Abstract
Werner Syndrome is a rare autosomal recessive condition characterized by premature aging and increased risk of malignancies due to gene mutations associated with DNA stability. We present the first case report of a 29-year-old Hispanic female with WS diagnosed with breast cancer. Diagnostic mammography and ultrasound, breast MRI and PET examinations revealed two lesions biopsy proven as invasive ductal carcinoma. The patient underwent neoadjuvant chemotherapy and radical mastectomy. Recurrence occurred 10 months postoperatively with molecular analysis demonstrating TP53 mutations. The multifactorial assessment of breast cancer in this case study is crucial towards optimizing screening, diagnosis and management of this disease in patients with WS.
Keywords: Werner syndrome, adult progeria, breast cancer, WRN mutation
CASE PRESENTATION
A 29-year-old female with a past medical history of Werner Syndrome (WS), fibromyalgia, hepatic steatosis, coronary artery disease (CAD), cataracts, hypertension, and type II diabetes mellitus presented with a chief complaint of right breast discomfort associated with a self-palpated mass.
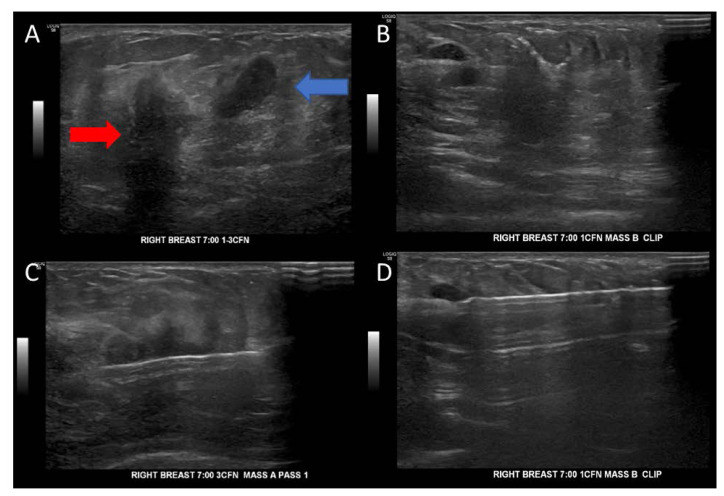
The patient underwent a targeted breast ultrasound, which revealed an anti-parallel irregular hypoechoic mass with posterior shadowing measuring 1.7 × 1.3 × 1.8 cm at the 7:00 position, 1 cm from the nipple. An adjacent anti-parallel irregular mass measuring 1.0 × 0.9 × 0.8 cm at the 7:00 position, 3 cm from the nipple was also identified, BI-RADS 4. The patient underwent ultrasound-guided core needle biopsies of the 2 suspicious masses in the right breast at 7:00 position, 1 cm from the nipple with placement of barrel-shaped clip and right breast at 7:00 position, 3 cm from the nipple with placement of ribbon-shaped biopsy clip with post biopsy mammogram demonstrating the clips in satisfactory position (Figure 1,2).
Figure 1.
Ultrasound findings reported a 1.7×1.3×1.8 cm3 antiparallel hypoechoic mass lesion at the 7:00 position 1 cm from the nipple of the right breast and another 1×0.9×0.8cm3 adjacent lesion noted. BI-RADS 4.
Figure 2.
Right breast mammography demonstrating round, indistinct hyperdensities with biopsy clip. right breast mass. BI-RADS 6.
Ultrasound-guided core biopsies of the lesions were then performed. The pathology reported invasive ductal carcinoma, Grade II, ER-positive (85%), PR-positive (15%) and HER2/neu equivocal.
MRI of the breast revealed 3 heterogeneous enhancing masses in the outer central right breast associated with biopsy clip and corresponding to the biopsy-proven invasive ductal carcinoma spanning a total area of 3.8 × 3.8 × 4.5 cm (Figure 3). There was linear nonmass enhancement extending toward the nipple, with the most posterior abnormal enhancement abutting the pectoralis major muscle, which was concerning for microinvasion. Additionally, a cortically thickened right axillary lymph node was identified for which targeted ultrasound and biopsy were recommended.
Figure 3.
Breast MRI with (a) axial STIR, (b) axial T1, (c) axial T1 Dynamic contrast enhanced, and (d) sagittal. MRI demonstrates three heterogenous enhancing masses in the outer central right breast with total area of 3.8×3.8×4.5cm with a linear nonmass enhancement extending towards the nipple. Posterior abnormal enhancement is abutting the pectoralis major muscle, concerning for microinvasion. Borderline right axillary lymph node with cortical thickening up to 0.4 cm. An indeterminate 0.8 cm enhancing mass in the posterior central left breast. Additional left breast nonmass enhancement measuring up to 2.3 cm.
Focused sonographic evaluation of the right axilla confirmed a cortically thickened lymph node measuring 1 × 0.6 × 0.9 cm, 9 cm from the nipple corresponding to the MRI finding, with biopsy confirming metastatic breast adenocarcinoma (Figure 4).
Figure 4.
(a) US and (b) Doppler of right axillary lymph node measuring 1.04cm × 0.63cm with cortical thickening, suggestive of reactive/reactive node
PET/CT was performed demonstrating the FDG Avid Right breast carcinoma and FDG avid metastatic right axillary lymph node. There was no FDG avid disease in the contralateral breast or distal metastasis (Figure 5).
Figure 5.
18FDG-PET performed and demonstrated FDG avid right breast carcinoma and axillary lymph node. Mild to moderate calcified atherosclerosis of the left anterior descending coronary artery.
Neoadjuvant chemotherapy was initiated with doxorubicin and tamoxifen. During this time patient complained of pressure-like pain in her chest and was evaluated by cardiology service. Cardiology service performed a stress test, which was positive with intermediate risk of ischemia. Due to her history and associated risk factors, a left heart catheterization was performed and showed 65–70% stenosis of the left anterior descending artery (LAD) (Figure 6). Patient developed abdominal pain during her chemotherapy treatment and was admitted due to gastroenteritis in which abdominal CT was performed. CT demonstrated hepatomegaly with steatosis and diffuse hypoattenuation for a young patient, but no acute findings (Figure 7). Follow-up ultrasound 8 weeks after initiation of neoadjuvant chemotherapy demonstrated a mild decrease in size of both the right breast mass and right axillary lymph node, consistent with early treatment response (Figure 8).
Figure 6.
Left Heart Catheterization with Coronary Angiography demonstrating left main coronary artery that is angiographically normal and gives rise to a Left anterior descending (LAD) and left circumflex artery (LCX). There is 65–70% stenosis of the LAD mid segment, and distal to D3 there is another focal 50% stenosis. The right coronary artery has ostial 40% stenosis. The posterior descending artery (PDA) is angiographically normal.
Figure 7.
Upper abdomen CT with contrast demonstrating hepatomegaly and steatosis with diffuse hepatic hypoattenuation.
Figure 8.
US of the right (a) breast and (b) axilla. Right breast mass decreased in size after 8 weeks of initiation of chemotherapy irregular hypoechoic mass with associated post-biopsy clip measuring 1.1 × 1 × 1.1 cm (previously measured (1.8 × 1 × 1 cm) and has decreased in size. Right axilla with 0.8×0.6×0.8cm lymph node with clip that previously measure 1×0.6×0.9cm.
After completion with four cycles of doxorubicin therapy, a right modified radical mastectomy was performed with no reported complications. The surgical pathology report confirmed invasive ductal carcinoma with one positive right axillary lymph node, and margins negative for malignancy, staging as pT1cN1a.
Ten months after right modified radical mastectomy, the patient experienced right chest discomfort with a palpable mass in close proximity to the mastectomy surgical scar. An ultrasound revealed a 1.5 × 1.0 × 1.3 cm oval mass with mixed echogenicity and increased vascularity extending into the dermis (Figure 9). A wide local excision confirmed recurrent breast cancer, determined to be ER-positive (91%–100%), PR− negative and HER2 equivocal. Within a year, follow-up PET/ CT ruled-out distant metastatic disease and demonstrated post-treatment changes with increased metabolic activity in the right axillary area, suggesting recurrence (Figure 10). The patient is currently undergoing trastuzumab therapy, as advanced molecular analysis revealed TP53 mutations.
Figure 9.
US of the right breast lateral to mastectomy scar demonstrating an oval mass measuring 1.5×1×1.3 with mixed echogenicity and increased vascularity extending into the dermis without axillary adenopathy.
Figure 10.
Chest CT showing asymmetrical breast densities, prominent on the right. Irregular 3 cm × 2.5 cm
DISCUSSION
ETIOLOGY & DEMOGRAPHICS
Werner syndrome (WS) is a rare autosomal recessive condition that presents with early onset of age-related diseases commonly associated to WRN gene mutation. The syndrome was first described in 1904 by Werner, but is now known to have over 1500 cases reported in the literature. The prevalence of the disease is reported to be 1:380,000 to 1:1,000,000 [1]. The onset of disease begins approximately at age of 10, but clinical manifestations usually appear by age 20 [2].
Due to the rarity of WS, there is limited literature regarding its association with different types of cancer. In a systematic review by Lauper et al, breast cancer was reported in just 2.8% of the female patients with WS included in the study [3]. Our case of breast cancer in a 29-year-old patient with WS adds to the limited body of knowledge and offers insights into the expression and management of breast cancer in this rare genetic syndrome.
CLINICAL & IMAGING FINDINGS
Clinical features of the WS include diseases typically associated with aging including diabetes mellitus, atherosclerosis, cataracts, and increased risk of malignancies. WS diagnosis is initially made in a clinical setting when patient presents with cardinal signs that include cataracts, short stature, premature greying and/or thinning of the scalp hair, “bird-like” facies and dermatological pathologies that include tight skin, atrophic skin, pigment alterations, ulcerations, hyperkeratosis and regional subcutaneous atrophy. WS requires a multidisciplinary team to monitor for associated conditions, such as diabetes mellitus, hypogonadism, cataracts and malignancies [4].
Molecular genetic testing confirms the diagnosis by identifying the WRN mutation [2,4]. WRN mutations have been found to be associated to malignancies, due to this gene’s role in DNA stability [5]. This gene is involved in creating the DNA-helicase for DNA repair and synthesis. WS has an increased predisposition to cancer due to impaired DNA damage repair and shortening of telomeres. Other malignancies commonly associated to WS are thyroid carcinoma, melanoma, meningioma, and soft tissue and bone sarcomas [6].
Patients diagnoses with breast cancer before 30 years-old represents 1% of all breast cancer cases in the general population [7]. In the past, the United States Preventive Services Taskforce (USPST) recommended breast cancer screening to start breast cancer screening in female patients at age 45 with risk factors and age 50 in all other patients. Currently, the USPST recommends that breast cancer screening should begin at age forty for all patients, including those with pre-existing risk factors [5,8,9]. However, the occurrence of breast cancer at a younger age, as observed in out WS patient, can lead to a delayed diagnosis and presentation with more advanced disease [10].
The typical presentation of breast cancer is a palpable mass, though more advanced cancers can present with skin dimpling with erythema, coined “peau d’orange”, nipple retraction and/ or discharge. Appropriate workup for palpable breast masses include imaging to rule-out cancer. (Table 2) includes breast mass diagnosis with their associated imaging findings [11–19]. The key factor in imaging studies is to identify findings associated to malignancy. BI-RADS is a risk assessment system, established by the ACR, that scores breast lesions from 0–6 based on imaging features associated to malignancy. Additional to the clinical context, BI-RADS scoring of lesions will help the physician determine if the patient would benefit from additional studies and/or biopsy if the lesion in question. BI-RADS 0 is used to define inconclusive imaging studies. BI-RADS 1 and 2 are used for benign findings, where 1 is a completely negative study and 2 is a study with benign findings. BI-RADS 3 is reported when a lesion is probably benign, but short interval surveillance is recommended. When a lesion is reported as BI-RADS 4 suspicion of malignancy is increased. This is further subdivided into 4a (2–10% malignancy likelihood), 4b (10–50% malignancy likelihood), and 4c (50–95% malignancy likelihood). BI-RADS 5 is assigned to lesions with ≥95% likelihood of malignancy. When a lesion has a biopsy-proven malignancy, BI-RADS 6 is used [20].
Table 2.
Differential diagnosis of breast masses with associated imaging findings.
| Ultrasound | Mammography | MRI | PET/CT | |||
|---|---|---|---|---|---|---|
| T1 | T2 | Contrast Enhancement | ||||
| Fibroadenoma | Well-circumscribed round homogenously hypoechoic lesion with round borders | Coarse “popcorn” calcifications | Hypointense lesion compared to surrounding breast tissue | Can present as hyperintense lesions with hypointense internal septations. | Slow initial contrast enhancement with persistent delayed phase. | Low FDG uptake |
| Breast Cyst | Well circumscribed anechoic lesion. May present internal echoes in complicated cysts | Well-circumscribed dense masses. | Simple: Iso- and hypointense on fat suppressed to breast parenchyma Complex: intermediate to high signal | Simple: Hyperintense to breast parenchyma Complex: variable depending on cyst contents |
No enhancement | No FDG uptake |
| Abscess | Hypoechoic collections of variable shape and size, Thick echogenic rim and increased vascularity | Asymmetric density and skin thickening with presence of a mass | Round or irregular mass with intermediate intensity | High signal within skin and breast parenchyma | Gradual and progressive without washout | Increased metabolic uptake relative to normal breast parenchyma |
| Fat necrosis | Unspecific and can vary depending on etiology and chronicity. | Varied. Can present with focal asymmetry, speculated borders, microcalcifications or coarse calcifications. | Decreased signal intensity on T1WI. Non-fat suppressed unenhanced show increased intensity of fat signal within lesion. | Decreased signal intensity | Rapid contrast enhancement | Increased metabolic uptake relative to normal breast parenchyma |
| Primary Breast Cancer | Irregular shape, microlobulated margins and hypo-homogenous echogenicity | Irregular Mass present with microcalcifications and obscured margins. “Oil cysts” observed within lesion. | Irregularly speculated mass with rim or heterogeneous enhancement; low signal intensity | Low or moderate signal intensity | Variable, restricted diffusion | Increased metabolic uptake relative to normal breast parenchyma |
| Metastatic Breast Cancer | Round or oval circumscribed mass, skin thickening, edema | High density, round mass, may contain calcifications | Round or oval masses with low signal intensity | Round or oval masses with intermediate signal intensity | Rapid contrast enhancement | Increased metabolic uptake relative to normal breast parenchyma |
In the case of our patient, she initially presented with a palpable right breast lesion which was categorized as a BI-RADS 4 lesion upon initial ultrasound imaging. This prompted a core-needle biopsy to be performed and was remarkable for grade 2 invasive ductal carcinoma. She presented to our institution after biopsy results were available, hence why our imaging studies categorized her with BI-RADS 6.
TREATMENT AND PROGNOSIS
Given that WS is characterized by premature aging, this gene has been studied to understand longevity and increased risk of age-related disease [21]. The average lifespan of patients with WS is 55 years [22]. WS patients have a strong predisposition to malignancy, making cancer one of the most common causes of death in this population [23].
Breast cancer, as an age-related malignancy, exhibits a directly proportional association of both incidence and mortality with age [3,23]. Consequently, individuals with WS may face an elevated risk of developing breast cancer at an early age, before the age recommendation for screening mammograms.
The case of our patient is not only a documented case of breast cancer in a rare genetic syndrome, but to our knowledge is the first case report of WS in a Hispanic patient. Notably, the gene variant Arg834Cys (rs3087425) has been found to have a two-fold increased risk for breast cancer. The prevalence of this variant in the Hispanic population may partly explain the association between breast cancer and WS in our patient. The uniqueness of our patient’s case as the first reported case of WS in a young Hispanic patient with breast cancer highlights the importance of documenting and reporting such cases. Another point to address in this case is the degree of stenosis in her coronary artery for the patient’s age. The prevalence of young CAD, patients younger than age 45, is 1.2% in which Asians are more vulnerable. Due to her condition, which increases the likelihood of developing CAD and Diabetes mellitus, may contribute to her degree of stenosis [24,25].
TEACHING POINT
In conclusion, this rare case highlights the importance of radiologic evaluation in diagnosing and managing malignancies in this unique population and underscores the need for further research to enable tailored approaches for screening and treatment in WS patients to improve clinical outcomes.
QUESTIONS: (ANSWERS IN BOLD)
-
Which of the following pathologies best presents as an anechoic well circumscribed lesion on ultrasound, but shows no contrast enhancement on MRI?
Breast cyst [Table 2]
Breast Abscess
Primary Breast Cancer
Phyllodes
Fibroadenoma
-
Which of the following statement is true?
Werner syndrome is autosomal dominant
Werner syndrome is autosomal recessive [Werner syndrome (WS) is a rare autosomal recessive condition that presents with early onset of age-related diseases commonly associated to WRN gene mutation.]
Werner syndrome is mitochondrial inheritance
The average lifespan is 80 years
Hispanics are commonly affected population of Werner syndrome
-
Which of the following statement is true?
WRN mutation halts DNA replication
WRN mutation impedes RNA transcription
WRN mutation lengthens Telomeres
WRN mutation halts the formation of the ribosomal complex
WRN creates DNA-helicase [This gene is involved in creating the DNA-helicase for DNA repair and synthesis. Mutations present in Werner syndrome disrupts this process.]
-
A homogenous, well circumscribed, round lesion was found on ultrasound, and mammography demonstrates coarse popcorn calcifications. What is the most likely diagnosis?
Phyllodes
Fibroadenoma [Table 2]
Breast Abscess
Breast cyst
Primary breast cancer
-
Which of the following is NOT a characteristic of Werner Syndrome?
“bird-like” facies
Short stature
Skin atrophy
Velvety skin [WS diagnosis is initially made in a clinical setting when patient presents with cardinal signs that include cataracts, short stature, premature greying and/or thinning of the scalp hair, “bird-like” facies and dermatological pathologies that include tight skin, atrophic skin, pigment alterations, ulcerations, hyperkeratosis and regional subcutaneous atrophy.]
Greying of skin
Table 1.
Summary table of Werner syndrome & Breast Cancer
| Werner Syndrome | Breast Cancer | Werner syndrome patients with Breast Cancer | |
|---|---|---|---|
| Etiology | WRN gene mutation | Breast Cancer is a multifactorial complex disease that causing DNA mutations that lead to malignant transformation. | Definitive etiology is unknown. Premature aging is pathognomonic in Werner syndrome and risk of breast cancer is directly proportional to age. |
| Incidence | 1 in 380,000 – 1,000,000 | 119 in 100,0001 | Rare2 |
| Gender Ratio | 1:1 | Women are 100 times more likely than man | Unknown, but no data to suggest different gender distribution compared to the general population. |
| Age Predilection | Progeria symptoms typically start to develop by age 25 | Most cases are diagnosed >50 years of age. | Unknown |
| Risk Factors | Werner syndrome has an autosomal recessive inheritance pattern | Most common risk factors include BRCA1 mutation in close relatives, obesity and prolonged estrogen exposure | Unknown, but no data to suggest different additional risk factors compared to the general population. |
| Treatment | Management of conditions associated to premature progeria | Treatment alternatives may include surgery, radiotherapy, chemotherapy or hormone therapy | Same recommendations apply as patients in the general population. Special consideration must be taken to concomitant health conditions. |
| Prognosis | Average lifespan is 55 years. | Prognosis depends on stage and molecular subtype. | Unknown |
| Findings on Imaging | Findings associated to age-related conditions | Categorized in BI-RADS classification. Solid lesions with irregular (spiculated) borders and microcalcifications. | No data to suggest associated additional findings compared to breast cancer patients without Werner Syndrome |
ABBREVIATIONS
- DNA
Deoxyribonucleic Acid
- WS
Werner syndrome
- CAD
Coronary Artery Disease
- BI-RADS
Breast-Imaging Reporting and Data System
- US
Ultrasound
- CT
Computed tomography
- MRI
Magnetic Resonance Imaging
- PET
Positron Emission Tomography
- FDG
Fluorodeoxyglucose-18F
- STIR
Short Tau Inversion Recovery
REFERENCES
- 1.Wang X, Liu S, Qin F, Liu Q, Wang Q. Werner syndrome presenting as early-onset diabetes: A case report. J Diabetes Investig. 2022;13(3):592–598. doi: 10.1111/jdi.13682. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 2.Oshima J, Martin GM, Hisama FM. Werner Syndrome. In: Adam MP, Mirzaa GM, Pagon RA, Wallace SE, Bean LJH, Gripp KW, Amemiya A, editors. GeneReviews((R)) Seattle (WA): 1993. [PubMed] [Google Scholar]
- 3.Lauper JM, Krause A, Vaughan TL, Monnat RJ., Jr Spectrum and risk of neoplasia in Werner syndrome: a systematic review. PLoS One. 2013;8(4):e59709. doi: 10.1371/journal.pone.0059709. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 4.Muftuoglu M, Oshima J, von Kobbe C, Cheng WH, Leistritz DF, Bohr VA. The clinical characteristics of Werner syndrome: molecular and biochemical diagnosis. Hum Genet. 2008;124(4):369–377. doi: 10.1007/s00439-008-0562-0. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 5.van Wietmarschen N, Nathan WJ, Nussenzweig A. The WRN helicase: resolving a new target in microsatellite unstable cancers. Curr Opin Genet Dev. 2021;71:34–38. doi: 10.1016/j.gde.2021.06.014. [DOI] [PubMed] [Google Scholar]
- 6.Chan EM, Shibue T, McFarland JM, et al. WRN helicase is a synthetic lethal target in microsatellite unstable cancers. Nature. 2019;568(7753):551–556. doi: 10.1038/s41586-019-1102-x. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 7.Giaquinto AN, Sung H, Miller KD, et al. Breast Cancer Statistics, 2022. CA Cancer J Clin. 2022;72(6):524–541. doi: 10.3322/caac.21754. [DOI] [PubMed] [Google Scholar]
- 8.Start Mammograms at 40, Not 50, USPSTF Suggests. Cancer Discov. 2023;13(7):1506. doi: 10.1158/2159-8290.CD-NB2023-0040. [DOI] [PubMed] [Google Scholar]
- 9.Albert L, Siu MMSPH on behalf ofthe U.S. Preventive Services Task Force. Screening for Breast Cancer: U.S. Preventive Services Task Force Recommendation Statement. Ann Intern Med. 2016;164(4):279–296. doi: 10.7326/M15-2886. [DOI] [PubMed] [Google Scholar]
- 10.Tesch ME, Partridge AH. Treatment of Breast Cancer in Young Adults. Am Soc Clin Oncol Educ Book. 2022;42:1–12. doi: 10.1200/EDBK_360970. [DOI] [PubMed] [Google Scholar]
- 11.Dhillon GS, Bell N, Ginat DT, Levit A, Destounis S, O'Connell A. Breast MR Imaging: What the Radiologist Needs to Know. J Clin Imaging Sci. 2011;1:48. doi: 10.4103/2156-7514.85655. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 12.Mahoney MC, Ingram AD. Breast Emergencies: Types, Imaging Features, and Management. AJR Am J Roentgenol. 2014;202(4):W390–W399. doi: 10.2214/AJR.13.11758. [DOI] [PubMed] [Google Scholar]
- 13.Bitencourt AGV, Gama RRM, Graziano L, et al. Breast metastases from extramammary malignancies: multimodality imaging aspects. Br J Radiol. 2017;90(1077):20170197. doi: 10.1259/bjr.20170197. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 14.Durhan G, Azizova A, Önder Ö, et al. Imaging Findings and Clinicopathological Correlation of Breast Cancer in Women under 40 Years Old. Eur J Breast Health. 2019;15(3):147–152. doi: 10.5152/ejbh.2019.4606. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 15.Radhakrishna S, Agarwal S, Parikh PM, et al. Role of magnetic resonance imaging in breast cancer management. South Asian J Cancer. 2018;7(2):69–71. doi: 10.4103/sajc.sajc_104_18. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 16.Adejolu M, Huo L, Rohren E, Santiago L, Yang WT. False-Positive Lesions Mimicking Breast Cancer on FDG PET and PET/CT. AJR Am J Roentgenol. 2012;198(3):W304–W314. doi: 10.2214/AJR.11.7130. [DOI] [PubMed] [Google Scholar]
- 17.Dong A, Wang Y, Lu J, Zuo C. Spectrum of the Breast Lesions With Increased 18F-FDG Uptake on PET/CT. Clin Nucl Med. 2016;41(7):543–557. doi: 10.1097/RLU.0000000000001203. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 18.Hines N, Slanetz PJ, Eisenberg RL. Cystic Masses of the Breast. American Journal of Roentgenology. 2010;194(2):W122–W133. doi: 10.2214/AJR.09.3688. [DOI] [PubMed] [Google Scholar]
- 19.Cloete DJ, Minne C, Schoub PK, Becker JHR. Magnetic resonance imaging of fibroadenoma-like lesions and correlation with Breast Imaging-Reporting and Data System and Kaiser scoring system. SA J Radiol. 2018;22(2):1532. doi: 10.4102/sajr.v22i2.1532. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 20.D’Orsi CJSE, Mendelson EB, Morris EA. ACR BI-RADS® Atlas, Breast Imaging Reporting and Data System. Reston, VA: American College of Radiology; 2013. [Google Scholar]
- 21.Lautrup S, Caponio D, Cheung H-H, et al. Studying Werner syndrome to elucidate mechanisms and therapeutics of human aging and age-related diseases. Biogerontology. 2019;20(3):255–269. doi: 10.1007/s10522-019-09798-2. [DOI] [PubMed] [Google Scholar]
- 22.Goto M, Ishikawa Y, Sugimoto M, Furuichi Y. Werner syndrome: a changing pattern of clinical manifestations in Japan (1917~2008) Biosci Trends. 2013;7(1):13–22. [PubMed] [Google Scholar]
- 23.Kato H, Koshizaka M, Kaneko H, Maezawa Y, Yokote K. Lifetime extension and the recent cause of death in Werner syndrome: a retrospective study from 2011 to 2020. Orphanet J Rare Dis. 2022;17(1):226. doi: 10.1186/s13023-022-02383-w. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 24.Juan-Salvadores P, Jiménez Díaz VA, Iglesia Carreño C, et al. Coronary Artery Disease in Very Young Patients: Analysis of Risk Factors and Long-Term Follow-Up. J Cardiovasc Dev Dis. 2022;9(3):82. doi: 10.3390/jcdd9030082. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 25.Aggarwal A, Srivastava S, Velmurugan M. Newer perspectives of coronary artery disease in young. World J Cardiol. 2016;8(12):728–734. doi: 10.4330/wjc.v8.i12.728. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 26.USCSW Group; U.S. Department of Health and Human Services CfDCaPaNCI, editor. U.S Cancer Statistics Data Visualizations Tool, based on 2022 submission data 1999–2020. Jun, 2023. https://www.cdc.gov/cancer/dataviz .