Abstract
Estuarine environments are critical to fish species and serve as nurseries for developing embryos and larvae. They also undergo daily fluctuations in salinity and act as filters for pollutants. Additionally, global climate change (GCC) is altering salinity regimes within estuarine systems through changes in precipitation and sea level rise. GCC is also likely to lead to an increased use of insecticides to prevent pests from damaging agricultural crops as their habitats and mating seasons change from increased temperatures. This underscores the importance of understanding how insecticide toxicity to fish changes under different salinity conditions. In this study, larval Inland Silversides (Menidia beryllina) were exposed to bifenthrin (1.1 ng/L), cyfluthrin (0.9 ng/L), or cyhalothrin (0.7 ng/L) at either 6 or 10 practical salinity units (PSU) for 96 h during hatching, with a subset assessed for end points relevant to neurotoxicity and endocrine disruption by testing behavior, gene expression of a select suite of genes, reproduction, and growth. At both salinities, directly exposed F0 larvae were hypoactive relative to the F0 controls; however, the indirectly exposed F1 larvae were hyperactive relative to the F1 control. This could be evidence of a compensatory response to environmentally relevant concentrations of pyrethroids in fish. Effects on development, gene expression, and growth were also observed. Overall, exposure to pyrethroids at 10 PSU resulted in fewer behavioral and endocrine disruptive effects relative to those observed in organisms at 6 PSU.
Keywords: insecticide, estuary, behavior, Inland Silverside, larvae, aquatic toxicity
Short abstract
Larval Inland Silversides directly exposed to pyrethroids (F0) exhibit the inverse activity level as indirectly exposed larvae (F1), suggesting a compensatory response.
1. Introduction
Increases in temperature and an earlier start to the growing season due to global climate change (GCC) are expected to result in earlier hatching rates, prolonged mating seasons, and wider home ranges for some insect species.1 These shifts are expected to result in an increase in insecticide application rates and widen application periods.2 Pyrethroids are neurotoxic insecticides commonly used in agriculture and urban environments that primarily enter the estuarine environment through runoff.3 They are favored over other insecticides, such as organophosphates, due to their low toxicity to mammals and birds and efficacy against insect pests.4 However, they can be highly toxic to nontarget aquatic organisms, including fish.5 Pyrethroids induce neurotoxicity by causing the continual firing of neurons through the prolonged opening of sodium voltage-gated ion channels, as well as other secondary modes of neurotoxicity such as inhibition of acetylcholinesterase and alteration of calcium and chloride channels.4,6 While pyrethroids are designed to act as neurotoxins at low environmentally relevant concentrations, they have also been found to act as endocrine-disrupting compounds (EDCs),7 and some pyrethroid metabolites can have stronger estrogenic-like properties than the parent compound.8,9 The endocrine-disrupting effects of pyrethroids lead to altered sex ratios, fecundity, and hatching success, which can have long-term consequences to a population.10,11 Under certain exposure scenarios, this may lead to population decline.12 Despite this, there are limited studies on the multigenerational effects of pyrethroids in fish.13 Multigenerational studies are beneficial since they allow for more population level end points to be measured and for generation-specific impacts to be accounted for, both of which are relevant to pyrethroid exposure, and more so to EDC exposure in general.
Estuaries are critical, sensitive habitats that provide ecosystem services to humans and wildlife.14 They function as filters of pollutants and sediment and provide aquatic species with habitats for nurseries and breeding grounds. Many fish species that are culturally and economically important to humans rely on estuaries at some point in their life histories. However, these ecosystem functions may be threatened under environmental stressors such as pollution and GCC.15 One impact of GCC on estuarine ecosystems is salinity intrusion, which can result from decreased precipitation and increased sea level rise.16 Estuaries in regions expected to experience decreased precipitation are at risk of increased salinity, which can impact fish habitat, breeding grounds, and water quality. Decreased river flow allows for more saline water to flow inland. On the other hand, increased precipitation and freshwater inputs into the estuary may decrease salinity.17 Salinity has been shown to alter the toxicity of environmental pollutants, including pyrethroids, but results can be varied across different species, end points, pollutants, and salinity ranges.18−27 Here, we expand upon existing studies and provide evidence for altered toxicity of pyrethroids at different salinities with end points across multiple biological levels.
The study presented here is an extension of a project where the goal was to compare toxicity to pyrethroids between a model fish, Inland Silverside (Menidia beryllina), and a nonmodel fish, Delta Smelt (Hypomesus transpacificus);24,25 both resident fishes of the San Francisco Bay and the Sacramento-Joaquin Delta (hereto referred to as the Bay-Delta), a large estuary in California undergoing salinity regime alterations due to GCC. Bifenthrin, cyfluthrin, and cyhalothrin were selected as candidates for the multigenerational study due to their high toxicities and their frequency of detection in the Bay-Delta. Here, the use of Inland Silverside for multigenerational testing was preferred over the Delta Smelt, which is more challenging to culture, a nonmodel species, and listed as threatened under the Federal Endangered Species Act.28 While there are limitations to using model organisms (i.e., toxicity may differ from more sensitive species), they are often the best option especially when culturing organisms until reproductive maturity. Overall, the larger project this study falls under was designed to document the differences in toxicity between the Inland Silverside and the Delta Smelt since Inland Silverside pesticide data have been used to model Delta Smelt risk.29 This study may be used to model the toxicity of pyrethroids to Delta Smelt and other estuarine and marine fishes at environmentally relevant concentrations and may be useful to risk assessors not just for the Bay-Delta but also for other estuaries around the USA and world.
Here, Inland Silversides were exposed to bifenthrin (1.1 ng/L), cyfluthrin (0.9 ng/L), and cyhalothrin (0.7 ng/L) at two salinities relevant to estuarine environments, 6 and 10 practical salinity units (PSU).30 These pyrethroids are commonly detected in the Bay-Delta, and with the exception of bifenthrin, there are very few fish toxicity data on them. The concentrations chosen for the exposures are environmentally relevant,31−33 and based on past studies in the laboratory, sublethal effects have been shown on Inland Silversides at these concentrations.24 F0 organisms were exposed as embryos that hatched into the exposure solution during the test duration. Following exposure, a subset of organisms were reared and spawned in clean water. F1 organisms were indirectly exposed to the pyrethroids through the germline. We assessed end points at molecular, organismal, and population levels, which allow comparison across a biological hierarchy. The aim of this study was to (1) assess the relative multigenerational toxicity of the three pyrethroids and (2) compare the toxicity between two salinities relevant to estuarine ecosystems.
2. Methods
2.1. Chemicals
Bifenthrin (part #: N-11203; CAS: 82657-04-3, purity 98.8%), cyfluthrin (part #: N-11130; CAS: 68359-37-5, purity 99.5%), and cyhalothrin (part #: N-12307; CAS: 91465-08-6, purity 99.5%) were purchased from Chem Service (West Chester, PA, USA). High-performance liquid chromatography grade methanol used to make stock solutions was purchased from Fisher Scientific (Waltham, MA, USA). Ellman’s reagent, 5,5′-dithio-bis(2-nitrobenzoic acid), cysteine hydrochloride monohydrate, bovine serum albumin, and Folin reagent were purchased from Millipore Sigma (Darmstadt, Germany).
2.2. Organisms and Husbandry
Adult Inland Silverside broodstock were housed and spawned at the Oregon State University (OSU), Hatfield Marine Science Center. Embryos were transferred to Corvallis, OR, where the experimental exposures were conducted under Animal Care and Use Program (ACUP) protocol #4999. For more information, see Supporting Information 1. At 4 days post fertilization (dpf), 15–17 embryos were placed into 250 mL beakers (n = 3) with 50 mL of either 6 or 10 PSU water made from artificial seawater (ASW) for a 24 h acclimation period. An additional subset of 28–30 embryos were placed into 1 L beakers (n = 3) with 100 mL of either 6 or 10 PSU to be reared to maturity. All experiments were conducted under OSU IACUC protocol #0035.
2.3. F0 Larvae Exposures
After the 24 h acclimation period, 50 or 100 mL of concentrated exposure solution was poured into the beakers to achieve nominal concentrations of 1 ng/L and a final volume of 100 or 200 mL/beaker, respectively. For the F0 96 h exposures, 15–17 embryos were kept in a 100 mL volume of solution in 250 mL beakers. For the F0 rear-out 96 h exposures, 28–30 embryos were kept in a 200 mL volume of solution in 1 L beakers. The rear-out beaker volume was doubled to account for the increase in organisms that was needed to ensure a large enough population per replicate for breeding. All exposure replicates (including controls) contained 0.01% methanol, which has been used as a carrier for pyrethroids in this species before.24 Organisms were exposed at two salinities, 6 and 10 PSU, to bifenthrin (1.1 ng/L), cyfluthrin (0.9 ng/L), or cyhalothrin (0.7 ng/L). There were three technical replicates per treatment combination. Exposures were conducted for 96 h using semistatic conditions. New exposure solutions were made daily, followed by a 50% water change. At this time, survival was assessed, and debris was removed. Organisms hatched during the exposure period, spending between 48 and 72 h as free-swimming larvae. pH, dissolved oxygen, salinity, temperature, and ammonia were recorded daily (Table S1). Organisms were maintained on a 14:10 light cycle.
F0 larvae to be reared until maturity were first maintained in 1 L beakers with 200 mL of exposure solution for 96 h. After the 96 h exposure period, the solution was exchanged with clean ASW at the respective salinity, and the fish were maintained in the 1 L beaker with 1 L of clean water until approximately 5 weeks posthatch. At this time, the fish were transferred into 13 L tanks at the appropriate salinity and maintained in a recirculating system until they reached reproductive maturity. pH, dissolved oxygen, salinity, and temperature were recorded daily, and ammonia, nitrate, and nitrite were measured weekly (Table S2). At approximately 8 months post hatch, Inland Silverside F0 adult fish were sexually mature and were spawned six times by placing a substrate into the tanks for 48 h at a time to ensure enough embryos were collected for each end point. See Supporting Information 1 for more details.
2.4. F1 Larvae Maintenance
After 48 h, the spawning substrate was removed from the F0 tanks, and the F1 embryos were removed using forceps and placed in 250 mL beakers with 100 mL of clean ASW at their respective salinities and checked for fertilization and development. The exposure water was replaced 50% every day when debris was removed and survival assessed. pH, dissolved oxygen, salinity, temperature, and ammonia were recorded daily (Table S3). Organisms were maintained on a 14:10 light cycle. At 9 dpf/∼2 dph, survival was assessed, behavioral assays were conducted, and the remaining organisms were collected for sample processing and analysis.
2.5. Molecular End Points
At the end of each exposure period, 5–10 larvae (dependent upon F0 adult spawning success for F1 larvae) were pooled per technical replicate (n = 3) and flash frozen in liquid nitrogen and stored at −80 °C until molecular end points were measured.
2.5.1. Acetylcholinesterase Inhibition
Acetylcholinesterase inhibition was measured in both F0 and F1 larvae. Acetylcholinesterase inhibition was measured using Ellman’s assay, and total protein was measured using the Lowry Assay. Both assays were measured using a Synergy LX Multimode Reader (Agilent, Santa Clara, California, USA).
2.5.2. Gene Expression
Eleven genes were selected for the quantitative polymerase chain reaction (qPCR), including two reference genes (Table S4). Genes were selected to represent a range of biological end points as determined in past studies. The goal in measuring gene expression was to test genes known to be involved in hormone regulation, reproduction, neurodevelopment, drug metabolism, growth, and metabolism. See Table S4 for details on each gene’s target function. See Supporting Information 2 for more methods on gene expression.
2.6. Behavioral Assay
At the end of the 96 h exposure period for F0 larvae and the end of the experiment for F1 larvae, behavior was measured using an assay modified from.24 There were three technical replicates for each treatment. A total of 1–6 individuals (dependent on the success of F0 spawning for F1 larvae) from each replicate were analyzed. Briefly, 24-well polystyrene plates were loaded randomly with one fish and 1 mL of salt water at the respective salinity per well. Fish were acclimated to the plate, keeping all other variables (light, temperature, and salinity) constant, for at least 45 min and then placed into a EthoVision Observation Chamber (Noldus, Wageningen, The Netherlands). An additional 15 min of acclimation occurred inside the observation chamber in the dark, followed by an alternating dark: light cycle with two 10 min periods of dark interspersed with one 10 min period of light. The dark and light cycles are termed dark1, light1, and dark2. Behavioral (total distance moved (TDM) (mm), velocity (mm/s), bursting (s), cruising (s), freezing (s), and thigmotaxis) tracking was conducted between 07:00 and 19:00 h, which encompassed the standard light period of the exposures. Behavior was recorded and tracked using a Basler Gen 1 Camera using Ethovision XT15 software, 1280 × 960 resolution, 10,000 lx of light, and a 25/s frame rate.
2.7. Growth and Development
For more information, see Supporting Information 3.
2.8. Analytical Chemistry
The concentrations tested in this study were below our limit of quantification. Therefore, we tested our stock solutions, which were diluted by 0.01% to make the final exposure solutions. To confirm that pyrethroid stock solutions were made correctly, a subset of each stock solution was stored at −20 °C until they were shipped to the USGS Organic Chemistry Research Laboratory (Sacramento, California). Pyrethroid concentrations were confirmed as described by Segarra et al. (2021). Briefly, pyrethroids concentrations were measured using solid-phase extraction followed by gas chromatography–mass spectrometry.34,35 The stock solutions contained 11 ng/mL bifenthrin, 9 ng/mL cyfluthrin, and 7 ng/mL cyhalothrin. Larval F0 exposure solutions contained 1.1 ng/L bifenthrin, 0.9 ng/L cyfluthrin, and 0.7 ng/L cyhalothrin.
2.9. Statistical Analysis
Statistical analysis was performed in R software version 4.0.3 (Vienna, Austria) and R Studio version 1.3.1093 (Boston, Massachusetts, USA). Molecular, growth, and survival data were normally distributed and were analyzed by one-way ANOVA followed by Dunnett’s test to compare treatment vs control (n = 3). F0 spawning success and F1 embryonic development scores were analyzed using a linear model with Poisson distribution (n = 3). For analysis, behavioral data were normalized between 0 and 1, and the dark and light cycles were compared within stimuli. Behavior data were not normally distributed and were analyzed with a Kruskal–Wallis and Dunn’s test (n = 3). Larval behavior was different in the controls between the two generations (p < 0.05, t test), so results were only compared within generations. For visualization of behavioral results, the z-score was calculated and normalized relative to the control to distribute the results above and below zero relative to the control z-score. Gene expression was analyzed using one-way ANOVA followed by Dunnett’s test to compare results to control values (n = 3). There were no differences in the controls across generations, but results were still normalized to their respective generation and salinity control. Gene expression is measured in fold change (log2) values normalized to the geometric mean of the housekeeping genes as described in.11 All results were considered statistically significant if p < 0.05.
3. Results and Discussion
3.1. F0 Larvae Were Hypoactive, While F1 Larvae Were Hyperactive
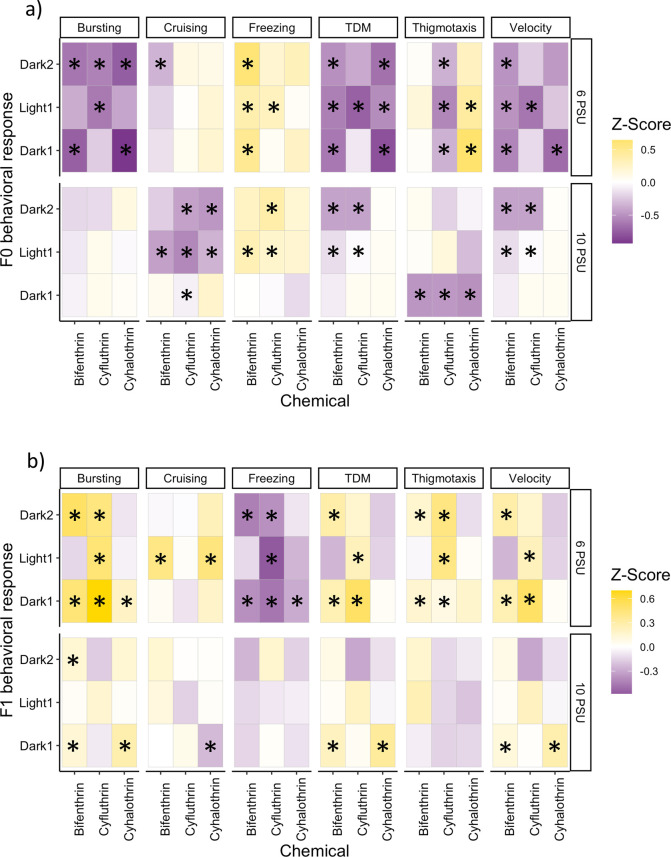
F0 larvae were directly exposed to bifenthrin (1.1 ng/L), cyfluthrin (0.9 ng/L), or cyhalothrin (0.7 ng/L), while F1 larvae were indirectly exposed through the germline. Overall, directly exposed larvae exhibited hypoactivity relative to their respective controls in both the 6 and 10 PSU exposures. This was demonstrated by the overall decrease in bursting, cruising, TDM, and velocity (Figure 1a). Bifenthrin had the most significant differences in the 6 PSU direct exposures, all dark:light cycles were significantly different for freezing, TDM, and velocity (p < 0.05, Dunn's test) (Figure 1a), which indicates that it had the strongest hypoactive effect after 96 h exposure at 6 PSU. Cyfluthrin and cyhalothrin 6 PSU exposures also caused hypoactivity through decreased bursting, TDM, and velocity and increased freezing (cyfluthrin only) (Figure 1a). Inland Silversides as a species exhibited a minor increase in activity during light cycles compared to that in dark, and there were no significant differences between the dark and light cycles in the control fish (data not shown). At 10 PSU, all the pyrethroid exposed treatments showed increased freezing and at least one significant decrease in bursting, cruising, TDM, and velocity (Figure 1a). Overall, the 10 PSU F0 exposures exhibited fewer behavioral effects than the 6 PSU F0 exposures, except cyfluthrin,which had 8 significant differences at 6 PSU and 10 at 10 PSU. This is in line with to our previous study, where pyrethroid toxicity decreased as salinity increased.36 The increased differences from cyfluthrin exposure on behavior at the higher salinity may indicate that the influence of salinity on toxicity is not always linear. It could also reflect different mechanisms of action between the pyrethroids.
Figure 1.
Heat map of behavioral results from the (a) F0 generation exposed directly to a methanol-only control, bifenthrin (1.1 ng/L), cyfluthrin (0.9 ng/L), and cyhalothrin (0.7 ng/L) and (b) F1 generation exposed indirectly to a methanol-only control, bifenthrin (1.1 ng/L), cyfluthrin (0.9 ng/L), and cyhalothrin (0.7 ng/L). End points are organized on the top panel and salinity (PSU) is on the right panel. The z-score is used for visualization purposes only. Yellow demonstrates an increase in the behavior and purple demonstrates a decrease. Behavior data consist of three technical replicates and 1–6 biological replicates per technical replicate, dependent on F0 adult spawning success for F1 offspring. An asterisk denotes p < 0.05.
The F1 indirectly exposed larvae displayed increased bursting, TDM, and velocity and decreased freezing relative to their respective controls (Figure 1b). At 6 PSU, freezing was decreased in all pyrethroids (p < 0.05, Dunn’s test) (Figure 1b). Bursting was also increased for all treatments at 6 PSU, and TDM and velocity were increased for bifenthrin and cyfluthrin (p < 0.05, Dunn’s test) (Figure 1b). Larvae indirectly exposed to cyfluthrin did not show any changes in behavior relative to the control at 10 PSU. Indirect exposure to bifenthrin caused increased bursting and decreased freezing at 10 PSU, and both bifenthrin and cyhalothrin caused increased TDM and velocity (p < 0.05, Dunn’s test) (Figure 1b). Despite the increased behavioral effects seen after direct 96 h exposure to cyfluthrin at 10 PSU, in the next generation, the larvae appear to have recovered. In some studies, it appears that toxicity is not observed until later, unexposed generations,9,11 and future studies that carry cyfluthrin fish to an unexposed (F2 or F3) generation would be able to investigate potential recovery further.
Thigmotaxis is often used as an indicator of anxiety-like behavior, wherein increased fear or anxiety-like responses in an organism causes them to swim closer to a wall/border. In contrast, antithigmotaxis behavior displays a potential decrease in anxiety and suggests an increase in boldness and risk-taking behavior. At 6 PSU, F0 larvae exposed to bifenthrin had no change in thigmotaxis behavior; meanwhile, cyfluthrin decreased thigmotaxis in all dark: light cycles, and cyhalothrin increased thigmotaxis in at least one dark cycle and the light cycle (p < 0.05 Dunn’s test) (Figure 1a). At 10 PSU, all F0 larvae demonstrated decreased thigmotaxis behavior relative to the control during the first dark cycle(p < 0.05 Dunn’s test) (Figure 1a). In the F1 generation, fish exposed indirectly to bifenthrin and cyfluthrin at 6 PSU displayed increased thigmotaxis, and at 10 PSU, there were no changes to thigmotaxis in the F1 larvae. Thigmotaxis behavior has been correlated to changes in neuron action potential in Zebra Danio (Danio rerio), commonly referred to as Zebrafish. Overall, it appears that the fish exhibited increased anxiety behavior in the F0 exposures, which is consistent with the decreased activity and decreased anxiety, less fear-like behavior, in the F1 larvae, with the increased activity also observed.
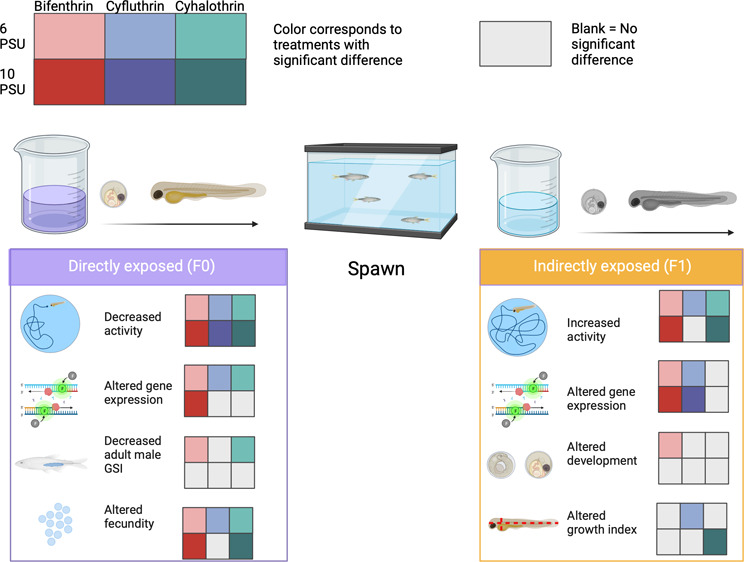
In the environment, concentrations of pyrethroids can range from nondetectable to hundreds of ng/L.3,31 The concentrations tested here are environmentally relevant, and these data can be used to inform ecological risk assessment and management. Overall, fish activity can be indicative of their overall fitness. Altered swimming speed may lead to increased predation rates, impact social behavior and mating success, and affect feeding and growth.37 Despite decades of research on fish swimming and behavior, there is still a large gap in the relationship between behavioral changes seen in the lab and what might be occurring in the environment.38 However, behavioral data have been growing in popularity and are considered to be important end points for ecological risk. Hypo- vs hyperactive responses have been described as “reactive” and “proactive”, respectively; reactive individuals are characterized as having low activity and aggression and characteristics of shyness, whereas proactive individuals are likely to have increased metabolic rates and increased aggression and to be more mobile and active.39 Whether these differences in traits would be apparent in wild populations is uncertain because there are few studies connecting laboratory findings to wild populations. These data demonstrate the directly exposed larvae display hypoactivity, which may be indicative of a reactive lifestyle, while the indirectly exposed larvae display a proactive lifestyle (Figure 2).
Figure 2.
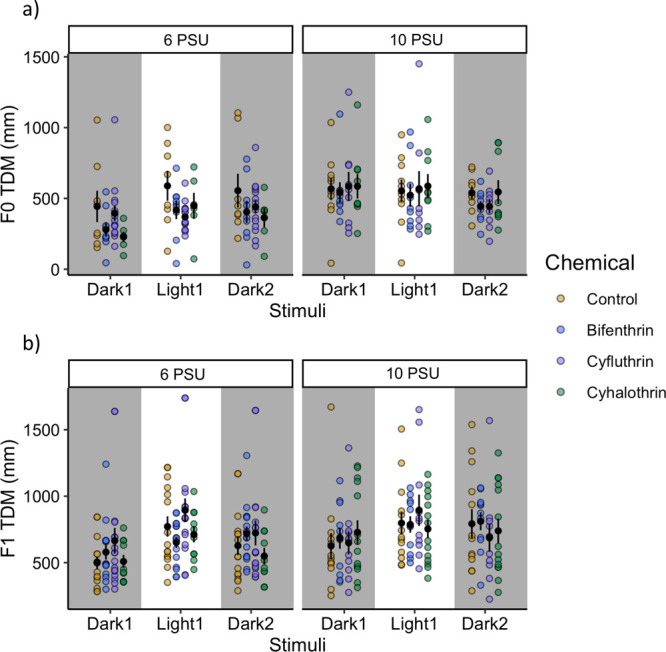
Spread of the total distance moved (TDM) data from the (top) F0 generation exposed directly to a methanol-only control, bifenthrin (1.1 ng/L), cyfluthrin (0.9 ng/L), and cyhalothrin (0.7 ng/L) and (bottom) F1 generation exposed indirectly. Behavior data consist of three technical replicates and 1–6 biological replicates per technical replicate, dependent on F0 adult spawning success for F1 offspring.
The stark difference in hypo- versus hyperactivity between the F0 and F1 generations, respectively, may be reflective of a compensatory, or over compensatory, response in the F1 generation (Figure 3). A compensatory response describes a reallocation of resources and energy to quickly respond to unfavorable conditions. In essence, a compensatory response is a way to fine-tune the molecular processes in an organism to better withstand its environment and limit long-term damage to the population.40 The F1 generation may be exhibiting instances of hyperactivity to counteract the hypoactivated effects of the pyrethroids from the F0 exposures. An organism’s ability to compensate for the energy required to tolerate and protect themselves from environmental stressors can influence population adaptation.40 The F1 generation, which was not directly exposed to the pyrethroids, was hyperactive relative to the unexposed F1 control larvae. If a true compensatory response occurred, then we could expect to not see any changes relative to the unexposed fish if the indirectly exposed F1 larvae were exposed directly to the pyrethroids, like the F0 larvae. However, overcompensation would occur if the F1 fish were exposed to the pyrethroids, which may induce hypoactivity upon direct exposure and were still hyperactive. Future studies should confirm this hypothesis; if true, this would be the first demonstration of a compensatory response in fish to pyrethroids. Compensatory mechanisms are frequently identified as adaptational responses in insects that develop resistance to pyrethroids.41 If Inland Silversides can compensate for low environmental concentrations of pyrethroids, this could suggest that they may be able to adapt to pyrethroid levels found in the environment. However, adaptation and compensation to pollution are not considered an advantageous outcome because they can divert resources from some processes to others and can lead to decreased genetic diversity, which leaves populations vulnerable to other stressors.
Figure 3.
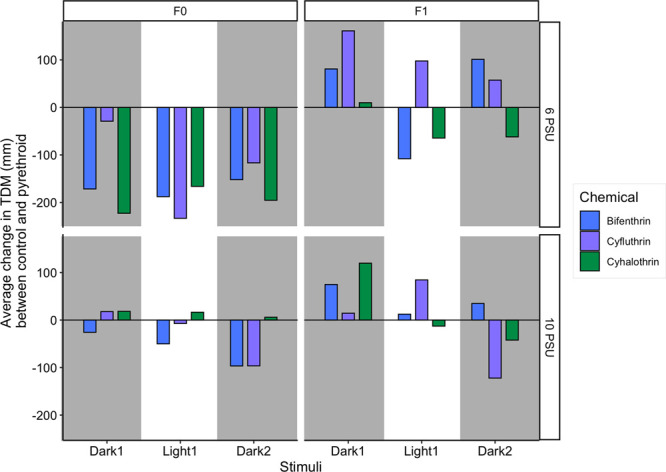
Average change in total distance moved results between each pyrethroid treatment and the respective control, separated by generation, salinity, and stimuli. (Left) F0 generation exposed directly to a methanol-only control, bifenthrin (1.1 ng/L), cyfluthrin (0.9 ng/L), and cyhalothrin (0.7 ng/L) and (right) F1 generation exposed indirectly.
3.2. Molecular Response to Pyrethroids Varied by Salinity and Generation
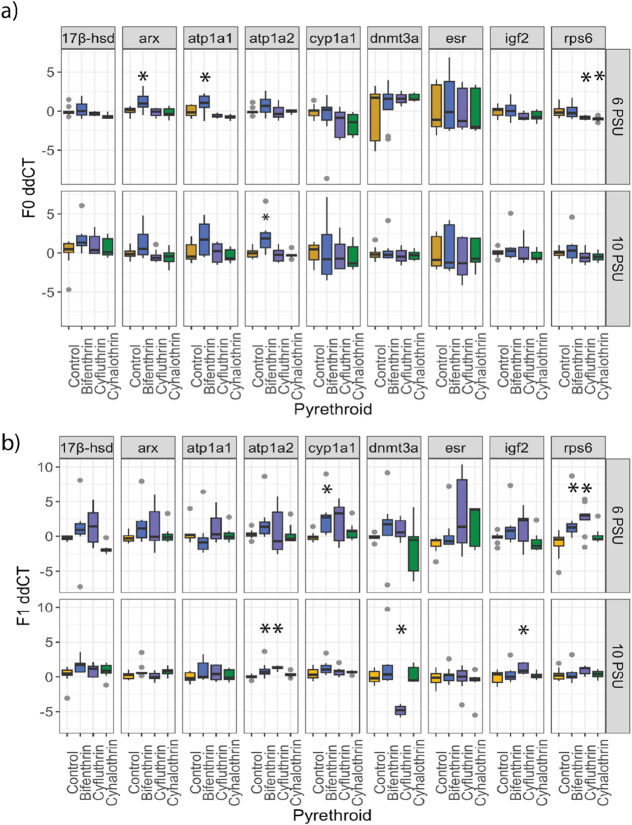
Pyrethroids are known for their effects on sodium-gated ion channels, but gene expression analysis indicates that other neurological pathways may also be affected. Genes for qPCR were selected from past studies that were the foundation of the current multigenerational study. The 40 s ribosomal protein s6 (rps6) gene was selected since it is a downstream target of the mTOR pathway, which is involved in dendritogenesis (nerve growth) and is linked to altered behavior.42,43 In our current study, cyfluthrin and cyhalothrin directly exposed larvae had decreased expression of rps6 in the F0 generation at 6 PSU (p < 0.05 Dunnett’s test) (Figure 4a). However, in the F1 generation, bifenthrin and cyfluthrin had increased expression of rps6 (p < 0.05 Dunnett’s test) (Figure 4b). Delayed disruption of the mTOR pathway has been seen following bifenthrin exposure43,44 Our findings also provide evidence that the mTOR pathway may also be affected following exposure to cyfluthrin and cyhalothrin. There is no evidence that any of the pyrethroids altered rps6 in the 10 PSU exposures. In the cyhalothrin treatments, a response occurs only after direct exposure (Figure 4a). The bifenthrin-exposed fish do not show any effect on rps6 until the F1 generation (Figure 4b). Following a seven day exposure to bifenthrin, Inland Silversides did not exhibit a change in rps6 expression until after a 14 day recovery period, and after the recovery period, there was a dose-dependent increase in expression.43 Disruption of the mTOR pathway has been linked to both neural developmental effects and changes in neuron function, as well as physiological impacts on skeletal muscle and muscle atrophy.44 Both neurological and physiological changes in developing larvae could explain the changes in behavior observed here. Rps6 is additionally involved in other pathways related to early life development; other mechanisms than those described here may also be influenced such as protein production and cellular apoptosis.45 Following a seven day exposure to bifenthrin, Inland Silversides did not exhibit a change in rps6 expression until after a 14 day recovery period, and after the recovery period, there was a dose-dependent increase in expression.43 Direct exposure to bifenthrin at 6 or 10 PSU and to cyfluthrin and cyhalothrin at 10 PSU may still alter the mTOR pathway, but future studies that measure more recovery time points with multiple pyrethroids would be needed.
Figure 4.
Boxplots showing gene expression in fold change (log2) values normalized to the geometric mean of the housekeeping genes. (a) F0 larvae exposed to control, bifenthrin (1.1 ng/L), cyfluthrin (0.9 ng/L), and cyhalothrin (0.7 ng/L) at 6 and 10 PSU. (b) F1 larvae indirectly exposed to control, bifenthrin (1.1 ng/L), cyfluthrin (0.9 ng/L), and cyhalothrin (0.7 ng/L) at 6 and 10 PSU. Upper and lower whiskers are the 95% upper and lower confidence limits, respectively, the box is the inter quantile range, the black horizontal bars represent the median, and points represent data beyond the 95% confidence limits. An asterisk denotes p < 0.05 (Dunnett’s test).
Na+-K+-ATPase (atp1a1 and atp1a2) transporter genes were upregulated following exposure to both bifenthrin and cyfluthrin. In the F0 generation, the 6 and 10 PSU bifenthrin larvae had increased expression of atp1a1 and atp1a2, respectively (p < 0.05 Dunnett’s test) (Figure 4a). In the F1 larvae, atp1a2 was significantly increased in the 10 PSU bifenthrin and cyfluthrin exposures (p < 0.05, Dunnett’s test) (Figure 4b). Altered atp1a1 and atp1a2 can increase extracellular potassium and glutamate concentrations in the central nervous system, contributing to neurodegenerative disease and altered behavior.46 Upregulation of Na+/K+-ATPases may also be linked to the increased expression of genes involved in aerobic metabolism. The increased expression of atp1a1 and atp1a2 may indicate that larvae allocate more energy toward metabolism, which may require them to sacrifice other processes to compensate. The alteration of atp1a2 in the F1 generation suggests that these effects may be delayed following cyfluthrin exposure and not appear until the F1 generation. For larvae exposed to bifenthrin at 6 PSU, altered expression on atp1a1 was not seen in the next generation, but at 10 PSU, increased atp1a2 was observed. Under short, low-dose scenarios, an organism may exhibit “metabolic compensation” where energy is reallocated to stress resistance and metabolism is altered, which we see evidence of here.
In some instances, pyrethroids have also been found to affect AChE activity. At the low concentrations used here, there was no effect from the pyrethroids at either salinity on AChE activity (p > 0.05, one-way ANOVA) (Figure S3). Cyhalothrin has been shown to alter AChE activity in freshwater fish; however, the concentrations used were much higher than those used in the present study.47 Our results establish that at concentrations found in the environment, AChE inhibition is unlikely to be a common mechanism of toxicity following pyrethroid exposure. Future studies should consider using RNA sequencing to characterize all neurological pathways involved in pyrethroid toxicity in fish.
3.3. Reproductive Effects Were Altered at the Different Salinities
In adult fish, the sex ratio was not altered in the F0 adults in the present study (p > 0.05, Dunnett’s test), which is consistent with other studies on bifenthrin.10 Condition factor was also not affected (p > 0.05, Dunnett’s test). We found gonadosomatic index (GSI) to be significantly reduced in the 6 PSU bifenthrin and cyhalothrin-exposed F0 adult males (p < 0.05, Dunnett’s test) (Figure 5). No differences in GSI were found in females or 10 PSU-exposed males. Similar findings have also been found in male Steelhead Trout (Oncorhynchus mykiss) exposed to bifenthrin at 0, 8, and 16 PSU, wherein the freshwater-exposed fish had a significant reduction in GSI, but the saltwater exposures did not.48 GSI was not changed in Inland Silverside adults after exposure to bifenthrin during embryonic and larval development (∼30 days) at 15 PSU.10 Evidence from the present study and others evaluating the effects of bifenthrin at varying salinities suggest that at low, environmentally relevant concentrations, this pyrethroid reduced GSI at lower salinities, but effects may be mitigated at higher salinities.
Figure 5.
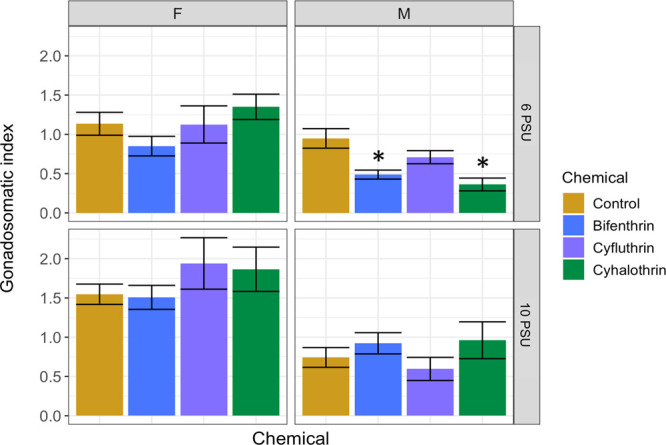
Bar plot of the gonadosomatic index of F0 adult Inland Silversides directly exposed to a methanol-only control, bifenthrin (1.1 ng/L), cyfluthrin (0.9 ng/L), and cyhalothrin (0.7 ng/L) at 6 and 10 PSU. Error bars represent standard error, and an asterisk denotes p < 0.05 (one-way ANOVA followed by Dunnett’s test).
Interestingly, fecundity was increased in all instances except in fish exposed to 6 PSU cyhalothrin, where fecundity was decreased (p < 0.05, Dunnett’s test), and fish exposed to 10 PSU cyfluthrin, where no effect was seen (p > 0.05) (Figure 6). Previous studies with Inland Silversides exposed to bifenthrin found a decrease in egg production;7,10,11,49 however, the exposures for the current study were shorter (96 h) and occurred at lower salinities and during an earlier developmental period. These differences in the study design could explain the conflicting results. It has also been shown that the bifenthrin metabolite has greater estrogenic properties than the parent compound.9 The pyrethroids bifenthrin and permethrin have been shown to act as estrogen receptor antagonists, whereas the metabolites act as agonists. At low concentrations, such as used here, the relative concentration of the parent compound may be more quickly surpassed by the metabolite, meaning the estrogenic effects of the metabolite are more influential than the parent compound. Bifenthrin and cyhalothrin’s metabolites have been shown to have estrogenic properties,50 while cyfluthrin has been shown to induce antiestrogenic properties. Since the parent and metabolite compounds are likely exerting their opposing effects simultaneously, the effects of parent versus metabolite are often challenging to interpret. Bifenthrin has been shown to have a life-stage dependent effect on estrogenicity; for example, in Zebrafish, bifenthrin caused an antiestrogenic effect in larvae but an estrogenic one in adults.51 We found that the larvae directly exposed to bifenthrin had an increase in the androgen receptor gene (arx) (p < 0.05, Dunnett’s test) (Figure 4a). It is possible that early life exposure to bifenthrin caused an increase in arx gene expression, but in adults, this effect is now reversed. In our study, early life exposure to bifenthrin at 6 PSU increased fecundity but also was associated with increased embryonic deformities in the indirectly exposed offspring. Increased fecundity is energetically demanding, and energy may have been diverted from egg production quality in order to increase quantity. Larvae indirectly exposed to cyfluthrin at 6 PSU had increased growth index relative to the control (p < 0.05, Dunnett’s test) (Figure S5); increased growth may be beneficial if increased biomass can increase survival in a stressful environment; however, this requires a diversion of resources from other processes, which can have a long-term detrimental effect. Larvae indirectly exposed to cyfluthrin at 10 PSU had a significant increase in igf2 gene expression (p < 0.05, Dunnett’s test) (Figure 4b) and an observable, but nonsignificant, reduction in survival (p = 0.06, Dunnett’s test) (Figure S1). Despite the increase in igft2 expression, the F1 cyfluthrin treatment had no changes in behavior at 10 PSU. Igf2 gene expression is involved in the insulin/IGF pathway, which has been found to precede the mTOR pathway in mammalian and Drosophila cells.52 Future studies on the relationship between increased igf2 expression and the observed recovery of F1 larvae could provide further insight into igf2’s role in early development and behavior.
Figure 6.
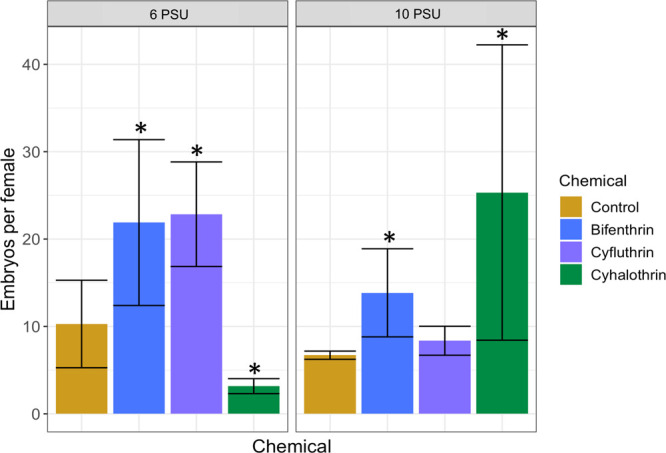
Bar plot showing F0 adult Inland Silversides fecundity after directly exposed to a methanol-only control, bifenthrin (1.1 ng/L), cyfluthrin (0.9 ng/L), and cyhalothrin (0.7 ng/L) at 6 and 10 PSU for 96 h from 5 to 9 dpf (2 dph) and reared in clean water. Error bars represent standard error, and an asterisk denotes p < 0.05 (one-way ANOVA followed by Dunnett’s test).
Studies have found that salinity can influence the production of metabolizing enzymes and lead to faster biotransformation of pyrethroids and other insecticides at higher salinities.23,53 Here, cyp1a1 was not different between the 6 and 10 PSU larvae for any pyrethroid (t test, p > 0.05). Cyp1a1 was significantly increased in the 6 PSU bifenthrin indirectly exposed F1 larvae compared to that in the unexposed control (p < 0.05, Dunnett’s test) (Figure 4b). While there were no changes in cyp1a1 in the F0 larvae, increased biotransformation at higher salinities cannot be ruled out. A larger number of metabolizing enzymes are involved in detoxification, and other genes not measured here may have been affected. Increased biotransformation would mean the parent compound is more quickly converted to the metabolite, but it may also mean the metabolite is more quickly excreted from the body. At the lower salinity, slower metabolism may mean that excretion of the metabolite is reduced and would explain why more reproductive effects are seen at the lower salinity for bifenthrin and cyhalothrin, which have known estrogenic metabolites. Pharmacokinetic and pharmacodynamic studies could test this hypothesis.
3.4. Implications for Future Generations
Exposure to contaminants is known to cause epigenetic changes that can have an effect on future generations. Epigenetic mechanisms control the expression of genes through processes other than changes to the DNA sequence. DNA methyltransferase 3 is the enzyme responsible for de novo methylation, which occurs after fertilization and again prior to differentiation of primordial germ cells.54 Following global erasure of DNA methylation patterns, dnmt3 enzymes establish de novo methylation patterns. In Japanese Medaka (Oryzias latipes) and Zebrafish, the final methylation pattern in embryos resembles the maternal and paternal patterns, respectively.55,56 The DNA methylation pattern of Inland Silversides following fertilization or germ cell differentiation is not currently known, although the pattern may influence the effect of environmental stressors. Altered gene expression of dnmt3a could indicate a change in the function of some dnmt3 enzymes. Here, we found that dnmt3a gene expression was significantly downregulated in the F1 larvae indirectly exposed to cyfluthrin at 10 PSU (p < 0.05 Dunnett’s test, Figure 4b). Dysregulation of dnmt3a has been found to have downstream effects on gene expression and behavior. In adult Zebrafish, dnmt3a knockout fish demonstrated decreased predator avoidance behavior and an overall reduction in fear behavior.57 We did not find any behavioral changes to the F1 10 PSU indirectly exposed cyfluthrin larvae; however, future studies that carry generations past F1 may find further effects.
In some scenarios, the unexposed or indirectly exposed generations may experience the most severe effects. Japanese medaka exposed to bisphenol A did not have reduced reproduction or larval survival until the F3 and F4 generations, respectively.58 Due to the longer generation time of fish compared to that of many invertebrates, many fish studies rarely go past the F2 or F3 larval generation. Studies in invertebrates can help inform on the risk to unexposed generations of fish, but uncertainty remains around how long it may take for an unexposed population to recover from pyrethroid exposure. When DNA replication was disrupted in nematodes (Caenorhabditis elegans) during embryogenesis, changes were caused to the chromatin structure for up to five generations before it was restored.59 In chironomids (Chironomus columbiensis), one generation reared in clean water was sufficient to show recovery in hatching success. Nevertheless, after two generations, fecundity remained low relative to the controls.60 Multiple and transgenerational fish studies using environmentally relevant concentrations of contaminants, such as the one presented here, are still limited in the literature. Understanding the ability of a fish to recover from past exposure or adapt to continuous environmental exposure is necessary to improve ecological risk assessment.
As discussed, the three pyrethroids (bifenthrin, cyfluthrin, and cyhalothrin) tested here altered fecundity at 6 PSU, while bifenthrin and cyhalothrin altered fecundity at 10 PSU. These findings support other studies that suggest that early life, low-level exposure to pyrethroids can impact the stability of populations. When larval Inland Silversides were exposed to bifenthrin at 10 PSU, fecundity was reduced in the F0 and F1 generations.11 When population dynamics were modeled in Inland Silversides following early life exposure to bifenthrin at concentrations like those tested here, a steady decline in population was predicted only one year post exposure.12 However, future studies should test whether population levels stop dropping or rebound if fish are able to adapt. No studies of fish have compared the effects of pyrethroids after multiple generations are exposed concurrently. These studies may more accurately represent a real-world environment where pesticide runoff occurs annually and offspring are unlikely to be raised in pristine conditions. A population would need to adapt quickly to a polluted environment to remain stable. Such rapid changes in tolerance are possible through epigenetic mechanisms but can also come at a cost to other biological processes such as growth.
3.5. Considerations for Risk Assessment
Scientists and government agencies have increasingly considered the idea of conducting risk assessments and decision making based upon contaminants as a class, rather than individual chemicals.61 This approach could provide the capacity to decrease the time to decision making, which is currently limited by the number of compounds on the market and could be reduced by considering compound classes instead of individual compounds. However, limitations of this approach may include the risk of placing overly conservative or liberal screening and threshold levels. For example, if a level is based on the most toxic compound in the class, this would ensure that the most conservative approach is taken. However, this may result in more restrictive levels that could impact agriculture production. An alternate approach could be to develop a system of chemical prioritization where the compounds with the most toxicological concern are further evaluated using a risk assessment model.62
As GCC continues to have increasingly severe effects on estuarine and other aquatic systems, consideration of abiotic factors and multiple stressor scenarios in risk assessment may be beneficial for science-based decision making. There is broad evidence in the literature that pyrethroids can both decrease and increase toxicity as salinity increases. This is likely dependent on the organism, life stage of exposure, concentration of exposure, and end points measured in the study. In the present study, the most end points had decreased effects at the higher salinity for both the F0 and F1 treatments (except cyfluthrin behavior in F0 larvae). When exposed to sublethal levels of pyrethroids from 5 dpf for 96 h at 0.5, 2, and 6 PSU, Inland Silverside behavioral toxicity consistently decreased as salinity increased. Permethrin may have also been an exception because its toxicity appeared to be slightly increased at the higher salinities.24 When Delta Smelt were exposed to bifenthrin and permethrin at concentrations and salinities similar those in this and our previous study,24 both pyrethroids had increased toxicity as salinity increased.25 When Inland Silversides were exposed to bifenthrin at 5 PSU and 15 PSU from 24 h post fertilization to 96 h post hatch, there was a nonsignificant increase in toxicity.26 The differences in responses to pyrethroid toxicity between Inland Silversides and Delta Smelt are important because Delta Smelt are an endangered species, and their risk is often modeled using the Inland Silverside.29 Given the variability of responses to increased salinity, how salinity is incorporated into risk assessments may likely need to be dependent on the system in question, the severity of pollution, and the species at risk. The data presented here also further highlight the variability of toxic responses within an insecticide class and demonstrate one of the challenges with taking a contaminant class approach to risk assessment.
Our results illustrate the potential for bifenthrin, cyfluthrin, and cyhalothrin to cause multigenerational effects at different salinities. We found that these effects can be dependent on exposure salinity, which may have implications for estuarine fish species. Our data indicate that overall pyrethroid toxicity decreased as salinity increases for Inland Silversides, which would suggest that in the wild these fish are at greater risk of toxic effects in lower salinity regions. Incorporation of different abiotic factors is important when considering risk assessments for estuarine systems given that they experience daily flux in water quality. This is likely to be exacerbated under GCC conditions, and our data provide insight into how pyrethroid toxicity is altered under different saline environments. Finally, we show evidence suggesting that low pyrethroid concentrations and a short exposure period could result in a compensatory behavioral response. Compensation responses should be further studied in fish and considered when conducting risk assessments as they have implications for the success of future generations and long-term population dynamics.63−65 Future work focusing on additional generational times with repeat exposures may provide more clarity into the compensatory behavior responses observed here.
Acknowledgments
The authors would like to thank Patrick Chappell for his assistance with qPCR and supplying the instrumentation needed to perform the analysis. They would also like to thank Julia Parker and Ingrid Bartlett for their assistance with the exposures and adult rearing/husbandry.
Data Availability Statement
Data and R code are available at https://doi.org/10.5281/zenodo.10471995.
Supporting Information Available
The Supporting Information is available free of charge at https://pubs.acs.org/doi/10.1021/acs.est.3c06234.
Fish husbandry, spawning, and experimental design methods; gene expression methods; growth and development methods; water quality tables; gene list; and survival, hatching success, AChE, developmental deformity rate, growth index, condition factor, and hepatosomatic index (PDF).
Author Contributions
S.J.H.: data curation, formal analysis, investigation, visualization, writing—original draft. S.S.: formal analysis, investigation, visualization, writing—review and editing. E.I.P.: data curation, investigation. C.Y.M.: data curation, investigation. A.S.: methodology, writing—review and editing. M.L.H.: funding acquisition, methodology, validation, writing—review and editing. R.E.C.: methodology, funding acquisition, writing—review and editing. S.M.B.: mentor to SJH, methodology, lead PI funding acquisition, resources, writing—review and editing.
This work was funded by the California Delta Stewardship Council, contract #18206 (to S.M.B., R.E.C., M.L.H.) which supported the bulk of this work, with additional support from EPA Science to Achieve Results grant #83950301-0 (to S.M.B.) and the Ivan Pratt Memorial Scholarship (S.J.H.). The ideas presented in this publication are those solely of the authors and do not necessarily reflect the opinions of Oregon State University, the University of California, Davis, the California Delta Stewardship Council, or the Environmental Protection Agency. Any use of trade, firm, or product names is for descriptive purposed only and does not imply endorsement by the U.S. Government.
The authors declare no competing financial interest.
Notes
This study was approved by the Oregon State University Institutional Animal Care and Use Committee (IACUC) protocol #0035, approved 19 October 2019. Adult brood stock was housed and spawned at the Oregon State University Hatfield Marine Science Center under the Animal Care and Use Program (ACUP) protocol #4999.
Supplementary Material
References
- Sattar Q.; Maqbool M. E.; Ehsan R.; Akhtar S. Review on climate change and its effect on wildlife and ecosystem. Open J. Environ. Biol. 2021, 6 (1), 8–14. 10.17352/ojeb.000021. [DOI] [Google Scholar]
- Tudi M.; Daniel Ruan H.; Wang L.; Lyu J.; Sadler R.; Connell D.; et al. Agriculture development, pesticide application and its impact on the environment. International journal of environmental research and public health. 2021, 18 (3), 1112. 10.3390/ijerph18031112. [DOI] [PMC free article] [PubMed] [Google Scholar]
- Weston D. P.; Lydy M. J. Urban and agricultural sources of pyrethroid insecticides to the Sacramento-San Joaquin Delta of California. Environmental science & technology. 2010, 44 (5), 1833–40. 10.1021/es9035573. [DOI] [PubMed] [Google Scholar]
- Ullah S.; Li Z.; Zuberi A.; Arifeen M. Z. U.; Baig M. M. F. A. Biomarkers of pyrethroid toxicity in fish. Environ. Chem. Lett. 2019, 17 (2), 945–973. 10.1007/s10311-018-00852-y. [DOI] [Google Scholar]
- Salako A. F.; Amaeze N. H.; Shobajo H. M.; Osuala F. I. Comparative acute toxicity of three pyrethroids (Deltamethrin, cypermethrin and lambda-cyhalothrin) on guppy fish (Poecilia reticulata peters, 1859). Scientific African. 2020, 9, e00504 10.1016/j.sciaf.2020.e00504. [DOI] [Google Scholar]
- Ullah S.; Li Z.; Arifeen M. Z. U.; Khan S. U.; Fahad S. Multiple biomarkers based appraisal of deltamethrin induced toxicity in silver carp (Hypophthalmichthys molitrix). Chemosphere 2019, 214, 519–533. 10.1016/j.chemosphere.2018.09.145. [DOI] [PubMed] [Google Scholar]
- Brander S. M.; Gabler M. K.; Fowler N. L.; Connon R. E.; Schlenk D. Pyrethroid pesticides as endocrine disruptors: molecular mechanisms in vertebrates with a focus on fishes. Environmental science & technology. 2016, 50 (17), 8977–92. 10.1021/acs.est.6b02253. [DOI] [PubMed] [Google Scholar]
- Tyler C. R.; Beresford N.; Van Der Woning M.; Sumpter J. P.; Tchorpe K. Metabolism and environmental degradation of pyrethroid insecticides produce compounds with endocrine activities. Environmental Toxicology and Chemistry: An International Journal. 2000, 19 (4), 801–9. 10.1002/etc.5620190404. [DOI] [Google Scholar]
- DeGroot B. C.; Brander S. M. The role of P450 metabolism in the estrogenic activity of bifenthrin in fish. Aquatic toxicology. 2014, 156, 17–20. 10.1016/j.aquatox.2014.07.007. [DOI] [PubMed] [Google Scholar]
- DeCourten B. M.; Brander S. M. Combined effects of increased temperature and endocrine disrupting pollutants on sex determination, survival, and development across generations. Sci. Rep. 2017, 7 (1), 9310. 10.1038/s41598-017-09631-1. [DOI] [PMC free article] [PubMed] [Google Scholar]
- DeCourten B. M.; Forbes J. P.; Roark H. K.; Burns N. P.; Major K. M.; White J. W.; et al. Multigenerational and transgenerational effects of environmentally relevant concentrations of endocrine disruptors in an estuarine fish model. Environmental Science & Technology. 2020, 54 (21), 13849–60. 10.1021/acs.est.0c02892. [DOI] [PubMed] [Google Scholar]
- Brander S. M.; White J. W.; DeCourten B. M.; Major K.; Hutton S. J.; Connon R. E.; et al. Accounting for transgenerational effects of toxicant exposure in population models alters predicted long-term population status. Environ. Epigenet. 2022, 8, dvac023 10.1093/eep/dvac023. [DOI] [PMC free article] [PubMed] [Google Scholar]
- Blanc M.; Cormier B.; Hyötyläinen T.; Krauss M.; Scherbak N.; Cousin X.; et al. Multi-and transgenerational effects following early-life exposure of zebrafish to permethrin and coumarin 47: Impact on growth, fertility, behavior and lipid metabolism. Ecotoxicology and Environmental Safety. 2020, 205, 111348 10.1016/j.ecoenv.2020.111348. [DOI] [PubMed] [Google Scholar]
- Barbier E. B.; Hacker S. D.; Kennedy C.; Koch E. W.; Stier A. C.; Silliman B. R. The value of estuarine and coastal ecosystem services. Ecological monographs. 2011, 81 (2), 169–93. 10.1890/10-1510.1. [DOI] [Google Scholar]
- Delorenzo M. E. Impacts of climate change on the ecotoxicology of chemical contaminants in estuarine organisms. Current Zoology. 2015, 61 (4), 641–52. 10.1093/czoolo/61.4.641. [DOI] [Google Scholar]
- Khojasteh D.; Glamore W.; Heimhuber V.; Felder S. Sea level rise impacts on estuarine dynamics: A review. Science of The Total Environment. 2021, 780, 146470 10.1016/j.scitotenv.2021.146470. [DOI] [PubMed] [Google Scholar]
- Du J.; Park K. Estuarine salinity recovery from an extreme precipitation event: Hurricane Harvey in Galveston Bay. Science of the total environment. 2019, 670, 1049–59. 10.1016/j.scitotenv.2019.03.265. [DOI] [PubMed] [Google Scholar]
- Amiri B. M.; Xu E. G.; Kupsco A.; Giroux M.; Hoseinzadeh M.; Schlenk D. The effect of chlorpyrifos on salinity acclimation of juvenile rainbow trout (Oncorhynchus mykiss). Aquatic Toxicology. 2018, 195, 97–102. 10.1016/j.aquatox.2017.12.011. [DOI] [PubMed] [Google Scholar]
- Giroux M.; Schlenk D. The effects of temperature and salinity on the endocrinology in two life stages of juvenile rainbow/steelhead trout (Oncorhynchus mykiss). Journal of Fish Biology. 2021, 99 (2), 513–23. 10.1111/jfb.14741. [DOI] [PubMed] [Google Scholar]
- Lavado R.; Maryoung L. A.; Schlenk D. Hypersalinity acclimation increases the toxicity of the insecticide phorate in coho salmon (Oncorhynchus kisutch). Environmental science & technology. 2011, 45 (10), 4623–9. 10.1021/es200451j. [DOI] [PMC free article] [PubMed] [Google Scholar]
- Moreira L. B.; Diamante G.; Giroux M.; Coffin S.; Xu E. G.; de Souza Abessa D. M.; Schlenk D.; et al. Impacts of Salinity and Temperature on the Thyroidogenic Effects of the Biocide Diuron in Menidia beryllina. Environ. Sci. Technol. 2018, 52 (5), 3146–3155. 10.1021/acs.est.7b04970. [DOI] [PubMed] [Google Scholar]
- Riar N.; Crago J.; Jiang W.; Maryoung L. A.; Gan J.; Schlenk D. Effects of salinity acclimation on the endocrine disruption and acute toxicity of bifenthrin in freshwater and euryhaline strains of Oncorhynchus mykiss. Environ. Toxicol. Chem. 2013, 32 (12), 2779–85. 10.1002/etc.2370. [DOI] [PMC free article] [PubMed] [Google Scholar]
- Derby A. P.; Fuller N. W.; Hartz K. E. H.; Segarra A.; Connon R. E.; Brander S. M.; et al. Trophic transfer, bioaccumulation and transcriptomic effects of permethrin in inland silversides, Menidia beryllina, under future climate scenarios. Environ. Pollut. 2021, 275, 116545 10.1016/j.envpol.2021.116545. [DOI] [PubMed] [Google Scholar]
- Hutton S. J.; Siddiqui S.; Pedersen E. I.; Markgraf C. Y.; Segarra A.; Hladik M. L.; et al. Comparative behavioral ecotoxicology of Inland Silverside larvae exposed to pyrethroids across a salinity gradient. Science of The Total Environment. 2023, 857, 159398 10.1016/j.scitotenv.2022.159398. [DOI] [PubMed] [Google Scholar]
- Segarra A.; Mauduit F.; Amer N. R.; Biefel F.; Hladik M. L.; Connon R. E.; et al. Salinity changes the dynamics of pyrethroid toxicity in terms of behavioral effects on newly hatched delta smelt larvae. Toxics. 2021, 9 (2), 40. 10.3390/toxics9020040. [DOI] [PMC free article] [PubMed] [Google Scholar]
- Hutton S. J.; St Romain S. J.; Pedersen E. I.; Siddiqui S.; Chappell P. E.; White J. W.; et al. Salinity Alters Toxicity of Commonly Used Pesticides in a Model Euryhaline Fish Species (Menidia beryllina). Toxics 2021, 9 (5), 114. 10.3390/toxics9050114. [DOI] [PMC free article] [PubMed] [Google Scholar]
- Siddiqui S.; Dickens J.; Cunningham B.; Hutton S.; Pedersen E.; Harper B.; et al. Internalization, reduced growth, and behavioral effects following exposure to micro and nano tire particles in two estuarine indicator species. Chemosphere. 2022, 296, 133934 10.1016/j.chemosphere.2022.133934. [DOI] [PMC free article] [PubMed] [Google Scholar]
- USEPA . Endangered Species Facts, U.S. Environmental Protection Agency, https://www.epa.gov/sites/default/files/2013-08/documents/delta-smelt_0.pdf, 2010. [Google Scholar]
- Lawrence E. J.; Elmstrom S. R.; Sharpe E. E.; Landis W. G.. Technical Memo: Incorporating Mixture Toxicity into Bayesian Networks to calculate risk to pesticides in the Upper San Francisco Estuary, 2021.
- Monismith S. G.; Kimmerer W.; Burau J. R.; Stacey M. T. Structure and flow-induced variability of the subtidal salinity field in northern San Francisco Bay. Journal of physical Oceanography. 2002, 32 (11), 3003–19. . [DOI] [Google Scholar]
- Oros D. R.; Werner I. Pyrethroid insecticides: an analysis of use patterns, distributions, potential toxicity and fate in the Sacramento-San Joaquin Delta and Central Valley; Citeseer, 2005. [Google Scholar]
- Weston D. P.; Chen D.; Lydy M. J. Stormwater-related transport of the insecticides bifenthrin, fipronil, imidacloprid, and chlorpyrifos into a tidal wetland, San Francisco Bay. California. Science of the Total Environment. 2015, 527, 18–25. 10.1016/j.scitotenv.2015.04.095. [DOI] [PubMed] [Google Scholar]
- Woudneh M. B.; Oros D. R. Pyrethroids, pyrethrins, and piperonyl butoxide in sediments by high-resolution gas chromatography/high-resolution mass spectrometry. Journal of Chromatography A 2006, 1135 (1), 71–7. 10.1016/j.chroma.2006.09.017. [DOI] [PubMed] [Google Scholar]
- Hladik M. L.; Kuivila K. M. Assessing the occurrence and distribution of pyrethroids in water and suspended sediments. Journal of agricultural and food chemistry. 2009, 57 (19), 9079–85. 10.1021/jf9020448. [DOI] [PubMed] [Google Scholar]
- Hladik M. L.; McWayne M. M.. Methods of Analysis, Determination of Pesticides in Sediment Using Gas Chromatography/mass Spectrometry, 2012.
- Hutton S. J.; Siddiqui S.; Pedersen E. I.; Markgraf C. Y.; Segarra A.; Hladik M. L.; et al. Comparative behavioral ecotoxicology of Inland Silverside larvae exposed to pyrethroids across a salinity gradient. Science of The Total Environment. 2023, 857, 159398 10.1016/j.scitotenv.2022.159398. [DOI] [PubMed] [Google Scholar]
- Kochhann D.; de Azevedo Brust S. M.; Domingos F. X. V.; Val A. L. Linking hematological, biochemical, genotoxic, and behavioral responses to crude oil in the Amazon fish Colossoma macropomum (Cuvier, 1816). Archives of environmental contamination and toxicology. 2013, 65, 266–75. 10.1007/s00244-013-9894-4. [DOI] [PubMed] [Google Scholar]
- Ågerstrand M.; Arnold K.; Balshine S.; Brodin T.; Brooks B. W.; Maack G.; et al. Emerging investigator series: use of behavioural endpoints in the regulation of chemicals. Environmental Science: Processes & Impacts. 2020, 22 (1), 49–65. 10.1039/C9EM00463G. [DOI] [PubMed] [Google Scholar]
- Réale D.; Garant D.; Humphries M. M.; Bergeron P.; Careau V.; Montiglio P.-O. Personality and the emergence of the pace-of-life syndrome concept at the population level. Philosophical Transactions of the Royal Society B: Biological Sciences. 2010, 365 (1560), 4051–63. 10.1098/rstb.2010.0208. [DOI] [PMC free article] [PubMed] [Google Scholar]
- Petitjean Q.; Jean S.; Gandar A.; Côte J.; Laffaille P.; Jacquin L. Stress responses in fish: From molecular to evolutionary processes. Sci. Total Environ. 2019, 684, 371–80. 10.1016/j.scitotenv.2019.05.357. [DOI] [PubMed] [Google Scholar]
- Perrier S.; Moreau E.; Deshayes C.; El-Adouzi M.; Goven D.; Chandre F.; et al. Compensatory mechanisms in resistant Anopheles gambiae AcerKis and KdrKis neurons modulate insecticide-based mosquito control. Commun. Biol. 2021, 4 (1), 665. 10.1038/s42003-021-02192-0. [DOI] [PMC free article] [PubMed] [Google Scholar]
- Skalecka A.; Liszewska E.; Bilinski R.; Gkogkas C.; Khoutorsky A.; Malik A. R.; et al. mTOR kinase is needed for the development and stabilization of dendritic arbors in newly born olfactory bulb neurons. Developmental neurobiology. 2016, 76 (12), 1308–27. 10.1002/dneu.22392. [DOI] [PMC free article] [PubMed] [Google Scholar]
- Frank D. F.; Miller G. W.; Harvey D. J.; Brander S. M.; Geist J.; Connon R. E.; et al. Bifenthrin causes transcriptomic alterations in mTOR and ryanodine receptor-dependent signaling and delayed hyperactivity in developing zebrafish (Danio rerio). Aquatic Toxicology. 2018, 200, 50–61. 10.1016/j.aquatox.2018.04.003. [DOI] [PMC free article] [PubMed] [Google Scholar]
- Bowling H.; Zhang G.; Bhattacharya A.; Pérez-Cuesta L. M.; Deinhardt K.; Hoeffer C. A.; et al. Antipsychotics activate mTORC1-dependent translation to enhance neuronal morphological complexity. Sci. Signaling 2014, 7 (308), ra4. 10.1126/scisignal.2004331. [DOI] [PMC free article] [PubMed] [Google Scholar]; –ra
- Meyuhas O. Ribosomal protein S6 phosphorylation: four decades of research. International review of cell and molecular biology. 2015, 320, 41–73. 10.1016/bs.ircmb.2015.07.006. [DOI] [PubMed] [Google Scholar]
- Kinoshita P. F.; Leite J. A.; Orellana A. M. M.; Vasconcelos A. R.; Quintas L. E.; Kawamoto E. M.; et al. The influence of Na+, K+-ATPase on glutamate signaling in neurodegenerative diseases and senescence. Front. Physiol. 2016, 7, 195. 10.3389/fphys.2016.00195. [DOI] [PMC free article] [PubMed] [Google Scholar]
- Kumar A.; Rai D. K.; Sharma B.; Pandey R. S. λ-cyhalothrin and cypermethrin induced in vivo alterations in the activity of acetylcholinesterase in a freshwater fish, Channa punctatus (Bloch). Pesticide biochemistry and physiology. 2009, 93 (2), 96–9. 10.1016/j.pestbp.2008.12.005. [DOI] [Google Scholar]
- Forsgren K. L.; Riar N.; Schlenk D. The effects of the pyrethroid insecticide, bifenthrin, on steroid hormone levels and gonadal development of steelhead (Oncorhynchus mykiss) under hypersaline conditions. General and comparative endocrinology. 2013, 186, 101–7. 10.1016/j.ygcen.2013.02.047. [DOI] [PubMed] [Google Scholar]
- Brander S. M.; Jeffries K. M.; Cole B. J.; DeCourten B. M.; White J. W.; Hasenbein S.; et al. Transcriptomic changes underlie altered egg protein production and reduced fecundity in an estuarine model fish exposed to bifenthrin. Aquat Toxicol. 2016, 174, 247–60. 10.1016/j.aquatox.2016.02.014. [DOI] [PubMed] [Google Scholar]
- Du G.; Shen O.; Sun H.; Fei J.; Lu C.; Song L.; et al. Assessing hormone receptor activities of pyrethroid insecticides and their metabolites in reporter gene assays. Toxicol. Sci. 2010, 116 (1), 58–66. 10.1093/toxsci/kfq120. [DOI] [PubMed] [Google Scholar]
- Bertotto L. B.; Richards J.; Gan J.; Volz D. C.; Schlenk D. Effects of bifenthrin exposure on the estrogenic and dopaminergic pathways in zebrafish embryos and juveniles. Environmental toxicology and chemistry. 2018, 37 (1), 236–46. 10.1002/etc.3951. [DOI] [PubMed] [Google Scholar]
- Hay N.; Sonenberg N. Upstream and downstream of mTOR. Genes & development. 2004, 18 (16), 1926–45. 10.1101/gad.1212704. [DOI] [PubMed] [Google Scholar]
- Lavado R.; Aparicio-Fabre R.; Schlenk D. Effects of salinity acclimation on the expression and activity of Phase I enzymes (CYP450 and FMOs) in coho salmon (Oncorhynchus kisutch). Fish physiology and biochemistry. 2014, 40 (1), 267–78. 10.1007/s10695-013-9842-2. [DOI] [PMC free article] [PubMed] [Google Scholar]
- Smallwood S. A.; Kelsey G. De novo DNA methylation: a germ cell perspective. Trends in Genetics. 2012, 28 (1), 33–42. 10.1016/j.tig.2011.09.004. [DOI] [PubMed] [Google Scholar]
- Wang X.; Bhandari R. K. DNA methylation dynamics during epigenetic reprogramming of medaka embryo. Epigenetics. 2019, 14 (6), 611–22. 10.1080/15592294.2019.1605816. [DOI] [PMC free article] [PubMed] [Google Scholar]
- Cavalieri V.; Spinelli G. Environmental epigenetics in zebrafish. Epigenet. Chromatin 2017, 10 (1), 46. 10.1186/s13072-017-0154-0. [DOI] [PMC free article] [PubMed] [Google Scholar]
- Lai Y.-H.; Audira G.; Liang S.-T.; Siregar P.; Suryanto M. E.; Lin H.-C.; et al. Duplicated dnmt3aa and dnmt3ab DNA methyltransferase genes play essential and non-overlapped functions on modulating behavioral control in zebrafish. Genes. 2020, 11 (11), 1322. 10.3390/genes11111322. [DOI] [PMC free article] [PubMed] [Google Scholar]
- Bhandari R. K.; Vom Saal F. S.; Tillitt D. E. Transgenerational effects from early developmental exposures to bisphenol A or 17α-ethinylestradiol in medaka, Oryzias latipes. Sci. Rep. 2015, 5 (1), 9303. 10.1038/srep09303. [DOI] [PMC free article] [PubMed] [Google Scholar]
- Klosin A.; Reis K.; Hidalgo-Carcedo C.; Casas E.; Vavouri T.; Lehner B. Impaired DNA replication derepresses chromatin and generates a transgenerationally inherited epigenetic memory. Sci. Adv. 2017, 3 (8), e1701143 10.1126/sciadv.1701143. [DOI] [PMC free article] [PubMed] [Google Scholar]
- Montaño-Campaz M. L.; Dias L. G.; Bacca T.; Toro-Restrepo B.; Oliveira E. E. Exposures to deltamethrin on immature Chironomus columbiensis drive sublethal and transgenerational effects on their reproduction and wing morphology. Chemosphere. 2022, 296, 134042 10.1016/j.chemosphere.2022.134042. [DOI] [PubMed] [Google Scholar]
- Fenner K.; Scheringer M. The need for chemical simplification as a logical consequence of ever-increasing chemical pollution. Environmental Science & Technology. 2021, 55 (21), 14470–2. 10.1021/acs.est.1c04903. [DOI] [PubMed] [Google Scholar]
- Luijten M.; Sprong R. C.; Rorije E.; van der Ven L. T. Prioritization of chemicals in food for risk assessment by integrating exposure estimates and new approach methodologies: A next generation risk assessment case study. Front. Toxicol. 2022, 4, 933197 10.3389/ftox.2022.933197. [DOI] [PMC free article] [PubMed] [Google Scholar]
- Rand G. M.; Newman J. R. The applicability of habitat evaluation methodologies in ecological risk assessment. Human and Ecological Risk Assessment: An International Journal. 1998, 4 (4), 905–29. 10.1080/10807039891284875. [DOI] [Google Scholar]
- Ankley G. T.; Bennett R. S.; Erickson R. J.; Hoff D. J.; Hornung M. W.; Johnson R. D.; et al. Adverse outcome pathways: a conceptual framework to support ecotoxicology research and risk assessment. Environmental Toxicology and Chemistry: An International Journal. 2010, 29 (3), 730–41. 10.1002/etc.34. [DOI] [PubMed] [Google Scholar]
- Perkins E. J.; Ashauer R.; Burgoon L.; Conolly R.; Landesmann B.; Mackay C.; et al. Building and applying quantitative adverse outcome pathway models for chemical hazard and risk assessment. Environ. Toxicol. Chem. 2019, 38 (9), 1850–65. 10.1002/etc.4505. [DOI] [PMC free article] [PubMed] [Google Scholar]
Associated Data
This section collects any data citations, data availability statements, or supplementary materials included in this article.
Supplementary Materials
Data Availability Statement
Data and R code are available at https://doi.org/10.5281/zenodo.10471995.