Abstract
Background:
The classical psychedelics psilocybin, peyote, ayahuasca/ N, N-dimethyltryptamine, and lysergic acid diethylamide can temporarily produce altered states of consciousness, characterized by changes in sensory perception, thought, mood, and the sense of self-reality and meaning. It is important to have reliable instruments for quantifying these altered states in trials, due to a plausible link between the acute subjective experience and treatment outcome.
Methods:
We conducted a review of outcome measures applied in research on classical psychedelics to assess one or more dimensions of the acute subjective psychedelic experience. Three relevant databases were searched electronically. Two reviewers independently conducted article selection and data extraction regarding the instruments, dimensions, geography, population, and psychedelic substance investigated in the included studies. We identified the five most utilized instruments for the most recent 6 years, as well as the five most utilized instruments for each psychedelic.
Results:
We included 93 papers, which reported on 93 unique trials and utilized 17 different rating scales. Of these, the most utilized were the Five-Dimensional Altered States of Consciousness Questionnaire, visual analog or Likert scales specially developed for the trials, the Hallucinogen Rating Scale, the States of Consciousness Questionnaire, and the Abnormer Psychischer Zustand.
Discussion:
Considerable variability was found in the instruments utilized in clinical trials on classical psychedelics. We advise and encourage the development of a core outcome set for psychedelic research to enable altered state comparisons across compounds, participants, and settings. We further advise that instruments be designed to assess the “setting” of a psychedelic experience.
Keywords: Psychedelics, LSD, DMT, ayahuasca, psilocybin
Introduction
The classical psychedelics psilocybin, lysergic acid diethylamide (LSD), N, N-dimethyltryptamine (DMT, the psychoactive ingredient in ayahuasca), and mescaline are serotonin (5-HT) receptor agonists, which dose dependently induce an altered state of consciousness (ASC) characterized by changes in sensory perception, thought, mood, and the sense of self-reality and meaning (Nichols, 2004; Nichols, 2016). As opposed to changes in the self and perception from self-disorders and psychotic symptoms seen in psychiatric diseases (Parnas et al., 2005), ASCs are short-lasting and self-limiting.
Although plants and fungi with psychedelic properties have been known to man for several millennia, it was not until the synthesis of LSD by Swiss chemist Albert Hofmann in 1938 that Western researchers became interested in psychedelics (Nichols, 2004; Nichols, 2016). Classical psychedelics were investigated for the treatment of various psychiatric illnesses, and several instruments were developed to assess their ability to produce ASCs. Research into these psychedelics was broadly shut down in the 1980s due to the political scheduling of the substances (Nichols, 2004), but recommenced with Strassman’s research into DMT in 1990, after a hiatus of two decades.
In this new era of psychedelic research, various new instruments have been designed to assess the acute subjective psychedelic experience, and the treatment outcomes have been discussed in multiple systematic reviews and meta-analyses (Castro Santos and Gama Marques, 2021; Hovmand et al., 2023; Kisely et al., 2023; van der Meer et al., 2023).
Assessing the subjective effects of psychedelics is essential since much research has indicated an association between the characteristic psychedelic experience and a positive effect on the symptomatology of several psychiatric illnesses. Most of this research has been carried out with the Revised Mystical Experience Questionnaire 30 (MEQ30), where a high score correlates with and has been suggested as a predictor of long-term positive therapeutic outcomes in various clinical and naturalistic studies of psychedelics. This is true in the treatment of people with alcohol use disorder (Bogenschutz et al., 2015; Garcia-Romeu et al., 2019) and depression and anxiety (Davis et al., 2020; Griffiths et al., 2016; Roseman et al., 2018; Ross et al., 2016), as well as for success with smoking cessation (Garcia-Romeu et al., 2014), done with primarily psilocybin (Bogenschutz et al., 2015; Garcia-Romeu et al., 2014; Griffiths et al., 2008; Roseman et al., 2018) but also a variety of psychedelic substances including DMT (Barsuglia et al., 2018; Bouso et al., 2016; Davis et al., 2019) and LSD (Liechti et al., 2017; Schmid and Liechti, 2018). High MEQ-30 scores have also been found to predict changes in personality-trait openness (MacLean et al., 2012) and positive changes in attitudes, mood, and behavior (Griffiths et al., 2011). Furthermore, the occurrence of a challenging psychedelic experience is influential in determining long-term responses to the experience (Barrett et al., 2016), and is inversely correlated with a negative outcome in psychiatric symptoms (Barrett et al., 2016; Carbonaro et al., 2016; Haijen et al., 2018; Roseman et al., 2018).
The advancement in psychedelic medicine is informed by clinical research; however, the interpretation of such evidence can be hampered by the heterogeneity of study design and the lack of an agreed-upon set of outcomes. This variation can act as a barrier when drawing comparisons between studies. One way to reduce the variation is by developing a core outcome set (COS), which represents a minimum required data set for randomized controlled trials—usually a data set using certain conditions, but in this case, using certain medicines (Williamson et al., 2012). A COS allows for easy comparison between studies, facilitates meta-analyses, improves the accuracy of data interpretation, reduces outcome reporting bias, and aims to reduce heterogeneity in both research and possible future clinical practice if, as expected, classical psychedelic compounds become widely available as medicine. The Core Outcome Measures in Effectiveness Trials (COMET) initiative aims to facilitate the development and application of COSs to overcome outcome reporting variation (Prinsen et al., 2014).
In this review, we will provide an overview of the available instruments specifically designed to assess the acute psychedelic experience and their frequency of use in clinical research—both in general, across countries, and for each specific classical psychedelic.
Material and methods
Data acquisition
We first attempted to identify all available human studies on classical psychedelics in a clinical setting, from 1990 until January 12th, 2022, in which one or more rating scales were used to assess one or more psychometric properties specific to the psychedelic experience. We chose 1990 as our beginning date because that was when Rick Strassman became the first to legally administer psychedelics to human subjects since the early 1970s.
Search strategy
Electronic searches were performed from January 1st, 1990 to May 22nd, 2023, using the PubMed, Embase, and APA PsycNet databases. See Supplemental Material 1 for the entire search strategy.
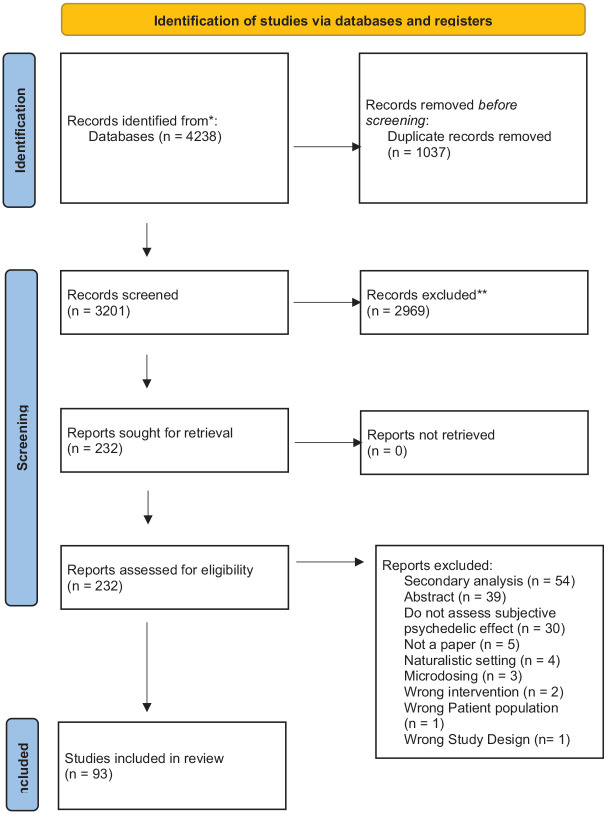
A total of 4238 hits were identified through electronic records and other sources, representing 3201 titles after the removal of duplicates. Two authors (ORH and EDP) screened all titles and abstracts to determine their relevance to the current study, with discrepancies resolved by a consensus decision. Titles and abstracts irrelevant to the current study were discarded, and the remaining 232 references were retrieved as full-text papers to evaluate inclusion and exclusion criteria.
Eligibility criteria
Retrieved papers were checked against the following criteria for the inclusion of studies relevant to this review: (1) complete articles published in peer-reviewed scientific journals, which were (2) randomized trials, non-randomized trials, or observational studies, in (3) clinical setting (4) with human subjects, (5) utilizing one of the classical psychedelics psilocybin, DMT/ayahuasca, LSD, or mescaline. The chosen papers were also all (6) using an instrument (whether standardized or developed for the study) designed to measure one or more psychometric properties of the psychedelic experience as a primary or secondary outcome, as judged by the authors of this review, and were (7) published in English or Danish. Emerging instruments that had yet to be used in clinical research but were judged to be relevant for discussion were also included in this review. These were found in the search and from a hand-search of the included trials.
Qualitative studies, abstracts, letters, reviews, meta-analyses, secondary analyses, conference abstracts, comments, study protocols, and editorials were excluded. We also excluded papers reporting on psychedelic microdosing and considered doses of less than 20 µg of LSD as microdoses, based on the principle that microdosing is 5–10% of a standard dose (Marschall et al., 2022).
After the evaluation of inclusion and exclusion criteria by two authors (ORH and EDP), with discrepancies resolved by a consensus decision, 93 papers were included in the final sample. A Preferred Reporting Items for Systematic Reviews and Meta-Analyses flow diagram illustrating the phases of the review is presented in Figure 1.
Figure 1.
PRISMA 2020 flow diagram with reasons for exclusion.
PRISMA: Preferred Reporting Items for Systematic Reviews and Meta-Analyses.
Data extraction
Two authors (ORH and EDP) independently extracted data regarding the title of each paper, names of authors, year of publication, study design, characteristics of the participants, type of intervention (drug, dose), and type of altered state patient-reported outcome measure(s) and recorded it for all included articles. A consensus decision resolved any discrepancies. EDP extracted data from the most recent editions on instrument items and dimensions or domains, and ORH extracted data factor structure and internal consistency from available psychometric studies. The latter was found by hand-searching the included trials.
Lastly, we coded the instruments and factors according to whether the instruments primarily captured the following themes (as judged by ORH and EDP): Overall strength of the experience (OST), which described the encounter’s overall intensity; psychological experience, which assessed themes relating to psychological insight and psychological themes occurring during the experience; subjective adverse reactions, which assessed anxiety, dysphoria, and other subjectively negative reactions; subjective positive reactions, which assessed euphoria and other subjectively positive reactions; mystical or spiritual nature, which assessed mystical phenomena; altered sensory input and thoughts, which assessed changes in sensory inputs, such as visual hallucinations; and other, which assessed factors which were either mixed or different from the other themes.
Results
Study selection
We identified 93 papers that reported on 93 unique trials (see Supplemental Material 2 for a table of the included papers). We further identified four emerging instruments.
The 93 trials utilized 17 different instruments to quantify one or more properties of the acute psychedelic experience. The included instruments had different response formats: visual analog scales (VAS), Likert scales, or dichotomous scales. VAS entail a line, most often ranging from 0 to 100, where the subject marks the level of the measured property. Likert scales utilize varying point ranges, such as a scale consisting of five points ranging from 1: “Strongly disagree” to 5: “Strongly agree.” Dichotomous scales offer two opposite categorical options from which the assessor must select.
Several studies employed unique scoring instruments created by individual researchers to measure the acute psychedelic experience. In this study, we combined these purpose-made VAS and Likert scales (PurposeM) into a single group to demonstrate the frequency of non-standardized methods utilized to measure the psychedelic experience. Furthermore, we considered the Five-Dimensional Altered States of Consciousness Questionnaire (5D-ASC) as a single instrument, irrespective of whether it was analyzed with the five dimensions (Bodmer, 1989) or the 11 dimensions (Studerus et al., 2010) subscale. For simplicity, we will refer to both analyses as the 5D-ASC.
Two included instruments, the Near-Death Experience Questionnaire (NDE) (Greyson, 1983) and the Clinician-Administered Dissociative States Scale (CADSS; Bremner et al., 1998), were initially designed for other purposes. The remaining instruments were specifically designed to be used in research on psychedelics or other psychotropic drugs and spontaneous ASCs.
The five most utilized instruments were the 5D-ASC, in which 54 trials (58%) were reported; VAS/Likert-type instruments developed specifically for their trial (PurposeM) in 27 trials (29%); the Hallucinogen Rating Scale, applied in 23 trials (25%); the 30-item MEQ, applied in 15 trials (16%); and the States of Consciousness Questionnaire (SOCQ), applied in 14 trials (15%).
Of these 93 trials, 62% were published within the most recent 8 years (2016–2023). In all, 10 instruments were solely applied in this recent subset. The five most utilized instruments in these recent trials were in descending order: the 5D-ASC (40 trials, 69%); PurposeM (20 trials, 34%); the MEQ30 (15 trials, 26%); and the HRS and SOCQ (nine trials each, 16%). See Table 1 for an overview of the usage of all instruments.
Table 1.
Most applied instruments for assessment of the acute psychedelic experience in clinical research on psychedelics from January 1st, 1990 to May 22nd, 2023.
| Instrument | N = 93 (1990–2023) | % of sample (1990–2023) | N = 58 (2016–2023) | % of sample (2016–2023) |
|---|---|---|---|---|
| 5D-ASC | 54 | 58.1 | 40 | 68.9 |
| VAS/Likert | 27 | 29.0 | 20 | 34.4 |
| HRS | 23 | 24.7 | 9 | 15.5 |
| MEQ30 | 15 | 16.1 | 15 | 25.9 |
| SOCQ/MEQ43 | 14 | 15.0 | 9 | 15.5 |
| ARCI | 11 | 11.8 | 4 | 6.9 |
| APZ | 10 | 10.7 | 2 | 3.4 |
| EDI | 7 | 7.5 | 7 | 12.1 |
| M-scale | 6 | 6.4 | 3 | 5.2 |
| CEQ | 5 | 5.4 | 5 | 8.6 |
| CADSS | 2 | 2.1 | 2 | 3.4 |
| DEQ | 2 | 2.1 | 2 | 3.4 |
| NDE | 1 | 1.1 | 1 | 1.7 |
| EBI | 1 | 1.1 | 1 | 1.7 |
| PSI | 1 | 1.1 | 1 | 1.7 |
| PIQ | 1 | 1.1 | 1 | 1.7 |
| PIS | 1 | 1.1 | 1 | 1.7 |
Overview of the entire sample and the 8 most recent years from 2016 to 2023.
APZ/OAV: Abnormer Psychischer Zustand; ARCI: Addiction Research Center Inventory; CADSS: Clinician-Administered Dissociative States Scale; CEQ: Challenging Experiences Questionnaire; DEQ: Drug Effects Questionnaire; EBI: Emotional Breakthrough Inventory; EDI: Ego Dissolution Inventory; HRS: Hallucinogen Rating Scale; MEQ30: Mystical Experience Questionnaire 30 items; M-scale: Hood Mysticism Scale; NDE: Near-Death Experience Questionnaire; PIS: Psychological Insight Scale; PIQ: Psychological Insight Questionnaire; PSI: Psychotomimetic States Inventory; SOCQ: States of Consciousness Questionnaire; VAS: Visual analog scales developed for the studies; 5D-ASC: Five-Dimension Altered States of Conscious Questionnaire.
Most (56%) of the included studies investigated psilocybin, while 25% investigated LSD and 17% investigated ayahuasca/DMT. The 5D-ASC was most extensively utilized in research on psilocybin and LSD (67% and 83% in the subsample of 52 and 23, respectively), while HRS was most extensively utilized in research on DMT/ayahuasca (81% of the subsample of 16). The APZ was the only applied instrument in the small subsample of two that investigated mescaline.
Table 2 provides an overview of the frequency of use across each classical psychedelic.
Table 2.
Five most utilized instruments for assessment of the acute psychedelic experience for each classical psychedelic from January 1st, 1990 to May 22nd, 2023.
| Psilocybin (N = 52) | 5D-ASC (67.3%) | HRS (19.2%) | SOCQ/MEQ43 (17.3%) | MEQ30 (17.3%) | VAS/Likert (15.4%) |
|---|---|---|---|---|---|
| LSD (N = 23) | 5D-ASC (82.6%) | VAS/Likert (60.9%) | SOCQ/MEQ43 (21.7%) | ARCI (17.4%) | MEQ30 (17.4%) |
| DMT/ayahuasca (N = 16) | HRS (81.2%) | VAS/Likert (31.2%) | ARCI (25.0%) | APZ (18.7%) | MEQ30, CADSS (12.5%) |
| Mescaline (N = 2) | APZ (100%) | NA | NA | NA | NA |
APZ: Abnormer Psychischer Zustand; ARCI: Addiction Research Center Inventory; CADSS: Clinician-Administered Dissociative States Scale; HRS: Hallucinogen Rating Scale; MEQ30: Mystical Experience Questionnaire 30 items; SOCQ: States of Consciousness Questionnaire; VAS: Visual analog scales developed for the studies; 5D-ASC: Five-Dimension Altered States of Conscious Questionnaire.
Switzerland, the United States, the UK, and Spain were the origin of most studies (80%). The most applied instrument in Switzerland and the United States was the 5D-ASC, and in Spain, it was the HRS. In the UK, PurposeM was the most common, while the MEQ30 and 5D-ASC were equally the most frequently applied standardized instruments. See Table 3 for an overview.
Table 3.
Most utilized instrument for the assessment of the acute psychedelic experience in clinical research for the four most contributing countries from Jan 1st, 1990 to May 22nd, 2023.
| Switzerland (N = 35) | 5D-ASC (91.4%) | VAS/Likert (25.7%) | SOCQ/MEQ43 (17.1%) | MEQ30 (8.5%) | ARCI (5.7) |
|---|---|---|---|---|---|
| USA (N = 23) | 5D-ASC (43.5%) | HRS (39.1%) | SOCQ/MEQ43 (34.8%) | M-scale (26.1%) | ARCI (21.7%) |
| UK (N = 9) | VAS/Likert (55.6%) | 5D-ASC, (33.3%) | MEQ30 (33.3%) | EDI (22.2%) | NDE, CADSS, EBI, PSI, HRS (11.1%) |
| Spain (N = 7) | HRS (100%) | ARCI (57.1%) | VAS/Likert (57.1%) | APZ (28.5%) | NA |
APZ/OAV: Abnormer Psychischer Zustand; ARCI: Addiction Research Center Inventory; CADSS: Clinician-Administered Dissociative States Scale; EBI: Emotional Breakthrough Inventory; EDI: Ego Dissolution Inventory; HRS: Hallucinogen Rating Scale; MEQ30: Mystical Experience Questionnaire 30 items; M-scale: Hood Mysticism Scale; NDE: Near-Death Experience Questionnaire; SOCQ: States of Consciousness Questionnaire; VAS: Visual analog scales developed for the studies; 5D-ASC: Five-Dimension Altered States of Conscious Questionnaire.
Table 4 provides a comprehensive overview of the response structure, size, and dimension of the various instruments. Table 5 provides the reliability of the included instruments and their factors. Table 5 shows a suggestion of how the different factors could be meaningfully grouped into seven domains, each of which describes a component of the acute psychedelic experience. Lastly, Table 6 shows the factors associated with each of these domains.
Table 4.
Instrument structure and item count.
| Instrument | Items | Dimensions/factors (no. scoring items) | Scoring | Paper |
|---|---|---|---|---|
| APZ | 158 | Oceanic Boundlessness, Dread of Ego Dissolution, Visionary Restructuralizationb | Dichotomous | Dittrich (1975) |
| OAV | 66 | Oceanic Boundlessness (27), Dread of Ego Dissolution (21), Visionary Restructuralization (18) | VAS | Bodmer (1989) |
| 5D-ASC | 94 | Oceanic Boundlessness (27), Dread of Ego Dissolution (21), Visionary Restructuralization (18), Auditory Alterations (16), Vigilance Reduction (12) | VAS | Dittrich (1999) |
| 5D-ASC, 11 subscales | 94 | Experience of unity (5), Spiritual Experience (3), Blissful State (3), Insightfulness (3), Disembodiment (3), Impaired Control and Cognition (7), Anxiety (6), Complex Imagery (3), Elementary Imagery (3), Audio-Visual Synesthesia (3), Changed Meaning of Percepts (3) | VAS | Studerus et al. (2010) |
| HRS, version 3.04a | 100 | Somaesthesia (15), Affect (18), Perception (16), Cognition (12), Volition (8), Intensity (5). | Likert (5 points) | Strassman et al. (1994) |
| SOCQ/MEQ43 | 100/43 | Internal Unity (6), External Unity (6), Transcendence of Time and Space (8), Ineffability and Paradoxicality (5), Sense of Sacredness (7), Noetic Quality (4), Deeply-Felt Positive Mood (7) | Likert (6 points) |
Pahnke (1969)
Richards (1975) |
| MEQ30 | 30 | Mystical (15), Positive Mood (6), Transcendence of Time and Space (6), Ineffability (3) | Likert (5 points) |
MacLean et al. (2012)
Barrett et al. (2015) |
| ARCI, LSD-scale | 14 | LSD Scale (14) | Dichotomous | Haertzen (1966) |
| M-Scale | 32 | Introvertive Factor (12), Extrovertive Factor (8), Interpretation Factor (12) | Likert (5 points) | Hood (1975) |
| EDI | 8 | Total Ego-Dissolution Inventory Score (8) | VAS | Nour et al. (2016) |
| CEQ | 26 | Fear (5), Grief (6), Physical Distress (5), Insanity (3), Isolation (3), Death (2), Paranoia (2) | Likert (6 points) | Barrett et al. (2016) |
| CADSS-1 | 28 | Subjective Items (23), Observer Items (5) | Likert (5 points) | Bremner et al. (1998) |
| NDE | 16 | Cognitive (4), Affective (4), Paranormal (4), Transcendental (4) | Likert (3 points) | Greyson (1983) |
| DEQ | 5 | Total Drug Effects Questionnaire Score (5) | VAS | Morean et al. (2013) |
| EBI | 6 | Total Emotional Breakthrough Score (6) | VAS | Roseman et al. (2019) |
| PSI | 48 | Delusory Thinking (8), Perceptual Distortions (9), Cognitive Disorganization (9), Anhedonia (7), Mania (6), Paranoia (8) | Likert (4 points) | Mason et al. (2008) |
| PIQ | 23 | Avoidance and Maladaptive Patterns Insights (14), Goals and adaptive Patterns Insights (9) | Likert (6 points) | Davis et al. (2021) |
| APEQ | 32 | Accepting Response (4), Relief (4), Pro-Acceptance Insights (4), Avoidant Response (4), Distress (4), Pro-Avoidance Insights (4), Introspection (4), Interaction (4) | VAS | Wolff et al. (2022) |
| PIS | 7 | Psychological Insight (7) | VAS | Peill et al. (2022) |
| ASEQ | 40 | Joy in Life (18), Relationship to the Sacred (15 items), Toxic Feelings (7) | Likert (5 points) | Harris and Gurel (2012) |
| MOS | 21 | Mysticism (21) | Likert (5 points) | Francis and Louden (2004) |
| SIMO | 9 | Mysticism (9) | Likert (5 points) | Francis and Louden (2004) |
a: Most recent version is available upon request from the authors; APEQ: The Acceptance/Avoidance-Promoting Experiences Questionnaire; APZ: Abnormer Psychischer Zustand; ARCI: Addiction Research Center Inventory; ASEQ: After the Spiritual Experience Questionnaire; b: data not found; CADSS: Clinician-Administered Dissociative States Scale; CEQ: Challenging Experiences Questionnaire; DEQ: Drug Effects Questionnaire; EBI: Emotional Breakthrough Inventory; EDI: Ego Dissolution Inventory; HRS: Hallucinogen Rating Scale; MEQ30: Mystical Experience Questionnaire 30 items; MOS: Mystical Orientation Scale; M-scale: Hood Mysticism Scale; NDE: Near-Death Experience Questionnaire; PIS: The Psychological Insight Scale; PIQ: Psychological Insight Questionnaire; PSI: Psychotomimetic States Inventory; SIMO: Short Index of Mystical Orientation; SOCQ: States of Consciousness Questionnaire; 5D-ASC: Five-Dimension Altered States of Conscious Questionnaire.
Table 5.
Assessed domains and sub-factor reliability of each instrument.
| Instrument | Dimensions/factors | Sample | Reliability (Cronbach’s α) | Paper |
|---|---|---|---|---|
| OAV | Oceanic Boundlessness [SPR] | 591 | 0.95 | Studerus et al. (2010) |
| Dread of Ego Dissolution [SAR] | 0.93 | |||
| Visionary Restructuralization [ASIT] | 0.91 | |||
| 5D-ASC | Oceanic Boundlessness [SPR] | 253 | 0.95 | Studerus (2012) |
| Dread of Ego Dissolution [SAR] | 0.93 | |||
| Visionary Reconstruction [ASIT] | 0.91 | |||
| Auditory Alterations [ASIT] | 0.93 | |||
| Vigilance Reduction [O] | 0.86 | |||
| 5D-ASC, 11 subscales | Experience of Unity [MSN] | 591 | 0.88 | Studerus et al. (2010) |
| Spiritual Experience [MSN] | 0.77 | |||
| Blissful State [MSN] | 0.82 | |||
| Insightfulness [PE] | 0.73 | |||
| Disembodiments [ASIT] | 0.82 | |||
| Impaired Control and Cognition [ASIT] | 0.85 | |||
| Anxiety [SAR] | 0.89 | |||
| Complex Imagery [ASIT] | 0.80 | |||
| Elemental Imagery [ASIT] | 0.84 | |||
| Audio-Visual Synesthesia [ASIT] | 0.91 | |||
| Changed Meaning of Percepts [PE] | 0.79 | |||
| HRS | Affect [PE] | 75 | 0.81 | Riba et al. (2001) |
| Cognition [ASIT] | 0.87 | |||
| Intensity [OS] | 0.33 | |||
| Perception [ASIT] | 0.88 | |||
| Somaesthesia [ASIT] | 0.82 | |||
| Volition [O] | 0.51 | |||
| MEQ30 | Mystical [MSN] | 195 | 0.97 | MacLean et al. (2012) |
| Positive Mood [SPR] | 0.92 | |||
| Ineffability [MSN] | 0.90 | |||
| Transcendence Time and Space [ASIT] | 0.86 | |||
| M-Scale | Introvertive Mysticism [MSN] | 1379 | 0.85 | Hood et al. (2001) |
| Extrovertive Mysticism [MSN] | 0.82 | |||
| Interpretive Factor [MSN] | 0.82 | |||
| EDI | Total Score [MSN] | 1828 | 0.93 | Nour et al. (2016) |
| CEQ | Fear [SAR] | 1052 | 0.84 | Barrett et al. (2016) |
| Grief [SAR] | 0.86 | |||
| Physical Distress [PE] | 0.81 | |||
| Insanity [SAR] | 0.76 | |||
| Isolation [SAR] | 0.77 | |||
| Death [SAR] | 0.85 | |||
| Paranoia [SAR] | 0.70 | |||
| CADSS | Subjective Items [ASIT] | 68 | 0.94 | Bremner et al. (1998) |
| Observer Items [O] | 0.90 | |||
| NDE | Cognitive [ASIT] | 74 | 0.75 | Greyson (1983) |
| Affective [SPR] | 0.86 | |||
| Paranormal [MSN] | 0.66 | |||
| Transcendental [MSN] | 0.76 | |||
| EBI | Total Emotional Breakthrough Score [PE] | 379 | 0.93 | Roseman et al. (2019) |
| PSI | Delusory Thinking [O] | 140 | 0.80 | Mason et al. (2008) |
| Perceptual Distortions [ASIT] | 0.78 | |||
| Cognitive Disorganization [ASIT] | 0.89 | |||
| Anhedonia [SAR] | 0.27 | |||
| Mania [O] | 0.67 | |||
| Paranoia [SAR] | 0.85 | |||
| PIQ | Avoidance and Maladaptive Patterns Insights [PE] | 1661 | 0.93 | Davis et al. (2021) |
| Goals and Adaptive Patterns [PE] | 0.85 | |||
| Insights [PE] | ||||
| APEC | Acceptance-related experience [PE] | 997 | 0.92 | Wolff et al. (2022) |
| Avoidance-related experience [PE] | 0.78 | |||
| PIS | Psychological Insight [PE] | 279 | 0.94 | Peill et al. (2022) |
| ASEQ | Joy in Life [MSN] | Harris and Gurel (2012) | ||
| Relationship to the Sacred [MSN] | ||||
| Toxic Feelings [O] | ||||
| MOS | Mysticism [MSN] | 1468 | 0.94 | Francis and Louden (2004) |
| SIMO | Mysticism [MSN] | 1468 | 0.86 | Francis and Louden (2004) |
| DEQ | Total Drug Effect Questionnaire Score [O] | Morean et al. (2013) |
We did not find any data on the psychometric abilities of the SOCQ/the MEQ-43, the ARCI, DEQ and APZ, ASEQ.
ASIT: Altered sensory input and thoughts; MSN: Mystical or spiritual nature; O: Other; OS: Overall strength of the experience; PE: Psychological experience; SAR: Subjective adverse reactions; SPR+: Subjective positive reactions.
Table 6.
Overview of factors associated with each domain.
| Domain | Instrument, factor |
|---|---|
| OS | HRS, Intensity |
| PE | 11D-ASC, Insightfulness 11D-ASC, Changed Meaning of Percepts HRS, Affect CEQ, Physical Distress EBI, Total Emotional Breakthrough Score PIQ, Avoidance and Maladaptive Patterns Insights PIQ, Goals and Adaptive Patterns PIQ, Insights APEQ, Acceptance-related experience APEQ, Avoidance-related experience PIS, Psychological Insight |
| SAR | OAV, Dread of Ego Dissolution 5D-ASC, Dread of Ego Dissolution 11D-ASC, Anxiety CEQ, Fear CEQ, Grief CEQ, Insanity CEQ, Isolation CEQ, Death CEQ, Paranoia PSI, Anhedonia PSI, Paranoia |
| SPR | OAV, Oceanic Boundlessness 5D-ASC, Oceanic Boundlessness MEQ30, Positive Mood NDE, Affective |
| MSN | 11D-ASC, Experience of Unity 11D-ASC, Spiritual Experience 11D-ASC, Blissful State MEQ30, Ineffability MEQ30, Mystical M-Scale, Introvertive Mysticism M-Scale, Extrovertive Mysticism M-Scale, Interpretive Factor NDE, Paranormal NDE, Transcendental ASEQ, Joy in Life ASEQ, Relationship to the Sacred MOS, Mysticism SIMO, Mysticism |
| ASIT | OAV, Visionary Restructuralization 5D-ASC, Visionary Reconstruction 5D-ASC, Auditory Alterations 11D-ASC, Disembodiments 11D-ASC, Impaired Control and Cognition 11D-ASC, Complex Imagery 11D-ASC, Elemental Imagery 11D-ASC, Audio-Visual Synesthesia HRS, Cognition HRS, Perception HRS, Somaesthesia MEQ30, Transcendence Time and Space CADSS, Subjective Items NDE, Cognitive PSI, Perceptual Distortions PSI, Cognitive Disorganization |
| O | 5D-ASC, Vigilance Reduction HRS, Volition EDI, Total Score CADSS, Observer Items PSI, Delusory Thinking PSI, Mania ASEQ, Toxic Feelings DEQ, Total Drug Effect Questionnaire Score |
5D-ASC: Five-Dimension Altered States of Conscious Questionnaire; ASIT: Altered sensory input and thoughts; ASEQ: After the Spiritual Experience Questionnaire; DEQ: Drug Effects Questionnaire; MSN: Mystical or spiritual nature; O: Other; OS: Overall strength of the experience; PE: Psychological experience; SAR: Subjective adverse reactions; SPR+: Subjective positive reactions; EDI: Ego Dissolution Inventory; HRS: Hallucinogen Rating Scale; MEQ30: Mystical Experience Questionnaire 30 items; M-scale: Hood Mysticism Scale; NDE: Near-Death Experience Questionnaire; PIS: Psychological Insight Scale; PIQ: Psychological Insight Questionnaire; PSI: Psychotomimetic States Inventory.
The most frequently used instruments entailed factors quantifying alterations in thought and sensory inputs as well as subjective positive reactions and feelings and mystical properties. In addition, we found that many of the recently developed instruments mainly captured what we described as the psychological experience associated with the psychedelic experience.
The following is a description of the individual instruments and their development. We describe the instruments chronologically because many of them are modifications of earlier instruments.
Addiction Research Center Inventory
One of the first of these instruments was the Addiction Research Center Inventory (ARCI), which was developed to differentiate subjective effects among several different classes of psychoactive drugs, including psychedelics. The items regarding LSD do, however, mainly focus on somatic effects (Haertzen, 1966). In its original form, it has 550 dichotomous “true/false” items which are developed specifically to measure subjective effects of drugs. It also exists in a short form with 49 true/false items. Both the long and the short form load on the following five scales: LSD, (a psychedelic-sensitive scale); pentobarbital, chlorpromazine, and alcohol group (a sedative-sensitive scale); benzedrine group; amphetamine; and morphine-benzedrine group (Griffiths et al., 2006; Jasinski, 1977; Martin et al., 1971). The long version of the ARCI has demonstrated good internal and external validity (Hill et al., 1963).
Abnormer Psychischer Zustand
Another pre-prohibition questionnaire is the Abnormer Psychischer Zustand (APZ, Eng: abnormal mental state) developed by Dittrich (1975) (Dittrich, 1975, 1998). It contains 158 dichotomous items covering a broad range of potential phenomena occurring during an ASC. Validation studies have found three oblique primary dimensions (Dittrich, 1996; Dittrich et al., 1985; Studerus et al., 2010): Oceanic Boundlessness (OBN), measuring positively experienced depersonalization and derealization, deeply felt positive mood, and experiences of unity, similar to spontaneous mystical experiences as described in the scientific literature on the psychology of religion (Stace, 1960); Visionary Restructuralization (VRS), measuring visual hallucinations, illusions, changes in the meaning of percepts and auditory-visual synesthesia; and Dread of Ego Dissolution (DED), measuring symptoms characteristic of a “bad trip,” such as negatively experienced derealization and depersonalization, catatonic symptoms, cognitive disturbances, paranoia, and loss of thought and body control. The secondary scale, General Altered States of Consciousness (G-ASC), is a general measure of consciousness alteration.
Five-Dimensional Altered States of Consciousness Questionnaire
The APZ was critiqued for being too crude to measure more subtle differences in an ASC, both due to its use of a binary item response format and because the OBN and VRS dimensions contained too few items (Studerus et al., 2010). Bodmer (1989) made a psychometrically improved version of the APZ, named the OAV (the abbreviation OAV stands for the German names of the three dimensions, OBN, DED, and VRS) (Bodmer, 1989). The OAV includes 66 items originating from the APZ, plus new items. It also changed from dichotomous items to a VAS scale.
An extended version of the OAV, called the 5D-ASC, was later developed and published by Dittrich (1998) (Dittrich et al., 2010). The latest refinement of Dittrich’s original questionnaire includes 94 items rated on a VAS scale. Two different ways of analyzing the 5D-ASC questionnaire are in use. The first contains five dimensions. The ratings of 66 items are combined to form three core dimensions which are believed to be shared by ASC experiences across different methods of induction: OBN, DED, and VRS. The remaining 28 items constitute two supplementary dimensions that are considered specific to particular methods of induction: Auditory Alterations and Vigilance Reduction (Dittrich et al., 2006, 2010). Another analytic approach entails 42 items belonging to the three core dimensions (OBN, DED, and VRS) and describes these items as loading onto 11 lower-order factors. These factors can be regarded as subscales of the three core dimensions and are as follows: (1) Experience of Unity, (2) Spiritual Experience, (3) Blissful State, (4) Insightfulness, (5) Disembodiment, (6) Impaired Control and Cognition, (7) Anxiety, (8) Complex Imagery, (9) Elementary Imagery, (10) Audio-Visual Synesthesia, and (11) Changed Meaning of Percepts (Studerus et al., 2010). Both the 5D-ASC (Studerus, 2012) and the 11 subscales (Studerus et al., 2010) interpretation have been found to have good psychometric properties.
States of Consciousness Questionnaire
Two other pre-prohibition instruments were developed independently based on Stace’s (1960) work on spontaneous mystical experiences and religion. They were not specifically designed to assess mystical experiences occasioned by psychedelic substances but also those occasioned by actions such as fasting and meditation. One was the 100-item SOCQ, developed by psychologist Richards and rated on a six-point Likert scale. Of the 100 items, 57 serve as distractor items, and the remaining 43 constitute the original Pahnke–Richards MEQ-43, which was used as a primary outcome measure in their contemporary Good Friday Experiment (Pahnke, 1963). It was later revised by Richards (1975) for use in the Maryland Psychiatric Center studies on psychedelics (Richards, 1975). It includes seven subscales, each representing a domain of the mystical experience: Internal Unity (pure awareness; a merging with ultimate reality); Transcendence of Time and Space; Ineffability and Paradoxicality (difficulty in describing the experience in words); Sense of Sacredness (awe); External Unity (unity of all things); Noetic Quality (a sense of intuitive knowledge of ultimate reality); and Feeling of Positive Mood (joy, love, and peace) (Griffiths et al., 2006). Pahnke (1969) stated that an individual had undergone a so-called “complete” mystical experience if the scores on each of the following scales were at least 0.6: Unity (either internal or external, whichever was greater), Transcendence of Time and Space, Ineffability and Paradoxicality, Sense of Sacredness, Noetic Quality, and Feeling of Positive Mood (Griffiths et al., 2006; Pahnke, 1969). We could not find any validation studies on the SOCQ or the MEQ-43.
Hood Mysticism Scale
The Hood Mysticism Scale (M-scale) was developed by psychologist Hood in 1975 (Hood, 1975) and is based on the work by Stace (Ai et al., 2021). It consists of 32 items and also exists in a shorter eight-item version (Ai et al., 2021). Respondents rate each item on a five-point Likert scale. Factor analysis has shown three distinct factors: “introvertive mysticism” (experiences primarily related to the internal world of the individual), “extrovertive mysticism” (experiences primarily related to the external world of the individual), and an “interpretive factor” (all items related to awe, divinity, sacrality, and ultimate reality) (Hood et al., 2001). Research has demonstrated excellent internal consistency for the M-scale as a whole, and good internal consistency for its three subscales (Hood et al., 2001).
The M-scale was the last of the major scales developed before clinical research into psychedelics was halted due to their change in legal status.
Hallucinogen Rating Scale
After a hiatus of two decades, the clinical research into psychedelics recommenced with Strassman’s research into DMT. Various new instruments have been designed to assess the psychedelic experience in this new era of investigation. The first was the Hallucinogen Rating Scale (HRS), a 75-item questionnaire which was designed by Strassman et al. (1994) specifically to evaluate the effects of DMT and ayahuasca (Strassman et al., 1994). It has since undergone several revisions. Its most recent full-length edition (3.04) contains 100 items, which are scored on a five-point Likert scale and distributed on the following six subscales: somaesthesia (reflecting somatic effects including interoceptive, visceral, and tactile effects); affect (sensitivity to emotional and affective responses), volition (indicating the subject’s capacity to willfully interact with his/her “self” and/or the environment), cognition (concerning alterations in thought processes or content), perception (measuring visual, auditory, gustatory and olfactory experiences) and intensity (measuring the strength of the overall experience) (Riba et al., 2001). The HRS has been validated in English-, Brazilian- and Spanish-speaking samples, where it has been shown to have good psychometric properties (Bouso et al., 2016; Riba et al., 2001; Suely et al., 2011).
Revised Mystical Experience Questionnaire
In the post-prohibition era of clinical research into psychedelics, several shorter instruments have been developed that aim to assess only a certain quality of the acute psychedelic experience. One such is the Revised MEQ30, developed by MacLean et al. (2012) (MacLean et al., 2012) from analyses of retrospective online surveys of mystical experiences occasioned by recreative use of psychedelics, assessed with the original MEQ-43 (Barrett et al., 2015; MacLean et al., 2012). The MEQ30 has 30 items which are scored using a five-point Likert scale and the following four dimensions: Unity, Noetic Quality, Sacredness (F1); Positive Mood (F2); Transcendence of Time/Space (F3); and Ineffability (F4) (MacLean et al., 2012). In studies on recreative users, it has been shown to have excellent internal consistency for the instrument as a whole (alpha = 0.93) and good internal consistency for the four dimensions (MacLean et al., 2012). It has further been translated into French and Finnish and validated among French- and Finnish-speaking recreative psychedelic users (Fauvel et al., 2022; Kangaslampi et al., 2020).
Ego Dissolution Inventory
Other recently developed questionnaires have focused on other aspects of the psychedelic experience. Nour et al. (2016) published the Ego Dissolution Inventory (EDI), an eight-item questionnaire that is scored on a VAS scale from 0 to 100. It aims to assess a particular effect of classical psychedelics: the subjective feeling of a compromised sense of “self,” termed ego dissolution, which is regarded as central to the acute psychedelic experience. Studies on recreative users show excellent internal consistency for the entire instrument (Nour et al., 2016).
Challenging Experience Questionnaire
Barrett et al. (2016) created the Challenging Experience Questionnaire (CEQ), which is designed to quantify challenging experiences under the influence of psychedelics (Barrett et al., 2016). The CEQ comprised of 26 items, each rated on a six-point Likert scale. Seven factors are assessed, including grief, fear, death, insanity, isolation, physical distress, and paranoia, to provide a phenomenological profile of challenging aspects of experiences with a classical psychedelic.
Emotional Breakthrough Inventory
A limitation of the CEQ is that it measures challenging emotions, memories, or both without addressing their potential resolution. Therefore, it cannot assess the potential value of overcoming any emotional challenges experienced under the influence of psychedelics (Watts et al., 2017). In 2019, Roseman published the eight-item Emotional Breakthrough Inventory (EBI), designed to assess the phenomenon of overcoming challenging emotions/memories, thereby experiencing emotional release while under the influence of psychedelics (Roseman et al., 2019). The EBI is scored on a VAS scale from 0 to 100 and has demonstrated good internal consistency (Roseman et al., 2019).
Psychological Insight Questionnaire
The Psychological Insight Questionnaire (PIQ) was developed by Davis et al. (2021) to measure new insights about one’s own personality, relationships, emotions, or behavioral patterns gained in the context of a discrete event (e.g., a psychedelic experience) (Davis et al., 2021). It consists of 23 items and is rated on a six-point Likert scale, which loads onto the following two subscales: (a) Avoidance and Maladaptive Patterns Insights and (b) Goals and Adaptive Patterns Insights (Davis et al., 2021). It has been found to have good psychometric properties (Davis et al., 2021).
Other instruments
In addition to the instruments explicitly developed to assess parts of the subjective psychedelic experience, some instruments designed to quantify other psychiatric phenomena are also employed in clinical research on psychedelics. One of these is the Psychotomimetic States Inventory (PSI) (Mason et al., 2008), which was originally developed to assess psychotomimetic states in the context of cannabis and ketamine use. The PSI includes the following six subscales: Delusory Thinking, Perceptual Distortions, Cognitive Disorganization, Anhedonia, Mania, and Paranoia. It has been found to have good psychometric properties (Mason et al., 2008).
The CADSS is designed to assess dissociative symptoms. It is a clinician-administered instrument and has been found to have good internal validity (Bremner et al., 1998).
The NDE (Greyson, 1983) also has good psychometric properties (Greyson, 1983).
Lastly, the Drug Effects Questionnaire (DEQ) has been designed to quickly assess whether the subject feels the effect of the drug being investigated, “feels high,” likes or dislikes the effects, and whether they want more of the substance. We found no data on the internal consistency of the DEQ; in addition, its format varies so widely across studies that it can hardly be regarded as a standardized instrument (Morean et al., 2013).
Purpose-made instruments
These instruments measure one or more aspects of the subjective psychedelic experience that the researchers deemed important to assess, such as “overall drug effect,” “good effects,” “bad effects,” “visual effects,” “dizzy,” “stimulated,” and “high.”
Emerging instruments
Our search identified several instruments that have yet to be utilized in clinical research but might be of use in the evaluation of outcomes for a COS:
The Acceptance/Avoidance-Promoting Experiences Questionnaire quantifies experiential acceptance (the ability to tolerate, allow, and engage with aversive feelings, emotions, or memories) and avoidance (the tendency to avoid said feelings and thoughts despite negative consequences) in the context of psychedelic use (Wolff et al., 2022). It includes six subscales (Accepting Response, Relief, Pro-Acceptance Insights, Avoidant Response, Distress, and Pro-Avoidance Insights) and two ancillary scales (Introspection and Interaction), which load onto two subscales, Acceptance-Related Experience and Avoidance-Related Experience and have been found to have good psychometric properties (Wolff et al., 2022).
The Psychological Insight Scale (Peill et al., 2022) was developed to quantify psychological insight gained in the time following a psychedelic experience, rather than during a psychedelic experience, as the PIQ does. It contains six items which inquire about psychological insight, and one item that enquires about any behavioral change occasioned by the experience. The instrument has been found to have good psychometric properties (Peill et al., 2022).
The After the Spiritual Experience Questionnaire (ASEQ), developed by Harris and Gurel (2012) (Harris and Gurel, 2012), is partly based on the Persisting Effects Questionnaire (Griffiths et al., 2006). It contains 40 items, which load onto the following three subscales: Joy in Life, Relationship to the Sacred, and Toxic Feelings. We are not aware of any studies that examine the psychometric properties of the questionnaire.
Finally, the 21-item Mystical Orientation Scale (MOS) and its shortened version, the nine-item Short Index of Mystical Orientation (SIMO) (Francis and Louden, 2004), were developed by Francis and Louden (2001) to assess mystical experiences, similar to the Hood Mysticism Scale. But instead of relying on Stace’s (1960) theoretical definition of mysticism, the MOS and SIMO rely on definitions by Happold (1963). Happold defined mysticism as including the following seven components: mysticism, ineffability, transiency, noesis, passivity, timelessness, oneness, and true ego. The MOS and SIMO were thus designed to capture each of these components. They have good psychometric properties (Francis and Louden, 2004).
Discussion
We found significant heterogeneity regarding the measures applied in the clinical research on classical psychedelic compounds. This heterogeneity was less pronounced when analyzing specific drugs, especially for research on DMT/ayahuasca. This is inherently so, as some instruments were designed specifically to assess the subjective effects of a specific psychedelic compound, as the HRS is for DMT.
Roughly half the sample utilized the 5D-ASC and one-fourth of the sample utilized the HRS. The heterogeneity was less pronounced in our analysis of the most utilized instruments for top contributing countries, which is probably due to an accumulation of research interest in specific psychedelic compounds in specific local research groups (e.g., DMT in Spain). This heterogeneity makes comparing studies troublesome, and it would be beneficial if one instrument were agreed upon as a standard.
We are not aware of any studies that, by factor analysis, compare the different instruments and examine to what extent—if any—they tap into the same domains and constructs. Therefore, we suggest seven domains and have coded the individual instruments and their associated factors according to which domain we believed they sought to capture. Many instruments (i.e., the MEQ30, the HRS, the M-scale, and ASEQ) do not measure negative experiences relating to the acute psychedelic experience. The use of these instruments could therefore possibly be combined with the CEQ or other instruments designed to capture any adverse reactions.
Several new instruments have been introduced during the most recent 6 years. Some are specifically designed to assess psychological changes and phenomena occasioned by psychedelic experiences, and could thus indicate an increased focus on the unique part the subjective experience plays in the beneficial effects of psychedelic medicine. This contrasts with conventional psychiatric medicine, which is thought to act independently of the subjective experience.
Beyond the pharmacological effects of the drug itself, two extra-pharmacological factors must be taken into account in addressing the total effect of the classical psychedelics: that is, the so-called set and setting (Haijen et al., 2018; Hartogsohn, 2016). Briefly explained, the “set” involves variables related to the subject who is ingesting the substance, such as their individual personality traits, life history, and what is currently happening in their life. The “setting” refers to the culture, place, and situation in which the consumption occurs, including other individuals (therapist, guides, peers), decor, what activities are being performed, and the beliefs shared among the group (Hartogsohn, 2016; Leary, 1962; Zinberg, 1984). The latter is important as regard consumption of ayahuasca or other psychedelics in a naturalistic and traditional setting with characteristic features arising from its origins in indigenous ritual practices from the Amazon Basin or Central and North America (Luna, 2011).
This review informed us on multiple applied instruments, which can be broadly grouped into two domains: “subjective experience” (e.g., 5D-ASC and MEQ30) and “psychological experience” (e.g., PIQ and EBI). However, acknowledging the unique extra-pharmacological factor of “setting” in psychedelic research, we speculate that a third domain termed “setting” might be necessary. We know that such an instrument has been developed specifically for ayahuasca sessions (de Deus Pontual et al., 2021), and we advise that a similar instrument be developed to more broadly assess the “setting” of a psychedelic experience.
The concept of psychedelic integration could also be beneficial to assess, as it is considered an important part of the specialized psychotherapy surrounding clinical trials with classical psychedelics (Guss et al., 2020). Briefly, psychedelic integration is the process by which an ASC, that is, a psychedelic experience, translates into positive changes in the ordinary state of consciousness in daily life. The Integration Engagement Scale and the Experienced Integration Scale have been proposed as instruments to assess psychedelic integration (Frymann et al., 2022).
While conducting this review, we encountered aspects of the 5D-ASC that require further clarification. The 5D-ASC is an extension of the OAV, with two additional dimensions (Auditory Alterations and Vigilance Reduction). The general dimension (G-ASC) is derived from the sum of all items in the questionnaire. Studerus et al. (2010) conducted a psychometric evaluation of the OAV dimensions and found them to be multidimensional. Their paper further included a critique of Dittrich’s original investigations, which argued that the OAV was etiology independent. To address their findings, the authors constructed 11 new lower-ordered factors from the OAV. They deemed these factors more suitable for most assessments, as they exhibit more homogeneity and are easier to interpret. This new interpretation included 44 items of the original 66-item OAV for analysis, but it is most often administered through the 94-item 5D-ASC. In the scientific literature, the informal term “11D-ASC” has been used to refer to the use of the 11 lower-ordered factors. However, researchers using this term often do not specify whether they employed the entire 5D-ASC, the OAV, or solely the 44 items associated with the lower-ordered factors. It is important to note that the 11 lowered-ordered factors of the OAV have not been validated by multiple group confirmatory factor analysis, as advised by the creators themselves. Considering the widespread use of these factors, we believe that such validation is overdue and strongly encourage researchers to undertake this step, as well as the formal establishment of a questionnaire that exclusively includes the 44 items for the 11 lower-ordered factors.
It was not the objective of this review to propose a COS for psychedelic research but to serve as a first step in the process by identifying currently available instruments. However, if we were to make recommendations at this stage, we would suggest that all trials employ the MEQ30 as a minimum. This is because the MEQ30 is currently the most well-investigated instrument, and has the most well-established association between score on the instrument and reductions in psychopathological symptoms in clinical trials. Furthermore, it is of reasonable length, with only 30 items compared to, for example, the 5D-ASC or HRS with 100 items, which could be burdensome for participants.
Our review has several shortcomings. First, we chose to exclude studies on psychedelic “microdosing.” We did so since microdosing, by popular definition, refers to the ingestion of a psychedelic substance that is sub-perceptual (Marschall et al., 2022) and, therefore, should not be detectable by any instrument designed to detect aspects of the psychedelic experience. However, by chance, we came across one study on psychedelic microdosing that applied the 5D-ASC and the EDI as in research on standard doses (Hutten et al., 2020). This study compared, under blinded conditions, the subjective effects of either placebo or doses of 5, 10, and 20 mcg LSD and found a statistically significant difference between placebo and 20 mcg LSD for the total 5D-ASC score, as well as in four main dimensions (OB, AED, VR, and RV, all p values < 0.01). Meanwhile, the 10 mcg dose only enhanced scores on the AED dimension compared to placebo (p = 0.04), and the 5 mcg dose was not statistically different from placebo. Furthermore, there was no statistical difference between the placebo and any LSD dose on the EDI. Judging from this study, it does seem feasible to measure an acute psychedelic effect from at least some microdoses, which might be better termed “minidosages.” We might have missed some pertinent questionnaires by excluding studies on psychedelic microdosing with a cutoff point of 20 mcg.
Second, we only included rating scales that we considered to assess altered state properties specific to the psychedelic experience. Therefore, we deemed some instruments, such as the Brief Psychiatric Rating Scale (Overall and Gorham, 1988) and the Adjective Mood Rating Scale (Janke and Debus, 1978) to be too generic for inclusion in our review.
Third, our description of the instruments’ validity is possibly incomplete, as it only includes the research we have gathered from hand-searching the included trials. Our description of emerging instruments is possibly incomplete for the same reasons. Furthermore, many of the early instruments included in the present review were initially developed in German and are inaccessible to us in terms of language. Future research could assess the quality of the instruments included in this review in more depth, according to the guidelines set forth by the Consensus-Based Standards for the Selection of Health Measurement Instruments (COSMIN) initiative (Muro-Culebras et al., 2021).
Fourth, our grouping of items into domains is limited, as it is solely based on our interpretation and not that of other stakeholders. Others might consider individual factors to be better described by other domains, or domains which we have not included.
Lastly, we did not systematically search for protocols or trial registers of the included trials. Therefore, due to the common practice of selectively reporting measures in clinical trials, other scales assessing subjective effects could have been used in the included studies, but not described in the primary paper. Hence, we may have underestimated the frequency of the instruments.
In summary, this review of questionnaires applied in psychedelics research found 17 standardized instruments assessing one or more aspects of the acute psychedelic experience. We advise that research should be conducted on how and if the instruments correlate and capture the same constructs. One could, for example, administer several instruments to subjects participating in a clinical trial on psychedelics and, by factor analysis, investigate which items tap into the same factor/construct. Second, we suggest that future research examine dose dependence and its association with changes in acute experiences. We further advise that instruments be developed to assess the “setting” of a psychedelic experience. Lastly, we propose that the research community seek to agree on a COS set in psychedelic research to enhance generalizability between studies. This review serves as the first step in the process suggested by the COMET Handbook (Williamson et al., 2017). It could be followed by a COSMIN review of the psychometric properties of the outcomes found in the present review. Following this, one or more Delphi processes could be conducted. In a Delphi process, relevant experts (stakeholders) are asked to share their opinions on the importance of different outcomes in sequential rounds. We suggest that relevant stakeholders could be trialists who have researched classical psychedelics, representatives from religious communities that have psychedelics as a sacrament, and representatives from select psychedelic-related societies worldwide.
Supplemental Material
Supplemental material, sj-docx-1-jop-10.1177_02698811231200019 for Assessment of the acute subjective psychedelic experience: A review of patient-reported outcome measures in clinical research on classical psychedelics by Oliver Rumle Hovmand, Emil Deleuran Poulsen and Sidse Arnfred in Journal of Psychopharmacology
Supplemental material, sj-docx-2-jop-10.1177_02698811231200019 for Assessment of the acute subjective psychedelic experience: A review of patient-reported outcome measures in clinical research on classical psychedelics by Oliver Rumle Hovmand, Emil Deleuran Poulsen and Sidse Arnfred in Journal of Psychopharmacology
Footnotes
Authors’ contributions: ORH and EDP conceptualized the review. ORH and EDP carried out literature screening, full-text reading, data extraction, and tabulation. ORH was responsible for writing the first draft manuscript, and it was finalized by ORH and EDP. SA contributed with significant comments and guidance. All authors have discussed, reviewed, and approved the manuscript.
The author(s) declared no potential conflicts of interest with respect to the research, authorship, and/or publication of this article.
Funding: The author(s) received no financial support for the research, authorship, and/or publication of this article.
ORCID iD: Oliver Rumle Hovmand  https://orcid.org/0000-0001-6928-6113
https://orcid.org/0000-0001-6928-6113
Supplemental material: Supplemental material for this article is available online.
References
- Ai AL, Wink P, Paloutzian RF, et al. (2021) Assessing Spirituality in a Diverse World. Switzerland: Springer. [Google Scholar]
- Barrett FS, Bradstreet MP, Leoutsakos JS, et al. (2016) The challenging experience questionnaire: Characterization of challenging experiences with psilocybin mushrooms. Psychopharmacology 30: 1279–1295. [DOI] [PMC free article] [PubMed] [Google Scholar]
- Barrett FS, Johnson MW, Griffiths RR. (2015) Validation of the revised Mystical Experience Questionnaire in experimental sessions with psilocybin. J Psychopharmacol 29: 1182–1190. [DOI] [PMC free article] [PubMed] [Google Scholar]
- Barsuglia J, Davis AK, Palmer R, et al. (2018) Intensity of mystical experiences occasioned by 5-MeO-DMT and comparison with a prior psilocybin study. Front Psychol 9: 2459. [DOI] [PMC free article] [PubMed] [Google Scholar]
- Bodmer I. (1989) Konstruktion des Fragebogens OAV zut quantitativen Erfassung auβergewöhnlicher Bewuβtseinszustände (ABZ). Zurich: University of Zurich. [Google Scholar]
- Bogenschutz MP, Forcehimes AA, Pommy JA, et al. (2015) Psilocybin-assisted treatment for alcohol dependence: A proof-of-concept study. J Psychopharmacol 29: 289–299. [DOI] [PubMed] [Google Scholar]
- Bouso JC, Pedrero-Pérez EJ, Gandy S, et al. (2016) Measuring the subjective: Revisiting the psychometric properties of three rating scales that assess the acute effects of hallucinogens. Hum Psychopharmacol 31: 356–372. [DOI] [PubMed] [Google Scholar]
- Bremner JD, Krystal JH, Putnam FW, et al. (1998) Measurement of dissociative states with the Clinician-Administered Dissociative States Scale (CADSS). J Trauma Stress 11: 125–136. [DOI] [PubMed] [Google Scholar]
- Carbonaro TM, Bradstreet MP, Barrett FS, et al. (2016) Survey study of challenging experiences after ingesting psilocybin mushrooms: Acute and enduring positive and negative consequences. J Psychopharmacol 30: 1268–1278. [DOI] [PMC free article] [PubMed] [Google Scholar]
- Castro Santos H, Gama Marques J. (2021) What is the clinical evidence on psilocybin for the treatment of psychiatric disorders? A systematic review. Porto Biomed J 6: e128. [DOI] [PMC free article] [PubMed] [Google Scholar]
- Davis AK, Barrett FS, Griffiths RR. (2020) Psychological flexibility mediates the relations between acute psychedelic effects and subjective decreases in depression and anxiety. J Contextual Behav Sci 15: 39–45. [DOI] [PMC free article] [PubMed] [Google Scholar]
- Davis AK, Barrett FS, So S, et al. (2021) Development of the Psychological Insight Questionnaire among a sample of people who have consumed psilocybin or LSD. J Psychopharmacol 35: 437–446. [DOI] [PMC free article] [PubMed] [Google Scholar]
- Davis AK, So S, Lancelotta R, et al. (2019) 5-Methoxy-N, N-dimeth- yltryptamine (5-MeO-DMT) used in a naturalistic group setting is associated with unintended improvements in depression and anxiety. Am J Drug Alcohol Abuse 45: 161–169. [DOI] [PMC free article] [PubMed] [Google Scholar]
- de Deus Pontual AA, Tófoli LF, Collares CF, et al. (2021) The setting questionnaire for the ayahuasca experience: Questionnaire development and internal structure. Front Psychol 12: 679016. [DOI] [PMC free article] [PubMed] [Google Scholar]
- Dittrich A. (1975) Zusammenstellung eines Fragebogens (APZ) zur Erfassung abnormer psychischer Zustände [Construction of a questionnaire (APZ) for assessing abnormal mental states]. Z Klin Psychol Psychiatr Psychother 23: 12–20. [Google Scholar]
- Dittrich A. (1996) Ätiologie-unabhängige Strukturen veränderter Wachbewusstseinszustände.Ergebnisse empirischer Untersuchungen über Halluzinogene I. und II. Ordnung, sensorische Deprivation, hypnagoge Zustände, hypnotische Verfahren sowie Reizüberflutung [Etiology-independent structures of altered states of consciousness. Results of empirical studies on hallucinogens of the first and second order, sensory deprivation, hypnagogic states, hypnotic procedures, and sensory overload]. Berlin, Germany: VWB. [Google Scholar]
- Dittrich A. (1998) The standardized psychometric assessment of altered states of consciousness (ASCs) in humans. Pharmacopsychiatry 31: 80–84. [DOI] [PubMed] [Google Scholar]
- Dittrich A, Lamparter D, Maurer M. (2006) 5D-ABZ: Fragebogen zur Erfassung Aussergewöhnlicher Bewusstseinszustände. Eine kurze Einführung [5D-ASC: Questionnaire for the assessment of altered states of consciousness. A short introduction]. Zürich, Switzerland. [Google Scholar]
- Dittrich A, Lamparter D, Maurer M. (2010) 5D-ASC: Questionnaire for the Assessment of Altered States of Consciousness. A Short Introduction. Zurich, Switzerland: Psin Plus. [Google Scholar]
- Dittrich A, von Arx S, Staub S. (1985) International study on altered states of consciousness (ISASC): Summary of the results. Ger J Psychol 9: 319–339. [Google Scholar]
- Fauvel B, Kangaslampi S, Strika-Bruneau L, et al. (2022) Validation of a French version of the mystical experience questionnaire with retrospective reports of the most significant psychedelic experience among French users. J Psychoactive Drugs 6: 1–10. [DOI] [PubMed] [Google Scholar]
- Francis LJ, Louden SH. (2001) The Francis-Louden Mystical Orientation Scale (MOS): A study among Roman Catholic priests. Res Social Sci Study Relig 11: 99–116. [Google Scholar]
- Francis LJ, Louden SH. (2004) A Short Index of Mystical Orientation (SIMO): A study among roman Catholic priests. Pastor Psychol 53: 49–51. [Google Scholar]
- Frymann T, Whitney S, Yaden DB, et al. (2022) The psychedelic integration scales: Tools for measuring psychedelic integration behaviors and experiences. Front Psychol 13: 863247. [DOI] [PMC free article] [PubMed] [Google Scholar]
- Garcia-Romeu A, Davis AK, Erowid F, et al. (2019) Cessation and reduction in alcohol consumption and misuse after psychedelic use. J Psychopharmacol 33: 1088–1101. [DOI] [PubMed] [Google Scholar]
- Garcia-Romeu A, Griffiths RR, Johnson MW. (2014) Psilocybin-occasioned mystical experiences in the treatment of tobacco addiction. Curr Drug Abuse Rev 7: 157–164. [DOI] [PMC free article] [PubMed] [Google Scholar]
- Greyson B. (1983) The near-death experience scale. Construction, reliability, and validity. J Nerv Ment Dis 171: 369–375. [DOI] [PubMed] [Google Scholar]
- Griffiths RR, Johnson MW, Carducci MA, et al. (2016) Psilocybin produces substantial and sustained decreases in depression and anxiety in patients with life-threatening cancer: A randomized double-blind trial. J Psychopharmacol 30: 1181–1197. [DOI] [PMC free article] [PubMed] [Google Scholar]
- Griffiths RR, Johnson MW, Richards WA, et al. (2011) Psilocybin occasioned mystical-type experiences: Immediate and persisting dose-related effects. Psychopharmacology 218: 649–665. [DOI] [PMC free article] [PubMed] [Google Scholar]
- Griffiths RR, Richards WA, Johnson MW, et al. (2008) Mystical-type experiences occasioned by psilocybin mediate the attribution of personal meaning and spiritual significance 14 months later. J Psychopharmacol 22: 621–632. [DOI] [PMC free article] [PubMed] [Google Scholar]
- Griffiths RR, Richards WA, McCann U, et al. (2006) Psilocybin can occasion mystical-type experiences having substantial and sustained personal meaning and spiritual significance. Psychopharmacology (Berl) 187: 268–283. [DOI] [PubMed] [Google Scholar]
- Guss J, Krause R, Sloshower J. (2020) The Yale Manual for Psilocybin-Assisted Therapy of Depression (Using Acceptance and Commitment Therapy as a Therapeutic Frame). New Haven, Connecticut: Yale University. [Google Scholar]
- Haertzen CA. (1966) Development of scales based on patterns of drug effects, using the addiction Research Center Inventory (ARCI). Psychol Rep 18: 163–194. [DOI] [PubMed] [Google Scholar]
- Haijen ECHM, Kaelen M, Roseman L, et al. (2018) Predicting responses to psychedelics: A prospective study. Front Pharmacol 9: 897. [DOI] [PMC free article] [PubMed] [Google Scholar]
- Happold FC. (1963) Mysticism: A Study and an Anthology. Harmondsworth: Penguin. [Google Scholar]
- Harris R, Gurel L. (2012) A study of ayahuasca use in North America. J Psychoactive Drugs 44: 209–215. [DOI] [PubMed] [Google Scholar]
- Hartogsohn I. (2016) Set and setting, psychedelics and the placebo response: An extra-pharmacological perspective on psychopharmacology. J Psychopharmacol 30: 1259–1267. [DOI] [PubMed] [Google Scholar]
- Hill HE, Haertzen CA, Wolbach AB, Jr, et al. (1963) The addiction research center inventory: Standardization of scales which evaluate subjective effects of morphine, amphetamine, pentobarbital, alcohol, LSD-25, pyrahexyl and chlorpromazine. Psychopharmacologia 4: 167–183. [DOI] [PubMed] [Google Scholar]
- Hood RW., Jr (1975) The construction and preliminary validation of a measure of reported mystical experience. J Sci Study Relig 14: 29–41. [Google Scholar]
- Hood RW, Jr, Ghorbani N, Watson PJ, et al. (2001) Dimensions of the mysticism scale: Confirming the three-factor structure in the United States and Iran. J Sci Study Relig 40: 691–705. [Google Scholar]
- Hovmand OR, Poulsen ED, Arnfred S, et al. (2023) Risk of bias in randomized clinical trials on psychedelic medicine: A systematic review. J Psychopharmacol 37: 649–659. [DOI] [PMC free article] [PubMed] [Google Scholar]
- Hutten NRPW, Mason NL, Dolder PC, et al. (2020) Mood and cognition after administration of low LSD doses in healthy volunteers: A placebo controlled dose-effect finding study. Eur Neuropsychopharmacol 41: 81–91. [DOI] [PubMed] [Google Scholar]
- Janke W, Debus G. (1978) Die Eigenschaftswrterliste (EWL-K)— Ein Verfahren zur Erfassung der Befindlichkeit. Gttingen: Hogrefe. [Google Scholar]
- Jasinski D. (1977) Assessment of the abuse potential of morphine-like drugs (methods used in man). In: Martin WR. (ed) Drug addiction. Berlin Heidelberg New York: Springer, pp. 197–258. [Google Scholar]
- Kangaslampi S, Hausen A, Rauteenmaa T. (2020) Mystical experiences in retrospective reports of first times using a psychedelic in Finland. J Psychoactive Drugs 52: 309–318. [DOI] [PubMed] [Google Scholar]
- Kisely S, Connor M, Somogyi AA, et al. (2023) A systematic literature review and meta-analysis of the effect of psilocybin and methylenedioxymethamphetamine on mental, behavioural or developmental disorders. Aust N Z J Psychiatry 57: 362–378. [DOI] [PubMed] [Google Scholar]
- Leary T, Alpert R. (1962) Foreword. Joyous Cosmol. Advent. Chem. Consciousn 1–3. [Google Scholar]
- Liechti ME, Dolder PC, Schmid Y. (2017) Alterations of consciousness and mystical-type experiences after acute LSD in humans. Psychopharmacology 234: 1499–1510. [DOI] [PMC free article] [PubMed] [Google Scholar]
- Luna LE. (2011) Indigenous and mestizo use of ayahuasca: An overview. Ethnopharmacol Ayahuasca 2: 1–21. [Google Scholar]
- Maclean KA, Leoutsakos JM, Johnson MW, et al. (2012) Factor analysis of the mystical experience questionnaire: A study of experiences occasioned by the hallucinogen psilocybin. J Sci Study Relig 51: 721. [DOI] [PMC free article] [PubMed] [Google Scholar]
- Marschall J, Fejer G, Lempe P, et al. (2022) Psilocybin microdosing does not affect emotion-related symptoms and processing: A preregistered field and lab-based study. J Psychopharmacol 36: 97–113. [DOI] [PMC free article] [PubMed] [Google Scholar]
- Martin WR, Sloan JW, Sapira JD, et al. (1971) Physiologic, subjective, and behavioral effects of amphetamine, methamphet- amine, ephedrine, phenmetrazine, and methylphenidate in man. Clin Pharmacol Ther 12: 245–258. [DOI] [PubMed] [Google Scholar]
- Mason OJ, Morgan CJ, Stefanovic A, et al. (2008) The Psychotomimetic States Inventory (PSI): Measuring psychotic-type experiences from ketamine and cannabis. Schizophr Res 103: 138–142. [DOI] [PubMed] [Google Scholar]
- Morean ME, de Wit H, King AC, et al. (2013) The drug effects questionnaire: Psychometric support across three drug types. Psychopharmacology (Berl) 227: 177–192. [DOI] [PMC free article] [PubMed] [Google Scholar]
- Muro-Culebras A, Escriche-Escuder A, Martin-Martin J, et al. (2021) Tools for evaluating the content, efficacy, and usability of mobile health apps according to the consensus-based standards for the selection of health measurement instruments: Systematic review. JMIR Mhealth Uhealth 9: e15433. [DOI] [PMC free article] [PubMed] [Google Scholar]
- Nichols DE. (2004) Hallucinogens. Pharmacol Ther 101: 131–181. [DOI] [PubMed] [Google Scholar]
- Nichols DE. (2016) Psychedelics. Pharmacol Rev 68: 264–355. [DOI] [PMC free article] [PubMed] [Google Scholar]
- Nour MM, Evans L, Nutt D, et al. (2016) Ego-dissolution and psychedelics: Validation of the Ego-Dissolution Inventory (EDI). Front Hum Neurosci 10: 269. [DOI] [PMC free article] [PubMed] [Google Scholar]
- Overall JE, Gorham DR. (1988) The Brief Psychiatric Rating Scale (BPRS): Recent developments in ascertainment and scaling. Psychopharmacology Bulletin 22: 97–99. [Google Scholar]
- Pahnke WN. (1963) Drugs and Mysticism: An Analysis of the Relationship Between Psychedelic Drugs and the Mystical Consciousness. Boston: MIA Harvard University, Press. [Google Scholar]
- Pahnke WN. (1969) Psychedelic drugs and mystical experience. Int Psychiatry Clin 5: 149–162. [PubMed] [Google Scholar]
- Parnas J, Møller P, Kircher T, et al. (2005) EASE: Examination of anomalous self-experience. Psychopathology 38: 236–258. [DOI] [PubMed] [Google Scholar]
- Peill JM, Trinci KE, Kettner H, et al. (2022) Validation of the psychological insight scale: A new scale to assess psychological insight following a psychedelic experience. J Psychopharmacol 36: 31–45. [DOI] [PMC free article] [PubMed] [Google Scholar]
- Prinsen CAC, Vohra S, Rose MR, et al. (2014) Core Outcome Measures in Effectiveness Trials (COMET) initiative: Protocol for an international Delphi study to achieve consensus on how to select outcome measurement instruments for outcomes included in a ‘core outcome set’. Trials 15: 247. [DOI] [PMC free article] [PubMed] [Google Scholar]
- Riba J, Rodríguez-Fornells A, Strassman RJ, et al. (2001) Psychometric assessment of the Hallucinogen Rating Scale. Drug Alcohol Depend 62: 215–223. [DOI] [PubMed] [Google Scholar]
- Richards WA. (1975) Counseling, peak experiences and the human encounter with death: An empirical study of the efficacy of DPT-assistance counseling in enhancing quality of life of persons with terminal cancer and their closest family members. PhD Thesis, Catholic University of America, Washington, DC. [Google Scholar]
- Roseman L, Haijen E, Idialu-Ikato K, et al. (2019) Emotional breakthrough and psychedelics: Validation of the emotional breakthrough inventory. J Psychopharmacol 33: 1076–1087. [DOI] [PubMed] [Google Scholar]
- Roseman L, Nutt DJ, Carhart-Harris RL. (2018) Quality of acute psychedelic experience predicts therapeutic efficacy of psilocybin for treatment-resistant depression. Front Pharmacol 8: 974. [DOI] [PMC free article] [PubMed] [Google Scholar]
- Ross S, Bossis A, Guss J, et al. (2016) Rapid and sustained symptom reduction following psilocybin treatment for anxiety and depression in patients with life-threatening cancer: A randomized controlled trial. J Psychopharmacol 30: 1165–1180. [DOI] [PMC free article] [PubMed] [Google Scholar]
- Schmid Y, Liechti ME. (2018) Long-lasting subjective effects of LSD in normal subjects. Psychopharmacology 235: 535–545. [DOI] [PMC free article] [PubMed] [Google Scholar]
- Stace WT. (1960) Mysticism and Philosophy. New York, NY: MacMillan Press. [Google Scholar]
- Strassman RJ, Qualls CR, Uhlenhuth EH, et al. (1994) Dose-response study of N,N-dimethyltryptamine in humans: II. Subjective effects and preliminary results of a new rating scale. Arch Gen Psychiatry 51: 98–108. [DOI] [PubMed] [Google Scholar]
- Studerus E. (2012) Tolerability, Assessment, and Prediction of Psilocybin-Induced Altered States of Consciousness. Zurich: University of Zurich. [Google Scholar]
- Studerus E, Gamma A, Vollenweider FX. (2010) Psychometric evaluation of the altered states of consciousness rating scale (OAV). PLoS One 5: e12412. [DOI] [PMC free article] [PubMed] [Google Scholar]
- Suely M, da Silveira DX, Barbosa PCR, et al. (2011) Hallucinogen Rating Scale (HRS) – Versão brasileira: Tradução e adaptação transcultural. Arch Clin Psychiatry (São Paulo) 38: 231–237. [Google Scholar]
- van der Meer PB, Fuentes JJ, Kaptein AA, et al. (2023) Therapeutic effect of psilocybin in addiction: A systematic review. Front Psychiatry 14: 1134454. [DOI] [PMC free article] [PubMed] [Google Scholar]
- Watts R, Day C, Krzanowski J, et al. (2017) Patients’ accounts of increased ‘Connectedness’ and ‘Acceptance’ after psilocybin for treatment-resistant depression. J Humanist Psychol 57: 520–564. [Google Scholar]
- Williamson PR, Altman DG, Bagley H, et al. (2017) The COMET handbook: Version 1.0. Trials 18(3): 280. [DOI] [PMC free article] [PubMed] [Google Scholar]
- Williamson PR, Altman DG, Blazeby JM, et al. (2012) Developing core outcome sets for clinical trials: Issues to consider. Trials 13: 132. [DOI] [PMC free article] [PubMed] [Google Scholar]
- Wolff M, Mertens LJ, Walter M, et al. (2022) The Acceptance/Avoidance-Promoting Experiences Questionnaire (APEQ): A theory-based approach to psychedelic drugs’ effects on psychological flexibility. J Psychopharmacol 36: 387–408. [DOI] [PMC free article] [PubMed] [Google Scholar]
- Zinberg NE. (1984) Drug, Set, and Setting: The Basis for Controlled Intoxicant Use. New Haven, CT: Yale University Press. [Google Scholar]
Associated Data
This section collects any data citations, data availability statements, or supplementary materials included in this article.
Supplementary Materials
Supplemental material, sj-docx-1-jop-10.1177_02698811231200019 for Assessment of the acute subjective psychedelic experience: A review of patient-reported outcome measures in clinical research on classical psychedelics by Oliver Rumle Hovmand, Emil Deleuran Poulsen and Sidse Arnfred in Journal of Psychopharmacology
Supplemental material, sj-docx-2-jop-10.1177_02698811231200019 for Assessment of the acute subjective psychedelic experience: A review of patient-reported outcome measures in clinical research on classical psychedelics by Oliver Rumle Hovmand, Emil Deleuran Poulsen and Sidse Arnfred in Journal of Psychopharmacology