Abstract
The cementum is a highly mineralized tissue that covers the tooth root. The regional differences among the types of cementum, especially in the extrinsic fibers that contribute to tooth support, remain controversial. Therefore, this study used second harmonic generation imaging in conjunction with automated collagen extraction and image analysis algorithms to facilitate the quantitative examination of the fiber characteristics and the changes occurring in these fibers over time. Acellular extrinsic fiber cementum (AEFC) was invariably observed in the superficial layer of the apical cementum in mouse molars, indicating that this region of the cementum plays a crucial role in supporting the tooth. The apical AEFC exhibited continuity and fiber characteristics comparable with the cervical AEFC, suggesting a common cellular origin for their formation. The cellular intrinsic fiber cementum present in the inner layer of the apical cementum showed consistent growth in the apical direction without layering. This study highlights the dynamic nature of the cementum in mouse molars and underscores the requirement for re-examining its structure and roles. The findings of the present study elucidate the morphophysiological features of cementum and have broader implications for the maintenance of periodontal tissue health.
Keywords: cementum, collagen, extrinsic fiber, periodontal ligament, second harmonic generation, Wnt/β-catenin signaling
Introduction
The cementum, a thin and highly mineralized tissue that covers the tooth root, anchors the tooth to the alveolar bone through the periodontal ligament (PDL), thereby playing an essential role in tooth support. 1 Although the cementum is often referred to as a bone-like tissue, unlike bone, it is avascular, does not undergo dynamic tissue remodeling, and continues to increase in thickness throughout life. 2 Moreover, the cementum has a distinct cellular origin 3 and a unique proteome profile 4 compared with that of bone, indicating the need to elucidate its unique properties.5,6
The cementum can be classified according to the status of two major elements: the presence or absence of cells (cementocytes) and the nature of the emb-edded fibers.2,7 Acellular extrinsic fiber cementum (AEFC), which contains extrinsic fibers but no embedded cementocytes, covers the cervical half of the root. The extrinsic fibers of AEFC are densely packed and connect to the principal fibers of the PDL nearly perpendicularly or oblique to the root surface, reflecting its essential role in tooth support.2,8,9 The apical half of the root and furcation area is covered by layers of cellular intrinsic fiber cementum (CIFC) and occasionally AEFC, the combination of which is referred to as cellular mixed stratified cementum (CMSC). CIFC often contains extrinsic fibers, unlike its name, and the density of these fibers varies across individuals. 10 The intrinsic fibers of the CIFC are aligned parallel to the root surface and at approximately right angles to the extrinsic fibers. CIFC with extrinsic fibers has not been distinctively classified and is considered a subtype of CIFC. 10 The presence of varying extrinsic fiber characteristics in CIFC makes understanding its functions, particularly in tooth support, difficult. Moreover, the formation of the layers of AEFC and CIFC in CMSC is unpredictable; consequently, particular root surfaces may remain unsupported when extrinsic fiber-free CIFC forms the outermost layer of the cementum. 11 Thus, CMSC of the apical region has been proposed to play a role in the adaptation of the tooth to occlusi-on rather than in tooth support. 7 Consequently, the detailed fiber characteristics and functional significance of the apical cementum remain elusive.
The cementum continues to grow throughout life and does not remodel; thus, the cementum in aged individuals provides an insight into its maintenance history. The mouse model is a versatile tool that is used for analyzing biological systems, such as gene function, cell lineage, and tissue properties; thus, understanding the growth process and morphophysiological changes in mouse cementum over time is of profound significance. Therefore, this study aimed to explore the morphological features of mouse molar cementum, with an emphasis on the extrinsic fibers owing to their crucial role in tooth support. Second harmonic generation (SHG) imaging in conjunction with automated collagen extraction and analysis algorithms was used to facilitate quantitative comparison of fiber characteristics. In addition, regional differences and age-related changes in Wnt/β-catenin signaling, which also play essential roles in the apposition of cementum, were analyzed via immunohistochemistry.
Materials and Methods
Ethics Statement
This study was approved by the Niigata University Animal Experiment Ethics Committee (approval number: SA00532). All animal experiments were conducted in accordance with the Animal Research: Reporting of In Vivo Experiments (ARRIVE) guidelines.
Animals
Wild-type mice (C57BL/6J, male) were obtained from Charles River Laboratories (Yokohama, Japan) and maintained in the animal facility at Niigata University.
Histological Analysis
The mice were sacrificed at 2, 6, and 12 months of age (n=3 for each time point), and the maxillary bones were fixed in 4% paraformaldehyde at 4°C for 24 hr. The specimens were decalcified in 10% ethylenediaminetetraacetic acid (EDTA) at 37°C for 10 days. The specimens were embedded in paraffin, and sections of 5 μm thickness were prepared subsequently. Hematoxylin and eosin (H&E) staining was performed for histological analysis. The cervical and apical regions of the cementum on the mesial side of the distal root of the first maxillary molar were the regions of interest (ROIs). A microscope (BX53; Olympus, Tokyo, Japan) equipped with a digital camera (DP80; Olympus) was used to acquire images. Image processing was performed using Fiji/ImageJ (NIH, Bethesda, MD).
Picrosirius Red Staining
Picrosirius red staining was performed as described in a previous study. 12 Briefly, the histological sections were deparaffinized and rehydrated. The sections were incubated in picrosirius red staining solution (1 % [w/v] Sirius Red S in saturated aqueous picric acid) for 30 min, washed thoroughly with deionized water, and dehydrated. A BX53 light microscope (Olympus) equipped with polarized filters was used to analyze the sections.
SHG Microscopy and Individual Fiber Analysis
Images were acquired using a custom-built multiphoton microscope (Zeiss, Oberkochen, Germany) at Niigata University. A tunable Ti-sapphire laser with an excitation wavelength of 800 nm was used to generate the SHG signal. 13 Morphological analysis of the SHG images was performed with a curvelet transform algorithm using CT-FIRE software with default settings 14 to assess the number, length, width, and angulation of individual collagen fibers. These parameters were expressed as pixels. 15
Immunohistochemistry
Immunohistochemistry was performed as described in previous studies.12,15,16 The following primary antibodies were used for the analysis: anti-dentin matrix protein-1 (anti-Dmp1; 1:800; M176; Takara Bio, Kusatsu, Japan), anti-osteopontin (1:200; 25715-1-AP; Prot-eintech, Rosemont, IL), anti-β-catenin (1:2,000; 51067-2-AP; Proteintech), and anti-mouse SOST/sclerostin antibody (1:50; AF1589; R&D Systems, Minneapolis, MN). Biotinylated anti-goat IgG (H+L) (1:200; BA-5000; Vector Laboratories, Burlingame, CA) and biotinyla-ted anti-rabbit IgG (H+L) (1:200; BA-1000; Vector Laboratories) were the secondary antibodies used. Immunoreactivity was detected using a VECTASTAIN Elite ABC kit (Vector Laboratories) and visualized with 3, 3-diaminobenzidine (DAB). The sections were counterstained with hematoxylin.
Statistical Analysis
Data regarding the number, width, length, and angulation of collagen fibers within a specific range were obtained using CT-FIRE. Three different samples were used for analysis. Kruskal-Wallis test and Mann-Whitney U test were used to perform statistical analyses. Statistical significance was set at p<0.05.
Results
Age-Related Changes in the Mouse Molar Cementum in the Cervical and Apical Regions
The alignment of the extrinsic fibers in the cementum and their conjunction with the principal fibers of the PDL play an essential role in tooth support.1,17,18 The extrinsic fibers of the AEFC in the cervical region integrate tightly with the principal fibers of the PDL; however, the attachment of the fibers in the apical cementum remains elusive. 11 Therefore, the fiber characteristics of mouse cementum and the age-related changes were analyzed in two ROIs: the cervical and apical regions on the mesial side of the distal root of the mouse upper first molar (Fig. 1A). Acellular cementum covered the tooth root in the cervical region at all ages, and the thickness increased in an age-dependent manner (Fig. 1B). In contrast, cellular cementum covered the apical end of the root, with the area increasing gradually with age (Fig. 1C).
Figure 1.
Morphological difference in mouse molar cementum in the cervical and apical regions with increase in age. (A) H&E staining of mouse upper first molar showing the regions of interest: (B) cervical region and (C) apical region. H&E staining of the bone-PDL-cementum complex from 2M to 12M in the cervical region (B) and apical region (C). Age-related increases in cementum thickness can be observed in the cervical and apical regions. *: cementum, bars: (A) 200 μm; (B, C) 50 μm. Abbreviation: H&E, hematoxylin and eosin; PDL, periodontal ligament.
In addition to H&E staining, cementocytes were detected via immunohistochemistry using the anti-Dmp1 antibody (Fig. 2A). Dmp1-positive cementocytes were not detected in the cervical region in any age group. Cementocytes were detected only in the inner layer in the apical region, and only a few cells were detected in the outermost layer of the cementum proper. The extension of the cementocyte dendritic process at the apical region became more prominent with age. The distribution of osteopontin (OPN), a major extracellular matrix component in the cementum, was also analyzed (Fig. 2B). OPN tended to be distributed on the PDL side of the cementum in the cervical region, whereas it was localized to the incremental lines in the bone. In contrast, OPN was distributed in the superficial layer in the apical region, and an intense signal was detected along with the extrinsic fiber-like structure, which was aligned perpendicular to the cementum surface.
Figure 2.
Immunohistochemistry of Dmp1 and osteopontin in mouse molar cementum. (A) Distribution of Dmp1-positive cells in the cervical and apical cementum of mouse molar. (B) Distribution of osteopontin (OPN) in the cervical and apical cementum of mouse molar. *: cementum; arrowheads: Dmp1-positive cementocytes; arrows: OPN-positive extrinsic fiber-like structure in the cementum; bar: 50 μm.
Quantitative Fiber Characterization of the Mouse Molar Cementum
The detailed characteristics of collagenous fibers in the cementum were characterized using picrosirius red staining (Fig. 3A) and SHG imaging (Fig. 3B). Picrosirius red staining revealed the presence of the extrinsic fibers of AEFC, which are continuous with the principal fibers of the PDL, at all ages in the cervical region. SHG imaging revealed the presence of dense fibrous structures aligned obliquely to the cementum surface in AEFC; however, the continuity with the principal fibers of the PDL could not be visualized with the same clarity as with picrosirius red staining. Picrosirius red staining revealed the presence of extrinsic fibers in the superficial layer of the cementum in the apical region. These fibers showed continuity with the principal fibers of the PDL. Notably, a clear boundary was observed between the superficial and inner layers of the apical cementum in the context of fiber orientation. The fibers in the superficial layer were aligned obliquely to the cementum surface, whereas those in the inner layer were aligned at right angles to the extrinsic fibers. The orientation of the fiber in the superficial layer visualized using SHG showed a pattern similar to that of picrosirius red staining, whereas the orientation of the fiber in the inner layer showed some continuity with the fibers of the superficial layer. Consistent with the findings of picrosirius red staining, a clear boundary was observed between the superficial and inner layers of the cementum. This boundary corresponded to the cell-free outer layer and cellular inner layers (Fig. 2A). These results clearly indicate that the apical region of the mouse molar cementum can be divided into superficial AEFC and inner CIFC.
Figure 3.
Picrosirius red staining and second harmonic generation (SHG) imaging of mouse molar cementum at cervical and apical regions. (A) Picrosirius-red-stained histology samples of mouse molar cementum analyzed under polarized light. (B) SHG imaging of mouse molar cementum analyzed using a multiphoton microscope. Abbreviation: CC, cellular cementum. *: Extrinsic fiber cementum; bar: 50 μm.
The collagenous fibers in each region of the cementum (cervical AEFC, apical AEFC, and apical CIFC) were quantitatively analyzed using CT-FIRE, a curvelet transform algorithm-based software, 14 based on SHG imaging. A significant increase in the number of fibers in the cervical AEFC was observed with an increase in age; however, no significant difference was observed in the apical AEFC (Fig. 4A). The fiber length was also analyzed; however, no significant differences were observed with an increase in age (Fig. 4B). An age-dependent change in the fiber width was observed in all regions (Fig. 4C). The histogram of the fiber angle revealed that each region of the cementum had a unique pattern and that their uniqueness tended to increase with age (Fig. 4D–F).
Figure 4.
Quantitative fiber characterization of the mouse molar cementum. Second harmonic generation (SHG) imaging is quantitatively analyzed using CT-FIRE, and the detected fiber number (A), fiber length (B), and fiber width (C) are shown. The patterns of fiber angulation in the cervical AEFC (D), apical AEFC (E), and apical CIFC (F) are shown. Abbreviations: AEFC, acellular extrinsic fiber cementum; CIFC, cellular intrinsic fiber cementum; *p<0.05.
Changes in Wnt/β-Catenin Signaling in the PDL and Cementum With Age
Age-related cementum apposition is regulated by Wnt/β-catenin signaling,3,19 and the transition of the cementum type may also be regulated by Wnt/β-catenin signaling. 20 Therefore, the age-related changes in β-catenin, an essential cytoplasmic mediator for Wnt/β-catenin signaling, were analyzed (Fig. 5A). β-catenin-positive cells were ubiquitously distributed in the PDL at 2 and 6M in the cervical region. The number of β-catenin-positive cells tended to decrease at 12M, and the β-catenin-positive cells were distributed near the surface of the bone and cementum. β-catenin-positive cells were distributed near the apex in the apical region, and no association with the surface of the bone or cementum was observed. The distribution of sclerostin, a negative mediator of Wnt/β-catenin signaling, was also analyzed 21 (Fig. 5B). Sclerostin-positive cells were primarily detected in the osteocytes in the cervical region, and the signal intensity in the osteocytes increased with age. Sclerostin-positive cementocytes were not observed at 2M in the apical region; however, sclerostin-positive osteoblasts were detected. Sclerostin-positive cementocytes were detectable at 6M, and the signal intensity further increased at 12M.
Figure 5.
Immunohistochemistry of β-catenin and sclerostin in mouse molar cementum. (A) Distribution of β-catenin-positive cells in the cervical and apical cementum of mouse molar. (B) Distribution of sclerostin-positive cells in the cervical and apical cementum of mouse molar. Abbreviation: CC, cellular cementum. *: Extrinsic fiber cementum; arrows: sclerostin-positive cementocytes; bar: 50 μm.
Discussion
The clinical significance of the cementum has been recognized since its discovery; however, its morphophysiological characteristics remain unclear, particularly in the apical cementum.1,2,5,6 Therefore, this study analyzed the regional differences in the fiber characteristics of mouse molar cementum and the changes with age. The data obtained in the present study demonstrate that AEFC is present in the superficial layer of the apical cementum in mouse molars. The apical cementum is considered to have a less-functional role in tooth attachment, 11 as CIFC in the CMSC in the apical region is often free of or deficient in extrinsic fibers. 1 However, the present study has revealed that AEFC is invariably present in the superficial layer of the apical cementum in mouse molars, indicating that this region of the cementum contributes to tooth support.
The AEFC of the apical cementum showed fiber characteristics similar to those of the cervical AEFC, in addition to continuity, regardless of age (Appendix Fig. 1). These observations indicate that the same developmental origin of the cervical and apical AEFCs as cementum apposition and root elongation progress toward the root apex during the developmental stage. 22 However, it has been proposed that the level of Wnt/β-catenin signaling determines the type of acellular or cellular cementum to be formed.3,20 The data obtained in the present study revealed that the distribution pattern of β-catenin-positive cells differed between the cervical and apical sides; however, the root surface was entirely covered by AEFC from the cervical region to the apex. Further investigations are required to understand the differences in the cell origins and formation processes of cementum in different regions.
Consistent with the findings of previous reports,7,23 lamellar structures were not observed in the apical cellular cementum of mouse molars in the present study. A clear partition was observed between the AEFC of the superficial layer and the inner CIFC. If the apical cementum is formed layer by layer, the CIFC will be secreted over the AEFC and vice versa, resulting in the formation of multiple lamellar lines. However, such a structure was not observed in any of the samples analyzed in this study. The time-course observations indicated that the apical cellular cementum of the mouse molar does not form layers but continues to grow in the apical direction even after root formation was completed (Fig. 6). Apposition of apical cellular cementum has been considered to rely on functional requirements; extrinsic fiber-rich CIFC or AEFC is formed when tooth anchorage is required, whereas extrinsic fiber-free or fiber-deficient CIFC is formed when reshaping, repair, or adaptation of the root surface is required.10,24 Although functional stimuli are thought to be responsible for cellular cementum apposition, 1 impacted teeth and erupted teeth without opposing teeth tend to have extremely thick cellular cementum compared with functional teeth.22,25 Thus, the findings of the present study support the notion that the formation of apical CIFC is regulated by an intrinsic mechanism rather than extrinsic stimuli such as occlusal force.
Figure 6.
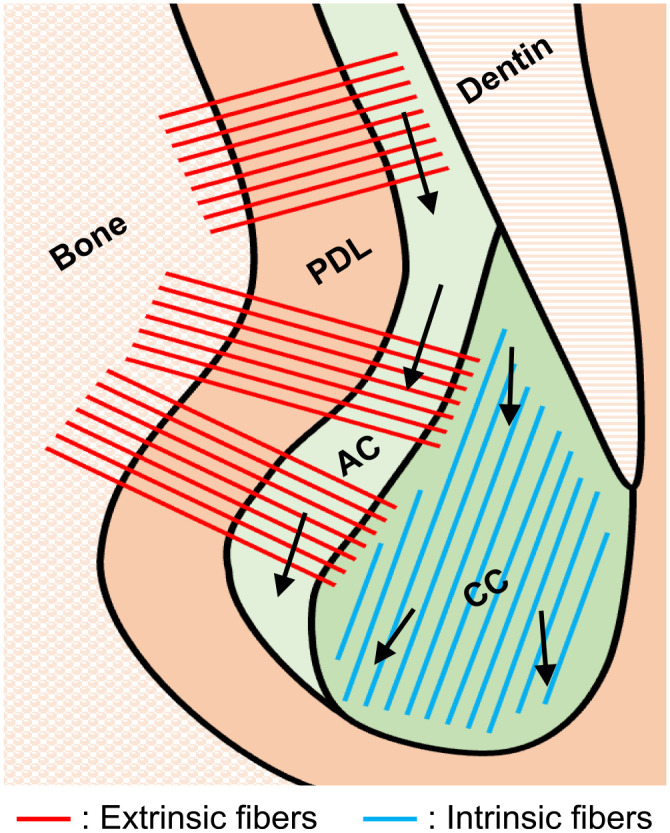
Schematic illustration of fiber alignment and age-related growth in mouse molar cementum. Abbreviations: PDL, periodontal ligament; AC, acellular cementum; CC, cellular cementum. Arrows: direction of cementum growth.
Another possible explanation for the apical growth of the cellular cementum is the wear compensation mechanism in the roots. The thickness of the cementum may increase at the apex to compensate for attritional wear on the occlusal surface in some species.26–28 Consistent with this assumption, mouse molars may have a hypsodont-like phenotype, which is characterized by a high crown and an ever-growing root and is considered a more evolved tooth type than the brachydont type of dentition. 29
Wnt/β-catenin signaling plays a vital role in cementum development and maintenance. 30 The constitutive activation of β-catenin in Wnt-responsive Gli1- or Axin2-lineage cells results in cementum hyperplasia,3,19 whereas the loss of β-catenin in these cells leads to cementum hypoplasia.19,31 Consistent with the findings of a previous study, 32 the present study revealed that the production of sclerostin in cementocytes increased with age, whereas the production of β-catenin in PDL cells remained unchanged. Occlusal unloading increases the production of sclerostin in osteocytes, resulting in periodontal tissue destruction due to the limited propagation of Gli1-lineage tissue stem cells. 33 Thus, the age-related increase of sclerostin in cementocytes may contribute to the age-related deterioration of periodontal tissue by negatively regulating the Wnt/β-catenin signaling. 21
The rapid growth of cellular cementum in rodent molars is advantageous for the analysis of cementum properties and changes. 7 However, interspecies variations in cementum apposition patterns hinder a comprehensive understanding of this tissue.2,7,24 Moreover, certain aspects of cementum formation and its morphophysiological characteristics may differ between humans and rodents owing to differences in tooth morphology, masticatory behavior, and lifespan.
To the best of our knowledge, this is the first study to demonstrate the invariable presence of AEFC in the superficial layer of the apical cementum in mouse molars, which plays a significant role in tooth support. The apical AEFC exhibited continuity and fiber characteristics comparable with the cervical AEFC, suggesting a common cellular origin for their formation. Notably, lamellar structures were not observed in the apical CIFC, indicating a uniform growth pattern. Although this study highlights the dynamic nature of the cementum and its functional significance in mouse molars, it also underscores the requirement for re-examining its structure and roles. This is because previous studies have not paid much attention to the presence of AEFC at the superficial layer of the apical cementum in mouse molars. The present findings elucidate the morphophysiological features of cementum and have broader implications for the maintenance of periodontal tissue health.
Appendix
Appendix Figure 1.
Morphological difference of mouse molar cementum with age. Low-magnification images of the distal root of the maxillary first molar. The continuity of the cervical and apical AEFC is maintained at all ages. Abbreviation: CC, cellular cementum. *: Extrinsic fiber-rich cementum; bar: 50 μm.
Footnotes
Author Contributions: All authors have contributed to this article as follows: study design and drafting the manuscript (HI, MK), acquiring the histological data (HI, LT), data analysis and interpretation (MM, MA, YO, KK), critical revision of the manuscript (IS, KU), and all the authors have read and approved the final manuscript.
The author(s) declared no potential conflicts of interest with respect to the research, authorship, and/or publication of this article.
Funding: The author(s) disclosed receipt of the following financial support for the research, authorship, and/or publication of this article: This study was supported by JSPS KAKENHI (grant no.: JP21H03127 and 21K19895 to MK) and the Center for the Coordination of Research Facilities (CCRF) at Niigata University.
ORCID iDs: Masaru Kaku  https://orcid.org/0000-0002-7988-6435
https://orcid.org/0000-0002-7988-6435
Isao Saito  https://orcid.org/0000-0003-2048-9248
https://orcid.org/0000-0003-2048-9248
Katsumi Uoshima  https://orcid.org/0000-0002-5615-8136
https://orcid.org/0000-0002-5615-8136
Contributor Information
Hajime Iwama, Division of Orthodontics, Faculty of Dentistry & Graduate School of Medical and Dental Sciences, Niigata University, Niigata, Japan.
Masaru Kaku, Division of Bio-Prosthodontics, Faculty of Dentistry & Graduate School of Medical and Dental Sciences, Niigata University, Niigata, Japan.
Lay Thant, Division of Orthodontics, Faculty of Dentistry & Graduate School of Medical and Dental Sciences, Niigata University, Niigata, Japan; Division of Dental Pharmacology, Faculty of Dentistry & Graduate School of Medical and Dental Sciences, Niigata University, Niigata, Japan; Center for Advanced Oral Science, Faculty of Dentistry & Graduate School of Medical and Dental Sciences, Niigata University, Niigata, Japan.
Masaru Mizukoshi, Division of Orthodontics, Faculty of Dentistry & Graduate School of Medical and Dental Sciences, Niigata University, Niigata, Japan.
Moe Arai, Division of Orthodontics, Faculty of Dentistry & Graduate School of Medical and Dental Sciences, Niigata University, Niigata, Japan.
Yoshiki Ono, Division of Bio-Prosthodontics, Faculty of Dentistry & Graduate School of Medical and Dental Sciences, Niigata University, Niigata, Japan.
Kohei Kitami, Division of Orthodontics, Faculty of Dentistry & Graduate School of Medical and Dental Sciences, Niigata University, Niigata, Japan.
Isao Saito, Division of Orthodontics, Faculty of Dentistry & Graduate School of Medical and Dental Sciences, Niigata University, Niigata, Japan.
Katsumi Uoshima, Division of Bio-Prosthodontics, Faculty of Dentistry & Graduate School of Medical and Dental Sciences, Niigata University, Niigata, Japan.
Literature Cited
- 1. Nanci A, Bosshardt DD. Structure of periodontal tissues in health and disease. Periodontol. 2006;40:11–28. [DOI] [PubMed] [Google Scholar]
- 2. Yamamoto T, Hasegawa T, Yamamoto T, Hongo H, Amizuka N. Histology of human cementum: its structure, function, and development. Jpn Dent Sci Rev. 2016;52(3):63–74. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 3. Xie X, Wang J, Wang K, Li C, Zhang S, Jing D, Xu C, Wang X, Zhao H, Feng JQ. Axin2(+)-mesenchymal PDL cells, instead of K14(+) epithelial cells, play a key role in rapid cementum growth. J Dent Res. 2019;98(11):1262–70. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 4. Salmon CR, Tomazela DM, Ruiz KG, Foster BL, Paes Leme AF, Sallum EA, Somerman MJ, Nociti FH., Jr. Proteomic analysis of human dental cementum and alveolar bone. J Proteomics. 2013;91:544–55. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 5. Foster BL. On the discovery of cementum. J Periodontal Res. 2017;52(4):666–85. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 6. Bosshardt DD. Are cementoblasts a subpopulation of osteoblasts or a unique phenotype? J Dent Res. 2005;84(5):390–406. [DOI] [PubMed] [Google Scholar]
- 7. Foster BL. Methods for studying tooth root cementum by light microscopy. Int J Oral Sci. 2012;4(3):119–28. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 8. Akiyoshi M, Inoue M. On the functional structure of the cementum. Bull Tokyo Med Dent Univ. 1963;10(1):41–59. [Google Scholar]
- 9. Sequeira P, Bosshardt DD, Schroeder HE. Growth of acellular extrinsic fiber cementum (AEFC) and density of inserting fibers in human premolars of adolescents. J Periodontal Res. 1992;27(2):134–42. [DOI] [PubMed] [Google Scholar]
- 10. Schroeder HE. Human cellular mixed stratified cementum: a tissue with alternating layers of acellular extrinsic- and cellular intrinsic fiber cementum. Schweiz Monatsschr Zahnmed. 1993;103(5):550–60. [PubMed] [Google Scholar]
- 11. Bosshardt DD, Schroeder HE. Cementogenesis reviewed: a comparison between human premolars and rodent molars. Anat Rec. 1996;245(2):267–92. [DOI] [PubMed] [Google Scholar]
- 12. Kaku M, Rosales Rocabado JM, Kitami M, Ida T, Akiba Y, Yamauchi M, Uoshima K. Mechanical loading stimulates expression of collagen cross-linking associated enzymes in periodontal ligament. J Cell Physiol. 2016;231(4):926–33. [DOI] [PubMed] [Google Scholar]
- 13. Chen X, Nadiarynkh O, Plotnikov S, Campagnola PJ. Second harmonic generation microscopy for quantitative analysis of collagen fibrillar structure. Nat Protoc. 2012;7(4):654–69. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 14. Bredfeldt JS, Liu Y, Pehlke CA, Conklin MW, Szulczewski JM, Inman DR, Keely PJ, Nowak RD, Mackie TR, Eliceiri KW. Computational segmentation of collagen fibers from second-harmonic generation images of breast cancer. J Biomed Opt. 2014;19(1):16007. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 15. Thant L, Kaku M, Kakihara Y, Mizukoshi M, Kitami M, Arai M, Kitami K, Kobayashi D, Yoshida Y, Maeda T, Saito I, Uoshima K, Saeki M. Extracellular matrix-oriented proteomic analysis of periodontal ligament under mechanical stress. Front Physiol. 2022;13:899699. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 16. Mizukoshi M, Kaku M, Thant L, Kitami K, Arai M, Saito I, Uoshima K. In vivo cell proliferation analysis and cell-tracing reveal the global cellular dynamics of periodontal ligament cells under mechanical-loading. Sci Rep. 2021;11(1):9813. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 17. Kaku M, Yamauchi M. Mechano-regulation of collagen biosynthesis in periodontal ligament. J Prosthodont Res. 2014;58(4):193–207. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 18. Kaku M, Thant L, Dobashi A, Ono Y, Kitami M, Mizukoshi M, Arai M, Iwama H, Kitami K, Kakihara Y, Matsumoto M, Saito I, Uoshima K. Multiomics analysis of cultured mouse periodontal ligament cell-derived extracellular matrix. Sci Rep. 2024;14(1):354. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 19. Xie X, Xu C, Zhao H, Wang J, Feng JQ. A biphasic feature of Gli1(+)-mesenchymal progenitors during cementogenesis that is positively controlled by Wnt/beta-catenin signaling. J Dent Res. 2021;100(11):1289–98. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 20. Bae CH, Choi H, You HK, Cho ES. Wnt activity is associated with cementum-type transition. J Periodontal Res. 2017;52(3):334–41. [DOI] [PubMed] [Google Scholar]
- 21. Liao C, Liang S, Wang Y, Zhong T, Liu X. Sclerostin is a promising therapeutic target for oral inflammation and regenerative dentistry. J Transl Med. 2022;20(1):221. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 22. Bosshardt DD, Selvig KA. Dental cementum: the dynamic tissue covering of the root. Periodontol. 1997;13:41–75. [DOI] [PubMed] [Google Scholar]
- 23. Yamamoto T, Hinrichsen KV. The development of cellular cementum in rat molars, with special reference to the fiber arrangement. Anat Embryol (Berl). 1993;188(6):537–49. [DOI] [PubMed] [Google Scholar]
- 24. Yamamoto T, Li M, Liu Z, Guo Y, Hasegawa T, Masuki H, Suzuki R, Amizuka N. Histological review of the human cellular cementum with special reference to an alternating lamellar pattern. Odontology. 2010;98(2):102–9. [DOI] [PubMed] [Google Scholar]
- 25. Azaz B, Ulmansky M, Moshev R, Sela J. Correlation between age and thickness of cementum in impacted teeth. Oral Surg Oral Med Oral Pathol. 1974;38(5):691–4. [DOI] [PubMed] [Google Scholar]
- 26. Ackermans NL, Clauss M, Winkler DE, Schulz-Kornas E, Kaiser TM, Müller DWH, Kircher PR, Hummel J, Hatt JM. Root growth compensates for molar wear in adult goats (Capra aegagrus hircus). J Exp Zool A Ecol Integr Physiol. 2019;331(2):139–48. [DOI] [PubMed] [Google Scholar]
- 27. Ackermans NL, Martin LF, Codron D, Kircher PR, Richter H, Clauss M, Hatt JM. Confirmation of a wear-compensation mechanism in dental roots of ruminants. Anat Rec (Hoboken). 2021;304(2):425–36. [DOI] [PubMed] [Google Scholar]
- 28. Pérez-Barbería FJ, Guinness FE, López-Quintanilla M, García AJ, Gallego L, Cappelli J, Serrano MP, Landete-Castillejos T. What do rates of deposition of dental cementum tell us? Functional and evolutionary hypotheses in red deer. Plos One. 2020;15(4):e0231957. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 29. Jernvall J, Thesleff I. Tooth shape formation and tooth renewal: evolving with the same signals. Development. 2012;139(19):3487–97. [DOI] [PubMed] [Google Scholar]
- 30. Tokavanich N, Wein MN, English JD, Ono N, Ono W. The role of Wnt signaling in postnatal tooth root development. Front Dent Med. 2021;2:769134. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 31. Ma R, Xie X, Xu C, Shi P, Wu Y, Wang J. Loss of beta-catenin causes cementum hypoplasia by hampering cementogenic differentiation of Axin2-expressing cells. J Periodontal Res. 2023;58(2):414–21. [DOI] [PubMed] [Google Scholar]
- 32. Lehnen SD, Götz W, Baxmann M, Jäger A. Immunohistochemical evidence for sclerostin during cementogenesis in mice. Ann Anat. 2012;194(5):415–21. [DOI] [PubMed] [Google Scholar]
- 33. Men Y, Wang Y, Yi Y, Jing D, Luo W, Shen B, Stenberg W, Chai Y, Ge WP, Feng JQ, Zhao H. Gli1+ periodontium stem cells are regulated by osteocytes and occlusal force. Dev Cell. 2020;54(5):639–54.e6. [DOI] [PubMed] [Google Scholar]








