimated. However, the use of these DMTs for MS management had remained limited in France by 2022. 33
In conclusion, this study highlights a form of normalization of pregnancy in women with MS that has occurred over the last decade with the advent of new DMTs. In this context, exposure during pregnancy to treatments whose safety profile has not yet been clearly established has increased sharply. These findings call for vigilance and for further studies to improve knowledge on the risk profile of these treatments for pregnant women and their exposed children, particularly for the most recent DMTs for which the available information is limited.
Supplemental Material
Supplemental material, sj-docx-1-msj-10.1177_13524585231223395 for Use of multiple sclerosis disease-modifying therapies during pregnancy in France: Nationwide study between 2010 and 2021 by Morgane Swital, Jérôme Drouin, Sara Miranda, Serge Bakchine, Jérémie Botton and Rosemary Dray-Spira in Multiple Sclerosis Journal
Footnotes
According to data protection and the French regulation, the authors cannot publicly release the data from the French National Health Data System (SNDS). However, any person or structure, public or private, for-profit or non-profit, is able to access SNDS data upon authorization from the French Data Protection Office (CNIL Commission Nationale de l’Informatique et des Libertés) to carry out a study, a research, or an evaluation of public interest (https://www.snds.gouv.fr/SNDS/Processus-d-acces-aux-donnees and https://www.indsante.fr/). Here is a non-author point of contact where data requests can be sent https://www.health-data-hub.fr/contact.
The author(s) declared no potential conflicts of interest with respect to the research, authorship, and/or publication of this article.
Funding: The author(s) received no financial support for the research, authorship, and/or publication of this article.
ORCID iDs: Morgane Swital  https://orcid.org/0009-0002-0159-0789
https://orcid.org/0009-0002-0159-0789
Jérémie Botton  https://orcid.org/0000-0002-4814-6370
https://orcid.org/0000-0002-4814-6370
Rosemary Dray-Spira  https://orcid.org/0000-0001-7646-3667
https://orcid.org/0000-0001-7646-3667
Supplemental Material: Supplemental material for this article is available online.
Contributor Information
Morgane Swital, EPI-PHARE Scientific Interest Group in Epidemiology of Health Products (French National Agency for the Safety of Medicines and Health Products-ANSM, French National Health Insurance-CNAM), Saint-Denis, France; French National Institute of Health and Medical Research (INSERM), Department of Social Epidemiology (ERES), Pierre Louis Institute for Epidemiology and Public Health (IPLESP), Paris, France.
Jérôme Drouin, EPI-PHARE Scientific Interest Group in Epidemiology of Health Products (French National Agency for the Safety of Medicines and Health Products-ANSM, French National Health Insurance-CNAM), Saint-Denis, France.
Sara Miranda, EPI-PHARE Scientific Interest Group in Epidemiology of Health Products (French National Agency for the Safety of Medicines and Health Products-ANSM, French National Health Insurance-CNAM), Saint-Denis, France.
Serge Bakchine, University of Reims Champagne Ardennes (URCA), Reims, France.
Jérémie Botton, EPI-PHARE Scientific Interest Group in Epidemiology of Health Products (French National Agency for the Safety of Medicines and Health Products-ANSM, French National Health Insurance-CNAM), Saint-Denis, France; Faculty of Pharmacy, Paris-Saclay University, Orsay, France.
Rosemary Dray-Spira, EPI-PHARE Scientific Interest Group in Epidemiology of Health Products (French National Agency for the Safety of Medicines and Health Products-ANSM, French National Health Insurance-CNAM), Saint-Denis, France.
References
- 1. Leray E, Moreau T, Fromont A, et al. Epidemiology of multiple sclerosis. Rev Neurol (Paris) 2016; 172: 3–13. [DOI] [PubMed] [Google Scholar]
- 2. McGinley MP, Goldschmidt CH, Rae-Grant AD. Diagnosis and treatment of multiple sclerosis: A review. JAMA 2021; 325: 765–779. [DOI] [PubMed] [Google Scholar]
- 3. Walton C, King R, Rechtman L, et al. Rising prevalence of multiple sclerosis worldwide: Insights from the Atlas of MS, third edition. Mult Scler 2020; 26(14): 1816–1821. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 4. Hauser SL, Cree BAC. Treatment of multiple sclerosis: A review. Am J Med 2020; 133: 1380–1390. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 5. Leblanc S, Lefort M, Le Page E, et al. Trends in disease-modifying therapy use in patients with multiple sclerosis using a 10-year population-based cohort study in France. Expert Rev Neurother 2022; 22(5): 411–418. [DOI] [PubMed] [Google Scholar]
- 6. Koch-Henriksen N, Magyari M. Apparent changes in the epidemiology and severity of multiple sclerosis. Nat Rev Neurol 2021; 17(11): 676–688. [DOI] [PubMed] [Google Scholar]
- 7. Vukusic S, Carra-Dalliere C, Ciron J, et al. Pregnancy and multiple sclerosis: 2022 recommendations from the French multiple sclerosis society. Mult Scler J 2023; 29: 11–36. [DOI] [PubMed] [Google Scholar]
- 8. Vukusic S, Michel L, Leguy S, et al. Pregnancy with multiple sclerosis. Rev Neurol (Paris) 2021; 177: 180–194. [DOI] [PubMed] [Google Scholar]
- 9. Dobson R, Dassan P, Roberts M, et al. UK consensus on pregnancy in multiple sclerosis: “Association of British Neurologists” guidelines. Pract Neurol 2019; 19(2): 106–114. [DOI] [PubMed] [Google Scholar]
- 10. Duchesneau ED, Kinlaw AC, Jonsson Funk M, et al. Trends in the use of disease-modifying therapies among reproductive-aged women with multiple sclerosis in the United States from 2010 to 2019. Pharmacoepidemiol Drug Saf 2022; 31(4): 481–487. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 11. Nguyen A-L, Havrdova EK, Horakova D, et al. Incidence of pregnancy and disease-modifying therapy exposure trends in women with multiple sclerosis: A contemporary cohort study. Mult Scler Relat Disord 2019; 28: 235–243. [DOI] [PubMed] [Google Scholar]
- 12. Toscano S, Chisari CG, Meli A, et al. Pregnancy planning and management for women with multiple sclerosis: What has changed over the last 15 years? An Italian single-center experience. Mult Scler Relat Disord 2023; 70: 104526. [DOI] [PubMed] [Google Scholar]
- 13. Fink K, Gorczyca A, Alping P, et al. Multiple sclerosis, disease-modifying drugs and risk for adverse perinatal and pregnancy outcomes: Results from a population-based cohort study. Mult Scler 2023; 29(6): 731–740 [DOI] [PMC free article] [PubMed] [Google Scholar]
- 14. Andersen JB, Sellebjerg F, Magyari M. Pregnancy outcomes after early fetal exposure to injectable first-line treatments, dimethyl fumarate, or natalizumab in Danish women with multiple sclerosis. Eur J Neurol 2023; 30(1): 162–171. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 15. Yeh WZ, Widyastuti PA, Van der Walt A, et al. Natalizumab, fingolimod, and dimethyl fumarate use and pregnancy-related relapse and disability in women with multiple sclerosis. Neurology 2021; 96: e2989–e3002. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 16. Blotière P-O, Weill A, Dalichampt M, et al. Development of an algorithm to identify pregnancy episodes and related outcomes in health care claims databases: An application to antiepileptic drug use in 4.9 million pregnant women in France. Pharmacoepidemiol Drug Saf 2018; 27(7): 763–770. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 17. Blotière P-O, Raguideau F, Weill A, et al. Risks of 23 specific malformations associated with prenatal exposure to 10 antiepileptic drugs. Neurology 2019; 93: e167–e180. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 18. Coste J, Blotiere P-O, Miranda S, et al. Risk of early neurodevelopmental disorders associated with in utero exposure to valproate and other antiepileptic drugs: A nationwide cohort study in France. Sci Rep 2020; 10: 17362. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 19. Meyer A, Fermaut M, Drouin J, et al. Drug use for gastrointestinal symptoms during pregnancy: A French nationwide study 2010–2018. PLoS ONE 2021; 16(1): e0245854. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 20. Tubiana S, Sibiude J, Herlemont P, et al. Trends in anti-infective use during pregnancy in France between 2010 and 2019: A nationwide population-based study. Br J Clin Pharmacol 2023; 89(5): 1629–1639. [DOI] [PubMed] [Google Scholar]
- 21. Meyer A, Neumann A, Drouin J, et al. Benefits and risks associated with continuation of anti–tumor necrosis factor after 24 weeks of pregnancy in women with inflammatory bowel disease. Ann Intern Med 2022; 175(10): 1374–1382. [DOI] [PubMed] [Google Scholar]
- 22. Lassalle M, Zureik M, Dray-Spira R. Proton pump inhibitor use and risk of serious infections in young children. JAMA Pediatr 2023; 177: 1028–1038. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 23. Tran A, Zureik M, Sibiude J, et al. Prevalence and associated factors of antibiotic exposure during pregnancy in a large French population-based study during the 2010–19 period. J Antimicrob Chemother 2023; 78: 2535–2543. [DOI] [PubMed] [Google Scholar]
- 24. Harding K, Williams O, Willis M, et al. Clinical outcomes of escalation vs early intensive disease-modifying therapy in patients with multiple sclerosis. JAMA Neurol 2019; 76: 536–541. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 25. Tillaut H, Degrémont A, Kerbrat S, et al. Pregnancy in women with multiple sclerosis in France from 2010 to 2015: Incidence, outcomes, and exposure to disease-modifying therapies. Mult Scler 2022; 28(5): 778–789. [DOI] [PubMed] [Google Scholar]
- 26. Thiel S, Ciplea AI, Gold R, et al. The German Multiple Sclerosis and Pregnancy Registry: Rationale, objective, design, and first results. Ther Adv Neurol Disord 2021; 14: 17562864211054956. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 27. Lu E, Dahlgren L, Sadovnick A, et al. Perinatal outcomes in women with multiple sclerosis exposed to disease-modifying drugs. Mult Scler 2012; 18(4): 460–467. [DOI] [PubMed] [Google Scholar]
- 28. Rae-Grant A, Day GS, Marrie RA, et al. Practice guideline recommendations summary: Disease-modifying therapies for adults with multiple sclerosis: Report of the Guideline Development, Dissemination, and Implementation Subcommittee of the American Academy of Neurology. Neurology 2018; 90: 777–788. [DOI] [PubMed] [Google Scholar]
- 29. Anna F, Rumbold Alice R, Grzeskowiak Luke E. Family planning and multiple sclerosis: A qualitative study of patient experiences to understand information needs and promote informed decision-making. Patient Educ Couns 2023; 110: 107673. [DOI] [PubMed] [Google Scholar]
- 30. Rasmussen PV, Magyari M, Moberg JY, et al. Patient awareness about family planning represents a major knowledge gap in multiple sclerosis. Mult Scler Relat Disord 2018; 24: 129–134. [DOI] [PubMed] [Google Scholar]
- 31. Foulon S, Maura G, Dalichampt M, et al. Prevalence and mortality of patients with multiple sclerosis in France in 2012: A study based on French health insurance data. J Neurol 2017; 264(6): 1185–1192. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 32. Barataud-Reilhac A, Kerbrat S, Roux J, et al. Teriflunomide-exposed pregnancies in a French cohort of patients with multiple sclerosis. Neurol Clin Pract 2020; 10(4): 287–297. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 33. OFSEP. Descriptif de la cohorte, https://www.ofsep.org/fr/la-cohorte-ofsep/descriptif-de-la-cohorte (accessed 8 February 2023).
Associated Data
This section collects any data citations, data availability statements, or supplementary materials included in this article.
Supplementary Materials
Supplemental material, sj-docx-1-msj-10.1177_13524585231223395 for Use of multiple sclerosis disease-modifying therapies during pregnancy in France: Nationwide study between 2010 and 2021 by Morgane Swital, Jérôme Drouin, Sara Miranda, Serge Bakchine, Jérémie Botton and Rosemary Dray-Spira in Multiple Sclerosis Journal