Abstract
Context:
Prostate cancer (PCa) is one of the most common cancers worldwide. Understanding the epidemiology and risk factors of the disease is paramount to improve primary and secondary prevention strategies.
Objective:
To systematically review and summarize the current evidence on the descriptive epidemiology, large screening studies, diagnostic techniques, and risk factors of PCa.
Evidence acquisition:
PCa incidence and mortality rates for 2020 were obtained from the GLOBOCAN database of the International Agency for Research on Cancer. A systematic search was performed in July 2022 using PubMed/MEDLINE and EMBASE biomedical databases. The review was conducted in accordance with the Preferred Reporting Items for Systematic Reviews and Meta-analyses guidelines and was registered in PROSPERO (CRD42022359728).
Evidence synthesis:
Globally, PCa is the second most common cancer, with the highest incidence in North and South America, Europe, Australia, and the Caribbean. Risk factors include age, family history, and genetic predisposition. Additional factors may include smoking, diet, physical activity, specific medications, and occupational factors. As PCa screening has become more accepted, newer approaches such as magnetic resonance imaging (MRI) and biomarkers have been implemented to identify patients who are likely to harbor significant tumors. Limitations of this review include the evidence being derived from a meta-analysis of mostly retrospective studies.
Conclusions:
PCa remains the second most common cancer among men worldwide. PCa screening is gaining acceptance and will likely reduce PCa mortality at the cost of overdiagnosis and overtreatment. Increasing use of MRI and biomarkers for the detection of PCa may mitigate some of the negative consequences of screening.
Patient summary:
Prostate cancer (PCa) remains the second most common cancer among men, and screening for PCa is likely to increase in the future. Improved diagnostic techniques can help reduce the number of men who need to be diagnosed and treated to save one life. Avoidable risk factors for PCa may include factors such as smoking, diet, physical activity, specific medications, and certain occupations.
Keywords: Prostate cancer, Epidemiology, Screening, Risk factors
Prostate cancer remains the second most common cancer among men. Screening is gaining acceptance, likely reducing mortality at the cost of overdiagnosis and overtreatment. Increasing use of magnetic resonance imaging and biomarkers may mitigate some of the negative consequences of screening.
1. Introduction
Prostate cancer (PCa) is one of the most common cancers worldwide, accounting for a large proportion of all cancer-related deaths [1,2]. Understanding the epidemiology and risk factors of the disease is of paramount importance. Identifying risk factors for the development of PCa is key to targeting primary and secondary prevention.
Age, race, family history, and germline mutations are well-established nonmodifiable risk factors for PCa, and metabolic syndrome, obesity, and smoking have been identified as possible modifiable risk factors [3]. In addition, an abundance of environmental, lifestyle, infectious, and dietary risk factors may play a role in the etiology of PCa, although the supporting evidence is generally weak [3].
In this update and systematic review, we aim to present and summarize contemporary evidence on the epidemiology of PCa, including modifiable and nonmodifiable risk factors for the disease, screening recommendations, and strategies to optimize screening.
2. Evidence acquisition
The International Agency for Research on Cancer (IARC) compiles and disseminates the estimates of the number of new cases of PCa and corresponding PCa deaths for 185 countries in the year 2020 as the GLOBOCAN database [4]. These were visualized geographically as age-adjusted incidence and mortality rates, standardized to the world standard population, using graphics available on IARC’s Global Cancer Observatory [5].
We conducted a systematic literature search in July 2022 using PubMed/MEDLINE and EMBASE biomedical databases to identify publications in English from the past 5 yr (Supplementary material). Published search hedges were applied to filter the search results to systematic reviews, meta-analyses, consensus statements, and guidelines. Comments, letters, editorials, meeting abstracts, and conference proceedings were excluded. Two reviewers (O.B. [Memorial Sloan Kettering Cancer Center] and K.R.P.) independently reviewed the titles and abstracts, facilitated by the software Covidence. Conflicts were discussed between reviewers, and if consensus could not be reached, it was resolved by a senior author (S.V.C.). Manuscripts then underwent full-text review to determine eligibility for inclusion and were categorized by section. One reviewer independently extracted the findings, and a second reviewer verified the extracted data. As this is an umbrella review of many publication types—most of which were already individually assessed for quality—no formal quality appraisal of the included manuscripts was performed.
The review is reported in accordance with the Preferred Reporting Items for Systematic Reviews and Meta-analyses (PRISMA) guidelines [6] and was registered a priori in PROSPERO (CRD42022359728).
3. Evidence synthesis
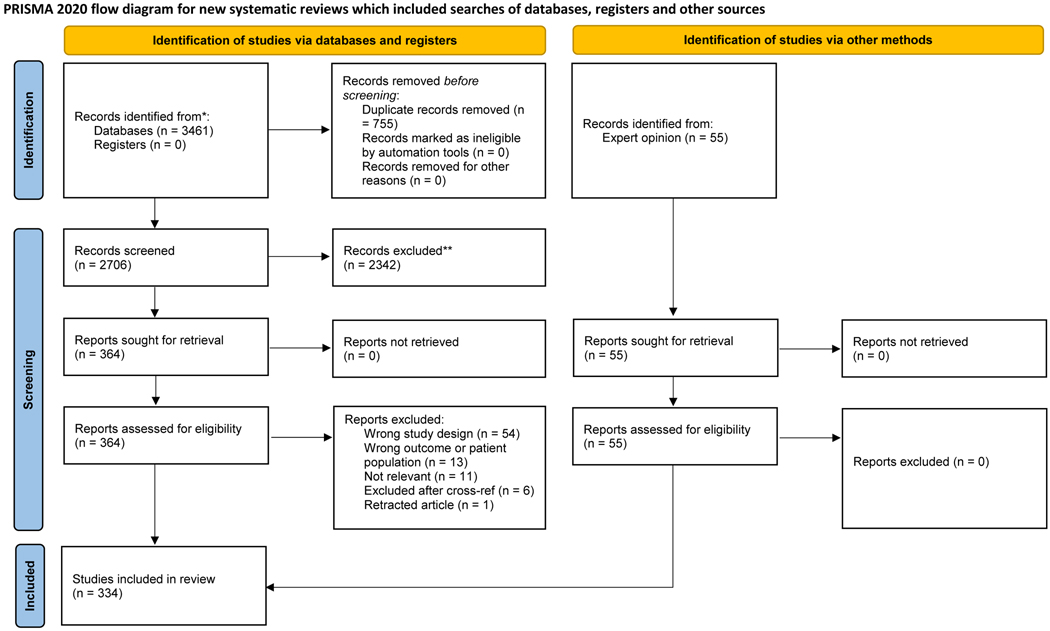
A total of 334 studies were included in the review and organized by section (Fig. 1, PRISMA flow diagram).
Fig. 1 –
PRISMA flow diagram. PRISMA = Preferred Reporting Items for Systematic Reviews and Meta-analyses.
3.1. Epidemiology
3.1.1. PCa incidence
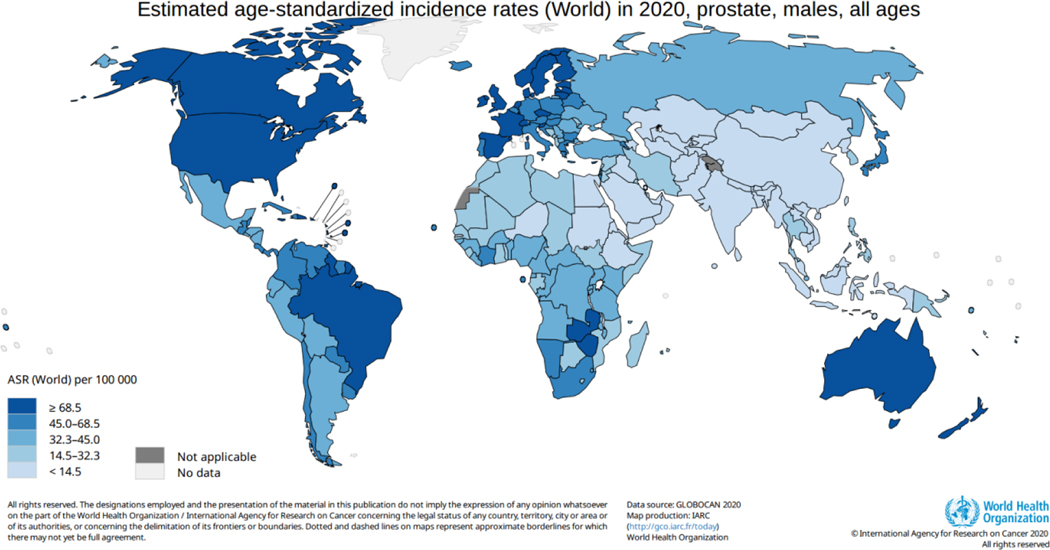
PCa is the most frequently diagnosed cancer among men in more than half of the countries in the world (112 of 185 countries/territories), with an estimated 1.4 million new cases in 2020. The highest age-standardized incidence rates are seen in Northern and Western Europe, the Caribbean, Australia/New Zealand, North and South America, and Southern Africa (Fig. 2).
Fig. 2 –
Estimated age-standardized prostate cancer incidence (world). ASR = age-standardized incidence rate.
3.1.2. PCa mortality
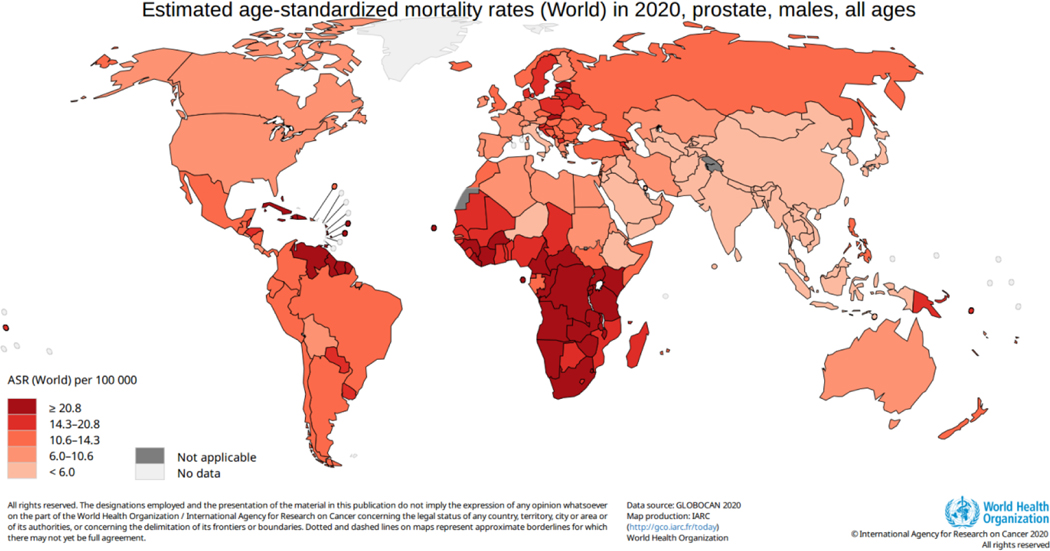
PCa is the leading cause of cancer death among men in a quarter of the world’s countries (48 of 185 countries), with an estimated 375 000 deaths in 2020. The highest age-standardized mortality rates are seen in the Caribbean (Barbados, Jamaica, and Haiti), parts of South America, and sub-Saharan Africa (Fig. 3). Global differences in PCa incidence and mortality can be attributed to differences in screening, imaging, access to care, and healthcare infrastructure [1,7]. In addition, there are emerging data to suggest that differences in germline genetic factors as well as lifestyle factors across populations may underlie geographic differences. PCa when diagnosed and treated at localized stages has a 97% 5-yr cancer-specific survival compared with 30% in the metastatic setting.[7]
Fig. 3 –
Estimated age-standardized prostate cancer mortality rate (world). ASR = age-standardized incidence rate.
3.1.3. Racial and ethnic disparities
In the USA, Black or African American men are 1.7 times more likely to be diagnosed with PCa than White men and 2.1 times more likely to die of the disease [7]. Reasons for this observation are unclear, given that race is a social construct. Underlying factors for this could include genetics associated with ancestry/geographic origin, environmental factors, or social determinants of health [8,9].
To elucidate the underlying causes of PCa as well as existent healthcare disparities, methods of categorizing populations can be helpful. Ancestry is one way of categorizing populations, as genetic traits can be passed down from one generation to another. Area of geographic origin is another way of categorizing populations. There are some known genetic traits that correlate with the area of geographic origin [10,11]. Assessment by the area of geographic origin is also complicated by admixture.
Race is a categorization of populations that may rely on a combination of ancestry, skin color, facial traits, and area of geographic origin. Within any one race, there are hundreds of areas of geographic origin [12]. In the USA and Europe, race as used today is a complex sociopolitical construct. Contrary to popular belief, race is not a biological categorization related to inherent biological traits.
Aspects often associated with certain races such as social deprivation, culture, and diet can influence biology and risk of disease, response to treatment, and risk of poor outcome. In the USA, African-American race generally correlates with a population more likely to be socioeconomically underprivileged. Social deprivation can lead to environmental exposures that increase the risk of some diseases including cancer. Social deprivation is closely linked to reduced access to quality health care [13–15].
The precise reasons for the higher incidence of PCa among Black and African American men are unknown, but it appears to be related more to genetic ancestry rather than race [16]. There is the suggestion of an increased risk of PCa among men with ancestry from northwestern sub-Saharan Africa, and it may be due to the higher prevalence of certain genetic factors [17]. It may also be due to environmental influences leading to inflammation and subsequent carcinogenesis [18].
Even though Black men are at a higher risk of developing the disease, there are substantial data to show that when Black and White men are diagnosed with similar grade and stage of PCa and are treated equally in equal-access healthcare systems, they have equivalent outcomes [19–21]. However, Black men are often diagnosed with more aggressive PCa. Using the National Cancer Database, a cancer registry drawing incidence and mortality data from a group of American cancer-accredited facilities, Black men with high-grade, localized PCa treated with radical prostatectomy had 50% higher mortality than White men (hazard ratio [HR] 1.51 [95% confidence interval {CI} 1.47–1.56]) [22]. Owing to social influences, the Black or African American population, in general, has a higher prevalence of comorbidities that can complicate treatment [19]. When adjusting for socioeconomic parameters and comorbidities, the disparity was reduced to 20% greater than that for White patients [22]. The coarse adjustment made here removed much of the disparity in mortality; however, it is rarely possible to fully adjust for socioeconomics and comorbidities.
3.1.4. Transgender
Three systematic reviews evaluated the risk of PCa in the transgender population receiving gender-affirming hormones. None of the studies found an increased risk of PCa. However, evidence is limited, and more long-term studies are needed. In the absence of evidence, transgender individuals with a prostate may be offered screening similarly to cis-gender males [23–25].
3.2. Risk factors
3.2.1. Nonmodifiable risk factors
3.2.1.1. Age
Age is a well-established risk factor for PCa. Recent US cancer statistics indicate that the probability of PCa increases from 1.8% in men 60–69 yr to 9.0% in men 70 yr and older for a lifetime probability of 12.5% [7]. Autopsy studies reveal that 40% of unscreened men older than 60 yr harbor PCa; this increases to 60% in men older than 80 yr. Notably, 32% of these cancers were of International Society of Urological Pathology grade group ≥2 [26].
3.2.1.2. Family history
Family history of PCa is well established as a risk factor for PCa incidence and may also increase the risk of fatal forms of PCa. However, determining the association between reported family history and PCa risk can be challenging, given family status, recall bias, and degree of screening. The risk of PCa is influenced by the number of afflicted relatives, closeness of the relative (ie, first degree vs second degree [27]), the family member’s age at diagnosis and age at death from PCa, high-grade disease, and other cancers (eg, breast or ovarian). Brothers and sons of men with PCa have approximately a 2.5-fold increased risk of being diagnosed with PCa [28], and there is evidence that a PCa family history increases the risk of fatal disease. A family history of high-grade or metastatic disease increases the risk of a similarly severe PCa more than a family history of low-grade, nonmetastatic disease [29,30]. A recent systematic review and meta-analysis showed that having a first-degree relative with breast cancer is associated with a 1.2-fold increased risk of PCa [31]. Recent data from the UK Genetic Prostate Cancer Study showed that a stronger family history was inversely associated with PCa mortality, attributable to greater awareness of screening and PCa among those with a familial risk [32]. Other studies have suggested the opposite, and so the context of family history and what it means need to be taken into account.
3.2.1.3. Genetic predisposition/germline variants
Twin studies suggest that PCa is more genetically heritable than other common cancers, with an estimate of heritability of 58% [33,34]. As with other cancers, genetic susceptibility can be classified into genetic factors with a low prevalence but high penetrance, and those with a higher prevalence but generally lower penetrance. The two most common alleles that confer autosomal dominant susceptibility to PCa are mutations in the HOXB13 and BRCA2 genes [35–37]. Still, the prevalence of these mutations in the general population is around 0.3%. There is increasing evidence of mutations in several DNA repair pathway genes, such as BRCA1, ATM, CHEK2, NBS1, and genes involved in Lynch syndrome (MLH1, MSH2, MSH6, and PMS2) are associated with PCa risk, with a higher prevalence in metastatic versus localized PCa; however, the associations with PCa incidence and disease aggressiveness are variable [35,38–40]. In a study of over 21 000 men from the UK Biobank, 1.4% carried a rare pathogenic mutation in at least one of the following three genes: BRCA2, HOXB13, and CHEK2 [41]. While rare in the general population and among patients with low-risk PCa, the prevalence of germline genetic mutations in DNA repair pathways is 10–20% in patients with advanced PCa.
A larger proportion of the underlying heritability in PCa can be explained through common genetic single nucleotide polymorphisms (SNPs) [42]. In multiethnic populations, genome-wide association studies in large international consortia have now validated 269 individual SNPs, each of which is associated with a PCa risk [43]. While each SNP contributes little to a man’s risk of developing PCa, the additive effects of multiple alleles are substantial when weighted and summed into polygenic risk scores (PRSs) [42–47]. Indeed, a higher PRS is associated with a substantially increased risk of incident PCa of any grade [48,49]. While the PRS is not able to differentiate between indolent and aggressive disease, that is, it is not prognostic, the PRS is associated with a higher risk of fatal PCa because it increases the overall risk [42,50]. Addition of a PRS to prostate-specific antigen (PSA) does not appear to contribute additional risk stratification [42].
3.2.1.4. Baldness
Four meta-analyses evaluated the relationship between male pattern baldness and PCa [51–54]. None of these studies found a relationship between overall male pattern baldness and the risk of PCa. However, in subgroup analyses, the meta-analyses found an association between vertex pattern baldness and an increased risk of PCa.
3.2.1.5. Height
Taller height is consistently associated with an increased risk of overall and high-grade PCa [55]. The potential mechanism underlying this association may be related to the levels of insulin-like growth factor during puberty.
3.2.2. Modifiable risk factors
3.2.2.1. Physical activity and sleep
There was no clear increased risk of PCa among those with sedentary behavior in multiple studies [56,57]. However, those with the highest versus lowest level of physical activity had a lower risk of PCa-specific mortality [58,59] and, among patients, longer cancer-free survival from PCa [60]. The inverse association is seen for both vigorous forms of physical activity as well as brisk walking. Most studies have focused on leisure time and recreational physical activity, whereas the effects of occupational physical activity are less established [58]. Long-term occupational physical activity may reduce PCa risk [59].
There does not appear to be an impact of sleep duration on the overall risk of PCa [61]. However, there are some studies that have found an association between sleep quality and risk of aggressive PCa [62].
3.2.2.2. Dietary intake
3.2.2.2.1. Specific diets—Mediterranean, vegetarian, semivegetarian, plant based
A Mediterranean diet was found to have a weak protective association for overall PCa among those with the highest adherence (relative risk [RR] 0.96, 95% CI 0.92–1.00), while another study found no association [63,64]. Vegetarians had a lower risk of PCa than meat eaters (HR 0.57, 95% CI 0.43–0.76) [65]. This was seen in other studies that demonstrated either mild protection or no association with plant-based, vegetarian, pesco-vegetarian, or semivegetarian diets [66,67]. In interventional studies, a plant-based diet showed positive results on short-term oncological outcomes among men with PCa [66].
3.2.2.2.2. Inflammatory and hyperinsulinemic diets
A proinflammatory diet defined by a high Dietary Inflammatory Index [68] is a risk factor for the development of PCa. Five studies evaluated a possible association, all of which found a positive association (ranging between odds ratio [OR] 1.31, 95% CI 1.04–1.57) and RR 1.74, 95% CI 1.24–2.43 for the highest vs lowest Dietary Inflammatory Index) [69–73]. Additionally, a hyperinsulinemic and inflammatory diet may be associated with an increased risk of aggressive PCa [74,75].
3.2.2.2.3. Macronutrients (protein, carbohydrates, and fat)
There was no association between overall protein intake and PCa risk [76,77]. On further investigation of protein intake, for dairy protein, an increased PCa risk was found (for men who consumed ≥30 g/d, RR 1.08, 95% CI 1–1.16). This was dose dependent, and with every 20 g/d increase in dairy protein, there was a pooled RR of 1.1 (95% CI 1.02–1.20). No increased risk was found for animal protein or plant-based protein [77].
Several studies have evaluated carbohydrate intake as a possible risk factor for PCa. Results were mixed, with two studies evaluating sugar-sweetened beverages finding an increased risk [78,79] ,while studies evaluating Glycemic Index or glycemic load found no or only weak associations [80–83].
Trans fatty acid intake, which are commonly found in the production of ultraprocessed food, was associated with an increased PCa risk (OR 1.49, 95% CI 1.13–1.95) [84].
3.2.2.2.4. Specific food intake (eg, meat, dairy, and tomato)
No associations were found for total fruit and vegetable intake and the risk of PCa [85,86]. Several articles assessed a possible association between red meat, processed meat, and total meat consumption [65,87–91]. Almost all found a positive association between meat consumption and PCa risk. However, associations were generally weak and mainly for processed meat [86–89]. No associations were found for fish consumption and fish-derived omega-3 fatty acid intake (both dietary and supplement) [86,92–94].
There were multiple studies on dairy consumption [95–99]. An umbrella review found an increase in PCa risk for dairy milk (RR 1.11, 95% CI 1.03–1.21) and an increased risk of PCa mortality (RR 1.50, 95% CI 1.03–2.17) [95]. The risk for fermented dairy milk products and yogurt may be similar to that of dairy milk [96].
Tomato, high in lycopene, as a protective factor for PCa has been debated extensively. Results were inconclusive: one meta-analysis as well as a systematic review pointed toward a protective effect [100–102], while a recent meta-analysis including only prospective studies showed no association [103].
Phytoestrogens are found in foods such as soybeans, lentils, tofu, peanuts, chickpeas, and kidney beans. One meta-analysis examined specific phytoestrogen subclasses and found that daidzein, genistein, and glycitein were possible protective factors [104]. Another meta-analysis found that soy was associated with a decreased risk of PCa (RR 0.71, 95% CI 0.58–0.85) [105]. One meta-analysis found a decreased PCa risk in men with high consumption of legumes, also high in phytoestrogens (RR 0.85, 95% CI 0.75–0.96) and with a 3.7% reduction in the risk of PCa with each 20 g increment of legume intake per day [106]. Another study evaluated circulating isoflavones and lignans and PCa risk, and found large differences based on population (Japan vs Europe) with a significant protective effect for Japanese men with high concentrations [107].
Tea consumption was evaluated as a possible protective factor; however, results were inconsistent, and no clear association was seen [86,108,109]. An umbrella review [110] and multiple meta-analyses [111,112] confirmed that coffee consumption was a probable protective factor for the development of localized PCa (RR 0.90, 95% CI 0.85–0.95) [110]; however, there was no association with advanced or fatal disease [110,112]. The most recent well-conducted large prospective study found no evidence of an association [113], so the effect is likely weak, if present (Table 1).
Table 1 –
Summary of modifiable risk factors and protective factors for prostate cancer
| Confirmed | Possible |
|---|---|
|
| |
| Modifiable risk factors | |
| Dietary | Dietary |
| Meat consumption | Sugar-sweetened beverages |
| Dietary inflammatory index | Dairy consumption |
| Trans fatty acid intake | |
| Medical diseases and treatments | Medical diseases and treatments |
| – | Vasectomy |
| Prostatitis | |
| Obesity and metabolic syndrome | |
| Periodontitis | |
| Human papillomavirus-16 | |
| Calcium channel blocker use | |
| Occupational factors | Occupational factors |
| – | Agricultural workers |
| Petroleum workers | |
| Rotating night shift work | |
| Lifestyle factors | Lifestyle factors |
| – | Smoking |
| Modifiable protective factors | Smokeless tobacco |
| Dietary | Dietary |
| – | Mediterranean diet |
| Plant-based diet | |
| Coffee consumption | |
| Dietary intake of phytoestrogens | |
| Medical diseases and treatments | Medical diseases and treatments |
| – | Aspirin |
| Nonsteroidal anti-inflammatory drugs | |
| Statins | |
| Selenium intake | |
| Lifestyle factors | Lifestyle factors |
| – | Physical activity |
| Ejaculation frequency | |
3.2.2.2.5. Alcohol
Alcohol intake did not appear to increase overall or aggressive PCa risk in most studies [86,114,115].
3.2.2.2.6. Smoking
The majority of epidemiology studies have found no association between cigarette smoking and overall PCa for smokers [86] or for those who used waterpipe tobacco (OR 7.0, 95% CI 0.9–56.9) [116]. However, previous reports have shown that smoking may be associated with an increased risk of lethal PCa [117,118], including a recent meta-analysis of cohort studies that found current smokers had an RR of 1.42 (95% CI 1.20–1.68) compared with nonsmokers [119]. There was an increased risk of PCa mortality for those who used smokeless tobacco (eg, snuff; OR 2.1, 95% CI 1.1–4.1) [120].
3.2.2.3. Diseases and treatments
3.2.2.3.1. Circumcision
A systematic review and random-effect meta-analysis found that circumcised men had a decreased risk of PCa (OR 0.87, 95% CI 0.76–1) [121], possibly due to differences in sexually transmitted infections and penile microbiome. However, circumcision patterns vary widely based on geographic and cultural norms.
3.2.2.3.2. Infertility and vasectomy
Four systematic reviews evaluated infertility and subfertility as a risk for PCa [122–125]. These were limited by substantial heterogeneity in study design, definitions of infertility, and limited length of follow-up. Both meta-analyses found a significant association between infertility and subfertility, and PCa (OR 1.65, 95% CI 1.17–2.40 [122] and OR 1.48, 95% CI 1.05–2.08 [125], respectively) compared with fertile men.
Multiple meta-analyses evaluated a possible association between vasectomy and PCa with varying results [126–130]. There was a weak significant association for any or localized PCa [128,129]; however, there was no association with high-grade PCa or PCa-specific mortality in studies that controlled for bias [128–130].
3.2.2.3.3. Prostatitis and prostate size
Several studies found a possible increased risk between prostatitis and PCa [131–134]. However, there was significant heterogeneity among included studies and definitions of prostatitis. In one analysis, there was a positive association (OR 2.05, 95% CI 1.64–2.57); however, this association disappeared when including only studies that had the most rigorous analyses to limit the detection bias (OR 1.16, 95% CI 0.77–1.74) [134]. Increasing prostate size was associated with a decreased PCa risk [135].
3.2.2.3.4. Autoimmune diseases—Sjogren’s syndrome and systemic lupus erythematous
Sjogren’s syndrome was associated with an overall increase in PCa risk and an increased standardized incidence ratio (SIR) of PCa 1.50 (95% CI 1.02–2.22) [136]. Systemic lupus erythematous was not associated with the risk of PCa [137,138].
3.2.2.3.5. Periodontitis
A possible association between periodontal disease and PCa was evaluated in five meta-analyses, all of which found a positive association [139–143]. However, the method of diagnosis of periodontal disease varied among included studies. The authors stated potential genetic predisposition to PCa or bacterial and viral pathogens to be responsible for the increased risk.
3.2.2.3.6. Inflammatory bowel disease
Seven studies evaluated whether men with inflammatory bowel disease (Crohn’s disease and ulcerative colitis) had an increased risk of PCa [144–150]. Results were mixed; three studies found a slightly increased risk, whereas two studies found no increased risk. In the subgroup of men with ulcerative colitis, results were more consistent with a clear association (SIR 1.58, 95% CI 1.08–2.30) [147].
3.2.2.3.7. Metabolic syndrome, nonalcoholic fatty liver disease and obesity, and elevated body mass index
Three meta-analyses evaluated a possible association between metabolic syndrome and PCa [151–153], and results were mixed. Two studies found a positive weak association. However, when looking specifically at men with higher-grade disease, a stronger association was found.
Similarly, seven studies evaluated a possible association between obesity and PCa [154–160]. No association was found regarding the overall risk of PCa, but several studies reported an increased risk of aggressive PCa (OR 1.06, 95% CI 1.00–1.12) [157]. One review article assessed the impact of body composition on PCa and found that a higher whole-body fat mass was associated with a lower risk of PCa [159].
Two studies evaluated a possible association between nonalcoholic fatty liver disease as the manifestation of metabolic syndrome in the liver and PCa. Results varied, and one meta-analysis that included more studies found no association [161], while a second meta-analysis found a weak positive association with the overall risk of PCa [162].
3.2.2.3.8. Type 2 diabetes, hyperglycemia, and high glycemic index
It has been suggested that type 2 diabetes is associated with a lower risk of PCa; however, results are mixed and it is unclear whether causal relationship exists [163–165]. Further, in the most updated meta-analysis, there was no association with PCa risk [166]. The studies that evaluated the impact of hyperglycemia, high glycemic index, and prediabetes were inconclusive [83,167].
3.2.2.3.9. Solid organ transplants
A meta-analysis that evaluated prospective observational studies of renal transplant recipients found an increase in the overall cancer risk but no association with PCa risk [168]. Another study evaluated the PCa risk in solid organ transplant recipients who were on immunosuppression and found no association with PCa risk [169].
3.2.2.3.10. Other medications
Multiple studies evaluated commonly used medications for patients with type 2 diabetes, and found no association between the use of metformin, thiazolidinediones, sulfonylureas, insulin, or dipeptidase-4 inhibitors and the risk of PCa [170–174].
Regarding hypertension medications, there was no association between PCa risk and the use of angiotensin converting enzyme inhibitors, angiotensin receptor blockers, diuretics, antiadrenergic agents, or beta-blockers [175,176]. Multiple studies identified an increased risk of PCa associated with calcium channel blocker use [175,177], whereas others found no association [176,178]. On subgroup analyses, some studies found an increased risk with longer durations of use [177] or longer follow-up [178], but this was not found in all studies [179].
No association was found between allopurinol [180] or acid suppressive use [181] and overall PCa.
3.2.2.4. Environmental and occupational factors
3.2.2.4.1. Specific environmental and chemical exposures
Two reviews and meta-analyses evaluated the risk of PCa among men exposed to asbestos. Results varied, and one article found no association [182], while the other found a weak association (effect size 1.10, 95% CI 1.05–1.15) [183]. No association was found for mixtures of persistent, bioaccumulative, and toxic chemicals; permethrin (a broad-spectrum insecticide); or work stress [184–186]. There was a possible positive association with cobalt exposure and hexavalent chromium [187,188].
3.2.2.4.2. Occupational factors
An increased risk of PCa was found among firefighters (RR 1.15, 95% CI 1.05–1.27) [189] and police workers (RR 1.14, 95% CI 1.02–1.28) [190]. However, there was significant heterogeneity among included studies and a probable higher rate of PSA testing in these populations. Additionally, there was an increased risk among workers with occupational chemical and pesticide exposures such as agricultural workers (SIR 1.06, 95% CI 1.01–1.12) [191] and petroleum workers (effect size 1.13, 95% CI 1.05–1.22) [192]. Increased occupational physical activity appeared to decrease PCa risk, possibly explaining the reduced risk seen in occupations such as those of coal miners [193,194]. There was an increased risk of PCa for shift work, specifically rotating shifts and rotating night shifts, but no increased risk of PCa for night shift work alone, except for men in Asian countries [195–198]. Possible mechanisms include unnatural light/melatonin suppression, disruption in circadian rhythm, and lifestyle factors [197].
3.2.2.5. Sexual activity
Older age at first intercourse was associated with a reduced risk of PCa (OR 0.96, 95% CI 0.92–0.99), and every ten female sexual partners were associated with an increased risk of PCa (OR 1.10, 95% CI 1.01–1.21) [199]. By contrast, men with >21 ejaculations per month had a reduced risk of both overall and aggressive PCa (OR 0.78, 95% CI 0.61–1.00 and OR 0.75, 95% CI 0.57–0.99, respectively) [200] and men with moderate ejaculation frequency (two to four times per week) had a lower risk of PCa (OR 0.91, 95% CI 0.87–0.96, p < 0.001) [199].
3.2.2.6. Infectious agents
A positive association between human papilloma virus (HPV) and overall PCa was demonstrated in multiple studies [201–204]. The risk of PCa was highest for men with HPV-16 (OR 1.38, 95% CI 1.16–1.64, p < 0.01) [201,204]. There may be an association between Neisseria gonorrhea, herpes simplex 1 and 2, Epstein-Barr virus, and Mycoplasma, and PCa, but results were conflicting [205]. Meanwhile, there was no association between hepatitis C virus infection [206], Trichomonas [207], Cytomegalovirus, Chlamydia, polyoma viruses, human immunodeficiency virus, and fungi, and overall PCa [205].
3.2.2.7. Marital status
Single men had an increased risk of high-grade PCa compared with married men (OR 1.21, 95% CI 1.00–1.50) [208]. Men who were widowed had an increased risk of PCa compared with men who were married or had a partner (OR 1.19, 95% CI 1.03–1.35) and more often had advanced disease at the time of diagnosis [208].
3.3. Primary prevention/chemoprevention
3.3.1. 5-Alpha reductase inhibitors
There is high-level evidence including randomized trials assessing the association between 5-alpha reductase inhibitors and PCa risk. One recent systematic review and meta-analysis [209] reported an approximate 30% reduction in the risk of PCa (OR 0.70, 95% CI 0.51–0.96), but a two-fold increase in the risk of high-grade disease (OR 2.10, 95% CI 1.85–2.38) associated with finasteride. Similar effect sizes have been reported with dutasteride [210]. Overall, the majority of studies assessing these drugs have shown a lower risk of overall, low-risk, and intermediate-risk PCa, but an increased rate of detection of high-grade disease [211]. However, studies that have examined 5-alpha reductase inhibitors and lethal PCa have found no association in populations with access to care and regular screening [212,213].
3.3.2. Anti-inflammatories
Consistent aspirin use has been found to be associated with a lower risk of development of PCa [214]. Regular aspirin use significantly reduced the risk of PCa (RR 0.92, 95% CI 0.86–0.98) [215,216]. Similarly, in a meta-analysis of 43 observational studies, long-term use of nonsteroidal anti-inflammatories was found to be protective against PCa (RR 0.88, 95% CI 0.79–0.99) [217].
3.4.3. Statins
The impact of statin use on the development of PCa remains controversial, although studies suggest that statins may lower the risk of aggressive PCa and, in patients, may reduce mortality. In one meta-analysis, statins were associated with a 12% reduction in the risk of overall PCa (RR 0.88, 95% CI 0.84–0.93) [218]. Another meta-analysis found statin use to be associated with a lower risk in advanced PCa, but found no impact of statins on localized disease [219]. A recent review meta-analysis found a lower risk of PCa with higher dose and duration of statin use [220]. By contrast, other studies have found limited to no association between statin use and the overall incidence of PCa [221].
3.3.4. Vitamins and supplements
Vitamin D has been shown to have no impact on PCa incidence overall [222]. However, higher circulating 25-hydroxyvitamin D concentration was associated with an increased risk of PCa (RR 1.15, 95% CI 1.06–1.24), and there was a nonlinear dose response [223]. In the VITAL randomized trial, men randomized to vitamin D had lower PCa mortality than those randomized to placebo [222]. This finding is important given the lower vitamin D levels in Black versus White men [224].
A meta-analysis found selenium to be protective against the development of PCa (RR 0.86, 95% CI 0.78–0.94) and particularly protective against the development of advanced PCa (RR 0.67, 95% CI 0.52–0.87) [225]. In the SELECT randomized trial, however, there was no association with PCa incidence overall; SELECT did not follow participants for mortality [226].
3.3.5. Miscellaneous medications
There are many other medications that have been suggested to have a potential impact on PCa, for example, phosphodiesterase-5 inhibitors [227], spironolactone [228], Glucagon-like peptide-1 receptor agonist [229], and vitamin K antagonist [230,231]; however, data are limited and of relatively poor quality.
3.4. Secondary prevention/screening and early detection
3.4.1. Guideline recommendations regarding screening
In December 2022, the EU Council recommended their member states to evaluate the feasibility and effectiveness of the implementation of organized screening programs for PCa with PSA testing in combination with magnetic resonance imaging (MRI) scanning as a follow-up test.
In 2021, the EAU-EANM-ESTRO-ESUR-SIOG revised their PCa guidelines on screening and recommended offering PSA testing to well-informed men. However, they strongly recommend to evaluate and stop testing based on life expectancy and performance status [232].
In 2018, the U.S. Preventive Services Task Force updated their recommendations regarding PSA screening. For men aged 55–69 yr, the decision to undergo periodic PSA-based screening for PCa should be individualized [233]. Given the increased PCa incidence and mortality rates for Black men in the USA, some groups suggest annual screening starting at age 40 yr for these men, based on modeling studies [234].
In summary, screening for PCa is gaining acceptance and is expected to increase in the future.
3.4.2. Large screening studies
In the 16-yr follow-up of the European Randomized study of Screening for PCa (ERSPC), the benefits of screening became more pronounced as data had matured and the number needed to invite to screen and the number needed to diagnose to prevent one PCa death continued to decrease to 570 and 18, respectively. The Prostate, Lung, Colorectal, and Ovarian (PLCO) Cancer Screening Trial published data from 17 yr of median follow-up in 2019 and found no differences in PCa mortality between the screening and control arms [235]. However, pooling the results of ERSPC and PLCO, and taking into account contamination of the control arms showed a reduction in PCa mortality by 30% [236].
3.4.3. Magnetic resonance imaging
In the PROMIS study [237], using prebiopsy prostate multiparametric MRI (mpMRI) to triage men reduce the number of patients having a primary biopsy by 27% and may result in 5% fewer clinically insignificant cancers as compared with the standard pathway of transrectal ultrasound (TRUS) biopsy. The PRECISION study further strengthened the recommendation for mpMRI as 28% of men in the MRI arm had normal MRI and avoided biopsy. Of the men who underwent biopsy, 38% in the MRI group compared with 26% of the standard TRUS biopsy group had clinically significant PCa (csPCa) [238]. Several meta-analyses have evaluated the role of prostate mpMRI [239–242] and estimated the negative predictive value to be around 90% for the detection of grade group ≥2 disease and a positive predictive value of 40%. For example, a recent study compared TRUS alone versus prebiopsy MRI pathways [242] and found that prebiopsy MRI with or without a biopsy was associated with a 57% improvement in the detection of csPCa, a 33% potential reduction in the number of biopsy procedures, and a 77% reduction in the number of cores taken. Two recently published randomized controlled trials have provided evidence for the use of MRI-targeted biopsy in PCa screening to reduce overdiagnosis [243,244].
3.4.4. Biomarkers
Several blood- and urine-based biomarkers can help better risk stratify before biopsy [245–248]. The 4 kallikrein panel, commercially known as the 4Kscore, or Prostate Health Index can be used to estimate the risk of csPCa on biopsy [249–251]. IsoPSA, which partitions isoforms of PSA, has also been found to predict the risk of csPCa, more so than PSA, in preliminary studies [252]. Proclarix, using plasma protein biomarkers PSA, free PSA, THBS1, and CTSD, outperformed total PSA and free PSA for men with larger prostates, ≥35 cc [253]. The Stockholm-3 test is a risk model that combines PSA, SNPs, clinical variables, as well as established and novel plasma protein biomarkers, and has been shown to outperform PSA alone in predicting csPCa [254].
Regarding urine-based biomarkers, ExoDx Prostate, a urine RNA exosome gene expression assay, and MyProstateScore predicts csPCa and can be used to aid in decision-making before biopsy [255–257]. SelectMDx is another urinary RNA biomarker, which was found in a meta-analysis to be comparable with predicting high-grade PCa, similar to mpMRI [258].
A recent systematic review did not yet recommend the use of biomarkers for reflex testing based on current evidence [259].
3.5. Limitations
The GLOBOCAN estimates assembled at IARC at the national level use the best available sources of recorded cancer incidence and mortality information within a given country. A strength of the estimates is their comprehensiveness based on routine data nationally, while an inherent weakness is that their validity is dependent on the degree of representativeness and quality of the source information [4]. At present, high-quality information on new cases of cancer from population-based cancer registries is available in only one of three countries worldwide, while the equivalent cause-of-death information from national vital registration systems is available in only one of four countries.
This systematic review performed a qualitative synthesis of the findings, with evidence mainly derived from systematic reviews and meta-analyses of retrospective studies and predominately from Europe and North America. While the strongest evidence for PCa risk factors lie in nonmodifiable risk factors, we highlight the emerging data for modest yet modifiable risk factors. Not all meta-analyses reported on clinical subtypes of PCa, such as by advanced stage, high grade, or lethal forms of PCa. PSA screening is an important confounder in epidemiology studies of PCa. Moreover, PSA screening increases the proportion of overall PCa cases that are low-grade, low-stage tumors. This review did not examine the extent to which studies accounted or did not account for PSA screening in the analyses.
4. Conclusions
PCa remains the second most common cancer among men with higher incidences and mortality in North and South America, Northern and Western Europe, Australia, and the Caribbean. Screening is gaining acceptance, which will likely reduce mortality at the cost of overdiagnosis. New techniques such as increasing use of MRI and biomarkers for the detection of PCa can help mitigate some of these issues. Herein, we have identified modifiable risk factors for PCa, which could be targeted to further reduce the incidence and mortality of PCa.
Supplementary Material
Funding/Support and role of the sponsor:
Oskar Bergengren, Kelly R. Pekala, Jonathan Fainberg, and Sigrid V. Carlsson are supported by a Cancer Center Support Grant from the National Cancer Institute made to Memorial Sloan Kettering Cancer Center (P30-CA008748). Sigrid V. Carlsson is supported by a National Institutes of Health/National Cancer Institute Transition Career Development Award (K22-CA234400), Kelly R. Pekala is supported by the Ruth L. Kirschstein National Research Service Award (T32-CA082088), and Todd M. Morgan is supported by the National Cancer Institute (5R01-CA240991). Oskar Bergengren is supported by the Hillevi Fries Research Foundation, Johanna Hagstrand and Sigfrid Linnérs Research Foundation, Nyströms America scholarship, and the Swedish Society of Medicine.
Appendix A: Search Methodology
- 1) Search strategy translated for PubMed/MEDLINE (NLM)
Search strategy component concepts 1 Prostate cancer ((“Prostatic Neoplasms”[Mesh] OR ((prostat*[ti] OR “Prostate”[Mesh]) AND (cancer* OR oncolog* OR “neoplasms”[MeSH Terms] OR neoplasm* OR carcinom* OR tumor* OR tumour* OR malignan* OR adenocarcinoma* OR adenoma*))) 2 Epidemiology/incidence/prevalence/mortality (“Epidemiologic Studies”[Mesh] OR “Epidemiology”[Mesh] OR “epidemiology” [Subheading] OR epidemiolog*[tiab] OR “Incidence”[Mesh] OR incidence[tiab] OR “Prevalence”[Mesh] OR prevalence[tiab] OR “Mortality”[Mesh] OR “mortality” [Subheading] OR “Morbidity”[Mesh] OR morbidit*[tiab] OR follow up studies[MeSH:noexp] OR prognos*[Text Word] OR predict*[Text Word] OR course*[Text Word] OR association*[Text Word])) 3 Risk factors/screening/socioeconomic/disparities ((risk*[tiab] OR risk*[MeSH:noexp] OR risk adjustment[MeSH:noexp] OR risk assessment[MeSH:noexp] OR risk factors[MeSH:noexp] OR risk management[MeSH:noexp] OR risk taking[MeSH:noexp]) OR (delivery of health care[MeSH:noexp] OR health behavior[MH] OR health knowledge, attitudes, practice[MH] OR health services accessibility[MH] OR health services, indigenous[MH] OR mass screening[MH] OR mass screening[TIAB] OR mass screenings[TIAB] OR health inequality[TIAB] OR health inequalities[TIAB] OR health inequities[TIAB] OR health inequity[TIAB] OR health services needs and demand[MH] OR patient acceptance of health care[MH] OR patient selection[MH] OR quality of health care[MAJR:noexp] OR quality of life[MH] OR quality of life[TIAB] OR social disparities[TIAB] OR social disparity[TIAB] OR social inequities[TIAB] OR social inequity[TIAB] OR Socioeconomic Factors[MAJR] OR socioeconomic factor[TIAB] OR socioeconomic factors[TIAB])) 4 Limit: Study Designs – Systematic reviews/meta-analyses/guidelines (((“Meta-Analysis as Topic”[MeSH] OR meta analy*[TIAB] OR metaanaly*[TIAB] OR “Meta-Analysis”[PT] OR “Systematic Review”[PT] OR “Systematic Reviews as Topic”[MeSH] OR systematic review*[TIAB] OR systematic overview*[TIAB] OR “Review Literature as Topic”[MeSH]) OR (cochrane[TIAB] OR embase[TIAB] OR psychlit[TIAB] OR psyclit[TIAB] OR psychinfo[TIAB] OR psycinfo[TIAB] OR cinahl[TIAB] OR cinhal[TIAB] OR “science citation index”[TIAB] OR bids[TIAB] OR cancerlit[TIAB]) OR (reference list*[TIAB] OR bibliograph*[TIAB] OR hand-search*[TIAB] OR “relevant journals”[TIAB] OR manual search*[TIAB]) OR ((“selection criteria”[TIAB] OR “data extraction”[TIAB]) AND “Review”[PT])) OR ((clinical[tiab] AND pathway[tiab]) OR (clinical[tiab] AND pathways[tiab]) OR (practice[tiab] AND parameter[tiab]) OR (practice[tiab] AND parameters[tiab]) OR algorithms[mesh:noexp] OR care pathway[tiab] OR care pathways[tiab] OR clinical protocols[mesh:noexp] OR Consensus[mesh:noexp] or consensus development conference[pt:noexp] OR “Consensus Development Conference, NIH”[pt:noexp] OR “Consensus Development Conferences as Topic”[Mesh:noexp] OR “Consensus Development Conferences, NIH as Topic”[Mesh:NoExp] OR critical pathway[mesh:noexp] OR guidance[tiab] OR guideline*[ti] OR guidelines as topic[mesh:noexp] or practice guidelines as topic[mesh:noexp] or Health Planning Guidelines[mesh:noexp] OR practice guideline[mesh:noexp])) 5 Limit: English, date range (english[Filter]) AND (y_5[Filter]) 6 Limit: Human ((“Animals”[MeSH]) NOT (“Animals”[MeSH] AND “Humans”[MeSH])) 7 Exclude: Publication types – Comments, letters, editorials, meeting/conference abstracts (“Comment”[PT] OR “Letter”[PT] OR “Editorial”[PT]) 8 Search strategy (1 AND 2 AND 3 AND 4 AND 5) NOT (6 OR 7) - 2) Search strategy translated for EMBASE (Elsevier)
Search strategy component concepts 1 Prostate cancer (‘prostate tumor’/exp OR ‘prostate cancer’/exp OR ‘prostate adenoma’/exp OR ((prostat*:ti OR ‘prostate’/exp) AND (cancer* OR oncolog* OR ‘neoplasm’/exp OR neoplasm* OR carcinom* OR tumor* OR tumour* OR malignan* OR adenocarcinoma* OR adenoma*))) 2 Epidemiology/incidence/prevalence/mortality (‘epidemiologic studies’ OR ‘epidemiology’/exp OR ‘epidemiology’/lnk OR epidemiolog*:ab,ti OR ‘incidence’/exp OR incidence:ab,ti OR ‘prevalence’/exp OR prevalence:ab,ti OR ‘mortality’/exp OR ‘mortality’/lnk OR ‘morbidity’/exp OR morbidit*:ab,ti OR ‘follow up’/exp OR prognos* OR predict* OR course* OR association*) 3 Risk factors/screening/socioeconomic/disparities (risk*:ab,ti OR ‘risk’/de OR ‘risk assessmen’/de OR ‘risk factor’/de OR ‘risk managemen’/de OR ‘high risk behavior’/de OR ((((((((((((((‘health care delivery’/de OR ‘health behavior’/exp OR ‘attitude to health’/exp OR ‘health care access’/exp OR ‘indigenous health care’/exp OR ‘mass screening’/exp OR mass) AND screening:ab,ti OR mass) AND screenings:ab,ti OR health) AND inequality:ab,ti OR health) AND inequalities:ab,ti OR health) AND inequities:ab,ti OR health) AND inequity:ab,ti OR ‘health service’/exp OR ‘patient attitude’/exp OR ‘patient selection’/exp OR ‘health care quality’/mj OR ‘quality of life’/exp OR quality) AND of AND life:ab,ti OR social) AND disparities:ab,ti OR social) AND disparity:ab,ti OR social) AND inequities:ab,ti OR social) AND inequity:ab,ti OR ‘socioeconomics’/exp/mj OR socioeconomic) AND factor:ab,ti OR socioeconomic) AND factors:ab,ti)) 4 Limit: Study Designs – Systematic reviews/meta-analyses/guidelines (‘meta analysis (topic)’/exp OR ‘meta analysis’/exp OR ((meta NEXT/1 analy*):ab,ti) OR metaanaly*:ab,ti OR ‘systematic review (topic)’/exp OR ‘systematic review’/exp OR ((systematic NEXT/1 review*):ab,ti) OR ((systematic NEXT/1 overview*):ab,ti) OR cancerlit:ab,ti OR cochrane:ab,ti OR embase:ab,ti OR psychlit:ab,ti OR psyclit:ab,ti OR psychinfo:ab,ti OR psycinfo:ab,ti OR cinahl:ab,ti OR cinhal:ab,ti OR ‘science citation index’:ab,ti OR bids:ab,ti OR ((reference NEXT/1 list*):ab,ti) OR bibliograph*:ab,ti OR ‘hand search*’:ab,ti OR ((manual NEXT/1 search*):ab,ti) OR ‘relevant journals’:ab,ti OR ((‘data extraction’:ab,ti OR ‘selection criteria‘:ab,ti) AND review/it) OR (((clinical:ab,ti AND pathway:ab,ti OR (clinical:ab,ti AND pathways:ab,ti) OR (practice:ab,ti AND parameter:ab,ti) OR (practice:ab,ti AND parameters:ab,ti) OR ‘algorithm’/de OR care) AND pathway:ab,ti OR care) AND pathways:ab,ti) OR ‘clinical protocol’/de OR ‘consensus’/de OR ‘consensus developmen’/de OR ‘clinical pathway’/de OR guidance:ab,ti OR guideline*:ti OR ‘practice guideline’/de OR ‘health care planning’/de) 5 Limit: English, date range ([english]/lim AND [2017–2022]/py) 6 Limit: Human (‘animal’/exp NOT (‘animal’/exp AND ‘human’/exp)) 7 Exclude: Publication types – Comments, letters, editorials, meeting/conference abstracts (letter/it OR editorial/it OR ‘conference abstrac’/it) 8 Search strategy (1 AND 2 AND 3 AND 4 AND 5) NOT (6 OR 7) Search filters consulted/used/adapted:PubMed’s Clinical Study Categories search filters https://pubmed.ncbi.nlm.nih.gov/help/#clinical-study-category-filtersPubMed’s Health Disparities search hedge (National Library of Medicine) https://www.nlm.nih.gov/services/queries/health_disparities_details.htmlSystematic Reviews study design filterAvau B, Van Remoortel H, De Buck E. Translation and validation of PubMed and Embase search filters for identification of systematic reviews, intervention studies, and observational studies in the field of first aid. J Med Libr Assoc. 2021 Oct 1;109(4):599–608. doi: 10.5195/jmla.2021.1219. PMID: 34858089; PMCID: PMC8608173. https://www.ncbi.nlm.nih.gov/pmc/articles/PMC8608173/Consensus statements/guidelines search filter (University of Texas School of Public Health) https://libguides.sph.uth.tmc.edu/search_filters/pubmed_filters
Footnotes
Financial disclosures: Oskar Bergengren certifies that all conflicts of interest, including specific financial interests and relationships and affiliations relevant to the subject matter or materials discussed in the manuscript (eg, employment/affiliation, grants or funding, consultancies, honoraria, stock ownership or options, expert testimony, royalties, or patents filed, received, or pending), are the following: None.
Publisher's Disclaimer: This is a PDF file of an unedited manuscript that has been accepted for publication. As a service to our customers we are providing this early version of the manuscript. The manuscript will undergo copyediting, typesetting, and review of the resulting proof before it is published in its final form. Please note that during the production process errors may be discovered which could affect the content, and all legal disclaimers that apply to the journal pertain.
References
- [1].Sung H, Ferlay J, Siegel RL, et al. Global cancer statistics 2020: GLOBOCAN estimates of incidence and mortality worldwide for 36 cancers in 185 countries. CA Cancer J Clin 2021;71:209–49. [DOI] [PubMed] [Google Scholar]
- [2].Culp MB, Soerjomataram I, Efstathiou JA, Bray F, Jemal A. Recent global patterns in prostate cancer incidence and mortality rates. Eur Urol 2020;77:38–52. [DOI] [PubMed] [Google Scholar]
- [3].Gandaglia G, Leni R, Bray F, et al. Epidemiology and prevention of prostate cancer. Eur Urol Oncol 2021;4:877–92. [DOI] [PubMed] [Google Scholar]
- [4].Ferlay J, Colombet M, Soerjomataram I, et al. Cancer statistics for the year 2020: an overview. Int J Cancer 2021;149:778–89. [DOI] [PubMed] [Google Scholar]
- [5].Ferlay J, Laversanne M, Lam F, et al. Global cancer observatory: cancer today. Lyon, France: International Agency for Research on Cancer; 2020. https://gco.iarc.fr/today [Google Scholar]
- [6].Page MJ, McKenzie JE, Bossuyt PM, et al. The PRISMA 2020 statement: an updated guideline for reporting systematic reviews. BMJ 2021;372:n71. [DOI] [PMC free article] [PubMed] [Google Scholar]
- [7].Siegel RL, Miller KD, Fuchs HE, Jemal A. Cancer statistics, 2022. CA Cancer J Clin 2022;72:7–33. [DOI] [PubMed] [Google Scholar]
- [8].Nair SS, Chakravarty D, Dovey ZS, Zhang X, Tewari AK. Why do African-American men face higher risks for lethal prostate cancer? Curr Opin Urol 2022;32:96–101. [DOI] [PMC free article] [PubMed] [Google Scholar]
- [9].Das H, Rodriguez R. Health care disparities in urologic oncology: a systematic review. Urology 2020;136:9–18. [DOI] [PubMed] [Google Scholar]
- [10].Howes RE, Dewi M, Piel FB, et al. Spatial distribution of G6PD deficiency variants across malaria-endemic regions. Malar J 2013;12:418. [DOI] [PMC free article] [PubMed] [Google Scholar]
- [11].Lucotte G, Hazout S. Geographic and ethnic distributions of the more frequent cystic fibrosis mutations in Europe show that a founder effect is apparent for several mutant alleles. Hum Biol 1995;67:562–76. [PubMed] [Google Scholar]
- [12].Wagner JK, Yu JH, Ifekwunigwe JO, Harrell TM, Bamshad MJ, Royal CD. Anthropologists' views on race, ancestry, and genetics. Am J Phys Anthropol 2017;162:318–27. [DOI] [PMC free article] [PubMed] [Google Scholar]
- [13].Coughlin SS. A review of social determinants of prostate cancer risk, stage, and survival. Prostate Int 2020;8:49–54. [DOI] [PMC free article] [PubMed] [Google Scholar]
- [14].Neupane S, Bray F, Auvinen A. National economic and development indicators and international variation in prostate cancer incidence and mortality: an ecological analysis. World J Urol 2017;35:851–8. [DOI] [PubMed] [Google Scholar]
- [15].Nyame YA, Cooperberg MR, Cumberbatch MG, et al. Deconstructing, addressing, and eliminating racial and ethnic inequities in prostate cancer care. Eur Urol 2022;82:341–51. [DOI] [PubMed] [Google Scholar]
- [16].Fujimura JH, Rajagopalan R. Different differences: the use of 'genetic ancestry' versus race in biomedical human genetic research. Soc Stud Sci 2011;41:5–30. [DOI] [PMC free article] [PubMed] [Google Scholar]
- [17].Mahal BA, Alshalalfa M, Kensler KH, et al. Racial differences in genomic profiling of prostate cancer. N Engl J Med 2020;383:1083–5. [DOI] [PMC free article] [PubMed] [Google Scholar]
- [18].Nelson WG, Brawley OW, Isaacs WB, et al. Health inequity drives disease biology to create disparities in prostate cancer outcomes. J Clin Invest 2022;132:e155031. [DOI] [PMC free article] [PubMed] [Google Scholar]
- [19].Dess RT, Hartman HE, Mahal BA, et al. Association of Black race with prostate cancer-specific and other-cause mortality. JAMA Oncol 2019;5:975–83. [DOI] [PMC free article] [PubMed] [Google Scholar]
- [20].Rude T, Walter D, Ciprut S, et al. Interaction between race and prostate cancer treatment benefit in the Veterans Health Administration. Cancer 2021;127:3985–90. [DOI] [PubMed] [Google Scholar]
- [21].McKay RR, Sarkar RR, Kumar A, et al. Outcomes of Black men with prostate cancer treated with radiation therapy in the Veterans Health Administration. Cancer 2021;127:403–11. [DOI] [PubMed] [Google Scholar]
- [22].Wen W, Luckenbaugh AN, Bayley CE, Penson DF, Shu XO. Racial disparities in mortality for patients with prostate cancer after radical prostatectomy. Cancer 2021;127:1517–28. [DOI] [PubMed] [Google Scholar]
- [23].Joint R, Chen ZE, Cameron S. Breast and reproductive cancers in the transgender population: a systematic review. BJOG 2018;125:1505–12. [DOI] [PubMed] [Google Scholar]
- [24].McFarlane T, Zajac JD, Cheung AS. Gender-affirming hormone therapy and the risk of sex hormone-dependent tumours in transgender individuals—a systematic review. Clin Endocrinol 2018;89:700–11. [DOI] [PubMed] [Google Scholar]
- [25].Sterling J, Garcia MM. Cancer screening in the transgender population: a review of current guidelines, best practices, and a proposed care model. Transl Androl Urol 2021;9:2771–85. [DOI] [PMC free article] [PubMed] [Google Scholar]
- [26].Zlotta AR, Egawa S, Pushkar D,, et al. Prevalence of prostate cancer on autopsy: cross-sectional study on unscreened Caucasian and Asian men. J Natl Cancer Inst 2013;105:1050–8. [DOI] [PubMed] [Google Scholar]
- [27].Clements MB, Vertosick EA, Guerrios-Rivera L, et al. Defining the impact of family history on detection of high-grade prostate cancer in a large multi-institutional cohort. Eur Urol 2022;82:163–9. [DOI] [PMC free article] [PubMed] [Google Scholar]
- [28].Johns LE, Houlston RS. A systematic review and meta-analysis of familial prostate cancer risk. BJU Int 2003;91:789–94. [DOI] [PubMed] [Google Scholar]
- [29].Jansson KF, Akre O, Garmo H, et al. Concordance of tumor differentiation among brothers with prostate cancer. Eur Urol 2012;62:656–61. [DOI] [PubMed] [Google Scholar]
- [30].Bratt O, Drevin L, Akre O, Garmo H, Stattin P. Family history and probability of prostate cancer, differentiated by risk category: a nationwide population-based study. J Natl Cancer Inst 2016;108:djw110. [DOI] [PubMed] [Google Scholar]
- [31].Ren ZJ, Cao DH, Zhang Q, et al. First-degree family history of breast cancer is associated with prostate cancer risk: a systematic review and meta-analysis. BMC Cancer 2019;19:871. [DOI] [PMC free article] [PubMed] [Google Scholar]
- [32].Brook MN, Ni Raghallaigh H, Govindasami K, et al. Family history of prostate cancer and survival outcomes in the UK Genetic Prostate Cancer Study. Eur Urol 2023;83:257–66. [DOI] [PubMed] [Google Scholar]
- [33].Mucci LA, Hjelmborg JB, Harris JR, et al. Familial risk and heritability of cancer among twins in Nordic countries. JAMA 2016;315:68–76. [DOI] [PMC free article] [PubMed] [Google Scholar]
- [34].Lichtenstein P, Holm NV, Verkasalo PK, et al. Environmental and heritable factors in the causation of cancer—analyses of cohorts of twins from Sweden, Denmark, and Finland. N Engl J Med 2000;343:78–85. [DOI] [PubMed] [Google Scholar]
- [35].Nyberg T, Tischkowitz M, Antoniou AC. BRCA1 and BRCA2 pathogenic variants and prostate cancer risk: systematic review and meta-analysis. Br J Cancer 2022;126:1067–81. [DOI] [PMC free article] [PubMed] [Google Scholar]
- [36].Nicolosi P, Ledet E, Yang S, et al. Prevalence of germline variants in prostate cancer and implications for current genetic testing guidelines. JAMA Oncol 2019;5:523–8. [DOI] [PMC free article] [PubMed] [Google Scholar]
- [37].Nyberg T, Govindasami K, Leslie G, et al. Homeobox B13 G84E mutation and prostate cancer risk. Eur Urol 2019;75:834–45. [DOI] [PMC free article] [PubMed] [Google Scholar]
- [38].Karlsson Q, Brook MN, Dadaev T, et al. Rare germline variants in ATM predispose to prostate cancer: a PRACTICAL consortium study. Eur Urol Oncol 2021;4:570–9. [DOI] [PMC free article] [PubMed] [Google Scholar]
- [39].Dominguez-Valentin M, Sampson JR, Seppala TT, et al. Cancer risks by gene, age, and gender in 6350 carriers of pathogenic mismatch repair variants: findings from the Prospective Lynch Syndrome Database. Genet Med 2020;22:15–25. [DOI] [PMC free article] [PubMed] [Google Scholar]
- [40].Bancroft EK, Page EC, Brook MN, et al. A prospective prostate cancer screening programme for men with pathogenic variants in mismatch repair genes (IMPACT): initial results from an international prospective study. Lancet Oncol 2021;22:1618–31. [DOI] [PMC free article] [PubMed] [Google Scholar]
- [41].Shi Z, Platz EA, Wei J, et al. Performance of three inherited risk measures for predicting prostate cancer incidence and mortality: a population-based prospective analysis. Eur Urol 2021;79:419–26. [DOI] [PubMed] [Google Scholar]
- [42].Klein RJ, Vertosick E, Sjoberg D, et al. Prostate cancer polygenic risk score and prediction of lethal prostate cancer. NPJ Precis Oncol 2022;6:25. [DOI] [PMC free article] [PubMed] [Google Scholar]
- [43].Siltari A, Lonnerbro R, Pang K, et al. How well do polygenic risk scores identify men at high risk for prostate cancer? Systematic review and meta-analysis. Clin Genitourin Cancer 2023;21:316.e1–11. [DOI] [PubMed] [Google Scholar]
- [44].Pagadala MS, Lynch J, Karunamuni R, et al. Polygenic risk of any, metastatic, and fatal prostate cancer in the Million Veteran Program. J Natl Cancer Inst 2023;115:190–9. [DOI] [PMC free article] [PubMed] [Google Scholar]
- [45].Schumacher FR, Al Olama AA, Berndt SI, et al. Association analyses of more than 140,000 men identify 63 new prostate cancer susceptibility loci. Nat Genet 2018;50:928–36. [DOI] [PMC free article] [PubMed] [Google Scholar]
- [46].Chen F, Darst BF, Madduri RK, et al. Validation of a multi-ancestry polygenic risk score and age-specific risks of prostate cancer: a meta-analysis within diverse populations. Elife 2022;11:e78304. [DOI] [PMC free article] [PubMed] [Google Scholar]
- [47].Nyberg T, Brook MN, Ficorella L, et al. CanRisk-Prostate: a comprehensive, externally validated risk model for the prediction of future prostate cancer. J Clin Oncol 2023;41:1092–104. [DOI] [PMC free article] [PubMed] [Google Scholar]
- [48].Plym A, Penney KL, Kalia S, et al. Evaluation of a multiethnic polygenic risk score model for prostate cancer. J Natl Cancer Inst 2022;114:771–4. [DOI] [PMC free article] [PubMed] [Google Scholar]
- [49].Plym A, Zhang Y, Stopsack KH, et al. A healthy lifestyle in men at increased genetic risk for prostate cancer. Eur Urol 2023;83:343–51. [DOI] [PMC free article] [PubMed] [Google Scholar]
- [50].Xu J, Resurreccion WK, Shi Z, et al. Inherited risk assessment and its clinical utility for predicting prostate cancer from diagnostic prostate biopsies. Prostate Cancer Prostatic Dis 2022;25:422–30. [DOI] [PubMed] [Google Scholar]
- [51].Liang W, Song L, Peng Z, Zou Y, Dai S. Possible association between androgenic alopecia and risk of prostate cancer and testicular germ cell tumor: a systematic review and meta-analysis. BMC Cancer 2018;18:279. [DOI] [PMC free article] [PubMed] [Google Scholar]
- [52].Jin T, Wu T, Luo Z, Duan X, Deng S, Tang Y. Association between male pattern baldness and prostate disease: a meta-analysis. Urol Oncol 2018;36:80.e7–15. [DOI] [PubMed] [Google Scholar]
- [53].Zhao J, Dong X, Sun B, et al. The relationship between male pattern baldness and prostate cancer risk: a meta-analysis. Int J Clin Exp Med 2019;12:13179–87. [Google Scholar]
- [54].He H, Xie B, Xie L. Male pattern baldness and incidence of prostate cancer: a systematic review and meta-analysis. Medicine (Baltimore) 2018;97:e11379. [DOI] [PMC free article] [PubMed] [Google Scholar]
- [55].Lophatananon A, Stewart-Brown S, Kote-Jarai Z, et al. Height, selected genetic markers and prostate cancer risk: results from the PRACTICAL consortium. Br J Cancer 2017;117:734–43. [DOI] [PMC free article] [PubMed] [Google Scholar]
- [56].Berger FF, Leitzmann MF, Hillreiner A, et al. Sedentary behavior and prostate cancer: a systematic review and meta-analysis of prospective cohort studies. Cancer Prev Res (Phila) 2019;12:675–88. [DOI] [PubMed] [Google Scholar]
- [57].Garcia L, Pearce M, Abbas A, et al. Non-occupational physical activity and risk of 22 cardiovascular disease, cancer, and mortality outcomes: a dose-response meta-analysis of large prospective studies. Br J Sports Med. In press. 10.1136/bjsports-2022-105669 [DOI] [PMC free article] [PubMed] [Google Scholar]
- [58].McTiernan A, Friedenreich CM, Katzmarzyk PT, et al. Physical activity in cancer prevention and survival: a systematic review. Med Sci Sports Exerc 2019;51:1252–61. [DOI] [PMC free article] [PubMed] [Google Scholar]
- [59].Benke IN, Leitzmann MF, Behrens G, Schmid D. Physical activity in relation to risk of prostate cancer: a systematic review and meta-analysis. Ann Oncol 2018;29:1154–79. [DOI] [PubMed] [Google Scholar]
- [60].Cuthbertson CC, Nichols HB, Tan X, et al. Associations of leisure-time physical activity and television viewing with life expectancy cancer-free at age 50: the ARIC study. Cancer Epidemiol Biomarkers Prev 2020;29:2617–25. [DOI] [PMC free article] [PubMed] [Google Scholar]
- [61].Liu R, Wu S, Zhang B, Guo M, Zhang Y. The association between sleep duration and prostate cancer: A systematic review and meta-analysis. Medicine (Baltimore) 2020;99:e21180. [DOI] [PMC free article] [PubMed] [Google Scholar]
- [62].Sigurdardottir LG, Valdimarsdottir UA, Fall K, et al. Circadian disruption, sleep loss, and prostate cancer risk: a systematic review of epidemiologic studies. Cancer Epidemiol Biomarkers Prev 2012;21:1002–11. [DOI] [PMC free article] [PubMed] [Google Scholar]
- [63].Cheng S, Zheng Q, Ding G, Li G. Mediterranean dietary pattern and the risk of prostate cancer: a meta-analysis. Medicine (Baltimore). 2019;98:e16341. [DOI] [PMC free article] [PubMed] [Google Scholar]
- [64].Schwingshackl L, Schwedhelm C, Galbete C, Hoffmann G. Adherence to Mediterranean diet and risk of cancer: an updated systematic review and meta-analysis. Nutrients 2017;9:1063. [DOI] [PMC free article] [PubMed] [Google Scholar]
- [65].Parra-Soto S, Ahumada D, Petermann-Rocha F, et al. Association of meat, vegetarian, pescatarian and fish-poultry diets with risk of 19 cancer sites and all cancer: findings from the UK Biobank prospective cohort study and meta-analysis. BMC Med 2022;20:79. [DOI] [PMC free article] [PubMed] [Google Scholar]
- [66].Gupta N, Patel HD, Taylor J, et al. Systematic review of the impact of a plant-based diet on prostate cancer incidence and outcomes. Prostate Cancer Prostatic Dis 2022;25:444–52. [DOI] [PMC free article] [PubMed] [Google Scholar]
- [67].Godos J, Bella F, Sciacca S, Galvano F, Grosso G. Vegetarianism and breast, colorectal and prostate cancer risk: an overview and meta-analysis of cohort studies. J Hum Nutr Diet 2017;30:349–59. [DOI] [PubMed] [Google Scholar]
- [68].Shivappa N, Steck SE, Hurley TG, Hussey JR, Hebert JR. Designing and developing a literature-derived, population-based dietary inflammatory index. Public Health Nutr 2014;17:1689–96. [DOI] [PMC free article] [PubMed] [Google Scholar]
- [69].Moradi S, Issah A, Mohammadi H, Mirzaei K. Associations between dietary inflammatory index and incidence of breast and prostate cancer: a systematic review and meta-analysis. Nutrition 2018;55–56:168–78. [DOI] [PubMed] [Google Scholar]
- [70].Mohseni R, Abbasi S, Mohseni F, Rahimi F, Alizadeh S. Association between dietary inflammatory index and the risk of prostate cancer: a meta-analysis. Nutr Cancer 2019;71:359–66. [DOI] [PubMed] [Google Scholar]
- [71].Lu DL, Ren ZJ, Zhang Q, et al. Meta-analysis of the association between the inflammatory potential of diet and urologic cancer risk. PLoS One 2018;13:e0204845. [DOI] [PMC free article] [PubMed] [Google Scholar]
- [72].Jayedi A, Emadi A, Shab-Bidar S. Dietary inflammatory index and site-specific cancer risk: a systematic review and dose-response meta-analysis. Adv Nutr 2018;9:388–403. [DOI] [PMC free article] [PubMed] [Google Scholar]
- [73].Zhu Y, Li Q, Xu X. Dietary inflammatory index and the risk of prostate cancer: a dose-response meta-analysis. Eur J Clin Nutr 2020;74:1001–8. [DOI] [PubMed] [Google Scholar]
- [74].Fu BC, Tabung FK, Pernar CH, et al. Insulinemic and inflammatory dietary patterns and risk of prostate cancer. Eur Urol 2021;79:405–12. [DOI] [PMC free article] [PubMed] [Google Scholar]
- [75].Langlais CS, Graff RE, Van Blarigan EL, et al. Postdiagnostic inflammatory, hyperinsulinemic, and insulin-resistant diets and lifestyles and the risk of prostate cancer progression and mortality. Cancer Epidemiol Biomarkers Prev 2022;31:1760–8. [DOI] [PMC free article] [PubMed] [Google Scholar]
- [76].Mao Y, Tie Y, Du J. Association between dietary protein intake and prostate cancer risk: evidence from a meta-analysis. World J Surg Oncol 2018;16:152. [DOI] [PMC free article] [PubMed] [Google Scholar]
- [77].Al-Zahrani MA, Ahmad M, Alkhamees M, et al. Dietary protein intake and prostate cancer risk in adults: a systematic review and dose-response meta-analysis of prospective cohort studies. Complement Ther Med 2022;70:102851. [DOI] [PubMed] [Google Scholar]
- [78].Llaha F, Gil-Lespinard M, Unal P, de Villasante I, Castañeda J, Zamora-Ros R. Consumption of sweet beverages and cancer risk. A systematic review and meta-analysis of observational studies. Nutrients 2021;13:1–35. [DOI] [PMC free article] [PubMed] [Google Scholar]
- [79].Li Y, Guo L, He K, Huang C, Tang S. Consumption of sugar-sweetened beverages and fruit juice and human cancer: a systematic review and dose-response meta-analysis of observational studies. J Cancer 2021;12:3077–88. [DOI] [PMC free article] [PubMed] [Google Scholar]
- [80].Fan LL, Su HX, Gu XJ, Chen YH, Nan CJ. Carbohydrate intake and the risk of prostate cancer. Clin Chim Acta 2018;484:60–71. [DOI] [PubMed] [Google Scholar]
- [81].Turati F, Galeone C, Augustin LSA, La Vecchia C. Glycemic index, glycemic load and cancer risk: an updated meta-analysis. Nutrients. 2019;11:2342. [DOI] [PMC free article] [PubMed] [Google Scholar]
- [82].Sadeghi A, Sadeghi O, Khodadost M, Pirouzi A, Hosseini B, Saedisomeolia A. Dietary Glycemic Index and glycemic load and the risk of prostate cancer: an updated systematic review and dose-response meta-analysis. Nutr Cancer 2020;72:5–14. [DOI] [PubMed] [Google Scholar]
- [83].Hatami Marbini M, Amiri F, Sajadi Hezaveh Z. Dietary glycemic index, glycemic load, insulin index, insulin load and risk of diabetes-related cancers: a systematic review of cohort studies. Clin Nutr ESPEN 2021;42:22–31. [DOI] [PubMed] [Google Scholar]
- [84].Michels N, Specht IO, Heitmann BL, Chajès V, Huybrechts I. Dietary trans-fatty acid intake in relation to cancer risk: a systematic review and meta-analysis. Nutr Rev 2021;79:758–76. [DOI] [PubMed] [Google Scholar]
- [85].Yan H, Cui X, Zhang P, Li R. Fruit and vegetable consumption and the risk of prostate cancer: a systematic review and meta-analysis. Nutr Cancer 2022;74:1235–42. [DOI] [PubMed] [Google Scholar]
- [86].Cirne F, Kappel C, Zhou S, et al. Modifiable risk factors for prostate cancer in low- and lower-middle-income countries: a systematic review and meta-analysis. Prostate Cancer Prostatic Dis 2022;25:453–62. [DOI] [PubMed] [Google Scholar]
- [87].Huang Y, Cao D, Chen Z, et al. Red and processed meat consumption and cancer outcomes: umbrella review. Food Chem 2021;356:129697. [DOI] [PubMed] [Google Scholar]
- [88].Nouri-Majd S, Salari-Moghaddam A, Aminianfar A, Larijani B, Esmaillzadeh A. Association between red and processed meat consumption and risk of prostate cancer: a systematic review and meta-analysis. Front Nutr 2022;9:801722. [DOI] [PMC free article] [PubMed] [Google Scholar]
- [89].Han MA, Zeraatkar D, Guyatt GH, et al. Reduction of red and processed meat intake and cancer mortality and incidence a systematic review and meta-analysis of cohort studies. Ann Intern Med 2019;171:711–20. [DOI] [PubMed] [Google Scholar]
- [90].Farvid MS, Sidahmed E, Spence ND, Mante Angua K, Rosner BA, Barnett JB. Consumption of red meat and processed meat and cancer incidence: a systematic review and meta-analysis of prospective studies. Eur J Epidemiol 2021;36:937–51. [DOI] [PubMed] [Google Scholar]
- [91].Grosso G, La Vignera S, Condorelli RA, et al. Total, red and processed meat consumption and human health: an umbrella review of observational studies. Int J Food Sci Nutr 2022;73:726–37. [DOI] [PubMed] [Google Scholar]
- [92].Aucoin M, Cooley K, Knee C, et al. Fish-derived omega-3 fatty acids and prostate cancer: a systematic review. Integr Cancer Ther 2017;16:32–62. [DOI] [PMC free article] [PubMed] [Google Scholar]
- [93].Dai Y, Bai Y, Zhang X. Fish consumption and prostate cancer risk: a meta-analysis of 37 studies. Int J Clin Exp Med 2017;10:9891–900. [Google Scholar]
- [94].Farrell SW, DeFina LF, Tintle NL, et al. Association of the omega-3 index with incident prostate cancer with updated meta-analysis: the Cooper Center Longitudinal Study. Nutrients 2021;13:384. [DOI] [PMC free article] [PubMed] [Google Scholar]
- [95].Zhang X, Chen X, Xu Y, et al. Milk consumption and multiple health outcomes: umbrella review of systematic reviews and meta-analyses in humans. Nutr Metab 2021;18:7. [DOI] [PMC free article] [PubMed] [Google Scholar]
- [96].Savaiano DA, Hutkins RW. Yogurt, cultured fermented milk, and health: a systematic review. Nutr Rev 2021;79:599–614. [DOI] [PMC free article] [PubMed] [Google Scholar]
- [97].Sargsyan A, Dubasi HB. Milk consumption and prostate cancer: a systematic review. World J Mens Health 2021;39:419–28. [DOI] [PMC free article] [PubMed] [Google Scholar]
- [98].López-Plaza B, Bermejo LM, Santurino C, Cavero-Redondo I, Álvarez-Bueno C, Gómez-Candela C. Milk and dairy product consumption and prostate cancer risk and mortality: an overview of systematic reviews and meta-analyses. Adv Nutr 2019;10:S212–23. [DOI] [PMC free article] [PubMed] [Google Scholar]
- [99].Jin S, Je Y. Dairy consumption and total cancer and cancer-specific mortality: a meta-analysis of prospective cohort studies. Adv Nutr 2022;13:1063–82. [DOI] [PMC free article] [PubMed] [Google Scholar]
- [100].Rowles JL 3rd, Ranard KM, Smith JW, An R, Erdman JW Jr. Increased dietary and circulating lycopene are associated with reduced prostate cancer risk: a systematic review and meta-analysis. Prostate Cancer Prostatic Dis 2017;20:361–77. [DOI] [PubMed] [Google Scholar]
- [101].Rowles JL 3rd, Ranard KM, Applegate CC, Jeon S, An R, Erdman JW Jr. Processed and raw tomato consumption and risk of prostate cancer: a systematic review and dose-response meta-analysis. Prostate Cancer Prostatic Dis 2018;21:319–36. [DOI] [PubMed] [Google Scholar]
- [102].Li N, Wu X, Zhuang W, et al. Tomato and lycopene and multiple health outcomes: umbrella review. Food Chem 2021;343:128396. [DOI] [PubMed] [Google Scholar]
- [103].Luo J, Ke D, He Q. Dietary tomato consumption and the risk of prostate cancer: a meta-analysis. Front Nutr 2021;8:625185. [DOI] [PMC free article] [PubMed] [Google Scholar]
- [104].Zhang Q, Feng H, Qluwakemi B, et al. Phytoestrogens and risk of prostate cancer: an updated meta-analysis of epidemiologic studies. Int J Food Sci Nutr 2017;68:28–42. [DOI] [PubMed] [Google Scholar]
- [105].Applegate CC, Rowles JL, Ranard KM, Jeon S, Erdman JW. Soy consumption and the risk of prostate cancer: an updated systematic review and meta-analysis. Nutrients 2018;10:40. [DOI] [PMC free article] [PubMed] [Google Scholar]
- [106].Li J, Mao Q. Legume intake and risk of prostate cancer: a meta-analysis of prospective cohort studies. Oncotarget 2017;8:44776–84. [DOI] [PMC free article] [PubMed] [Google Scholar]
- [107].Perez-Cornago A, Appleby PN, Boeing H, et al. Circulating isoflavone and lignan concentrations and prostate cancer risk: a meta-analysis of individual participant data from seven prospective studies including 2,828 cases and 5,593 controls. Int J Cancer 2018;143:2677–86. [DOI] [PMC free article] [PubMed] [Google Scholar]
- [108].Guo Y, Zhi F, Chen P, et al. Green tea and the risk of prostate cancer: a systematic review and meta-analysis. Medicine 2017;96:e6426. [DOI] [PMC free article] [PubMed] [Google Scholar]
- [109].Filippini T, Malavolti M, Borrelli F, et al. Green tea (Camellia sinensis) for the prevention of cancer. Cochrane Database Syst Rev 2020;3:CD005004. [DOI] [PMC free article] [PubMed] [Google Scholar]
- [110].Grosso G, Godos J, Galvano F, Giovannucci EL. Coffee, caffeine, and health outcomes: an umbrella review. Annu Rev Nutr 2017;37:131–56. [DOI] [PubMed] [Google Scholar]
- [111].Chen X, Zhao Y, Tao Z, Wang K. Coffee consumption and risk of prostate cancer: a systematic review and meta-analysis. BMJ Open 2021;11:e038902. [DOI] [PMC free article] [PubMed] [Google Scholar]
- [112].Xia J, Chen J, Xue JX, Yang J, Wang ZJ. An up-to-date meta-analysis of coffee consumption and risk of prostate cancer. Urol J 2017;14:4079–88. [PubMed] [Google Scholar]
- [113].Sen A, Papadimitriou N, Lagiou P, et al. Coffee and tea consumption and risk of prostate cancer in the European Prospective Investigation into Cancer and Nutrition. Int J Cancer 2019;144:240–50. [DOI] [PubMed] [Google Scholar]
- [114].Hong S, Khil H, Lee DH, Keum N, Giovannucci EL. Alcohol consumption and the risk of prostate cancer: a dose-response meta-analysis. Nutrients. 2020;12:2188. [DOI] [PMC free article] [PubMed] [Google Scholar]
- [115].Vartolomei MD, Kimura S, Ferro M, et al. The impact of moderate wine consumption on the risk of developing prostate cancer. Clin Epidemiol 2018;10:431–44. [DOI] [PMC free article] [PubMed] [Google Scholar]
- [116].Waziry R, Jawad M, Ballout RA, Akel MA, Akl EA. The effects of waterpipe tobacco smoking on health outcomes: an updated systematic review and meta-analysis. Int J Epidemiol 2017;46:32–43. [DOI] [PubMed] [Google Scholar]
- [117].Islami F, Moreira DM, Boffetta P, Freedland SJ. A systematic review and meta-analysis of tobacco use and prostate cancer mortality and incidence in prospective cohort studies. Eur Urol 2014;66:1054–64. [DOI] [PMC free article] [PubMed] [Google Scholar]
- [118].Kenfield SA, Stampfer MJ, Chan JM, Giovannucci E. Smoking and prostate cancer survival and recurrence. JAMA 2011;305:2548–55. [DOI] [PMC free article] [PubMed] [Google Scholar]
- [119].Al-Fayez S, El-Metwally A. Cigarette smoking and prostate cancer: a systematic review and meta-analysis of prospective cohort studies. Tob Induc Dis 2023;21:19. [DOI] [PMC free article] [PubMed] [Google Scholar]
- [120].Gupta S, Gupta R, Sinha DN, Mehrotra R. Relationship between type of smokeless tobacco & risk of cancer: a systematic review. Indian J Med Res Suppl 2018;148:56–76. [DOI] [PMC free article] [PubMed] [Google Scholar]
- [121].Morris BJ, Matthews JG, Pabalan N, Moreton S, Krieger JN. Male circumcision and prostate cancer: a meta-analysis revisited. Can J Urol 2021;28:10768–76. [PubMed] [Google Scholar]
- [122].Del Giudice F, Kasman AM, De Berardinis E, Busetto GM, Belladelli F, Eisenberg ML. Association between male infertility and male-specific malignancies: systematic review and meta-analysis of population-based retrospective cohort studies. Fertil Steril 2020;114:984–96. [DOI] [PubMed] [Google Scholar]
- [123].Del Giudice F, Kasman AM, Ferro M, et al. Clinical correlation among male infertility and overall male health: a systematic review of the literature. Investig Clin Urol 2020;61:355–71. [DOI] [PMC free article] [PubMed] [Google Scholar]
- [124].Laukhtina E, Mori K, Pradere B, Shariat SF. Association between male infertility and prostate cancer: a systematic review and meta-analysis. Curr Opin Urol 2021;31:346–53. [DOI] [PubMed] [Google Scholar]
- [125].Behboudi-Gandevani S, Bidhendi-Yarandi R, Panahi MH, Vaismoradi M. A systematic review and meta-analysis of male infertility and the subsequent risk of cancer. Front Oncol 2021;11:696702. [DOI] [PMC free article] [PubMed] [Google Scholar]
- [126].Wu T, Duan X, Chen S, Hu C, Yu R, Cui S. Does vasectomy increase prostate cancer risk? An updated meta-analysis and systematic review of cohort studies. Int J Clin Exp Med 2018;11:2978–87. [Google Scholar]
- [127].Cheng S, Yang B, Xu L, Zheng Q, Ding G, Li G. Vasectomy and prostate cancer risk: a meta-analysis of prospective studies. Carcinogenesis 2021;42:31–7. [DOI] [PubMed] [Google Scholar]
- [128].Bhindi B, Wallis CJD, Nayan M, et al. The association between vasectomy and prostate cancer: a systematic review and meta-analysis. JAMA Intern Med 2017;177:1273–86. [DOI] [PMC free article] [PubMed] [Google Scholar]
- [129].Baboudjian M, Rajwa P, Barret E, et al. Vasectomy and risk of prostate cancer: a systematic review and meta-analysis. Eur Urol Open Sci 2022;41:35–44. [DOI] [PMC free article] [PubMed] [Google Scholar]
- [130].Xu Y, Li L, Yang W, et al. Association between vasectomy and risk of prostate cancer: a meta-analysis. Prostate Cancer Prostatic Dis 2021;24:962–75. [DOI] [PubMed] [Google Scholar]
- [131].Ding H, Fan S, Zhang L, Hao Z, Liang C. Does prostatitis increase the risk of prostate cancer? A meta-analysis. Int J Clin Exp Med 2017;10:4798–808. [Google Scholar]
- [132].Perletti G, Monti E, Magri V, et al. The association between prostatitis and prostate cancer. Systematic review and meta-analysis. Arch Ital Urol Androl 2017;89:259–65. [DOI] [PubMed] [Google Scholar]
- [133].Zhang L, Wang Y, Qin Z, et al. Correlation between prostatitis, benign prostatic hyperplasia and prostate cancer: a systematic review and meta-analysis. J Cancer 2020;11:177–89. [DOI] [PMC free article] [PubMed] [Google Scholar]
- [134].Langston ME, Horn M, Khan S, et al. A systematic review and meta-analysis of associations between clinical prostatitis and prostate cancer: new estimates accounting for detection bias. Cancer Epidemiol Biomarkers Prev 2019;28:1594–603. [DOI] [PMC free article] [PubMed] [Google Scholar]
- [135].Moolupuri A, Camacho J, de Riese WT. Association between prostate size and the incidence of prostate cancer: a meta-analysis and review for urologists and clinicians. Int Urol Nephrol 2021;53:1955–61. [DOI] [PubMed] [Google Scholar]
- [136].Zhong H, Liu S, Wang Y, et al. Primary Sjögren's syndrome is associated with increased risk of malignancies besides lymphoma: a systematic review and meta-analysis. Autoimmun Rev 2022;21:103084. [DOI] [PubMed] [Google Scholar]
- [137].Song L, Wang Y, Zhang J, Song N, Xu X, Lu Y. The risks of cancer development in systemic lupus erythematosus (SLE) patients: a systematic review and meta-analysis. Arthritis Res Ther 2018;20:270. [DOI] [PMC free article] [PubMed] [Google Scholar]
- [138].Gu D, Tang M, Wang Y, et al. The causal relationships between extrinsic exposures and risk of prostate cancer: a phenome-wide Mendelian randomization study. Front Oncol 2022;12:829248. [DOI] [PMC free article] [PubMed] [Google Scholar]
- [139].Shi J, Leng W, Zhao L, et al. Tooth loss and cancer risk: a dose-response meta analysis of prospective cohort studies. Oncotarget 2018;9:15090–100. [DOI] [PMC free article] [PubMed] [Google Scholar]
- [140].Ma H, Zheng J, Li X. Potential risk of certain cancers among patients with periodontitis: a supplementary meta-analysis of a large-scale population. Int J Med Sci 2020;17:2531–43. [DOI] [PMC free article] [PubMed] [Google Scholar]
- [141].Corbella S, Veronesi P, Galimberti V, Weinstein R, Fabbro MD, Francetti L. Is periodontitis a risk indicator for cancer? A meta-analysis. PLoS One 2018;13:e0195683. [DOI] [PMC free article] [PubMed] [Google Scholar]
- [142].Guo Z, Gu C, Li S, et al. Periodontal disease and the risk of prostate cancer: a meta-analysis of cohort studies. Int Braz J Urol 2021;47:1120–30. [DOI] [PMC free article] [PubMed] [Google Scholar]
- [143].Wei Y, Zhong Y, Wang Y, Huang R. Association between periodontal disease and prostate cancer: a systematic review and meta-analysis. Med Oral Patol Oral Cir Bucal 2021;26:e459–65. [DOI] [PMC free article] [PubMed] [Google Scholar]
- [144].Feng D, Yang Y, Wang Z, Wei W, Li L. Inflammatory bowel disease and risk of urinary cancers: A systematic review and pooled analysis of population-based studies. Transl Androl Urol 2021;10:1332–41. [DOI] [PMC free article] [PubMed] [Google Scholar]
- [145].Chen M, Yuan C, Xu T. An increase in prostate cancer diagnosis during inflammatory bowel disease: A systematic review and meta-analysis. Clin Res Hepatol Gastroenterol 2020;44:302–9. [DOI] [PubMed] [Google Scholar]
- [146].Carli E, Caviglia GP, Pellicano R, et al. Incidence of prostate cancer in inflammatory bowel disease: a meta-analysis. Medicina (Kaunas) 2020;56:285. [DOI] [PMC free article] [PubMed] [Google Scholar]
- [147].Ge Y, Shi Q, Yao W, Cheng Y, Ma G. The association between inflammatory bowel disease and prostate cancer risk: a meta-analysis. Prostate Cancer Prostatic Dis 2020;23:53–8. [DOI] [PubMed] [Google Scholar]
- [148].Haddad A, Al-Sabbagh MQ, Al-Ani H, et al. Inflammatory bowel disease and prostate cancer risk: a systematic review. Arab J Urol 2020;18:207–12. [DOI] [PMC free article] [PubMed] [Google Scholar]
- [149].Zhang C, Liu S, Peng L, et al. Does inflammatory bowel disease increase the risk of lower urinary tract tumors: a meta-analysis. Transl Androl Urol 2021;10:164–73. [DOI] [PMC free article] [PubMed] [Google Scholar]
- [150].Shen X, Wan Q, Zhao R, et al. Inflammatory bowel diseases and the risk of adverse health outcomes: umbrella review of meta-analyses of observational studies. Digest Liver Dis 2021;53:809–16. [DOI] [PubMed] [Google Scholar]
- [151].Motterle G DE Zorzi L, Zecchini G, et al. Metabolic syndrome and risk of prostate cancer: a systematic review and meta-analysis. Panminerva Med 2022;64:337–43. [DOI] [PubMed] [Google Scholar]
- [152].Guo ZL, Weng XT, Chan FL, et al. Serum C-peptide concentration and prostate cancer: a meta-analysis of observational studies. Medicine (Baltimore) 2018;97:e11771. [DOI] [PMC free article] [PubMed] [Google Scholar]
- [153].Gacci M, Russo GI, Sebastianelli A, et al. Meta-analysis of metabolic syndrome and prostate cancer. Prostate Cancer Prostatic Dis 2017;20:146–55. [DOI] [PubMed] [Google Scholar]
- [154].Harrison S, Tilling K, Turner EL, et al. Systematic review and meta-analysis of the associations between body mass index, prostate cancer, advanced prostate cancer, and prostate-specific antigen. Cancer Causes Control 2020;31:431–49. [DOI] [PMC free article] [PubMed] [Google Scholar]
- [155].Burton AJ, Gilbert R, Tilling K, et al. Circulating adiponectin and leptin and risk of overall and aggressive prostate cancer: a systematic review and meta-analysis. Sci Rep 2021;11:320. [DOI] [PMC free article] [PubMed] [Google Scholar]
- [156].Choi EK, Park HB, Lee KH, et al. Body mass index and 20 specific cancers: re-analyses of dose-response meta-analyses of observational studies. Ann Oncol 2018;29:749–57. [DOI] [PubMed] [Google Scholar]
- [157].Fang X, Wei J, He X, et al. Quantitative association between body mass index and the risk of cancer: a global Meta-analysis of prospective cohort studies. Int J Cancer 2018;143:1595–603. [DOI] [PubMed] [Google Scholar]
- [158].Xie B, Zhang G, Wang X, Xu X. Body mass index and incidence of nonaggressive and aggressive prostate cancer: a dose-response meta-analysis of cohort studies. Oncotarget 2017;8:97584–92. [DOI] [PMC free article] [PubMed] [Google Scholar]
- [159].Purcell SA, Oliveira CLP, Mackenzie M, Robson P, Lewis J, Prado CM. Body composition and prostate cancer risk: a systematic review of observational studies. Adv Nutr 2022;13:1118–30. [DOI] [PMC free article] [PubMed] [Google Scholar]
- [160].Shi J, Zhao L, Gao Y, et al. Associating the risk of three urinary cancers with obesity and overweight: an overview with evidence mapping of systematic reviews. Syst Rev 2021;10:58. [DOI] [PMC free article] [PubMed] [Google Scholar]
- [161].Mantovani A, Petracca G, Beatrice G, et al. Non-alcoholic fatty liver disease and increased risk of incident extrahepatic cancers: a meta-analysis of observational cohort studies. Gut 2022;71:778–88. [DOI] [PubMed] [Google Scholar]
- [162].Liu SS, Ma XF, Zhao J, et al. Association between nonalcoholic fatty liver disease and extrahepatic cancers: a systematic review and meta-analysis. Lipids Health Dis 2020;19:118. [DOI] [PMC free article] [PubMed] [Google Scholar]
- [163].Miller EA, Pinsky PF. Examining the relationship between diabetes and prostate cancer through changes in screening guidelines. Cancer Causes Control 2020;31:1105–13. [DOI] [PubMed] [Google Scholar]
- [164].Amadou A, Freisling H, Jenab M, et al. Prevalent diabetes and risk of total, colorectal, prostate and breast cancers in an ageing population: meta-analysis of individual participant data from cohorts of the CHANCES consortium. Br J Cancer 2021;124:1882–90. [DOI] [PMC free article] [PubMed] [Google Scholar]
- [165].Ling S, Brown K, Miksza JK, et al. Association of type 2 diabetes with cancer: a meta-analysis with bias analysis for unmeasured confounding in 151 cohorts comprising 32 million people. Diabetes Care 2020;43:2313–22. [DOI] [PubMed] [Google Scholar]
- [166].Ling S, Brown K, Miksza JK, et al. Risk of cancer incidence and mortality associated with diabetes: a systematic review with trend analysis of 203 cohorts. Nutr Metab Cardiovasc Dis 2021;31:14–22. [DOI] [PubMed] [Google Scholar]
- [167].Jayedi A, Djafarian K, Rezagholizadeh F, Mirzababaei A, Hajimohammadi M, Shab-Bidar S. Fasting blood glucose and risk of prostate cancer: a systematic review and meta-analysis of dose-response. Diabetes Metab 2018;44:320–7. [DOI] [PubMed] [Google Scholar]
- [168].Wang Y, Lan GB, Peng FH, Xie XB. Cancer risks in recipients of renal transplants: a meta-analysis of cohort studies. Oncotarget 2018;9:15375–85. [DOI] [PMC free article] [PubMed] [Google Scholar]
- [169].Bao JM, Zhu HL, Yang GS, et al. No significant association between immunosuppression in solid organ transplantation and prostate cancer risk: a meta-analysis of cohort studies. Transl Cancer Res 2019;8:939–49. [DOI] [PMC free article] [PubMed] [Google Scholar]
- [170].Cui H, Wang Y, Yang S, et al. Antidiabetic medications and the risk of prostate cancer in patients with diabetes mellitus: a systematic review and meta-analysis. Pharmacol Res 2022;177:106094. [DOI] [PubMed] [Google Scholar]
- [171].Feng Z, Zhou X, Liu N, Wang J, Chen X, Xu X. Metformin use and prostate cancer risk: a meta-analysis of cohort studies. Medicine (Baltimore) 2019;98:e14955. [DOI] [PMC free article] [PubMed] [Google Scholar]
- [172].Chen CB, Eskin M, Eurich DT, Majumdar SR, Johnson JA. Metformin, Asian ethnicity and risk of prostate cancer in type 2 diabetes: a systematic review and meta-analysis. BMC Cancer 2018;18:65. [DOI] [PMC free article] [PubMed] [Google Scholar]
- [173].Wang Y, Liu X, Yan P, et al. Effect of metformin on the risk of prostate cancer in patients with type 2 diabetes by considering different confounding factors: a meta-analysis of observational studies. Eur J Cancer Prev 2020;29:42–52. [DOI] [PubMed] [Google Scholar]
- [174].Nath M, Nath S, Choudhury Y. The impact of thiazolidinediones on the risk for prostate cancer in patients with type 2 diabetes mellitus: a review and meta-analysis. Meta Gene 2021;27:100840. [Google Scholar]
- [175].Cao L, Zhang S, Jia CM, et al. Antihypertensive drugs use and the risk of prostate cancer: a meta-analysis of 21 observational studies. BMC Urol 2018;18:17. [DOI] [PMC free article] [PubMed] [Google Scholar]
- [176].Datzmann T, Fuchs S, Andree D, Hohenstein B, Schmitt J, Schindler C. Systematic review and meta-analysis of randomised controlled clinical trial evidence refutes relationship between pharmacotherapy with angiotensin-receptor blockers and an increased risk of cancer. Eur J Intern Med 2019;64:1–9. [DOI] [PubMed] [Google Scholar]
- [177].Yang H, Yu Y, Hu X, et al. Association between the overall risk of prostate cancer and use of calcium channel blockers: a systematic review and meta-analysis. Clin Ther 2020;42:1715–27.e2. [DOI] [PubMed] [Google Scholar]
- [178].Thakur AA, Wang X, Garcia-Betancourt MM, Forse RA. Calcium channel blockers and the incidence of breast and prostate cancer: a meta-analysis. J Clin Pharm Ther 2018;43:519–29. [DOI] [PubMed] [Google Scholar]
- [179].Rotshild V, Rabkin N, Matok I. The risk for prostate cancer with calcium channel blockers: a systematic review, meta-analysis, and meta-regression. Ann Pharmacother 2023;57:16–28. [DOI] [PubMed] [Google Scholar]
- [180].Lai SW, Hwang BF, Kuo YH, Liu CS, Liao KF. A meta-analysis of allopurinol therapy and the risk of prostate cancer. Medicine (Baltimore) 2022;101:e28998. [DOI] [PMC free article] [PubMed] [Google Scholar]
- [181].Song HJ, Jeon N, Squires P. The association between acid-suppressive agent use and the risk of cancer: a systematic review and meta-analysis. Eur J Clin Pharmacol 2020;76:1437–56. [DOI] [PubMed] [Google Scholar]
- [182].Godono A, Clari M, Franco N, et al. The association between occupational asbestos exposure with the risk of incidence and mortality from prostate cancer: a systematic review and meta-analysis. Prostate Cancer Prostatic Dis 2022;25:604–14. [DOI] [PubMed] [Google Scholar]
- [183].Dutheil F, Zaragoza-Civale L, Pereira B, et al. Prostate cancer and asbestos: a systematic review and meta-analysis. Perm J 2020;24:19.086. [DOI] [PMC free article] [PubMed] [Google Scholar]
- [184].Fernández-Martínez NF, Ching-López A, Olry de Labry Lima A, et al. Relationship between exposure to mixtures of persistent, bioaccumulative, and toxic chemicals and cancer risk: a systematic review. Environ Res 2020;188:109787. [DOI] [PubMed] [Google Scholar]
- [185].Yang T, Qiao Y, Xiang S, Li W, Gan Y, Chen Y. Work stress and the risk of cancer: a meta-analysis of observational studies. Int J Cancer 2019;144:2390–400. [DOI] [PubMed] [Google Scholar]
- [186].Boffetta P, Desai V. Exposure to permethrin and cancer risk: a systematic review. Crit Rev Toxicol 2018;48:433–42. [DOI] [PubMed] [Google Scholar]
- [187].Holy CE, Zhang S, Perkins LE, et al. Site-specific cancer risk following cobalt exposure via orthopedic implants or in occupational settings: a systematic review and meta-analysis. Regul Toxicol Pharmacol 2022;129:105096. [DOI] [PubMed] [Google Scholar]
- [188].Deng Y, Wang M, Tian T, et al. The effect of hexavalent chromium on the incidence and mortality of human cancers: a meta-analysis based on published epidemiological cohort studies. Front Oncol 2019;9:24. [DOI] [PMC free article] [PubMed] [Google Scholar]
- [189].Jalilian H, Ziaei M, Weiderpass E, Rueegg CS, Khosravi Y, Kjaerheim K. Cancer incidence and mortality among firefighters. Int J Cancer 2019;145:2639–46. [DOI] [PubMed] [Google Scholar]
- [190].Sritharan J, Pahwa M, Demers PA, Harris SA, Cole DC, Parent ME. Prostate cancer in firefighting and police work: a systematic review and meta-analysis of epidemiologic studies. Environ Health 2017;16:124. [DOI] [PMC free article] [PubMed] [Google Scholar]
- [191].Togawa K, Leon ME, Lebailly P, et al. Cancer incidence in agricultural workers: findings from an international consortium of agricultural cohort studies (AGRICOH). Environ Int 2021;157:106825. [DOI] [PMC free article] [PubMed] [Google Scholar]
- [192].Onyije FM, Hosseini B, Togawa K, Schüz J, Olsson A. Cancer incidence and mortality among petroleum industry workers and residents living in oil producing communities: a systematic review and meta-analysis. Int J Environ Res Public Health 2021;18:4343. [DOI] [PMC free article] [PubMed] [Google Scholar]
- [193].Alif SM, Sim MR, Ho C, Glass DC. Cancer and mortality in coal mine workers: a systematic review and meta-analysis. Occup Environ Med 2022;79:347–57. [DOI] [PubMed] [Google Scholar]
- [194].Krstev S, Knutsson A. Occupational risk factors for prostate cancer: a meta-analysis. J Cancer Prev 2019;24:91–111. [DOI] [PMC free article] [PubMed] [Google Scholar]
- [195].Du HB, Bin KY, Liu WH, Yang FS. Shift work, night work, and the risk of prostate cancer: a meta-analysis based on 9 cohort studies. Medicine (Baltimore) 2017;96:e8537. [DOI] [PMC free article] [PubMed] [Google Scholar]
- [196].Gan Y, Li L, Zhang L, et al. Association between shift work and risk of prostate cancer: a systematic review and meta-analysis of observational studies. Carcinogenesis 2018;39:87–97. [DOI] [PubMed] [Google Scholar]
- [197].Dun A, Zhao X, Jin X, et al. Association between night-shift work and cancer risk: Updated systematic review and meta-analysis. Front Oncol 2020;10:1–15. [DOI] [PMC free article] [PubMed] [Google Scholar]
- [198].Rivera-Izquierdo M, Martínez-Ruiz V, Castillo-Ruiz EM, Manzaneda-Navío M, Pérez-Gómez B, Jiménez-Moleón JJ. Shift work and prostate cancer: an updated systematic review and meta-analysis. Int J Environ Res Public Health 2020;17:1345. [DOI] [PMC free article] [PubMed] [Google Scholar]
- [199].Jian Z, Ye D, Chen Y, Li H, Wang K. Sexual activity and risk of prostate cancer: a dose-response meta-analysis. J Sex Med 2018;15:1300–9. [DOI] [PubMed] [Google Scholar]
- [200].Crocetto F, Arcaniolo D, Napolitano L, et al. Impact of sexual activity on the risk of male genital tumors: a systematic review of the literature. Int J Environ Res Public Health 2021;18:8500. [DOI] [PMC free article] [PubMed] [Google Scholar]
- [201].Russo GI, Calogero AE, Condorelli RA, Scalia G, Morgia G, La Vignera S. Human papillomavirus and risk of prostate cancer: a systematic review and meta-analysis. Aging Male 2020;23:132–8. [DOI] [PubMed] [Google Scholar]
- [202].Yin B, Liu W, Yu P, Liu C, Chen Y, Duan X, et al. Association between human papillomavirus and prostate cancer: a meta-analysis. Oncol Lett 2017;14:1855–65. [DOI] [PMC free article] [PubMed] [Google Scholar]
- [203].Lawson JS, Glenn WK. Evidence for a causal role by human papillomaviruses in prostate cancer—a systematic review. Infect Agent Cancer 2020;15:41. [DOI] [PMC free article] [PubMed] [Google Scholar]
- [204].Moghoofei M, Keshavarz M, Ghorbani S, et al. Association between human papillomavirus infection and prostate cancer: a global systematic review and meta-analysis. Asia Pac J Clin Oncol 2019;15:e59–67. [DOI] [PubMed] [Google Scholar]
- [205].Lawson JS, Glenn WK. Multiple pathogens and prostate cancer. Infect Agent Cancer. 2022;17:23. [DOI] [PMC free article] [PubMed] [Google Scholar]
- [206].Ma Y, Huang Z, Jian Z, Wei X. The association between hepatitis C virus infection and renal cell cancer, prostate cancer, and bladder cancer: a systematic review and meta-analysis. Sci Rep 2021;11:10833. [DOI] [PMC free article] [PubMed] [Google Scholar]
- [207].Najafi A, Chaechi Nosrati MR, Ghasemi E, et al. Is there association between Trichomonas vaginalis infection and prostate cancer risk? A systematic review and meta-analysis. Microb Pathog 2019;137:103752. [DOI] [PubMed] [Google Scholar]
- [208].Salmon C, Song L, Muir K, et al. Marital status and prostate cancer incidence: a pooled analysis of 12 case-control studies from the PRACTICAL consortium. Eur J Epidemiol 2021;36:913–25. [DOI] [PubMed] [Google Scholar]
- [209].Wang L, Lei Y, Gao Y, et al. Association of finasteride with prostate cancer: a systematic review and meta-analysis. Medicine (Baltimore) 2020;99:e19486. [DOI] [PMC free article] [PubMed] [Google Scholar]
- [210].Min GE, Kim H, Lee DE, Kim K. Effects of dutasteride on prostate cancer incidence: a systematic review and meta-analysis. Int J Res Pharm Sci 2020;11:7266–70. [Google Scholar]
- [211].Hu X, Wang YH, Yang ZQ, Shao YX, Yang WX, Li X. Association of 5-alpha-reductase inhibitor and prostate cancer incidence and mortality: a meta-analysis. Transl Androl Urol 2020;9:2519–32. [DOI] [PMC free article] [PubMed] [Google Scholar]
- [212].Vaselkiv JB, Ceraolo C, Wilson KM, et al. 5-Alpha reductase inhibitors and prostate cancer mortality among men with regular access to screening and health care. Cancer Epidemiol Biomarkers Prev 2022;31:1460–5. [DOI] [PMC free article] [PubMed] [Google Scholar]
- [213].Bjornebo L, Nordstrom T, Discacciati A, et al. Association of 5alpha-reductase inhibitors with prostate cancer mortality. JAMA Oncol 2022;8:1019–26. [DOI] [PMC free article] [PubMed] [Google Scholar]
- [214].Qiao Y, Yang T, Gan Y, et al. Associations between aspirin use and the risk of cancers: a meta-analysis of observational studies. BMC Cancer 2018;18:288. [DOI] [PMC free article] [PubMed] [Google Scholar]
- [215].Wang L, Zhang R, Yu L, et al. Aspirin use and common cancer risk: a meta-analysis of cohort studies and randomized controlled trials. Front Oncol 2021;11:690219. [DOI] [PMC free article] [PubMed] [Google Scholar]
- [216].Sauer CM, Myran DT, Costentin CE, et al. Effect of long term aspirin use on the incidence of prostate cancer: a systematic review and meta-analysis. Crit Rev Oncol Hematol 2018;132:66–75. [DOI] [PubMed] [Google Scholar]
- [217].Shang Z, Wang X, Yan H, et al. Intake of non-steroidal anti-inflammatory drugs and the risk of prostate cancer: a meta-analysis. Front Oncol 2018;8:437. [DOI] [PMC free article] [PubMed] [Google Scholar]
- [218].Chen J, Zhang B, Chen D, Zhuang W. The association of statin use with risk of kidney, bladder and prostate cancer: a systematic review and meta-analysis of cohort studies. Int J Clin Exp Med 2018;11:8873. [Google Scholar]
- [219].Li Y, Cheng X, Zhu JL, et al. Effect of statins on the risk of different stages of prostate cancer: a meta-analysis. Urol Int 2022;106:869–77. [DOI] [PubMed] [Google Scholar]
- [220].Xu MY, An Y, Liu CQ, et al. Association of statin use with the risk of incident prostate cancer: a meta-analysis and systematic review. J Oncol 2022;2022:7827821. [DOI] [PMC free article] [PubMed] [Google Scholar]
- [221].Jeong GH, Lee KH, Kim JY, Eisenhut M, et al. Effect of statin on cancer incidence: an umbrella systematic review and meta-analysis. J Clin Med 2019;8:819. [DOI] [PMC free article] [PubMed] [Google Scholar]
- [222].Manson JE, Bassuk SS, Buring JE. Principal results of the VITamin D and OmegA-3 TriaL (VITAL) and updated meta-analyses of relevant vitamin D trials. J Steroid Biochem Mol Biol 2020;198:105522. [DOI] [PMC free article] [PubMed] [Google Scholar]
- [223].Gao J, Wei W, Wang G, Zhou H, Fu Y, Liu N. Circulating vitamin D concentration and risk of prostate cancer: a dose-response meta-analysis of prospective studies. Ther Clin Risk Manag 2018;14:95–104. [DOI] [PMC free article] [PubMed] [Google Scholar]
- [224].Giovannucci E, Liu Y, Willett WC. Cancer incidence and mortality and vitamin D in black and white male health professionals. Cancer Epidemiol Biomarkers Prev 2006;15:2467–72. [DOI] [PubMed] [Google Scholar]
- [225].Sayehmiri K, Azami M, Mohammadi Y, Soleymani A, Tardeh Z. The association between selenium and prostate cancer: a systematic review and meta-analysis. Asian Pac J Cancer Prev 2018;19:1431–7. [DOI] [PMC free article] [PubMed] [Google Scholar]
- [226].Lippman SM, Klein EA, Goodman PJ, et al. Effect of selenium and vitamin E on risk of prostate cancer and other cancers: the Selenium and Vitamin E Cancer Prevention Trial (SELECT). JAMA 2009;301:39–51. [DOI] [PMC free article] [PubMed] [Google Scholar]
- [227].Aoun F, Slaoui A, Walid AHO, et al. Association between phosphodiesterase type 5 inhibitors and prostate cancer: a systematic review. Prog Urol 2018;28:560–6. [DOI] [PubMed] [Google Scholar]
- [228].Bommareddy K, Hamade H, Lopez-Olivo MA, Wehner M, Tosh T, Barbieri JS. Association of spironolactone use with risk of cancer: a systematic review and meta-analysis. JAMA Dermatol 2022;158:275–82. [DOI] [PMC free article] [PubMed] [Google Scholar]
- [229].Lin C, Cai X, Yang W, Lv F, Nie L, Ji L. Glycemic control and the incidence of neoplasm in patients with type 2 diabetes: a meta-analysis of randomized controlled trials. Endocrine 2020;70:232–42. [DOI] [PubMed] [Google Scholar]
- [230].Luo JD, Luo J, Lai C, Chen J, Meng HZ. Is use of vitamin K antagonists associated with the risk of prostate cancer? A meta-analysis. Medicine (Baltimore) 2018;97:e13489. [DOI] [PMC free article] [PubMed] [Google Scholar]
- [231].Kristensen KB, Jensen PH, Skriver C, Friis S, Pottegård A. Use of vitamin K antagonists and risk of prostate cancer: meta-analysis and nationwide case-control study. Int J Cancer 2019;144:1522–9. [DOI] [PubMed] [Google Scholar]
- [232].Mottet N, van den Bergh RCN, Briers E, al. EAU-EANM-ESTRO-ESUR-SIOG guidelines on prostate cancer—2020 update. Part 1: screening, diagnosis, and local treatment with curative intent. Eur Urol 2021;79:243–62. [DOI] [PubMed] [Google Scholar]
- [233].Force USPST, Grossman DC, Curry SJ, et al. Screening for prostate cancer: US Preventive Services Task Force recommendation statement. JAMA 2018;319:1901–13. [DOI] [PubMed] [Google Scholar]
- [234].Nyame YA, Gulati R, Heijnsdijk EAM, et al. The impact of intensifying prostate cancer screening in Black men: a model-based analysis. J Natl Cancer Inst 2021;113:1336–42. [DOI] [PMC free article] [PubMed] [Google Scholar]
- [235].Pinsky PF, Miller E, Prorok P, Grubb R, Crawford ED, Andriole G. Extended follow-up for prostate cancer incidence and mortality among participants in the Prostate, Lung, Colorectal and Ovarian randomized cancer screening trial. BJU Int 2019;123:854–60. [DOI] [PMC free article] [PubMed] [Google Scholar]
- [236].Tsodikov A, Gulati R, Etzioni R. Reconciling the effects of screening on prostate cancer mortality in the ERSPC and PLCO trials. Ann Intern Med 2018;168:608–9. [DOI] [PubMed] [Google Scholar]
- [237].Ahmed HU, El-Shater Bosaily A, Brown LC, et al. Diagnostic accuracy of multi-parametric MRI and TRUS biopsy in prostate cancer (PROMIS): a paired validating confirmatory study. Lancet (London, England) 2017;389:815–22. [DOI] [PubMed] [Google Scholar]
- [238].Kasivisvanathan V, Emberton M, Moore CM. MRI-targeted biopsy for prostate-cancer diagnosis. N Engl J Med 2018;379:589–90. [DOI] [PubMed] [Google Scholar]
- [239].Goldberg H, Ahmad AE, Chandrasekar T, et al. Comparison of magnetic resonance imaging and transrectal ultrasound informed prostate biopsy for prostate cancer diagnosis in biopsy naïve men: a systematic review and meta-analysis. J Urol 2020;203:1085–93. [DOI] [PubMed] [Google Scholar]
- [240].Woo S, Suh CH, Eastham JA, et al. Comparison of magnetic resonance imaging-stratified clinical pathways and systematic transrectal ultrasound-guided biopsy pathway for the detection of clinically significant prostate cancer: a systematic review and meta-analysis of randomized controlled trials. Eur Urol Oncol 2019;2:605–16. [DOI] [PMC free article] [PubMed] [Google Scholar]
- [241].Zhen L, Liu X, Yegang C, et al. Accuracy of multiparametric magnetic resonance imaging for diagnosing prostate cancer: a systematic review and meta-analysis. BMC Cancer 2019;19:1244. [DOI] [PMC free article] [PubMed] [Google Scholar]
- [242].Elwenspoek MMC, Sheppard AL, McInnes MDF, et al. Comparison of multiparametric magnetic resonance imaging and targeted biopsy with systematic biopsy alone for the diagnosis of prostate cancer: a systematic review and meta-analysis. JAMA Netw Open 2019;2:e198427. [DOI] [PMC free article] [PubMed] [Google Scholar]
- [243].Eklund M, Jaderling F, Discacciati A, et al. MRI-targeted or standard biopsy in prostate cancer screening. N Engl J Med 2021;385:908–20. [DOI] [PubMed] [Google Scholar]
- [244].Hugosson J, Mansson M, Wallstrom J, et al. Prostate cancer screening with PSA and MRI followed by targeted biopsy only. N Engl J Med 2022;387:2126–37. [DOI] [PMC free article] [PubMed] [Google Scholar]
- [245].Latosinska A, Frantzi M, Merseburger AS, Mischak H. Promise and implementation of proteomic prostate cancer biomarkers. Diagnostics (Basel) 2018;8:57. [DOI] [PMC free article] [PubMed] [Google Scholar]
- [246].Cucchiara V, Cooperberg MR, Dall'Era M, et al. Genomic markers in prostate cancer decision making. Eur Urol 2018;73:572–82. [DOI] [PubMed] [Google Scholar]
- [247].Anceschi U, Tuderti G, Lugnani F, et al. Novel diagnostic biomarkers of prostate cancer: an update. Curr Med Chem 2019;26:1045–58. [DOI] [PubMed] [Google Scholar]
- [248].McNally CJ, Ruddock MW, Moore T, McKenna DJ. Biomarkers that differentiate benign prostatic hyperplasia from prostate cancer: a literature review. Cancer Manag Res 2020;12:5225–41. [DOI] [PMC free article] [PubMed] [Google Scholar]
- [249].Vickers A, Vertosick EA, Sjoberg DD, et al. Value of intact prostate specific antigen and human kallikrein 2 in the 4 kallikrein predictive model: an individual patient data meta-analysis. J Urol 2018;199:1470–4. [DOI] [PMC free article] [PubMed] [Google Scholar]
- [250].Vertosick EA, Zappala S, Punnen S, et al. Individual patient data meta-analysis of discrimination of the four kallikrein panel associated with the inclusion of prostate volume. Urology 2021;157:102–6. [DOI] [PMC free article] [PubMed] [Google Scholar]
- [251].Lazzeri M, Haese A, de la Taille A, et al. Serum isoform [-2]proPSA derivatives significantly improve prediction of prostate cancer at initial biopsy in a total PSA range of 2–10 ng/ml: a multicentric European study. Eur Urol 2013;63:986–94. [DOI] [PubMed] [Google Scholar]
- [252].Klein EA, Chait A, Hafron JM, et al. The Single-parameter, structure-based IsoPSA assay demonstrates improved diagnostic accuracy for detection of any prostate cancer and high-grade prostate cancer compared to a concentration-based assay of total prostate-specific antigen: a preliminary report. Eur Urol 2017;72:942–9. [DOI] [PubMed] [Google Scholar]
- [253].Campistol M, Morote J, Regis L, Celma A, Planas J, Trilla E. Proclarix, a new biomarker for the diagnosis of clinically significant prostate cancer: a systematic review. Mol Diagn Ther 2022;26:273–81. [DOI] [PubMed] [Google Scholar]
- [254].Ström P, Nordström T, Aly M, Egevad L, Grönberg H, Eklund M. The Stockholm-3 model for prostate cancer detection: algorithm update, biomarker contribution, and reflex test potential. Eur Urol. 2018;74:204–10. [DOI] [PubMed] [Google Scholar]
- [255].McKiernan J, Donovan MJ, Margolis E, et al. A prospective adaptive utility trial to validate performance of a novel urine exosome gene expression assay to predict high-grade prostate cancer in patients with prostate-specific antigen 2–10 ng/ml at initial biopsy. Eur Urol 2018;74:731–8. [DOI] [PubMed] [Google Scholar]
- [256].Margolis E, Brown G, Partin A, et al. Predicting high-grade prostate cancer at initial biopsy: clinical performance of the ExoDx (EPI) Prostate Intelliscore test in three independent prospective studies. Prostate Cancer Prostatic Dis 2022;25:296–301. [DOI] [PMC free article] [PubMed] [Google Scholar]
- [257].Tomlins SA, Day JR, Lonigro RJ, et al. Urine TMPRSS2:ERG Plus PCA3 for individualized prostate cancer risk assessment. Eur Urol 2016;70:45–53. [DOI] [PMC free article] [PubMed] [Google Scholar]
- [258].Sari Motlagh R, Yanagisawa T, Kawada T, et al. Accuracy of SelectMDx compared to mpMRI in the diagnosis of prostate cancer: a systematic review and diagnostic meta-analysis. Prostate Cancer Prostatic Dis 2022;25:187–98. [DOI] [PubMed] [Google Scholar]
- [259].Chang EK, Gadzinski AJ, Nyame YA. Blood and urine biomarkers in prostate cancer: are we ready for reflex testing in men with an elevated prostate-specific antigen? Asian J Urol 2021;8:343–53. [DOI] [PMC free article] [PubMed] [Google Scholar]
Associated Data
This section collects any data citations, data availability statements, or supplementary materials included in this article.