Abstract
This study compares optical genome mapping (OGM) performed at multiple sites with current standard-of-care (SOC) methods used in clinical cytogenetics. This study included 50 negative controls and 359 samples from individuals (patients) with suspected genetic conditions referred for cytogenetic testing. OGM was performed using the Saphyr system and Bionano Access software version 1.7. Structural variants, including copy number variants, aneuploidy, and regions of homozygosity, were detected and classified according to American College of Medical Genetics and Genomics guidelines. Repeated expansions in FMR1 and contractions in facioscapulohumeral dystrophy 1 were also analyzed. OGM results were compared with SOC for technical concordance, clinical classification concordance, intrasite and intersite reproducibility, and ability to provide additional, clinically relevant information. Across five testing sites, 98.8% (404/409) of samples yielded successful OGM data for analysis and interpretation. Overall, technical concordance for OGM to detect previously reported SOC results was 99.5% (399/401). The blinded analysis and variant classification agreement between SOC and OGM was 97.6% (364/373). Replicate analysis of 130 structural variations was 100% concordant. On the basis of this demonstration of the analytic validity and clinical utility of OGM by this multisite assessment, the authors recommend this technology as an alternative to existing SOC tests for rapid detection and diagnosis in postnatal constitutional disorders.
Constitutional disorders—including birth defects and neurodevelopmental disorders—warrant genetic/genomic testing for accurate disease diagnosis and clinical management. Developmental disabilities, including attention-deficit/hyperactivity disorder, autism spectrum disorder, blindness, cerebral palsy, moderate to profound hearing loss, learning disability, intellectual disability, seizures, stuttering or stammering, and other developmental delays, affect about 17% of the pediatric population in the United States.1 Different classes of structural variations (SVs) in the genome, such as aneuploidies, translocations, inversions, interstitial deletions/duplications, repeated expansions/contractions, and complex rearrangements, contribute to a significant number of developmental disability cases.2 Before 2010, G-banded karyotype was the recommended genetic testing method for individuals with developmental disabilities to detect gross unbalanced rearrangements as well as balanced events with a diagnostic yield of approximately 5%.3,4 In the mid-2000s, chromosomal microarray analysis (CMA) was established as an effective clinical tool for the analysis of whole-genome copy number variations (CNVs) with a diagnostic yield of up to 15% to 20%.4 Subsequently, in 2010, CMA was recommended as the first-tier clinical diagnostic test by several medical organizations and societies [including the American College of Medical Genetics and Genomics (ACMG),5 the American Academy of Neurology,6 and the American Academy of Pediatrics7,8] for individuals with developmental disabilities and neurodevelopmental disorders. However, CMA has several technical limitations, including inability to detect balanced structural rearrangements, orientation and location of duplicated segments, and low sensitivity for low-level mosaicism.9 In addition, fragile X syndrome (FXS) testing is recommended in the diagnostic workup for individuals with developmental delay/intellectual disability and autism spectrum disorder,10 and neither chromosome analysis nor CMA is able to detect repeated expansions responsible for FXS.
Recent advances in genome sequencing technologies have enabled laboratories to evaluate and offer targeted gene panels, whole-exome sequencing (ES), and whole-genome sequencing for genetic testing of individuals with clinically presenting developmental disorders. In a recent meta-analysis, ES presented a diagnostic yield of approximately 35% and is currently being offered as a reflex test after a normal CMA test result.11 However, detection of SVs with ES/whole-genome sequencing incurs technical problems at repetitive sequences in the genome because of ambiguous mapping of short reads. Whole-genome sequencing/ES can detect repeated expansions but cannot size repeated expansions above approximately 300 bp,12 and neither whole-genome sequencing nor ES can detect macrosatellite contractions of D4Z4, the cause of facioscapulohumeral dystrophy 1 (FSHD1). Ancillary methods, such as Southern blot analysis or PCR testing, are required for repeated expansion/contraction analysis, although they are time-consuming and have low accuracy (Southern blot analysis) or have a low sizing range (PCR and next-generation sequencing). Nevertheless, a cumulative diagnostic yield with all these technologies is estimated to be >50% for individuals with developmental delay/intellectual disability and >25% for individuals with primarily autism spectrum disorder.9,13
Optical genome mapping (OGM) is an emerging next-generation technology that enables comprehensive cytogenomic analysis of the genome.14 OGM is performed on the Saphyr system (Bionano Genomics, Inc., San Diego, CA), which scans ultra–high-molecular-weight DNA molecules (≥150 kbp) labeled at a 6 bp sequence motif (CTTAAG) throughout the genome. The images of the labeled DNA molecules are used to generate a de novo assembly of the banding patterns that can be compared with a reference genome to identify several classes of SVs15 [CNVs, deletions, duplications, balanced and unbalanced events (inversions, insertions, and translocations), and triploidy], including absence of heterozygosity (AOH) regions. A separate coverage-based algorithm allows for the detection of CNVs similar to CMA. OGM also provides sizing of CGG repeated expansions in the FMR1 gene (FXS) as well as D4Z4 repeated contractions with haplotype (4qA or 4qB haplotype), of which 4qA is the disease-permissive haplotype in FSHD1 cases.16 In several recent validation studies, OGM has demonstrated near-perfect concordance to standard-of-care (SOC) testing.17,18 Thus, OGM has been validated and is clinically offered in the United States for FSHD1 testing19 (University of Iowa Hospitals and Clinics, Iowa City, IA; PerkinElmer Genetics, Pittsburgh, PA; University of Augusta, Augusta, GA; and Praxis Genomics, Atlanta, GA) as well as genome-wide SV analysis (Praxis Genomics and University of Augusta). More important, the OGM workflow is comparable to SOC test protocols, providing results within 3 to 5 days.20, 21, 22
The aim of this study was to evaluate and validate the performance of OGM compared with SOC technologies (CMA, karyotyping, fluorescence in situ hybridization, Southern blot analysis, and PCR), in a large cohort of 409 samples. The performance of the OGM workflow at five clinical sites (multiple operators and instruments) was assessed to demonstrate the robustness and consistency of the technology. Last, a consensus workflow and interpretation protocol was developed to efficiently filter and classify variants detected by OGM, to establish concordance with SOC results.
Materials and Methods
Cohort Design
This retrospective, double-blinded, observational study was approved through multiple institutional review boards (IRBs) and included 409 independent de-identified samples representing 233 unique individuals. This study included individuals suspected of having a genetic condition and having received a previous genetic test and parents of those individuals who are suspected to be carriers of a pathogenic/likely pathogenic structural variant. The study intent was to include a variety of SV classes and AOH result types (eg, deletion, duplication, insertion, aneuploidy, inversion, translocation, repeated expansion in FMR1, repeated contraction of DUX4, and other complex variants) so that the classes of variants and results identified between SOC and OGM technology can be maximized. Exclusion criteria were established for the following variant types: single-nucleotide variants and insertions/deletions, robertsonian translocations, balanced centromeric translocations, balanced variants with breakpoints in large low-copy repeats, and mosaic cases at <20% cellular fraction. In addition, 58 samples were excluded from the analysis because they were parents of probands with no known clinically relevant SVs (n = 31), sample mix-up occurred during the process (n = 17), or they did not meet inclusion criteria on analysis (n = 10). This study has been registered at https://clinicaltrials.gov, identifier: NCT05295277 (last accessed December 13, 2022). Raw data are available on request.
Ethics Declaration
The study was conducted according to the guidelines of the Declaration of Helsinki and approved by the following IRBs: A-BIOMEDICAL I (IRB registration number 00000150), Augusta University; Human Assurance Committee IRB number 611298; Western IRB–Copernicus Group, study number 20203726 (University of Rochester Medical Center, Rochester, NY; Medical College of Wisconsin, Milwaukee, WI; Columbia University Medical Center, New York, NY; Greenwood Genetic Center, Greenwood, SC; Augusta University; Praxis Genomics; and University of Iowa Health Clinics); the Self Regional Healthcare IRB numbers Pro00085001 and Pro00107951 (University of Rochester Medical Center, Medical College of Wisconsin, Columbia University Medical Center, Greenwood Genetic Center, Augusta University, Praxis Genomics, and University of Iowa Health Clinics); and the Columbia University Human Subjects IRB, protocol number AAAT9083/M00Y01. This includes informed consent provided by individuals with newly collected samples or waived authorization for use of de-identified, banked samples. All protected health information was removed, and data were anonymized (coded and double blinded) before accessioning for the study.
Sample Processing
Ultra–high-molecular-weight DNA was isolated and labeled using Bionano Prep SP version 2 and DLS kits (Bionano Genomics, Inc.). Briefly, aliquots of frozen EDTA-stabilized blood samples or cryopreserved cells were thawed and counted. A total of 1.5 million cells were pelleted from supernatant, resuspended, and digested with lysis buffer and Proteinase K. DNA was precipitated with isopropanol and bound to a nanobind magnetic disk, washed successively, and then eluted into buffer overnight. Solubilized DNA was quantitated fluorometrically, then 750 ng was labeled at a 6-bp motif (CTTAAG) using a Direct Label and Stain assay. Labeled DNA solution was quantitated for quality control (QC), and then loaded on Saphyr chips for imaging. The fluorescently labeled DNA molecules were imaged sequentially across nanochannel arrays on a Saphyr instrument. An 800-Gbp throughput target was set for data collection for each sample.
Assay QC
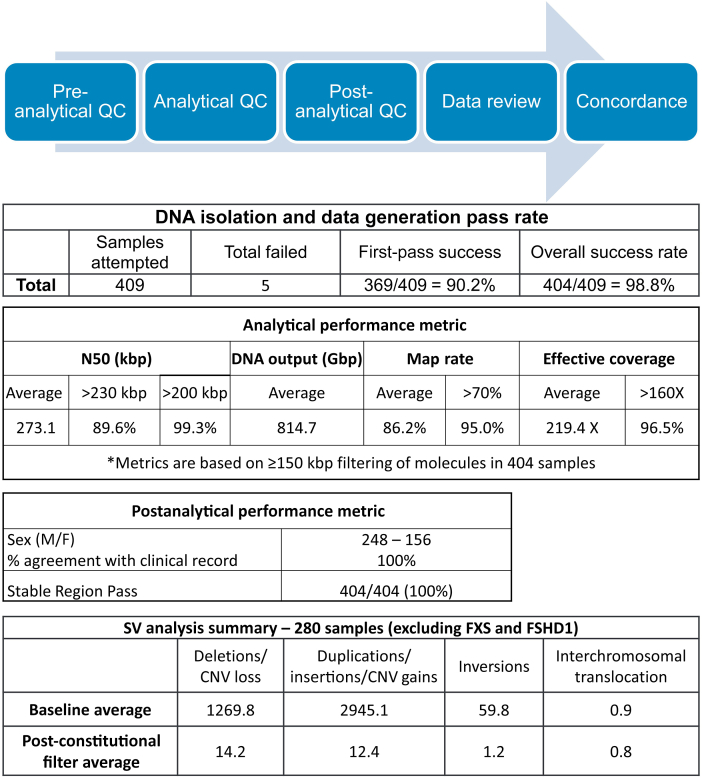
Ultra–high-molecular-weight DNA quality was assessed on the basis of observation of viscosity, and then concentration and homogeneity (36 to 150 ng/μL; CV for triplicate measurements <30%). The first-pass assay success rate, which includes DNA isolation, labeling, and Saphyr chip run, was defined on the basis of the output data meeting set QC thresholds on the initial assay run. The final pass rate was defined on the basis of output data meeting set QC thresholds, where one or more rounds of the assay were performed. A molecule quality report was generated for each data set and included three key metrics to evaluate sample QC: molecule N50 (≥150 kbp), map rate, and effective coverage. Molecule N50 is a weighted average of all molecules according to their sequence length and is used herein to assess the size distribution of DNA ≥150 kbp. The map rate metric is calculated as the fraction of the DNA molecules aligned to GRCh38. The effective coverage is calculated by the coverage depth of molecules aligned to the reference genome (GRCh38). Analytical QC targets were set to achieve 230 kbp of molecule N50 (≥150 kbp), ≥70% map rate, and ≥160× effective coverage of the genome.
Postanalytical quality control performance was determined by assessing sample sex and measurement of performance at stable regions of the genome. The EnFocus Fragile X Analysis pipeline (Bionano Genomics, Inc.) infers sex of each sample as well as pass/fail criteria for postanalytical quality metrics. The stable regions include one from each autosome, which has been established as stable in control populations. On the basis of expected sizing errors, the absolute percentage differences between the OGM map and the reference should not exceed 1.2%. The pipeline requires that at least 90% of the stable regions be under this threshold.15
Data Analysis
Genome analysis was performed using Bionano Solve software version 3.7, and OGM-specific pipelines were managed via Bionano Access software version 1.7 (Bionano Genomics, Inc.). De novo assembly was based on the overlap-layout-consensus paradigm. Initial consensus genome maps were generated by pairwise comparing all DNA molecules.15 Genome maps were further refined and extended with the best matching molecules. SVs were called on the basis of alignment between the de novo assembled genome maps and GRCh38. If the assembled map did not align contiguously to the reference but instead was punctuated by internal or end alignment gaps, then a putative SV was identified. Fractional copy number analyses were performed from the alignment of molecules and labels against GRCh38. A sample’s raw label coverage was normalized against relative coverage from human controls and segmented, and baseline copy number state was estimated from calculating mode of coverage of all labels.15 If chromosome Y molecules were present, baseline coverage in sex chromosomes was halved. With a baseline estimated, copy number states of segmented genomic intervals were assessed for significant increase/decrease from the baseline. Aneuploidies are called using normalized coverage comparisons between chromosomes. Simultaneously, AOH is also called when SV homozygosity segments are larger than expected compared with the distribution in a control sample database, and they will be detected when >25 Mbp and at germline fraction.
Genomic variants consistent with FXS and FSHD1 were accessed using Bionano EnFocus pipelines.15 For FXS analysis, the workflow uses bayesian probability to compute the CGG repeat size in the 5′ untranslated region of FMR1. The number of CGG repeats is inferred on the basis of the measured distance between two neighboring labels on the assembled map that contains FMR1. By incorporating a known set of control map measurements, the pipeline estimates the likeliest CGG repeat count with corresponding 99% credible interval (CI) lower/upper bounds, and a probabilistic model estimating that the expansion exceeds the pathogenic threshold of >200 repeat units.
More important, this assay is designed to estimate the FMR1 expansion length but is not precise enough for conclusive differentiation of normal-, intermediate-, and premutation-sized alleles or full expansions near the threshold length. Thus, this assay is not suitable for identification of individuals with premutations but can give an indication for follow-up. Analysts and laboratory directors used the following criteria to interpret FXS alleles in this study: if the upper bound of the 99% CI is <200 CGG repeats, the allele is categorized as negative for the full expansion; if the lower bound of the 99% CI is >200 CGG repeats, the allele is categorized as positive for the full expansion; and if the upper and lower bounds of the 99% CI straddle 200 CGG repeats, the allele is categorized as inconclusive. Visualization of the maps was done in all FXS cases to ensure fidelity of the allele and case classification. More important, inconclusive in this context refers only to the patient’s clinical status for full-expansion fragile X syndrome rather than failure of the assay. Diagnostic fragile X syndrome testing would be recommended for follow-up.
For FSHD1 analysis, molecules aligning to the D4Z4 regions of interest on chromosomes 4 and 10 were extracted and assembled. The resulting consensus maps were used to calculate D4Z4 repeat size and assigned genotype of permissive (4qA) and nonpermissive (4qB) alleles, as well as assembling and distinguishing D4Z4-spanning regions of 10q (which is not implicated in FSHD1).
SV Interpretation
Bionano Access software version 1.7 was used for filtering and classifying variants from the de novo assembly outputs. Variants were first filtered through a default set of baseline quality control filters, to remove calls with low support and possible technical artifacts. Laboratory analysts then applied a defined set of guideline filters to focus on the most relevant variants. These criteria excluded SVs at >1% population frequency in an OGM-specific controls database of >300 phenotypically normal individuals, excluded SVs not overlapping genes in GRCh38 within 3 kbp, excluded SVs <1.5 kbp, and included SVs and CNVs overlapping masked regions of GRCh38. After adding these variants to a curated list, analysts searched for intrafusion and translocation breakpoints with the GRCh38 gene overlap criterion removed. Any newly passing fusion would be suggestive of a large rearrangement, and would be successively included among curated variants regardless of gene overlap at the breakpoint.
SVs passing filtration steps were analyzed and classified into one of the following categories: pathogenic, likely pathogenic, variant of uncertain significance (VUS), likely benign, or benign, using ACMG guidelines.23 Classifications were based broadly around known pathogenic variants, size, gene or exon overlap, and/or potential severity in context of the given clinical indication. Variant classification was performed in Bionano Access software by two reviewers: first by the laboratory analyst (N.S.S., K.A., A.S., and G.S.), followed by a more advanced user/laboratory director (R.S., A.B., P.L.N., R.K., N.S.S., M.A.I., U.B., and B.L.) with visibility to the analyst’s review. Directors also included large-scale abnormalities (such as potential aneuploidies or AOH regions) not integrated into the curation and classification process described above, into the final summary.
Concordance Assessment
Unblinded Technical Concordance
OGM data were compared with the respective SOC test results to determine concordance of OGM with the SOC method. SOC test results and clinical information/phenotype were unblinded to a small analyst team not involved in the blinded clinical interpretation. Variant concordance was assessed by software calling, visual inspection, and manual curation. For control samples and undiagnosed cases, technical concordance was done by checking samples for any large events that would be detected on the respective negative SOC method (eg, no deletion/duplication syndromes in undiagnosed individuals who underwent CMA).
Blinded Technical Concordance
OGM data were interpreted as previously described through the dual-reviewer process by individuals who did not have access to SOC results. This step measures the ability of the algorithm and process to deliver the variant for classification without prior knowledge of the expected result.
Classification Agreement
Variants delivered through the dual-reviewer and blinded technical concordance step were classified as pathogenic, likely pathogenic, variant of uncertain significance, or likely benign. Reviewers had access to clinical indication for testing and sex but not SOC test results. Cases were considered concordant or in agreement if the SOC and dual review yielded the same clinical classification. Only pathogenic/likely pathogenic classifications, which were lumped for clinical purposes, were considered. Specifically, an SOC result of pathogenic with an analyst/director sign off of likely pathogenic was considered concordant, whereas an SOC result of VUS and an analyst/director classification of likely pathogenic was considered discordant or in disagreement.
Overall, comparative analysis with SOC testing was performed for three metrics: i) unblinded technical concordance, defined as OGM's ability to detect what the SOC test identified; ii) blinded technical concordance, defined as reporting the variant from SOC through blinded analyst/laboratory director review process; and iii) clinical concordance, defined as a matching classification for the variant. In addition, intrasite and intersite replicate analysis was performed.
Average Unique SV Counts by Type
The de novo assembly analysis pipeline generates annotated variants, which are then assessed in Bionano Access software version 1.7. Herein, the SVs from a single sample are subjected to a baseline set of filters, which pass thousands of variants in an average sample and include polymorphic variants. Subsequently, an analyst can use the constitutional variant filtering guidelines to generate a relatively small number of variants that require review and classification, shown in the post-filter row. The workflow requires variants to be ≥1.5 kbp, nearly absent in a control database (≤1% presence), and to overlap a gene within 3 kbp. The data below are from 280 samples whose SVs were analyzed genome-wide (excluding FXS and FSHD1 cases) (Table 1).
Table 1.
Average Unique SV Counts by Type
| SV analysis summary | Deletions/CNV loss | Insertions/duplications/CNV gains | Inversion breakpoints | Interchromosomal translocation breakpoints |
|---|---|---|---|---|
| Baseline average (prefilter) | 1269.8 | 2945.1 | 59.8 | 0.9 |
| Post-constitutional filter average | 14.2 | 12.4 | 1.2 | 0.8 |
CNV, copy number variation; SV, structural variation.
Results
Cohort Description and SOC Testing
Of the 409 samples (233 unique cases) included in the study, 404 (5 failed assay) samples underwent variant analysis. Of the 404 samples, 228 were unique cases and 176 were additional replicate runs that were fully analyzed and classified. Samples in this double-blinded study were grouped into three categories: i) cases with a genetic diagnosis (n = 332), ii) cases without a genetic diagnosis (n = 22), and iii) unaffected controls (n = 50). The undiagnosed category included affected individuals without a known genetic diagnosis after receiving negative or nondiagnostic results from CMA, karyotype, fluorescence in situ hybridization, and/or other methods. Figure 1 shows the variant classes according to SOC testing that were assessed for concordance evaluation in this study (Supplemental Table S1). SOC testing was primarily performed by karyotyping, fluorescence in situ hybridization, CMA, Southern blot analysis, or PCR.
Figure 1.
Sample cohort. A: The pie chart shows the variant classes according to standard-of-care (SOC) testing that were assessed for concordance evaluation in this study. B: The techniques used in SOC testing. n = 404 (B). CMA, chromosomal microarray analysis; FISH, fluorescence in situ hybridization; FSHD1, facioscapulohumeral dystrophy 1; NGS, next-generation sequencing.
QC Performance
All samples passed initial preanalytical requirements, including a cell count of at least 1.5 million cells per aliquot for blood and cell culture samples measured using a HemoCue WBC Analyzer (HemoCue Holding AB, Ängelholm, Sweden) and hemocytometer, respectively. During the study, 11 cell samples failed as a result of having low viability and were excluded from the study. For subsequent samples, cell viability was assessed as a preanalytical QC metric.
First-pass success rate was evaluated across five sites (Figure 2): 369 of 409 (90.2%) sample assessments met data generation guidelines on the first attempt (one DNA preparation, one labeling reaction, and one flow cell run). Of the samples requiring repeated sample preparations, all but five were finally successful, resulting in an overall success rate of 404 of 409 (98.8%). For specific molecule QC targets, among the 404 samples, 89.6% achieved the N50 target of >230 kbp and 99.3% had an N50 of >200 kbp, 95.0% of samples met or exceeded 70% map rate, and 96.5% had effective coverage >160× (Figure 2). The final average analytical QC metrics were as follows: N50 (>150 kbp) of 273.1 kbp, map rate of 86.2%, and average effective coverage of 219.4×.
Figure 2.
Success rates, analytical quality control (QC) metrics, postanalytical QC metrics, and structural variation (SV) calling. First-pass success rate is defined as data meeting approximate QC standards with a single round of laboratory work (extraction, labeling, and chip flow cell). The overall success rate is defined as data meeting approximate QC standards in which one or more rounds of laboratory work were required. The results of analytical and postanalytical QC metrics, as defined in Materials and Methods, are shown. Average unique SV counts by type are provided in Table 1. F, female; M, male; CNV, copy number variation; FSHD1, facioscapulohumeral dystrophy 1; FXS, fragile X syndrome.
For postanalytical QC, a pass/fail criterion was used to assess assembly of 22 stable regions of the genome (one region per autosome) as internal controls. All 404 samples passed this QC of stable regions and measurement accuracy. A corresponding postanalytical inference of sex determined 248 male and 156 female data sets (Figure 2), which was 100% concordant with the accessioning information for these samples.
Whole-Genome SV Calls and SV Filtering
SV calls in each sample were made by running the de novo assembly pipeline. Approximately 800 Gbp of data were collected from each sample, and at that depth of coverage, mosaic variants down to approximately 20% mosaic cell fraction can be detected.24,25 For samples that underwent whole-genome analysis (280; excluding FXS and FSHD1 indication cases), the average number of SVs detected was 4275.6 variants per sample (Figure 2). These variants were filtered on the basis of size, frequency, and relative position to genes defined in an SV filtration protocol devised for this study. Size cutoff for SVs was 1500 bp, and 500 kbp for CNVs based on the molecule coverage algorithm. The study focused on variants with <1% frequency in the OGM control sample SV database, which were overlapping or near genes (SVs within 3 kbp). In the same 280 samples, this filtering scheme generated an average of 28.6 SVs per sample that required clinical assessment (Figure 2).
FXS and FSHD1 Analysis
In total, there were 93 samples classified as positive for an FMR1 full expansion, one of which was manually determined to be positive after reflex to whole-genome analysis and subsequent detection of an expansion by that pipeline. A second case was calculated to have 2841 repeats but, on manual inspection, it was deemed to be an incorrect call resulting from a missing label and was manually assigned negative for full expansion. All other samples were automatically called negative for full expansion or inconclusive. Two inconclusive samples were from cases with SOC classification of premutation (PUBKH-00294) or full mutation near 200 repeats (PUBKH-00251). A final inconclusive case with a 99% CI lower bound of 198 repeats had an SOC result for a male patient of 800 and 300 to 1000 (PUBKH-00247). In this blinded analysis, 100% of samples with >200 repeats from SOC were positive for full expansion or inconclusive (Supplemental Table S2).
For FSHD1 analysis, molecules aligning across the D4Z4 regions of chromosomes 4 and 10 were extracted and assembled. The resulting consensus maps were used to calculate D4Z4 repeat size and assign genotype of the permissive (4qA) and nonpermissive (4qB) alleles, as well as assembling and distinguishing D4Z4-spanning regions of 10q (which is not implicated in FSHD1). Also, copy number gains and losses overlapping the SMCHD1 gene on chromosome 18 are also interrogated and were negative in these analyses. In total, 16 samples had clinical indications of FSHD1, and results from the OGM analysis were 100% concordant.
Concordance of OGM with SOC Testing Results
Technical Concordance
This study reveals 99.5% (399/401) of samples were fully concordant (Figure 3, Figure 4, Figure 5) with SOC (three samples that were inconclusive for FMR1 expansions were not considered concordant or discordant) based on an unblinded assessment of the presence or absence of variants initially called by SOC. The remaining two samples were partially concordant (finding pathogenic/likely pathogenic but missing VUS). The two missed VUS CNVs were as follows: PUBKH-00009, a terminal deletion of Xq28 pseudoautosomal region 2. The OGM result was instead a CNV loss of pseudoautosomal region 2 on Yq12, and the map of Xq28 was intact, suggesting a true Yq12 deletion that the microarray could not accurately distinguish between the highly homologous pseudoautosomal region 2 of the X and Y chromosomes. Also, PUBKH-00113, a terminal duplication of 19p13.3 was not called but could be observed as part of a derivative chromosome der(11)t(11;19). An additional two cases deserve further discussion: PUBKH-00211, a 300-kb duplication of Xq25 (hg19, chromosome X:125,128,547-125,510,298; and hg38, chromosome X:125,994,565-126,376,315) was called by a low-resolution microarray (OGT X chromosome exon array; OGT, Tarrytown, NY) but was not detected by OGM. However, this case was reanalyzed by Illumina (San Diego, CA) Infinium Global Screening Array version 2.0, and it was negative for this duplication, suggesting a false positive on the OGT array. Also, PUBKH-00212, a mosaic 20q13.33 terminal deletion (40% cells) reported by clinical fluorescence in situ hybridization testing (CytoCell number LPT 20R, OGT), was not detected by OGM. A follow-up test by CMA (Illumina Global Screening Array version 2.0) did not confirm this 20q deletion, and most likely suggests a sample mix up. Because CMA and OGM could not detect the deletion, the results were deemed concordant.
Figure 3.
Representative examples of concordant microduplication and microdeletions. A: A case with a 4.3-Mbp deletion at chromosome (Chr) 10q11.22q11.23 (Chr10:45,794,475-50,135,024; hg38) identified and highlighted (red) in the copy number variation (CNV) track. The deletion was also captured by Sample Map 1, generated by the de novo assembly algorithm. The bottom shows a diagram of the chromosomal structures predicted by the assembled maps illustrated by blocks, with arrowhead indicating direction. B: The concordant chromosomal microarray analysis (CMA) result of the Chr10 deletion (Chr10:46,900,000-51,800,000; hg19) from the same sample (visualized by NxClinical software version 6.0; BioDiscovery, a Bionano Genomics Company, El Segundo, CA). C and D: A case with 22q11.2 low copy repeat (LCR) D-F (distal type I) deletion of 2.2-Mbp deletion (Chr22:21312519-23542560; hg38) as shown in the CNV track and de novo assembly (Sample Map 1). C: At the bottom, LCR D-F deletion is illustrated by the loss of blocks B through E. A complex configuration of the LCR22 D segment was also noted in the predicted genotype 2. D: The CMA result (generated by ChAS software version 4.3.0.71; ThermoFisher Scientific, Santa Clara, CA) shows the concordant Chr 22q11.2 deletion (Chr22:21,922,619-23,654,064; hg19). E: A case with a 19.7-Mbp copy number gain of Chr 7q21.3q31.2 (Chr7:96198684-115928803; hg38) is highlighted in blue, and the tandem duplication of this region is depicted by Sample Map 1 and illustrated at the bottom (F). The concordant CMA result of the Chr7 duplication (Chr7:95,731,159-115,400,069; hg19; NxClinical software version 6.0). BAF, B allele frequency; Ref, reference.
Figure 4.
Representative examples of concordant structural variations (SVs). A: Left panel: A gain of chromosome (Chr) X is shown. Right panel: The whole-genome view copy number variation (CNV) track depicting the gain of Chr X. B: Left panel: Rearrangement between Chr11 and Chr22 from an individual with Emmanuel syndrome [47, XX,+der(22)t(11;22)(q23;q11)] is shown in the circos plot. Right panel: The unbalanced translocation was captured by Sample Map 1, also showing the copy number gain resulting from a der(22) chromosome (CNV tracks support the gains of Chr11 and Chr22). C: Left panel: A circos plot showing a balanced translocation detected between chromosomes 4q27 and 15q11.2, as indicated by a magenta line between Chr4 and Chr15. Right panel: The same translocation event was captured by Sample Map 1. D: An insertional translocation event of a 419-kbp region of 1p35.1-p34.3 (Chr1:34203802-34622531; hg38; partially including C1orf94 gene) is inserted into Xq21.31 (ChrX:88,741,968-88,777,061; hg38; near CPXCR1 gene). Ref, reference.
Figure 5.
Representative examples of concordant structural variations (SVs). A: A 275-kbp tandem duplication (red arrows) was identified involving multiple genes, including PPARG, TSEN2, MKRN2, and RAF1, shown in Sample Map 1. B: A 345-kbp intragenic deletion involving exons 1 to 14 of the KMT2C gene is shown in Sample Map 1. C: A 29.8-kbp single-exon deletion involving exon 2 of the PAFAH1B1 gene was identified and indicated (red line) in the SV track. D: A 174-kbp hemizygous deletion involving exon 45 of the DMD gene is shown in Sample Map 1. Chr, chromosome; CNV, copy number variation; Ref, reference.
Blinded Variant Filtering and Calling Concordance
In double-blinded analysis of 401 cases, 390 were fully concordant, 5 were partially concordant, and 6 were discordant. Excluding one likely benign variant, and four cases with errors made during variant reporting, 98.5% (390/396 cases) were fully concordant in the double-blinded analysis of variant detection and classification. Comprehensive details of discrepancies and corrective actions can be found in Supplemental Table S1. Briefly, reasons for missing variants were as follows: i) One case with an Xq28 deletion was discussed above and is likely benign (PUBKH-00009). ii) Four cases contained unreported variants as a result of oversights during variant analysis: two cases with AOH were called by the software but not recorded in the results; one case (PUBKH-00208) of t(X;1) was called by the software but not recorded in the results; and one case (PUBKH-00113) of der(11)t(11;19) was called by the software but only partially recorded in the results, as discussed above. iii) Six cases had missed variants, not detected or filtered out, and were not reported. There were two cases (PUBKH-00110 and PUBKH-00154) of MECP2 duplications (76 and 280 kbp) on chromosome Xq28. These duplications were present in the unfiltered data and would have been detected by a targeted analysis (ie, carrier testing). Another case (PUBKH-00202) harboring a pathogenic recessive variant at 15q15.3 was filtered out on the basis of 2.3% frequency in the Bionano control database, which would have been assessed on the basis of phenotype-driven analysis. A case with an inverted duplication at chromosome 15q11.1q13.2 (PUBKH-00007) demonstrated the duplication by OGM but did not reveal the orientation of the duplication (albeit visible by manual inspection). In a case with a pathogenic approximately 500 kbp telomeric 19p13.3 deletion (PUBKH-00143), the variant calling required visual inspection and reporting. PUBKH-00200 contained an AOH region of 25 Mbp that was called, but the confidence score was below the default threshold.
Blinded Classification Concordance
Agreement in classification (matching of variant classification from this study to SOC) was assessed for 373 samples, excluding FXS inconclusive (n = 3) and cases where SOC classification was not given (n = 28). Of these cases, 364 (97.6%) were in classification agreement (considering only pathogenic and likely pathogenic variants and binning both groups together), 3 (0.8%) were in partial agreement, and 6 (1.6%) were discordant (Table 2). For more detail, Supplemental Tables S1 and S2 contain information regarding the differences for each of the unblinded, blinded, and classification agreement steps, and FXS results, respectively. Classification differences were as a result of missing/not reporting variants, as discussed above, as well as variants that were called but classified differently because of new genomic information and ACMG classification guidelines compared with the initial clinical testing.
Table 2.
Concordance of OGM with SOC Testing Results
| Variable | Unblinded technical concordance | Classification agreement |
|---|---|---|
| Unclassified | NA | 28 |
| Concordant | 399/401 (99.5) | 364/373 (97.6) |
| Partially concordant | 2/401 (0.5) | 3/373 (0.8) |
| Discordant | 0 | 6/373 (1.6) |
| Inconclusive (excluded from concordance rate) | 3 | 3 |
| Total | 404 | 404 |
Data are given as number or number/total (percentage). Pathogenic variants, likely pathogenic variants, and some reported variants of uncertain significance, detected by Southern blot analysis, PCR, next-generation sequencing, karyotype, fluorescence in situ hybridization, and/or chromosomal microarray analysis, were evaluated in this data set. Technical concordance: samples were assessed for each variant according to the SOC results using software calling, visual inspection, and manual curation. Classification agreement: samples were assessed in a blinded manner by dual-analyst/reviewer process without knowledge of SOC result and only sex and indication for testing. Classification resulting from blind analysis and classification of each case were compared with the SOC (for this purpose, pathogenic and likely pathogenic classifications were grouped together). Details are in Supplemental Table S1.
NA, not applicable; OGM, optical genome mapping; SOC, standard of care.
Novel SV Findings by OGM
A total of 41 of 228 (18.0%) samples with full genome evaluation (excluding control, FXS, and FSHD1 samples) had additional clinically relevant findings or pertinent information provided by OGM. For the undiagnosed disease cohort, 6 of 22 (27.3%) cases had compelling candidate variants. Clinical correlation and/or orthogonal confirmatory testing is underway, and details can be found in Supplemental Tables S1 and S3.
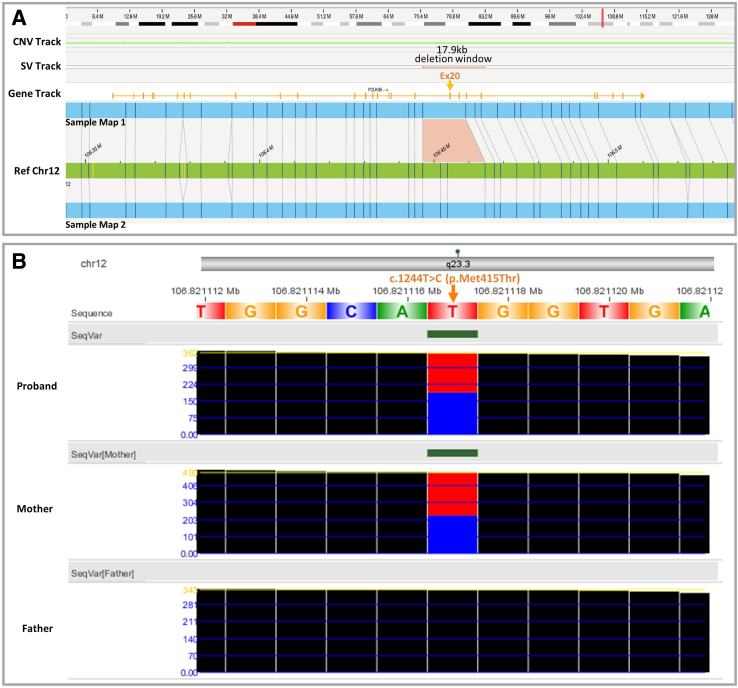
In one particular case, OGM revealed an approximately 5.5 kbp deletion at 12q23.3 [ogm(GRCh38) 12q23.3(106446738_106464666)x1] (Figure 6). The 12q23.3 deletion is likely inclusive of coding regions of the POLR3B gene, associated with an autosomal recessive leukodystrophy syndrome characterized by mildly delayed or normal early development, ataxia, and cerebellar atrophy (Mendelian Inheritance in Man number 614381). Interestingly, when initially referred for genetic testing, the individual was a <5-year–old boy presenting with ataxia, hypotonia, cerebellar atrophy, and speech delay. Early development was normal, with concerns beginning around 18 months. To determine the role of POLR3B gene disruption due to the deletion, RNA sequencing was performed, which suggested POLR3B exon 20 skipping. More important, the patient's prior whole-exome sequencing data identified a maternally inherited, likely pathogenic missense variant in POLR3B gene (exon 13). In addition, the CNV analysis of the exome and genome sequencing data in NxClinical version 6.0 software (BioDiscovery, a Bionano Genomics Company, El Segundo, CA) confirmed the exon 20 deletion to be paternally inherited. These findings are most likely consistent with a diagnosis of autosomal recessive leukodystrophy.
Figure 6.
A: Representative novel finding by optical genome mapping: a 5.5-kb deletion of 12q23.3, likely including exon 20 of the POLR3B gene [ogm(GRCh38) 12q23.3(106446738_106464666)x1]. The predicted 5.5-kb deletion is located within the 17.9-kb window indicated in the structural variation (SV) track. This gene is associated with an autosomal recessive leukodystrophy syndrome that matches the patient phenotype. B: Trio exome sequencing data revealed a maternally inherited sequence variant [exon 13; c.1244T>C(p.Met413Thr)] and could confirm a paternally inherited exon 20 deletion. Also, RNA sequencing results support the exon 20 deletion. Chr, chromosome; CNV, copy number variation; Ref, reference; SeqVar, sequencing variants.
Replicate Analysis (Intrarun, Interrun, and Intersite)
There were 242 data sets from a total of 66 unique cases used to assess the reproducibility of the assay and workflow.
FMR1 full expansion cases were represented by 33 unique cases and 93 data sets. Expansions >200 repeats were detected in 93 of 93 measurements, showing 100% concordance with the full expansion by SOC. Of the 33 unique FXS cases run in replicates, the expansion measurements had an SD of 46 CGG repeats. There were two cases that deserve more attention: In a female patient with two replicate runs (PUBKH-00251 and PUBKH-00252), the CGG repeat measurements were 89 and 301 for her two alleles and classified as positive for full expansion. The other run contained two alleles at 166 repeats each, suggesting that her alleles were erroneously averaged. Another sample (PUBKH-00271) from a female patient harboring a full expansion was run in triplicate, where the EnFocus Fragile X software successfully called the full expansions in two of the three replicates but erroneously averaged the two alleles into a single one in the third replicate. In both of these cases, examination of the de novo assembly revealed the full expansion; hence, they are considered concordant.
There were two FSHD1 cases represented by seven data sets. The pathogenic contractions were detected in all replicates, demonstrating 100% concordance (Supplemental Table S4 and Supplemental Figure S1).
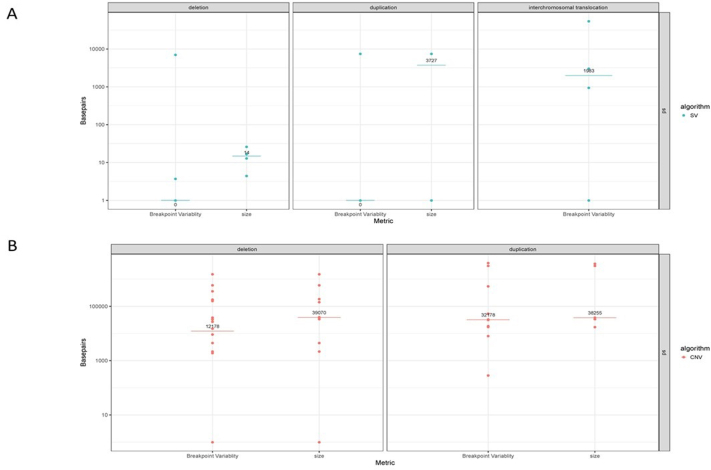
The authors evaluated the precision of SV and CNV sizes and breakpoints using 23 known pathogenic variants, which amounted to 130 total replicated variants (74 deletions, 39 duplications, and 17 translocations). Initially, nine replicates did not show concordance; they all localized to the same region [19.6 Mbp duplication at 7q21.3q31.2 (chromosome 7:96,154,726-115,831,386)], and on manual inspection/rereview, it was observed that the expected duplication was fragmented into two adjacent calls that spanned the entire known pathogenic region. Manually merging the two adjacent calls into one call revised the reproducibility to 100%. Some SVs can be detected by the SV pipeline (when a unique fusion is generated and can be uniquely read), others can be detected only by the CNV coverage pipeline (eg, numerical aberrations), and others can be detected by both. The replicate analysis shows that SVs detected by the CNV pipeline have lower resolution and size agreement (median positional SD = 38,359 bp; size SD = 22,939 bp) compared with those detected by the SV/fusion pipeline (median positional SD = 0 bp; size SD = 137 bp) (Supplemental Table S4 and Supplemental Figure S1).
Discussion
Several medical organizations and societies (including but not limited to the ACMG, American Academy of Pediatrics, and American Academy of Neurology) recommend CMA as the first-tier test in the genetic workup for individuals with multiple congenital anomalies and neurodevelopmental disorders, such as developmental delay, intellectual disability, and autism spectrum disorder.4, 5, 6, 7, 8, 9 Sequencing-based assays, which can complement CMA by detection of pathogenic/likely pathogenic single-nucleotide variants and insertions/deletions, are often performed on negative first-tier assessment. Because next-generation sequencing methods are refractory to the detection of many SVs due to challenges in mapping to nonunique genomic regions,26 other SV tools are still essential. As a result of the various limitations of current technologies, the SOC genetic testing workup often includes several cytogenetic and molecular testing modalities to reach a conclusive genetic diagnosis, which can be a tedious and time-consuming process, causing significant economic and emotional burden to the patients and their families. Despite using a battery of genomics tests, a significant percentage of cases (approximately 50%) still remain genetically undiagnosed.27 Recently, long-read technologies have emerged as promising tools that reveal the genomic complexity with much higher resolution and accuracy compared with SOC methods. However, a recent side-by-side comparison study of OGM with long-read sequencing technology (PacBio HiFi reads; PacBio, Menlo Park, CA) by the Human Genome Structural Variation Consortium revealed a higher sensitivity of OGM for detecting large SVs.28
OGM has demonstrated the potential to consolidate multiple SOC methods because of its ability to detect several classes of SVs with high sensitivity and specificity.14,15 Recently, published studies have demonstrated 100% concordance between OGM and SOC methods.21,22 However, the technical performance of OGM across multiple sites with large data sets has not yet been assessed. In this study, 409 samples representative of diverse constitutional disorders with varying clinical indications and chromosomal abnormalities were tested by OGM. The analytical validity and technical performance of OGM were assessed across five different Clinical Laboratory Improvement Amendments–certified laboratories in the United States. The robustness of OGM is demonstrated by a first-pass success rate (requiring no repreparations) of 90.2% and an overall success rate of 98.8%, observed across sites, which is in line with clinical cytogenetic testing by karyotype and chromosomal microarray.
One important aspect of this study was to define key specification and performance indicators to evaluate and assess the performance of this novel technology. The following key analytical QC metrics for OGM were assessed and set as targets through the evaluation of samples in this study (Figure 2):
-
•
Minimum map rate of molecules aligning to the reference was set at 70% and was achieved with 95.0% of samples in this study;
-
•
Average number of molecules mapped to the genome versus total molecules (ie, effective coverage was set to 160× and was achieved with 96.5% of samples);
-
•
Molecule N50 (≥150 kbp), a weighted average of molecule length distribution, was >200 kbp for 99.3% of samples.
Samples that had performance metrics below these levels were also analyzed and included in this study and were determined to be successful for SOC concordance. However, some deficiencies are expected when assay performance is significantly below the established targets. For example, low map rate could lead to higher noise for the coverage-based CNV algorithm, and interpretation of those CNV calls needs to be scrutinized. Also, lower effective coverage could potentially reduce the sensitivity for mosaic variants.
In addition, in a College of American Pathologists/Clinical Laboratory Improvement Amendments setting, the postanalytical QC metrics allow for assessing the fidelity of the assay. The postanalytical QC assessment measured known stable regions (defined by having no SVs in control samples); and in all 404 tested samples, these metrics were passed (Figure 2). The second aspect of this QC metric was the inference of the sex of the cases, and, in this study, all sex inferences matched the original accession information. These measurements not only serve as an internal control for the assay, but also provide relevant information for the possibility of chain-of-custody errors, such as sample mix-ups.
Although FXS testing is recommended for individuals with autism spectrum disorder/developmental delay/intellectual disability,9 the diagnostic yield is only 0.5% to 2%.10 Considering that CMA is also recommended and often done in parallel with FXS testing, it would be highly advantageous to combine FXS testing with SV testing. The authors ran OGM's EnFocus Fragile X tool for postanalytical QC and to assess potential CGG expansions in the 5′ untranslated region of the FMR1 gene. Of 404 samples, 401 in this study resulted with a conclusive clinical classification of not full expansion or full expansion. The SOC testing for these cases was in 100% agreement (Supplemental Table S2). Regarding the cases with collapsed (erroneously averaged) alleles with the EnFocus Fragile X analysis, it is always recommended to also review the fragile X locus in the de novo annotation pipeline results, which will greatly minimize the possibility of false-negative results from the fragile X pipeline alone. Of the three samples classified as inconclusive, SOC indicated premutation or full expansion near the 200-unit threshold. For these samples, reflex testing by another method, like PCR, would be recommended. OGM is not suitable for identification of individuals with permutations but can give an indication for follow-up testing, similar to the SOC for PCR-based testing when repeat lengths are large, is to reflex to Southern blot analysis.
For 280 samples undergoing full-genome review (excluding FXS and FSHD), there were an average of 4276 unique SVs per sample. It has been well established that SVs called by OGM are highly accurate and precise.22,26,27,29 However, they are mostly polymorphic and/or benign; therefore, a filtering strategy was devised to prioritize clinically relevant SVs. On the basis of a standardized variant filtration strategy (a maximum 1% population frequency, confidence thresholds, proximity to genes, and a minimum SV size), the number of variants was significantly reduced to 28.6 per sample (Figure 2), thus filtering out >99% of the initial variants. Remaining variants could be classified in the Bionano Access graphical user interface, which enables the categorization of variants into ACMG classifications (pathogenic, likely pathogenic, VUS, likely benign, and benign). The analysis of each case was facilitated by the ability to use several readily available tools, such as an integrated comparison to the Database of Genomic Variants, disease-related loci, as well as link out to University of California, Santa Cruz, genome browser for each SV. Classification was done in Bionano Access by a variant analyst, followed by final review by an American Board of Medical Genetics and Genomics– or American Board of Medical Specialties–certified laboratory director or equivalent to make the final variant classification and generate The International System for Human Cytogenomic Nomenclature (ISCN) for each case.
Of the 401 samples evaluated (after exclusion of three inconclusive fragile X results) for technical concordance (unblinded), 399 (99.5%) were completely concordant with SOC results. Blinded detection and classification agreement to the SOC was 97.6% (364/373). Reasons for missing variants can be summarized into the following categories: variants not called because they lie in complex regions of the genome, where the reference is poorly assembled; user errors, which can be reduced by improving the analysis software to better capture important variants; and increasing the number of samples in the built-in control database to assist in interrogating some variants that lie in large repetitive regions of the genome. Manual review of certain regions of the genome, such as Xq28, and reviewing the CNV and AOH profiles is strongly recommended to alleviate some of the discrepancies identified in this study. This process is analogous to the standard practice followed by analysts and directors while reporting whole-genome CNV or AOH by CMAs.
This study has primarily focused on genetically diagnosed cases and the ability of OGM to detect SVs concordant with SOC, and assess OGM performance across multiple sites, instruments, and operators. In 22 cases with no genetic diagnosis by SOC, candidate SVs were detected that could potentially help to explain the genetic cause for the conditions of these six cases (Supplemental Table S3). Future studies will more extensively characterize the ability of OGM to detect clinically relevant variants refractory by SOC testing and to provide higher resolution and better characterization of variants in undiagnosed cases as a continuation of this study and others.30, 31, 32, 33 OGM was able to detect all classes of structural variation and repeated expansion/contraction disorder-related mechanisms. Overall, OGM provides a simplified and streamlined workflow that tackles the diagnostic odyssey of individuals with rare diseases in an efficient and effective manner.32
Besides single-nucleotide variants, insertions/deletions, and other small variants, OGM has some known limitations for structural variation that were considered in the design of this study (ie, inability to detect balanced centromeric translocations and balanced robertsonian translocations). The authors also used a workflow with approximately 800 Gbp of data, which provided an expected limit of detection of 20% mosaics. These balanced translocations are not known to cause constitutional disorders but are of concern from a reproductive health outcome because there is the possibility of having an offspring with an unbalanced genomic rearrangement. This study also identified cases where a more in-depth analysis can help in the accurate detection of variants falling in highly homologous regions of the genome, such as pseudoautosomal region 2 (Xq28/Yq12). Improvements in the control databases and potential incorporation of an alternative reference based on the telomere to telomere complete genome assembly34 are expected to improve performance of OGM.
Conclusions
Our results from this study demonstrate exceptional technical performance of the OGM workflow from DNA isolation through data analysis in a large cohort of postnatal cases. OGM could potentially replace the current first-tier techniques, and therefore enhance SV detection as part of the first step in the diagnostic workup. Furthermore, the intersite, interrun, and intrarun performance in this study demonstrates the reproducibility of the OGM technique for easy adoption and validation as a laboratory-developed test by Clinical Laboratory Improvement Amendments laboratories. The novelty of OGM is not limited to CNV analysis alone, as it is also able to resolve balanced structural rearrangements and size both FMR1 repeated expansions and D4Z4 repeated contractions. Thus, in a single assay, OGM allows genomic testing laboratories to provide a comprehensive analysis of the genome via a single diagnostic result in a cost- and time-efficient manner while also reducing the economic burden on health care systems, affected individuals, and their families. Further expansion of the data set presented in this study is ongoing, and collaboration between researchers, clinicians, and families is underway to demonstrate the maximum benefit of OGM methods.
Acknowledgments
We thank the Greenwood Genetic Center, its clinical testing laboratory, and its affiliated physicians for participation and clinical acumen; without this expertise, many of these diagnoses would not have been made to enable the validation of new genomic technologies. In addition, we thank several other groups who assisted in patient recruitment for known genomic findings, including June Kinoshita (Facioscapulohumeral Muscular Dystrophy Society), the 4p- Society, the 5p- Society, the Balanced Translocation Support Group, the University of Utah Maternal-Fetal Medicine Center, and Jill Rosenfeld Mokry (Baylor's Undiagnosed Diseases Network); the following team at Bionano Laboratories (previously Lineagen, Inc.; Clinical Laboratory Improvement Amendments number 46D2042721): genetic counselors for the recruitment of consented samples, accessioning staff for double blinding and shipping the specimens as per the institutional review board protocol, scientific affairs team that collaborated with the investigators at each site for data analysis, and for the final compilation of the multisite data for publication; the technical staff at each of the participating sites; and the children and families who submitted samples (which are often difficult to collect) to further genomic discovery.
Footnotes
Supported by Bionano Genomics, including coverage of chips and reagents as well as project management, bioinformatics, and analyst efforts; the Childrens Research Institute; Childrens Wisconsin; and the Department of Pathology and Laboratory Medicine, University of Rochester Medical Center (Rochester, NY).
M.A.I., U.B., B.L., R.S., and A.B. contributed equally to this work.
Disclosures: R.K. has received honoraria, and/or travel funding, and/or research support from Illumina, Asuragen, Qiagen, Perkin Elmer Inc., Bionano Genomics, Agena, Agendia, PGDx, Thermo Fisher Scientific, Cepheid, and BMS. N.S.S. owns a limited number of personal stocks of Bionano Genomics. P.L.N. owns a limited number of personal stocks in Bionano. All other authors declare no conflicts of interest.
Supplemental material for this article can be found at http://doi.org/10.1016/j.jmoldx.2022.12.005.
Contributor Information
Peter L. Nagy, Email: plnagy@praxisgenomics.com.
Ravindra Kolhe, Email: rkolhe@augusta.edu.
Author Contributions
B.L., R.S., A.B., P.L.N., and R.K. conceptualized the study; M.A.I., U.B., B.L., S.S., N.S.S., V.R., A.S., K.A., G.S., C.S., R.S., A.B., P.L.N., and R.K. curated and analyzed data, developed methodology, and visualized the data; N.S.S., R.S., P.L.N., and R.K. wrote the manuscript; and M.A.I., U.B., B.L., S.S., N.S.S., V.R., A.S., K.A., G.S., C.S., R.S., A.B., P.L.N., and R.K. wrote, reviewed, and edited the manuscript. All authors have read and agreed to the published version of the article.
Supplemental Data
Supplemental Figure S1.
Replicate analysis. A: Breakpoint variability of position 1 and position 2 for deletions (breakpoints = 30, samples = 15, and cases = 4), duplications (breakpoints = 18, samples = 9, and cases = 2), and intrachromosomal translocations (breakpoints = 34, samples = 17, and cases = 2) of the structural variation (SV) algorithm. B: Breakpoint variability of position 1 and position 2 for deletions (breakpoints = 118, samples = 59, and cases = 10) and duplications (breakpoints = 42, samples = 21, and cases = 5) of the copy number variation (CNV) algorithm. Medial SD is indicated by the horizontal line, and value is written.
References
- 1.Zablotsky B., Black L.I., Maenner M.J., Schieve L.A., Danielson M.L., Bitsko R.H., Blumberg S.J., Kogan M.D., Boyle C.A. Prevalence and trends of developmental disabilities among children in the United States: 2009-2017. Pediatrics. 2019;144:e20190811. doi: 10.1542/peds.2019-0811. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 2.Sudmant P.H., Rausch T., Gardner E.J., Handsaker R.E., Abyzov A., Huddleston J., et al. An integrated map of structural variation in 2,504 human genomes. Nature. 2015;526:75–81. doi: 10.1038/nature15394. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 3.Martin C.L., Ledbetter D.H. Chromosomal microarray testing for children with unexplained neurodevelopmental disorders. JAMA. 2017;317:2545–2546. doi: 10.1001/jama.2017.7272. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 4.Miller D.T., Adam M.P., Aradhya S., Biesecker L.G., Brothman A.R., Carter N.P., Church D.M., Crolla J.A., Eichler E.E., Epstein C.J., Faucett W.A., Feuk L., Friedman J.M., Hamosh A., Jackson L., Kaminsky E.B., Kok K., Krantz I.D., Kuhn R.M., Lee C., Ostell J.M., Rosenberg C., Scherer S.W., Spinner N.B., Stavropoulos D.J., Tepperberg J.H., Thorland E.C., Vermeesch J.R., Waggoner D.J., Watson M.S., Martin C.L., Ledbetter D.H. Consensus statement: chromosomal microarray is a first-tier clinical diagnostic test for individuals with developmental disabilities or congenital anomalies. Am J Hum Genet. 2010;86:749–764. doi: 10.1016/j.ajhg.2010.04.006. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 5.Manning M., Hudgins L., Professional Practice and Guidelines Committee Array-based technology and recommendations for utilization in medical genetics practice for detection of chromosomal abnormalities [published correction appears in Genet Med. 2020 Dec;22(12):2126] Genet Med. 2010;12:742–745. doi: 10.1097/GIM.0b013e3181f8baad. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 6.Shevell M., Ashwal S., Donley D., Flint J., Gingold M., Hirtz D., Majnemer A., Noetzel M., Sheth R.D., Quality Standards Subcommittee of the American Academy of Neurology; Practice Committee of the Child Neurology Society Practice parameter: evaluation of the child with global developmental delay: report of the Quality Standards Subcommittee of the American Academy of Neurology and The Practice Committee of the Child Neurology Society. Neurology. 2003;60:367–380. doi: 10.1212/01.wnl.0000031431.81555.16. [DOI] [PubMed] [Google Scholar]
- 7.Hyman S.L., Levy S.E., Myers S.M. Identification, evaluation, and management of children with autism spectrum disorder. Pediatrics. 2020;145:e20193447. doi: 10.1542/peds.2019-3447. [DOI] [PubMed] [Google Scholar]
- 8.Moeschler J.B., Shevell M., Committee on Genetics Comprehensive evaluation of the child with intellectual disability or global developmental delays. Pediatrics. 2014;134:e903–e918. doi: 10.1542/peds.2014-1839. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 9.Savatt J.M., Myers S.M. Genetic testing in neurodevelopmental disorders. Front Pediatr. 2021;9:526779. doi: 10.3389/fped.2021.526779. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 10.Abrams L., Cronister A., Brown W.T., Tassone F., Sherman S.L., Finucane B., McConkie-Rosell A., Hagerman R., Kaufmann W.E., Picker J., Coffey S., Skinner D., Johnson V., Miller R., Berry-Kravis E. Newborn, carrier, and early childhood screening recommendations for fragile X. Pediatrics. 2012;130:1126–1135. doi: 10.1542/peds.2012-0693. [DOI] [PubMed] [Google Scholar]
- 11.Manickam K., McClain M.R., Demmer L.A., Biswas S., Kearney H.M., Malinowski J., Massingham L.J., Miller D., Yu T.W., Hisama F.M. Exome and genome sequencing for pediatric patients with congenital anomalies or intellectual disability: an evidence-based clinical guideline of the American College of Medical Genetics and Genomics (ACMG) Genet Med. 2021;23:2029–2037. doi: 10.1038/s41436-021-01242-6. [DOI] [PubMed] [Google Scholar]
- 12.Dolzhenko E., Deshpande V., Schlesinger F., Krusche P., Petrovski R., Chen S., Emig-Agius D., Gross A., Narzisi G., Bowman B., Scheffler K., van Vugt J.J.F.A., French C., Sanchis-Juan A., Ibáñez K., Tucci A., Lajoie B.R., Veldink J.H., Raymond F.L., Taft R.J., Bentley D.R., Eberle M.A. ExpansionHunter: a sequence-graph-based tool to analyze variation in short tandem repeat regions. Bioinformatics. 2019;35:4754–4756. doi: 10.1093/bioinformatics/btz431. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 13.Álvarez-Mora M.I., Sánchez A., Rodríguez-Revenga L., Corominas J., Rabionet R., Puig S., Madrigal I. Diagnostic yield of next-generation sequencing in 87 families with neurodevelopmental disorders. Orphanet J Rare Dis. 2022;17:60. doi: 10.1186/s13023-022-02213-z. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 14.Barseghyan H., Tang W., Wang R.T., Almalvez M., Segura E., Bramble M.S., Lipson A., Douine E.D., Lee H., Délot E.C., Nelson S.F., Vilain E. Next-generation mapping: a novel approach for detection of pathogenic structural variants with a potential utility in clinical diagnosis. Genome Med. 2017;9:90. doi: 10.1186/s13073-017-0479-0. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 15.Chan S., Lam E., Saghbini M., Bocklandt S., Hastie A., Cao H., Holmlin E., Borodkin M. Structural variation detection and analysis using Bionano optical mapping. Methods Mol Biol. 2018;1833:193–203. doi: 10.1007/978-1-4939-8666-8_16. [DOI] [PubMed] [Google Scholar]
- 16.Dai Y., Li P., Wang Z., Liang F., Yang F., Fang L., Huang Y., Huang S., Zhou J., Wang D., Cui L., Wang K. Single-molecule optical mapping enables quantitative measurement of D4Z4 repeats in facioscapulohumeral muscular dystrophy (FSHD) J Med Genet. 2020;57:109–120. doi: 10.1136/jmedgenet-2019-106078. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 17.Stinnett V., Jiang L., Haley L. Adoption of optical genome mapping in clinical cancer cytogenetic laboratory: a stepwise approach. J Clin Anat Pathol. 2021;6:117. [Google Scholar]
- 18.Khan W.A., Toledo D.M. Applications of optical genome mapping in next-generation cytogenetics and genomics. Adv Mol Pathol. 2021;4:27–36. [Google Scholar]
- 19.Stence A.A., Thomason J.G., Pruessner J.A., Sompallae R.R., Snow A.N., Ma D., Moore S.A., Bossler A.D. Validation of optical genome mapping for the molecular diagnosis of facioscapulohumeral muscular dystrophy. J Mol Diagn. 2021;23:1506–1514. doi: 10.1016/j.jmoldx.2021.07.021. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 20.Sahajpal N.S., Barseghyan H., Kolhe R., Hastie A., Chaubey A. Optical genome mapping as a next-generation cytogenomic tool for detection of structural and copy number variations for prenatal genomic analyses. Genes (Basel) 2021;12:398. doi: 10.3390/genes12030398. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 21.Yang H., Garcia-Manero G., Rush D., Montalban-Bravo G., Mallampati S., Medeiros L.J., Levy B., Luthra R., Kanagal-Shamanna R. Application of optical genome mapping for comprehensive assessment of chromosomal structural variants for clinical evaluation of myelodysplastic syndromes. medRxiv. 2021 doi: 10.1101/2021.01.13.21249611. [Preprint] doi: [DOI] [Google Scholar]
- 22.Mantere T., Neveling K., Pebrel-Richard C., Benoist M., van der Zande G., Kater-Baats E., Baatout I., van Beek R., Yammine T., Oorsprong M., Hsoumi F., Olde-Weghuis D., Majdali W., Vermeulen S., Pauper M., Lebbar A., Stevens-Kroef M., Sanlaville D., Dupont J.M., Smeets D., Hoischen A., Schluth-Bolard C., El Khattabi L. Optical genome mapping enables constitutional chromosomal aberration detection. Am J Hum Genet. 2021;108:1409–1422. doi: 10.1016/j.ajhg.2021.05.012. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 23.Riggs E.R., Andersen E.F., Cherry A.M., Kantarci S., Kearney H., Patel A., Raca G., Ritter D.I., South S.T., Thorland E.C., Pineda-Alvarez D., Aradhya S., Martin C.L. Technical standards for the interpretation and reporting of constitutional copy-number variants: a joint consensus recommendation of the American College of Medical Genetics and Genomics (ACMG) and the Clinical Genome Resource (ClinGen) Genet Med. 2020;22:245–257. doi: 10.1038/s41436-019-0686-8. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 24.Sahajpal N.S., Mondal A.K., Fee T., Hilton B., Layman L., Hastie A.R., Chaubey A., DuPont B.R., Kolhe R. Clinical validation and diagnostic utility of optical genome mapping in prenatal diagnostic testing. medRxiv. 2022 doi: 10.1101/2022.05.11.22274975. [Preprint] doi: [DOI] [PubMed] [Google Scholar]
- 25.Sahajpal N.S., Mondal A.K., Tvrdik T., Hauenstein J., Shi H., Deeb K.K., Saxe D., Hastie A.R., Chaubey A., Savage N.M., Kota V., Kolhe R. Clinical validation and diagnostic utility of optical genome mapping for enhanced cytogenomic analysis of hematological neoplasms. J Mol Diagn. 2022;24:1279–1291. doi: 10.1016/j.jmoldx.2022.09.009. [DOI] [PubMed] [Google Scholar]
- 26.Chaisson M.J.P., Sanders A.D., Zhao X., Malhotra A., Porubsky D., Rausch T., et al. Multi-platform discovery of haplotype-resolved structural variation in human genomes. Nat Commun. 2019;10:1784. doi: 10.1038/s41467-018-08148-z. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 27.Mahmoud M., Gobet N., Cruz-Dávalos D.I., Mounier N., Dessimoz C., Sedlazeck F.J. Structural variant calling: the long and the short of it. Genome Biol. 2019;20:246. doi: 10.1186/s13059-019-1828-7. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 28.Mak A.C., Lai Y.Y., Lam E.T., Kwok T.P., Leung A.K., Poon A., Mostovoy Y., Hastie A.R., Stedman W., Anantharaman T., Andrews W., Zhou X., Pang A.W., Dai H., Chu C., Lin C., Wu J.J., Li C.M., Li J.W., Yim A.K., Chan S., Sibert J., Džakula Ž., Cao H., Yiu S.M., Chan T.F., Yip K.Y., Xiao M., Kwok P.Y. Genome-wide structural variation detection by genome mapping on nanochannel arrays. Genetics. 2016;202:351–362. doi: 10.1534/genetics.115.183483. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 29.Ebert P., Audano P.A., Zhu Q., Rodriguez-Martin B., Porubsky D., Bonder M.J., et al. Haplotype-resolved diverse human genomes and integrated analysis of structural variation. Science. 2021;372:eabf7117. doi: 10.1126/science.abf7117. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 30.Shieh J.T., Penon-Portmann M., Wong K.H.Y., Levy-Sakin M., Verghese M., Slavotinek A., Gallagher R.C., Mendelsohn B.A., Tenney J., Beleford D., Perry H., Chow S.K., Sharo A.G., Brenner S.E., Qi Z., Yu J., Klein O.D., Martin D., Kwok P.Y., Boffelli D. Application of full-genome analysis to diagnose rare monogenic disorders [published correction appears in NPJ Genom Med. 2021 Oct 12;6(1):88] NPJ Genom Med. 2021;6:77. doi: 10.1038/s41525-021-00241-5. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 31.Sabatella M., Mantere T., Waanders E., Neveling K., Mensenkamp A.R., van Dijk F., Hehir-Kwa J.Y., Derks R., Kwint M., O'Gorman L., Tropa Martins M., Gidding C.E., Lequin M.H., Küsters B., Wesseling P., Nelen M., Biegel J.A., Hoischen A., Jongmans M.C., Kuiper R.P. Optical genome mapping identifies a germline retrotransposon insertion in SMARCB1 in two siblings with atypical teratoid rhabdoid tumors. J Pathol. 2021;255:202–211. doi: 10.1002/path.5755. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 32.Fadaie Z., Neveling K., Mantere T., Derks R., Haer-Wigman L., den Ouden A., Kwint M., O'Gorman L., Valkenburg D., Hoyng C.B., Gilissen C., Vissers L.E.L.M., Nelen M., Cremers F.P.M., Hoischen A., Roosing S. Long-read technologies identify a hidden inverted duplication in a family with choroideremia. Hum Genet Genomics Adv. 2021;2:100046. doi: 10.1016/j.xhgg.2021.100046. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 33.Cope H., Barseghyan H., Bhattacharya S., Fu Y., Hoppman N., Marcou C., Walley N., Rehder C., Deak K., Alkelai A., Undiagnosed Diseases Network. Vilain E., Shashi V. Detection of a mosaic CDKL5 deletion and inversion by optical genome mapping ends an exhaustive diagnostic odyssey. Mol Genet Genomic Med. 2021;9:e1665. doi: 10.1002/mgg3.1665. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 34.Nurk S., Koren S., Rhie A., Rautiainen M., Bzikadze A.V., Mikheenko A., et al. The complete sequence of a human genome. Science. 2022;376:44–53. doi: 10.1126/science.abj6987. [DOI] [PMC free article] [PubMed] [Google Scholar]
Associated Data
This section collects any data citations, data availability statements, or supplementary materials included in this article.