Abstract
Innate immunity has been shown to be associated with schizophrenia (Sch). This study explored the relationship between symptoms and neutrophil-to-lymphocyte ratio (NLR) (a marker of innate immunity) in patients with Sch. Ninety-seven first-episode medication-naïve (FEMN) patients with Sch and 65 healthy controls were recruited in this study. We measured the complete blood count and assessed the clinical symptoms using the PANSS scales. We found higher NEU counts and NLR in patients with Sch compared with control subjects. Male patients showed a higher NEU count than female patients. In addition, FEMN patients with higher NLR and NEU values showed higher PANSS-p, PANSS-g, and PANSS-total scores (all p < 0.05). Regression analysis revealed that NLR was a predictor for PANSS total scores in patients with Sch. Higher NLR value was observed in patients with Sch and the significant associations between NLR and psychotic symptoms indicate that an imbalance in inflammation and innate immune system may be involved in the pathophysiology of Sch.
Subject terms: Schizophrenia, Biomarkers
Introduction
Schizophrenia (Sch) is a complex psychiatric disorder manifesting as psychotic symptoms, negative symptoms, and cognitive impairments. The pathophysiological mechanism underlying Sch remains unknown and is partially due to its heterogeneous genetic, neurobiological, and phenotypic profile. Current antipsychotic medications have been reported to be beneficial in only about 30% of patients with Sch1–3. The vulnerability-stress-inflammation model has received increasing interest and suggests that prenatal exposure to influenza virus, toxoplasmosis, herpes viruses or other viruses is considered to be an environmental neurodevelopmental trigger for psychotic symptoms and that infection during childhood and adolescence may trigger episodes or relapses of Sch in genetically susceptible people4–11.
Inflammation is a natural and essential response of the body to foreign pathogens to activate immune cells and molecular mediators, such as cytokines and complement proteins12,13. Although peripheral cytokines can cross the blood-brain barrier and invade the central nervous system14, the brain may be attacked by inflammation through the activation of microglia, the innate immune cells in the brain15,16. It is now generally accepted that microglia are primed during early brain development and then switch to a pro-inflammatory state in response to stress during critical periods of development, which in turn leads to neurotransmission abnormalities, synaptic pruning, and structural damage to neurons17. The roots of immune abnormality as a potential predictive biomarker for Sch lie in the observed association between microglia-mediated inflammatory processes and the development of psychosis. There is accumulating evidence for the presence of abnormal immune system markers, including chronic immunity and inflammatory biomarkers in the pathophysiology of Sch in postmortem studies and studies examining cytokines in cerebrospinal fluid of patients18–21. In particular, the previously identified genetic locus of vulnerability to Sch includes gene-coding immune-related components22,23. Moreover, anti-inflammatory agents in combination with antipsychotics have shown better efficacy in improving clinical symptoms as compared with treatment with antipsychotic drugs alone24. Furthermore, recent studies have shown that first-degree relatives of patients with Sch have altered levels of inflammatory markers, increasing the likelihood of inflammation-related abnormalities as a promising endophenotype for Sch.
Peripheral white blood cells are important components of the immune system. Complete blood cell count is a widely used non-specific marker of inflammatory state25. Altered complete blood cell counts have been reported in patients with Sch, especially lymphocyte (LYM) and neutrophils (NEU), compared to healthy controls. NEU reflects non-specific inflammation and plays a role in the body’s innate immunity, resulting in a rapid response to infection26. LYM indicates specific immunity and is important for the body’s adaptive immunity27,28. Increased NEU counts have been reported in patients with first-episode Sch and were associated with PANSS total scores29,30. In addition, a recent meta-analysis also revealed an increase in LYM counts in first-episode patients31. In addition to these two indicators, the neutrophil-to-lymphocyte ratio (NLR) is also a measure of the overall inflammatory reaction and reflects the balance between two opposing but complementary pathways32. Thus, NLR has recently been shown to be a novel marker for systemic inflammation derived from routine blood cell counts. In addition, increased NLR values were found to be correlated with an elevation in cytokines and CRP and have been widely used as a proxy of systemic inflammation in recent studies33–35. A recent meta-analysis of 804 patients with Sch and 671 controls reported elevated NLR in first-episode patients with Sch36. Moreover, the authors found that antipsychotic medication affected patients’ NLR36. However, the findings of the relationship between NLR and clinical symptoms in patients with first-episode Sch were controversial37.
Considering that NLR is a relatively new biomarker in Sch and few studies have investigated the potential involvement in the disease pathophysiology, this study was designed to assess whether NLR was associated with the clinical symptoms evaluated by the PANSS in first patients with Sch after controlling for the confounding factors. This study would answer the following questions: 1) Were there differences in NEU, LYM, and NLR, between first-episode medication-naive (FEMN) patients and healthy controls? and 2) Is there an association between NEU, LYM and NLR and clinical symptoms in FEMN patients with Sch?
Methods
Participants
This study was designed as a multi-center and cross-sectional observational study of the FEMN patients. We conducted this study at Hebei Province Veterans Hospital and Beijing Huilongguan Hospital. The diagnostic eligibility of participants was determined using the SCID38. The research protocol was approved by the Institutional Review Board of Beijing Huilongguan Hospital and written informed consent was obtained from the participants or their parent/legal guardian/next of kin to participate in the study.
The inclusion criteria were: 1) Han ethnicity; 2) age between 16 and 45 years; 3) duration of illness≤ 5 years; and 4) no previous treatment or cumulative use of oral antipsychotic medication ≤ 14 days. Exclusion criteria included: 1) substance abuse/dependence; 2) severe somatic diseases; 3) self-injury, intrusive agitation, destructive behaviour, or suicide; 4) use of oral anti-immune agents; and 5) with ongoing infections, allergies or history of autoimmune disorders. Finally, a total of 97 FEMN patients with Sch were recruited in this study.
A total of 65 control subjects were recruited in our study. Healthy controls were excluded if they were diagnosed with major Axis I disorder or had a family history of psychiatric disorders. Medical histories and physical examinations were obtained from each participant.
Clinical assessment
The clinical symptoms were assessed using the Positive and Negative Syndrome Scale (PANSS)39. Experienced interviewers assessed the symptoms of patients. They received comprehensive training and the intra-class correlation coefficients (ICCs) for the total score exceeded 0.8.
Determination of blood cell counts
Blood samples were extracted from each patient between 7:00 and 8:00 am. Complete blood count was conducted using the SYSMEX XN-3000 assembly line (Sysmex Corporation, Japan). The measure was carried out in strict accordance with the operating manual and was quality-controlled. LYM and NEU count data for each patient was obtained from the laboratory electronic system at the hospital.
Statistical analysis
Sample size power was calculated based on expected differences in NLR values. The sample size in our study was considered to achieve significance with a large effect size (ES) (d = 0.50), a power of 80%, and α = 0.05. Demographic data, baseline PANSS scores, LYM and NEU counts were compared in patients and healthy controls. The normality of the distribution of LYM and NEU counts and NLR values was assessed using the Shapiro-Wilk test. The normally distributed continuous variable was reported by mean ± standard deviation (SD) and was compared using the univariate analysis of variance. Non-normally distributed continuous variables were expressed median and interquartile ranges and were compared using the nonparametric Wilcoxon tests. The categorical variable was reported as absolute numbers and percentages, and was compared using the X2 test.
Since NLR was not normally distributed in patients and controls, Spearman’s nonparametric bivariate correlations analyses were conducted to analyze the associations of LYM and NEU counts and NLR values with clinical symptoms and demographic and clinical data in patients. Further regression analysis was performed to investigate the potential relevant variables for the clinical symptoms and the following variables were included in the models: demographic data and blood cell count.
Bonferroni correction was conducted to adjust for multiple comparisons. All analyses were performed using the SPSS version 20.0. P < 0.05 was set as the threshold for significance.
Results
Comparisons between patients and healthy controls
The values of NEU and LYM, NRI, sex, age, education, age of onset and BMI of the patients and controls are shown in Table 1. No significant differences were observed in age, sex, age of onset and BMI between the two groups.
Table 1.
Demographic data and clinical characteristics between patients and controls at baseline by using univariate analysis of variance, nonparametric analysis and chi-square.
| Sch patients | Healthy controls | ||
|---|---|---|---|
| (n = 97) | (n = 65) | F or Z or X2 (p) | |
| Age (ys) | 24.1 ± 6.9 | 24.8 ± 6.1 | 0.4 (0.51) |
| Education (ys) | 8.1 ± 2.8 | 11.5 ± 3.4 | 46.5 (<0.001) |
| Sex (Male, %) | 55 (56.7%) | 42 (64.6%) | 1.0 (0.31) |
| BMI (kg/m2) | 21.3 ± 3.4 | 21.4 ± 3.2 | 0.2 (0.68) |
| Onset age (ys) | 23.4 ± 6.9 | ||
| NLR, median (IQR) | 2.1 (1.6, 3.3) | 1.6 (1.3, 2.2) | 3.0 (0.003) |
| LYM count (109/L) | 2.0 ± 0.8 | 2.3 ± 0.7 | 3.3 (0.07) |
| NEU count (109/L) | 4.4 ± 1.7 | 3.9 ± 1.4 | 4.1 (0.045) |
Note: BMI Body mass index, ys years, LYM lymphocytes, NEU neutrophils, NLR neutrophil/lymphocyte ratio.
The median NLR values for the patient group were 2.1 (IQR: 1.6, 3.3, 95% CI: 2.2–2.8) and the median NLR values for the control group were 1.6 (IQR: 1.3, 2.2, 95% CI: 1.7–2.1). The mean NEU counts were 4.4 (SD: 1.7, 95% CI: 1.9–2.2) in the patient group and 3.9 (SD: 1.4, 95% CI: 3.6–4.3) in the control group. NLR values and NEU counts were higher in FEMN patients with Sch compared with controls (for NLR: Z = 3.0, p = 0.003; for NEU counts: F = 4.1, p = 0.045). After Bonferroni corrections, the difference in NLR remained significant (Bonferroni corrected p = 0.009). There was no significant difference in LYM counts between the two groups.
Correlation analysis revealed no significant associations between age, age of onset, BMI, and counts of NEU and LYM or NLR values in patients with Sch (all p > 0.05).
Association between NLR and clinical symptoms
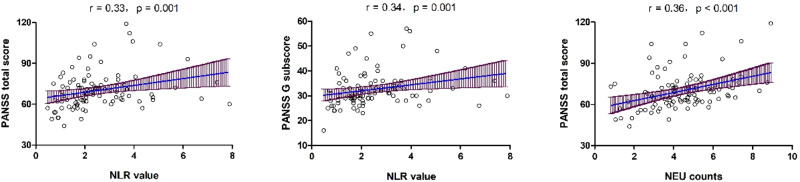
As shown in Table 2, Spearman nonparametric bivariate correlation analyses revealed significant positive associations between NLR and negative symptoms (r = 0.25, p = 0.01), general psychopathology (r = 0.34, p = 0.001), and total scores (r = 0.33, p = 0.001) (Table 2). Additionally, we found that NEU counts were also positively associated with negative symptoms (r = 0.27, p = 0.008), general psychopathology (r = 0.27, p = 0.008) and total scores (r = 0.36, p < 0.001). After Bonferroni corrections, correlations between NLR or NEU counts and PANSS total scores (NLR: Bonferroni corrected p = 0.012 and NEU: Bonferroni corrected p = 0.0036), as well as between NLR and general psychopathology remained significant (Bonferroni corrected p = 0.012).
Table 2.
Correlations between NLR and clinical symptoms using Spearman nonparametric bivariate correlations analyses.
| P subscale | N subscale | G subscale | Total score | |||||
|---|---|---|---|---|---|---|---|---|
| r | p | r | p | r | p | r | p | |
| NLR | 0.16 | 0.12 | 0.25 | 0.01 | 0.34 | 0.001* | 0.33 | 0.001* |
| LYM | 0.02 | 0.82 | -0.06 | 0.56 | -0.15 | 0.14 | -0.05 | 0.61 |
| NEU | 0.16 | 0.12 | 0.27 | 0.008 | 0.27 | 0.008 | 0.36 | <0.001* |
Note: *After Bonferroni correction, p < 0.05. Bold p values mean significant.
Because of the close relationship between age and age of onset (r = 0.98), and to avoid including completely collinear covariates in the multivariate analysis, we chose age for the regression analysis. After covarying for confounding factors such as age, sex, and years of education, the regression analysis revealed that NLR was a predictor for PANSS total scores in FEMN patients with Sch (β = 2.929, t = 3.489, p = 0.001; 95% CI: 1.261–4.598) (R2 = 0.247) (Table 3 and Fig. 1). In addition, NEU counts were also an independent predictor for clinical symptoms in patients (β = 2.894, t = 4.007, p < 0.001; 95% CI: 1.459–4.329) (R2 = 0.274) (Table 3 and Fig. 1).
Table 3.
Regression analysis of the associations between NLR and NEU counts and clinical symptoms.
| Regression analysis | |||||
|---|---|---|---|---|---|
| NLR values | B (95% CI) | SE | β | t | p |
| Sex | 0.566(−4.380 to 5.512) | 2.489 | 0.021 | 0.227 | 0.821 |
| Age | −0.581(−0.942 to −0.219) | 0.182 | −0.294 | −3.191 | 0.002 |
| Education years | −1.449(−2.315 to −0.582) | 0.436 | −0.305 | −3.321 | 0.001 |
| NLR values | 2.929(1.261 to 4.598) | 0.840 | 0.328 | 3.489 | 0.001 |
| NEU counts | |||||
| Sex | 1.210(−3.691 to 6.111) | 2.467 | 0.046 | 0.490 | 0.625 |
| Age | −0.396(−0.755 to −0.036) | 0.181 | −0.201 | −2.187 | 0.031 |
| Education years | −1.281(−2.132 to −0.429) | 0.429 | −0.270 | −2.988 | 0.004 |
| NEU counts | 2.894(1.459 to 4.329) | 0.722 | 0.377 | 4.007 | <0.001 |
Note: NEU neutrophils, NLR neutrophil/lymphocyte ratio, B unstandardized regression coefficient, β standardized regression coefficient, t t statistic, SE standard error.
For NLR: ANOVA, F = 7.370; p < 0.001; adjusted R2 = 0.247.
For NEU counts: ANOVA, F = 8.507; p < 0.001; adjusted R2 = 0.274.
Fig. 1.
There were significant associations between NEU counts or NLR values and clinical symptoms assessed by PANSS in first-episode and drug-naïve patients with schizophrenia.
Discussion
We revealed significant increases in NEU counts and NLR values in FEMN patients relative to healthy controls. NLR and NEU counts correlated with clinical symptoms assessed by the PANSS scale after controlling for sex, age, BMI, and age of onset.
Our finding of higher NEU counts in patients compared to healthy controls is consistent with previous studies in patients with Sch25,29,30, suggesting the involvement of increased NEU counts in the pathogenesis of Sch. The immune hypothesis of Sch has become increasingly prominent in the study of its etiology40–42, including maternal viral infection and immune activation, as well as variants of immune genes that contributed to Sch risk. NEUs are the most abundant leukocytes in peripheral blood cells and are considered to be the first line of defense of the innate immune system26. In addition to its phagocytosis, NEU secretes more than 70 different pro-inflammatory compounds, including cytokines, chemokines, and growth factors, which provoke inflammatory responses and oxidative stress, resulting in a rapid response to infection43,44. It should be noted that there was no difference in LYM counts between patients and controls in our study, indicating that adaptive immunity was not involved in the onset of this disease. Our findings provide further evidence for the role of innate immune cells as modulators of acute disease severity.
Furthermore, we found that NLR was increased in patients with Sch relative to healthy controls and that increased NLR was positively correlated with psychotic illness severity score. The NLR, calculated by dividing the absolute NEU count by the absolute LYM count, is an important inflammatory marker that is easily measured, repeatable, and inexpensive from routine blood testing44. It is a more reliable indicator of the intensity of stress and systemic inflammation. In line with our findings, two recent meta-analyses have shown evidence of higher NLR values in individuals with non-affective psychosis36,45. In addition, NEU counts have been reported to be influenced by age, sex, nicotine, and BMI46. Notably, in the present study, patients were well-matched and specifically controlled for several confounders, such as sex, age, years of education, and BMI. We also assessed the effects of sex, years of education, age, and BMI on NLR in patients and found no associations. Therefore, the results of increased NLR found in our study were not correlated with these confounding factors, but maybe with Sch itself.
Consistent with previous studies, we found that NEU counts and NLR were associated with clinical symptoms30,47,48, suggesting that NEU may be involved in the pathophysiology of Sch. The potential mechanisms that may be behind the increased NLR values in Sch, primarily cytokine system disorders and their impact on the activation of the immune system. A growing and compelling body of evidence demonstrates that patients suffering from Sch have the baseline changes in proinflammatory and anti-inflammatory cytokine levels throughout the disease and immunologic dysfunctions as a key element in its pathophysiology20,49,50. However, inconsistent findings from previous studies regarding the associations between NLR values and clinical symptom severity scores have limited the conclusions. For example, two studies reported no significant association with the severity of clinical symptoms assessed by the Brief Psychiatric Rating Scale, Clinical Global Impression, PANSS, or disease duration51,52. Possible explanations for these controversial findings may be that the patients recruited were at different stages of Sch or the type and dose of antipsychotic medication or treatment duration. Previous studies have shown the potential impact of antipsychotics on NLR values53. The patients in our study were FEMN patients, while other studies recruited patients treated with antipsychotics.
The present study benefited from a sample of drug-naïve Sch patients, given the inherent challenges of recruiting FEMN patients. In particular, patients and controls were well-matched in most demographic characteristics. However, several limitations should also be noted in this study. Firstly, given the cross-sectional design we used and we only examined our patients once, this study cannot draw any causal relationship regarding the associations of NLR and clinical symptoms in Sch. A further longitudinal study and comparisons between naïve and long-term medicated states could likely better demonstrate the relationship between them. Secondly, although we controlled for some confounding factors, some other factors associated with immune activation and inflammation were neglected. These factors are particularly important for first-episode patients with Sch, as many of these factors were exacerbated when patients experience their first episodes, such as depression, anxiety, stress, and related sleep abnormalities. However, these data were not collected and would need to be investigated in future short- or long-term longitudinal studies. Thirdly, we only measured the NEU and LYM counts in white blood cells, and we did not measure other immune cells or related cytokines, chemokines, and components. As previous studies have shown that NEU and LYM were significantly correlated with cytokines, chemokines, and growth factors, the interrelationships between white blood cells and cytokines need further investigation in further studies. Forth, the detailed duration of the disease of recruited patients was not collected in our study. However, all recruited participants were first-episode patients and their duration of the disease was <= 5 years to meet the inclusion/exclusion criteria. Fifth, NEU counts can be influenced by nicotine consumption, and nicotine addiction is very prevalent in patients with Sch. However, we did not report the smoking rates in our study, nor did we control for this variable in the comparisons.
Conclusion
The present study demonstrates that increased NEU counts result in the high NLR value in patients with Sch. Our findings provide further evidence for the existence of increased NLR in Sch. More importantly, our results revealed that increased NLR was associated with psychotic symptoms at the first episode of symptoms. Notably, our findings may not be generalized to other patients with different ethnic and chronic Sch receiving antipsychotic medication.
Acknowledgements
We would like to thank the participants in the study and their families. This study was funded by the the Natural Science Foundation of Guangdong Province (2023A1515011728), Guangzhou Municiple Health Commission (2023C-TS26), Plan on enhancing scientific research in GMU (02-410-230221XM), Project of Guangzhou Municipal Science and Technology Bureau (2023A03J0835), Opening Foundation of Jiangsu Key Laboratory of Neurodegeneration, Nanjing Medical University (KF202202), Open Project Program of State Key Laboratory of Virtual Reality Technology and Systems, Beihang University (VRLAB2022 B02), and Shanghai Key Laboratory of Psychotic Disorders Open Grant (21-K03), Guangzhou High-level Clinical Key Specialty, and Guangzhou Research-oriented Hospital. All funding had no role in study design, data analysis, paper submission and publication. The funding for this study had no further role in study design, data analysis, and the decision to submit the paper for publication.
Author contributions
XW and ZL were responsible for study design, statistical analysis and manuscript preparation. XW, XC, XG and ZL were responsible for recruiting the patients, performing the clinical rating and collecting the clinical data. XW and ZL were evolving the ideas and editing the manuscript. XW and ZL were involved in writing the protocol, and cowrote the paper. All authors have contributed to and have approved the final manuscript.
Data availability
The data that support the findings of this study are available on request from the corresponding author. The data are not publicly available due to privacy or ethical restrictions.
Competing interests
The authors declare no competing interests.
Footnotes
Publisher’s note Springer Nature remains neutral with regard to jurisdictional claims in published maps and institutional affiliations.
These authors contributed equally: Xuan Wang, Xiaofang Chen.
References
- 1.Lieberman JA, et al. Effectiveness of antipsychotic drugs in patients with chronic schizophrenia. N. Engl. J. Med. 2005;353:1209–1223. doi: 10.1056/NEJMoa051688. [DOI] [PubMed] [Google Scholar]
- 2.Huhn M, et al. Comparative efficacy and tolerability of 32 oral antipsychotics for the acute treatment of adults with multi-episode schizophrenia: a systematic review and network meta-analysis. Lancet. 2019;394:939–951. doi: 10.1016/S0140-6736(19)31135-3. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 3.Peng S, Li W, Lv L, Zhang Z, Zhan X. BDNF as a biomarker in diagnosis and evaluation of treatment for schizophrenia and depression. Discov. Med. 2018;26:127–136. [PubMed] [Google Scholar]
- 4.Steiner J, et al. Bridging the gap between the immune and glutamate hypotheses of schizophrenia and major depression: Potential role of glial NMDA receptor modulators and impaired blood-brain barrier integrity. World J. Biol. Psychiatry. 2012;13:482–492. doi: 10.3109/15622975.2011.583941. [DOI] [PubMed] [Google Scholar]
- 5.Brown AS. The risk for schizophrenia from childhood and adult infections. Am. J. Psychiatry. 2008;165:7–10. doi: 10.1176/appi.ajp.2007.07101637. [DOI] [PubMed] [Google Scholar]
- 6.Nielsen PR, Benros ME, Mortensen PB. Hospital contacts with infection and risk of schizophrenia: a population-based cohort study with linkage of Danish national registers. Schizophr. Bull. 2014;40:1526–1532. doi: 10.1093/schbul/sbt200. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 7.Müller N, Weidinger E, Leitner B, Schwarz MJ. The role of inflammation in schizophrenia. Frontiers in neuroscience. 2015;9:372. doi: 10.3389/fnins.2015.00372. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 8.Saatci D, van Nieuwenhuizen A, Handunnetthi L. Maternal infection in gestation increases the risk of non-affective psychosis in offspring: a meta-analysis. J. Psychiatr. Res. 2021;139:125–131. doi: 10.1016/j.jpsychires.2021.05.039. [DOI] [PubMed] [Google Scholar]
- 9.Selten JP, Termorshuizen F. The serological evidence for maternal influenza as risk factor for psychosis in offspring is insufficient: critical review and meta-analysis. Schizophr. Res. 2017;183:2–9. doi: 10.1016/j.schres.2016.11.006. [DOI] [PubMed] [Google Scholar]
- 10.Soheila R, Arezoo B, Galavani H, Saber R. Comments on “Genetic characterization and phylogenetic analysis of Fasciola species based on ITS2 gene sequence, with first molecular evidence of intermediate Fasciola from water buffaloes in Aswan, Egypt”. Ann. Parasitol. 2021;67:351–352. doi: 10.17420/ap6702.350. [DOI] [PubMed] [Google Scholar]
- 11.Davies C, et al. Prenatal and perinatal risk and protective factors for psychosis: a systematic review and meta-analysis. Lancet. Psychiatry. 2020;7:399–410. doi: 10.1016/S2215-0366(20)30057-2. [DOI] [PubMed] [Google Scholar]
- 12.Kotas ME, Medzhitov R. Homeostasis, inflammation, and disease susceptibility. Cell. 2015;160:816–827. doi: 10.1016/j.cell.2015.02.010. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 13.Tu Z, et al. Design of therapeutic biomaterials to control inflammation. Nat. Rev. Mater. 2022;7:557–574. doi: 10.1038/s41578-022-00426-z. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 14.Banks WA. The blood-brain barrier in psychoneuroimmunology. Immunol. Allergy Clin. North Am. 2009;29:223–228. doi: 10.1016/j.iac.2009.02.001. [DOI] [PubMed] [Google Scholar]
- 15.Munn NA. Microglia dysfunction in schizophrenia: an integrative theory. Med. Hypotheses. 2000;54:198–202. doi: 10.1054/mehy.1999.0018. [DOI] [PubMed] [Google Scholar]
- 16.Monji A, Kato T, Kanba S. Cytokines and schizophrenia: Microglia hypothesis of schizophrenia. Psychiatry Clin. Neurosci. 2009;63:257–265. doi: 10.1111/j.1440-1819.2009.01945.x. [DOI] [PubMed] [Google Scholar]
- 17.Howes OD, McCutcheon R. Inflammation and the neural diathesis-stress hypothesis of schizophrenia: a reconceptualization. Transl. Psychiatry. 2017;7:e1024. doi: 10.1038/tp.2016.278. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 18.Bora E. Peripheral inflammatory and neurotrophic biomarkers of cognitive impairment in schizophrenia: a meta-analysis. Psychol. Med. 2019;49:1971–1979. doi: 10.1017/S0033291719001685. [DOI] [PubMed] [Google Scholar]
- 19.Dunleavy C, Elsworthy RJ, Upthegrove R, Wood SJ, Aldred S. Inflammation in first-episode psychosis: The contribution of inflammatory biomarkers to the emergence of negative symptoms, a systematic review and meta-analysis. Acta Psychiatr. Scand. 2022;146:6–20. doi: 10.1111/acps.13416. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 20.Halstead S, et al. Alteration patterns of peripheral concentrations of cytokines and associated inflammatory proteins in acute and chronic stages of schizophrenia: a systematic review and network meta-analysis. Lancet Psychiatry. 2023;10:260–271. doi: 10.1016/S2215-0366(23)00025-1. [DOI] [PubMed] [Google Scholar]
- 21.Zhang Y, et al. Peripheral cytokine levels across psychiatric disorders: A systematic review and network meta-analysis. Prog. Neuropsychopharmacol. Biol. Psychiatry. 2023;125:110740. doi: 10.1016/j.pnpbp.2023.110740. [DOI] [PubMed] [Google Scholar]
- 22.Sekar A, et al. Schizophrenia risk from complex variation of complement component 4. Nature. 2016;530:177–183. doi: 10.1038/nature16549. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 23.Consortium, S.W.G.o.t.P.G. Biological insights from 108 schizophrenia-associated genetic loci. Nature. 2014;511:421–427. doi: 10.1038/nature13595. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 24.Çakici N, van Beveren NJM, Judge-Hundal G, Koola MM, Sommer IEC. An update on the efficacy of anti-inflammatory agents for patients with schizophrenia: a meta-analysis. Psychol. Med. 2019;49:2307–2319. doi: 10.1017/S0033291719001995. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 25.Jackson AJ, Miller BJ. Meta-analysis of total and differential white blood cell counts in schizophrenia. Acta Psychiatr. Scand. 2020;142:18–26. doi: 10.1111/acps.13140. [DOI] [PubMed] [Google Scholar]
- 26.Rosales C. Neutrophil: A Cell with Many Roles in Inflammation or Several Cell Types? Front. Physiol. 2018;9:113. doi: 10.3389/fphys.2018.00113. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 27.Mayadas TN, Cullere X, Lowell CA. The multifaceted functions of neutrophils. Annu. Rev. Pathol. 2014;9:181–218. doi: 10.1146/annurev-pathol-020712-164023. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 28.Orakpoghenor O, Avazi DO, Markus TP, Olaolu OS. Lymphocytes: a brief review. Sci. J. Immunol. Immunotherapy. 2019;3:4–8. [Google Scholar]
- 29.Zorrilla EP, Cannon TD, Gur RE, Kessler J. Leukocytes and organ-nonspecific autoantibodies in schizophrenics and their siblings: markers of vulnerability or disease? Biol. Psychiatry. 1996;40:825–833. doi: 10.1016/0006-3223(95)00598-6. [DOI] [PubMed] [Google Scholar]
- 30.Núñez C, et al. Neutrophil Count Is Associated With Reduced Gray Matter and Enlarged Ventricles in First-Episode Psychosis. Schizophr. Bull. 2019;45:846–858. doi: 10.1093/schbul/sby113. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 31.Miller BJ, Gassama B, Sebastian D, Buckley P, Mellor A. Meta-analysis of lymphocytes in schizophrenia: clinical status and antipsychotic effects. Biol. Psychiatry. 2013;73:993–999. doi: 10.1016/j.biopsych.2012.09.007. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 32.Trtica Majnarić L, Guljaš S, Bosnić Z, Šerić V, Wittlinger T. Neutrophil-to-lymphocyte ratio as a cardiovascular risk marker may be less efficient in women than in men. Biomolecules. 2021;11:528. doi: 10.3390/biom11040528. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 33.Lin L, et al. Prognostic nomogram incorporating neutrophil-to-lymphocyte ratio for early mortality in decompensated liver cirrhosis. Int. Immunopharmacol. 2018;56:58–64. doi: 10.1016/j.intimp.2018.01.007. [DOI] [PubMed] [Google Scholar]
- 34.Sanguinete MMM, et al. Serum IL-6 and IL-8 Correlate with Prognostic Factors in Ovarian Cancer. Immunol. Invest. 2017;46:677–688. doi: 10.1080/08820139.2017.1360342. [DOI] [PubMed] [Google Scholar]
- 35.Ackland GL, et al. Autonomic regulation of systemic inflammation in humans: A multi-center, blinded observational cohort study. Brain Behav. Immun. 2018;67:47–53. doi: 10.1016/j.bbi.2017.08.010. [DOI] [PubMed] [Google Scholar]
- 36.Karageorgiou V, Milas GP, Michopoulos I. Neutrophil-to-lymphocyte ratio in schizophrenia: A systematic review and meta-analysis. Schizophr. Res. 2019;206:4–12. doi: 10.1016/j.schres.2018.12.017. [DOI] [PubMed] [Google Scholar]
- 37.Garcia-Rizo C, et al. Metabolic syndrome or glucose challenge in first episode of psychosis? Eur. Psychiatry. 2017;41:42–46. doi: 10.1016/j.eurpsy.2016.10.001. [DOI] [PubMed] [Google Scholar]
- 38.Phillips MR, et al. Prevalence, treatment, and associated disability of mental disorders in four provinces in China during 2001-05: an epidemiological survey. Lancet. 2009;373:2041–2053. doi: 10.1016/S0140-6736(09)60660-7. [DOI] [PubMed] [Google Scholar]
- 39.Kay SR, Fiszbein A, Opler LA. The positive and negative syndrome scale (PANSS) for schizophrenia. Schizophr. Bull. 1987;13:261–276. doi: 10.1093/schbul/13.2.261. [DOI] [PubMed] [Google Scholar]
- 40.Zamanpoor M. Schizophrenia in a genomic era: a review from the pathogenesis, genetic and environmental etiology to diagnosis and treatment insights. Psychiatric Genet. 2020;30:1–9. doi: 10.1097/YPG.0000000000000245. [DOI] [PubMed] [Google Scholar]
- 41.Buckley PF. Neuroinflammation and schizophrenia. Curr. Psychiatry Rep. 2019;21:1–3. doi: 10.1007/s11920-019-1050-z. [DOI] [PubMed] [Google Scholar]
- 42.van Mierlo HC, Schot A, Boks MP, de Witte LD. The association between schizophrenia and the immune system: Review of the evidence from unbiased ‘omic-studies. Schizophr. Res. 2020;217:114–123. doi: 10.1016/j.schres.2019.05.028. [DOI] [PubMed] [Google Scholar]
- 43.Sheshachalam A, Srivastava N, Mitchell T, Lacy P, Eitzen G. Granule protein processing and regulated secretion in neutrophils. Front. Immunol. 2014;5:448. doi: 10.3389/fimmu.2014.00448. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 44.Bhikram T, Sandor P. Neutrophil-lymphocyte ratios as inflammatory biomarkers in psychiatric patients. Brain Behav. Immun. 2022;105:237–246. doi: 10.1016/j.bbi.2022.07.006. [DOI] [PubMed] [Google Scholar]
- 45.Mazza MG, Lucchi S, Rossetti A, Clerici M. Neutrophil-lymphocyte ratio, monocyte-lymphocyte ratio and platelet-lymphocyte ratio in non-affective psychosis: A meta-analysis and systematic review. World J. Biol. Psychiatry. 2020;21:326–338. doi: 10.1080/15622975.2019.1583371. [DOI] [PubMed] [Google Scholar]
- 46.Xu Y, et al. Cigarette smoke (CS) and nicotine delay neutrophil spontaneous death via suppressing production of diphosphoinositol pentakisphosphate. Proc. Natl. Acad. Sci. USA. 2013;110:7726–7731. doi: 10.1073/pnas.1302906110. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 47.Steiner J, et al. Innate Immune Cells and C-Reactive Protein in Acute First-Episode Psychosis and Schizophrenia: Relationship to Psychopathology and Treatment. Schizophr. Bull. 2020;46:363–373. doi: 10.1093/schbul/sbz068. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 48.Kulaksizoglu B, Kulaksizoglu S. Relationship between neutrophil/lymphocyte ratio with oxidative stress and psychopathology in patients with schizophrenia. Neuropsychiatr. Dis. Treat. 2016;12:1999–2005. doi: 10.2147/NDT.S110484. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 49.Misiak B, et al. Immune-inflammatory markers and psychosis risk: A systematic review and meta-analysis. Psychoneuroendocrinology. 2021;127:105200. doi: 10.1016/j.psyneuen.2021.105200. [DOI] [PubMed] [Google Scholar]
- 50.Dawidowski, B. et al. The Role of Cytokines in the Pathogenesis of Schizophrenia. J. Clin. Med. 2021;10. [DOI] [PMC free article] [PubMed]
- 51.Yüksel RN, Ertek IE, Dikmen AU, Göka E. High neutrophil-lymphocyte ratio in schizophrenia independent of infectious and metabolic parameters. Nord. J. Psychiatry. 2018;72:336–340. doi: 10.1080/08039488.2018.1458899. [DOI] [PubMed] [Google Scholar]
- 52.Varsak N, AYDIN M, İbrahim E. The evaluation of neutrophil-lymphocyte ratio in patients with first episode psychosis. Family Pract. Palliat. Care. 2017;1:65–69. doi: 10.22391/920.287411. [DOI] [Google Scholar]
- 53.Dawidowski, B. et al. Effect of Antipsychotic Treatment on Neutrophil-to-Lymphocyte Ratio during Hospitalization for Acute Psychosis in the Course of Schizophrenia-A Cross-Sectional Retrospective Study. J. Clin. Med. 2021;11. [DOI] [PMC free article] [PubMed]
Associated Data
This section collects any data citations, data availability statements, or supplementary materials included in this article.
Data Availability Statement
The data that support the findings of this study are available on request from the corresponding author. The data are not publicly available due to privacy or ethical restrictions.