Abstract
Background
Management guidelines for obesity suggest maintaining a minimum of 5% body weight reduction to help prevent or lower the risk of developing conditions such as hypertension and type 2 diabetes. However, achieving long‐term weight control is difficult with lifestyle modification alone, making it essential to combine pharmacotherapy with diet and exercise in individual cases. Semaglutide 2.4 mg has demonstrated significant reductions in body weight and cardiometabolic risk factors in clinical trials, but information on outcomes in a real‐world setting is limited.
Objective
To assess changes in body weight and other clinical outcomes at 6‐month follow‐up among adults on semaglutide 2.4 mg in a real‐world setting in the United States (US).
Methods
Observational and retrospective cohort study of patients initiating treatment between 15 June 2021, and 31 March 2022, using a large US claims‐linked electronic health record database.
Results
Mean (±SD) body mass index (BMI) of the 343 patients included in the analysis was 37.9 ± 5.5 kg/m2. After 6 months, mean body weight change was −10.5 ± 6.8 kg (95% CI: −11.2; −9.8, p < 0.001) and mean percentage body weight change was −10.0% ± 6.6% (95% CI: −10.7; −9.3, p < 0.001). Most (79.0%) patients had ≥5% body weight reduction, 48.1% had ≥10% body weight reduction, and 19.0% had ≥15% body weight reduction. Among patients with available data, the mean change in HbA1c (n = 30) was −0.6% ± 1.2% (95% CI: −1.0; −0.1, p = 0.016) and nearly two‐thirds of patients with prediabetes or diabetes at baseline reverted to normoglycemia. Mean reductions of −4.4 ± 12.3 mmHg (95% CI: −5.7; −3.0, p < 0.001) and −1.7 ± 8.4 mmHg (95% CI: −2.6; −0.7, p < 0.001) were observed in systolic and diastolic blood pressure, respectively (n = 307). Statistically significant reductions in mean total cholesterol (−12.2 ± 38.8 mg/dl [95% CI: −24.3 to −0.06, p < 0.049]) and triglycerides (−18.3 ± 43.6 mg/dl [95% CI: −4.7; −31.9, p < 0.009]) were also observed (n = 42).
Conclusions
This study demonstrated the effectiveness of semaglutide 2.4 mg in reducing body weight and improving cardiometabolic parameters in adults with overweight or obesity in a real‐world clinical practice setting, showing a significant mean body weight reduction and improvements in biomarkers like blood pressure and HbA1c over a 6‐month period. These findings, aligning with previous clinical trials at comparable time points, highlight the clinical relevance of semaglutide as an effective therapeutic option for obesity.
Keywords: anti‐obesity agents, body mass index, evidence‐based practice, obesity, weight loss
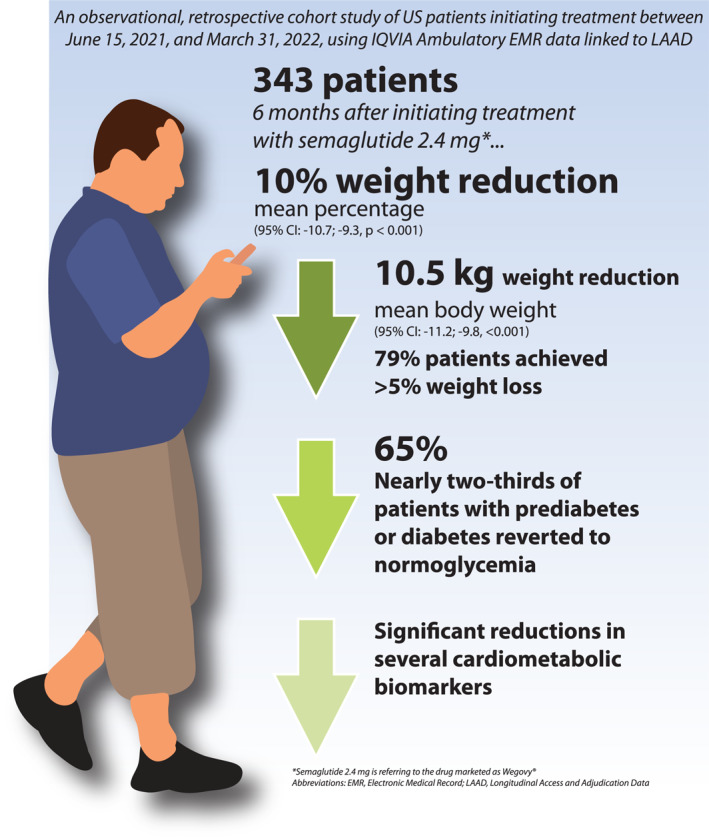
Changes in body weight and cardiometabolic biomarkers were evaluated at 6‐month after initiating treatment with semaglutide 2.4 mg in a real‐world setting in the United States using data from the IQVIA Ambulatory Electronic Medical Record linked to Longitudinal Access and Adjudication Data. The study found that a 6‐month use of semaglutide 2.4 mg resulted in a 10% decrease in weight and a reduction of 10.5 kg in adult participants with overweight/obesity. Nearly two‐thirds of patients with prediabetes or diabetes reverted to normoglycemia, and statistically significant reductions in total cholesterol and triglycerides were also observed.
1. INTRODUCTION
Consensus‐based guidelines for the management of obesity recommend targeting sustained body weight reduction of at least 5% body weight. 1 , 2 Reductions of this magnitude have been linked to delaying the onset of, or reducing the likelihood of developing, a variety of conditions, including hypertension, hyperlipidemia, type 2 diabetes (T2D), and osteoarthritis. 3 , 4 However, it is difficult to successfully manage long‐term obesity with lifestyle changes alone. 5 , 6 , 7
Multiple evidence‐ and consensus‐based clinical guidelines for the treatment of obesity recommend the use of pharmacotherapy in combination with diet and exercise in patients with body mass index (BMI) ≥ 30 kg/m2 or a BMI of ≥27 kg/m2 and at least one weight‐related comorbidity. 1 , 2 Anti‐obesity medications (AOMs) in conjunction with lifestyle modification have been shown to be significantly more effective for chronic weight management in this population than lifestyle modification alone. 8 , 9 , 10 To date, however, few such pharmacological interventions are available for patients with obesity, and accessibility remains a challenge, particularly due to limited health insurance coverage. 11 , 12 , 13 Semaglutide is a glucagon‐like peptide‐1 receptor agonist (GLP‐1 RA) that can be administered subcutaneously once weekly. Semaglutide 2.4 mg was approved by the United States (US) Food and Drug Administration (FDA) in June 2021 for chronic weight management in adults with obesity or overweight who have at least one weight‐related condition (e.g., hypertension, dyslipidemia, or T2D). 14 In this paper, the drug marketed as Wegovy® was referred to as “semaglutide 2.4 mg” to differentiate it from other brands of semaglutide (i.e., Ozempic®, Rybelsus®) that have varying dosages, indications, and/or routes of administration.
Reduction in body weight was demonstrated in the Semaglutide Treatment Effect in People with Obesity (STEP) clinical trials. 15 , 16 In the STEP 1 study, patients assigned to treatment with semaglutide 2.4 mg achieved approximately 15% reduction in body weight at 68 weeks 16 During the course of treatment, mean body weight reduction was approximately 6% at 3 months and approximately 12% at 7 months 16 In a recent retrospective cohort study from a single health system, Ghusn et al. observed an overall body weight reduction of 10.9% at 6 months among patients taking semaglutide 2.4 mg, 17 demonstrating real‐world effectiveness that was comparable to changes in body weight reported in clinical trials. 15 , 16
The present study seeks to expand the real‐world evidence base for semaglutide 2.4 mg by examining its effectiveness for chronic weight management in a larger population across diverse practice settings and multiple health systems. In addition to changes in body weight, changes in BMI and cardiometabolic biomarkers (where available) were reported over the course of a 6‐month follow‐up period among US adult patients who escalated to the maintenance dose of semaglutide at 2.4 mg per FDA label during the study time.
2. MATERIALS AND METHODS
2.1. Study design and patient population
This was an observational, retrospective cohort study using IQVIA Ambulatory Electronic Medical Record (AEMR) data 18 linked to Longitudinal Access and Adjudication Data (LAAD) 19 in the US. The AEMR database comprises approximately 75 million US patient records that are sourced from an ‘opt‐in’ provider research network and includes key demographic and clinical variables such as age, sex, race/ethnicity, height, body weight, BMI, risk factors, laboratory tests, diagnoses, prescription drugs prescribed or administered, procedures performed, and patient care encounters (i.e., health care visits, appointments, correspondence). The aggregated database comprises records collected across 40,000 physicians from large practices and physician networks across the US. Approximately 50% of the contributing physicians were primary care practitioners and the remaining were specialists. The records were available starting in 2006 and were updated monthly. The LAAD database captures information on dispensed prescriptions sourced from retail, mail, long‐term care, and specialty pharmacies, as well as information on medical claims, including patient diagnoses and procedures. Information was provided on a daily or weekly basis and represented more than 90% of pharmacy claims (including more than 70% of mail order claims) and up to 60% of medical claim coverage.
Patients 18 years of age or older with obesity or overweight with at least one weight‐related condition (e.g., hypertension, dyslipidemia, or T2D), who started semaglutide 2.4 mg for chronic weight management between 15 June 2021, and 31 March 2022, were identified. The date of 15 June 2021 was chosen as the start date of the patient identification period as this was when semaglutide 2.4 mg became available in the US market. The date at the first prescription fill (claim) of any dose less than 2.4 mg of the drug (0.25, 0.5, 1.0, or 1.7 mg) was designated as the index date. Patients who started the drug at the 2.4 mg dose were excluded as these patients may have had prior exposure to a GLP‐1 RA (unable to capture and verify using the database). Thus, only patients who initiated at a dose lower than 2.4 mg and escalated to the 2.4 mg dose at any point during the follow‐up period were included in the analysis. Data 6 months prior to the index date (baseline period) and 6 months post‐index date (follow‐up period) were examined for a total study period from 15 December 2020 to 30 September 2022 (Figure 1).
FIGURE 1.
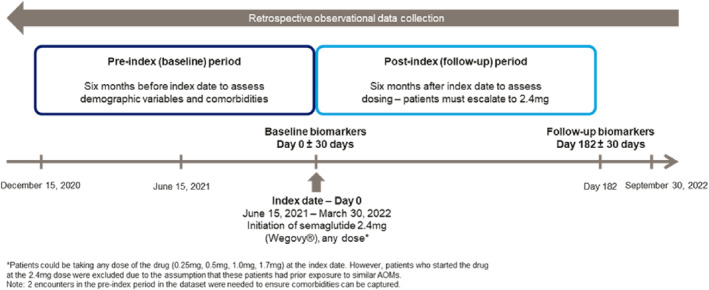
Study design. *Patients could be taking any dose of the drug (0.25, 0.5, 1.0, 1.7 mg) at the index date. However, patients who started the drug at the 2.4 mg dose were excluded due to the assumption that these patients had prior exposure to similar AOMs. 2 encounters in the pre‐index period in the dataset were needed to ensure comorbidities can be captured.
Eligible patients also had to have available body weight and/or BMI values in the data at the index date ±30 days and at the end of study follow‐up (182 days post the index date ±30 days). Additionally, patients had to have had at least one health care encounter (defined as a health care office visit, telehealth appointment, virtual consult, etc.) in the baseline period in the data; this encounter served as a proxy for continuous enrollment in the database and allowed for assessment of baseline comorbidities.
Patients were excluded if there was a history of bariatric surgery or use of a branded AOM that was approved by the FDA for chronic use (phentermine/topiramate [Qsymia®], bupropion/naltrexone [Contrave®], liraglutide [Saxenda®] or orlistat [Xenical®]) or other GLP‐1 RAs (liraglutide [Victoza®], dulaglutide [Trulicity®], exenatide [Byetta®, Bydureon®, Bydureon BCise®] or lixisenatide [Adlyxin®]) during the baseline period. Patients with normal body weight BMI (<25 kg/m2) were excluded from the analysis. Patients with evidence of pregnancy at any point during the baseline or follow‐up periods were also excluded. Additional exclusion criteria included any personal or family history of medullary thyroid carcinoma or multiple endocrine neoplasia syndrome type 2 in the baseline period.
As this study was a retrospective analysis of deidentified patient medical and prescription records, institutional review board approval and patient informed consent were not required. This study was carried out in accordance with relevant guidelines and regulations including the Declaration of Helsinki. This study followed the Strengthening the Reporting of OBservational Studies in Epidemiology (STROBE) guidelines regarding the conduct and reporting of observational studies.
2.2. Variables and outcomes
Baseline demographic characteristics (age, age group, sex, race/ethnicity, payer type, geographic region) as of the index date (month and year) were captured. Clinical characteristics captured included the index dose of semaglutide, comorbidities of interest identified through diagnostic codes (International Classification of Diseases, Tenth Revision, Clinical Modification [ICD‐10‐CM]), 20 Charlson Comorbidity Index (CCI), 21 and cardiometabolic biomarkers (total cholesterol [TC], low‐density lipo‐protein cholesterol [LDL‐C], high‐density lipoprotein cholesterol [HDL‐C], glycated hemoglobin [HbA1c] and blood pressure [BP]). Comorbidities were assessed in the full 182‐day baseline period and included the index and cardiometabolic‐related outcomes were assessed for the overall patient cohort at the baseline by taking the value closest [absolute] to the index date and again after six months of follow‐up (182 days post‐index ± 30 days, taking the value closest to day 182). Changes in body weight (kg and % of initial body weight) and BMI from baseline to the end of the 6‐month follow‐up period were calculated. Reductions in baseline body weight of ≥5%, ≥10%, and ≥15% were also evaluated categorically. Changes in cardiometabolic markers (if available for both baseline and follow‐up for a given patient) were calculated; these included systolic blood pressure (SBP), diastolic blood pressure (DBP), HbA1c, and lipids (TC, LDL‐C, HDL‐C, triglycerides). The change in glycemic category was determined for patients with both pre‐ and post‐index HbA1c values. Patients were considered to have normoglycemia, prediabetes, or diabetes with HbA1c levels <5.7%, 5.7%–6.4%, and ≥6.5%, respectively. 22 This classification was determined independently of concomitant anti‐hyperglycemic medication use. For all variables except BP, changes were evaluated by subtracting the baseline value from the post‐index value. Because BP may fluctuate day to day, if more than one BP value was available within the respective baseline and follow‐up periods, the mean value of 0±30 days and mean of 182±30 days were calculated separately, and the change was calculated as the difference in means.
Baseline and post‐index body weight and/or BMI were required for all patients. The baseline BMI value was used to determine the baseline BMI category. Patient subgroups were defined by baseline BMI categories: overweight (BMI 25.0 to 29.9 kg/m2), obesity class 1 (BMI 30.0–34.9 kg/m2), obesity class 2 (BMI 35.0 to 39.9 kg/m2); and obesity class 3 (BMI ≥40 kg/m2).
2.3. Statistical analysis
Descriptive statistics were reported for all study measures for the overall cohort, and for the subgroups of interest. Continuous and count variables were presented using mean, standard deviation (SD), median, interquartile range (IQR), and minimum and maximum values. Continuous variables were categorized into intervals as relevant. Categorical measures were presented using frequency and percentage of study patients observed in each category. Outcomes were compared between the 6‐month baseline period and the 6‐month follow‐up period within the overall cohort or subgroup. Dependent within‐group comparisons were examined using appropriate statistical testing: paired t‐tests were conducted on the means, Wilcoxon signed‐rank tests on the medians (if the assumptions of a t‐test were violated) for continuous variables, and McNemar's test for categorical variables. A p‐value of <0.05 was considered statistically significant.
3. RESULTS
3.1. Baseline characteristics of the study population
A total of 343 patients who met all study eligibility criteria were identified (Figure 2). The mean age (±SD) of the study population was 48.0 (±10.5) years. Most of the study cohort was female (85%), White (74%), from the Southern region of the US (67%) and had commercial health insurance coverage (87%). Mean (±SD) BMI was 37.9 (±5.5) kg/m2 and mean (±SD) body weight was 106.8 (±20.7 kg). Approximately two‐thirds of the study population had class 2 (28%) or class 3 obesity (39%). An index dose of 0.25 mg of semaglutide was reported for 63% of the cohort. Hypertension and dyslipidemia were the most common obesity‐related comorbidities among the patient cohort (44% and 42%, respectively). Baseline demographic and clinical characteristics are shown in Tables 1 and 2, respectively.
FIGURE 2.
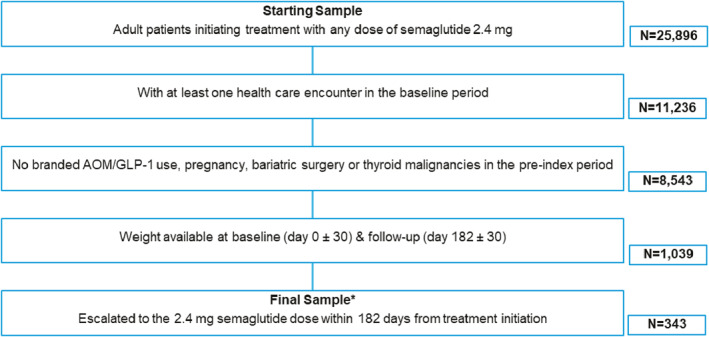
Study flow diagram. *Patients who started the drug at the 2.4 mg dose were excluded. AOM, anti‐obesity medication.
TABLE 1.
Baseline demographic characteristics.
| Total (N = 343) | |
|---|---|
| Age (years), mean ± SD (range) | 48.0 ± 10.5 (20.0–73.0) |
| Age group, n (%) | |
| 18–34 | 32 (9.3%) |
| 35–44 | 78 (22.7%) |
| 45–54 | 122 (35.6%) |
| 55–64 | 94 (27.4%) |
| ≥65 | 17 (5.0%) |
| Sex, n (%) | |
| Female | 292 (85.1%) |
| Male | 51 (14.9%) |
| Race, n (%) | |
| White | 253 (73.8%) |
| Black or African American | 44 (12.8%) |
| Other | 9 (2.6%) |
| Unknown | 37 (10.8%) |
| Region, n (%) | |
| South | 230 (67.1%) |
| Midwest | 65 (19.0%) |
| Northeast | 20 (5.8%) |
| West | 28 (8.2%) |
| Payer type, n (%) | |
| Commercial | 297 (86.6%) |
| Cash | 37 (10.8%) |
| Other | 9 (2.6%) |
TABLE 2.
Baseline clinical characteristics.
| N | Total | |
|---|---|---|
| Semaglutide index dose, n (%) | 343 | |
| 0.25 mg | 215 (62.7%) | |
| 0.5 mg | 89 (25.9%) | |
| 1.0 mg | 26 (7.6%) | |
| 1.7 mg | 13 (3.8%) | |
| BMI (kg/m2), mean ± SD | 343 | 37.9 ± 5.5 |
| BMI category, n (%) | 343 | |
| Overweight (25.0 to 29.9 kg/m2) | 35 (10.2%) | |
| Obesity class 1 (30.0–34.9 kg/m2) | 77 (22.4%) | |
| Obesity class 2 (35.0 to 39.9 kg/m2) | 97 (28.3%) | |
| Obesity class 3 (BMI ≥40 kg/m2) | 134 (39.1%) | |
| Body weight (kg), mean ± SD | 343 | 106.8 ± 20.7 |
| Systolic BP at baseline (mmHg), mean ± SD | 307 | 126.4 ± 11.5 |
| Diastolic BP at baseline (mmHg), mean ± SD | 307 | 79.4 ± 7.3 |
| HbA1c at baseline (%), mean ± SD | 30 | 6.1 ± 1.2 |
| Total cholesterol at baseline (mg/dL), mean ± SD | 42 | 184.2 ± 42.7 |
| LDL‐C [mg/dL], mean ± SD | 44 | 107.0 ± 43.1 |
| HDL‐C [mg/dL], mean ± SD | 33 | 49.0 ± 11.6 |
| Triglycerides at baseline [mg/dL], mean ± SD | 42 | 119.0 ± 66.6 |
| Charlson comorbidity index, mean ± SD | 343 | 0.4 ± 0.8 |
| Obesity‐related comorbidities, n (%) | ||
| Hypertension | 343 | 152 (44.3%) |
| Dyslipidemia | 343 | 144 (42.0%) |
| Musculoskeletal pain | 343 | 125 (36.4%) |
| Prediabetes | 343 | 79 (23.0%) |
| GERD | 343 | 75 (21.9%) |
| Obstructive/mixed sleep apnea | 343 | 66 (19.2%) |
| Asthma | 343 | 40 (11.7%) |
| Depression | 343 | 34 (9.9%) |
| Type 2 diabetes | 343 | 26 (7.6%) |
| Knee osteoarthritis | 343 | 17 (5.0%) |
| Psoriasis | 343 | 9 (2.6%) |
| HFpEF | 343 | 2 (0.6%) |
| PCOS | 292 a | 22 (7.5%) |
| Urinary incontinence | 292 a | 6 (2.1%) |
Abbreviations: BMI, body mass index; BP, blood pressure; GERD, gastroesophageal reflux disease; HbA1c, glycated hemoglobin; HDL, high‐density lipoprotein cholesterol; HFpEF, heart failure with preserved ejection fraction; LDL‐C, low‐density lipoprotein cholesterol; PCOS, polycystic ovary syndrome.
Reported proportion of female patients only.
3.2. Body weight and BMI outcomes
In the total study population, the mean (±SD) change in body weight was −10.5 (±6.8) kg (Table 3) at the 6‐month follow‐up, representing an average (±SD) change of −10.0% (±6.6%) (95% CI: −10.7; −9.3, p < 0.001) from baseline body weight. A total of 79% of the patients reduced body weight by ≥ 5%, 48% by ≥ 10%, and 19% by ≥ 15% at the 6‐month follow‐up (Figure 3). The mean (±SD) change in BMI was −3.7 ± 2.4 kg/m2 (Table 3). Absolute (kg) and relative (%) changes in body weight by BMI classification are presented in Table 4. Mean (±SD) body weight change across baseline BMI categories ranged from −8.5 (±6.5) kg for patients with overweight to −11.3 (±7.3) kg for patients with class 3 obesity. Close to a quarter of patients with a BMI <40 kg/m2 achieved ≥15% body weight reduction compared to that of 10% for patients with BMI ≥40 kg/m2 at baseline.
TABLE 3.
Changes in body weight and BMI measures between baseline and 6‐month follow‐up.
| Body weight and BMI measures | N | Mean baseline measure ± SD | Mean 6‐month follow‐up measure ± SD | Mean change ± SD (baseline to 6‐month follow‐up measure) | 95% CI | p‐value a |
|---|---|---|---|---|---|---|
| Body weight (kg) | 343 | 106.8 ± 20.7 | 96.3 ± 21.0 | −10.5 ± 6.8 | −11.2; −9.8 | <0.001 |
| BMI (kg/m2) b | 321 c | 37.9 ± 5.5 | 34.4 ± 6.2 | −3.7 ± 2.4 d | −4.0; −3.4 | <0.001 |
Note: The bold signifies the difference between the mean baseline measure and the mean 6‐month follow‐up measure.
Abbreviations: BMI, body mass index; CI, confidence interval.
Statistical significance of change from baseline to the 6‐month follow‐up.
Change in BMI was not calculated for patients with baseline and follow‐up BMI measures of 45 or greater.
BMI at baseline, N = 343.
Mean change in BMI does not equal to 3.7 kg/m2 because the change was calculated for only 321 out of 343 patients.
FIGURE 3.
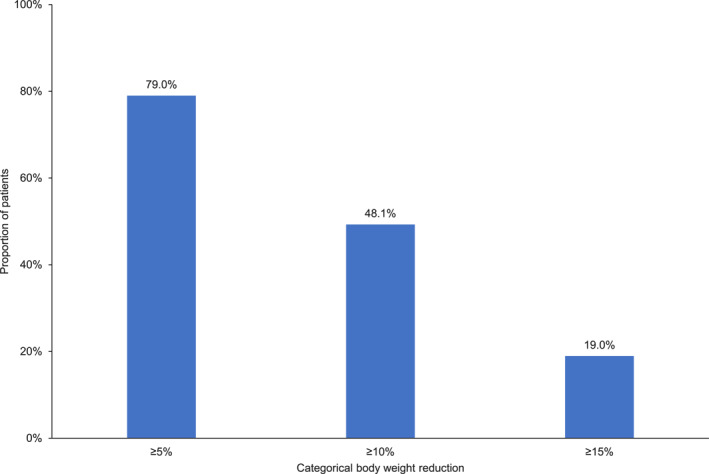
Proportion of patients reaching body weight reduction thresholds at 6‐month follow‐up (change from baseline).
TABLE 4.
Changes in body weight and BMI measures between baseline and 6‐month follow‐up, by BMI category.
| Body weight and BMI measures | Overweight (n = 35) | Obesity class 1 (n = 77) | Obesity class 2 (n = 97) | Obesity class 3 (n = 134) |
|---|---|---|---|---|
| Change in body weight (kg), mean ± SD | −8.5 ± 6.5 | −10.3 ± 5.9 | −10.3 ± 6.9 | −11.3 ± 7.3 |
| Change in body weight (%), mean ± SD | −10.4 ± 7.6 | −11.6 ± 6.7 | −9.9 ± 6.6 | −9.2 ± 6.0 |
| Percentage body weight reduction, n (%) | ||||
| ≥5% | 25 (71.4%) | 66 (85.7%) | 79 (81.4%) | 101 (75.4%) |
| ≥10% | 22 (62.9%) | 44 (57.1%) | 43 (44.3%) | 56 (41.8%) |
| ≥15% | 9 (25.7%) | 19 (24.7%) | 23 (23.7%) | 14 (10.4%) |
Note: Overweight: BMI 25.0 to 29.9 kg/m2, obesity class 1: BMI 30.0–34.9 kg/m2, obesity class 2: BMI 35.0 to 39.9 kg/m2, obesity class 3: BMI ≥40 kg/m2.
Abbreviation: BMI, body mass index.
3.3. Cardiometabolic outcomes
For the 307 patients with available baseline and follow‐up BP values, statistically significant differences were observed. The mean (±SD) changes in SBP and DBP were −4.4 (±12.3) (p < 0.001) mmHg and −1.7 (±8.4) (p < 0.001) mmHg, respectively (Table 5). Patients with overweight (n = 35) experienced a greater change in mean (±SD) SBP than patients who had class 1 (n = 62), class 2 (n = 92), or class 3 (n = 118) obesity (−6.4 ± 10.8 vs. −4.0 ± 13.4, −5.0 ± 12.1, and −3.5 ± 12.4, respectively). DBP changes were more pronounced in patients with class 1 or class 2 obesity with mean (±SD) changes from baseline to follow‐up of −2.6 (±8.3) and −2.6 (±8.8), respectively, compared with patients with overweight −0.9 (±7.9) and patients with class 3 obesity −0.8 (±8.0). Statistically significant changes in TC (−12.2 ± 38.8 mg/dl) and triglycerides (−18.3 ± 43.6 mg/dl) were found among patients with available baseline and follow‐up lipid measures. Changes in mean (±SD) LDL‐C −5.6 mg/dl (±39.5) and mean (±SD) HDL‐C 0.2 mg/dl (±6.9) were not statistically significant (Table 5). Among the 30 patients with available baseline and follow‐up HbA1c data, the mean (±SD) change was −0.6% (±1.2%) (p = 0.016) (Table 5). At the end of the 6‐month follow‐up period, nearly two‐thirds of patients with prediabetes or diabetes at baseline reverted to normoglycemia, specifically 57.1% patients with diabetes and 70% patients with prediabetes at baseline had normoglycemia at 6‐month follow‐up (Figure 4). All patients with normoglycemia remained in normoglycemia at the 6‐month follow‐up.
TABLE 5.
Changes in cardiometabolic measures between baseline and 6‐month follow‐up.
| Cardiometabolic measure | N | Mean baseline measure ± SD | Mean 6‐month follow‐up measure ± SD | Mean change ± SD (baseline to 6‐month follow‐up measure) | 95% CI | p‐value a |
|---|---|---|---|---|---|---|
| Systolic BP (mmHg) | 307 | 126.4 ± 11.5 | 122.0 ± 11.0 | −4.4 ± 12.3 | −5.7; −3.0 | <0.001 |
| Diastolic BP (mmHg) | 307 | 79.4 ± 7.3 | 77.7 ± 7.2 | −1.7 ± 8.4 | −2.6; −0.7 | <0.001 |
| HbA1c (%) | 30 | 6.1 ± 1.2 | 5.6 ± 1.1 | −0.6 ± 1.2 | −1.0; −0.1 | 0.016 |
| Total cholesterol (mg/dl) | 42 | 184.2 ± 42.7 | 172.0 ± 45.5 | −12.2 ± 38.8 | −24.3; −0.06 | 0.049 |
| LDL‐C (mg/dl) | 44 | 107.0 ± 43.1 | 101.5 ± 37.8 | −5.6 ± 39.5 | −17.6; 6.4 | 0.355 |
| HDL‐C (mg/dl) | 33 | 49.0 ± 11.6 | 49.3 ± 12.0 | 0.2 ± 6.9 | −2.2; 2.7 | 0.844 |
| Triglycerides (mg/dl) | 42 | 119.0 ± 66.6 | 100.7 ± 52.3 | −18.3 ± 43.6 | 31.9; −4.7 | 0.009 |
Note: The bold signifies the difference between the mean baseline measure and the mean 6‐month follow‐up measure.
Abbreviations: BMI, body mass index; BP, blood pressure; CI, confidence interval; HbA1c, glycated hemoglobin; HDL, high‐density lipoprotein cholesterol; LDL‐C, low‐density lipoprotein cholesterol.
Statistical significance of change from baseline to the 6‐month follow‐up.
FIGURE 4.
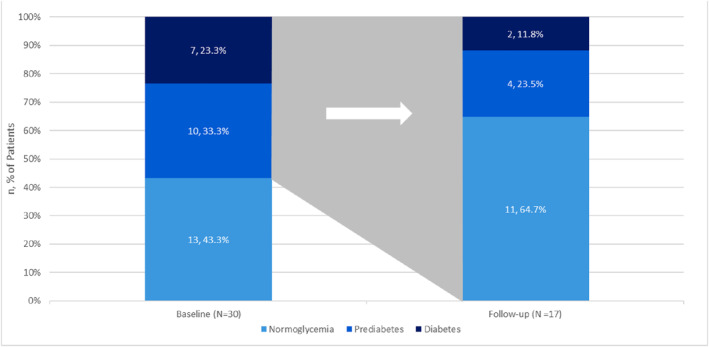
The glycemic status change among proportions of patients with prediabetes or type 2 diabetes from baseline to 6 months follow‐up. The change in glycemic category was determined for patients with both pre‐ and post‐index glycated hemoglobin (HbA1c) values (N = 30). Patients were considered to have normoglycemia, prediabetes, or diabetes with HbA1c levels <5.7%, 5.7%–6.4%, and ≥6.5%, respectively. All patients with normoglycemia at baseline (N = 13) remained in normoglycemia at the 6‐month follow‐up.
4. DISCUSSION
Randomized placebo‐controlled trials, required to establish the safety and efficacy of new therapies, are conducted under controlled conditions and involve selected populations, which may not fully reflect real‐world clinical practice. The present analysis corroborates evidence from clinical trials and demonstrates the real‐world effectiveness of semaglutide 2.4 mg for reducing body weight and improving some cardiometabolic parameters in adults with overweight or obesity over 6 months. This study found that, in the real‐world, patients treated with semaglutide 2.4 mg had a mean change of −10.5 kg or −10% of body weight from baseline to the end of the 6‐month follow‐up period, corresponding to a BMI reduction of −3.7 kg/m2. After the 6‐month follow‐up period, nearly 8 in 10 patients reduced baseline body weight by at least 5%, nearly half reduced body weight 10% or more, and almost one‐fifth reduced body weight by at least 15% from baseline.
Semaglutide 2.4 mg was found to have induced an overall mean body weight reduction of 14.9% for patients without diabetes in the STEP 1 trial and 9.6% for patients with diabetes in the STEP 2 trial at 68 weeks 16 , 23 The present study had slightly higher proportions of patients who were female, overweight, had class 3 obesity, or who had baseline comorbidities of hypertension, dyslipidemia, and obstructive sleep apnea compared to the STEP 1 trial. 16 Moreover, individuals with type 2 diabetes, who have been shown to experience less weight reduction than those without diabetes, were not excluded from the analysis. Nevertheless, the body weight outcomes from this real‐world study align with the results from the STEP 1 trial at a comparable time point. 16 , 23
To date, there is only one published study examining real‐world body weight change outcomes of patients with overweight or obesity treated with semaglutide 2.4 mg. The study by Ghusn and colleagues was a retrospective cohort analysis using electronic medical records that found a mean 6‐month body weight reduction of 10.9% among 102 patients who were treated with 1.7 or 2.4 mg of semaglutide at a specialty weight management clinic in a large health system. 17 The modestly greater body weight reduction observed in the study may be attributed to the specialized weight management center setting compared to the present analysis, which encompasses patients from various health systems and practice settings. This study expanded upon Ghusn et al. by adopting a real‐world evidence‐based approach outside a single health system, yielding a diverse and inclusive patient cohort across multiple clinical settings, not confined to the standard protocols and practices of one specific health system. In addition, this study, compared to Ghusn et al., provides real‐world findings not only in changes in body weight but also changes in other cardiometabolic biomarkers including lipids, BP and HbA1c. There were statistically significant reductions in BP, HbA1c, TC, and triglycerides at the 6‐month follow‐up, albeit in a small subset of the study population with relevant information recorded in the database.
The results showed that nearly two‐thirds of patients with prediabetes or diabetes at baseline who were treated with semaglutide 2.4 mg had normoglycemia at 6‐month. This finding was noteworthy considering the short follow‐up period. In comparison, prior research details prediabetes reversion rates ranging from 31% to 50% following 1–3 years of lifestyle intervention, 24 , 25 , 26 about 20% after around 4 years of metformin treatment, 27 35% around 3 years of acarbose treatment, 28 approximately 50% after 2.4 years of pioglitazone treatment, 29 66% following 3 years of treatment with a combination of liraglutide and lifestyle intervention. 30 , 31 It is important to note that while direct comparison of the effect sizes of these studies was not feasible due to disparities in factors such as patient attributes, study configuration, and various follow‐up durations, the robustness of the impact of semaglutide on glycemic status remained evident.
The findings of the study add to the literature supporting the use of AOMs as an effective treatment modality for obesity. When used with diet and exercise, AOMs increase the likelihood of achieving clinically meaningful (≥5%) body weight reduction as compared to placebo. 32 Additionally, body weight reduction has been associated with an improvement in, or delayed onset of, cardiovascular risk factors. 3 , 33 , 34 , 35 Additional research is needed with a larger patient population and a longer follow‐up period to determine whether long‐term body weight change outcomes in a real‐world setting are similar to that seen in the STEP 1 trial demonstrating a significant and sustained body weight reduction over a 68‐week period 16 and if changes in body weight and cardiometabolic endpoints can be sustained long‐term.
This study has some limitations including that the number of individuals for whom cardiometabolic measures were available in the AEMR and LAAD databases was quite low, with the exception of BP, which prevented us from evaluating the other cardiometabolic outcomes for the BMI and HbA1c subgroups. This limitation is inherent in all secondary database analyses that rely on laboratory or biometric data. The availability of such data, however, is non‐deterministic with respect to the research objective of the study, thereby precluding any systematic bias in study results for patients with or without these data. Despite the relatively small sample size, the available data suggest the potential impact of semaglutide 2.4 mg on reducing cardiovascular risk factors in a real‐world setting.
As AEMR and LAAD are open‐source databases, continuous enrollment could not be confirmed; hence, proxies were used to best address this limitation and determine periods of continuous patient ‘visibility’ in these data sources. The study sample primarily consisted of white females. However, this study aimed to extend the real‐world evidence base for semaglutide 2.4 mg by assessing its effectiveness in managing obesity across multiple health systems and practice settings, with no specific focus on racial or ethnic differences. In order to understand the real‐world effectiveness within a particular race or ethnicity, conducting future studies with a focus on these racial or ethnic populations is warranted.
The study, while focusing on participants naive to GLP1‐RA, did not investigate the effects of other anti‐hyperglycemic medications, warranting additional research to evaluate the effect of concomitant anti‐hyperglycemic medication use on HbA1c levels. Furthermore, the distribution of contributing physicians in the study, approximately 50% primary care practitioners and 50% specialists, presents a notable point of interest due to the potential disparities in the care each group may offer and merits further investigation.
Results from observational and retrospective studies must be interpreted with caution and can only establish associations and not cause‐and‐effect relationships, which is an inherent limitation to the administrative nature of data or retrospective study design. This study only had a 6‐month follow‐up period due to data availability at the time the study commenced. Additionally, the study period overlapped with a period of shortage of semaglutide 2.4 mg; thus, it is unknown how the variation in the availability of different dose strengths affected patients' ability to escalate to the 2.4 mg dosage. Further research examining body weight and cardiometabolic risk factors over an extended period of time such as 12‐month is warranted.
This analysis of nearly 350 patients with a follow‐up period of 6 months was a robust real‐world assessment. The study size largely exceeded the required sample size to gain sufficient statistical power to conduct the real‐world assessment done in this study and was larger than the sample sizes evaluated in similar studies. 17 , 36 Another advantage of this study was the reliability of LAAD data, given its broad recognition and usage as a database. Furthermore, the generalizability of the data compared to previous publications and the inclusion of patients across multiple health systems and practice settings were all considered strengths of the study.
5. CONCLUSIONS
Patients using semaglutide 2.4 mg in a real‐world setting achieved a significant mean body weight reduction as well as a significant improvement in several cardiometabolic biomarkers including BP, HbA1c, triglycerides and total cholesterol at the 6‐month follow‐up. Further research with longer follow‐up periods is needed to underline the long‐term effectiveness of semaglutide 2.4 mg in the real‐world.
AUTHOR CONTRIBUTIONS
Aleksandrina Ruseva, Wojciech Michalak, Zhenxiang Zhao, Anthony Fabricatore, and Bríain Ó. Hartaigh were responsible for study concept and design, data collection, data interpretation and analysis, drafting/revising the manuscript, and reviewing/approving the final version for submission. Wojciech Michalak conducted the statistical analyses. Devika Umashanker was responsible for data interpretation and analysis, drafting/revising the manuscript, and reviewing/approving the final version for submission.
CONFLICT OF INTEREST STATEMENT
AR, WM, ZZ, AF, and BO are employees of Novo Nordisk Inc. and are shareholders of Novo Nordisk A/S. DU has served as a consultant for Novo Nordisk Inc.
ACKNOWLEDGMENTS
The authors thank Rebecca Hahn, MPH and Ladan Panahi, PharmD of KJT Group, Inc., Rochester, NY for providing medical writing support, which was funded by Novo Nordisk Inc., Plainsboro, NJ in accordance with Good Publication Practice (GPP 2022) and International Committee of Medical Journal Editors (ICMJE 2023) guidelines.
Ruseva A, Michalak W, Zhao Z, Fabricatore A, Hartaigh BÓ, Umashanker D. Semaglutide 2.4 mg clinical outcomes in patients with obesity or overweight in a real‐world setting: a 6‐month retrospective study in the United States (SCOPE). Obes Sci Pract. 2024;e737. 10.1002/osp4.737
This work was previously presented in part at the International Society for Pharmacoeconomics and Outcomes Research (ISPOR) conference May 7–10, 2023 in Boston, MA, USA.
REFERENCES
- 1. Garvey WT, Mechanick JI, Brett EM, et al. American Association of Clinical Endocrinologists and American College of Endocrinology comprehensive clinical practice guidelines for medical care of patients with obesity. Endocr Pract. 2016;22(Suppl 3):1‐203. 10.4158/ep161365.Gl [DOI] [PubMed] [Google Scholar]
- 2. Jensen MD, Ryan DH, Apovian CM, et al. 2013 AHA/ACC/TOS guideline for the management of overweight and obesity in adults: a report of the American College of Cardiology/American Heart Association task force on practice guidelines and the obesity society. Circulation. 2014;129(25 (Suppl 2)):S102‐S138. 10.1161/01.cir.0000437739.71477.ee [DOI] [PMC free article] [PubMed] [Google Scholar]
- 3. Bailey‐Davis L, Wood GC, Benotti P, et al. Impact of sustained weight loss on cardiometabolic outcomes. Am J Cardiol. 2022;162:66‐72. 10.1016/j.amjcard.2021.09.018 [DOI] [PubMed] [Google Scholar]
- 4. Wood GC, Bailey‐Davis L, Benotti P, et al. Effects of sustained weight loss on outcomes associated with obesity comorbidities and healthcare resource utilization. PLoS One. 2021;16(11):e0258545. 10.1371/journal.pone.0258545 [DOI] [PMC free article] [PubMed] [Google Scholar]
- 5. Ghanemi A, Yoshioka M, St‐Amand J. Broken Energy homeostasis and obesity pathogenesis: the surrounding concepts. J Clin Med. 2018;7(11):453. 10.3390/jcm7110453 [DOI] [PMC free article] [PubMed] [Google Scholar]
- 6. Sumithran P, Prendergast LA, Delbridge E, et al. Long‐term persistence of hormonal adaptations to weight loss. N Engl J Med. 2011;365(17):1597‐1604. 10.1056/NEJMoa1105816 [DOI] [PubMed] [Google Scholar]
- 7. Wing RR, Phelan S. Long‐term weight loss maintenance. Am J Clin Nutr. 2005;82(1 Suppl):222s‐225s. 10.1093/ajcn/82.1.222S [DOI] [PubMed] [Google Scholar]
- 8. Gadde KM, Allison DB, Ryan DH, et al. Effects of low‐dose, controlled‐release, phentermine plus topiramate combination on weight and associated comorbidities in overweight and obese adults (CONQUER): a randomised, placebo‐controlled, phase 3 trial. Lancet. 2011;377(9774):1341‐1352. 10.1016/s0140-6736(11)60205-5 [DOI] [PubMed] [Google Scholar]
- 9. Greenway FL, Fujioka K, Plodkowski RA, et al. Effect of naltrexone plus bupropion on weight loss in overweight and obese adults (COR‐I): a multicentre, randomised, double‐blind, placebo‐controlled, phase 3 trial. Lancet. 2010;376(9741):595‐605. 10.1016/s0140-6736(10)60888-4 [DOI] [PubMed] [Google Scholar]
- 10. Pi‐Sunyer X, Astrup A, Fujioka K, et al. A randomized, controlled trial of 3.0 mg of liraglutide in weight management. N Engl J Med. 2015;373(1):11‐22. 10.1056/NEJMoa1411892 [DOI] [PubMed] [Google Scholar]
- 11. Bessesen DH, Van Gaal LF. Progress and challenges in anti‐obesity pharmacotherapy. Lancet Diabetes Endocrinol. 2018;6(3):237‐248. 10.1016/s2213-8587(17)30236-x [DOI] [PubMed] [Google Scholar]
- 12. Baum C, Andino K, Wittbrodt E, Stewart S, Szymanski K, Turpin R. The challenges and opportunities associated with reimbursement for obesity pharmacotherapy in the USA. Pharmacoeconomics. 2015;33(7):643‐653. 10.1007/s40273-015-0264-0 [DOI] [PMC free article] [PubMed] [Google Scholar]
- 13. Gomez G, Stanford FC. US health policy and prescription drug coverage of FDA‐approved medications for the treatment of obesity. Int J Obes. 2018;42(3):495‐500. 10.1038/ijo.2017.287 [DOI] [PMC free article] [PubMed] [Google Scholar]
- 14. U.S. Food and Drug Administration . FDA approves new drug treatment for chronic weight management, first since 2014. Accessed June 7, 2023. https://www.fda.gov/news‐events/press‐announcements/fda‐approves‐new‐drug‐treatment‐chronic‐weight‐management‐first‐2014
- 15. Wadden TA, Bailey TS, Billings LK, et al. Effect of subcutaneous semaglutide vs placebo as an adjunct to intensive behavioral therapy on body weight in adults with overweight or obesity: the STEP 3 randomized clinical trial. JAMA. 2021;325(14):1403‐1413. 10.1001/jama.2021.1831 [DOI] [PMC free article] [PubMed] [Google Scholar]
- 16. Wilding JPH, Batterham RL, Calanna S, et al. Once‐weekly semaglutide in adults with overweight or obesity. N Engl J Med. 2021;384(11):989‐1002. 10.1056/NEJMoa2032183 [DOI] [PubMed] [Google Scholar]
- 17. Ghusn W, De la Rosa A, Sacoto D, et al. Weight loss outcomes associated with semaglutide treatment for patients with overweight or obesity. JAMA Netw Open. 2022;5(9):e2231982. 10.1001/jamanetworkopen.2022.31982 [DOI] [PMC free article] [PubMed] [Google Scholar]
- 18. IQVIA . IQVIA Ambulatory Electronic Medical Record (AEMR) Data. IQVIA; 2023. https://www.iqvia.com/locations/united‐states/library/fact‐sheets/iqvia‐ambulatory‐emr‐us [Google Scholar]
- 19. IQVIA . Advanced Analytics Solution for the Longitudinal Access and Adjudication Data Set. IQVIA; 2023. https://www.iqvia.com/locations/united‐states/library/fact‐sheets/advanced‐analytics‐solution‐for‐the‐longitudinal‐access‐and‐adjudication‐data‐set [Google Scholar]
- 20. World Health Organization. International Classification of Diseases, Tenth Revision, Clinical Modification (ICD‐10‐CM). World Health Organization. https://icd.who.int/browse10/2019/en [Google Scholar]
- 21. Quan H, Sundararajan V, Halfon P, et al. Coding algorithms for defining comorbidities in ICD‐9‐CM and ICD‐10 administrative data. Med Care. 2005;43(11):1130‐1139. 10.1097/01.mlr.0000182534.19832.83 [DOI] [PubMed] [Google Scholar]
- 22. American Diabetes Association . 2. Classification and diagnosis of diabetes: standards of medical care in diabetes‐2021. Diabetes Care. 2021;44(Suppl 1):S15‐s33. 10.2337/dc21-S002 [DOI] [PubMed] [Google Scholar]
- 23. Davies M, Færch L, Jeppesen OK, et al. Semaglutide 2·4 mg once a week in adults with overweight or obesity, and type 2 diabetes (STEP 2): a randomised, double‐blind, double‐dummy, placebo‐controlled, phase 3 trial. Lancet. 2021;397(10278):971‐984. 10.1016/s0140-6736(21)00213-0 [DOI] [PubMed] [Google Scholar]
- 24. Nah EH, Chu J, Kim S, Cho S, Kwon E. Efficacy of lifestyle interventions in the reversion to normoglycemia in Korean prediabetics: one‐year results from a randomised controlled trial. Prim Care Diabetes. 2019;13(3):212‐220. 10.1016/j.pcd.2018.11.017 [DOI] [PubMed] [Google Scholar]
- 25. Giráldez‐García C, Cea‐Soriano L, Albaladejo R, et al. The heterogeneity of reversion to normoglycemia according to prediabetes type is not explained by lifestyle factors. Sci Rep. 2021;11(1):9667. 10.1038/s41598-021-87838-z [DOI] [PMC free article] [PubMed] [Google Scholar]
- 26. Röhling M, Kempf K, Banzer W, et al. Prediabetes conversion to normoglycemia is superior adding a low‐carbohydrate and energy deficit formula diet to lifestyle intervention‐A 12‐month subanalysis of the ACOORH trial. Nutrients. 2020;12(7):2022. 10.3390/nu12072022 [DOI] [PMC free article] [PubMed] [Google Scholar]
- 27. Knowler WC, Barrett‐Connor E, Fowler SE, et al. Reduction in the incidence of type 2 diabetes with lifestyle intervention or metformin. N Engl J Med. 2002;346(6):393‐403. 10.1056/NEJMoa012512 [DOI] [PMC free article] [PubMed] [Google Scholar]
- 28. Chiasson JL, Josse RG, Gomis R, Hanefeld M, Karasik A, Laakso M. Acarbose for prevention of type 2 diabetes mellitus: the STOP‐NIDDM randomised trial. Lancet. 2002;359(9323):2072‐2077. 10.1016/s0140-6736(02)08905-5 [DOI] [PubMed] [Google Scholar]
- 29. DeFronzo RA, Tripathy D, Schwenke DC, et al. Pioglitazone for diabetes prevention in impaired glucose tolerance. N Engl J Med. 2011;364(12):1104‐1115. 10.1056/NEJMoa1010949 [DOI] [PubMed] [Google Scholar]
- 30. le Roux CW, Astrup A, Fujioka K, et al. 3 years of liraglutide versus placebo for type 2 diabetes risk reduction and weight management in individuals with prediabetes: a randomised, double‐blind trial. Lancet. 2017;389(10077):1399‐1409. 10.1016/s0140-6736(17)30069-7 [DOI] [PubMed] [Google Scholar]
- 31. Sallar A, Dagogo‐Jack S. Regression from prediabetes to normal glucose regulation: state of the science. Exp Biol Med. 2020;245(10):889‐896. 10.1177/1535370220915644 [DOI] [PMC free article] [PubMed] [Google Scholar]
- 32. Yanovski SZ, Yanovski JA. Long‐term drug treatment for obesity: a systematic and clinical review. JAMA. 2014;311(1):74‐86. 10.1001/jama.2013.281361 [DOI] [PMC free article] [PubMed] [Google Scholar]
- 33. Ryan DH, Yockey SR. Weight loss and improvement in comorbidity: differences at 5%, 10%, 15%, and over. Curr Obes Rep. 2017;6(2):187‐194. 10.1007/s13679-017-0262-y [DOI] [PMC free article] [PubMed] [Google Scholar]
- 34. Wing RR, Lang W, Wadden TA, et al. Benefits of modest weight loss in improving cardiovascular risk factors in overweight and obese individuals with type 2 diabetes. Diabetes Care. 2011;34(7):1481‐1486. 10.2337/dc10-2415 [DOI] [PMC free article] [PubMed] [Google Scholar]
- 35. Cohen JB. Hypertension in obesity and the impact of weight loss. Curr Cardiol Rep. 2017;19(10):98. 10.1007/s11886-017-0912-4 [DOI] [PMC free article] [PubMed] [Google Scholar]
- 36. Ghusn W, Fansa S, Nicolalde B, et al. Semaglutide Weight Loss and Metabolic Parameters Outcomes: A 1‐year Multicentered Study. Presented at 30th European Congress on Obesity. May 2023; Dublin Ireland. PO4.099. Obesity Facts; 2023;282. 10.1159/000530456 [DOI]


