Abstract
Cognitive reappraisal is a well-studied emotion regulation technique that involves changing the meaning of stimuli. To be useful in everyday life, reappraisal’s effects would ideally generalize from previously reappraised stimuli to novel, but similar stimuli, saving individuals from needing to generate novel interpretations for similar stimuli. Here, 41 participants were asked to use reappraisal to down-regulate their response to negative pictures from one category (e.g., snakes), and to view negative pictures from another category (e.g., guns) as well as neutral pictures (e.g., plants). In a subsequent task, participants passively viewed novel pictures from all three categories (e.g., snakes, guns, and plants). EEG and subjective ratings of valence and arousal were collected in both tasks. In the reappraisal task, we did not find an effect of reappraisal on the LPP or arousal ratings, but reappraisal reduced ratings of picture unpleasantness. In the second task, negative pictures from the previously reappraised category elicited smaller LPPs than negative pictures from the previously viewed category, though there was no evidence that reappraisal generalized to subjective ratings of pictures. Therefore, at the electrocortical level, cognitive reappraisal may generalize to similar but novel stimuli encountered outside of the reappraisal context. Moreover, meaning change might be more effective in modulating electrocortical response following a delay and in the absence of deliberate attempts to down-regulate emotional response. Nonetheless, reappraisal’s effects appear to differ across levels of affective response when similar stimuli are encountered in the absence of willful attempts at reappraisal.
Keywords: cognitive reappraisal, emotion regulation, generalization, event-related potential (ERP), late positive potential (LPP), time
1. Introduction
In the early 2000s, the first lab-based studies on emotion regulation were performed. At first, investigations of emotion regulation aimed to determine whether people could willfully modulate their emotions using techniques like cognitive reappraisal, which involves changing the meaning of stimuli (Gross, 1998). Early work on reappraisal showed that after a few minutes of training, people could reduce their subjective and neurobiological response to negative stimuli (Hajcak & Nieuwenhuis, 2006; Moser et al., 2006; Ochsner et al., 2002, 2004). More recently, it has become apparent that while people can often reduce their negative emotions in the lab, effective application of emotion regulation in everyday life may be more challenging (e.g., Goldin et al., 2009; Kivity & Huppert, 2019). This has prompted a new generation of emotion regulation work that has aimed to understand the factors that modulate emotion regulation success and might affect its utility in everyday life. For example, in an applied context (i.e., outside of the lab), reappraisal is probably only useful if it can create lasting change in the emotional salience of a stimulus so that an individual does not have to enact reappraisal again at each stimulus encounter. Moreover, reappraisal’s usefulness in everyday life probably also depends on its ability to generalize to other, similar stimuli. That is, once an individual has learned how to think differently about a situation (e.g., speaking to strangers at a party), it would be useful if this reinterpretation persisted, so that the individual experienced less negative emotion in response to a subsequent, similar situation (e.g., another party). Without generalization, people would need to continually generate novel meanings that were only applicable to specific stimuli, limiting the utility of emotion regulation in everyday life.
The late positive potential (LPP) is a centroparietally maximal event-related potential that starts around 400 ms following stimulus onset and is larger for emotional compared to neutral stimuli (Cuthbert et al., 2000; Hajcak et al., 2010). In a number of studies, reappraisal has been shown to reduce the LPP and subjective ratings elicited by negative stimuli (Hajcak & Nieuwenhuis, 2006; MacNamara et al., 2009; Moran et al., 2013; Parvaz et al., 2012), though some studies have failed to observe an effect of reappraisal on the LPP (Bernat et al., 2011; Cao et al., 2020; Gan et al., 2015; Kudinova et al., 2016; Langeslag & Surti, 2017). The LPP has also been used to investigate the lasting effects of reappraisal. This work found that when participants performed a reappraisal task and then viewed the same pictures 30 minutes later without instructions to reappraise (i.e., passive view), previously reappraised pictures elicited smaller LPPs and more pleasant and less arousing ratings than pictures that had not been reappraised (MacNamara et al., 2011). Therefore, the effects of reappraisal appeared to persist across time to affect the same pictures at subsequent encounter. These results are in keeping with work from the fMRI literature, which has also suggested that the effects of reappraisal are durable (e.g., up to 24 hours later; Denny et al., 2015; Hermann et al., 2017). Critically, however, it is unknown whether reappraisal’s effects would persist across time to affect neurobiological response to similar but previously unseen stimuli – in other words, whether reappraisal would generalize.
Prior research on the generalizability of reappraisal has been limited to subjective ratings and has yielded contradictory findings. One study examined the generalizability of implementation intentions (Huang et al., 2020), which are a type of reappraisal in which participants form an intention to change the meaning of a particular stimulus if they encounter it. For example, participants in Huang and colleagues’ (2020) study formed the following implementation intention prior to viewing pictures of disgusting, bloody scenes: “If I see blood, then I will take the perspective of a physician” (Huang et al., 2020, pg. 2). While participants in the reappraisal group showed reduced valence and arousal ratings for bloody scenes, they also showed reduced valence and arousal ratings for disgusting, non-bloody scenes. In a second experiment, generalization was not observed for more dissimilar pictures (i.e., non-bloody frightening pictures). Therefore, this study suggested that when participants are engaged in reappraisal of one type of stimuli, effects might also be observed for closely related stimuli presented in the same task. In another study, participants reappraised negative pictures from one category (e.g., crying) and viewed negative pictures from another category (e.g., violence), prior to performing a passive picture viewing task that incorporated both previously viewed and novel stimuli from the reappraised category and from the viewed category (Adam et al., 2014). Novel pictures from the reappraised categories elicited similar ratings as pictures from the previously viewed categories in the passive viewing task, indicating no evidence of generalization to novel pictures from the previously reappraised category.
Therefore, using subjective ratings, there is limited evidence that reappraisal can generalize to unseen, similar stimuli that are encountered in the absence of an emotion regulation goal (e.g., in a subsequent, passive picture viewing task). Moreover, despite the large body of work on the electrocortical effects of reappraisal (e.g., Hajcak & Nieuwenhuis, 2006; Imburgio & MacNamara, 2019; MacNamara et al., 2009; Parvaz et al., 2012), prior work has not investigated whether reappraisal can generalize at the electrocortical level. Here, we set out to determine whether reappraisal would generalize to affect the LPP and subjective response to previously unseen pictures of the same category (i.e., with similar pictorial content) as those that had been reappraised, compared to previously unseen pictures from a category that had been viewed, but not reappraised. Based on prior work showing that reappraisal can reduce the LPP and subjective ratings of negative pictures (e.g., Hajcak & Nieuwenhuis, 2006; MacNamara et al., 2009; Parvaz et al., 2012), we expected that reappraisal would reduce the LPP and subjective ratings of picture unpleasantness and arousal in the reappraisal task. Based on prior work showing that the effect of reappraisal can persist across time to affect the same pictures when seen later in a passive picture viewing task (MacNamara et al., 2011), we also expected that pictures from the same category as those that had been reappraised would elicit smaller LPPs in the generalization task. In addition, based on prior work demonstrating that reappraisal’s effects on picture ratings might generalize to similar negative, non-reappraised stimuli (Huang et al., 2020) and that reappraisal’s effects on ratings are durable across time (MacNamara et al., 2011), we expected that in the generalization task, we would observe less unpleasant and less arousing ratings for pictures that resembled those that had previously been reappraised compared to those that had been previously viewed normally. If our hypotheses were confirmed, this would suggest that when people learn new ways of thinking about negative stimuli, these reappraisals can generalize to affect response to other, subsequently encountered, similar stimuli without the need for additional, novel interpretations of stimulus meaning.
2. Materials and Methods
2.1. Participants
Participants were 41 undergraduates who completed the study for course credit; sample size was determined by the a priori decision to run the study for two semesters. Data was not available for one participant due to technical difficulties, leaving 40 participants available for analyses (24 female; M = 18.65 years, SD = .86). Post-hoc sensitivity analyses using G*Power (Faul et al., 2009) showed that with a final sample size of n = 40, power 1- β = .80, and α = .05, the study was powered to detect a small-to-medium effect size of f = .17.
Study procedures were in compliance with the Helsinki Declaration of 1975 (as revised in 1983) and approved by the Texas A&M Institutional Board.
2.2. Materials
Forty-eight images1 were selected from the International Affective Picture Systems (IAPS; Lang et al., 2008) and Emotional Picture Set (EmoPicS; Wessa et al., 2010). Sixteen of these images were neutral and depicted pictures of plants and 32 of these images were negative, with 16 of them depicting guns and 16 depicting snakes2.
Participants used the Self-Assessment Manikin 9-point scales (SAM; Bradley & Lang, 1994) to rate the valence and arousal level of each picture, with higher numbers indicating that pictures were more pleasant and more arousing.
2.3. Procedure
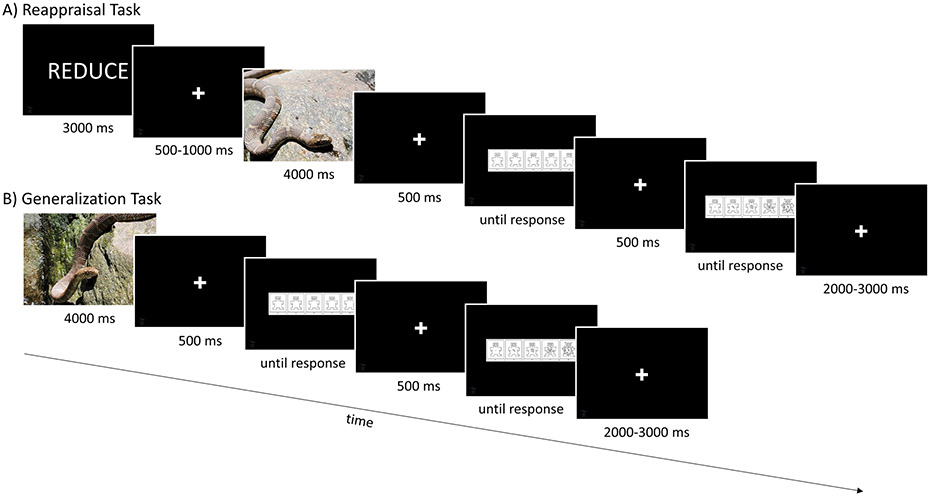
While continuous electroencephalography (EEG) was recorded, participants performed reappraisal task followed immediately by a generalization (i.e., passive picture viewing) task (Figure 1). In between the two tasks, the experimenter entered the room and read instructions for the next task. Pictures were presented in color and filled the computer monitor (measured diagonally at 48.26 cm). Participants sat approximately 60 cm from the monitor, with the picture covering around 40 degrees of visual angle (i.e., horizontally and vertically).
Figure 1.
Example trials from the A) reappraisal task and B) generalization task.
2.3.1. Reappraisal Task
The reappraisal task was modeled after those previously used (Hajcak & Nieuwenhuis, 2006; Imburgio & MacNamara, 2019). Participants were told that they would be viewing neutral and negative pictures. On each trial, participants saw the word, “VIEW,” “NORMAL,” or “REDUCE” for 3000 ms. Next, they viewed a white fixation cross on a black background for 500-1000 ms. This was followed by the presentation of a negative or neutral picture for 4000 ms. Participants were told that when they saw the word “VIEW” or “NORMAL”, they should view the upcoming picture as they normally would, without trying to change their emotional response. They were told that if they saw the word “REDUCE”, they should try to reduce their negative feelings about the picture by changing its meaning. For example, if viewing a picture of a man pointing a gun at someone, participants could tell themselves that this is a scene from a movie. Alternatively, they could tell themselves that the man calmed down and no one was hurt. Similarly, if viewing a picture of a snake, participants could tell themselves that the snake was not real or that it was not a dangerous snake. At the beginning of the task, participants completed six practice trials to become familiar with the paradigm. On each “REDUCE” trial, the experimenter asked the participant how they had attempted to reduce their emotional response and provided guidance or feedback as necessary (i.e., to ensure participants understood directions and were using cognitive reappraisal; Parvaz et al., 2012).
The task was broken into three blocks; in each block, participants performed eight trials in which neutral pictures were preceded by the word “VIEW” (neutral view); eight trials in which negative pictures were preceded by the word “NORMAL” (negative normal) and eight trials in which negative pictures were preceded by the word “REDUCE” (negative reduce). After picture offset, participants rated the valence and arousal of each picture; picture ratings were self-paced. Negative picture categories (i.e., snakes, guns) were assigned to condition and counterbalanced across two versions of the task. That is, in one version of the task, pictures of snakes were assigned to the negative normal condition and pictures of guns were assigned to the negative reduce condition. In the other version of the task, pictures of guns were assigned to the negative normal condition and pictures of snakes were assigned to the negative reduce condition. In both versions, pictures of plants were assigned to the neutral view condition. Each picture (i.e., eight guns, eight snakes, eight plants) was shown three times, with conditions presented in randomized order, resulting in 24 neutral view trials, 24 negative normal trials and 24 negative reduce trials. In between trials, participants viewed a white fixation cross presented on a black background for 2000-3000 ms. Participants received a self-paced break following every block of 24 trials.
2.3.2. Generalization Task
The generalization task was a passive picture viewing task in which participants viewed neutral and negative pictures, prior to rating each picture on valence and arousal. Task timing and structure was the same as in the reappraisal task, except that participants were asked to simply view pictures and rate them; no instructions (i.e., REDUCE, NORMAL, VIEW) were presented.
Participants viewed novel pictures from each picture category (i.e., guns, plants, and snakes) in the reappraisal task. Pictures were presented for 4000 ms and participants viewed a white fixation cross presented on a black background for 2000-3000 ms in between each trial. Although participants were simply asked to view all pictures (i.e., no reappraisal instructions were given), we classified trials according to picture category assignment to condition in the reappraisal task. That is, if pictures of snakes had been assigned to the negative normal condition in the reappraisal task, trials with pictures of snakes were considered negative normal trials in the generalization task. Likewise, if pictures of guns had been assigned to the negative reduce condition in the reappraisal task, they were considered negative reduce trials in the generalization task. This allowed us to examine whether pictures that were similar to those that had previously been reappraised would elicit smaller LPPs and more pleasant and less arousing ratings than pictures that were dissimilar/resembled pictures that had not been previously reappraised. Pictures of plants were considered neutral view trials, as in the reappraisal task. Following picture offset, participants rated each picture on valence and arousal (ratings were self-paced). Each picture (i.e., eight guns, eight snakes, eight plants) was shown three times, resulting in 24 neutral view trials, 24 negative normal trials and 24 negative reduce trials. The task was broken into three blocks, with eight trials of each condition shown in each block. Participants received a self-paced break following every block of 24 trials.
2.4. EEG Recording and Data Processing
Continuous EEG was recorded using an ActiCap and the ActiCHamp amplifier system (Brain Products GmbH, Gilching Germany). Thirty-two electrode sites were used based on the 10/20 system. The electrooculogram (EOG) was recorded from four facial electrodes: two that were placed approximately 1 cm above and below the right eye, forming a bipolar channel to measure vertical eye movement and blinks and two that were placed approximately 1 cm beyond the outer edges of each eye, forming a bipolar channel to measure horizontal eye movements. The EEG data were digitized at 24-bit resolution and a sampling rate of 1000 Hz.
EEG data were processed offline using BrainVision Analyzer 2 software (Brain Products GmbH, Gilching Germany). Data were processed separately for the reappraisal and generalization tasks. For each task, data were segmented for each trial beginning 200 ms prior to stimulus onset and lasting throughout the entire duration of picture presentation (4200 ms in total); baseline correction for each trial was performed using the 200 ms pre-stimulus period. The signal from each electrode was re-referenced to the average of the left and right mastoids (TP9/10) and band-pass filtered with high-pass and low-pass filters of 0.01 and 30 Hz, respectively. Eyeblink and ocular corrections used the method developed by Miller and colleagues (1988). Artifact analysis was used to identify a voltage step of more than 50.0 μV between sample points, a voltage difference of 300.0 μV within a trial, and a maximum voltage difference of less than 0.50 μV within 100ms intervals. Trials were also inspected visually for any remaining artifacts, and data from individual channels containing artifacts were rejected on a trial-to-trial basis. The LPP during both tasks was scored at electrodes, Cz and Pz, from 600-4000 ms following picture onset (Moser et al., 2010; Qi et al., 2020). LPP amplitude was calculated using the mean amplitude export function in BrainVision Analyzer 2. The average percent of trials rejected per condition and electrode in the reappraisal task was as follows: neutral view Cz, M = 7.60%, SD = 10.75; negative normal Cz, M = 7.08%, SD = 10.04; negative reduce Cz, M = 5.52%, SD = 8.56; neutral view Pz, M = 8.54%, SD = 11.48%; negative normal Pz, M = 7.50%, SD = 10.97; negative reduce Pz, M = 6.04%, SD = 10.88. The average percent of trials rejected per condition and electrode in the generalization task was as follows: neutral view Cz, M = 6.35%, SD = 9.58; negative normal Cz, M = 6.15%, SD = 8.39; negative reduce Cz, M = 8.13%, SD = 9.05; neutral view Pz, M = 7.08%, SD = 13.15; negative normal Pz, M = 6.46%, SD = 10.20; negative reduce Pz, M = 9.06%, SD = 13.27. Percent of trials rejected did not significantly differ by condition in the reappraisal task (Cz, p = .102; Pz, p = .050) or in the generalization task (Cz, p = .117; Pz, p = .059).
2.5. Data Analyses
The LPP was analyzed using a 3 (condition: neutral view, negative normal, negative reduce) X 2 (electrode: Cz, Pz) repeated measures analysis of variance (ANOVA), performed separately for each task. Significant effects were followed up using repeated measures ANOVAs and Bonferroni-corrected paired t-tests, as appropriate, with effect sizes presented as partial eta squared (ηp2) for ANOVA results and Cohen’s d for follow-up t-tests. Only effects pertinent to the aim of this study are reported – i.e., main effects or interactions involving the factor, condition. Valence and arousal ratings were analyzed using separate repeated measures ANOVAs, with the single factor, condition (neutral view, negative normal, negative reduce). Greenhouse–Geisser correction was used when necessitated by violation of the assumption of sphericity. Bonferroni-corrected correlations were performed between subjective ratings and the LPP, separately for each task and condition. Internal consistency of the LPP was assessed using even-odd reliability, in which correlations were performed between averages created separately for even and odd trials, and for each condition and task. The Spearman-Brown formula was used to correct these correlations (Nunnally, 1978). All analyses were performed using SPSS statistical software version 25.0 (IBM, Armonk, NY).
3. Results
Table 1 presents means and standard deviations for the LPP and ratings, shown separately for each task and condition. Figure 2 depicts grand-averaged waveforms at Cz and Pz, where the LPP was scored, as well as headmaps showing the scalp distribution of condition difference scores from 600-4000 ms following picture onset, shown separately for the reappraisal and generalization tasks.
Table 1.
Means (and standard deviations) for the LPP (at Cz and Pz), valence and arousal ratings, shown separately for each condition and task.
| Reappraisal Task | Generalization Task | |||||
|---|---|---|---|---|---|---|
| Neutral View | Negative Normal | Negative Reduce | Neutral View | Negative Normal | Negative Reduce | |
| Cz (μV) | 2.91 (6.42) | 10.09 (6.97) | 9.83 (7.14) | .90 (6.71) | 8.56 (4.88) | 5.91 (6.09) |
| Pz (μV) | .08 (5.94) | 7.42 (6.07) | 6.27 (5.62) | −.25 (6.02) | 7.64 (4.79) | 6.98 (6.28) |
| Valence | 6.14 (1.12) | 4.27 (.92) | 4.69 (1.17) | 5.98 (1.16) | 4.06 (.99) | 4.03 (1.45) |
| Arousal | 1.49 (.60) | 3.00 (1.20) | 2.83 (1.15) | 1.52 (.71) | 3.05 (1.50) | 3.46 (1.79) |
Note: Valence and arousal were rated from 1 to 9, with larger numbers indicating more pleasant and more arousing ratings.
Figure 2.
Grand-averaged waveforms at central and parietal electrodes where the LPP was scored, and headmaps depicting condition differences, shown separately for the A) reappraisal task and B) generalization task.
Even-odd reliability for the LPP at Cz and Pz, computed separately for each task and condition, ranged from r = .48 to .75, ps < .046, with the exception of the following conditions and electrodes: reappraisal task, neutral view Pz, r = .46, p = .064 and negative normal Pz, r = .46, p = .061; generalization task, negative normal Cz, r = .44, p = .079 and negative normal Pz, r = .42, p = .095.
3.1. Reappraisal Task
3.1.1. LPP
There was a main effect of condition, F(2, 78) = 41.63, p <.001, ηp2 = .516. Using a Bonferroni-corrected p value of .05/3 = .017, follow-up t-tests showed that negative normal pictures, t(39) = 8.14, p <.001, d =1.29 and negative reduce pictures, t(39) = 8.06, p <.001, d = 1.28 elicited larger LPPs than neutral view pictures (negative reduce versus negative normal pictures, p = .448). There was no significant interaction between condition X electrode, p = .483.
3.1.2. Ratings
There was a main effect of condition for valence ratings, F(1.64, 63.92) = 38.76, p <.001, ηp2 = .50. Using a Bonferroni-corrected p value of .05/3 = .017, follow-up t-tests showed that negative reduce pictures were rated as less unpleasant than negative normal pictures, t(39) = 2.60, p = .013, d = .41. In addition, negative normal pictures, t(39) = 7.78, p <.001, d =1.23, and negative reduce pictures, t(39) = 5.70, p <.001, d = .90 were rated as more unpleasant than neutral view pictures.
There was a main effect of condition for arousal ratings, F(2, 78) = 40.70, p <.001, ηp2 = .51. Using a Bonferroni-corrected p value of .05/3 = .017, follow-up t-tests revealed that negative normal pictures, t(39) = 7.73, p <.001, d =1.22, and negative reduce pictures, t(39) = 6.76, p <.001, d =1.07, were rated as more emotionally arousing than neutral view pictures (negative reduce versus negative normal pictures, p = .272).
Using a Bonferroni-corrected p value of .05/6 = .008, there were no significant correlations between either valence or arousal ratings and the LPP (ps > .023).
3.2. Generalization task
3.2.1. LPP
There was a main effect of condition, F(2, 78) = 49.51, p <.001, ηp2 = .56. This was qualified by a significant interaction between electrode X condition, F(2, 78) = 4.05, p =.021, ηp2 = .09. Follow-up ANOVAs performed separately at Cz and Pz showed that there was a significant effect of condition at Cz, F(2, 78) = 35.79, p <.001, ηp2 = .48. Using a Bonferroni-corrected p value of .05/3 = .017, follow-up t-tests showed negative reduce pictures elicited smaller LPPs than negative normal pictures, t(39) = 3.01, p =.005, d = .48. Both negative normal pictures, t(39) = 8.08, p <.001, d = 1.28 and negative reduce pictures, t(39) = 5.39, p <.001, d = .85, elicited larger LPPs than neutral view pictures at Cz. There was also an effect of condition at Pz, F(2, 78) = 43.55, p <.001, ηp2 = .53, but no significant difference between the LPP elicited by negative normal and negative reduce pictures, p = .493. Bonferroni-corrected t-tests using corrected p value of .05/3 = .017 indicated that both negative normal pictures, t(39) = 8.69, p <.001, d = 1.37, and negative reduce pictures, t(39) = 7.61, p <.001, d = 1.20, elicited larger LPPs than neutral view pictures at Pz.
3.2.2. Ratings
There was a main effect of condition for valence ratings, F(1.53, 59.46) = 35.45, p <.001, ηp2 = .48. Using a Bonferroni-corrected p value of .05/3 = .017, follow-up t-tests showed that negative normal pictures, t(39) = 7.45, p <.001, d = 1.18, and negative reduce pictures t(39) = 5.99, p <.001, d = .95 were rated as more unpleasant than neutral view pictures (negative normal versus negative reduce, p = .846).
There was a main effect of condition for arousal ratings, F(1.51, 58.85) = 35.16, p <.001, ηp2 = .47. Using a Bonferroni-corrected p value of .05/3 = .017, follow-up t-tests showed that negative normal pictures, t(39) = 6.47, p <.001, d = 1.02 and negative reduce pictures, t(39) = 6.45, p <.001, d = 1.02 were rated as more arousing than neutral view pictures. The difference between ratings for negative reduce pictures and negative normal pictures did not reach significance when corrected for multiple comparisons, p = .030.
Using a Bonferroni-corrected p value of .05/6 = .008, there were no significant correlations between either valence or arousal ratings and the LPP (ps > .020).
4. Discussion
The current study sought to determine whether reappraisal would reduce the LPP and subjective ratings of similar but previously unseen pictures encountered in a passive picture viewing task. First, participants were asked to reappraise negative pictures from one category (e.g., snakes) and to view negative pictures from another category (e.g., guns), as well as neutral pictures (plants). Next, participants performed a passive viewing task in which they saw unseen pictures from the previously reappraised category (e.g., snakes) and unseen pictures from the previously viewed category (e.g., guns), as well as unseen pictures from the neutral category (plants). Results showed that LPP was reduced for novel pictures from the previously reappraised category compared to novel pictures from the previously viewed category. Therefore, we observed reduced processing of pictures that resembled those that had previously been reappraised. This suggests that at least at the electrocortical level, people can spontaneously apply cognitive reappraisal to future encounters with similar stimuli, without the need for additional training or interpretations.
Although we observed an effect of reappraisal in the passive viewing/generalization task, we did not observe an effect of reappraisal on the LPP during the initial reappraisal task. Reappraisal also failed to modulate arousal ratings in the first task, even though it did reduce picture unpleasantness (valence) ratings (Li et al., 2018; Sarlo et al., 2013; Van Cauwenberge et al., 2017). One possible explanation is that pictures in the negative reduce condition may have taken on increased significance related to their unique meaning within the reappraisal task. For example, during tasks in which participants are asked to count a particular type of stimuli (Schupp et al., 2007) or press a button each time a certain type of stimulus is presented (Ferrari et al., 2008; Weinberg et al., 2012), the LPP is increased to those “target” stimuli (see also Gable & Adams, 2013; for a review, see Hajcak & Foti, 2020). Therefore, because they were the only pictures that required participants to respond (by attempting to reduce elicited negative emotion), negative reduce pictures may have taken on a target-like status in the current task, which could have led to an increase in the LPP that may have counteracted any down-regulatory effect of meaning change. Though prior studies have observed an effect of reappraisal on the LPP (Krompinger et al., 2008; MacNamara et al., 2009; Moser et al., 2006; Parvaz et al., 2012; Thiruchselvam et al., 2011), several other studies have also failed to observe an effect of reappraisal on the LPP (Bernat et al., 2011; Cao et al., 2020; Gan et al., 2015; Kudinova et al., 2016; Langeslag & Surti, 2017). Nonetheless, while the present study was well powered to detect small-to-medium effects (as suggested by sensitivity analyses), future work may wish to replicate our results in order to confirm the absence of an effect of reappraisal on the LPP in the initial task, and also to determine whether – even if reappraisal significantly modulates the LPP in the initial task – the effect of reappraisal might be larger in a subsequent passive viewing task with similar pictures, in which effortful reappraisal is not required.
While we found an effect of reappraisal for the LPP in the generalization task, we did not observe an effect for valence or arousal ratings, despite evidence that reappraisal had successfully modulated valence in the first task. The discrepancy between results observed for the LPP versus subjective ratings underscores that these measures reflect different levels of affective response. Subjective ratings are more downstream from electrocortical response: ratings are made after picture offset, and reflect a number of intervening, higher order cognitive processes that may not be evident in psychophysiological responses that are more proximal to stimulus presentation. Therefore, subjective ratings necessarily reflect other cognitive and affective processes in addition to picture-elicited affective response, which could also explain why other prior work also failed to observe generalization of reappraisal down-regulation using subjective ratings (Adam et al., 2014). Future work may wish to measure additional levels of affective response (e.g., skin conductance or eyeblink startle; other types of subjective ratings) in order to obtain a more complete picture of the ways in which reappraisal generalizes. In addition, given that reappraisal has been shown to modulate arousal ratings in prior work (e.g., Hajcak & Nieuwenhuis, 2006; Thiruchselvam et al., 2011) and such an effect was not observed here, conclusions regarding reappraisal’s failure to generalize at the subjective level should be tempered and assessed again in the context of a reappraisal task that successfully modulates arousal ratings. Of note, arousal ratings for negative pictures were rather low in the current task (averaging around 3, whereas the normed ratings for the same images were around 6), potentially because all of the stimuli in a given condition depicted similar picture content (i.e., all snakes or all guns). Low arousal ratings may have led to a floor effect that impeded our ability to observe an effect of reappraisal on arousal (i.e., ratings have been too low to permit an observable reduction in arousal during reappraisal).
Only one study to-date found that reappraisal generalizes (Huang et al., 2020), as measured using valence and arousal ratings. However, this study only examined generalization to similar types of stimuli within a single reappraisal session (i.e., not in a subsequent, passive viewing task). Furthermore, this study examined reappraisal implementation intentions rather than reappraisal per se. That is, participants in this study were trained to set an overarching reappraisal goal, rather than to generate novel interpretations for each stimulus. Therefore, it is possible that for reappraisal to generalize at the level of subjective response, it must be implemented in a broader, less stimulus-specific manner. More flexible forms of reappraisal also appear to generalize to a wider array of stimulus content (i.e., stimulus categories that are farther afield; Huang et al., 2020). As such, broader reappraisal goals and/or other ways of bolstering reappraisal (e.g., longer training sessions with more varied types of stimuli) might help extend the benefits of reappraisal to more dissimilar stimuli and to multiple levels of affective response.
Although reappraisal is a cognitively demanding emotion regulation technique that requires the generation of novel stimulus interpretations and maintenance of these interpretations in working memory over time (Gan et al., 2017), the current results suggest that it may result in generalizable changes to stimulus interpretation. Moreover, meaning change may have a stronger effect on stimulus processing when participants are not trying to reduce their emotional response to stimuli, and have already practiced enacting changes to stimulus meaning. Therefore, initial investment of effort in reappraisal should translate into lasting effects that extend to novel situations that have not previously been reappraised (at least at the electrocortical level). More research is needed to understand the boundary conditions under which generalization of cognitive reappraisal occurs, as well as the durability of generalization effects (e.g., whether reappraisal’s effects decay at a slower rate for trained versus novel items). Additionally, more work is needed to understand whether reappraisal generalizes at the subjective level. Nonetheless, generalization of cognitive reappraisal to novel but similar stimuli appears to be possible, as evidenced by the LPP.
Supplementary Material
Acknowledgements
Annmarie MacNamara was supported in part by NIMH R01 MH1250983 during the preparation and editing of this manuscript.
Footnotes
The numbers of the IAPS pictures used were the following: negative (1022, 1026, 1030, 1033, 1040, 1050, 1051, 1052, 1080, 1090, 1110, 1113, 1114, 1120, 6190, 6200, 6210, 6230, 6231, 6240, 6241, 6242, 6243, 6244, 6250, 6260, 6410, 6560, 6571, 6610). The numbers of the EmoPics pictures used were the following: negative (322, 324) and neutral (334, 335, 336, 338, 339, 340, 342, 343, 345, 346, 350, 352, 354, 355, 356).
Although picture category assignment to condition was counterbalanced across participants (i.e., n = 20 participants reappraised pictures of snakes and n = 20 participants reappraised pictures of guns), we ran one-way analyses of variance (ANOVA) to determine any differences in normed valence and arousal ratings between picture categories. Results showed a significant effect of picture category for arousal ratings, F(2,47) = 255.09, p < .001. Post hoc analyses using a Bonferroni corrected p value of .05/3 = .017 indicated that normed ratings were more arousing for pictures of snakes [M = 6.02, SD = .48; t(20.55) = 26.00, p < .001] and guns (M = 5.96, SD = .75; t(17.45) = 17.59, p < .001] compared to ratings for pictures of plants (M = 2.55, SD = .21). There was no significant difference in normed arousal ratings for pictures of snakes compared to guns (p = .809). At the omnibus level, there was also a significant effect of picture category for valence ratings, F(2,47) = 151.87, p < .001. Post hoc analyses using a Bonferroni corrected p value of .05/3 = .017 also indicated that ratings were more unpleasant for pictures of snakes (M = 3.98, SD = .32; t(22.02) = 13.54, p < .001] and pictures of guns [M = 2.95, SD = .52; t(17.84) = 16.52, p < .001] compared to pictures of plants (M = 5.19, SD = .16). In addition, normed ratings were more unpleasant for pictures of guns compared to snakes [t(25.04) = 6.73, p < .001]. The Supplementary Material section presents the analyses including task version as a factor.
References
- Adam R, Schönfelder S, Forneck J, & Wessa M (2014). Regulating the blink: Cognitive reappraisal modulates attention. Frontiers in Psychology, 5, 143. 10.3389/fpsyg.2014.00143 [DOI] [PMC free article] [PubMed] [Google Scholar]
- Bernat EM, Cadwallader M, Seo D, Vizueta N, & Patrick CJ (2011). Effects of instructed emotion regulation on valence, arousal, and attentional measures of affective processing. Developmental Neuropsychology, 36(4), 493–518. 10.1080/87565641.2010.549881 [DOI] [PubMed] [Google Scholar]
- Bradley MM, & Lang PJ (1994). Measuring emotion: The self-assessment manikin and the semantic differential. Journal of Behavior Therapy and Experimental Psychiatry, 25(1), 49–59. 10.1016/0005-7916(94)90063-9 [DOI] [PubMed] [Google Scholar]
- Cao D, Li Y, & Niznikiewicz MA (2020). Neural characteristics of cognitive reappraisal success and failure: An ERP study. Brain and Behavior, 10(4), e01584. 10.1002/brb3.1584 [DOI] [PMC free article] [PubMed] [Google Scholar]
- Cuthbert BN, Schupp HT, Bradley MM, Birbaumer N, & Lang PJ (2000). Brain potentials in affective picture processing: Covariation with autonomic arousal and affective report. Biological Psychology, 52, 95–111. https://doi.org/doi: 10.1016/S0301-0511(99)00044-7 [DOI] [PubMed] [Google Scholar]
- Denny BT, Inhoff MC, Zerubavel N, Davachi L, & Ochsner KN (2015). Getting Over It: Long-Lasting Effects of Emotion Regulation on Amygdala Response. Psychological Science, 26(9), 1377–1388. 10.1177/0956797615578863 [DOI] [PMC free article] [PubMed] [Google Scholar]
- Faul F, Erdfelder E, Buchner A, & Lang A-G (2009). Statistical power analyses using G*Power 3.1: Tests for correlation and regression analyses. Behavior Research Methods, 41(4), 1149–1160. 10.3758/BRM.41.4.1149 [DOI] [PubMed] [Google Scholar]
- Ferrari V, Codispoti M, Cardinale R, & Bradley MM (2008). Directed and motivated attention during processing of natural scenes. Journal of Cognitive Neuroscience, 20, 1753–1761. [DOI] [PubMed] [Google Scholar]
- Gable PA, & Adams DL (2013). Nonaffective motivation modulates the sustained LPP (1,000–2,000 ms). Psychophysiology, 50(12), 1251–1254. 10.1111/psyp.12135 [DOI] [PubMed] [Google Scholar]
- Gan S, Yang J, Chen X, & Yang Y (2015). The electrocortical modulation effects of different emotion regulation strategies. Cognitive Neurodynamics, 9(4), 399–410. 10.1007/s11571-015-9339-z [DOI] [PMC free article] [PubMed] [Google Scholar]
- Gan S, Yang J, Chen X, Zhang X, & Yang Y (2017). High working memory load impairs the effect of cognitive reappraisal on emotional response: Evidence from an event-related potential study. Neuroscience Letters, 639, 126–131. 10.1016/j.neulet.2016.12.069 [DOI] [PubMed] [Google Scholar]
- Goldin PR, Manber T, Hakimi S, Canli T, & Gross JJ (2009). Neural bases of social anxiety disorder: Emotional reactivity and cognitive regulation during social and physical threat. Archives of General Psychiatry, 66, 170–180. [DOI] [PMC free article] [PubMed] [Google Scholar]
- Gross JJ (1998). Antecedent-and response-focused emotion regulation: Divergent consequences for experience, expression, and physiology. Journal of Personality and Social Psychology, 74, 224–237. [DOI] [PubMed] [Google Scholar]
- Hajcak G, & Foti D (2020). Significance?... Significance! Empirical, methodological, and theoretical connections between the late positive potential and P300 as neural responses to stimulus significance: An integrative review. Psychophysiology, 57(7), e13570. 10.1111/psyp.13570 [DOI] [PubMed] [Google Scholar]
- Hajcak G, MacNamara A, & Olvet DM (2010). Event-related potentials, emotion, and emotion regulation: An Integrative Review. Developmental Neuropsychology, 35, 129–155. https://doi.org/doi: 10.1080/87565640903526504 [DOI] [PubMed] [Google Scholar]
- Hajcak G, & Nieuwenhuis S (2006). Reappraisal modulates the electrocortical response to unpleasant pictures. Cognitive, Affective & Behavioral Neuroscience, 6, 291–297. https://doi.org/doi: 10.3758/CABN.6.4.291 [DOI] [PubMed] [Google Scholar]
- Hermann A, Kress L, & Stark R (2017). Neural correlates of immediate and prolonged effects of cognitive reappraisal and distraction on emotional experience. Brain Imaging and Behavior, 11(5), 1227–1237. 10.1007/s11682-016-9603-9 [DOI] [PubMed] [Google Scholar]
- Huang X, Chen S, Gao W, Yang J, & Yuan J (2020). Emotion regulation by implementation intention is generalizable to unspecified situations: The nature of the underlying goal matters. Acta Psychologica, 210, 103144. 10.1016/j.actpsy.2020.103144 [DOI] [PubMed] [Google Scholar]
- Imburgio MJ, & MacNamara A (2019). Cognitive reappraisal in an unpredictable world: Prior context matters. International Journal of Psychophysiology: Official Journal of the International Organization of Psychophysiology, 146, 173–179. 10.1016/j.ijpsycho.2019.09.003 [DOI] [PMC free article] [PubMed] [Google Scholar]
- Kivity Y, & Huppert JD (2019). Emotion regulation in social anxiety: A systematic investigation and meta-analysis using self-report, subjective, and event-related potentials measures. Cognition & Emotion, 33(2), 213–230. 10.1080/02699931.2018.1446414 [DOI] [PubMed] [Google Scholar]
- Krompinger JW, Moser JS, & Simons RF (2008). Modulations of the electrophysiological response to pleasant stimuli by cognitive reappraisal. Emotion, 8, 132–137. [DOI] [PubMed] [Google Scholar]
- Kudinova AY, Owens M, Burkhouse KL, Barretto KM, Bonanno GA, & Gibb BE (2016). Differences in emotion modulation using cognitive reappraisal in individuals with and without suicidal ideation: An ERP study. Cognition & Emotion, 30(5), 999–1007. 10.1080/02699931.2015.1036841 [DOI] [PubMed] [Google Scholar]
- Lang PJ, Bradley MM, & Cuthbert BN (2008). International affective picture system (IAPS): Affective ratings of pictures and instruction manual, Technical Report A-8. University of Florida. [Google Scholar]
- Langeslag SJE, & Surti K (2017). The effect of arousal on regulation of negative emotions using cognitive reappraisal: An ERP study. International Journal of Psychophysiology: Official Journal of the International Organization of Psychophysiology, 118, 18–26. 10.1016/j.ijpsycho.2017.05.012 [DOI] [PubMed] [Google Scholar]
- Li F, Yin S, Feng P, Hu N, Ding C, & Chen A (2018). The cognitive up- and down-regulation of positive emotion: Evidence from behavior, electrophysiology, and neuroimaging. Biological Psychology, 136, 57–66. 10.1016/j.biopsycho.2018.05.013 [DOI] [PubMed] [Google Scholar]
- MacNamara A, Foti D, & Hajcak G (2009). Tell me about it: Neural activity elicited by emotional stimuli and preceding descriptions. Emotion, 9, 531–543. [DOI] [PubMed] [Google Scholar]
- MacNamara A, Ochsner KN, & Hajcak G (2011). Previously reappraised: The lasting effect of description type on picture-elicited electrocortical activity. Social Cognitive and Affective Neuroscience, 6(3), 348–358. 10.1093/scan/nsq053 [DOI] [PMC free article] [PubMed] [Google Scholar]
- Miller GA, Gratton G, & Yee CM (1988). Generalized implementation of an eye movement correction procedure. Psychophysiology, 25, 241–243.https://doi.org/doi: 10.1111/j.1469-8986.1988.tb00999.x [DOI] [Google Scholar]
- Moran TP, Jendrusina AA, & Moser JS (2013). The psychometric properties of the late positive potential during emotion processing and regulation. Brain Research, 1516, 66–75. 10.1016/j.brainres.2013.04.018 [DOI] [PubMed] [Google Scholar]
- Moser JS, Hajcak G, Bukay E, & Simons RF (2006). Intentional modulation of emotional responding to unpleasant pictures: An ERP study. Psychophysiology, 43, 292–296. https://doi.org/doi: 10.1111/j.1469-8986.2006.00402.x [DOI] [PubMed] [Google Scholar]
- Moser JS, Most SB, & Simons RF (2010). Increasing negative emotions by reappraisal enhances subsequent cognitive control: A combined behavioral and electrophysiological study. Cognitive, Affective & Behavioral Neuroscience, 10(2), 195–207. 10.3758/CABN.10.2.195 [DOI] [PubMed] [Google Scholar]
- Nunnally JC (1978). Psychometric theory. McGraw-Hill. [Google Scholar]
- Ochsner KN, Bunge SA, Gross JJ, & Gabrieli JDE (2002). Rethinking Feelings: An fMRI Study of the Cognitive Regulation of Emotion. Journal of Cognitive Neuroscience, 14(8), 1215–1229. 10.1162/089892902760807212 [DOI] [PubMed] [Google Scholar]
- Ochsner KN, Ray RD, Cooper JC, Robertson ER, Chopra S, Gabrieli JDE, & Gross JJ (2004). For better or for worse: Neural systems supporting the cognitive down- and up-regulation of negative emotion. NeuroImage, 23(2), 483–499. 10.1016/j.neuroimage.2004.06.030 [DOI] [PubMed] [Google Scholar]
- Parvaz MA, MacNamara A, Goldstein RZ, & Hajcak G (2012). Event-related induced frontal alpha as a marker of lateral prefrontal cortex activation during cognitive reappraisal. Cognitive, Affective, & Behavioral Neuroscience, 12, 730–740. https://doi.org/doi: 10.3758/s13415-012-0107-9 [DOI] [PMC free article] [PubMed] [Google Scholar]
- Qi S, Basanovic J, Wang L, Xiang S, Hu W, & Yi X (2020). Regulation of negative emotions through positive reappraisal and distancing in high-trait-anxious women. Journal of Affective Disorders, 267, 191–202. 10.1016/j.jad.2020.02.027 [DOI] [PubMed] [Google Scholar]
- Sarlo M, Übel S, Leutgeb V, & Schienle A (2013). Cognitive reappraisal fails when attempting to reduce the appetitive value of food: An ERP study. Biological Psychology, 94(3), 507–512. 10.1016/j.biopsycho.2013.09.006 [DOI] [PubMed] [Google Scholar]
- Schupp HT, Stockburger J, Codispoti M, Junghöfer M, Weike AI, & Hamm AO (2007). Selective Visual Attention to Emotion. Journal of Neuroscience, 27(5), 1082–1089. 10.1523/JNEUROSCI.3223-06.2007 [DOI] [PMC free article] [PubMed] [Google Scholar]
- Thiruchselvam R, Blechert J, Sheppes G, Rydstrom A, & Gross JJ (2011). The temporal dynamics of emotion regulation: An EEG study of distraction and reappraisal. Biological Psychology, 87(1), 84–92. 10.1016/j.biopsycho.2011.02.009 [DOI] [PubMed] [Google Scholar]
- Van Cauwenberge V, El Kaddouri R, Hoppenbrouwers K, & Wiersema JR (2017). To make a molehill out of a mountain: An ERP-study on cognitive reappraisal of negative pictures in children with and without ADHD. Clinical Neurophysiology: Official Journal of the International Federation of Clinical Neurophysiology, 128(4), 529–537. 10.1016/j.clinph.2017.01.008 [DOI] [PubMed] [Google Scholar]
- Weinberg A, Hilgard J, Bartholow BD, & Hajcak G (2012). Emotional Targets: Evaluative categorization as a function of context and content. International Journal of Psychophysiology, 84, 149–154. [DOI] [PubMed] [Google Scholar]
- Wessa M, Kanske P, Neumeister P, Bode K, Heissler J, & Schönfelder S (2010). EmoPics: Subjektive und psychophysiologische Evaluation neuen Bildmaterials für die klinisch-biopsychologische Forschung. Zeitschrift Für Klinische Psychologie Und Psychotherapie, Supplementum, 1(11), 77. [Google Scholar]
Associated Data
This section collects any data citations, data availability statements, or supplementary materials included in this article.