Abstract
Background:
Monoamine oxidase (MAO) inhibitors can interact with selective serotonin reuptake inhibitors (SSRIs)/serotonin–norepinephrine reuptake inhibitors (SNRIs). There is clinical interest surrounding use of ozanimod with SSRIs/SNRIs because the major metabolites of ozanimod are weak inhibitors of MAO-B in vitro.
Objective:
To evaluate the incidence of treatment-emergent adverse events (TEAEs) potentially related to serotonin accumulation (SA) during concomitant ozanimod and SSRI/SNRI use by performing analyses of data from an open-label, oral ozanimod 0.92 mg trial (DAYBREAK; NCT02576717).
Methods:
SA narrow (serotonin syndrome, neuroleptic malignant syndrome, and hyperthermia malignant) and broad (terms potentially associated with SA) MedDRA v24.0 searches were performed using TEAE data from participants with relapsing multiple sclerosis who entered DAYBREAK from phase 3 studies (cutoff February 1, 2022). Incidences of TEAEs matching terms from each search were stratified by SSRI/SNRI use.
Results:
Of 2257 DAYBREAK participants, 274 (12.1%) used an SSRI/SNRI. No participants had TEAEs matching the SA narrow search terms. There was no significant difference in the percentage of participants with ⩾1 TEAE matching the SA broad search for those on versus off SSRIs/SNRIs (on: 12.4%, n = 34/274; off: 15.6%, n = 310/1982, nominal p = 0.1630).
Conclusion:
MedDRA searches showed no increase in TEAEs potentially associated with SA with concomitant SSRI/SNRI and ozanimod use.
Keywords: Multiple sclerosis, sphingosine 1-phosphate receptor modulators, selective serotonin reuptake inhibitors, serotonin–norepinephrine reuptake inhibitors
Introduction
Depression and anxiety are prevalent comorbidities occurring in up to 54% of people with multiple sclerosis (MS) 1 ; thus, patients with MS may receive selective serotonin reuptake inhibitors (SSRIs) or serotonin–norepinephrine reuptake inhibitors (SNRIs) as a first-line treatment for major depressive and generalized anxiety disorders.2,3 Although SSRIs and SNRIs are generally well tolerated, there are potential drug–drug interactions, particularly when other serotonergic agents are used concomitantly.4,5 For example, serotonin accumulation (SA) may occur when SSRIs/SNRIs are used in combination with tramadol, high-dose triptans, or linezolid through the inhibition of monoamine oxidase (MAO). 5 Of the drugs that inhibit MAO, SA is more likely with MAO-A inhibition than MOA-B inhibition because MAO-A is more involved in serotonin breakdown than MAO-B. 6
Ozanimod is a sphingosine 1-phosphate (S1P) receptor 1 and 5 modulator approved in multiple countries for the treatment of adults with relapsing forms of MS (RMS) or moderately to severely active ulcerative colitis.7,8 One major active metabolite of ozanimod, CC112273, is a substrate of MAO-B, and both major active metabolites (CC112273 and CC1084037) are weak inhibitors of MAO-B, but not MAO-A, in vitro.9,10 In a multiple-dose pharmacokinetic study, ozanimod treatment did not lead to the inhibition of human platelet MAO-B activity in vivo. 10 Considering MAO-B inhibition reduces the deamination of norepinephrine, leading to a hypothetically enhanced pressor effect of pseudoephedrine, the potential pharmacokinetic interactions of ozanimod and pseudoephedrine were also assessed in healthy participants. 10 Steady-state exposure to the major active metabolites of ozanimod did not potentiate the effect of pseudoephedrine on blood pressure, further supporting the lack of inhibitory effects on MAO-B in humans. 10 A statement in the April 2023 version of the ozanimod prescribing information describes the potential for serious adverse reactions, including hypertensive crisis, during coadministration of ozanimod with drugs that increase serotonin or norepinephrine such as SSRIs and SNRIs. 8
To address the concern identified from other MAO-A and MAO-B inhibitors that concomitant use with SSRIs/SNRIs could lead to SA, this analysis was conducted to evaluate the incidence of treatment-emergent adverse events (TEAEs) that could be related to SA during concomitant SSRI/SNRI use in ozanimod-treated participants with RMS from the DAYBREAK open-label extension (OLE) trial.
Methods
Study design
This analysis included participants with RMS who entered DAYBREAK (NCT02576717) from two phase 3 trials (SUNBEAM (NCT02294058; duration: ⩾12 months) and RADIANCE (NCT02047734; duration: 24 months)). RADIANCE 11 and SUNBEAM 12 were multicenter, randomized, double-blind, double-dummy, active-controlled, phase 3 trials that assessed the efficacy and safety of ozanimod in participants with RMS. Participants who completed these trials were eligible to enroll in the ongoing, single-arm, multicenter, OLE DAYBREAK trial, aimed to characterize the long-term safety and efficacy of ozanimod 0.92 mg in RMS. 13 The DAYBREAK study began on 16 October 2015, 13 and data here were generated using a 1 February 2022 cutoff. Participants in SUNBEAM, RADIANCE, and DAYBREAK were allowed concurrent SSRI/SNRI use. In this analysis, participants from SUNBEAM or RADIANCE who were taking SSRIs/SNRIs in DAYBREAK were compared with those who did not take an SSRI/SNRI concomitantly. Although formally a serotonin antagonist and reuptake inhibitor, trazodone was also included because it may also cause SA 14 and was commonly used by participants enrolled in DAYBREAK.
The institutional review board or ethics committee at each site approved the protocols of the RADIANCE 11 and SUNBEAM 12 trials, which conformed to Good Clinical Practice guidelines and Declaration of Helsinki principles. All participants who entered DAYBREAK reconsented by written agreement before entering the OLE trial. 13
Study procedures and outcomes
A primary SA narrow Medical Dictionary for Regulatory Activities (MedDRA (version 24.0)) search was conducted using TEAE data from DAYBREAK with the following preferred terms: serotonin syndrome, neuroleptic malignant syndrome, and hyperthermia malignant. This search was supplemented with a secondary, broader SA MedDRA search based on a standardized MedDRA query for neuroleptic malignant syndrome (substantial overlap of potential symptoms with SA) that was supplemented with SA-specific terms (see Supplemental Table 1 for all search terms used in this analysis). Hypertension was included in the SA broad search terms as a key manifestation of neuroleptic malignant syndrome and the potential autonomic hyperactivity that can accompany increased serotonin levels, while also being a known effect of S1P receptor modulators via impaired endothelial-dependent vasodilation.15–17 Participants were counted at most once per system organ class or preferred term for multiple occurrences.
A TEAE was defined as an adverse event with a start date on or after the date of the first dose of ozanimod in DAYBREAK. A participant was categorized as on an SSRI/SNRI for a TEAE preferred term if ⩾1 TEAE occurred while taking an SNRI/SSRI. A participant was counted as not on an SSRI/SNRI for a TEAE preferred term if they did not receive an SSRI/SNRI in DAYBREAK.
Statistical analysis
Duration of exposure to SSRIs/SNRIs was defined as the period when participants were exposed to ⩾1 SSRI/SNRI and ozanimod during the analysis period.
The percentage of participants who had ⩾1 TEAE matching the terms from each MedDRA search was determined and stratified by SSRI/SNRI use and compared using a chi-square test. All statistical comparisons are considered as hypothesis generating rather than declarative, and p-values are nominal. Incidences of TEAEs from individual terms from the SA broad search were also stratified by SSRI/SNRI use and captured >1 day from the start date of concomitant treatment with ozanimod (broad window). To assess whether potential SA-related TEAEs were clustered around the start of concomitant use of an SSRI/SNRI and ozanimod, an additional analysis was performed to identify broad search events from >1 day to ≤6 weeks of the start date of concomitant use (narrow window), as steady-state plasma concentrations of commonly used SSRIs/SNRIs are achieved within that period.14,18
Results
Study population
A total of 2257 participants from the phase 3 studies (SUNBEAM (1203/2257; 53.3%); RADIANCE (1054/2257; 46.7%)) entered DAYBREAK. Participants were a mean (standard deviation (SD)) age of 37.3 (9.1) years and most were women (66.5%), white (99.4%), and from Eastern Europe (90.9%) (Table 1). The mean (SD) age at MS symptom onset was 29.3 (8.8) years, age at MS diagnosis was 32.4 (9.0) years, and Expanded Disability Status Scale score was 2.6 (1.1). Mean (SD) exposure to ozanimod during DAYBREAK was 56.1 (15.3) months and total exposure was 10,540 person-years. Of the 2256 participants in DAYBREAK who received study drug, 274 (12.1%) used an SSRI/SNRI. Trazodone hydrochloride, escitalopram, sertraline hydrochloride, and escitalopram oxalate were among the most frequently (⩾10%) used SSRI/SNRIs (Table 1). The 274 participants were exposed to ⩾1 SSRI/SNRI for a mean (SD) of 37.0 (24.6) months during DAYBREAK; the total concomitant SSRI/SNRI and ozanimod exposure was 845 person-years.
Table 1.
Demographics and clinical characteristics of participants from the phase 3 SUNBEAM and RADIANCE studies who enrolled in the DAYBREAK open-label extension trial.
| Characteristic | Ozanimod 0.92 mg (N = 2257 a ) |
|---|---|
| Age, years | |
| Mean (SD) | 37.3 (9.1) |
| Sex | |
| Men | 755 (33.5) |
| Women | 1502 (66.5) |
| Race | |
| White | 2244 (99.4) |
| Black | 9 (0.4) |
| Asian | 1 (<0.1) |
| Other | 3 (0.1) |
| BMI, b kg/m2 | |
| Mean (SD) | 24.2 (4.7) |
| BMI category b | |
| <30 kg/m2 | 2022 (89.6) |
| Time since MS symptom onset, years | |
| Mean (SD) | 6.8 (6.2) |
| Concomitant SSRI/SNRI use | 274 (12.1) |
| Trazodone hydrochloride | 54 (19.7) c |
| Escitalopram | 52 (19.0) c |
| Sertraline hydrochloride | 49 (17.9) c |
| Escitalopram oxalate | 30 (11.0) c |
| Venlafaxine hydrochloride | 25 (9.1) c |
| Sertraline | 24 (8.8) c |
| Fluoxetine hydrochloride | 23 (8.4) c |
| Fluoxetine | 22 (8.0) c |
| Paroxetine | 22 (8.0) c |
| Venlafaxine | 16 (5.8) c |
| Duloxetine | 14 (5.1) c |
| Citalopram | 13 (4.7) c |
| Paroxetine hydrochloride | 12 (4.4) c |
| Citalopram hydrobromide | 11 (4.0) c |
| Duloxetine hydrochloride | 5 (1.8) c |
| Vortioxetine hydrobromide | 4 (1.5) c |
| Desvenlafaxine | 2 (0.7) c |
| Fluvoxamine maleate | 2 (0.7) c |
| Trazodone | 1 (0.4) c |
| Vortioxetine | 1 (0.4) c |
SD: standard deviation; BMI: body mass index; MS: multiple sclerosis; SSRI: selective serotonin reuptake inhibitor; SNRI: serotonin–norepinephrine reuptake inhibitor.
Data are presented as n (%) at DAYBREAK baseline, unless otherwise indicated.
One participant did not receive study drug.
At parent study baseline.
Percentage based on participants on an SSRI/SNRI medication.
TEAEs included in the SA narrow MedDRA search
No participant had a TEAE matching the SA narrow search terms.
TEAEs included in the SA broad MedDRA search
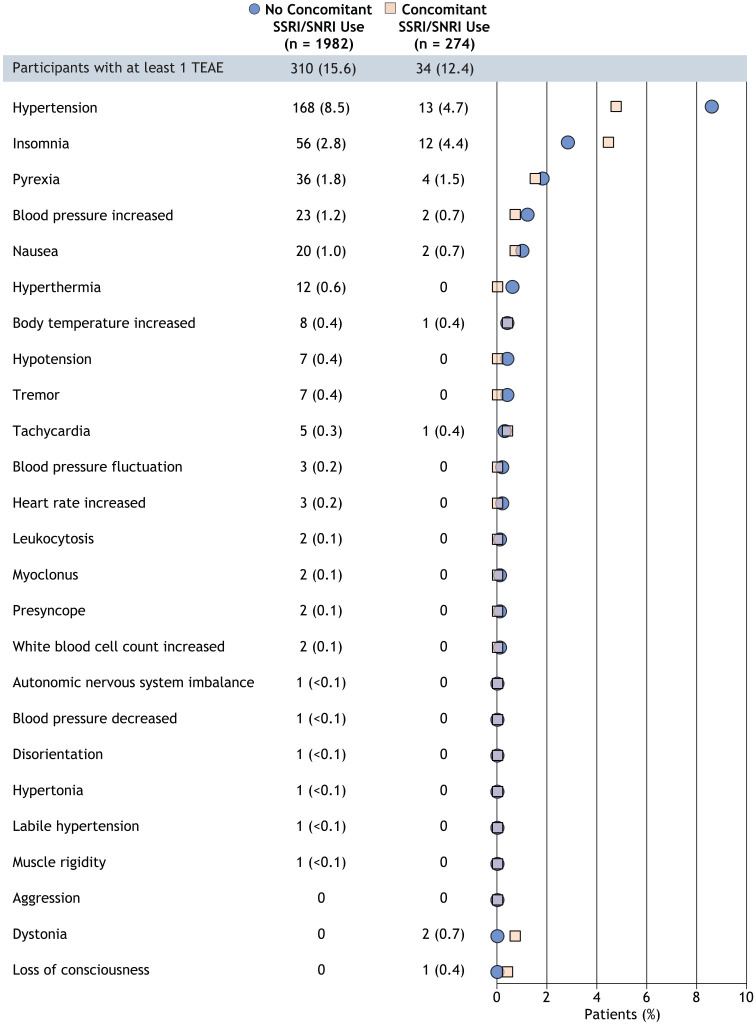
The proportion of DAYBREAK participants with ⩾1 TEAE matching the SA broad search and broad timing window with terms potentially related to SA was 34/274 (12.4%) for those with concomitant SSRI/SNRI use and 310/1982 (15.6%) for those without concomitant SSRI/SNRI use (Figure 1); the difference (95% confidence interval) in the proportions of participants with ⩾1 TEAE (−3.2% (−7.5, 1.0)) was not nominally significant (p = 0.1630). In the SA broad search, hypertension was the most common TEAE identified in participants with (4.7%) and without (8.5%) concomitant SSRI/SNRI use (Figure 1). Using the SA broad search criteria, the incidence of new TEAEs potentially related to SA remained relatively similar from >1 day to ≤6 weeks after the start date of combination ozanimod and SSRI/SNRI treatment (0%–0.4% of participants had ⩾1 TEAE, respectively) (Table 2). The incidence of most potentially SA-related TEAEs (12.0% of participants with concomitant SSRI/SNRI use) was >6 weeks after the start of concomitant use, the most common being hypertension (4.7%) followed by insomnia (4.0%) (Table 2). Two SA broad search TEAEs (hypertension, hypotension) led to or prolonged hospitalization in the group of participants who did not use an SSRI/SNRI concomitantly with ozanimod; no TEAEs required or prolonged hospitalization in the group of participants concomitantly using an SSRI/SNRI with ozanimod.
Figure 1.
Overall incidence of TEAEs identified using the SA broad MedDRA search. Data are presented as n (%).
MedDRA: Medical Dictionary for Regulatory Activities; SA: serotonin accumulation; SNRI: serotonin–norepinephrine reuptake inhibitor; SSRI: selective serotonin reuptake inhibitor; TEAE: treatment-emergent adverse event.
Table 2.
Incidence of new TEAEs over time from >1 day after the start of concomitant SSRI/SNRI and ozanimod use per the SA broad MedDRA search.
| TEAE preferred term | Concomitant SSRI/SNRI use (n = 274) | |||
|---|---|---|---|---|
| >1 day to ≤2 weeks | >2 weeks to ≤4 weeks | >4 weeks to ≤6 weeks | >6 weeks | |
| Number of participants with ⩾1 TEAE | 0 | 0 | 1 (0.4) | 33 (12.0) |
| Hypertension | 0 | 0 | 0 | 13 (4.7) |
| Insomnia | 0 | 0 | 1 (0.4) | 11 (4.0) |
| Pyrexia | 0 | 0 | 0 | 4 (1.5) |
| Blood pressure increased | 0 | 0 | 0 | 2 (0.7) |
| Dystonia | 0 | 0 | 0 | 2 (0.7) |
| Nausea | 0 | 0 | 0 | 2 (0.7) |
| Body temperature increased | 0 | 0 | 0 | 1 (0.4) |
| Loss of consciousness | 0 | 0 | 0 | 1 (0.4) |
| Tachycardia | 0 | 0 | 0 | 1 (0.4) |
TEAE, treatment-emergent adverse event; SSRI, selective serotonin reuptake inhibitor; SNRI, serotonin–norepinephrine reuptake inhibitor; SA, serotonin accumulation; MedDRA, Medical Dictionary for Regulatory Activities.
Data are presented as n (%).
When considering the treatment of participants prior to DAYBREAK among the participants who did not use an SSRI/SNRI concomitantly with ozanimod in DAYBREAK, 103/638 (16.1%) who received interferon (IFN) β-1a and 207/1344 (15.4%) who received ozanimod in SUNBEAM or RADIANCE prior to entering the DAYBREAK OLE experienced a TEAE matching the SA broad search. Of the participants who used an SSRI/SNRI concomitantly with ozanimod in DAYBREAK, 15/98 (15.3%) who received IFN β-1a and 19/176 (10.8%) who received ozanimod in RADIANCE or SUNBEAM prior to entering the DAYBREAK OLE experienced a TEAE matching the SA broad search.
Discussion
In this analysis of ozanimod-treated participants in the DAYBREAK OLE study, SA narrow and broad MedDRA searches did not identify any evidence of increased incidence of TEAEs potentially related to SA with concomitant SSRI/SNRIs use compared with those not using SSRI/SNRIs. TEAEs potentially related to SA were not clustered around the start of concomitant use.
Serotonin reuptake inhibitors and MAO inhibitors increase serotonin concentrations by preventing the transport of serotonin from the synapse back into the presynaptic terminal to be degraded or slowing the breakdown of serotonin, respectively. 6 Although MAO-A inhibitors are more likely to cause serotonin toxicity, 6 there is hypothetical concern of SA with use of SSRIs/SNRIs and MAO-B inhibitors. Clinical evidence of an interaction between serotonergic agents and MAO-B inhibitors is weak, and the incidences of toxic SA are exceedingly low, with concomitant use of these agents (rasagiline and selegiline) being generally well tolerated.19–23 Importantly, these agents were developed as MAO-B inhibitors as their primary mechanism of action.
The current analysis has limitations. First, a small subset of participants in DAYBREAK used an SSRI/SNRI and use was not systematic. The majority of the study population was white; therefore, these results may not be applicable to all patients, considering differences in the susceptibility of different racial groups to SA. 24 In addition, there is a potential for overestimation of risk based on the number of sensitive but nonspecific preferred terms in the SA broad MedDRA search. Ozanimod, along with the other S1P receptor modulators, has a known risk of increasing blood pressure and is associated with hypertension in a subset of patients. Finally, DAYBREAK was not designed to specifically assess a potential interaction between ozanimod and SSRI/SNRI use; therefore, the current analysis should be interpreted as a descriptive observation of TEAEs that occurred without a prespecified study hypothesis regarding this topic.
In summary, symptoms related to SA were not associated with concomitant use of ozanimod and SSRI/SNRIs in the DAYBREAK participant cohort.
Supplemental Material
Supplemental material, sj-docx-1-msj-10.1177_13524585231216854 for Concurrent administration of serotonergic antidepressants and ozanimod in participants with relapsing multiple sclerosis from the open-label extension DAYBREAK trial by Robert T Naismith, Jeffrey A Cohen, Amit Bar-Or, Giancarlo Comi, Krzysztof W Selmaj, Hans-Peter Hartung, James K Sheffield, Anthony Krakovich, Daniel Tatosian, Chun-Yen Cheng, Jennifer Reardon, Vadim Khaychuk, Jon V Riolo, Diego Silva and Bruce AC Cree in Multiple Sclerosis Journal
Acknowledgments
The authors acknowledge Noud van Helmond, MD, PhD, of Peloton Advantage, an OPEN Health company, for providing writing and editorial assistance.
Footnotes
Author Contributions: All authors contributed to and approved this manuscript.
Data Sharing: BMS policy on data sharing may be found at https://www.bms.com/researchers-and-partners/independent-research/data-sharing-request-process.html.
The author(s) declared the following potential conflicts of interest with respect to the research, authorship, and/or publication of this article: R.T.N. consulted for Abata Therapeutics, Banner Life Sciences, BeiGene, Biogen, Bristol Myers Squibb, Celltrion, Genentech, Genzyme, GW Therapeutics, Horizon Therapeutics, Janssen Pharmaceuticals, Lundbeck, NervGen, and TG Therapeutics. J.A.C. received personal compensation for consulting for Astoria, Bristol Myers Squibb, Convelo, EMD Serono, Find Therapeutics, INmune, and Sandoz, and serving as an editor of Multiple Sclerosis Journal. A.B.-O. received fees for advisory board participation and/or consulting from Accure, Atara Biotherapeutics, Biogen, BMS/Celgene/Receptos, GlaxoSmithKline, Gossamer Bio, Janssen/Actelion, MedImmune, Merck/EMD Serono, Novartis, Roche/Genentech, Sanofi-Genzyme and has received grant support to the University of Pennsylvania from Biogen Idec, Merck/EMD Serono, Novartis, and Roche/Genentech. G.C. reports compensation for consulting and/or speaking activities from Almirall, Biogen, Celgene, EXCEMED, Forward Pharma, Genzyme, Merck, Novartis, Roche, Sanofi, and Teva. K.W.S. reports consulting for Biogen, Celgene, Genzyme, Merck, Novartis, Ono Pharma, Roche, Synthon, and Teva. H.-P.H. reports personal fees for consulting, serving on steering committees, and speaking from Bayer Healthcare, Biogen, Celgene, GeNeuro, Genzyme, MedImmune, Merck, Novartis, Octapharma, Roche, Sanofi, and Teva. J.K.S., A.K., D.T., C.-Y.C., J.R., V.K., J.V.R., and D.S. are employees and/or shareholders of Bristol Myers Squibb. B.A.C.C. reports personal compensation for consulting for Alexion, Atara, Autobahn, Avotres, Biogen, Boston Pharma, EMD Serono, Gossamer Bio, Hexal/Sandoz, Horizon Therapeutics, Immunic AG, Neuron23, Novartis, Sanofi, Siemens, and TG Therapeutics and received research support from Genentech.
Funding: The author(s) disclosed receipt of the following financial support for the research, authorship, and/or publication of this article: This study was sponsored by Bristol Myers Squibb.
ORCID iDs: Robert T Naismith  https://orcid.org/0000-0003-0520-4283
https://orcid.org/0000-0003-0520-4283
Jeffrey A Cohen  https://orcid.org/0000-0001-9245-9772
https://orcid.org/0000-0001-9245-9772
Giancarlo Comi  https://orcid.org/0000-0002-6989-1054
https://orcid.org/0000-0002-6989-1054
Bruce AC Cree  https://orcid.org/0000-0001-7689-2533
https://orcid.org/0000-0001-7689-2533
Supplemental Material: Supplemental material for this article is available online.
Contributor Information
Robert T Naismith, Washington University School of Medicine, St. Louis, MO, USA.
Jeffrey A Cohen, Mellen Center for MS Treatment and Research, Cleveland Clinic, Cleveland, OH, USA.
Amit Bar-Or, Center for Neuroinflammation and Experimental Therapeutics, and Department of Neurology, Perelman School of Medicine, University of Pennsylvania, Philadelphia, PA, USA.
Giancarlo Comi, Vita-Salute San Raffaele University and Casa di Cura Igea, Milan, Italy.
Krzysztof W Selmaj, Center for Neurology, Łódź, Poland; Collegium Medicum, Department of Neurology, University of Warmia and Mazury in Olsztyn, Olsztyn, Poland.
Hans-Peter Hartung, Department of Neurology, Medical Faculty, Heinrich-Heine University Düsseldorf, Düsseldorf, Germany; Brain and Mind Centre, The University of Sydney, Sydney, NSW, Australia; Department of Neurology, Medical University of Vienna, Vienna, Austria; Palacký University Olomouc, Olomouc, Czech Republic.
James K Sheffield, Bristol Myers Squibb, Princeton, NJ, USA.
Anthony Krakovich, Bristol Myers Squibb, Princeton, NJ, USA.
Daniel Tatosian, Bristol Myers Squibb, Princeton, NJ, USA.
Chun-Yen Cheng, Bristol Myers Squibb, Princeton, NJ, USA.
Jennifer Reardon, Bristol Myers Squibb, Princeton, NJ, USA.
Vadim Khaychuk, Bristol Myers Squibb, Princeton, NJ, USA.
Jon V Riolo, Bristol Myers Squibb, Princeton, NJ, USA.
Diego Silva, Bristol Myers Squibb, Princeton, NJ, USA.
Bruce AC Cree, Weill Institute for Neurosciences, Department of Neurology, University of California San Francisco, San Francisco, CA, USA.
References
- 1. Boeschoten RE, Braamse AMJ, Beekman ATF, et al. Prevalence of depression and anxiety in multiple sclerosis: A systematic review and meta-analysis. J Neurol Sci 2017; 372: 331–341. [DOI] [PubMed] [Google Scholar]
- 2. Garakani A, Murrough JW, Freire RC, et al. Pharmacotherapy of anxiety disorders: Current and emerging treatment options. Front Psychiatry 2020; 11: 595584. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 3. Ghaffari Darab M, Hedayati A, Khorasani E, et al. Selective serotonin reuptake inhibitors in major depression disorder treatment: An umbrella review on systematic reviews. Int J Psychiatry Clin Pract 2020; 24(4): 357–370. [DOI] [PubMed] [Google Scholar]
- 4. Chu A, Wadhwa R. Selective serotonin reuptake inhibitors. Treasure Island, FL: StatPearls Publishing, 2022. [PubMed] [Google Scholar]
- 5. Gelenberg AJ, Freeman MP, Markowitz JC, et al. Practice guideline for the treatment of patients with major depressive disorder. Washington, DC: American Psychiatric Association.2010. [Google Scholar]
- 6. Foong AL, Grindrod KA, Patel T, et al. Demystifying serotonin syndrome (or serotonin toxicity). Can Fam Physician 2018; 64(10): 720–727. [PMC free article] [PubMed] [Google Scholar]
- 7. Zeposia (package insert). Princeton, NJ: Bristol Myers Squibb, 2023. [Google Scholar]
- 8. Zeposia (summary of product characteristics). Utrecht: Celgene Distribution B.V., 2023. [Google Scholar]
- 9. Surapaneni S, Yerramilli U, Bai A, et al. Absorption, metabolism, and excretion, in vitro pharmacology, and clinical pharmacokinetics of ozanimod, a novel sphingosine 1-phosphate receptor agonist. Drug Metab Dispos 2021; 49: 405–419. [DOI] [PubMed] [Google Scholar]
- 10. Tran JQ, Zhang P, Walker S, et al. Multiple-dose pharmacokinetics of ozanimod and its major active metabolites and the pharmacodynamic and pharmacokinetic interactions with pseudoephedrine, a sympathomimetic agent, in healthy subjects. Adv Ther 2020; 37(12): 4944–4958. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 11. Cohen JA, Comi G, Selmaj KW, et al. Safety and efficacy of ozanimod versus interferon beta-1a in relapsing multiple sclerosis (RADIANCE): A multicentre, randomised, 24-month, phase 3 trial. Lancet Neurol 2019; 18(11): 1021–1033. [DOI] [PubMed] [Google Scholar]
- 12. Comi G, Kappos L, Selmaj KW, et al. Safety and efficacy of ozanimod versus interferon beta-1a in relapsing multiple sclerosis (SUNBEAM): A multicentre, randomised, minimum 12-month, phase 3 trial. Lancet Neurol 2019; 18(11): 1009–1020. [DOI] [PubMed] [Google Scholar]
- 13. Cree BA, Selmaj KW, Steinman L, et al. Long-term safety and efficacy of ozanimod in relapsing multiple sclerosis: Up to 5 years of follow-up in the DAYBREAK open-label extension trial. Mult Scler 2022; 28: 1944–1962. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 14. Common questions about trazodone . London: National Health Services, 2022. [Google Scholar]
- 15. Prakash S, Rathore C, Rana KK, et al. Refining the clinical features of serotonin syndrome: A prospective observational study of 45 patients. Ann Indian Acad Neurol 2019; 22(1): 52–60. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 16. Cantalupo A, Gargiulo A, Dautaj E, et al. S1PR1 (sphingosine-1-phosphate receptor 1) signaling regulates blood flow and pressure. Hypertension 2017; 70(2): 426–434. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 17. Tse L, Barr AM, Scarapicchia V, et al. Neuroleptic malignant syndrome: A review from a clinically oriented perspective. Curr Neuropharmacol 2015; 13(3): 395–406. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 18. Lexapro (package insert). Irvine, CA: Allergan, 2017. [Google Scholar]
- 19. Richard IH, Kurlan R, Tanner C, et al. Serotonin syndrome and the combined use of deprenyl and an antidepressant in Parkinson’s disease. Parkinson study group. Neurology 1997; 48(4): 1070–1077. [DOI] [PubMed] [Google Scholar]
- 20. Hilli J, Korhonen T, Laine K. Lack of clinically significant interactions between concomitantly administered rasagiline and escitalopram. Prog Neuropsychopharmacol Biol Psychiatry 2009; 33: 1526–1532. [DOI] [PubMed] [Google Scholar]
- 21. Laine K, Anttila M, Heinonen E, et al. Lack of adverse interactions between concomitantly administered selegiline and citalopram. Clin Neuropharmacol 1997; 20(5): 419–433. [DOI] [PubMed] [Google Scholar]
- 22. Panisset M, Chen JJ, Rhyee SH, et al. Serotonin toxicity association with concomitant antidepressants and rasagiline treatment: Retrospective study (STACCATO). Pharmacotherapy 2014; 34(12): 1250–1258. [DOI] [PubMed] [Google Scholar]
- 23. Aboukarr A, Giudice M. Interaction between monoamine oxidase B inhibitors and selective serotonin reuptake inhibitors. Can J Hosp Pharm 2018; 71(3): 196–207. [PMC free article] [PubMed] [Google Scholar]
- 24. Williams RB, Marchuk DA, Gadde KM, et al. Serotonin-related gene polymorphisms and central nervous system serotonin function. Neuropsychopharmacology 2003; 28(3): 533–541. [DOI] [PubMed] [Google Scholar]
Associated Data
This section collects any data citations, data availability statements, or supplementary materials included in this article.
Supplementary Materials
Supplemental material, sj-docx-1-msj-10.1177_13524585231216854 for Concurrent administration of serotonergic antidepressants and ozanimod in participants with relapsing multiple sclerosis from the open-label extension DAYBREAK trial by Robert T Naismith, Jeffrey A Cohen, Amit Bar-Or, Giancarlo Comi, Krzysztof W Selmaj, Hans-Peter Hartung, James K Sheffield, Anthony Krakovich, Daniel Tatosian, Chun-Yen Cheng, Jennifer Reardon, Vadim Khaychuk, Jon V Riolo, Diego Silva and Bruce AC Cree in Multiple Sclerosis Journal