Abstract
Studies in mice and cross-sectional studies in humans support the premise that cellular senescence is a contributing mechanism to age-associated deficits in physical function. We tested the hypotheses that circulating proteins secreted by senescent cells are (i) associated with the incidence of major mobility disability (MMD), the development of persistent mobility disability (PMMD), and decrements in physical functioning in older adults, and (ii) influenced by physical activity (PA). Using samples and data obtained longitudinally from the Lifestyle Interventions in Elders Study clinical trial, we measured a panel of 27 proteins secreted by senescent cells. Among 1 377 women and men randomized to either a structured PA intervention or a healthy aging (HA) intervention, we observed significant associations between several senescence biomarkers, most distinctly vascular endothelial growth factor A (VEGFA), tumor necrosis factor receptor 1 (TNFR1), and matrix metallopeptidase 7 (MMP7), and the onset of both MMD and PMMD. Moreover, VEGFA, GDF15, osteopontin, and other senescence biomarkers were associated with reductions in short physical performance battery scores. The change in senescence biomarkers did not differ between PA and HA participants. In the whole cohort, higher levels of PA were associated with significantly greater reductions in 10 senescence-related proteins at 12 and/or 24 months. These data reinforce cellular senescence as a contributing mechanism of age-associated functional decline and the potential for PA to attenuate this hallmark of aging.
Clinical Trials Registration Number: NCT01072500
Keywords: Aging, Biomarkers, Frailty, Physical function, Sarcopenia, Senescence-associated secretory phenotype
The loss of physical function with advancing age substantially increases the risk for adverse health outcomes, such as falls, fractures, institutionalization, and mortality (1–4). Yet, the most effective intervention that has been shown to meaningfully improve physical functioning among older adults remains physical exercise (5–7). Advancing the mechanistic understanding of how aging drives and exercise attenuates, deficits in physical functioning may reveal new therapeutic strategies to optimize the health and independence of older adults.
Cellular senescence, a cell fate in response to multiple forms of genotoxic, proteotoxic, metabolic, and inflammatory stress, is a hallmark of aging (8,9). Senescent cells accumulate in many tissues with advancing age and mediate inflammation, deterioration, and cellular remodeling, and, simultaneously, reduce regenerative capacity, both locally and systemically (10). The deleterious effects of senescent cells are partly mediated by their marked and multifaceted senescence-associated secretory phenotype (SASP), comprised of cytokines, chemokines, matrix remodeling proteins, growth factors, and a host of other bioactive molecules (11). Previously, we and others leveraged protein components of the SASP as biomarkers of senescent cell burden, and observed cross-sectional associations between their circulating concentrations and chronological age as well as clinical signs of advanced biological age, including frailty, compromised mobility, and impaired pulmonary function, in humans (12–16). Whether senescence biomarkers are predictive of changes in physical function or disability onset remains unknown, but would advance the concept that senescence plays a causal role and support their utility for risk stratification.
There is great interest in discovering behavioral and/or pharmacological interventions to modify senescent cell burden. Prior work has shown that a structured exercise program lowers the abundance of blood-based biomarkers of senescence in healthy older adults (17). Moreover, endurance exercise training has been shown to counter senescence cell burden in mice (18,19). Whether lifestyle interventions have the potential to mitigate this hallmark of aging in persons with advanced chronological age and clinical manifestations of advanced biological age (eg, multiple chronic conditions and functional limitations) is unknown.
To address these research gaps, we used longitudinal data and blood samples obtained from participants in the Lifestyle Interventions in Elders (LIFE) Study, a large, single-blind, multicenter, randomized trial comparing a structured physical activity (PA) program to a healthy aging (HA) education-based program in older adults with mobility limitations (20). The PA program significantly reduced both incident and persistent major mobility disability (MMD) compared to the HA program (6). We tested the hypotheses that baseline plasma biomarkers of cellular senescence would predict functional decline in LIFE Study participants, defined by incidence of MMD and persistent MMD (PMMD) and greater reductions on the short physical performance battery (SPPB), and that those randomized to PA would demonstrate lower biomarker levels at the 12- and 24-month time points than those randomized to HA.
Method
Design
The LIFE Study was a randomized controlled trial of a group-based PA program compared to a HA education program, which was conducted from February 2010 to December 2013, enrolling 1 635 sedentary older adults (20). Recruitment occurred at 8 centers in the United States (University of Florida, Gainesville and Jacksonville, Florida; Northwestern University, Chicago, Illinois; Pennington Biomedical Research Center, Baton Rouge, Louisiana; University of Pittsburgh, Pittsburgh, Pennsylvania; Stanford University, Stanford, California; Tufts University, Boston, Massachusetts; Wake Forest School of Medicine, Winston-Salem, North Carolina; and Yale University, New Haven, Connecticut).
To be included in the study, females and males aged 70–89 years had to meet the following inclusion criteria: (i) sedentary lifestyle (defined as reporting <20 min/day of regular PA and <125 min/week of moderate PA); (ii) at high risk of disability based upon a score between 4 and 10 on the SPPB; (iii) ability to complete the 400-meter (400-m) walk test without an assistive device within 15 minutes; (iv) absence of cognitive impairment; and (v) willingness to consent to randomization. Study exclusions were unstable chronic disease and factors that would likely affect adherence to the intervention or underlying conditions that might limit survival. Participants were randomized (1:1) to either PA or HA using block randomization stratified by field center and participant sex. Institutional Review Board approval and informed consent were obtained from all participants.
Interventions
The PA intervention consisted of group-based walking, aerobic, resistance, flexibility, and balance training in a supervised setting (2 times per week) with additional home-based PA goals (21). Individual goals were established to progress participants toward a goal of at least 150 minutes per week of moderate-intensity PA. Each PA session consisted primarily of walking, with participants also completing a 10-minute lower extremity resistance training program with ankle weights, balance exercises, and lower extremity stretching. Individualized home-based walking goals were prescribed to supplement the supervised program.
The HA intervention was designed to deliver age-specific health information about “successful aging.” Health education included workshops on topics that were of interest to older adults (eg, cancer screening, elder abuse, nutrition, etc.). Classes met weekly for the first 26 weeks, and from week 27 on the program was offered 2 times per month and participants were prompted to attend at least once per month.
Mobility Disability and Physical Function Outcome Measures
The 400-m walk test was performed at baseline and every 6 months for the duration of the study. Participants were asked to walk 400 m at their usual pace, without overexertion, for 10 laps on a course defined by 2 cones placed 20 m apart. They could stop and rest for up to 1 minute for fatigue or related symptoms.
Incident MMD over the 24-month study period was defined as the inability to complete the 400-m walk test within 15 minutes without sitting and without the help of another person or walker (20). Use of a cane was acceptable. When MMD could not be objectively measured because the participant was not able to come to the clinic and lacked a suitable walking course at their home, institution, or hospital, an alternative adjudication of the outcome was based on directly observed inability to walk 4 m in <10 seconds, or self-, proxy-, or medical record-reported inability to walk across a room. If participants met these alternative criteria, it was considered that they would also not be able to complete the 400-m walk within 15 minutes.
PMMD over the 24-month study period was defined as 2 consecutive MMD assessments, or MMD followed by death.
The SPPB, which consists of a standing balance test, a usual pace 4-m walk, and 5 timed repeated chair stands (1), was performed at baseline and every 6 months of the study. Each performance measure in the SPPB is assigned a categorical score ranging from 0 (inability to complete the test) to 4 (best performance possible) and a summary score ranging from 0 (worst performance) to 12 (best performance) was calculated. The analyses here focused on change in SPPB score at 6 months, given the PA intervention had a significantly more positive effect on this measure than the HA intervention at this time point (22).
Objective Measurement of PA
Participants were instructed to wear an accelerometer (Actigraph GT3X) on their hip for 7 consecutive days except during sleep, showering/bathing, and swimming.
Movement was captured along the vertical axis in 1-minute epochs, and nonwear time was defined as 90 minutes of consecutive zero counts (23). PA patterns were characterized by intensity and bout lengths. For the current study, the minutes of activity categorized as high light PA or greater, defined as equal to or greater than 760 counts/min (24,25), was used for analysis. Data from a total of 1 131 participants (566 from the PA intervention, 565 from the HA intervention) who had a baseline measure and at least one of the follow-up measure were analyzed.
Measurement of Circulating Senescence Biomarkers
During the trial, plasma specimens were collected from participants at the baseline, 12- and 24-month visits after a minimum of an 8-hour fast. Plasma samples were collected in ethylenediaminetetraacetic acid (EDTA) tubes, immediately placed on ice (4°C) for no more than 30 minutes, centrifuged, aliquoted, and then stored at −80°C. For the current study, archived specimens were requested and shipped from the National Institute of Aging (NIA) Aging Research Biobank to Mayo Clinic for analysis.
A panel of senescence-related proteins was initially developed based on the secretome of several different senescent human cell types in vitro. Prior work has demonstrated these biomarkers are reliably detected in human blood, are elevated in human conditions (eg, advanced chronological age, frailty, disease) and genetically modified mice with increased senescent cell burden, and/or are components of senescence-focused gene sets (12–15,26–28).
Here, the concentrations of the following senescence biomarkers in plasma samples were determined with commercially available multiplex magnetic bead-based immunoassays (R&D Systems, Minneapolis, MN) on the Luminex xMAP multianalyte profiling platform and analyzed on a MAGPIX System (Merck Millipore, Burlington, MA) according to the standard manufacturer’s protocols: a disintegrin-like metalloprotease domain with thrombospondin type 1 motif 13 (ADAMTS13), eotaxin, Fas cell surface death receptor (Fas), growth differentiation factor 15 (GDF15), intercellular adhesion molecule 1 (ICAM1), interleukin (IL) 6 (IL6), IL7, IL8, IL10, IL15, monocyte chemoattractant protein-1 (MCP1), macrophage-derived chemokine (MDC), matrix metallopeptidase (MMP)-1 (MMP1), MMP7, MMP9, myeloperoxidase (MPO), osteopontin (OPN), plasminogen activator inhibitor 1 (PAI1), pulmonary and activation-regulated chemokine (PARC), receptor foradvanced glycationendproducts (RAGE), regulated on activation, normal T cell expressed and secreted (RANTES), sclerostin (SOST), tumor necrosis factor (TNF) receptor 1 (TNFR1), TNFR2, TNFα, and vascular endothelial growth factor A (VEGFA). Activin A concentration was measured using a Quantikine ELISA Kit (R&D Systems) according to the manufacturer’s specifications.
Assay performance parameters have been reported previously (13). In cases where a variable was below the limit of detection (LOD) in a sample, a value of half of the lowest measured value for that analyte was assigned as described previously (12). Specifically, among the 3 886 samples analyzed, eotaxin was below the LOD in 16 (0.41%) samples, OPN in 9 (0.23%) samples, and TNFα in 4 (0.10%) samples. ICAM1, IL7, and IL6 were each below the LOD in 3 (0.08%) samples, MMP7, IL8, and IL15 were each below the LOD in 2 (0.05%) samples, and SOST and VEGF were each below the LOD in 1 sample.
Data Analysis
Continuous variables were summarized using mean (standard deviation [SD]) and compared using the Wilcoxon rank sum. Variables were compared pairwise using unadjusted and adjusted Spearman correlations. Biomarkers were evaluated for skewed distributions and if appropriate were log-transformed. Then all biomarkers were standardized by subtracting the mean and dividing by the SD, then K-nearest neighbor imputation was applied to impute the small number of missing values. The impact of activity level on change from baseline in biomarkers was assessed at the 12- and 24-month time point. Quartile intervals of activity level were determined by using activity measures at all time points. Activity level at each time point was then grouped according to these overall intervals. Mixed-effects models were used to assess at 12 and 24 months the difference in biomarker values by activity quartile after adjusting for baseline biomarker values, intervention, age, sex, and race. The activity level effect was allowed to vary by visit. Similar models were fit to assess the impact of intervention at the 12- and 24-month time points (including an interaction between visit and intervention) ignoring activity level; overall intervention effect was also assessed excluding the interaction.
Cox regression models were used to associate individual baseline biomarkers with MMD and PMMD after adjusting for intervention, age, sex, body mass index (BMI), education, and race and stratified by clinical site. Hazard ratios (HRs) for the biomarkers are shown for a 1-SD change (assuming a linear association) and biomarkers categorized into quartiles with comparisons to the lowest quartile. Similarly, mixed-effects models were used to associate individual baseline biomarkers with change in SPPB at the 6-month time point after adjusting for baseline SPPB, intervention, age, sex, BMI, and race, stratified by clinical site.
Gradient boosting machine (GBM) learning using the gbm3 package in R was used to create a predictive model for time to MMD and PMMD using the “coxph” distribution and change in SPPB using the “gaussian” distribution (29). Model hyperparameters (depth of the tree, number of trees, and size of the shrinkage parameter) were determined using cross-validation using the training data set. Models were fit using (i) all the variables, (ii) the top 10 baseline biomarkers plus intervention, age, sex, BMI, and race, and (iii) intervention, age, sex, BMI, and race. All models predicting MMD and PMMD also included clinical site as a stratification variable. Models for change in SPPB focused on the 6-month time point. All analyses were conducted using R version 4.1.2 (30).
Data Availability
LIFE Study data sets are available through the NIA Aging Research Biobank, https://agingresearchbiobank.nia.nih.gov/studies/life/. Biomarker data presented in the current study will be made available by the investigative team upon reasonable request.
Results
Demographic and Clinical Characteristics of Participants
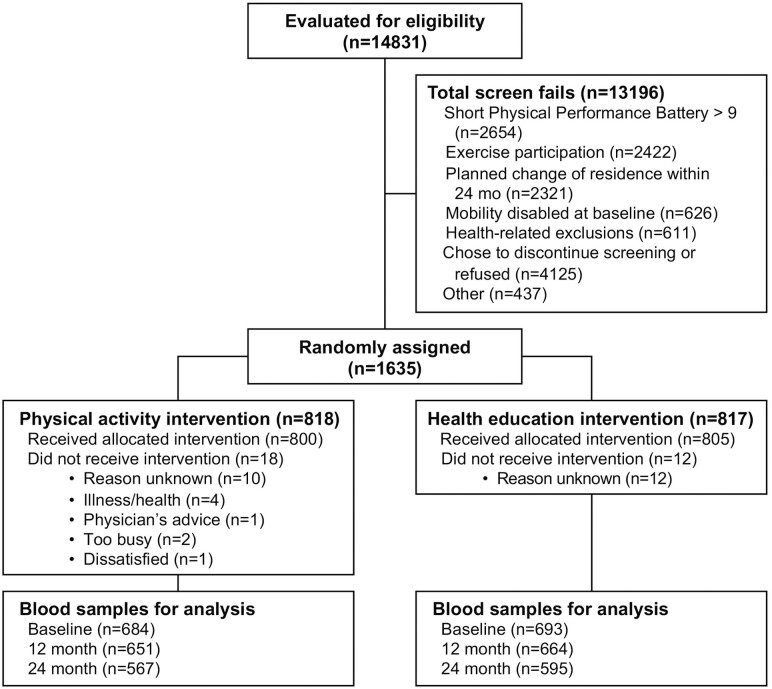
Potential LIFE Study participants were screened for enrollment between February 2010 and December 2011 (Figure 1). Those who qualified were randomized to either a HA or a PA intervention group. We studied 1 377 of the 1 635 participants with baseline blood samples and outcome data available for analysis. Similar to the overall cohort, baseline demographic characteristics were not different between randomized groups (Table 1).
Figure 1.
Consolidated Standards of Reporting Trials (CONSORT) flow diagram of the randomized Lifestyle Interventions in Elders Study clinical trial.
Table 1.
Demographic and Clinical Characteristics and Physical Function Measures in Study Participants by Randomized Group (Physical Activity or Healthy Aging)
| Physical Activity (N = 684) | Healthy Aging (N = 693) | p Value | |
|---|---|---|---|
| Age, years | 78.6 (5.2) | 79.1 (5.3) | .108 |
| Sex | .688 | ||
| Female | 449 (65.6%) | 462 (66.7%) | |
| Male | 235 (34.4%) | 231 (33.3%) | |
| Race and ethnicity, n (%) | .387 | ||
| White | 517 (75.6%) | 546 (78.8%) | |
| Black or African American | 127 (18.6%) | 99 (14.3%) | |
| Hispanic | 24 (3.5%) | 26 (3.8%) | |
| Asian | 6 (0.9%) | 8 (1.2%) | |
| Other | 8 (1.2%) | 11 (1.6%) | |
| Missing | 2 (0.3%) | 3 (0.4%) | |
| Education | .457 | ||
| High school or less | 236 (34.5%) | 226 (32.6%) | |
| College or more | 448 (65.5%) | 467 (67.4%) | |
| Body mass index, kg/m2 | 30.2 (5.8) | 30.3 (6.1) | .735 |
| Health conditions, n (%) | |||
| High blood pressure | 483 (71.1%) | 492 (71.6%) | .844 |
| Diabetes | 183 (26.9%) | 193 (28.0%) | .649 |
| Cancer | 159 (23.3%) | 165 (23.8%) | .829 |
| Chronic lung disease | 127 (18.6%) | 104 (15.1%) | .079 |
| MI/heart attack | 55 (8.1%) | 73 (10.6%) | .111 |
| Stroke | 56 (8.2%) | 46 (6.7%) | .266 |
| Physical function | |||
| SPPB score (0–12) | 7.4 (1.6) | 7.3 (1.6) | .198 |
| 400-m walk, s | 503.0 (110.8) | 507.4 (111.6) | .412 |
| Gait speed, m/s | 0.8 (0.2) | 0.8 (0.2) | .690 |
| Repeated chair stand, s | 16.6 (4.6) | 17.0 (6.2) | .247 |
| Grip strength, kg | 24.9 (9.8) | 24.6 (9.4) | .615 |
| Frailty, n (%) | 130 (19.1) | 138 (20.0) | .671 |
| Incident MMD, n | 200 (29.2%) | 233 (33.6 %) | |
| Persistent MMD, n | 103 (15.0 %) | 145 (20.9 %) | |
Notes: MI = myocardial infarction; MMD = major mobility disability; SPPB = short physical performance battery. Data reported as mean (standard deviation) unless otherwise noted.
Senescence Biomarkers as Predictors of Major and Persistent Mobility Disability
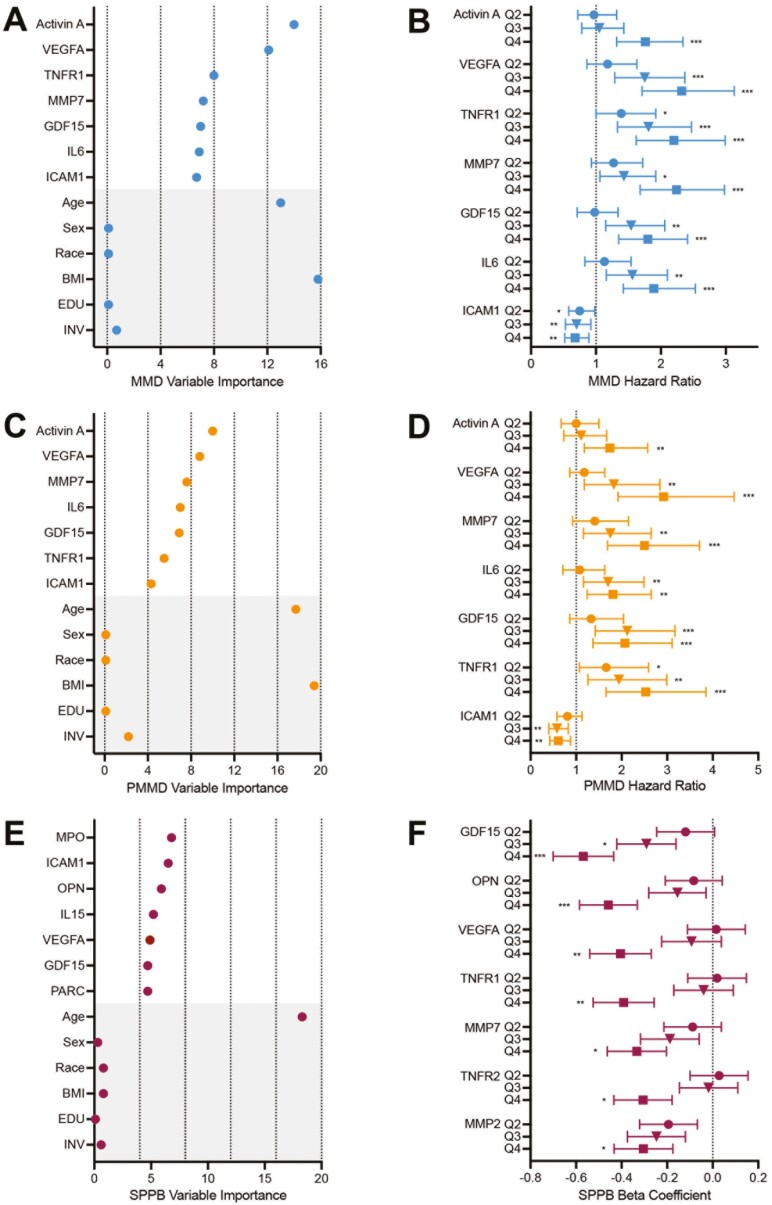
First, we evaluated whether baseline concentrations of 27 senescence-related proteins (Supplementary Table 1 and (12,13)) were associated with incident MMD over the 24-month study period. Incident MMD occurred in 200 participants randomized to PA and in 230 participants randomized to HA. Univariate Cox models adjusted for intervention, age, sex, BMI, education, and race revealed higher concentrations of 14 biomarkers were associated with significantly higher risk for incident MMD, with VEGFA (HR = 1.35, p < .001), TNFR1 (HR = 1.32, p < .001), and MMP7 (HR = 1.32, p < .001) having the highest HRs for incident MMD (Supplementary Table 2). Additionally, higher ICAM1 was associated with a significantly lower risk for onset of MMD (HR = 0.89, p = .026). GBM modeling identified activin A, VEGFA, TNFR1, MMP7, GDF15, IL6, and ICAM1, along with age and BMI, as variables of greatest importance (Figure 2A). When each of these biomarkers were grouped into quartiles (adjusted for intervention, age, sex, BMI, education, and race and stratified by clinical site), HRs for incident MMD progressively increased from the lowest to highest quartile, except for ICAM1, for which the HR decreased (Figure 2B).
Figure 2.
Biomarkers of cellular senescence identify risk for onset of incident and persistent mobility disability, and change in short physical performance battery. The top 7 biomarkers along with age (years), sex (female, male), race (White, Black or African American, Hispanic, Asian, or Other), body mass index (BMI; kg/m2), education (EDU; high school or less, or college or more), and intervention assignment (INV; physical activity, or health education) selected by gradient boost modeling for: (A) onset of incident mobility disability (MMD) and (C) onset of persistent major mobility disability (PMMD) over the entire study period, and (E) change in short physical performance battery (SPPB) score at 6 months. Quartiles of the top 7 biomarker concentrations at baseline and association with: (B) onset of incident mobility disability compared to quartile 1, (D) onset of persistent mobility disability, (F) change in SPPB score. *p < .05; **p < .01; ***p < .001 compared to quartile 1. All models were adjusted for intervention, age, sex, BMI, education, and race and stratified by clinical site.
We also studied the associations between senescence biomarkers and incident MMD in women (295 events) and men (136 events) separately. Consistent with the overall analyses, univariate Cox models adjusted for intervention, age, BMI, education, and race showed that higher concentrations of activin A, GDF15, IL6, IL15, MMP7, TNFR1, and VEGFA were associated with significantly higher risk for incident MMD in both women and men (Supplementary Table 2). In women, associations between higher levels of MMP1, OPN, TNFα, and TNFR2 and higher risk of incident MMD were also significant, while in men, associations between higher concentrations of eotaxin and lower concentrations of ICAM1 and higher risk of incident MMD were significant.
PMMD was identified in 103 participants randomized to PA and 145 participants randomized to HA. Univariate Cox models adjusted for intervention, age, sex, BMI, education, and race revealed significant positive HRs for 8 proteins. Similar to incident MMD, VEGFA (HR = 1.38, p < .001), MMP7 (HR = 1.37, p < .001), and TNFR1 (HR = 1.29, p < .001) exhibited the greatest HRs for PMMD, along with GDF15 (HR = 1.29, p < .001; Supplementary Table 3). GBM modeling again revealed activin A, VEGFA, MMP7, IL6, GDF15, TNFR1, and ICAM1, along with age and BMI, as the top biomarker variables of importance (Figure 2C). When these biomarkers were grouped into quartiles (adjusted for intervention, age, sex, BMI, education, and race and stratified by clinical site), HRs for PMMD increased from quartile 1 to quartile 4, except for ICAM1, for which the HR decreased (Figure 2D).
We also examined associations between senescence biomarkers and risk for PMMD in women (169 events) and men (79 events) separately. Consistent with the analysis of both sexes combined, univariate Cox models adjusted for intervention, age, BMI, education, and race revealed higher concentrations of activin A, GDF15, TNFR1, and VEGFA were associated with significantly higher risk for PMMD in both women and men (Supplementary Table 3). In women, higher concentrations of MMP1 MMP7, OPN, TNFα, IL15, and IL6 were associated with significantly higher risk of PMMD. None of the biomarkers exhibited significant associations with risk of PMMD exclusively in men.
Senescence Biomarkers and Change in Physical Function
Next, we examined associations between baseline senescence biomarker levels and change in SPPB score at 6 months. Univariate mixed-effects modeling adjusted for intervention, age, sex, BMI, education, and race and stratified by site identified significant negative associations—higher biomarker, greater decline in SPPB score—between 9 proteins, most notably GDF15 (beta coefficient = −0.193, p < .001), OPN (beta coefficient = −0.136, p = .003), MMP1 (beta coefficient = −0.122, p = .008), and VEGFA (beta coefficient = −0.123, p = .012), and change in SPPB score (Supplementary Table 4). GBM modeling for the change in SPPB revealed MPO, ICAM1, OPN, IL15, GDF15, PARC, and VEGFA as the top biomarker variables of importance, and age as the top covariate of importance (Figure 2E). When each of the top biomarkers were grouped into quartiles adjusted for age as well as sex, BMI, education, race, and intervention and stratified by clinical site, beta coefficients for a greater decline in SPPB score progressively increased from the lowest to the highest quartile (Figure 2F).
To examine associations between senescence biomarkers and risk of decline in SPPB in women and men separately, univariate mixed models adjusted for intervention, age, BMI, education, and race were used. Higher concentrations of only GDF15 and OPN were associated with significantly greater decline in SPPB score (GDF15 and OPN) in both women and men (Supplementary Table 4). In women, higher concentrations of MMP2, MMP1, and PARC were associated with greater decline in SPPB, while in men, only lower concentrations of ICAM1 were associated with a greater decline.
PA Intervention and Biomarkers of Senescence
To compare the effects of the PA and HA interventions on senescence biomarkers, we measured the panel of 27 proteins in participant baseline, 12- and 24-month plasma samples. There were no significant differences in the changes in individual biomarker levels from either baseline to 12 months or baseline to 24 months between PA and HA groups (Supplementary Table 5).
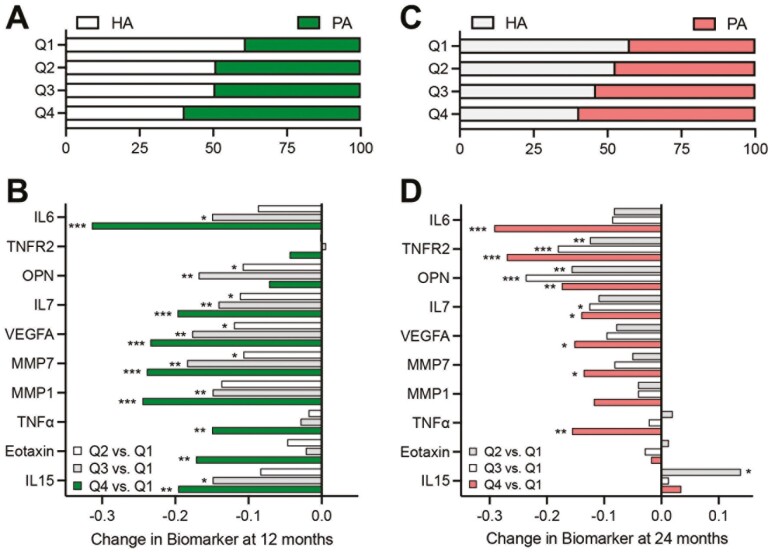
We examined whether LIFE Study participant total PA levels influenced senescence biomarker concentrations at 12 and 24 months compared to baseline. Participants were divided into quartiles based on the amount of accelerometry-based PA >760 activity counts per minute at 12 and 24 months, separately (quartile 1 = lowest physical activity, quartile 4 = highest physical activity; Figures 3A and C). Participants in the higher quartiles of PA at each time point, the majority of whom were randomized to the PA intervention, had significantly greater reductions in 10 senescence biomarkers at 12 months (Figure 3B) and/or 24 months (Figure 3D) compared to those in the lowest quartile of PA. Significant reductions in TNFα, MMP7, VEGFA, IL7, OPN, TNFR2, and IL6 were evident in the higher quartiles at both 12 and 24 months.
Figure 3.
Biomarkers of cellular senescence are associated with performance of moderate-intensity physical activity (PA). Percentage of PA intervention versus healthy aging (HA) intervention participants in each quartile (Q1 to Q4 lowest to highest) of accelerometry-measured high light or greater PA at 12 (A) and 24 months (C). Change in biomarker concentrations from baseline at 12 months (B) and 24 months (D) by quartile of accelerometry-measured high light or greater PA. *p < .05; **p < .01; ***p < .001 compared to quartile 1.
Discussion
The development of mobility disability and declines in physical functioning are major contributors to the loss of independence among older adults. Despite their high prevalence and socioeconomic impact, the underlying biological drivers remain elusive. Cellular senescence, a recognized hallmark of biological aging, contributes to age-related inflammation and the deterioration, aberrant remodeling, and impaired regeneration of tissues through the SASP, a biologically diverse and active set of molecules that act locally and systemically. Using circulating protein components of the SASP as biomarkers of senescent cell burden, we observed significant associations with the incidence of mobility disability, the development of permanent mobility disability, and decrements in physical function within a large cohort of community-dwelling older adults.
Although the PA intervention was sufficient to reduce the incidence on MMD (6), it did not significantly reduce the level of any of the senescence biomarkers. Importantly, however, the levels of several senescence biomarkers at 12 and 24 months were significantly reduced in participants who were most physically active relative to those who were least physically active. Collectively, our data further support cellular senescence as a mediator of age-associated limitations in physical functioning and PA as an effective means to counter the biology of aging.
Mobility disability affects approximately 20% of community-dwelling adults over 65 years (31). Development of mobility disability, defined as the inability to walk 400 m or ¼ of a mile, is associated with multimorbidity, increased healthcare costs and utilization, and mortality (2,32,33). Several senescence biomarkers, the strongest of which were VEGFA, TNFR1, and MMP7, were associated with incident MMD. Using GBM we determined the importance of the top biomarkers and the traditional predictors of disability such as age and BMI. Interestingly, after adjusting for age and BMI as well as sex, education, race, and intervention group, the risk for incident MMD progressively increased from quartile 1 (lowest concentration) to quartile 4 (highest concentration) of activin A, VEGFA, TNFR1, MMP7, GDF15, and IL6. These results extend and amplify our previous cross-sectional observations showing strong relationships between activin A, VEGFA, TNFR1, and MMP7 and gait speed during the 400-m walk (13). Of note, these biomarkers also showed significant univariate associations with MMD after adjusting for covariates when women and men were studied separately. Whether circulating proteins such as OPN uniquely confer risk in women, and others such as eotaxin uniquely confer risk in men, is worthy of further investigation. We note that 445 (46%) fewer men than women participated in LIFE, which limited our statistical power to examine sex differences and our ability to suggest biological underpinnings.
Persistent mobility disability, operationalized as 2 consecutive 400-m walk failures or a single failure followed by death, reflects a more sustained state of disablement or failure to recover following an acute illness or event. A similar panel of senescence biomarkers were found to be related to risk for PMMD including VEGFA, TNFR1, and MMP7, along with age and BMI. The top biomarkers that predicted PMMD—activin A, VEGFA, TNFR1, MMP7, GDF15, IL6, and ICAM1—were identical to those that predicted incident MMD. Moreover, activin A, VEGFA, TNFR1, and GDF15 demonstrated significant univariate associations with PMMD after adjustment for covariates in women and men separately.
Evidence in support of senescence biomarkers as potential mediators of mobility disability and functional decline is varied. Activin A, a member of the transforming growth factor-β family, is recognized as a negative regulator of skeletal muscle mass, which, like growth and differentiation factor 8 (GDF8), acts via the activin receptor type IIB (ActRIIB). Combined antibody-mediated inhibition of activin A and GDF8 synergistically increases muscle mass in both mice and cynomolgus monkeys and muscle force in mice, with a magnitude of effects similar to that induced by treatment with a soluble form of ActRIIB fused to human immunoglobulin g Fc fragment (34). Recent data have also suggested a direct effect of GDF15, a stress response protein that plays diverse roles in multiple tissues, on skeletal muscle, including an increase in oxidative stress, activation of catabolic proteins, and down-regulation of miRNAs (35,36). Circulating GDF15 levels are elevated in the context of inflammation and chronic disease, and prior studies have reported associations with incident mobility disability as defined in our study (37,38). Similarly, there is clear evidence that the pro-inflammatory factors IL6 and TNFR1 can compromise skeletal muscle health and function (39,40). Higher circulating levels of IL6 have been associated with increased risk for declines in gait speed (41) and, in 2 large cohorts of older adults, higher blood concentrations of TNFR1 have been shown confer risk for incident mobility disability and accelerated functional decline (42,43). It is less clear if VEGFA and MMP7 mediate mobility disability and functional decline through skeletal muscle or other organ systems or instead, as biomarkers of systemic senescent cell burden, are more general indicators of biological age.
Notably, each quartile of increasing ICAM1 concentration was associated with a lower risk of both incident and persistent mobility disability. ICAM1, a cell surface glycoprotein expressed on the surface of endothelial cells, fibroblasts, and immune cells, promotes cell adhesion, movement of leukocytes to sites of inflammation, and activation of immune cells (44). ICAM1 expression is induced by p53 and NFκB signaling making it a component of the SASP secreted by senescent endothelial and epithelial cells and preadipocytes (12). Circulating levels of ICAM1 have previously been associated with cardiovascular disease onset and progression (45). The association and potential influence of ICAM1 on late-life function and mobility warrants further study.
The SPPB is a well-validated and extensively utilized composite measure of physical functioning. As a composite measure, the SPPB integrates the capacity and physiological reserve of multiple, integrated systems, for example, cardiovascular, neuromuscular, and vestibular/balance. Previously, we identified a large number of circulating SASP proteins that were cross-sectionally associated with the SPPB score (13). In the present study, we extend these findings by demonstrating that several circulating SASP proteins are predictive of the change in SPPB score with GDF15, OPN, and VEGFA showing the strongest associations. Higher concentrations of GDF15 and OPN also exhibited significant adjusted univariate associations with reductions in SPPB score in women and men separately.
Despite the robust clinically meaningful and dose-dependent effects of the PA intervention in the LIFE Study on changes in the SPPB score and the reduction in incident and persistent mobility disability (6,22,46), we failed to observe any significant changes in our panel of circulating SASP biomarkers at year 1 or year 2 in participants randomized to the PA compared to the HA “control” group. This may imply that the associations between senescence biomarkers and physical function reported here and previously are merely correlative, and PA interventions improve physical function independent of an influence on the abundance or behavior (ie, secretory phenotype) of senescent cells (12–16). The absence of change in the biomarkers among PA participants may also challenge the utility or temporal response of the biomarker panel for detecting a “senotherapeutic” effect of an intervention. This may be more specifically addressed in clinical trials of drugs being developed or repurposed to effectively target senescent cells or the SASP. Our observations may also indicate the intensity, volume, or type of PA in the LIFE trial was not sufficient to alter the burden of senescent cells. It is also possible that PA is not sufficient to reverse or mitigate senescent cell burden in older adults with more advanced biological age. We previously reported that 12 weeks of a more intensive structured strength, and endurance training reduced the expression of well-described markers of the senescence program including P16, P21, cGAS, and TNFα in peripheral blood CD3+ T cells and lowered the circulating concentrations of several SASP proteins in healthy older (~67 years) adults (17).
Using accelerometry, we found that individuals with the highest levels of “high light” intensity PA (a cut point associated with moderately demanding activities of daily living for older adults (47)) had significant reductions in senescence biomarkers at the 12- and 24-month study time points. Of note, several of the biomarkers reduced by higher levels of PA, including VEGFA, MMP7, and IL6, were most predictive of incident and persistent MMD. These results generate interest in a clinical trial to test the hypothesis that higher-level PA interventions reduce senescent cell burden and, correspondingly, circulating levels of senescence-related proteins.
Limitations of the Study
The present study has many strengths including the robust randomized sample of older males and females with mobility limitations and multiple chronic diseases, rigorous assessment of physical functioning and disability, a carefully controlled PA intervention, and an evidence-based and a validated platform of senescence-associated proteins. However, several limitations need to also be considered. We realize that while there is a biological basis for senescent cells to be a likely source of the circulating biomarkers assessed, they may not be the only source. Senescent cells may indirectly contribute to circulating concentrations of the measured proteins through multiple mechanisms (48). Although well-controlled and supervised, the PA intervention reduced the incidence of MMD, it may not have provided a sufficient stimulus to reduce the burden of cellular senescence. Finally, participants in the LIFE Study volunteered for study enrollment and thus may represent a unique population of older adults.
In summary, we show that a consistent panel of senescence proteins is associated with the onset of acute and persistent mobility disability and declines in physical functioning. An intervention that reduced MMD did not change levels of the proteins. These same senescence proteins were reduced in those who had higher levels of total PA participation. Given the current interest in developing senescent cell-targeting therapies, our data may also be leveraged to serve as surrogate end points for future clinical trials targeting improvements in healthspan.
Supplementary Material
Contributor Information
Roger A Fielding, Nutrition, Exercise Physiology and Sarcopenia Laboratory, Jean Mayer USDA Human Nutrition Research Center on Aging, Tufts University, Boston, Massachusetts, USA.
Elizabeth J Atkinson, Department of Quantitative Health Sciences, Mayo Clinic, Rochester, Minnesota, USA.
Zaira Aversa, Robert and Arlene Kogod Center on Aging, Mayo Clinic, Rochester, Minnesota, USA; Department of Physical Medicine and Rehabilitation, Mayo Clinic, Rochester, Minnesota, USA.
Thomas A White, Robert and Arlene Kogod Center on Aging, Mayo Clinic, Rochester, Minnesota, USA.
Amanda A Heeren, Robert and Arlene Kogod Center on Aging, Mayo Clinic, Rochester, Minnesota, USA.
Michelle M Mielke, Department of Epidemiology and Prevention, Wake Forest University School of Medicine, Winston-Salem, North Carolina, USA.
Steven R Cummings, Departments of Medicine, Epidemiology and Biostatistics, University of California San Francisco, San Francisco, California, USA; Research Institute, California Pacific Medical Center, San Francisco, California, USA.
Marco Pahor, Institute on Aging, University of Florida, Gainesville, Florida, USA.
Christiaan Leeuwenburgh, Institute on Aging, University of Florida, Gainesville, Florida, USA.
Nathan K LeBrasseur, Robert and Arlene Kogod Center on Aging, Mayo Clinic, Rochester, Minnesota, USA; Department of Physical Medicine and Rehabilitation, Mayo Clinic, Rochester, Minnesota, USA.
Gustavo Duque, (Biological Sciences Section).
Funding
We are grateful for the support of the National Institutes of Health, National Institute on Aging for grants R01 AG055529, R56 AG060907, and P01 AG062413 to N.K.L., U54 AG044170 to M.M.M. This work was also supported by the Glenn Foundation for Medical Research and the Pritzker Foundation (N.K.L.). R.A.F. is partially supported by the U.S. Department of Agriculture (USDA), under agreement no. 58-8050-9-004, by NIH Boston Claude D. Pepper Center (OAIC; 1P30AG031679), and by NIH University of Florida Claude D. Pepper Center (P30AG028740). The LIFE Study was supported by the NIH grant U01AG022376. Any opinions, findings, conclusions, or recommendations expressed in this publication are those of the authors and do not necessarily reflect the view of the USDA.
Conflict of Interest
E.J.A., T.A.W., and N.K.L. have intellectual property related to this research, which has been reviewed by the Mayo Clinic Conflict of Interest Review Board and was conducted in compliance with Mayo Clinic Conflict of Interest policies.
Author Contributions
Conceptualization: N.K.L., R.A.F., C.L., M.P.; Methodology: N.K.L., T.A.W.; Investigation: R.A.F., T.A.W., A.A.H., M.P., C.L., N.K.L.; Resources: R.A.F., M.P., C.L., N.K.L.; Writing—original draft: R.A.F., E.J.A., N.K.L.; Writing—reviewing & editing: R.A.F., Z.A., M.M.M., S.R.C., M.P., C.L., N.K.L.; Supervision: N.K.L.; Funding acquisition: N.K.L.
References
- 1. Guralnik JM, Simonsick EM, Ferrucci L, et al. A short physical performance battery assessing lower extremity function: association with self-reported disability and prediction of mortality and nursing home admission. J Gerontol. 1994;49:M85–M94. 10.1093/geronj/49.2.m85 [DOI] [PubMed] [Google Scholar]
- 2. Newman AB, Simonsick EM, Naydeck BL, et al. Association of long-distance corridor walk performance with mortality, cardiovascular disease, mobility limitation, and disability. JAMA. 2006;295:2018–2026. 10.1001/jama.295.17.2018 [DOI] [PubMed] [Google Scholar]
- 3. Rantanen T, Guralnik JM, Foley D, et al. Midlife hand grip strength as a predictor of old age disability. JAMA. 1999;281:558–560. 10.1001/jama.281.6.558 [DOI] [PubMed] [Google Scholar]
- 4. Cawthon PM, Manini T, Patel SM, et al. Putative cut-points in sarcopenia components and incident adverse health outcomes: an SDOC analysis. J Am Geriatr Soc. 2020;68:1429–1437. 10.1111/jgs.16517 [DOI] [PMC free article] [PubMed] [Google Scholar]
- 5. Pahor M, Blair SN, Espeland M, et al. ; LIFE Study Investigators. Effects of a physical activity intervention on measures of physical performance: results of the Lifestyle Interventions and Independence for Elders Pilot (LIFE-P) study. J Gerontol A Biol Sci Med Sci. 2006;61:1157–1165. 10.1093/gerona/61.11.1157 [DOI] [PubMed] [Google Scholar]
- 6. Pahor M, Guralnik JM, Ambrosius WT, et al. ; LIFE Study Investigators. Effect of structured physical activity on prevention of major mobility disability in older adults: the LIFE study randomized clinical trial. JAMA. 2014;311:2387–2396. 10.1001/jama.2014.5616 [DOI] [PMC free article] [PubMed] [Google Scholar]
- 7. Bernabei R, Landi F, Calvani R, et al. ; SPRINTT Consortium. Multicomponent intervention to prevent mobility disability in frail older adults: randomised controlled trial (SPRINTT project). BMJ. 2022;377:e068788. 10.1136/bmj-2021-068788 [DOI] [PMC free article] [PubMed] [Google Scholar]
- 8. LeBrasseur NK, Tchkonia T, Kirkland JL. Cellular senescence and the biology of aging, disease, and frailty. Nestle Nutr Inst Workshop Ser. 2015;83:11–18. 10.1159/000382054 [DOI] [PMC free article] [PubMed] [Google Scholar]
- 9. Munoz-Espin D, Serrano M. Cellular senescence: from physiology to pathology. Nat Rev Mol Cell Biol. 2014;15:482–496. 10.1038/nrm3823 [DOI] [PubMed] [Google Scholar]
- 10. Kirkland JL, Tchkonia T. Cellular senescence: a translational perspective. EBioMedicine. 2017;21:21–28. 10.1016/j.ebiom.2017.04.013 [DOI] [PMC free article] [PubMed] [Google Scholar]
- 11. van Deursen JM. The role of senescent cells in ageing. Nature. 2014;509:439–446. 10.1038/nature13193 [DOI] [PMC free article] [PubMed] [Google Scholar]
- 12. Schafer MJ, Zhang X, Kumar A, et al. The senescence-associated secretome as an indicator of age and medical risk. JCI Insight. 2020;5:e133668. 10.1172/jci.insight.133668 [DOI] [PMC free article] [PubMed] [Google Scholar]
- 13. Fielding RA, Atkinson EJ, Aversa Z, et al. Associations between biomarkers of cellular senescence and physical function in humans: observations from the Lifestyle Interventions for Elders (LIFE) study. Geroscience. 2022;44:2757–2770. 10.1007/s11357-022-00685-2 [DOI] [PMC free article] [PubMed] [Google Scholar]
- 14. Aversa Z, Atkinson EJ, Carmona EM, et al. Biomarkers of cellular senescence in idiopathic pulmonary fibrosis. Respir Res. 2023;24:101. 10.1186/s12931-023-02403-8 [DOI] [PMC free article] [PubMed] [Google Scholar]
- 15. Basisty N, Kale A, Jeon OH, et al. A proteomic atlas of senescence-associated secretomes for aging biomarker development. PLoS Biol. 2020;18:e3000599. 10.1371/journal.pbio.3000599 [DOI] [PMC free article] [PubMed] [Google Scholar]
- 16. Tanaka T, Basisty N, Fantoni G, et al. Plasma proteomic biomarker signature of age predicts health and life span. Elife. 2020;9:e61073. 10.7554/eLife.61073 [DOI] [PMC free article] [PubMed] [Google Scholar]
- 17. Englund DA, Sakamoto AE, Fritsche CM, et al. Exercise reduces circulating biomarkers of cellular senescence in humans. Aging Cell. 2021;20:e13415. 10.1111/acel.13415 [DOI] [PMC free article] [PubMed] [Google Scholar]
- 18. Schafer MJ, White TA, Evans G, et al. Exercise prevents diet-induced cellular senescence in adipose tissue. Diabetes. 2016;65:1606–1615. 10.2337/db15-0291 [DOI] [PMC free article] [PubMed] [Google Scholar]
- 19. Zhang X, Englund DA, Aversa Z, Jachim SK, White TA, LeBrasseur NK. Exercise counters the age-related accumulation of senescent cells. Exerc Sport Sci Rev. 2022;50:213–221. 10.1249/JES.0000000000000302 [DOI] [PMC free article] [PubMed] [Google Scholar]
- 20. Fielding RA, Rejeski WJ, Blair S, et al. ; LIFE Research Group. The Lifestyle Interventions and Independence for Elders study: design and methods. J Gerontol A Biol Sci Med Sci. 2011;66:1226–1237. 10.1093/gerona/glr123 [DOI] [PMC free article] [PubMed] [Google Scholar]
- 21. Rejeski WJ, Axtell R, Fielding R, et al. ; LIFE Study Investigator Group. Promoting physical activity for elders with compromised function: the Lifestyle Interventions and Independence for Elders (LIFE) study physical activity intervention. Clin Interv Aging. 2013;8:1119–1131. 10.2147/CIA.S49737 [DOI] [PMC free article] [PubMed] [Google Scholar]
- 22. Santanasto AJ, Glynn NW, Lovato LC, et al. ; LIFE Study Group. Effect of physical activity versus health education on physical function, grip strength and mobility. J Am Geriatr Soc. 2017;65:1427–1433. 10.1111/jgs.14804 [DOI] [PMC free article] [PubMed] [Google Scholar]
- 23. Bann D, Hire D, Manini T, et al. ; LIFE Study Group. Light intensity physical activity and sedentary behavior in relation to body mass index and grip strength in older adults: cross-sectional findings from the Lifestyle Interventions and Independence for Elders (LIFE) study. PLoS One. 2015;10:e0116058. 10.1371/journal.pone.0116058 [DOI] [PMC free article] [PubMed] [Google Scholar]
- 24. Rejeski WJ, Marsh AP, Brubaker PH, et al. ; LIFE Study Investigators. Analysis and interpretation of accelerometry data in older adults: the LIFE study. J Gerontol A Biol Sci Med Sci. 2015;71:521–528. 10.1093/gerona/glv204 [DOI] [PMC free article] [PubMed] [Google Scholar]
- 25. Wanigatunga AA, Tudor-Locke C, Axtell RS, et al. Effects of a long-term physical activity program on activity patterns in older adults. Med Sci Sports Exerc. 2017;49:2167–2175. 10.1249/MSS.0000000000001340 [DOI] [PMC free article] [PubMed] [Google Scholar]
- 26. Englund DA, Jolliffe A, Aversa Z, et al. p21 induces a senescence program and skeletal muscle dysfunction. Mol Metab. 2023;67:101652. 10.1016/j.molmet.2022.101652 [DOI] [PMC free article] [PubMed] [Google Scholar]
- 27. Yousefzadeh MJ, Zhao J, Bukata C, et al. Tissue specificity of senescent cell accumulation during physiologic and accelerated aging of mice. Aging Cell. 2020;19:e13094. 10.1111/acel.13094 [DOI] [PMC free article] [PubMed] [Google Scholar]
- 28. Saul D, Kosinsky RL, Atkinson EJ, et al. A new gene set identifies senescent cells and predicts senescence-associated pathways across tissues. Nat Commun. 2022;13:4827. 10.1038/s41467-022-32552-1 [DOI] [PMC free article] [PubMed] [Google Scholar]
- 29. Greenwell B, Cunningham J, Developers G. gbm: Generalized Boosted Regression Models. R Package Version 2.1.8. 2020. [Google Scholar]
- 30. Team RC. R: A Language and Environment for Statistical Computing. Vienna, Austria: RFfS Computing; 2021. [Google Scholar]
- 31. Taylor DM. Americans with Disabilities 2014. Currrent Population Reports. Washington, DC: U.S. Census Bureau; 2018:70–152. [Google Scholar]
- 32. Lampinen P, Heikkinen E. Reduced mobility and physical activity as predictors of depressive symptoms among community-dwelling older adults: an eight-year follow-up study. Aging Clin Exp Res. 2003;15:205–211. 10.1007/BF03324501 [DOI] [PubMed] [Google Scholar]
- 33. Hardy SE, Kang Y, Studenski SA, Degenholtz HB. Ability to walk 1/4 mile predicts subsequent disability, mortality, and health care costs. J Gen Intern Med. 2011;26:130–135. 10.1007/s11606-010-1543-2 [DOI] [PMC free article] [PubMed] [Google Scholar]
- 34. Latres E, Mastaitis J, Fury W, et al. Activin A more prominently regulates muscle mass in primates than does GDF8. Nat Commun. 2017;8:15153. 10.1038/ncomms15153 [DOI] [PMC free article] [PubMed] [Google Scholar]
- 35. Tang H, Inoki K, Brooks SV, et al. mTORC1 underlies age-related muscle fiber damage and loss by inducing oxidative stress and catabolism. Aging Cell. 2019;18:e12943. 10.1111/acel.12943 [DOI] [PMC free article] [PubMed] [Google Scholar]
- 36. Bloch SA, Lee JY, Syburra T, et al. Increased expression of GDF-15 may mediate ICU-acquired weakness by down-regulating muscle microRNAs. Thorax. 2015;70:219–228. 10.1136/thoraxjnl-2014-206225 [DOI] [PMC free article] [PubMed] [Google Scholar]
- 37. Baek SJ, Eling T. Growth differentiation factor 15 (GDF15): a survival protein with therapeutic potential in metabolic diseases. Pharmacol Ther. 2019;198:46–58. 10.1016/j.pharmthera.2019.02.008 [DOI] [PMC free article] [PubMed] [Google Scholar]
- 38. Osawa Y, Semba RD, Fantoni G, et al. Plasma proteomic signature of the risk of developing mobility disability: a 9-year follow-up. Aging Cell. 2020;19:e13132. 10.1111/acel.13132 [DOI] [PMC free article] [PubMed] [Google Scholar]
- 39. Belizario JE, Fontes-Oliveira CC, Borges JP, Kashiabara JA, Vannier E. Skeletal muscle wasting and renewal: a pivotal role of myokine IL-6. Springerplus. 2016;5:619. 10.1186/s40064-016-2197-2 [DOI] [PMC free article] [PubMed] [Google Scholar]
- 40. Li J, Yi X, Yao Z, Chakkalakal JV, Xing L, Boyce BF. TNF receptor-associated factor 6 mediates TNFalpha-induced skeletal muscle atrophy in mice during aging. J Bone Miner Res. 2020;35:1535–1548. 10.1002/jbmr.4021 [DOI] [PMC free article] [PubMed] [Google Scholar]
- 41. Verghese J, Holtzer R, Oh-Park M, Derby CA, Lipton RB, Wang C. Inflammatory markers and gait speed decline in older adults. J Gerontol A Biol Sci Med Sci. 2011;66:1083–1089. 10.1093/gerona/glr099 [DOI] [PMC free article] [PubMed] [Google Scholar]
- 42. Vasunilashorn S, Ferrucci L, Crimmins EM, Bandinelli S, Guralnik JM, Patel KV. Association of inflammation with loss of ability to walk 400 meters: longitudinal findings from the Invecchiare in Chianti Study. J Am Geriatr Soc. 2013;61:1743–1749. 10.1111/jgs.12446 [DOI] [PMC free article] [PubMed] [Google Scholar]
- 43. Dhamoon MS, Cheung YK, Moon YP, et al. Association between serum tumor necrosis factor receptor 1 and trajectories of functional status: the Northern Manhattan study. Am J Epidemiol. 2017;186:11–20. 10.1093/aje/kwx035 [DOI] [PMC free article] [PubMed] [Google Scholar]
- 44. Scott DW, Patel RP. Endothelial heterogeneity and adhesion molecules N-glycosylation: implications in leukocyte trafficking in inflammation. Glycobiology. 2013;23:622–633. 10.1093/glycob/cwt014 [DOI] [PubMed] [Google Scholar]
- 45. Ridker PM, Hennekens CH, Roitman-Johnson B, Stampfer MJ, Allen J. Plasma concentration of soluble intercellular adhesion molecule 1 and risks of future myocardial infarction in apparently healthy men. Lancet. 1998;351:88–92. 10.1016/S0140-6736(97)09032-6 [DOI] [PubMed] [Google Scholar]
- 46. Fielding RA, Guralnik JM, King AC, et al. ; LIFE Study Group. Dose of physical activity, physical functioning and disability risk in mobility-limited older adults: results from the LIFE study randomized trial. PLoS One. 2017;12:e0182155. 10.1371/journal.pone.0182155 [DOI] [PMC free article] [PubMed] [Google Scholar]
- 47. Matthew CE. Calibration of accelerometer output for adults. Med Sci Sports Exerc. 2005;37:S512–S522. 10.1249/01.mss.0000185659.11982.3d [DOI] [PubMed] [Google Scholar]
- 48. Chambers ES, Vukmanovic-Stejic M, Shih BB, et al. Recruitment of inflammatory monocytes by senescent fibroblasts inhibits antigen-specific tissue immunity during human aging. Nature Aging. 2021;1:101–113. 10.1038/s43587-020-00010-6 [DOI] [PubMed] [Google Scholar]
Associated Data
This section collects any data citations, data availability statements, or supplementary materials included in this article.
Supplementary Materials
Data Availability Statement
LIFE Study data sets are available through the NIA Aging Research Biobank, https://agingresearchbiobank.nia.nih.gov/studies/life/. Biomarker data presented in the current study will be made available by the investigative team upon reasonable request.