Abstract
Background
Chronic cough affects up to 10% of the general population and was previously perceived as a comorbidity of underlying conditions, but is nowadays classified as a disease in its own entity that could confer increased risk of morbidity and mortality. We tested the hypothesis that chronic cough is associated with increased risk of COPD exacerbation, pneumonia and all-cause mortality in the general population.
Methods
We identified 2801 individuals with chronic cough, defined as cough lasting >8 weeks, among 44 756 randomly selected individuals from the Copenhagen General Population Study, and recorded COPD exacerbations, pneumonia and all-cause mortality during follow-up.
Results
During up to 5.9 years of follow-up (median 3.4 years), 173 individuals experienced COPD exacerbation, 767 experienced pneumonia and 894 individuals died. Individuals with chronic cough versus those without had cumulative incidences at age 80 years of 12% versus 3% for COPD exacerbation, 30% versus 15% for pneumonia, and 25% versus 13% for death from all causes. After adjustment for age, sex and smoking, individuals with chronic cough versus those without had adjusted hazard ratios of 4.6 (95% CI 2.9–7.2) for COPD exacerbation, 2.2 (1.7–2.7) for pneumonia and 1.7 (1.4–2.0) for all-cause mortality. Among current smokers aged >60 years with airflow limitation, those with versus without chronic cough had an absolute 5-year risk of 10% versus 4% for COPD exacerbation, 16% versus 8% for pneumonia and 19% versus 12% for all-cause mortality.
Conclusion
Chronic cough is associated with higher risks of COPD exacerbation, pneumonia and death, independent of airflow limitation and smoking.
Tweetable abstract
Chronic cough is associated with 4.6-fold increased risk of COPD exacerbation, 2.2-fold of pneumonia and 1.7-fold of death https://bit.ly/47QUTcg
Introduction
Chronic cough is defined as a cough lasting >8 weeks and affects up to 10% of the general population [1, 2]. Although chronic cough previously was perceived as a comorbidity of underlying conditions, it is nowadays classified as a disease in its own entity [3]. Risk factors for chronic cough include bronchiectasis, asthma, airflow limitation, gastroesophageal reflux disease, upper airway cough syndrome, smoking and obesity [1, 4].
Recently different mechanisms of cough were suggested between patients with COPD and chronic refractory cough [5], suggesting drugs for the treatment of chronic cough may potentially be beneficial for some patients with chronic cough and COPD and not others. Cough as a defence mechanism of aspiration is important for the pathogenesis of chronic cough, and prandial aspiration may occur more often in COPD contributing to susceptibility to acute exacerbations and pneumonia in some of the patients [6]. Chronic cough in individuals with COPD has been associated with a more severe disease phenotype in terms of more accompanying respiratory symptoms and lower lung function [7–9], and it has been suggested that chronic cough could be an important predictor of acute exacerbations and perhaps other future COPD-related outcomes. If patients with chronic cough have a higher susceptibility for future COPD-related outcomes like COPD exacerbation, pneumonia and mortality, then these patients should perhaps be treated more intensely.
We tested the hypothesis that chronic cough is associated with increased risk of COPD exacerbation, acute pneumonia and all-cause mortality in the general population. For this purpose, we identified individuals with chronic cough from 44 756 randomly selected individuals from the Copenhagen General Population Study and followed them for up to 5.9 years. Risk was also investigated according to age, airflow limitation and current smoking, as these covariates are important factors for COPD severity and prognosis.
Materials and methods
Study design and participants
The Copenhagen General Population Study is a Danish contemporary population-based cohort initiated in 2003 with ongoing enrolment (e-Figure 1). All individuals in Denmark are assigned a unique identification number at birth/immigration and recorded in the national Danish Civil Registration System. By using this unique number, individuals aged 20–100 years are randomly selected and invited from the national Danish Civil Registration System to reflect the adult Danish population. The participation rate of the Copenhagen General Population Study is 49.3%. From 1 January 2013 to 14 September 2018, using a nested cohort design, 44 756 consecutive individuals were asked about presence of chronic cough and completed a clinical questionnaire, underwent a physical health examination and gave blood for biochemical analyses (supplementary e-Figure 1) [1, 7]. Questionnaires were reviewed at the day of attendance by a healthcare professional together with the participant. The study was approved by Herlev and Gentofte Hospital and a Danish ethical committee and was conducted according to the Declaration of Helsinki (identification no.: H-KF-01-144/01). All participants provided written informed consent.
Chronic cough and clinical outcomes
Chronic cough was defined as an affirmative response to the question: “Do you have a cough lasting for more than 8 weeks?” in accordance with the clinical recommendations from the American College of Chest Physicians, British Thoracic Society and the European Respiratory Society (ERS) [3, 10–12]. Participants were also asked about sputum production (=phlegm from the lungs in the morning and/or during the day for as long as 3 consecutive months each year). Productive chronic cough was defined in those who had chronic cough with sputum production. Non-productive chronic cough was defined in those who had chronic cough without sputum production.
COPD exacerbations (International Classification of Diseases (ICD)-10: J41–44) and pneumonia (ICD-10: J12–18) were defined as acute emergency department visits or hospital admissions according to the national Danish Patient Registry, which is a complete register of all public and private hospital contacts in Denmark. Information on all-cause mortality and cause-specific mortality (COPD, ICD10: J41–44; respiratory diseases, ICD10: J00–99; ischaemic heart disease, ICD10: I20–25; cardiovascular diseases, ICD10: I00–I99) was retrieved from the national Danish Register of Causes of Death.
Forced expiratory volume in 1 s (FEV1) and forced vital capacity (FVC) were measured pre-bronchodilator as described in detail elsewhere [13]. Airflow limitation was defined as FEV1/FVC <0.70. From 2014 to 2018 individuals with airflow limitation with a pre-bronchodilator FEV1/FVC <0.70 were asked to undergo reversibility testing in which post-bronchodilator lung function was measured 15 min following inhalation of 400 µg of salbutamol (Ventoline Diskus, GlaxoSmithKline) [7]. Smoking status was reported as never, former and current smoking. Former and current smokers provided information on type and daily amount of consumed tobacco, and cumulative tobacco consumption was calculated in pack-years: 1 pack-year corresponded to 20 cigarettes or equivalent (cigars, cheroots or pipe tobacco) consumed daily for a year.
Statistical analyses
All statistical analyses were conducted using STATA/SE version 12.1 for Windows (StataCorp), and a two-tailed p-value <0.05 was considered as statistically significant. The number of individuals with missing values were few and random among study groups, and we chose not to fill in missing values in the analysis. All participants were included for the baseline cross-sectional analysis using Pearson's χ2-squared tests for comparisons of categorical attributes, and t-tests and Wilcoxon's rank-sum tests, respectively, for comparisons of normally and non-normally distributed continuous attributes. Participants who had a COPD diagnosis prior to the baseline survey were excluded from the cumulative incidences and Cox regression analyses. End of study follow-up was 31 December 2018. Cumulative incidences of COPD exacerbation and pneumonia were calculated and graphed using Fine–Gray competing risk models with death as competing event. Cumulative incidence of all-cause mortality was calculated and graphed using Kaplan–Meier estimated probability of survival. Hazard ratios (HRs) for COPD exacerbations and pneumonias were calculated using modified Cox regressions according to the method of Anderson–Gill [14]. HR for all-cause mortality was calculated using regular Cox regression. Age, sex and smoking were included in adjusted analyses, and interactions between airflow limitation and current smoking with chronic cough were investigated. For COPD exacerbation and pneumonia, absolute 5-year risks were calculated using Fine–Gray competing risk models with death as competing event, and for all-cause mortality, we used regular Kaplan–Meier analysis, all with study entry as left truncation. These data were presented as estimated incidence rates (number of events per 5 years) in per cent.
Results
During up to 5.9 years follow-up (median: 3.4 years) (supplementary e-Figure 1), 173 (0.4%) individuals experienced an exacerbation of COPD, 767 (1.7%) experienced pneumonia, and 894 (2.0%) individuals died. Baseline characteristics of individuals with and without a subsequent clinical outcome are shown in table 1. As expected, individuals with a future outcome were older, had lower FEV1 % predicted, had more often airflow limitation, and were more often current smokers or ex-smokers and had higher tobacco consumption compared with individuals who remained free from outcomes during follow-up. Prevalence of chronic cough was higher at baseline examination in individuals with a future outcome (table 1). Individuals with chronic cough at baseline versus those without had higher risk of spirometric COPD (supplementary e-Figure 2).
TABLE 1.
Baseline characteristics according to clinical COPD outcomes in individuals in the Copenhagen General Population Study
| None | COPD exacerbation | Pneumonia | Death | Any | |
| Subjects | 43 158 (96) | 173 (0.4) | 767 (1.7) | 894 (2.0) | 1598 (3.6) |
| Male sex | 18 981 (44) | 76 (44) | 430 (56)* | 526 (59)* | 888 (56)* |
| Age years | 59 (50–69) | 73 (66–79)* | 72 (63–80)* | 76 (68–82)* | 73 (65–80)* |
| FEV1 % predicted | 99±15 | 59±21* | 86±24* | 93±24* | 88±25* |
| Airflow limitation # | 8545 (20) | 140 (83)* | 324 (43)* | 366 (42)* | 694 (44)* |
| Current smoker | 4674 (11) | 52 (30)* | 133 (18)* | 168 (19)* | 298 (19)* |
| Ex-smoker | 18 148 (42) | 104 (60)* | 410 (53)* | 458 (51)* | 829 (52)* |
| Never-smoker | 20 025 (46) | 17 (10)* | 218 (28)* | 263 (29)* | 461 (29)* |
| Tobacco consumption pack-years | 14 (5–26) | 38 (25–50)* | 24 (10–41)* | 25 (11–43)* | 25 (11–43)* |
| Chronic cough | 2587 (6) | 40 (23)* | 113 (15)* | 113 (13)* | 214 (13)* |
Data are presented as n (%), median (interquartile range) or mean±sd. FEV1: forced expiratory volume in 1 s; FVC: forced vital capacity. #: airflow limitation defined as FEV1/FVC <0.7. *: p<0.05 versus subjects without any outcome during follow-up using Pearson's χ2, t-test or Wilcoxon's rank-sum test.
The cumulative incidences of COPD exacerbation, pneumonia and all-cause mortality were higher in those with chronic cough compared with those without chronic cough (figure 1). At age 80 years, 12% of those with chronic cough at baseline had experienced a COPD exacerbation versus 3.2% in those without chronic cough. Corresponding values were 30% versus 15% for pneumonia and 25% versus 13% for all-cause mortality. As expected, the cumulative incidences for clinical COPD outcomes were higher in those with versus without airflow limitation and in current smokers versus nonsmokers (figure 2).
FIGURE 1.
Cumulative incidence of a) COPD exacerbation, b) pneumonia and c) all-cause mortality according to chronic cough. Cumulative incidences of COPD exacerbation and pneumonia were obtained from Fine–Gray competing risk model, while cumulative incidence of all-cause mortality was obtained from Kaplan–Meier analysis. Dashed lines highlight absolute risk at the age of 80 years.
FIGURE 2.
Cumulative incidence of COPD exacerbation, pneumonia and all-cause mortality according to chronic cough, stratified analysis. Cumulative incidences of COPD exacerbation and pneumonia were obtained from Fine–Gray competing risk model, while cumulative incidence of all-cause mortality was obtained from Kaplan–Meier analysis. Dashed lines highlight absolute risk at age of 80 years.
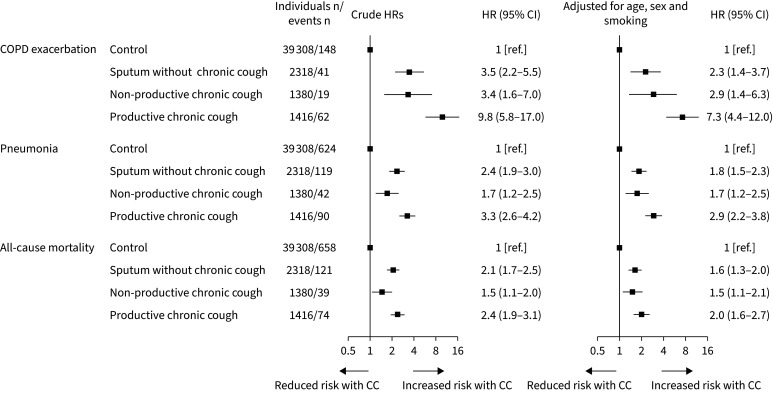
During a median of 3.4 years of follow-up, 81 individuals with chronic cough (3%) experienced a COPD exacerbation, 132 (5%) experienced pneumonia and 113 (4%) died. After statistical adjustment for age, sex and smoking, individuals with chronic cough versus those without had an increased HR for COPD exacerbation of 4.6 (95% confidence interval: 2.9–7.2) (figure 3). Corresponding HRs were 2.2 (1.7–2.7) for pneumonia and 1.7 (1.4–2.0) for all-cause mortality, respectively. Results were similar after stratification for airflow limitation and current smoking, and no statistically significant interactions were observed between these parameters and chronic cough (figure 3). This suggests that the increased risks of COPD outcomes in individuals with chronic cough versus those without were independent of influences caused by airflow limitation and current smoking. When the analysis was stratified by chronic cough type, the HRs for COPD exacerbation and pneumonia were higher in individuals with productive chronic cough versus individuals with sputum production alone (p=0.001 and p=0.01) or non-productive chronic cough (p=0.05 and p<0.03) (figure 4). A similar trend was seen for all-cause mortality, but results were not statistically significant (p-values ≥0.15). When the analysis was stratified by cause-specific mortality, individuals with chronic cough versus those without had increased HRs of 6.7 (3.2–14) for COPD-specific mortality, 3.1 (2.1–4.7) for respiratory disease-related mortality and 1.9 (1.3–2.8) for cardiovascular disease-related mortality (supplementary e-Figure 3)
FIGURE 3.
Risk of a) COPD exacerbation, b) pneumonia and c) all-cause mortality in individuals with chronic cough versus those without chronic cough, stratified analysis. Hazard ratios (HRs) with 95% confidence intervals (CIs) were obtained from Cox proportional hazards models. Individuals: number of individuals with chronic cough; Events: number of individuals with chronic cough who had an event during follow-up. Individuals without chronic cough was used as reference group in the analyses. Adjustment for smoking status was omitted when stratifying for current smoking. AL: airflow limitation; CC: chronic cough.
FIGURE 4.
Risk of COPD exacerbations, pneumonia and all-cause mortality in individuals with sputum production, non-productive chronic cough and productive chronic cough. Hazard ratios (HRs) with 95% confidence intervals (CIs) were obtained from Cox proportional hazards models. Sputum production: phlegm from the lungs in the morning and/or during the day for as long as 3 consecutive months each year. Non-productive chronic cough was chronic cough without sputum production. Productive chronic cough was chronic cough with sputum production. CC: chronic cough.
The lowest 5-year absolute risk for COPD exacerbation was 0.1% and could be observed in nonsmokers without chronic cough aged <60 years without airflow limitation (figure 5). Corresponding values for pneumonia and all-cause mortality were 0.8% and 0.5%, respectively. The absolute risks increased with presence of chronic cough, current smoking, increasing age and airflow limitation. The highest 5-year absolute risk for COPD exacerbation was 10% in current smokers with chronic cough aged ≥60 years with airflow limitation (figure 5). Corresponding values for pneumonia and all-cause mortality were 16% and 19%, respectively.
FIGURE 5.
Absolute 5-year risk of COPD exacerbation, pneumonia and all-cause mortality for different combinations of risk factors and chronic cough. The Fine–Gray regression model was implemented when accounting for competing risk. AL: airflow limitation.
Discussion
We tested the hypothesis that chronic cough is associated with increased risk of COPD exacerbation, pneumonia and all-cause mortality in the general population using a contemporary Danish population-based cohort with 44 756 randomly selected individuals. We found that chronic cough is a strong predictor of future COPD exacerbation, pneumonia and death, and that this is independent of age, sex, smoking and airflow limitation. To our knowledge this is the first study investigating clinical COPD outcomes in the general population using a chronic cough definition in accordance with the current recommendations and guidelines [3].
Supportive findings have been reported in previous studies focused either on chronic cough in patients with COPD [8] or using the term chronic bronchitis [15, 16]. Koo et al. [8] demonstrated that presence of chronic cough for 3 months in COPD patients was associated with lower lung function, more severe dyspnoea and frequent acute exacerbations, defined as worsening of symptoms which required medical treatment. We corroborated such findings in a previous study showing that chronic cough in COPD was associated with a more severe disease phenotype in terms of more accompanying respiratory symptoms and lower lung function [7]. Çolak et al. [14] showed that chronic respiratory symptoms are associated with increased respiratory hospitalisations and death in individuals with normal spirometry from the Copenhagen General Population Study both in never- and ever-smokers, separately. Our study adds to these previous findings and shows that chronic cough is an independent predictor of future clinical COPD outcomes, including COPD exacerbations, pneumonia and death in the general population.
Balte et al. [17] recently found that chronic bronchitis was associated with increased risk of respiratory hospitalisations and death in individuals without airway obstruction in the National Heart, Lung and Blood Institute Pooled Cohort Study; however, the risk of death was only significantly affected in ever-smokers. Similar findings were reported when looking at the cough component of chronic bronchitis (with and without phlegm) with HR for COPD-related outcomes (mortality and hospitalisations) of 2.0 (95% CI 1.3–2.9) in never-smokers and 1.7 (1.5–2.1) in ever-smokers. Our study expands on these findings in several ways. We found similar prevalence of chronic cough (6% versus 5%) and higher risks of respiratory outcomes with chronic cough but with shorter follow-up time. In addition, we restricted our analyses to the clinical definition of chronic cough in a cohort of ∼45 000 randomly selected adults, including individuals with airflow limitation, and investigated the risks conferred by chronic cough on COPD exacerbation, pneumonia and all-cause mortality.
Cough is a major symptom of COPD linked with exacerbation frequency and clinical deterioration. Acute exacerbations of COPD are associated with cough as a symptom [18]. Deslee et al. [19] showed that assessing cough in the past week was superior to the usual chronic bronchitis definition in identifying cough-associated impairment of health-related quality of life using the St. George's Respiratory Questionnaire in individuals with COPD. This finding is supported by recent cross-sectional studies in Japan and the USA showing that chronic cough negatively impacts quality of life [20, 21].
In our study, airflow limitation and current smoking were important in that cumulative incidences for all clinical COPD outcomes at age 80 years were indeed higher in the presence of these covariates. Nonetheless, airflow limitation and current smoking still had no influence on risk of outcomes, i.e. no effect modification or interaction. Moreover, the absolute 5-year risks reveal additive effects of both risk factors: airflow limitation being most important for respiratory-related outcomes including COPD exacerbation and pneumonia, and current smoking impacting more massively on all-cause mortality.
When stratifying the analysis by chronic cough type, the risks for clinical COPD outcomes were nominally higher in those with productive chronic cough as opposed to sputum production alone or non-productive chronic cough indicating productive chronic cough was the stronger predictor of future clinical outcomes in the population. A possible mechanism underlying the relationship between productive chronic cough and subsequent COPD exacerbation could be a higher prevalence of aspiration in these patients [6, 22, 23]. Indeed cough is a defence mechanism of aspiration, and aspiration occurs more often in COPD contributing to susceptibility to exacerbations and pneumonia [6, 23]. This could also contribute to higher risk of COPD mortality in the affected individuals as was observed in the current study.
Strengths of the present study include the use of a large contemporary population-based cohort with randomly selected individuals, the use of chronic cough defined as a cough >8 weeks, and the fact that the main findings regarding increased risks of future clinical COPD outcomes and death were replicated in subgroup analyses with regard to airflow limitation and current smoking. A potential limitation is that we did not have post-bronchodilator spirometry for all participants for the cross-sectional analysis of COPD in individuals with chronic cough at baseline versus those without. However, results were similar for pre-bronchodilator and post-bronchodilator defined COPD [7], and thus we do not think that this substantially biased our findings. Because 99.9% of the participants were of white Caucasian descent, the generalisability of our results could potentially be constrained; however, this may also make our results less dependent on influences caused by ethnic differences. Some of the individuals with the most severe types of chronic cough may not have attended the physical examination and participated in the Copenhagen General Population Study. This could theoretically tend to bias the results towards the null hypothesis and lead us to underestimate some of the associations observed between chronic cough and subsequent COPD outcomes.
Since chronic cough has been associated with increased accompanying respiratory symptoms and low lung function in individuals with COPD [7], and in the present study also with increased risk of adverse clinical outcomes, targeting chronic cough with effective drugs could potentially improve disease burden and prognosis in some patients. The highest risk for any events was observed in patients with chronic productive cough. However, in these patients the effectiveness of therapy with novel antitussive drugs such as P2X3 antagonists has not been proved yet. Instead, the results of this study are good premises to treat patients (especially COPD patients) with productive chronic cough more intensely with mucolytics, antibiotics or bronchodilators [3, 24–26]. We have an existing therapy, azithromycin, which has been shown in several studies to reduce exacerbation rates, and it is recommended in the ERS and CHEST cough guidelines [3, 25]. It works as an antibacterial drug and promotility agent (an agonist of motilin) thus reducing aspiration events. The presence of chronic cough in COPD should be an indication for this existing treatment. Azithromycin has also been tested for other chronic cough-related diseases beyond COPD, e.g. in sarcoidosis cough, idiopathic pulmonary fibrosis cough and asthmatic cough, but with differing results [24, 27–29].
In conclusion, chronic cough is a strong and independent predictor of future clinical outcomes in the general population and associated with 4.6-fold risk of COPD exacerbation, 2.2-fold risk of pneumonia and 1.7-fold risk of death. The highest absolute risks were observed in older current smokers with chronic cough and airflow limitation, but the presence of chronic cough was associated with the adverse outcomes also in nonsmokers with normal lung function.
Supplementary material
Please note: supplementary material is not edited by the Editorial Office, and is uploaded as it has been supplied by the author.
Figure S1 00697-2023.SUPPLEMENT (82.8KB, tif)
Figure S2 00697-2023.SUPPLEMENT2 (93.5KB, tif)
Figure S3 00697-2023.SUPPLEMENT3 (117.4KB, tif)
Footnotes
Provenance: Submitted article, peer reviewed.
Author contributions: E.M. Landt, Y. Çolak and M. Dahl had full access to all data in the study and had final responsibility for the decision to submit for publication. E.M. Landt, Y. Çolak, B.G. Nordestgaard, P. Lange and M. Dahl contributed to the study concept and design. E.M. Landt, Y. Çolak, B.G. Nordestgaard, P. Lange and M. Dahl collected, analysed or interpreted the data. E.M. Landt wrote the draft manuscript and performed the statistical analyses. E.M. Landt, Y. Çolak, B.G. Nordestgaard, P. Lange and M. Dahl revised the manuscript for important intellectual content. B.G. Nordestgaard, P. Lange and M. Dahl obtained funding and provided administrative, technical or material support. M. Dahl supervised the study.
Support statement: This study was supported by the Danish Lung Association, Danish Cancer Society, Novo Nordisk Foundation, Department of Clinical Biochemistry and Department of Internal Medicine, Copenhagen University Hospital – Herlev and Gentofte, and Department of Public Health, University of Copenhagen. The sponsors did not participate in the design and conduct of the study; collection, management, analysis or interpretation of the data; in the preparation, review or approval of the manuscript; or the decision to submit the manuscript for publication. Funding information for this article has been deposited with the Crossref Funder Registry.
Ethics statement: The study was approved by Herlev and Gentofte Hospital and a Danish ethical committee, and was conducted according to the Declaration of Helsinki (identification number H-KF-01-144/01). All participants provided written informed consent.
Conflict of interest: B.G. Nordestgaard has consultancies with Amarin, Akcea, Amgen, AstraZeneca, Denka Seiken, Kowa, Novartis, Novo Nordisk and Silence Therap. No conflicts of interest exist for E.M. Landt, Y. Çolak, P. Lange and M. Dahl
References
- 1.Çolak Y, Nordestgaard BG, Laursen LC, et al. Risk factors for chronic cough among 14,669 individuals from the general population. Chest 2017; 152: 563–573. doi: 10.1016/j.chest.2017.05.038 [DOI] [PubMed] [Google Scholar]
- 2.Song WJ, Chang YS, Faruqi S, et al. The global epidemiology of chronic cough in adults: a systematic review and meta-analysis. Eur Respir J 2015; 45: 1479–1481. doi: 10.1183/09031936.00218714 [DOI] [PubMed] [Google Scholar]
- 3.Morice AH, Millqvist E, Bieksiene K, et al. ERS guidelines on the diagnosis and treatment of chronic cough in adults and children. Eur Respir J 2020; 55: 1901136. doi: 10.1183/13993003.01136-2019 [DOI] [PMC free article] [PubMed] [Google Scholar]
- 4.Landt EM, Çolak Y, Nordestgaard BG,et al. Risk and impact of chronic cough in obese individuals from the general population. Thorax 2022; 77: 223–230. doi: 10.1136/thoraxjnl-2020-216351 [DOI] [PubMed] [Google Scholar]
- 5.Cho PSP, Fletcher HV, Patel IS, et al. Cough hypersensitivity and suppression in COPD. Eur Respir J 2021; 57: 2003569. doi: 10.1183/13993003.03569-2020 [DOI] [PubMed] [Google Scholar]
- 6.Cvejic L, Guiney N, Nicholson T, et al. Aspiration and severe exacerbations in COPD: a prospective study. ERJ Open Res 2021; 7: 00735-2020. doi: 10.1183/23120541.00735-2020 [DOI] [PMC free article] [PubMed] [Google Scholar]
- 7.Landt E, Çolak Y, Lange P, et al. Chronic cough in individuals with COPD a population-based cohort study. Chest 2020; 157: 1446–1454. doi: 10.1016/j.chest.2019.12.038 [DOI] [PubMed] [Google Scholar]
- 8.Koo HK, Park SW, Park JW, et al. Chronic cough as a novel phenotype of chronic obstructive pulmonary disease. Int J COPD 2018; 13: 1793–1801. doi: 10.2147/COPD.S153821 [DOI] [PMC free article] [PubMed] [Google Scholar]
- 9.Çolak Y, Afzal S, Lange P, et al. Role and impact of chronic cough in individuals with asthma from the general population. J Allergy Clin Immunol Pract 2019; 7: 1783–1792. doi: 10.1016/j.jaip.2019.02.021 [DOI] [PubMed] [Google Scholar]
- 10.Irwin RS, French CL, Chang AB, et al. Classification of cough as a symptom in adults and management algorithms. Chest 2018; 153: 196–209. doi: 10.1016/j.chest.2017.10.016 [DOI] [PMC free article] [PubMed] [Google Scholar]
- 11.Smith JE, Woodcock A. Chronic cough. N Engl J Med 2016; 375: 1544–1551. doi: 10.1056/NEJMcp1414215 [DOI] [PubMed] [Google Scholar]
- 12.Irwin RS, Baumann MH, Bolser DC, et al. Diagnosis and management of cough executive summary: ACCP evidence-based clinical practice guidelines. Chest 2006; 129: Suppl 1, 1S–23S. doi: 10.1378/chest.129.1_suppl.1S [DOI] [PMC free article] [PubMed] [Google Scholar]
- 13.Løkke A, Marott JL, Mortensen J, et al. New Danish reference values for spirometry. Clin Respir J 2013; 7: 153–167. doi: 10.1111/j.1752-699X.2012.00297.x [DOI] [PubMed] [Google Scholar]
- 14.Çolak Y, Nordestgaard BG, Vestbo J, et al. Prognostic significance of chronic respiratory symptoms in individuals with normal spirometry. Eur Respir J 2019; 54: 1900734. doi: 10.1183/13993003.00734-2019 [DOI] [PubMed] [Google Scholar]
- 15.Vestbo J, Prescott E, Lange P. Association of chronic mucus hypersecretion with FEV1 decline and chronic obstructive pulmonary disease morbidity. Copenhagen City Heart Study Group. Am J Respir Crit Care Med 1996; 153: 1530–1535. doi: 10.1164/ajrccm.153.5.8630597 [DOI] [PubMed] [Google Scholar]
- 16.Lahousse L, Seys LJM, Joos GF, et al. Epidemiology and impact of chronic bronchitis in chronic obstructive pulmonary disease. Eur Respir J 2017; 50: 1602470. doi: 10.1183/13993003.02470-2016 [DOI] [PMC free article] [PubMed] [Google Scholar]
- 17.Balte PP, Chaves PHM, Couper DJ, et al. Association of nonobstructive chronic bronchitis with respiratory health outcomes in adults. JAMA Intern Med 2020; 180: 676–686. doi: 10.1001/jamainternmed.2020.0104 [DOI] [PMC free article] [PubMed] [Google Scholar]
- 18.Crooks MG, Brown T, Morice AH. Is cough important in acute exacerbations of COPD. Respir Physiol Neurobiol 2018; 257: 30–35. doi: 10.1016/j.resp.2018.02.005 [DOI] [PubMed] [Google Scholar]
- 19.Deslee G, Burgel PR, Escamilla R, et al. Impact of current cough on health-related quality of life in patients with COPD. Int J COPD 2016; 11: 2091–2097. doi: 10.2147/COPD.S106883 [DOI] [PMC free article] [PubMed] [Google Scholar]
- 20.Kubo T, Tobe K, Okuyama K, et al. Disease burden and quality of life of patients with chronic cough in Japan: a population-based cross-sectional survey. BMJ Open Respir Res 2021; 8: e000764. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 21.Meltzer EO, Zeiger RS, Dicpinigaitis P, et al. Prevalence and burden of chronic cough in the United States. J Allergy Clin Immunol Pract 2021; 9: 4037–4044. doi: 10.1016/j.jaip.2021.07.022 [DOI] [PubMed] [Google Scholar]
- 22.Cvejic L, Guiney N, Lau KK, et al. Swallow patterns associated with aspiration in COPD: a prospective analysis. ERJ Open Res 2021; 7: 00170-2021. doi: 10.1183/23120541.00170-2021 [DOI] [PMC free article] [PubMed] [Google Scholar]
- 23.Zheng Z, Wu Z, Liu N, et al. Silent aspiration in patients with exacerbation of COPD. Eur Respir J 2016; 48: 570–573. doi: 10.1183/13993003.00007-2016 [DOI] [PubMed] [Google Scholar]
- 24.Hirons B, Rhatigan K, Kesavan H, et al. Cough in chronic lung disease: a state of the art review. J Thoracic Dis 2023; 15: 5823–5843. doi: 10.21037/jtd-22-1776 [DOI] [PMC free article] [PubMed] [Google Scholar]
- 25.Malesker MA, Callahan-Lyon P, Madison M, et al. Chronic cough due to stable chronic bronchitis. Chest 2020; 158: 705–718. doi: 10.1016/j.chest.2020.02.015 [DOI] [PubMed] [Google Scholar]
- 26.Morice A, Dicpinigaitis P, McGarvey L, et al. Chronic cough: new insights and future prospects. Eur Respir Rev 2021; 30: 210127. doi: 10.1183/16000617.0127-2021 [DOI] [PMC free article] [PubMed] [Google Scholar]
- 27.Zhou J, Yi F, Xu Z, et al. The efficacy and safety of azithromycin in chronic respiratory diseases related cough. Ann Palliat Med 2020; 9: 1488–1496. doi: 10.21037/apm-20-119 [DOI] [PubMed] [Google Scholar]
- 28.Fraser SD, Thckray-Nocera S, Shepherd M, et al. Azithromycin for sarcoidosis cough: an open-label exploratory clinical trial. ERJ Open Res 2020; 6: 00534-2020. doi: 10.1183/23120541.00534-2020 [DOI] [PMC free article] [PubMed] [Google Scholar]
- 29.Guler SA, Clarenbach C, Brutsche M, et al. Azithromycin for the treatment of chronic cough in idiopathic pulmonary fibrosis. A randomized controlled crossover trial. Ann Am Thorac Soc 2021; 18: 2018–2026. doi: 10.1513/AnnalsATS.202103-266OC [DOI] [PubMed] [Google Scholar]
Associated Data
This section collects any data citations, data availability statements, or supplementary materials included in this article.
Supplementary Materials
Please note: supplementary material is not edited by the Editorial Office, and is uploaded as it has been supplied by the author.
Figure S1 00697-2023.SUPPLEMENT (82.8KB, tif)
Figure S2 00697-2023.SUPPLEMENT2 (93.5KB, tif)
Figure S3 00697-2023.SUPPLEMENT3 (117.4KB, tif)