Abstract
Liposarcoma is a tumor derived from primitive cells that undergo adipose differentiation. They comprise 10%–35% of all soft tissue sarcomas. We present a 46-year-old female patient with a 4-year history of a giant swelling on the lateral aspect of the left thigh. Wide local excision of the mass was performed and histopathological analysis revealed a well-differentiated liposarcoma. Liposarcomas commonly appear in the extremities and have distinct features on imaging studies. Surgical treatment and histology are the most important prognostic factors for patients with lipomatous tumors, as complete surgical excision reduces local recurrence rate.
Keywords: Adipose tissue, giant, lipoma, liposarcoma, surgery
Introduction
Lipomas are benign tumors composed of mature lipocytes and are said to be the most prevalent tumors with an estimated incidence of 10%. 1 They can be located in any part of the body, usually weigh a few grams and are usually less than 2 cm.1,2 Liposarcomas comprise of 10%–35% of all soft tissue sarcomas, specifically, 24% of all extremity and 45% of all retroperitoneal soft-tissue sarcomas, and can occur wherever fat is present.1,3 They are histologically diverse and have a wide range of imaging appearances and behavior patterns, whereby the aggressive ones need a combination therapy of surgery, chemotherapy, and radiation. 4 Differentiation between the sub-types is vital due to the therapeutic and prognostic implications. 4 Liposarcomas have a tendency to occur in people aged 40–60 years of age with equal male and female predisposition. 5 Giant liposarcomas are rare; hence, we present a case of a giant liposarcoma in an African female which was excised whole successfully.
Case presentation
A 46-year-old, a known HIV-positive female on antiretroviral therapy was referred to our surgical clinic with a 4-year history of a swelling on the lateral aspect of her left thigh that gradually increased in size. The swelling was painless and was not associated with any discharge or ulceration, but it did lead to inability to use the affected limb due to the mass effect it was causing on the thigh. She did not complain of loss of sensation or tingling sensation on the affected limb.
On physical examination, she was clinically stable with vitals within normal range. On local examination, there was a gross swelling on the lateral aspect of the left thigh, firm to smooth consistency on palpation approximately 40 cm × 30 cm with no palpable pulsations, not tender, not mobile, no temperature change on the skin over mass, no inguinal swelling.
Her laboratory findings revealed a normal leukocyte count 4.73 × 109/L; normal erythrocyte count and hemoglobin of 14.1 g/L; normal platelet count 254 × 109/L. A normal serum sodium of 139.6 mol/L, normal potassium of 4.7 mmol/L, with liver enzymes and renal functions within normal limits. A trucut biopsy of the swelling showed mature adipocyte, features suggestive of a lipoma. Due to financial constrains Magnetic resonance imaging was requested but not done to further assess the lesion.
After counseling the patient, she was planned for an elective excision surgery of the thigh mass whereby a crescent incision was made and was extended deep to the fascia. Blunt dissection was done, the lipoma was identified, mobilized whole, resected and taken for histological analysis (Figure 1). The mass was approximately 30 cm in greatest diameter and weight approximately 4 kg. The histology results revealed atypical proliferation of normal and abdominal lipoblasts with areas of sclerosis, features of a well-differentiated liposarcoma (WDL; Figure 2). Due to financial constraints, no imaging was done to aid for possible metastasis of the liposarcoma despite postoperative counseling. The patient was nursed in the surgical unit for 7 days and was discharged after an uneventful stay. She was reviewed after 8 months whereby her surgery site had healed with no clinical signs and symptoms of recurrence or metastasis (Figure 3). She continued to maintain normal limb function. She was also reviewed by the oncology team whereby a computed tomographic (CT)-scan was ordered to rule out metastasis and an MRI of the thigh however she was lost to follow-up.
Figure 1.

Photograph showing the resected giant liposarcoma.
Figure 2.

Histopathology of atypical lipomatous neoplasia/well-differentiated liposarcoma displaying mature fat with variably sized adipocytes and bands of fibrotic stroma containing spindle cells with enlarged, hyperchromatic nuclei with focal cytologic atypia (Hematoxylin & Eosin staining 10× original magnification).
Figure 3.
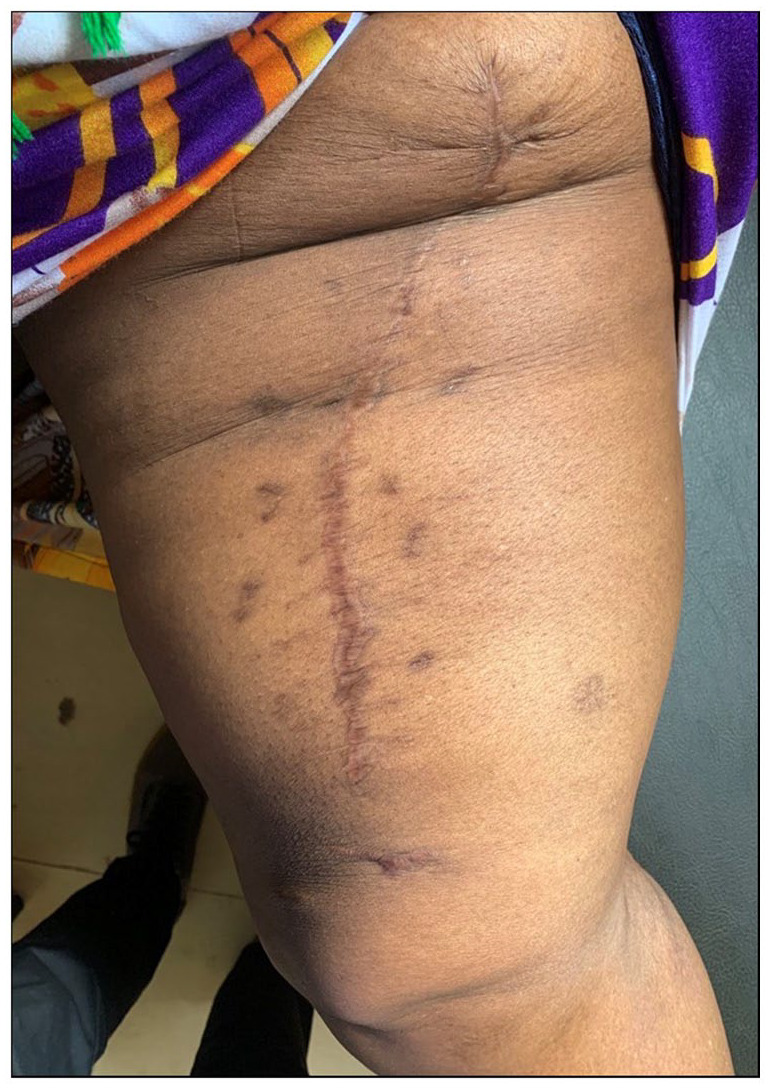
Eight months postoperative photograph showing healed incision site and no evidence of recurrence.
Discussion
Liposarcomas appear to originate from primitive mesenchymal cells rather than mature adipose tissue, and most commonly occurs in the extremities accounting for 52%, followed by retroperitoneum (19%) and inguinal (12%) regions. WHO classifies liposarcomas into five histologic types; well-differentiated, myxoid, round cell, pleomorphic, and dedifferentiated. 5 WDLs are also known as “atypical lipomatous tumor” (ALT) due to identical morphology and genetic make-up. 6 ATL frequently occur in the deep soft tissues of proximal extremities and are usually deep seated, painless, and grow slowly to reach a large size. 6 The challenge is identification of potential dedifferential components of ALTs and WDL or of alternative subtypes that would alter the management whereby neoadjuvant therapy can be considered; hence, management in a tertiary institute with availability of a multidisciplinary team is recommended. 4
Dedifferentiated liposarcoma (DDL) is a biphasic neoplasm with WDL and a non-adipocytic cellular sarcoma, of generally high grade. They are usually at least 3 cm and lack macroscopic fat signal intensity in the dedifferentiated element. 4 The usual dedifferentiated component histologically frequently resembles high-grade fibrosarcoma or undifferentiated pleomorphic sarcoma although other lines have been reported. 4 The major pitfalls in diagnosis to liposarcomas can be divided into three. Accurate diagnosis cannot be achieved without gross specimen orientation and margin analysis. Another challenge in the analysis include presence of intranuclear vacuoles in normal adipocytes (Lockhern cells), which may cause diagnostic confusion. Brown fat cells, which contain numerous fine intracytoplasmic lipid droplets, are another potential mimic of lipoblasts. 4 However, lipoblasts can also be seen in other benign lesions like lipoblastoma, chondroid lipoma, or spindle cell lipoma. Lastly, use of core biopsies, as in the index case, can also pose a challenge in making a diagnosis. 7
Dedifferentiated liposarcoma is much less aggressive than other types of high-grade pleomorphic sarcoma despite its high-grade morphology. Dedifferentiation is associated with a 15%–20% metastatic rate; however, mortality is associated more with local recurrence. 7 Not all dedifferentiated liposarcomas exhibit “high-grade” morphology but have higher tendency of recurrences, and distant metastasis is uncommon.7,8 Dedifferentiated liposarcomas have similar genetic abnormalities to WDL with high-level amplifications of chromosome 12q14-15, including the MDM2 and CDK4 cell cycle oncogenes, and additional co-amplifications of 6q23 and 1p32. 8 WDLs have certain diagnostic features on CT or magnetic resonance images. They present as a largely lipomatous mass (>75% of the lesion) with non-lipomatous components in thick septa or focal nodules. 9
Although WDL may have local recurrences, they have no metastatic potential unless dedifferentiation is present. 10 For subcutaneously located lesions such as in the index case, surgical excision with wide resection margins is sufficient. For WDL, located deeper in the body, the local recurrence risk is high due to difficulties in obtaining negative surgical margins. 11 Studies have shown that the local recurrence rate is 43% for lesions located in the extremities, 70% in the groin, and 91% for retroperitoneal lesions. 12 In these instances, radiotherapy may be used as adjunctive therapy to decrease relapse. 13 Retroperitoneal and mediastinal lesions have higher morbidity and mortality due to their aggressive features and higher rates of recurrence. 14 Currently, there is no set guidelines on the most appropriate surgical margin for ALTs and WDL of the trunk and extremities. Some authors suggest wide margin excision, whereas others suggest marginal excision is sufficient for these tumors of low metastatic potential. 14 Mortality associated with multiple complications of local recurrence is significant when WDLs are located in the retroperitoneal (33%) or inguinal (14%) areas and is insignificant for extremities lesions, if dedifferentiation does not appear. 12
There are current development of novel treatment including MDM2-targeted therapy, CDK4-targeted therapy, Exportin 1 inhibitors, and Aurora Kinases inhibitors. These however cannot obtain satisfactory level of clinical outcomes; hence, further clinical trials are necessary. 15
In a series by Terzioglu et al. 1 the authors mention that for a lipoma to be termed “giant” it should be at least 10 cm and weigh 1000 g, and the mechanism for such large growths remain unclear. Very large ones have been reported according to the authors, but are very rare. 1 The role of trauma on the causative mechanism of liposarcoma is also unclear. The authors continue to state that lower extremities are common for liposarcomas, especially myxoid type. 1 In another study by Ortiz-Ibáñez et al. 16 stated that WDL of the lower extremities are painless and slow growing with locally aggressive behavior, and no metastatic potential unless they are DDL. Poorer prognosis is associated with larger size tumors, metastasis, and advanced patient age. Lesions that are located in the extremities have favorable prognosis among young candidates and adjuvant chemotherapy yields greater relapse-free survival in those over 30 years of age. The primary treatment for high-risk patients is surgical resection and local control with adjuvant radiotherapy. 16
According to Ng and Tan 17 liposarcomas of the extremities are uncommon and are usually managed by limb-sparing surgery, with or without radio- and/or chemotherapy. Adequate clinical and radiological assessment is important to assess the resectability of the lesion. Larger tumors may need second look excision surgery to clear positive margins. In their study, most tumors were also in the thigh. Role of chemotherapy and radiotherapy is still controversial. 17
Conclusion
Malignant adipocytic tumors account for about 20% of all sarcomas whereby liposarcoma denotes a heterogeneous group of typical lesions that pose several diagnostic difficulties. Liposarcomas are usually well-differentiated tumors with non-metastatic potential, especially if they are located in the extremities. It is difficult to differentiate between giant lipoma and WDL with physical examination and imaging. Histopathologic examination in uncertain conditions and the above precursor cases will decrease morbidity and mortality from the more aggressive forms of liposarcomas.
Acknowledgments
The authors would like to thank the patient for his permission to share his medical history for educational purposes and publication.
Footnotes
Author contributions: J.S. and J.L. conceptualized and drafted the manuscript. J.T. and A.M. reviewed the medical records, and A.M. performed histopathology investigations. All authors have read and approved the final script.
Availability of data and material: We have not shared patient’s hospital records as they contain personal identification information.
The authors declared no potential conflicts of interest with respect to the research, authorship, and/or publication of this article.
Funding: The authors received no financial support for the research, authorship, and/or publication of this article.
Ethical approval: Our institution does not require ethical approval for reporting individual cases or case series.
Informed consent: Written informed consent was obtained from the patient(s) for their anonymized information to be published in this article.
ORCID iDs: Alex Mremi  https://orcid.org/0000-0001-7226-0168
https://orcid.org/0000-0001-7226-0168
Jay Lodhia  https://orcid.org/0000-0002-3373-5762
https://orcid.org/0000-0002-3373-5762
References
- 1. Terzioglu A, Tuncali D, Yuksel A, et al. Giant lipomas: a series of 12 consecutive cases and a giant liposarcoma of the thigh. Dermatol Surg 2004; 30(3): 463–467. [DOI] [PubMed] [Google Scholar]
- 2. Sanchez MR, Golomb FM, Moy JA, et al. Giant lipoma: case report and review of the literature. J Am Acad Dermatol 1993; 28: 266–268. [DOI] [PubMed] [Google Scholar]
- 3. Henze J, Bauer S. Liposarcomas. Hematol Oncol Clin North Am 2013; 27(5): 939–955. [DOI] [PubMed] [Google Scholar]
- 4. Rizer M, Singer AD, Edgar M, et al. The histological variants of liposarcoma: predictive MRI findings with prognostic implications, management, follow-up, and differential diagnosis. Skeletal Radiol 2016;4 5: 1193–1204. [DOI] [PubMed] [Google Scholar]
- 5. Hashimoto Y, Hatakeyama S, Tachiwada T, et al. Surgical treatment of a giant liposarcoma in a Japanese man. Adv Urol 2010; 2010: 943073. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 6. Burusapat C, Wongprakob N, Wanichjaroen N, et al. Atypical lipomatous tumor/well-differentiated liposarcoma with intramuscular lipoma-like component of the thigh. Case Rep Surg 2020; 2020: 8846932. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 7. Dei Tos AP. Liposarcomas: diagnostic pitfalls and new insights. Histopathology 2014; 64(1): 38–52. [DOI] [PubMed] [Google Scholar]
- 8. Thway K, Jones RL, Noujaim J, et al. Dedifferentiated liposarcoma: updates on morphology, genetics, and therapeutic strategies. Adv Anat Pathol 2016; 23(1): 30–40. [DOI] [PubMed] [Google Scholar]
- 9. Murphey MD, Arcara LK, Fanburg-Smith J. From the archives of the AFIP: imaging of musculoskeletal liposarcoma with radiologic-pathologic correlation. Radiographics 2005; 25(5): 1371–1395. [DOI] [PubMed] [Google Scholar]
- 10. Forus A, Larramendy ML, Meza–Zepeda LA, et al. Dedifferentiation of a well-differentiated liposarcoma to a highly malignant metastatic osteosarcoma: amplification of 12q14 at all stages and gain of 1q22–q24 associated with metastases. Cancer Genet Cytogenet 2001; 125: 100–111. [DOI] [PubMed] [Google Scholar]
- 11. Kim HS, Lee J, Yi SY, et al. Liposarcoma: exploration of clinical prognostic factors for risk based stratification of therapy. BMC Cancer 2009; 9: 205. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 12. Kindblom LG, Angervall L, Svendsen P. Liposarcoma a clinicopathologic, radiographic and prognostic study. Acta Pathol Microbiol Scand Suppl 1975; 253: 1–71. [PubMed] [Google Scholar]
- 13. Chung PW, Deheshi BM, Ferguson PC, et al. Radiosensitivity translates into excellent local control in extremity myxoid liposarcoma: a comparison with other soft tissue sarcomas. Cancer 2009; 115: 3254–3261. [DOI] [PubMed] [Google Scholar]
- 14. Choi KY, Jost E, Mack L, et al. Surgical management of truncal and extremities atypical lipomatous tumors/well-differentiated liposarcoma: a systematic review of the literature. Am J Surg 2020; 219(5): 823–827. [DOI] [PubMed] [Google Scholar]
- 15. Mashima E, Sawada Y, Nakamura M. Recent advancement in atypical lipomatous tumor research. Int J Mol Sci 2021; 22(3): 994. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 16. Ortiz-Ibáñez B, Amaya JV, Baixauli F, et al. Surgical resection of massive liposarcomas at the extremities: a 10-year experience in a referral musculoskeletal sarcoma unit. World J Surg Oncol 2015; 13(1): 206. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 17. Ng YC, Tan MH. Liposarcoma of the extremities: a review of the cases seen and managed in a major tertiary hospital in Singapore. Singapore Med J 2009; 50(9): 857–861. [PubMed] [Google Scholar]


