Abstract
Background
A post hoc analysis of the MERGE trial was conducted, to investigate whether sex differences are evident at the mildest end of the disease spectrum, for symptoms associated with obstructive sleep apnoea (OSA) and the response to continuous positive airway pressure (CPAP) treatment.
Methods
MERGE participants with mild OSA (apnoea–hypopnoea index 5–15 events·h−1; American Academy of Sleep Medicine 2012 criteria) were randomised to either CPAP plus standard care (sleep hygiene counselling) or standard care alone for 3 months. Quality of life (QoL) was measured by questionnaires completed before and after the 3 months. This post hoc analysis of participants of the MERGE trial compared the symptom presentation, and response to CPAP, between the sexes.
Results
233 patients were included; 71 (30%) were female. Females were more symptomatic at baseline in all QoL questionnaires. Specifically, females had lower 36-item Short-Form Health Survey (SF-36) Vitality scores (mean±sd 39.1±10.1 versus 44.8±10.3) and higher Epworth Sleepiness Scale (ESS) scores (mean±sd 11.0±4.2 versus 9.5±4.4). Both sexes experienced snoring, but more females reported fatigue and more males reported witnessed apnoeas. All symptoms improved with CPAP for both sexes; however, females had larger improvements in SF-36 Vitality scores, which was the primary outcome of the MERGE trial (mean change 9.4 (95% CI 6.8–12.0) versus 6.0 (95% CI 4.3–7.7); p=0.034), and ESS (mean change −4.1 (95% CI −5.1– −3.0) versus −2.5 (95% CI −3.1– −1.8); p=0.015), after adjustment for baseline scores and CPAP usage.
Conclusions
Sex differences are apparent in patients with mild OSA. Females experience worse QoL symptoms than males at presentation to the sleep clinic; however, these improve significantly with CPAP treatment.
Tweetable abstract
Females presenting to sleep clinics with mild obstructive sleep apnoea (OSA) experienced reduced QoL compared with males; symptoms improved significantly with treatment. It is important for clinicians to recognise and refer females with mild OSA. https://bit.ly/48jz8kX
Introduction
Mild obstructive sleep apnoea (OSA) is more common in females than males, whereas moderate-to-severe OSA is more common in males [1]. Differences also exist between the sexes in the presentation of OSA. Notably, up to 40% of females with moderate-to-severe disease do not report the classic symptoms of OSA, such as excessive daytime sleepiness, snoring and witnessed apnoeas [2, 3]. Common symptoms in females with OSA include fatigue, insomnia, lack of energy, headaches, muscle pain, depression and anxiety [2–5]. Moreover, a recent study has shown that females present with a higher symptom burden and lower disease severity than males [4]. Objectively, females present with fewer apnoeas and hypopnoeas per hour (i.e. a lower apnoea–hypopnoea index (AHI)) and shorter respiratory events, with less severe oxygen desaturations than males [5]. Females tend to have more episodes of upper airway resistance and flow limitation than males, rather than overt airway obstruction [5]. To investigate whether symptom differences are attributed to sex, or to severity of OSA, we performed a post hoc analysis on data from patients with mild OSA, who participated in the MERGE clinical trial [6]. We hypothesised that differences in symptoms between the sexes would be evident even in patients presenting to sleep clinics with mild OSA.
Continuous positive airway pressure (CPAP) is the recommended treatment for OSA [7, 8]; however, until the MERGE trial, there were scarce data available on CPAP treatment in patients with mild OSA, and no data from mild patients defined using updated 2012 scoring criteria from the American Academy of Sleep Medicine (AASM) [9]. The MERGE trial showed that the SF-36 Vitality score significantly improved after 3 months of CPAP treatment in patients with mild OSA [6]. Similar QoL improvements were seen in sleepiness, fatigue and insomnia. This post hoc analysis of MERGE data also investigated any sex differences in the response to CPAP treatment.
Methods
The MERGE trial
The MERGE trial was a multicentre, parallel group, randomised controlled trial comparing CPAP treatment plus standard care with standard care alone. It was approved by a UK central ethics committee (REC 16/SC/0387) and all patients gave written informed consent. The trial was prospectively registered at ClinicalTrials.gov with identifier number NCT02699463. Full methodology has previously been described and primary outcomes presented [6].
Subjects
Participants were recruited from 11 sites across the UK Respiratory Sleep Network. Patients who were referred to their local sleep centre with suspicion of OSA were investigated by home polygraphy sleep tests (ApneaLink Air; ResMed, Sydney, Australia). Automated scoring using both the AASM 2007 and 2012 criteria was applied. AASM 2007 scoring was performed using commercially available automated scoring software (AirView; ResMed). AASM 2012 scoring was performed using an automated algorithm which utilises surrogate measures to identify arousals from sleep associated with hypopnoeas [10]. Adult patients who were found to have mild OSA (defined as AHI 5–15 events·h−1) were eligible. Exclusions included: body mass index (BMI) ≥40 kg·m−2, unstable cardiac disease, use of supplemental oxygen, secondary sleep pathology (e.g. periodic limb movement syndrome, narcolepsy, circadian disorder and obesity hypoventilation syndrome), previous CPAP usage, Epworth Sleepiness Scale (ESS) score ≥15, concerns over driving while sleepy or an inability to tolerate a 1-h CPAP tolerance test. There were no entry criteria that would have contributed to bias between the sexes.
Study design
Patients with mild OSA were randomised to a 3-month trial comparing CPAP treatment plus standard care with standard care alone. Randomisation was 1:1 and minimised by age, sex and BMI.
Methods
Eligible patients attended two outpatient visits at their local sleep centre: at baseline and at 3 months. At the baseline visit, demographics, medical history and reasons for participants’ referral to the sleep clinic were recorded. Participants also completed a range of self-administered QoL questionnaires which they repeated at the completion of the 3-month trial. The questionnaires measured general QoL (36-item Short-Form Health Survey (SF-36) and EuroQol 5 Dimensions (EQ-5D)), sleepiness and fatigue (ESS and Fatigue Severity Scale (FSS)), the impact of sleep disorders on everyday living and changes with treatment (Functional Outcomes of Sleep Questionnaire (FOSQ)), mental health (Hospital Anxiety and Depression Scale (HADS)) and insomnia (Insomnia Severity Index (ISI)).
Standard care consisted of standardised sleep hygiene counselling. For patients randomised to the CPAP group, CPAP was provided by an auto-adjusting mode (AirSense 10 AutoSet or AirSense 10 AutoSet for Her; ResMed), with wireless CPAP data collected in a centralised trial account (AirView; ResMed). Standardised, centralised follow-up was provided to all patients after 3 days plus further follow-up as determined by the protocol.
The primary outcome in the MERGE trial was change from baseline to 3 months in the Vitality score of the SF-36 questionnaire in patients with mild OSA, diagnosed using the AASM 2012 scoring criteria.
Post hoc analysis of the MERGE trial
Patients who were diagnosed with mild OSA (AHI 5–15 events·h−1 using the AASM 2012 criteria) were the primary analysis population for the MERGE trial and were included in this post hoc analysis.
Baseline demographics, comorbidities, disease symptoms and severity, and QoL were summarised descriptively and compared by sex using the t-test, Wilcoxon rank sum test or Fisher's exact test, as appropriate. Mean change in QoL scores was compared between treatment groups using ANCOVA adjusting for baseline QoL score, sex and treatment-by-sex interaction. Missing 3-month scores were conservatively replaced with baseline scores using a last observation carried forward (LOCF) approach. Statistically significant treatment-by-sex interactions highlighted potential differences between females and males in treatment response. These differences were also highlighted in the plots of mean QoL with associated 95% confidence intervals at each time-point stratified by sex and treatment. To further investigate these differences, mean change in QoL scores was compared between sexes specifically within the CPAP treatment group. This subgroup analysis was performed using the aforementioned LOCF ANCOVA approach, with adjustment for baseline QoL score, sex and average daily CPAP usage. Additional analyses were performed to investigate the effects of baseline factors with significant differences between females and males.
Results
The MERGE trial
A total of 301 participants were randomised in the MERGE trial, of which 233 had mild OSA (AHI 5–15 events·h−1) when scored with AASM 2012 scoring criteria [6]. These patients were the primary analysis population for the MERGE trial, the results of which have been previously published [6].
Post hoc analysis
The baseline characteristics of the participants included in the MERGE trial are presented by sex in table 1. 71 (30%) participants were female. Females tended to be older and had a higher BMI than males, but smaller neck circumference. Both sexes had similar disease severity assessed using AHI and the 3% oxygen desaturation index.
TABLE 1.
Baseline characteristics and reasons for referral
| Females (n=71) | Males (n=162) | p-value | |
| Age (years) | 51.9±10.4 | 49.8±12.2 | 0.20 |
| Ethnicity | 0.82 | ||
| White | 63 (88.7) | 145 (89.5) | |
| Non-White | 8 (11.3) | 17 (10.5) | |
| BMI (kg·m−2) | 32.2±5.0 | 29.4±3.7 | <0.0001 |
| Weight (kg) | 85.7±15.7 | 92.2±12.5 | 0.003 |
| Neck circumference (cm) | 37.8±3.7 | 41.4±3.9 | <0.0001 |
| Medical history | |||
| Diabetes | 13 (18.3) | 8 (4.9) | 0.002 |
| Heart disease | 3 (4.2) | 14 (8.6) | 0.28 |
| Hypertension | 22 (31.0) | 43 (26.5) | 0.53 |
| AHI (AASM 2012) (events·h−1) | 9.6 (6.5–12.4) | 10.3 (7.1–13.2) | 0.24 |
| Obstructive apnoea index | 0.57 (0.22–1.86) | 0.84 (0.25–2.17) | |
| Central apnoea index | 0.15 (0.00–0.39) | 0.11 (0.00–0.49) | |
| Hypopnoea index | 8.07 (5.39–10.37) | 8.01 (5.76–10.52) | |
| Hypopnoea with 3% desaturation | 6.77 (4.94–8.82) | 6.94 (5.18–8.82) | |
| Hypopnoea with arousal | 0.72 (0.30–1.38) | 0.87 (0.30–1.54) | |
| ODI (4%) (events·h−1) | 6.20 (4.10–8.70) | 7.00 (4.80–9.40) | 0.15 |
| ODI (3%) (events·h−1) | 12.20 (10.00–15.20) | 13.50 (10.10–16.10) | 0.41 |
| Flow limitation (%) | 17.41 (6.46–39.08) | 19.33 (7.17–40.14) | 0.37 |
| Snore total (n breaths) | 612.5 (225.5–1333.0) | 711.5 (285.0–1546.0) | 0.33 |
| Quality of life | |||
| Lower scores indicate better status | |||
| ESS | 11.0±4.2 | 9.5±4.4 | 0.017 |
| FSS | 42.0±12.8 | 34.4±13.5 | <0.0001 |
| HADS Anxiety | 8.6±4.7 | 6.4±3.8 | 0.0006 |
| HADS Depression | 7.4±4.2 | 4.8±3.8 | <0.0001 |
| ISI | 15.5±5.6 | 11.8±5.3 | <0.0001 |
| Higher scores indicate better status | |||
| FOSQ | 14.7±2.8 | 16.8±2.5 | <0.0001 |
| EQ-5D Index | 0.66±0.21 | 0.80±0.16 | <0.0001 |
| EQ-5D VAS | 59.9±21.4 | 72.5±15.9 | <0.0001 |
| SF-36 Vitality | 39.1±10.1 | 44.8±10.3 | 0.0001 |
| SF-36 Physical | 43.0±11.2 | 49.7±9.1 | <0.0001 |
| SF-36 Mental | 41.8±13.0 | 47.3±10.9 | 0.001 |
| Reason(s) for referral# | |||
| Snoring | 48 (72.7) | 122 (85.3) | 0.036 |
| Fatigue | 31 (47.0) | 50 (35.0) | 0.13 |
| Daytime sleepiness | 30 (45.5) | 55 (38.5) | 0.37 |
| Witnessed apnoeas | 21 (31.8) | 88 (61.5) | <0.0001 |
| Frequent nocturia | 8 (12.1) | 19 (13.3) | 1.00 |
| Insomnia | 4 (6.1) | 6 (4.2) | 0.73 |
| Screening (pre-operative or due to a comorbidity) | 1 (1.5) | 2 (1.4) | 1.00 |
| Other | 10 (15.2) | 16 (11.2) | 0.50 |
Data are presented as mean±sd, n (%) or median (interquartile range), unless otherwise stated. BMI: body mass index; AHI: apnoea–hypopnoea index; AASM: American Academy of Sleep Medicine; ODI: oxygen desaturation index; ESS: Epworth Sleepiness Scale; FSS: Fatigue Severity Score; HADS: Hospital Anxiety and Depression Scale; ISI: Insomnia Sleep Index; FOSQ: Functional Outcomes of Sleep Questionnaire; EQ-5D: EuroQol 5 Dimensions Questionnaire; VAS: visual analogue scale; SF-36: 36-item Short-Form Health Survey. #: n=66 females and n=143 males; subjects may have more than one reason for referral (percentages are based on the number of subjects who had reason for referral assessed). p-values from the t-test, Wilcoxon rank sum test or Fisher's exact test, as appropriate.
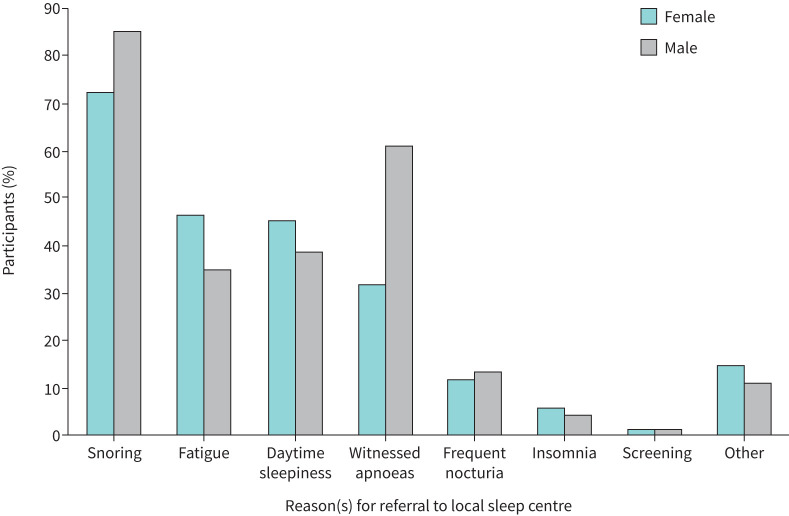
QoL at baseline was significantly worse in female participants, compared with males, across all questionnaires, including SF-36 Vitality scores (table 1). Further analysis of the FSS showed that 73.2% of females had elevated FSS scores (score ≥36), indicating problematic fatigue, compared with 45.1% of males. Additionally, 16.9% of females had a normal ESS score <10 at baseline, but an elevated FSS score ≥36. The most common reasons for referral in female participants were snoring, fatigue and daytime sleepiness; in male participants the most common reasons were snoring, witnessed apnoeas and daytime sleepiness (figure 1). Females tended to be less often referred for snoring and witnessed apnoeas, and more often referred for daytime sleepiness and fatigue than males.
FIGURE 1.
Reason(s) for referral to local sleep centre. At the baseline visit, participants were asked for their self-reported reason(s) for their referral to their local sleep centre. Participants could report more than one reason.
All participants
QoL at baseline is presented by treatment group and sex in table 2. All QoL scores improved with CPAP use compared with standard care alone. The analyses of change in QoL scores accounting for sex showed greater improvements in all QoL scores for females compared with males (table 3). Because significant treatment-by-sex interactions were observed for SF-36 Vitality and ESS scores, further exploration of the relationship between these QoL measures and sex was indicated within the CPAP group alone. Figures 2 (SF-36 Vitality score) and 3 (ESS) show the difference in treatment response between females and males within the CPAP group.
TABLE 2.
Quality of life at baseline by treatment group and sex (all participants)
| CPAP | Standard care | |||
| Females (n=34) | Males (n=81) | Females (n=37) | Males (n=81) | |
| Lower scores indicate better status | ||||
| ESS | 11.5±4.3 | 9.3±4.5 | 10.6±4.1 | 9.8±4.2 |
| FSS | 41.3±13.8 | 32.9±13.4 | 42.6±12.1 | 35.9±13.7 |
| HADS Anxiety | 8.0±5.3 | 6.0±3.8 | 9.1±4.1 | 6.8±3.8 |
| HADS Depression | 6.3±4.1 | 4.5±3.8 | 8.3±4.1 | 5.1±3.8 |
| ISI | 14.1±5.1 | 11.8±5.7 | 16.8±5.8 | 11.7±4.9 |
| Higher scores indicate better status | ||||
| FOSQ | 14.9±3.0 | 17.1±2.6 | 14.5±2.6 | 16.5±2.5 |
| EQ-5D Index | 0.68±0.23 | 0.80±0.16 | 0.64±0.19 | 0.79±0.17 |
| EQ-5D VAS | 63.2±21.3 | 74.6±16.4 | 56.8±21.3 | 70.4±15.1 |
| SF-36 Vitality | 40.0±11.3 | 45.9±10.2 | 38.3±8.9 | 43.7±10.4 |
| SF-36 Physical | 44.5±10.9 | 49.5±8.8 | 41.6±11.5 | 49.8±9.4 |
| SF-36 Mental | 42.7±13.6 | 48.7±9.4 | 40.9±12.5 | 45.9±12.0 |
Data are presented as mean±sd. CPAP: continuous positive airway pressure; ESS: Epworth Sleepiness Scale; FSS: Fatigue Severity Score; HADS: Hospital Anxiety and Depression Scale; ISI: Insomnia Sleep Index; FOSQ: Functional Outcomes of Sleep Questionnaire; EQ-5D: EuroQol 5 Dimensions Questionnaire; VAS: visual analogue scale; SF-36: 36-item Short-Form Health Survey.
TABLE 3.
Quality of life changes from baseline (all participants)
| Treatment difference |
Treatment effect
p-value |
Sex effect
p-value |
Treatment-by-sex interaction
p-value |
||
| Females (n=71) | Males (n=162) | ||||
| Decrease in scores indicates improvement | |||||
| ESS | −5.2 (−6.7– −3.6) | −2.0 (−3.0– −1.0) | <0.0001 | 0.38 | 0.001 |
| FSS | −11.9 (−16.2– −7.5) | −7.0 (−10.0– −4.1) | <0.0001 | 0.19 | 0.07 |
| HADS Anxiety | −1.0 (−2.2–0.3) | −0.8 (−1.6–0.0) | 0.020 | 0.45 | 0.79 |
| HADS Depression | −2.4 (−3.6– −1.2) | −1.3 (−2.1– −0.5) | <0.0001 | 0.88 | 0.13 |
| ISI | −5.5 (−7.5– −3.5) | −3.6 (−4.9– −2.3) | <0.0001 | 0.06 | 0.11 |
| Increase in scores indicates improvement | |||||
| FOSQ | 1.9 (1.1–2.7) | 1.1 (0.5–1.6) | <0.0001 | 0.10 | 0.08 |
| EQ-5D Index | 0.05 (−0.02–0.11) | 0.03 (−0.02–0.07) | 0.06 | 0.77 | 0.57 |
| EQ-5D VAS | 6.5 (−0.3–13.3) | 2.7 (−2.1–7.5) | 0.031 | 0.21 | 0.36 |
| SF-36 Vitality | 11.7 (7.9–15.5) | 5.6 (3.1–8.1) | <0.0001 | 0.26 | 0.008 |
| SF-36 Physical | 2.3 (−0.6–5.3) | 1.3 (−0.7–3.2) | 0.045 | 0.19 | 0.56 |
| SF-36 Mental | 5.7 (2.1–9.3) | 4.5 (2.2–6.9) | <0.0001 | 0.14 | 0.59 |
Treatment difference (CPAP−standard care) is presented as adjusted mean (95% CI). Adjusted means and 95% CIs are from an ANCOVA model of change from baseline, adjusting for treatment, sex, baseline score and treatment-by-sex interaction. ESS: Epworth Sleepiness Scale; FSS: Fatigue Severity Score; HADS: Hospital Anxiety and Depression Scale; ISI: Insomnia Sleep Index; FOSQ: Functional Outcomes of Sleep Questionnaire; EQ-5D: EuroQol 5 Dimensions Questionnaire; VAS: visual analogue scale; SF-36: 36-item Short-Form Health Survey.
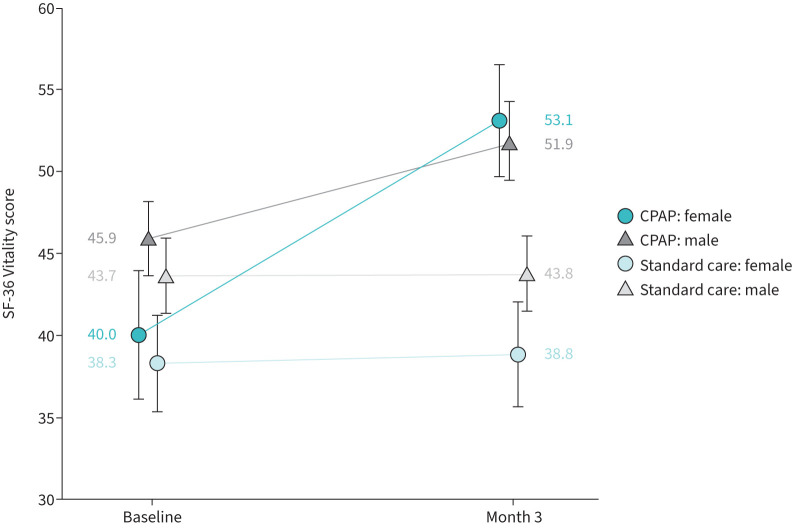
FIGURE 2.
36-item Short-Form Health Survey (SF-36) Vitality scores. Unadjusted mean (95% CI) SF-36 Vitality scores by treatment group and sex, highlighting the difference between females and males in treatment response. An increase in score indicates improvement. Statistical analysis of the continuous positive airway pressure (CPAP) group showed that females had a greater improvement in the SF-36 Vitality score than males (mean change 9.4 (95% CI 6.8–12.0) versus 6.0 (95% CI 4.3–7.7); p=0.0343), after adjustments for baseline SF-36 Vitality score and average daily usage (table 4).
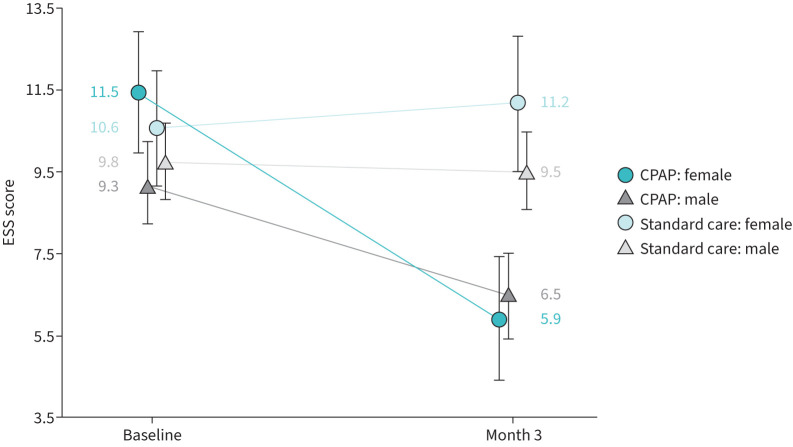
FIGURE 3.
Epworth Sleepiness Scale (ESS) scores. Unadjusted mean (95% CI) ESS scores by treatment group and sex, highlighting the difference between females and males in treatment response. A decrease in score indicates improvement. Statistical analysis of the continuous positive airway pressure (CPAP) group showed that females had a greater reduction in sleepiness than males, as measured by the ESS (mean change −4.1 (95% CI −5.1– −3.0) versus −2.5 (95% CI −3.1– −1.8); p=0.0153), after adjustments for baseline ESS score and average daily usage (table 4).
CPAP participants
All symptoms improved with CPAP use in both sexes; however, females had greater improvements than males (table 4), even after accounting for the differences in baseline scores (table 2) and CPAP usage (table 5). As shown in table 4, females, compared with males, experienced significantly greater increases in SF-36 Vitality (mean change 9.4 (95% CI 6.8–12.0) versus 6.0 (95% CI 4.3–7.7); p=0.034) and greater reductions in sleepiness ESS (mean change −4.1 (95% CI −5.1– −3.0) versus −2.5 (95% CI −3.1– −1.8); p=0.015). While there were sex differences at baseline in BMI and self-reported history of diabetes, as well as baseline QoL scores for insomnia (ISI), anxiety (HADS) and depression (HADS), these were not confounded with the sex effect on change in SF-36 Vitality and ESS scores.
TABLE 4.
Quality of life changes from baseline (continuous positive airway pressure participants)
| Change from baseline | Sex difference | |||
| Females (n=34) | Males (n=81) | Female versus male | p-value | |
| Decrease in scores indicates improvement | ||||
| ESS | −4.1 (−5.1– −3.0) | −2.5 (−3.1– −1.8) | −1.6 (−2.9– −0.3) | 0.015 |
| FSS | −9.1 (−12.3– −6.0) | −5.7 (−7.7– −3.7) | −3.5 (−7.3–0.3) | 0.07 |
| HADS Anxiety | −0.7 (−1.6–0.2) | −0.4 (−0.9–0.2) | −0.3 (−1.4–0.7) | 0.53 |
| HADS Depression | −1.3 (−2.2– −0.4) | −1.0 (−1.6– −0.4) | −0.3 (−1.4–0.8) | 0.58 |
| ISI | −4.9 (−6.2– −3.5) | −3.5 (−4.3– −2.6) | −1.4 (−3.0–0.2) | 0.09 |
| Increase in scores indicates improvement | ||||
| FOSQ | 1.8 (1.2–2.3) | 1.2 (0.8–1.5) | 0.6 (−0.0–1.3) | 0.06 |
| EQ-5D Index | 0.04 (−0.01–0.08) | 0.02 (−0.01–0.05) | 0.01 (−0.04–0.07) | 0.63 |
| EQ-5D VAS | 5.7 (0.8–10.6) | 0.5 (−2.8–3.9) | 5.2 (−0.8–11.2) | 0.09 |
| SF-36 Vitality | 9.4 (6.8–12.0) | 6.0 (4.3–7.7) | 3.4 (0.3–6.5) | 0.034 |
| SF-36 Physical | 0.6 (−1.2–2.4) | 1.1 (−0.0–2.2) | −0.5 (−2.6–1.7) | 0.66 |
| SF-36 Mental | 4.7 (2.2–7.2) | 3.4 (1.9–5.0) | 1.3 (−1.7–4.2) | 0.40 |
Data are presented as adjusted mean (95% CI), unless otherwise stated. Sex difference (females−males) is presented as the difference in adjusted mean change from baseline (95% CI). Adjusted means and 95% CIs are from an ANCOVA model of change from baseline, adjusting for sex, baseline score and average daily continuous positive airway pressure usage (h). Missing scores at month 3 were replaced with baseline scores using a last observation carried forward approach. ESS: Epworth Sleepiness Scale; FSS: Fatigue Severity Score; HADS: Hospital Anxiety and Depression Scale; ISI: Insomnia Sleep Index; FOSQ: Functional Outcomes of Sleep Questionnaire; EQ-5D: EuroQol 5 Dimensions Questionnaire; VAS: visual analogue scale; SF-36: 36-item Short-Form Health Survey. p-value for the test of difference between females and males.
TABLE 5.
Continuous positive airway pressure usage summary
| Females (n=34) | Males (n=81) | p-value | |
| Average usage (h per night) | 4.7 (2.0–6.1) | 3.5 (1.6–5.6) | 0.27 |
| Median pressure (cmH2O) | 7.6 (6.4–9.0) | 7.2 (6.2–8.7) | 0.23 |
| Median mask leak (L·min−1) | 1.0 (0.3–2.5) | 1.8 (0.6–4.7) | 0.11 |
Data are presented as median (interquartile range), unless otherwise stated. p-values from the Wilcoxon rank sum test.
Females required slightly higher median pressures than males, and their median mask leak was slightly lower. CPAP usage was greater in females than males (median (interquartile range) 4.7 (2.0–6.1) versus 3.5 (1.6–5.6) h per night, respectively). The difference in usage was accounted for in the analysis of sex differences within the CPAP group.
Discussion
This analysis of patients with mild OSA found important sex differences in presentation at the sleep clinic and in treatment responses. Females presented with worse subjective symptoms and QoL than males. Treatment with CPAP improved sleepiness, vitality and other QoL measures in both sexes, but to a greater extent in females, even after adjustment for differences in baseline QoL and CPAP usage.
Previous studies have shown that females generally report less daytime sleepiness, and when present, typically report lower levels of sleepiness, compared with males [4]. In the current analysis, females were more symptomatic than males across all QoL questionnaire scores at baseline, including higher levels of sleepiness, for similar objective severity of OSA. However, all patients that presented to sleep clinics had relatively high sleepiness scores compared with population-based studies [11]. It is possible, therefore, that milder OSA patients, and in particular females, may reach a higher symptom burden before seeking clinical help. Alternatively, the high sleepiness levels might represent diagnostic bias, with only those patients with overt sleepiness being referred for sleep assessments; furthermore, this referral bias may differ between males and females.
The ability to differentiate between sleepiness and other symptoms including fatigue and tiredness is challenging for patients and sleep professionals. The ESS questionnaire defines sleepiness as “the likelihood of falling asleep in certain situations”. While easy to quantify, it may not capture other feelings of daytime tiredness. The FSS questionnaire is designed to measure how feelings of fatigue impact an individual's daily life [12]. The FSS was included in the MERGE trial, based on previous reports that females with OSA experience more fatigue than sleepiness [13]. The post hoc analysis showed high levels of fatigue in all participants; however, females were significantly (p<0.001) more fatigued than males at baseline. 73.2% of females had an elevated FSS score, indicating problematic fatigue, compared with 45.1% of males. Additionally, 16.9% of females did not report sleepiness (ESS score <10) but had elevated fatigue (FSS score ≥36). This suggests the need to assess a wide variety of symptoms, and not rely solely on the ESS when determining effects of OSA on daytime functioning.
Snoring was the commonest reason that both females and males sought help. It has been suggested that snoring may be less frequently observed in females [14] or that females may be embarrassed to seek help for snoring [15]. However, in the current analysis 73% of female participants listed snoring as a reason they were referred for further assessment. This finding is consistent with snoring being the strongest and most sensitive predictor of OSA in both sexes [16], and highlights the importance of supporting females to discuss the risk factor of snoring during clinical consultations.
Insomnia frequently accompanies OSA and is particularly prevalent in females [17]. Comorbid insomnia and OSA (COMISA) has recently been associated with increased risk of all-cause mortality [18]. A recent meta-analysis found the prevalence of insomnia to be significantly higher in females than males [19]. However, in the MERGE post hoc analysis only 6% of female participants listed insomnia as a reason for sleep clinic referral. Despite low numbers reporting insomnia as a presenting symptom, the average baseline ISI score was higher in the female group compared with the male group. Because insomnia is such a significant global health problem, participants may have under-reported it, considering it to be “normal”. Another possibility is that some physicians are not aware of the links between insomnia as a symptom of OSA, particularly in females [17, 20], and therefore do not refer patients with insomnia to sleep clinics for further evaluation.
It has previously been shown that OSA is associated with anxiety and depressive symptoms, and that female patients with OSA have a higher incidence of these affective disorders than do males [13], and a higher likelihood of developing them [21]. In the current analysis, females reported significantly worse anxiety and depression than males at baseline; however, on average the group scores were <10 points, which is the recognised clinical threshold for diagnosis. Moreover, it has been reported that depressive symptoms are independently related to AHI [22] and therefore may be less prominent in a mild population. CPAP treatment has been shown to significantly reduce anxiety and depression [22], and in this group of mild OSA patients, anxiety and depressive scores were decreased significantly with CPAP treatment, although there were no significant sex differences in the decrease.
In this analysis, we cannot determine whether the sex differences in the presentation of OSA which were observed reflect biological differences in the nature of, and response to, sleep disruption or social factors leading to differences in the way care is accessed. While these data show that mild OSA female patients can be overtly symptomatic, it remains unanswered whether females wait until they reach a higher threshold before seeking help or whether they perceive and describe symptoms differently, as in other respiratory diseases [23].
Response to treatment
CPAP is the recommended treatment for OSA; however, there is scarce data in females, and although existing data suggest a positive effect of CPAP on sleep, symptoms and mood as well as neurocognitive and cardiovascular outcomes, these results are limited to females with moderate-to-severe OSA [24–26]. In the current analysis, CPAP treatment improved all symptoms for both sexes. Female patients with mild OSA had larger improvements in sleepiness and vitality, even after adjustment for worse baseline scores and greater CPAP usage, compared with males. While baseline conditions such as BMI, diabetes, insomnia, anxiety and depression may be related to sleepiness and vitality, sex remained a strong predictor of response to CPAP treatment.
Limitations
Previous studies have shown that females are able to achieve good long-term compliance to CPAP therapy [27] and that there is a linear dose response to CPAP usage and positive outcomes, with reductions in healthcare utilisation seen with as little as 1–2 h usage per night [28], and improved sleepiness starting at as little as 2.5 h [29], whereas memory and executive function may require at least 6 h per night to see substantial improvements [29–31]. However, this notion is based on data from patients with moderate-to-severe OSA. It is unknown if the same principles can be applied to mild patients. Interestingly, one study found no correlation between usage hours and the normalisation of sleepiness, and also noted that it was more difficult for female patients than male patients with high levels of sleepiness to achieve normalisation [32]. In this post hoc analysis we did not investigate the dose response to CPAP treatment.
In this post hoc analysis the groups were not matched on baseline characteristics. Females were older and had higher BMI than males. Obesity, even without comorbid OSA, is associated with anxiety, depression and sleepiness [33]. It is, therefore, possible that this has contributed to the increased symptoms seen in these female patients. Sleep quality and associated daytime mood are also known to vary during the menstrual cycle, which was not controlled for in this trial.
While this post hoc analysis has shown that females with mild OSA are more symptomatic than males, it does not provide a mechanism. Sex differences have been observed in the sleep architecture of healthy patients, showing that females in general appear to require more sleep, take longer to fall asleep, and have fewer awakenings and more slow wave sleep overall [34, 35]. Additionally, females experience less sleep disturbance from external stimuli than males [34]. The exact mechanisms for these sex differences are not fully understood; however, it is theorised that the hormone progesterone enhances sleep quality, depth and duration [36]. It could therefore be postulated that as arousability levels are higher in females, more sympathetic disruption is required to rouse them from sleep, thus leading to higher daytime symptomatic burden as a result of breathing-related arousals from sleep.
Conclusions
This post hoc analysis supports the existence of sex differences in the characteristics of patients with untreated mild OSA. While moderate-to-severe OSA in female patients has previously been associated with poor QoL [37], these data support the notion that even in mild patients, females report worse QoL symptoms than males, which improve significantly with CPAP treatment.
Footnotes
Provenance: Submitted article, peer reviewed.
MERGE Trial Investigators: Alison J. Wimms, Julia L. Kelly, Christopher D. Turnbull, Alison McMillan, Sonya E. Craig, John F. O'Reilly, Annabel H. Nickol, Sophie D. West, Anita K. Simonds, Shirmila Withana, Justin C. Pepperell, Neil R. Ward, Brian D. Kent, Timothy Quinnell, Mohammad Paracha, William Anderson, Emma L. Hedley, Meredith D. Decker, Leslee A. Willes, Peter M.A. Calverley, Adam V. Benjafield, John R. Stradling and Mary J. Morrell.
This article reports a post hoc analysis of a clinical trial that is registered at ClinicalTrials.gov with identifier number NCT02699463.
Ethics statement: This study was approved by a UK central ethics committee (REC 16/SC/0387) and all patients gave written informed consent.
Conflict of interest: A.J. Wimms is an employee of ResMed.
Conflict of interest: J.L. Kelly has received personal fees from Sunrise, outside the scope of this work.
Conflict of interest: C.D. Turnbull has received personal fees from Stowood, outside the scope of this work.
Conflict of interest: A. McMillan has no conflict of interest.
Conflict of interest: S.E. Craig has no conflict of interest.
Conflict of interest: J.F. O'Reilly has no conflict of interest.
Conflict of interest: A.H. Nickol has no conflict of interest.
Conflict of interest: M.D. Decker confirms that they have no conflicts of interest (presence or absence) that might raise the question of bias in the work reported or the conclusions, implications or opinions stated in this manuscript, with the exception that they are working as an independent paid statistical consultant for ResMed Corporation.
Conflict of interest: L.A. Willes confirms that they have no conflicts of interest (presence or absence) that might raise the question of bias in the work reported or the conclusions, implications or opinions stated in this manuscript, with the exception that they are working as an independent paid statistical consultant for ResMed Corporation.
Conflict of interest: P.M.A. Calverley has no conflict of interest.
Conflict of interest: A.V. Benjafield is an employee of ResMed.
Conflict of interest: J.R. Stradling has received personal fees from ResMed UK, and a grant and personal fees from Bayer during the course of, but unrelated to, this study.
Conflict of interest: M.J. Morrell has no conflict of interest.
Support statement: This study was funded by ResMed Ltd. Funding information for this article has been deposited with the Crossref Funder Registry.
Contributor Information
on behalf of the MERGE Trial Investigators:
J. Wimms Alison, L. Kelly Julia, D. Turnbull Christopher, McMillan Alison, E. Craig Sonya, F. O'Reilly John, H. Nickol Annabel, D. West Sophie, K. Simonds Anita, Withana Shirmila, C. Pepperell Justin, R. Ward Neil, D. Kent Brian, Quinnell Timothy, Paracha Mohammad, Anderson William, L. Hedley Emma, D. Decker Meredith, A. Willes Leslee, M.A. Calverley Peter, V. Benjafield Adam, R. Stradling John, and J. Morrell Mary
References
- 1.Heinzer R, Vat S, Marques-Vidal P, et al. Prevalence of sleep-disordered breathing in the general population: the HypnoLaus study. Lancet Respir Med 2015; 3: 310–318. doi: 10.1016/S2213-2600(15)00043-0 [DOI] [PMC free article] [PubMed] [Google Scholar]
- 2.Valipour A, Lothaller H, Rauscher H, et al. Gender-related differences in symptoms of patients with suspected breathing disorders in sleep: a clinical population study using the sleep disorders questionnaire. Sleep 2007; 30: 312–319. doi: 10.1093/sleep/30.3.312 [DOI] [PubMed] [Google Scholar]
- 3.Bouloukaki I, Mermigkis C, Markakis M, et al. Cardiovascular effect and symptom profile of obstructive sleep apnea: does sex matter? J Clin Sleep Med 2019; 15: 1737–1745. doi: 10.5664/jcsm.8074 [DOI] [PMC free article] [PubMed] [Google Scholar]
- 4.Van Eycken S, Neu D, Newell J, et al. Sex-related differences in sleep-related PSG parameters and daytime complaints in a clinical population. Nat Sci Sleep 2020; 12: 161–171. doi: 10.2147/NSS.S235642 [DOI] [PMC free article] [PubMed] [Google Scholar]
- 5.Ware JC, McBrayer RH, Scott JA. Influence of sex and age on duration and frequency of sleep apnea events. Sleep 2000; 23: 165–170. doi: 10.1093/sleep/23.2.1c [DOI] [PubMed] [Google Scholar]
- 6.Wimms AJ, Kelly JL, Turnbull CD, et al. Continuous positive airway pressure versus standard care for the treatment of people with mild obstructive sleep apnoea (MERGE): a multicentre, randomised controlled trial. Lancet Respir Med 2020; 8: 349–358. doi: 10.1016/S2213-2600(19)30402-3 [DOI] [PubMed] [Google Scholar]
- 7.Jacobowitz O, Afifi L, Penzel T, et al. Endorsement of: “Treatment of adult obstructive sleep apnea with positive airway pressure: an American Academy of Sleep Medicine Clinical Practice Guideline” by World Sleep Society. Sleep Med 2022; 89: 19–22. doi: 10.1016/j.sleep.2021.10.007 [DOI] [PubMed] [Google Scholar]
- 8.National Institute for Health and Care Excellence . Obstructive sleep apnoea/hypopnoea syndrome and obesity hypoventilation syndrome in over 16s. NICE guideline NG202. 2021. www.nice.org.uk/guidance/ng202 Date last accessed: 22 December 2023.
- 9.Berry RB, Abreu AR, Krishnan V, et al. A transition to the American Academy of Sleep Medicine-recommended hypopnea definition in adults: initiatives of the Hypopnea Scoring Rule Task Force. J Clin Sleep Med 2022; 18: 1419–1425. doi: 10.5664/jcsm.9952 [DOI] [PMC free article] [PubMed] [Google Scholar]
- 10.Berry RB, et al. , Purdy S, Kantner G, et al. Validation of a home sleep apnea testing device for the diagnosis of sleep disordered breathing based on AASM 2012 guidelines. Sleep 2019; 42: Suppl. 1, A186. doi: 10.1093/sleep/zsz067.462 [DOI] [Google Scholar]
- 11.Takegami M, Sokejima S, Yamazaki S, et al. [An estimation of the prevalence of excessive daytime sleepiness based on age and sex distribution of Epworth Sleepiness Scale scores: a population based survey]. Nihon Koshu Eisei Zasshi 2005; 52: 137–145. [PubMed] [Google Scholar]
- 12.Krupp LB, LaRocca NG, Muir-Nash J, et al. The Fatigue Severity Scale. Application to patients with multiple sclerosis and systemic lupus erythematosus. Arch Neurol 1989; 46: 1121–1123. doi: 10.1001/archneur.1989.00520460115022 [DOI] [PubMed] [Google Scholar]
- 13.Lin CM, Davidson TM, Ancoli-Israel S. Gender differences in obstructive sleep apnea and treatment implications. Sleep Med Rev 2008; 12: 481–496. doi: 10.1016/j.smrv.2007.11.003 [DOI] [PMC free article] [PubMed] [Google Scholar]
- 14.Redline S, Kump K, Tishler PV, et al. Gender differences in sleep disordered breathing in a community-based sample. Am J Respir Crit Care Med 1994; 149: 722–726. doi: 10.1164/ajrccm.149.3.8118642 [DOI] [PubMed] [Google Scholar]
- 15.Wimms A, Woehrle H, Ketheeswaran S, et al. Obstructive sleep apnea in women: specific issues and interventions. Biomed Res Int 2016; 2016: 1764837. doi: 10.1155/2016/1764837 [DOI] [PMC free article] [PubMed] [Google Scholar]
- 16.Young T, Hutton R, Finn L, et al. The gender bias in sleep apnea diagnosis. Are women missed because they have different symptoms? Arch Intern Med 1996; 156: 2445–2451. doi: 10.1001/archinte.1996.00440200055007 [DOI] [PubMed] [Google Scholar]
- 17.Ragnoli B, Pochetti P, Raie A, et al. Comorbid insomnia and obstructive sleep apnea (COMISA): current concepts of patient management. Int J Environ Res Public Health 2021; 18: 9248. doi: 10.3390/ijerph18179248 [DOI] [PMC free article] [PubMed] [Google Scholar]
- 18.Lechat B, Appleton S, Melaku YA, et al. Comorbid insomnia and sleep apnoea is associated with all-cause mortality. Eur Respir J 2022; 60: 2101958. doi: 10.1183/13993003.01958-2021 [DOI] [PubMed] [Google Scholar]
- 19.Zeng LN, Zong QQ, Yang Y, et al. Gender difference in the prevalence of insomnia: a meta-analysis of observational studies. Front Psychiatry 2020; 11: 577429. doi: 10.3389/fpsyt.2020.577429 [DOI] [PMC free article] [PubMed] [Google Scholar]
- 20.Bonsignore MR, Saaresranta T, Riha RL. Sex differences in obstructive sleep apnoea. Eur Respir Rev 2019; 28: 190030. doi: 10.1183/16000617.0030-2019 [DOI] [PMC free article] [PubMed] [Google Scholar]
- 21.Kim JY, Ko I, Kim DK. Association of obstructive sleep apnea with the risk of affective disorders. JAMA Otolaryngol Head Neck Surg 2019; 145: 1020–1026. doi: 10.1001/jamaoto.2019.2435 [DOI] [PMC free article] [PubMed] [Google Scholar]
- 22.Edwards C, Mukherjee S, Simpson L, et al. Depressive symptoms before and after treatment of obstructive sleep apnea in men and women. J Clin Sleep Med 2015; 11: 1029–1038. doi: 10.5664/jcsm.5020 [DOI] [PMC free article] [PubMed] [Google Scholar]
- 23.Celli B, Vestbo J, Jenkins CR, et al. Sex differences in mortality and clinical expressions of patients with chronic obstructive pulmonary disease. The TORCH experience. Am J Respir Crit Care Med 2011; 183: 317–322. doi: 10.1164/rccm.201004-0665OC [DOI] [PubMed] [Google Scholar]
- 24.Campos-Rodriguez F, Martinez-Garcia M, Cruz-Moron I, et al. Cardiovascular mortality in women with OSA with or without CPAP treatment – a cohort study. Ann Intern Med 2012; 156: 115–122. [DOI] [PubMed] [Google Scholar]
- 25.Campos-Rodriguez F, Queipo-Corona C, Carmona-Bernal C, et al. Continuous positive airway pressure improves quality of life in women with OSA. A randomized-controlled trial. Am J Respir Crit Care Med 2016; 194: 1286–1294. doi: 10.1164/rccm.201602-0265OC [DOI] [PubMed] [Google Scholar]
- 26.Ye L, Pien G, Ratcliffe S, et al. Gender differences in obstructive sleep apnea and treatment responses to continuous positive airway pressure. J Clin Sleep Med 2009; 5: 512–518. doi: 10.5664/jcsm.27650 [DOI] [PMC free article] [PubMed] [Google Scholar]
- 27.Campos-Rodriguez F, Martinez-Garcia MA, Reyes-Nuñez N, et al. Long-term CPAP compliance in females with obstructive sleep apnoea. Eur Respir J 2013; 42: 1255–1262. doi: 10.1183/09031936.00165812 [DOI] [PubMed] [Google Scholar]
- 28.Malhotra A, Sterling KL, Cistulli PA, et al. Dose-response relationship between obstructive sleep apnea therapy adherence and healthcare utilization. Ann Am Thorac Soc 2023; 20: 891–897. doi: 10.1513/AnnalsATS.202208-738OC [DOI] [PMC free article] [PubMed] [Google Scholar]
- 29.Barnes M, Houston D, Worsnop CJ, et al. A randomized controlled trial of continuous positive airway pressure in mild obstructive sleep apnea. Am J Respir Crit Care Med 2002; 165: 773–780. doi: 10.1164/ajrccm.165.6.2003166 [DOI] [PubMed] [Google Scholar]
- 30.Antic NA, Catcheside P, Buchan C, et al. The effect of CPAP in normalizing daytime sleepiness, quality of life, and neurocognitive function in patients with moderate to severe OSA. Sleep 2011; 34: 111–119. doi: 10.1093/sleep/34.1.111 [DOI] [PMC free article] [PubMed] [Google Scholar]
- 31.Weaver TE, Maislin G, Dinges DF, et al. Relationship between hours of CPAP use and achieving normal levels of sleepiness and daily functioning. Sleep 2007; 30: 711–719. doi: 10.1093/sleep/30.6.711 [DOI] [PMC free article] [PubMed] [Google Scholar]
- 32.Scharf MT, Zhang P, Walker NA, et al. Sex differences in Epworth Sleepiness Scale normalization with continuous positive airway pressure. J Clin Sleep Med 2022; 18: 2273–2279. doi: 10.5664/jcsm.10048 [DOI] [PMC free article] [PubMed] [Google Scholar]
- 33.Araghi MH, Jagielski A, Neira I, et al. The complex associations among sleep quality, anxiety-depression, and quality of life in patients with extreme obesity. Sleep 2013; 36: 1859–1865. doi: 10.5665/sleep.3216 [DOI] [PMC free article] [PubMed] [Google Scholar]
- 34.Bixler EO, Papaliaga MN, Vgontzas AN, et al. Women sleep objectively better than men and the sleep of young women is more resilient to external stressors: effects of age and menopause. J Sleep Res 2009; 18: 221–228. doi: 10.1111/j.1365-2869.2008.00713.x [DOI] [PMC free article] [PubMed] [Google Scholar]
- 35.Valencia-Flores M, Bliwise DL, Guilleminault C, et al. Gender differences in sleep architecture in sleep apnoea syndrome. J Sleep Res 1992; 1: 51–53. doi: 10.1111/j.1365-2869.1992.tb00009.x [DOI] [PubMed] [Google Scholar]
- 36.Pengo MF, Won CH, Bourjeily G. Sleep in women across the life span. Chest 2018; 154: 196–206. doi: 10.1016/j.chest.2018.04.005 [DOI] [PMC free article] [PubMed] [Google Scholar]
- 37.Wimms A, Woehrle H, Topfer V, et al. Improvements in quality of life in female obstructive sleep apnea patients using a gender specific positive airway pressure device. J Sleep Disor Treat Care 2020; 9: 1. [Google Scholar]