Abstract
Ewing sarcoma (ES) was first reported by Ewing in 1921. It is the second largest malignant bone tumor in children and adolescents, typically occurring in the bones of trunk or limbs . Extraskeletal Ewing sarcoma (EES) was first reported by Tefft et al. in 1969 and is extremely rare, accounting for less than 1% of all sarcomas. It can occur in any part of soft tissue, mostly in the trunk and lower limbs, and rarely in the pleura. We report a 22-year-old case of extraosseous Ewing sarcoma of pleural origin discovered and pathologically confirmed by physical examination. We report its CT manifestations and pathological results, and review the literature to summarize and analyze the clinical and imaging characteristics of extraosseous Ewing sarcoma, in order to improve our understanding of the disease.
CASE REPORT
BASIC INFORMATION
The patient, a 22 year old male, was admitted due to a "right lung mass of 2+months" during physical examination. The patient's pre admission physical examination revealed a lump in the lower lobe of the right lung, without any discomfort such as coughing, expectoration, tightness of breath, or coughing up blood. Therefore, the patient sought medical attention at West China Hospital. Physical examination: No abnormalities were found in the chest, bilateral respiratory movements were uniform and symmetrical, without enhancement or weakening, and no pleural friction was felt. The percussion of both lungs showed clear sounds, with low respiratory sounds in both lungs and no dry or wet rales heard. The heart boundary was normal, the heart rhythm was uniform, and no murmurs were heard in each valve area.
LABORATORY INSPECTION
Serum neuron specific enolase (NSE) level 25.70ng/ ml (reference value<20.4); Carcinoembryonic antigen, glycoantigen-125, glycoantigen-199, cytokeratin-19 fragments, liver and kidney function, blood routine, and coagulation parameters are all within the normal range.
IMAGING EXAMINATION
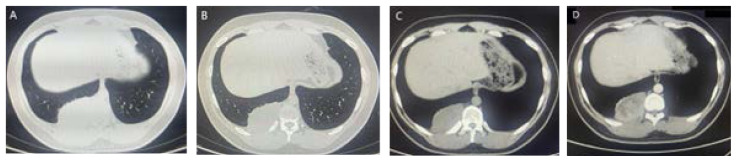
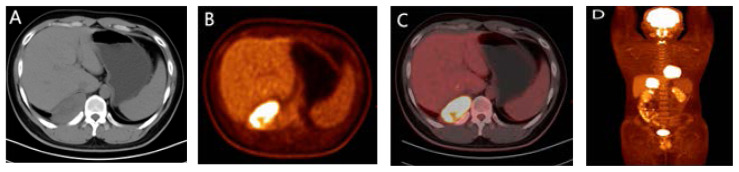
Chest CT manifestations: After admission, chest CT plain scan and enhanced examination were performed. The CT image showed a soft tissue density mass with a size of approximately 9.0 × 7.9 × 4.8cm in the posterior basal segment of the right lower lobe of the lung, adjacent to the spine, with a smooth edge. The average CT value of plain scan was 38HU, while heterogeneous contrast enhancement with an average CT value of 71HU. The periphery was stronger than the center, and the lesion had a wide base that was close to the pleura and unclear boundary (Figure 1). The lung tissue around the mass was slightly compressed, The boundary with the adjacent chest wall muscles is unclear, and the bone density of the adjacent right 11th rib dorsal segment is unevenly reduced or increased; No abnormalities were found in the remaining structures of the chest. The imaging diagnosis is: a soft tissue mass adjacent to the spinal column in the basal segment of the right lower lobe of the lung, multiple malignant tumors originating from the pleura, involving the right 11th rib.PET-CT shows a pleural mass in the right posterior rib, invading the right lower lobe of the lung, the 11th posterior rib, and the 12th intercostal muscle. The lesion uptake of 18F-FDG is increased (Figure 2), with a maximum SUV of 10.84, which is consistent with the appearance of malignant tumors; No signs of tumor metastasis were observed in other parts of the body; There is a slight atelectasis in the lower lobe of the right lung.
Figure 1.
Cross-sectional CT scan of the chest. Figures A and B show the lung window, while C and D show the plain and enhanced images of the mediastinal window. They show a soft tissue mass in the posterior basal segment of the right lower lobe of the lung, under the pleura and adjacent to the spinal column, with a smooth edge that is close to the broad base of the pleura, unclear boundary, heterogeneous contrast enhancement, and stronger periphery than the center.
Figure 2.
PET-CT scan appearance: Cross section CT plain scan (Figure A) shows a pleural mass in the right posterior inferior rib, PET (Figure B) shows a high uptake area of 18F-FDG, and PET-CT (Figures C, D) fusion shows a pleural hypermetabolic mass invading the right 11th posterior rib.
PATHOLOGICAL EXAMINATION AND DIAGNOSIS
After admission, a puncture biopsy of the right lower chest mass was performed, and the histopathological findings showed infiltration of small round cell tumors; Immunohistochemical staining showed CD99 (+), desmin (−), myogenin (−), WT-1 (−), ERG (−), SATB2 (−), EMA (+), TLE-1 (−), LCA (−), FLI (−), NSE (−), NKX2.2 (+). FISH: Detection of EWSR1 gene translocation; Detect EWSR1-FL11 gene fusion; ESWR1-WT1 gene fusion was not detected. Gene mutation detection: No DICER1 gene mutation was detected. Diagnosis: Ewing sarcoma.
CHEMOTHERAPY AND SURGICAL FINDINGS
After a clear diagnosis and improvement of the baseline examination, we fully communicated with the patient and chose the VDC regimen (Vincristine+doxorubicin/epirubicin/ liposome doxorubicin+cyclophosphamide) alternating with the IE regimen (etoposide+ifosfamide) for treatment. At the same time, symptomatic support treatments such as acid suppression, gastric protection, and antiemesis were given. After completing 3VDC+2IE, a "thoracoscopic assisted free thoracic mass and partial chest wall resection" was performed under general anesthesia. During the surgery, a hard mass, approximately 8.0 × 9.0cm in size, was found in the wall layer near the rib spinal angle of the right lower thoracic cavity, protruding towards the chest cavity. The base was tightly adhered to the 10th to 12th ribs and chest wall tissue, with intact pleura and obvious local edema; The section of the package changes in a fish like manner.
Follow up: After 1 month of surgery, the third IE regimen chemotherapy was completed. After 6 months of surgery, CT scan showed no recurrence or metastasis, and the patient's condition was good.
DISCUSSION
Ewing's sarcoma (ES) is a small round cell malignant tumor of neuroectodermal origin, first described by James Ewing in 1921. ES represents a group of tumors with similar morphology, including "classic" Ewing osteosarcoma, exoskeletal Ewing sarcoma, small cell tumors in the chest and lung region (Askin tumor), and soft tissue based primitive neuroectodermal tumors (PNET) [1]. These sarcomas all originate from unique mesenchymal progenitor cells and have similar histological and immunohistochemical characteristics. They are composed of densely arranged small circular to elliptical cells under a microscope. Immunohistochemical examination shows that the fusion oncogene EWSR1: FLI1 produced by chromosomal translocation between chr11 and chr22 has specificity [2].
ES is the second most common malignant bone tumor in children and adolescents, accounting for 10% to 15% of all primary bone tumors and approximately 3% of all malignant tumors in children [3]. It most commonly occurs in the pelvis, axial bones, and femur. A very small number of ES originating and developing from exoskeletons are classified as extraskeletal Ewing's sarcoma (EES). The incidence of EES has no difference in gender and race. According to statistics, the incidence rate is 0.4 per million people [4]. EES mostly occurs in people aged 10 to 30 years old, with a peak age of onset around 20 years old [5]. EES occurs in the head and neck, retroperitoneum, omentum, paravertebral, orbit, skin, chest wall, pelvis, and lower limbs, as well as in the gastrointestinal tract, pancreas, omentum, kidneys, and adrenal glands.
EES originating from the pleura is very rare. Using the keywords "sarcoma, Juventus, Juventus sarcoma" and "pleura, chest cavity, chest wall", a search was conducted for relevant literature published in the PubMed and CNKI China National Knowledge Infrastructure medical databases in the past 20 years. A total of 7 cases of EES originating from the pleura were found [7–13] (Table 1). Among these 7 patients, 4 were females and 3 were males. In addition, one male case was reported in this case, indicating no gender difference in incidence. The average age of 8 patients was 18.9 years old, with a median age of 18 years old, There were 4 cases of pleural tumors on both sides.
Table 1.
Extraosseous Ewing's sarcoma of the pleura
| First author | Publication date | Age (years) | Gender | Primary location | Clinical manifestations | Diagnosis method | Therapy | Outcome |
|---|---|---|---|---|---|---|---|---|
| Wu Y | 2023 | 11 | Male | Left Pleura | Left Shoulder Pain | needle biopsy | Chemotherapy | Pain Relief |
| Zou X | 2021 | 14 | Male | Right Pleura | Fever, difficulty breathing | Thoracoscopic biopsy | radio-therapy, chemo-therapy | Death |
| Bhaskaran A | 2021 | 34 | Female | Right Pleura | Fever, cough with phlegm, right chest and upper back pain | needle biopsy | Surgery, radio-therapy, chemo-therapy | NS |
| Mathew D | 2019 | 7 | Male | Left Pleura | expiratory dyspnea | needle biopsy of the tumor | Chemotherapy | NS |
| Tsunezuka Y | 2012 | 27 | Female | Left Pleura | No obvious symptoms | Thoracoscopic resection and pathological examination | Thoracoscopic resection | No recurrence was found one year after surgery |
| Karatziou C | 2011 | 21 | Female | Left Pleura | Fever,productive cough | Thoracoscopic examination and biopsy | Chemotherapy | NS |
| Ozge C | 2004 | 18 | Female | Right Pleura | Back pain, cough, expiratory dyspnea | Pleural needle biopsy | NS | NS |
NS: Not Stated
Clinical manifestations: Among the 8 cases including this one, 6 had symptoms without specificity, and 2 had no clinical manifestations. This case was discovered by chance during physical examination.
Imaging features: A total of 8 cases reported in the literature and this case, with 3 cases indicating tumor size, were as follows: Among them, 1 case had a right pleural mass of 13×11×15 cm; On CT enhanced images, all showed unevenly enhanced soft tissue masses under the pleura, with a wide base attached to the pleura and no calcification. Six cases described liquefaction and necrosis areas within the lesion, four cases had a large amount of pleural effusion, three cases had adjacent rib erosion, one case had thoracic vertebral invasion, four cases had adjacent bone abnormalities, and adjacent bone invasion accounted for 50%; The protruding edge towards the lung field is relatively smooth, indicating that the adjacent lung tissue shows compression rather than infiltration sign. Overall, CT findings can indicate pleural origin tumors, and based on the enhancement method, it may be judged as malignant tumors. However, if there is no invasion or distant metastasis of adjacent structures, it is difficult to determine the nature. Therefore, further examination is needed in this case to assist in diagnosis.
18 fluorodeoxyglucose positron emission tomography (PET-CT) is helpful in distinguishing between benign and malignant lesions. Masses with high SUV values can indicate the presence of malignant tumors [14]. In this case, PET showed active glucose metabolism in the pleural mass, with a maximum SUV of 10.84, indicating pleural malignancy. No metastatic lesions were found in the rest of the body, and it was determined that the lesion was not in the late stage. Two other reports also showed high uptake of 18F-FDG in the lesion area, both cases with bone scan showed tracer concentration.
All 8 cases were diagnosed with EES through puncture, thoracoscopic biopsy, or postoperative histopathology and immunohistochemistry. The diagnosis relies on pathological examination, mainly based on the histopathology and immunohistochemistry of tumor specimens. The histological feature of EES is the presence of small blue circular or elliptical cells containing solid substances separated by fibers. It should be distinguished from primitive neuroectodermal tumors (PNET), embryonic rhabdomyosarcoma, neuroblastoma, and lymphoma. Clinical features combined with immunohistochemical staining and cytogenetic analysis are helpful for diagnosis. According to reports, immunohistochemical staining has detected positive for CD99, while S-100, Demin, factor 8 cytokines, and neurofilament, as well as negative for T cells, B cells, LCA, CD43, and CD68 [15]. Combined with fluorescence in situ hybridization (FISH) to detect translocation of 22q12 (EWSR1 gene) [16], the diagnosis of EES can be confirmed.
The treatment of EES is similar to that of ES, with a combination of cytotoxic drug therapy and local surgery or radiotherapy [17]. After local control, the tumor should be completely removed as much as possible. Among the 8 cases, 6 underwent chemotherapy, 1 did not receive treatment after thoracoscopic resection, and 1 did not specify the treatment method. This patient underwent tumor resection after completing 3 rounds of VDC+2 rounds of IE alternating chemotherapy. The third IE regimen chemotherapy was completed 1 month after surgery, and no CT scan or transfer was found after 6 months of surgery.
CONCLUSIONS
This case reports primary pleural Ewing's sarcoma, which is a very rare type of extraosseous Ewing's sarcoma. It has a high degree of malignancy, lacks specificity in clinical and imaging manifestations, and is prone to misdiagnosis when it appears in rare areas. However, its imaging findings can indicate a tendency towards malignancy, providing a basis for further clinical treatment, and final diagnosis requires pathological diagnosis.
REFERENCES
- 1.Ludwig JA. Ewing sarcoma: historical perspectives, current state-of-the-art, and opportunities for targeted therapy in the future. Curr Opin Oncol. 2008;20(4):412–418. doi: 10.1097/CCO.0b013e328303ba1d. [DOI] [PubMed] [Google Scholar]
- 2.Yu L, Davis IJ, Liu P. Regulation of EWSR1-FLI1 Function by Post-Transcriptional and Post-Translational Modifications. Cancers (Basel) 2023;15(2):382. doi: 10.3390/cancers15020382. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 3.Haveman LM, van Ewijk R, van Dalen EC, et al. High-dose chemotherapy followed by autologous haematopoietic cell transplantation for children, adolescents, and young adults with first recurrence of Ewing sarcoma. Cochrane Database Syst Rev. 2021;9(9):CD011406. doi: 10.1002/14651858.CD011406.pub2. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 4.Abboud A, Masrouha K, Saliba M, et al. Extraskeletal Ewing sarcoma: Diagnosis, management and prognosis. Oncol Lett. 2021;21(5):354. doi: 10.3892/ol.2021.12615. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 5.Iwamoto Y. Diagnosis and treatment of Ewing's sarcoma. Jpn J Clin Oncol. 2007;37(2):79–89. doi: 10.1093/jjco/hyl142. [DOI] [PubMed] [Google Scholar]
- 6.Gurria JP, Dasgupta R. Rhabdomyosarcoma and Extraosseous Ewing Sarcoma. Children (Basel) 2018;5(12):165. doi: 10.3390/children5120165. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 7.Wu Y, Xie CB, Huang Q, Zhao KF. Case report: Primary pleural giant extraskeletal Ewing sarcoma in a child. Front Oncol. 2023;13:1137586. doi: 10.3389/fonc.2023.1137586. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 8.Zou X, Chang W, Gao H. A primary Ewing's sarcoma of pleura: Case report and literature review. Respir Med Case Rep. 2021;34:101516. doi: 10.1016/j.rmcr.2021.101516. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 9.Bhaskaran A, Sethi P, Toi PC, Penumadu P. Dilemma in diagnosis and management of rare primary pleural Ewing's sarcoma with synchronous limited metastatic disease. BMJ Case Rep. 2021;14(6):e243495. doi: 10.1136/bcr-2021-243495. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 10.Mathew D, Prince DN, Mahomed N. Extra-skeletal Ewing Sarcoma of the chest wall in a child. SA J Radiol. 2019;23(1):1733. doi: 10.4102/sajr.v23i1.1733. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 11.Tsunezuka Y, Furusawa T, Yachi T, Kurumaya H. Rapidly growing intrathoracic extraskeletal Ewing's sarcoma. Interact Cardiovasc Thorac Surg. 2012;14(1):117–119. doi: 10.1093/icvts/ivr023. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 12.Karatziou C, Pitta X, Stergiouda T, Karadimou V, Termentzis G. A case of extraskeletal Ewing sarcoma originating from the visceral pleura. Hippokratia. 2011;15(4):363–365. [PMC free article] [PubMed] [Google Scholar]
- 13.Ozge C, Calikoglu M, Cinel L, Apaydin FD, Ozgür ES. Massive pleural effusion in an 18-year-old girl with Ewing sarcoma. Can Respir J. 2004;11(5):363–365. doi: 10.1155/2004/103637. [DOI] [PubMed] [Google Scholar]
- 14.Harrison DJ, Parisi MT, Shulkin BL. The Role of 18F-FDG-PET/CT in Pediatric Sarcoma. Semin Nucl Med. 2017;47(3):229–241. doi: 10.1053/j.semnuclmed.2016.12.004. [DOI] [PubMed] [Google Scholar]
- 15.Lee WS, Kim YH, Chee HK, et al. Multimodal Treatment of Primary Extraskeletal Ewing's Sarcoma of the Chest Wall: Report of 2 Cases. Cancer Res Treat. 2009;41(2):108–112. doi: 10.4143/crt.2009.41.2.108. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 16.Javalgi AP, Karigoudar MH, Palur K. Blue cell tumour at unusual site: Retroperitoneal Ewings sarcoma. J Clin Diagnostic Res. 2016;10(4):19–20. doi: 10.7860/JCDR/2016/18302.7618. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 17.Eaton BR, Claude L, Indelicato DJ, et al. Ewing sarcoma. Pediatr Blood Cancer. 2021;68(2):e28355. doi: 10.1002/pbc.28355. [DOI] [PubMed] [Google Scholar]