Abstract
Background:
En bloc removal of the kidney with tumor thrombus excision in a multidisciplinary team remains the standard treatment for renal cell carcinoma (RCC) with tumor thrombus extension. In order to minimize the hemodynamic impact of the surgical blood loss, intraoperative cell salvage (IOCS) techniques can decrease the need for allogeneic blood and prevent blood transfusion related complications.
Objective:
In this article, we evaluated the safety of IOCS during radical nephrectomy with inferior vena cava thrombectomy under cardiopulmonary bypass with or without deep hypothermic circulatory arrest.
Design and method:
In this retrospective comparative multicenter analysis, clinical characteristics of 27 consecutive patients who underwent surgery with or without IOCS between 2012 and 2022 in three referral care units were collected into a database. The need for an allogenic blood transfusion (ABT) was also recorded, defined as any transfusion that occurred either intraoperatively or during the hospital stay.
Results:
The need for ABT in the cell saver arm was significantly smaller due to the reinfusion of rescued blood (p < 0.015). In multivariate analysis, no cell saver usage was an independent predictor for complications ⩾3 Clavien 3a [odds ratio (OR) 18.71, 95% CI 1.056–331.703, p = 0.046]. No usage of IOCS was an independent predictor for a lower risk of death (OR 0.277, 95% CI 0.062–0.825, p = 0.024). During follow-up, patients who received salvaged blood did not experience an increased risk for developing local recurrence or distant metastases.
Conclusion:
Transfusion of autologous blood is safe and can be using during nephrectomy and thrombectomy for advanced RCC.
Keywords: advanced renal cell carcinoma, blood management, cavoatrial extension, intraoperative cell salvage techniques, oncological outcomes
Plain language summary
Role of intraoperative cell salvage techniques in the management of renal tumors with advanced caval extension
En bloc removal of the kidney with tumor thrombus excision in a multidisciplinary team remains the standard treatment for RCC with tumor thrombus extension. Intraoperative cell salvage techniques (IOCS) can decrease the need for allogeneic blood and prevent blood transfusion related complications. In this article we demonstrated that transfusion of autologous blood is safe and can be using during nephrectomy and thrombectomy for advanced renal cell carcinoma.
Introduction
Renal cell carcinoma (RCC) represents approximately 2–3% of all tumors and has a unique propensity of intraluminal growth into the venous circulation. 1 Inferior vena cava (IVC) tumor thrombosis can be detected in 4–15% of patients, with extension into the right atrium in approximately 1%.1,2
In cases with suprahepatic or cavoatrial tumor thrombus extension, en bloc removal of the kidney with IVC thrombectomy in a multidisciplinary team represents the main treatment option. 1 In a non-metastatic setting, complete surgical excision in may offer a 5-year survival benefit in 40–69% of cases.2,3 However, perioperative mortality rates are significant (3–16%), with a perioperative morbidity reported up to 70% mainly due to significant blood loss, renal or liver dysfunction, and cardiovascular complications.4,5
Intraoperative cell salvage (IOCS) techniques can decrease the need for allogeneic blood and prevent blood transfusion-related complications. Despite the routine use of salvaged blood in benign surgery (e.g. cardiac surgery), the usage of IOCS techniques in oncological surgery is still under debate. Concern exists that the usage of blood collected intraoperatively will result in a reinfusion of tumor cells and subsequently influence oncological outcomes. 6
In this article, we evaluated the safety of IOCS during open radical nephrectomy with IVC thrombectomy under cardiopulmonary bypass (CPB) using a combined approach for RCC with suprahepatic or cavoatrial tumor thrombus extension.
Material and method
Data collection
In this retrospective comparative multicenter analysis, clinical characteristics of consecutive patients who underwent surgery with or without IOCS between 2012 and 2022 in three Referral Centers were collected into a database in accordance with the local ethical standards and the declaration of Helsinki. The reporting of this study conforms to the Strengthening the Reporting of Observational Studies in Epidemiology (STROBE) checklist. 7
All patients aged 18 years and older, with a diagnosis of primary renal mass with IVC tumor thrombus extension, were included in the analysis. Patients with level 1 or 2 tumor thrombus (Mayo Clinic classification) or with distant metastasis on contrast enhanced CT and/or MRI were excluded. 2
In order to perform a comparative analysis regarding the safety of IOCS techniques, the patients were divided into two groups, depending upon the intraoperative IOCS usage. The decision to use these devices was made preoperatively on a patient-based approach depending on the complexity of the case (large tumor, bulky lymph nodes, presence of enlarged collateral veins, bland tumor thrombosis) and availability of the device in the center. The XTRA Autotransfusion system (LivaNova PLC, London, UK) was used in all cases where IOCS techniques were performed. The decision to autotransfuse was made by the anesthesiologists based upon the collected volume and hemoglobin level. The need for an allogenic blood transfusion was also recorded, defined as any transfusion that occurred either intraoperatively or during the hospital stay.
We recorded data regarding symptoms, maximal tumor diameter and final pathology, lymph node invasion, presence of bland thrombosis, or contralateral vein involvement. We performed a comparative analysis of perioperative outcomes (operating time, blood loss, transfusion rates), as well as intraoperative and complications rates using a Clavien Dindo scale. Cancer recurrence was defined as any new radiologic metastatic lesion recorded during follow-up using imaging (CT/MRI).
The surgical techniques for open radical nephrectomy with cavoatrial thrombectomy have been previously described. 8 All procedures were performed in a combined team with significant experience in the management of complex oncological cases with more than 80 patients treated in the last 20 years (cardiovascular: C.P., C.O., O.S., V.P.R., H.M., I.S.; urologists: I.S., C.S., C.G., M.H., C.B.).
Statistical analysis
Medians and interquartile ranges are presented for continuous variables and frequencies and proportions for categorical variables. Chi-squared test and Mann–Whitney U-test were used for group comparison between the groups. A multivariate binary and linear regression analysis was performed (forward selection). The survival time was calculated from the date of operation to the date of death or last follow-up (when the patient was confirmed to be alive) and the date of newly diagnosed metastases, respectively. The Kaplan–Meier method was used to analyze the survival curve and differences between groups were compared using the log-rank test. For survival parameters a multivariate cox proportional-hazard model was calculated. Significance level was set to p < 0.05. Statistical analysis was performed using IBM SPSS Statistics Version 20 (IBM Corp., Armonk, NY).
Results
Clinical characteristics of the 27 patients included in the analysis are presented in Table 1. There were no significant differences between groups for any demographic or pathologic variables. Median age in our cohort was 67 years. In 10 cases (37%), the tumor was located on the left, whereas 17 (63%) patients presented a right-sided tumor, with a median size of 8.7 cm (interval 6.8–10.4). In six cases (22.2%), the IVC tumor thrombosis was below the diaphragm but above the suprahepatic veins (level 3), whereas 21 (77.8%) patients presented atrial involvement (level 4).
Table 1.
Clinical baseline characteristics of the study cohort and group comparison between the groups with or without cell saver usage.
| Variable | Cell saver (11) | No cell saver (16) | All patients (n = 27) | p Value |
|---|---|---|---|---|
| Median age (range) – years | 65 (62–72) | 68 (60–75) | 67.0 (60.0–77.0) | 0.748 |
| Sex – no. (%) | 0.383 | |||
| Female | 2 (18.2) | 5 (31.3) | 7 (25.9) | |
| Male | 9 (81.8) | 11 (68.8) | 20 (74.1) | |
| Laterality | ||||
| Left | 4 (36.4) | 6 (37.5) | 10 (37.0) | 0.637 |
| Right | 7 (63.6) | 10 (62.5) | 17 (63.0) | |
| Median BMI, IQR (kg/m2) | 21 (18–22) | 23.4 (20.87–26.62) | 22 (19–25) | 0.012 |
| ECOG performance status | 0.222 | |||
| 0 | 0 | 1 (6.3) | 1 (3.7) | |
| 1 | 4 (36.4) | 2 (12.5) | 6 (22.2) | |
| 2 | 4 (36.4) | 5 (31.3) | 9 (33.3) | |
| 3 | 3 (27.3) | 7 (43.8) | 10 (37.0) | |
| 4 | 0 | 1 (6.3) | 1 (3.7) | |
| Median Charlson comorbidity score | 5 (4–8) | 8 (6–9) | 6 (5–8) | 0.032 |
| Clinical symptoms | 0.469 | |||
| Urinary | 6 (54.5) | 11 (68.8) | 17 (63.0) | |
| Systemic | 1 (9.1) | 1 (6.3) | 2 (7.4) | |
| Local and systemic | 4 (36.4) | 3 (25.0) | 8 (29.6) | |
| Median preoperative Hb (range) – g/dL | 13.0 (12.0–14.0) | 12.8 (10.63–14.52) | 13.0 (11.6–14.3) | 0.586 |
| Median preoperative serum creatinine (range) – mg/dL | 1.00 (1.00–1.00) | 1.32 (1.00–1.50) | 1.0 (1.00–1.45) | 0.105 |
| Median maximum tumor size (range) – cm | 8.5 (7.4–10.2) | 8.8 (6.8–10.4) | 8.7 (6.8–10.2) | 0.902 |
| Preoperative embolization of the renal artery | ||||
| Yes | 1 (9.1) | 3 (18.3) | 0.455 | |
| No | 10 (90.9) | 13 (81.3) | ||
| Histology | 0.473 | |||
| Clear cell RCC | 10 (90.9) | 13 (81.3) | 23 (85.2) | |
| Urothelial cancer | 0 | 1 (6.3) | 1 (3.7) | |
| Unclassified | 1 (9.1) | 0 | 1 (3.7) | |
| Clear cell and papillary RCC | 0 | 1 (6.3) | 1 (3.7) | |
| Clear cell RCC and unclassified | 0 | 1 (6.3) | 1 (3.7) | |
| Fuhrman grade | 0.688 | |||
| 2 | 3 (30.0) | 2 (13.3) | 5 (20) | |
| 3 | 3 (30.0) | 11 (73.3) | 14 (56.0) | |
| 4 | 4 (40.0) | 2 (13.3) | 6 (24.0) | |
| pT-Stage | 0.315 | |||
| pT3c | 5 (45.5) | 10 (62.5) | 15 (55.6) | |
| pT4 | 6 (54.5) | 6 (37.5) | 12 (44.4) | |
| Lymph node metastases | 0.436 | |||
| N0 | 6 (54.5) | 7 (43.8) | 13 (48.1) | |
| N1 | 5 (45.5) | 9 (56.3) | 14 (51.9) | |
| IVC involvement | 0.189 | |||
| Thrombus above diaphragm | 1 (9.1) | 5 (31.3) | 6 (22.2) | |
| Atrial thrombus | 10 (90.9) | 11 (68.8) | 21 (77.8) | |
| Cruoric thrombosis | 0.528 | |||
| Yes | 2 (18.2) | 5 (31.3) | 7 (25.9) | |
| No | 9 (81.8) | 12 (75.0) | 21 (77.8) | |
| IVC invasion | 0.545 | |||
| Yes | 2 (18.2) | 2 (12.5) | 4 (14.8) | |
| No | 9 (81.8) | 14 (87.5) | 23 (85.2) | |
| Contralateral renal vein thrombosis | 0.643 | |||
| Yes | 1 (9.1) | 2 (12.5) | 3 (11.1) | |
| No | 10 (90.9) | 14 (87.4) | 24 (88.9) | |
BMI, body mass index; ECOG = Eastern Cooperative Oncology Group; Hb, hemoglobin; IVC, inferior vena cava; IQR= interquartile range; pT, pathological stage T; RCC, renal cell carcinoma.
Mean duration of surgery was similar in both groups [320 min (interval: 280–380)]. There were no significant differences between groups in the median durations of cardiopulmonary bypass, deep hypothermic circulatory arrest (DHCA) and contralateral renal vein clamping (Table 2). IVC was ligated below the renal veins in five cases (31.3%) due to extensive bland thrombosis.
Table 2.
Perioperative clinical characteristics of the study cohort and group comparison between the groups with or without cell saver usage.
| Variable | Cell saver (11) | No cell saver (16) | All patients (n = 27) | p-Value |
|---|---|---|---|---|
| Median duration of surgery (range) – min | 310 (270–360) | 330 (285–402) | 320 (280–380) | 0.322 |
| Median duration of CBP (range) – min | 35 (30–45) | 31 (30–44) | 32 (30–45) | 0.780 |
| Median duration of DHCA (range) – min | 20 (16–25) | 18 (15–22) | 20 (15–24) | 0.367 |
| Median duration of clamping of contralateral renal vein (range) – min | 10 (10–15) | 12 (10–18) | 10 (10–18) | 0.649 |
| IVC ligation | ||||
| Yes | 0 | 5 (31.3) | 5 (18.5) | 0.054 |
| No | 11 (100) | 11 (86.8) | 22 (81.5) | |
| Median estimated blood loss (range) – mL | 2500 (1800–6400) | 2100 (1850–4100) | 2200 (1800–6400) | 0.535 |
| Median units of allogenic whole blood transfusion (range) – n | 2 (0–2) | 4 (2–6) | 2 (0–6) | 0.015 |
| Median units of allogenic plasma (range) – n | 0 (0–1) | 1 (0–4) | 0 (0–1) | 0.190 |
| Median estimated rescued blood (range) – mL | 1200 (840–2800) | 0.535 | ||
| Median postoperative Hb (range) – g/dL | 10.0 (9.0–10.0) | 9.72 (7.60–10.60) | 9.74 (8.21–10.60) | 0.621 |
| Intraoperative complications | 0.977 | |||
| grade1 | 5 (45.5) | 8 (53.3) | 13 (50.0) | |
| grade2 | 6 (54.5) | 5 (33.3) | 11 (42.3) | |
| grade3 | 0 | 2 (13.3) | 2 (7.7) |
ABT, allogenic blood transfusion; CBP, cardiopulmonary bypass; DHCA, Deep hypothermic circulatory arrest; Hb, hemoglobin; IVC, inferior vena cava.
Although there was no significant difference in the median estimated blood loss between groups, the need for intraoperative ABT in the cell saver arm was significantly smaller due to the reinfusion of rescued blood (p < 0.015). The salvaged blood was filtered through the XTRA systems prior to infusion and rescued an average of 1200 ml (interval: 840–2800 ml) of autologous blood, decreasing the need of perioperative allogenic transfusion with a median of 2 units/case.
Overall intraoperative complication rate was 62.8%, with no significant differences between groups. No intraoperative death was recorded, but two patients (7.7%) developed cardiac arrest after CBP that required intraoperative defibrillation. Overall postoperative complication rate was 48.14%, with no significant differences between groups (Table 3). Clavien grade ⩾3b complication rate in our cohort was 18.51%, with one death recorded in the no cell saver arm due to hemorrhagic shock after massive hemomediastinum.
Table 3.
Clinical outcome characteristics of the study cohort and group comparison.
| Variable | Cell saver (11) | No cell saver (16) | All patients (n = 27) | p-Value |
|---|---|---|---|---|
| Postoperative complications | 0.373 | |||
| None | 4 (36.4) | 3 (18.8) | 7 (25.9) | |
| Clavien-Dindo 2 | 3 (27.3) | 2 (12.5) | 5 (18.5) | |
| Clavien-Dindo 3a | 1 (9.1) | 4 (25.0) | 5 (18.5) | |
| Clavien-Dindo 3b | 0 | 2 (12.5) | 2 (7.4) | |
| Clavien-Dindo 4a | 2 (18.2) | 1 (6.3) | 3 (11.1) | |
| Clavien-Dindo 4b | 1 (9.1) | 3 (18.8) | 4 (14.8) | |
| Clavien-Dindo 5 | 0 | 1 (6.3) | 1 (3.7) | |
| Median length of hospital stay, (range) – days | 14 (10–18) | 24 (18–31) | 20 (10–28) | 0.010 |
| Median length of ICU stay (days) | 2 (2–6) | 9 (4–15) | 6 (4–12) | 0.003 |
| Local recurrence | ||||
| Yes | 0 | 4 (25.0) | 4 (14.8) | |
| No | 11 (100) | 12 (75.0) | 23 (85.2) | |
| Systemic metastasis | ||||
| Yes | 6 (54.5) | 9 (56.3) | 15 (55.5) | |
| No | 5 (45.5) | 7 (43.8) | 12 (44.5) | |
| Adjuvant treatment | 0.467 | |||
| Yes | 3 (27.2) | 5 (31.2) | 8 (29.6) | |
| No | 8 (72.7) | 11 (68.7) | 19 (70.3) | |
| Alive at last follow-up | ||||
| Yes | 7 (63.6) | 9 (56.3) | 16 (59.3) | |
| No | 4 (36.4) | 7 (43.8) | 11 (40.7) |
ICU, intensive care unit.
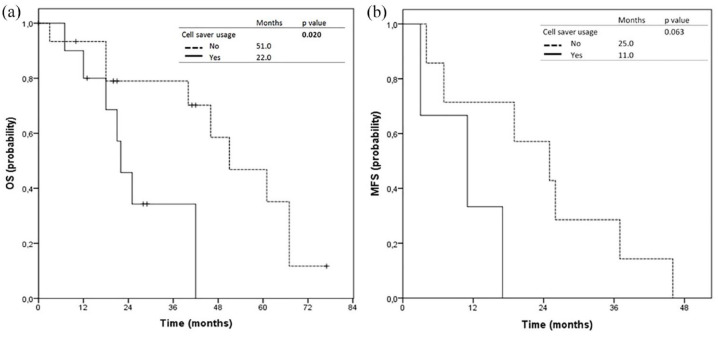
Median length of hospital stay recorded for the entire cohort was 20 days (interval 10–28 days), with a median ICU stay of 6 days (interval 4–12 days). Median metastasis-free survival showed no significant difference (p = 0.063) and was 25 months for the no cell saver arm and 11 months for the cell saver arm, respectively. Median overall survival was 51 months in the no cell saver arm and 22 months in the cell saver arm (p = 0.02; Figure 1).
Figure 1.
Kaplan–Meier curves for (a) overall survival (OS) and (b) metastasis-free survival (MFS) comparing the cohorts with (solid line) and without (dashed lines) cell saver usage.
In multivariate logistic regression analysis, no cell saver usage was an independent predictor for postoperative complications ⩾3 Clavien 3a (OR 18.71, 95% CI 1.056–331.703, p = 0.046) (Supplemental Table 1). The usage of cell saver was an independent predictor for shorter length of ICU stay (coefficient = −0.355, 95% CI 1.056–331.703, p = 0.046) and for a lower risk of death (OR 0.277, 95% CI 0.062–0.825, p = 0.024) (Table 4).
Table 4.
Multivariate cox regression analyses of preoperative and intraoperative factors to predict OS and MFS.
| Risk factors | End points | |||||
|---|---|---|---|---|---|---|
| OS | MFS | |||||
| HR | 95% CI | p Value | HR | 95% CI | p Value | |
| Cell saver usage | ||||||
| Yes | 1 (ref.) | 1 (ref.) | ||||
| No | 0.277 | (0.062–0.825) | 0.024 | 0.173 | (0.024–1.1256) | 0.083 |
| BMI | 0.964 | (0.804–1.157) | 0.696 | 1.198 | (0.880–1.631) | 0.252 |
| Maximum tumor size | 0.989 | (0,964–1.014) | 0.378 | 0.961 | (0.961–1.043) | 0.952 |
| Preoperative Hb | 0.819 | (0.556–1.204) | 0.309 | 0.573 | (0.266–1.230) | 0.153 |
BMI, body mass index; CI, confidence interval; Hb, hemoglobin; HR, hazard ratio; MFS, metastasis free survival; OS, overall survival.
During follow-up, 4 (14.8%) patients developed local recurrence in the no cell saver arm, which was excised in two cases. A total of 15 patients (55%) developed distant metastasis, with no statistical differences between groups. We recorded three cases with solitary lesion in the contralateral suprarenal gland who underwent surgery. Adjuvant treatment with targeted therapy was recommended in 8 (29.62%) cases.
Discussions
Cavoatrial tumor thrombus extension in RCC represents a complex and challenging scenario in which the surgery has to resolve a locally advanced cancer that associates a significant central venous obstruction. Although the perioperative morbidity and mortality rates are high, mainly due to increased blood loss and thrombus migration, the oncological outcomes reported are reasonable, with up to 64% of cases with no lymph node metastasis alive at 5 years after surgery.2,5,9,10
Our study included six patients with level three IVC tumor thrombus excision that underwent open radical nephrectomy with IVC thrombectomy under CBP bypass. Although there are several articles demonstrating that an abdominal approach to the supradiaphragmatic vena cava is feasible, there are several important drawbacks to this approach (such as the need for Pringle maneuver on the hepatic pedicle, possible damage to the posterior wall of the inferior vena cava, uncontrolled bleeding), patients with a friable thrombus tip may be at risk of intraoperative embolization.9,11,12 Although the complications of a CBP bypass are not neglectable, we consider that these cases should be performed under CBP bypass with or without DHCA in order to minimize the risk of intraoperative tumor thrombus embolization.
There is an increasing body of literature on the application of minimally invasive methods (robotic, laparoscopic) for IVC thrombectomy and radical nephrectomy. According to several studies, for level II thrombi, laparoscopy or robotics may be a safe substitute for open surgery and for level III/IV thrombi, laparoscopic nephrectomy with open venotomy can be performed safely.13–15 Minimally invasive services were unavailable in our centers during the study period.
In order to minimize the hemodynamic impact of the surgical blood loss, transfusions with allogeneic blood (ABT) are currently the main option for perioperative blood conservation.16,17 The usage of ABT presents several drawbacks, with multiple studies demonstrating the negative impact of this strategy on clinical and oncological outcomes and increased risk of death due to transfusion related side effects.6,18 In addition, perioperative homologous blood transfusion may have an immunosuppressive effect, increasing the perioperative mortality risk or adverse oncological outcomes.19–21 Autologous intraoperative blood transfusions using the cell saver systems have been used in different scenarios where a significant blood loss is to be expected (vascular, cardiothoracic, orthopedic, etc.). The usage of allogenic blood transfusion for cancer patients was considered unsafe after the American Medical Council advised in 1986 against using autologous saved blood during oncologic surgery. 22 Following this statement, several systematic reviews and meta-analysis of clinical reinfusion and nonreinfusion studies (in which salvaged blood is not reinfused to patients) were published, indicating that salvaged blood presents a good safety profile with similar or better oncological outcomes reported for patients receiving IOCS during cancer surgery compared to patients with ABT or nontransfused patients.6,17,23,24
In uro-oncology, several authors have demonstrated that the usage of IOCS during radical cystectomy or retropubic prostatectomy does not increase the risks of distant metastasis or biochemical recurrence.25–27 The National Institute for Health and Clinical Excellence (NICE) approved the use of autologous blood transfusion in patients undergoing prostate or bladder cancer surgery. 28
The evidence regarding the safety of autologous blood transfusions during kidney cancer surgery is scarce and limited to small case series or case reports.29–32 To the best of our knowledge, this is the first case control series to report the oncological outcomes after IOCS usage in patients undergoing radical nephrectomy with cavoatrial thrombectomy.
In terms of postoperative complications, our study also supports the safety of IOCS usage. The shorter length of stay and decreased median number of days in the intensive care unit in the cell saver group (14 versus 24 and 2 versus 9, respectively) are not correlated to the IOCS usage in the multivariate analysis (Supplementary Table 2), but may reflect the general trend of a shorter hospital stay in recent years.
Reported mortality rates after radical nephrectomy with cavoatrial thrombectomy range from 2.7% to 13% in contemporary series and may be dependent on comorbidities.2,33 In our study, overall mortality was 3.7% and has decreased significantly from our last report. 34 Further refinement of our surgical technique, together with the increasing surgical experience and better case selection, as well as advances in perioperative care, may have helped in reducing the perioperative morbidity and mortality.
Although modern systemic treatment with immune checkpoint inhibitors and tyrosine kinase inhibitors has considerably improved survival for metastatic RCC, the usage of these drugs in an adjuvant setting after radical treatments is controversial and is not reimbursed in most of the European countries.1,12 Several trials, such as Keynote 564 (NCT03142334) or Checkmate 914 (NCT03138512), are now being conducted to evaluate these drugs in the adjuvant context for non-metastatic patients who are at high risk of recurrence, such as those with IVC tumor thrombus.35,36 In our cohort, only six patients received adjuvant treatment with targeted therapy, and the impact of these treatments on the overall survival could not be assessed.
In terms of oncological outcomes, our findings do not support the hypothesis that intraoperative autotransfusion can influence the development of systemic metastases. Even though, the cohort with the usage of IOCS showed decreased overall survival, no significant difference regarding metastasis free survival on Kaplan–Meier or multivariate Cox regression analyses could be seen (Table 4).
Several studies have shown that patients with caval tumor thrombosis present a high level of circulating tumor cells (CTCs) and have raised concerns that the reinfusion of viable tumor cells from intraoperative recovered blood may result in tumor spread and metastasis.37,38 However, a postoperative cytological examination revealed that the tumor cells were only detected on the internal surface of the heart–lung machine arterial filters. 31 According to Karczewski et al., 39 62% of tumor cells became nonviable after processing with an intraoperative blood salvage device, whereas the remaining tumor cells underwent irreversible morphologic changes. These results support the hypothesis that tumor cells are rendered nonviable during collection and processing in the cell saver system.
Our study presents several limitations inherent to the retrospective nature of the study and small number of patients included. However, enrolling a large number of patients fit enough to undergo surgery in a prospective study may be challenging due to the low incidence of cavoatrial thrombus extension in RCC and the narrow window of opportunity for curative surgery since these patients are at high risk of metastatic spread. Second, since the present series spans over a decade, the surgical techniques and the perioperative protocols have constantly improved, which may have biased the oncological outcome analysis. In addition, the impact of adjuvant treatment was not evaluated due to the small number of patients that received targeted therapy in our cohort.
Conclusion
Transfusion of autologous blood using intraoperative blood salvage techniques is safe and can be used during nephrectomy and cavoatrial thrombectomy for advanced RCC. Centralization of these patients in high volume centers with large experience is paramount in order to maximize oncological outcomes and decrease perioperative morbidity.
Supplemental Material
Supplemental material, sj-docx-1-tau-10.1177_17562872241229248 for Current role of intraoperative cell salvage techniques in the management of renal tumors with level III and IV inferior vena cava thrombus extension by Cristian Surcel, Robert Dotzauer, Cristian Mirvald, Calin Popa, Cosmin Olariu, Catalin Baston, Mihai Harza, Constantin Gangu, Igor Tsaur and Ioanel Sinescu in Therapeutic Advances in Urology
Supplemental material, sj-docx-2-tau-10.1177_17562872241229248 for Current role of intraoperative cell salvage techniques in the management of renal tumors with level III and IV inferior vena cava thrombus extension by Cristian Surcel, Robert Dotzauer, Cristian Mirvald, Calin Popa, Cosmin Olariu, Catalin Baston, Mihai Harza, Constantin Gangu, Igor Tsaur and Ioanel Sinescu in Therapeutic Advances in Urology
Acknowledgments
The authors appreciate their colleagues for all the invaluable contribution to this article: O. Stiru, I. Socoteanu, H. Moldovan, and V. P. Rasvan from “C.C. Iliescu” Institute for Cardiovascular Diseases, Bucharest, Romania; V. Cerempei and A. Danilov from the Department of Urology, Fundeni Clinical Institute, “Carol Davila” University of Medicine and Pharmacy, Bucharest, Romania; A. Dermengiu from Cardiac Center, Monza Hospital, Bucharest, Romania; and Oana Muntiu from Medlife Polisano Hospital, Sibiu, Romania.
Footnotes
ORCID iDs: Robert Dotzauer  https://orcid.org/0000-0003-1532-4385
https://orcid.org/0000-0003-1532-4385
Cristian Mirvald  https://orcid.org/0000-0002-7278-5551
https://orcid.org/0000-0002-7278-5551
Supplemental material: Supplemental material for this article is available online.
Contributor Information
Cristian Surcel, Department of Urology, Fundeni Clinical Institute, Bucharest, Romania; “Carol Davila” University of Medicine and Pharmacy, Bucharest, Romania.
Robert Dotzauer, Department of Urology, Johannes Gutenberg University Medical Center, Mainz, Germany.
Cristian Mirvald, Department of Urology, Fundeni Clinical Institute, “Carol Davila” University of Medicine and Pharmacy, 258 Fundeni Street, 2nd District, Bucharest, Romania.
Calin Popa, Cardiac Center, Monza Hospital, Bucharest, Romania.
Cosmin Olariu, Medlife Polisano Hospital, Sibiu, Romania.
Catalin Baston, Department of Urology, Fundeni Clinical Institute, Bucharest, Romania; “Carol Davila” University of Medicine and Pharmacy, Bucharest, Romania.
Mihai Harza, Department of Urology, Fundeni Clinical Institute, Bucharest, Romania; “Carol Davila” University of Medicine and Pharmacy, Bucharest, Romania.
Constantin Gangu, Department of Urology, Fundeni Clinical Institute, Bucharest, Romania; “Carol Davila” University of Medicine and Pharmacy, Bucharest, Romania.
Igor Tsaur, Department of Urology, Johannes Gutenberg University Medical Center, Mainz, Germany.
Ioanel Sinescu, Department of Urology, Fundeni Clinical Institute, Bucharest, Romania; “Carol Davila” University of Medicine and Pharmacy, Bucharest, Romania.
Declarations
Ethics approval and consent to participate: All procedures performed in studies involving human participants were in accordance with the ethical standards of the institutional and/or national research committee and with the 1964 Helsinki Declaration and its later amendments or comparable ethical standards. The present study was approved by the “Fundeni” Clinical Institute ethics review board (IRB approval no. 16536/2023). In accordance to local laws, for retrospective studies, no inform consent from the patients is required.
Consent for publication: Not Applicable.
Author contributions: Cristian Surcel: Conceptualization; Data curation; Formal analysis; Methodology; Project administration; Supervision; Writing – review & editing.
Robert Dotzauer: Conceptualization; Investigation; Methodology; Software; Visualization; Writing – original draft; Writing – review & editing.
Cristian Mirvald: Conceptualization; Data curation; Validation; Writing – original draft; Writing – review & editing.
Calin Popa: Data curation; Methodology; Supervision; Writing – original draft.
Cosmin Olariu: Conceptualization; Data curation; Investigation; Methodology; Writing – review & editing.
Catalin Baston: Data curation; Methodology; Validation; Writing – original draft; Writing – review & editing.
Mihai Harza: Data curation; Formal analysis; Investigation; Methodology.
Constantin Gangu: Data curation; Formal analysis; Investigation; Methodology; Supervision; Writing – review & editing.
Igor Tsaur: Conceptualization; Supervision; Validation; Writing – review & editing.
Ioanel Sinescu: Conceptualization; Formal analysis; Investigation; Methodology; Project administration; Supervision; Writing – review & editing.
Funding: The authors received no financial support for the research, authorship, and/or publication of this article.
The authors declare that there is no conflict of interest.
Availability of data and materials: All relevant data will be freely available to any researcher wishing to use them for non-commercial purposes, without breaching participant confidentiality.
References
- 1. Ljungberg B, Albiges L, Abu-Ghanem Y, et al. European association of urology guidelines on renal cell carcinoma: the 2022 update. Eur Urol 2022; 82: 399–410. [DOI] [PubMed] [Google Scholar]
- 2. Blute ML, Leibovich BC, Lohse CM, et al. The Mayo clinic experience with surgical management, complications and outcome for patients with renal cell carcinoma and venous tumour thrombus. BJU Int 2004; 94: 33–41. [DOI] [PubMed] [Google Scholar]
- 3. Wotkowicz C, Libertino JA, Sorcini A, et al. Management of renal cell carcinoma with vena cava and atrial thrombus: minimal access vs median sternotomy with circulatory arrest. BJU Int 2006; 98: 289–297. [DOI] [PubMed] [Google Scholar]
- 4. Kirkali Z, Van Poppel H. A critical analysis of surgery for kidney cancer with vena cava invasion. Eur Urol 2007; 52: 658–662. [DOI] [PubMed] [Google Scholar]
- 5. Novick AC, Kaye MC, Cosgrove DM, et al. Experience with cardiopulmonary bypass and deep hypothermic circulatory arrest in the management of retroperitoneal tumors with large vena caval thrombi. Ann Surg 1990; 212: 472–476; discussion 476–477. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 6. Frietsch T, Steinbicker AU, Horn A, et al. Safety of intraoperative cell salvage in cancer surgery: an updated meta-analysis of the current literature. Transfus Med Hemother 2022; 49: 143–157. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 7. Cuschieri S. The STROBE guidelines. Saudi J Anaesth 2019; 13: S31–S34. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 8. Lawindy SM, Kurian T, Kim T, et al. Important surgical considerations in the management of renal cell carcinoma (RCC) with inferior vena cava (IVC) tumour thrombus. BJU Int 2012; 110: 926–939. [DOI] [PubMed] [Google Scholar]
- 9. Ciancio G, Livingstone AS, Soloway M. Surgical management of renal cell carcinoma with tumor thrombus in the renal and inferior vena cava: the University of Miami experience in using liver transplantation techniques. Eur Urol 2007; 51: 988–994; discussion 994–985. [DOI] [PubMed] [Google Scholar]
- 10. Kakoti S, Jena R, Sureka SK, et al. Experience with management of renal cell carcinoma with inferior vena cava/right atrial tumor thrombus. Indian J Urol 2021; 37: 234–240. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 11. Shchukin D, Lesovoy V, Garagatiy I, et al. Surgical approaches to supradiaphragmatic segment of IVC and right atrium through abdominal cavity during intravenous tumor thrombus removal. Adv Urol 2014; 2014: 924269. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 12. Ray S, Singer EA, Dason S. Inferior vena cava thrombectomy for renal cell carcinoma: perioperative systemic therapy, cytoreductive nephrectomy, and complex cases. Ann Transl Med 2023; 11: 239. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 13. Chopra S, Simone G, Metcalfe C, et al. Robot-assisted level II-III inferior vena cava tumor thrombectomy: step-by-step technique and 1-year outcomes. Eur Urol 2017; 72: 267–274. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 14. Kundavaram C, Abreu AL, Chopra S, et al. Advances in robotic vena cava tumor thrombectomy: intracaval balloon occlusion, patch grafting, and vena cavoscopy. Eur Urol 2016; 70: 884–890. [DOI] [PubMed] [Google Scholar]
- 15. Shao P, Li J, Qin C, et al. Laparoscopic radical nephrectomy and inferior vena cava thrombectomy in the treatment of renal cell carcinoma. Eur Urol 2015; 68: 115–122. [DOI] [PubMed] [Google Scholar]
- 16. Spahn DR, Shander A, Hofmann A. The chiasm: transfusion practice versus patient blood management. Best Pract Res Clin Anaesthesiol 2013; 27: 37–42. [DOI] [PubMed] [Google Scholar]
- 17. Zaw AS, Bangalore Kantharajanna S, Kumar N. Is autologous salvaged blood a viable option for patient blood management in oncologic surgery? Transfus Med Rev 2017; 31: 56–61. [DOI] [PubMed] [Google Scholar]
- 18. Li L, Zhu D, Chen X, et al. Perioperative allogenenic blood transfusion is associated with worse clinical outcome for patients undergoing gastric carcinoma surgery: a meta-analysis. Medicine (Baltimore) 2015; 94: e1574. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 19. Soubra A, Zabell JR, Adejoro O, et al. Effect of perioperative blood transfusion on mortality for major urologic malignancies. Clin Genitourin Cancer 2015; 13: e173–e181. [DOI] [PubMed] [Google Scholar]
- 20. Wang YL, Jiang B, Yin FF, et al. Perioperative blood transfusion promotes worse outcomes of bladder cancer after radical cystectomy: a systematic review and meta-analysis. PLoS One 2015; 10: e0130122. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 21. Kang HW, Seo SP, Kim WT, et al. Intraoperative allogeneic blood transfusion is associated with adverse oncological outcomes in patients with surgically treated non-metastatic clear cell renal cell carcinoma. Int J Clin Oncol 2020; 25: 1551–1561. [DOI] [PubMed] [Google Scholar]
- 22. Autologous blood transfusions. Council on scientific affairs. JAMA 1986; 256: 2378–2380. [PubMed] [Google Scholar]
- 23. Kumar N, Chen Y, Zaw AS, et al. Use of intraoperative cell-salvage for autologous blood transfusions in metastatic spine tumour surgery: a systematic review. Lancet Oncol 2014; 15: e33–e41. [DOI] [PubMed] [Google Scholar]
- 24. Waters JH, Yazer M, Chen YF, et al. Blood salvage and cancer surgery: a meta-analysis of available studies. Transfusion 2012; 52: 2167–2173. [DOI] [PubMed] [Google Scholar]
- 25. Hart OJ, III, Klimberg IW, Wajsman Z, et al. Intraoperative autotransfusion in radical cystectomy for carcinoma of the bladder. Surg Gynecol Obstet 1989; 168: 302–306. [PubMed] [Google Scholar]
- 26. Nieder AM, Manoharan M, Yang Y, et al. Intraoperative cell salvage during radical cystectomy does not affect long-term survival. Urology 2007; 69: 881–884. [DOI] [PubMed] [Google Scholar]
- 27. Gray CL, Amling CL, Polston GR, et al. Intraoperative cell salvage in radical retropubic prostatectomy. Urology 2001; 58: 740–745. [DOI] [PubMed] [Google Scholar]
- 28. Bouras I, Mingo O. Should cell salvage be used in oncological surgery? Br J Hosp Med (Lond) 2010; 71: 57. [DOI] [PubMed] [Google Scholar]
- 29. Zeng H, Rong XY, Zhang XQ, et al. [Application of intraoperative cell salvage combined with leukocyte depletion filter on radical nephrectomy for renal carcinoma with inferior vena cava tumor thrombus: 2 case reports]. Beijing Da Xue Xue Bao Yi Xue Ban 2017; 49: 736–739. [PubMed] [Google Scholar]
- 30. Moskowitz DM, Perelman SI, Cousineau KM, et al. Multidisciplinary management of a Jehovah’s Witness patient for the removal of a renal cell carcinoma extending into the right atrium. Can J Anaesth 2002; 49: 402–408. [DOI] [PubMed] [Google Scholar]
- 31. Zhang X, Guo X, Zong Y, et al. CTCs detection from intraoperative salvaged blood in RCC-IVC thrombus patients by negative enrichment and iFISH identification: a preliminary study. BMC Urol 2021; 21: 89. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 32. Lyon TD, Ferroni MC, Turner RM, et al. Short-term outcomes of intraoperative cell saver transfusion during open partial nephrectomy. Urology 2015; 86: 1153–1158. [DOI] [PubMed] [Google Scholar]
- 33. Casey RG, Raheem OA, Elmusharaf E, et al. Renal cell carcinoma with IVC and atrial thrombus: a single centre’s 10 year surgical experience. Surgeon 2013; 11: 295–299. [DOI] [PubMed] [Google Scholar]
- 34. Drăgan A, Sinescu I. Which risk score better predicts in-hospital mortality in radical nephrectomies with cavo-atrial thrombectomy. Romanian J Cardiol 2019; 29: p571. [Google Scholar]
- 35. Powles T, Tomczak P, Park SH, et al. Pembrolizumab versus placebo as post-nephrectomy adjuvant therapy for clear cell renal cell carcinoma (KEYNOTE-564): 30-month follow-up analysis of a multicentre, randomised, double-blind, placebo-controlled, phase 3 trial. Lancet Oncol 2022; 23: 1133–1144. [DOI] [PubMed] [Google Scholar]
- 36. Motzer RJ, Russo P, Grunwald V, et al. Adjuvant nivolumab plus ipilimumab versus placebo for localised renal cell carcinoma after nephrectomy (CheckMate 914): a double-blind, randomised, phase 3 trial. Lancet 2023; 401: 821–832. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 37. Ferroni MC, Correa AF, Lyon TD, et al. The use of intraoperative cell salvage in urologic oncology. Rev Urol 2017; 19: 89–96. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 38. Ge L, Song Y, Yang F, et al. Clinical significance of circulating tumor cells detection in renal cell carcinoma with thrombus: a STROBE-compliant study. Medicine (Baltimore) 2020; 99: e20615. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 39. Karczewski DM, Lema MJ, Glaves D. The efficiency of an autotransfusion system for tumor cell removal from blood salvaged during cancer surgery. Anesth Analg 1994; 78: 1131–1135. [DOI] [PubMed] [Google Scholar]
Associated Data
This section collects any data citations, data availability statements, or supplementary materials included in this article.
Supplementary Materials
Supplemental material, sj-docx-1-tau-10.1177_17562872241229248 for Current role of intraoperative cell salvage techniques in the management of renal tumors with level III and IV inferior vena cava thrombus extension by Cristian Surcel, Robert Dotzauer, Cristian Mirvald, Calin Popa, Cosmin Olariu, Catalin Baston, Mihai Harza, Constantin Gangu, Igor Tsaur and Ioanel Sinescu in Therapeutic Advances in Urology
Supplemental material, sj-docx-2-tau-10.1177_17562872241229248 for Current role of intraoperative cell salvage techniques in the management of renal tumors with level III and IV inferior vena cava thrombus extension by Cristian Surcel, Robert Dotzauer, Cristian Mirvald, Calin Popa, Cosmin Olariu, Catalin Baston, Mihai Harza, Constantin Gangu, Igor Tsaur and Ioanel Sinescu in Therapeutic Advances in Urology