Abstract
In a previous paper we demonstrated that human polymorphonuclear cells (PMN) in the presence of normal human serum (NHS) secrete proinflammatory cytokines in response to Cryptococcus neoformans or its major capsular component, glucuronoxylomannan (GXM). The hypothesis that activation of the complement system could be responsible for the observed phenomenon is supported by the fact that encapsulated and acapsular C. neoformans isolates are activators of the complement system and, in particular, large encapsulated isolates are powerful activators. In the present study we demonstrate that (i) interleukin-8 (IL-8) release in response to acapsular or encapsulated strains of C. neoformans is significantly reduced in the presence of heat-inactivated serum rather than NHS and is completely abrogated in the absence of human serum; (ii) GXM-induced IL-8 release is strictly dependent on the presence of NHS, is inhibited by specific antibodies to either C3a and C5 complement components, and is completely abrogated by the combined use of these antibodies; (iii) the addition of purified C3a and C5a directly stimulates IL-8 release by PMN; and (iv) monoclonal antibody to GXM in combination with GXM or encapsulated C. neoformans potentiates IL-8 release by PMN. These data shed light on the mechanism involved in GXM-induced IL-8 secretion by PMN, provide an additional potential role for complement in the control of C. neoformans infections, and suggest a complex interplay between the complement system, humoral immunity, and cytokine regulation.
Cryptococcus neoformans is an opportunistic encapsulated yeast-like microorganism which causes cryptococcosis in 5 to 10% of adults (13) and 1% of children (23) with AIDS. Infection is believed to occur via inhalation of thinly encapsulated C. neoformans strains with subsequent synthesis of a large capsule in vivo. The capsule represents a major virulence factor that has a variety of effects on the host immune system (18, 19, 28, 32, 38). C. neoformans has a polysaccharide capsule which is antiphagocytic and interferes with antigen presentation and lymphocyte proliferation by reducing internalization of yeast cells by phagocytic cells (36). In addition, capsular polysaccharide is shed into tissue where it can have direct deleterious effects on host immune cells (7, 37). The argument for the latter effect is supported by recent observations that an adhesion molecule of the integrin family on polymorphonuclear cells (PMN) is a possible molecular target for glucuronoxylomannan (GXM), the major component of C. neoformans capsular material (8).
PMN are professional phagocytic cells which have been shown to be potent anticryptococcal effector cells in vitro (6, 28, 29, 35). Cooperation between PMN and humoral defense mechanisms is demonstrated by the fact that killing of C. neoformans by neutrophils is most efficient when complement-derived opsonins (3, 6) or capsule-binding antibodies (28) are present. PMN are part of the inflammatory response to C. neoformans infection and are sometimes found in close association with yeast cells (12, 25, 39). Evidence for the importance of PMN in protection against C. neoformans comes from mouse studies showing that PMN depletion enhances susceptibility to infection (15) whereas administration of recombinant granulocyte colony-stimulating factor enhances resistance to infection (14). Hence, there is strong evidence that PMN are involved in host defense against cryptococcal infection.
High titers of GXM are commonly found in body fluids of AIDS patients with cryptococcosis. Direct interaction of GXM with PMN has been reported as a regulator of tumor necrosis factor receptor expression and l-selectin shedding, which may prevent accumulation of neutrophils in infected tissues (7). Additional data on the interaction of GXM with human neutrophils, probably mediated by complement activation, have been provided by our group (34). Using an in vitro experimental system we found that encapsulated C. neoformans or purified GXM induced proinflammatory cytokine release by human PMN. The potent complement-activating potential of the cryptococcal capsule (20, 22, 41) suggests that induction of proinflammatory cytokine in PMN is a function of activation of the complement system and release of biologically active complement cleavage fragments.
For example, C3a and/or C5a have been involved in the activation of phagocytic cells. Interleukin-8 (IL-8) synthesis by human monocytes (11) and by PMN (1) has been documented. In this study, the possible contributions of C3a and C5a in the activation of human PMN induced by (i) GXM, (ii) encapsulated C. neoformans, or (iii) acapsular strains of C. neoformans were evaluated. The results indicate a critical role for C3a and C5a in C. neoformans-dependent induction of IL-8 secretion by PMN.
MATERIALS AND METHODS
C. neoformans and cryptococcal polysaccharide.
CBS 6995 is a thinly encapsulated strain of C. neoformans var. neoformans serotype A (CBS 6995 = NIH 37 [National Institutes of Health, Bethesda, Md.]); CBS 7698 is an acapsular mutant of C. neoformans var. neoformans (CBS 7698 = NIH B4131). Strains 6995 and 7698 were obtained from J. Orendi (Delft, The Netherlands). The cultures were maintained by serial passage on Sabouraud agar (BioMerieux, Lyon, France) and harvested by suspending a single colony in RPMI 1640; the cells were washed twice, counted on a hemacytometer, and adjusted to the desired concentration. C. neoformans strains (CBS 6995 and CBS 7698) were inactivated by autoclaving. GXM was isolated from culture supernatant fluid of a serotype A isolate (ATCC 24064) by differential precipitation with ethanol and cetyltrimethylammonium bromide (2).
Reagents.
RPMI 1640 and fetal bovine serum were obtained from Eurobio Laboratories (Paris, France). Normal human serum (NHS) was obtained from Biosource International (Camarillo, Calif.). In selected experiments, NHS was heated to 56°C for 30 min to inactivate complement components. Lipopolysaccharide (LPS) from Escherichia coli O55:135 was obtained from Difco Laboratories (Detroit, Mich.). RPMI 1640 and C. neoformans cells (approximately 5 × 108) were tested for endotoxic contamination by the Limulus amebocyte lysate assay (Sigma Chemical Co., St. Louis, Mo.), which has a sensitivity of approximately 0.05 to 0.1 ng of E. coli LPS per ml. All reagents tested negative for endotoxic contamination.
Human complement C3a (Cortex Biochem, San Leandro, Calif.), human recombinant C5a (C5a; Fluka Chimica, Division Sigma Aldrich, Milan, Italy), monoclonal antibody (MAb) anti-human C3a (MAb anti-C3a [catalog no. A203]; Quidel, San Diego, Calif.) and goat anti-human C5 (catalog no. A306; Quidel) were purchased from the indicated sources. The polyclonal anti-human C5 shows strong reactivity to human C5a (33). MAb 2H1, a murine immunoglobulin G1 (IgG1) that binds to capsular GXM, was isolated from ascites fluid as previously described (31). A control irrelevant mouse IgG1 was obtained from Sigma (catalog no. M 5284).
Preparation of PMN.
Heparinized venous blood, obtained from healthy donors, was diluted with RPMI 1640, and mononuclear cells were separated by Ficoll-Hypaque density gradient centrifugation (34). The pellet containing PMN and erythrocytes was treated with hypotonic saline to lyse the erythrocytes. Granulocytes were collected by centrifugation, washed twice in RPMI 1640, counted, and adjusted to the desired concentration. The purity of PMN isolated by this method was always >98%, as determined by Giemsa staining. PMN preparations contained about 0.5 to 1% eosinophils. PMN viability evaluated after 18 h of incubation was >98% in all determinations performed, as assessed by a trypan blue dye exclusion test.
Production of PMN culture supernatants.
Isolated PMN were distributed in 1-ml volumes into 24-well flat-bottom tissue culture plates (Becton Dickinson, Oxnard, Calif.) at 4 × 106 cells per ml and incubated for 18 h at 37°C under a 5% CO2 atmosphere with RPMI containing 10% NHS, 10% heat-inactivated serum (HIS), or RPMI 1640 singly or in various combinations with (i) LPS (10 μg/ml), (ii) heat-inactivated C. neoformans 6995 or 7698 at an effector-to-target cell ratio (E:T) of 1:5, (iii) GXM (250 μg/ml), (iv) various doses of anti-C3a and/or anti-C5, or (v) C3a and/or C5a. Supernatant fluids were harvested at the indicated times and stored at −20°C until use.
Cytokine level determination.
Cytokine levels in culture supernatant fluids were measured with an enzyme-linked immunosorbent assay kit for human IL-8 (Biosource International).
Statistical analysis.
Statistical analysis was performed by using Student’s t test.
RESULTS
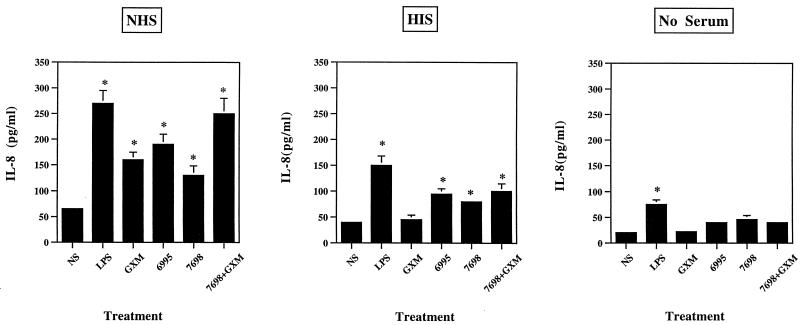
In our previous study we established that PMN from normal subjects induce proinflammatory cytokines in response to stimulation with C. neoformans or purified capsular material in the presence of NHS (34). To verify the contribution of NHS in the observed phenomenon, PMN were cultured for 18 h with or without stimuli in the presence or absence of NHS or HIS. Experimental variables included stimulation of PMN with (i) GXM alone, (ii) encapsulated cryptococci (6995), and (iii) acapsular C. neoformans (7698) with or without GXM. The results reported in Fig. 1 show that maximum IL-8 levels were detected when PMN were incubated with various stimulants in the presence of NHS. Substitution of HIS for NHS produced a substantial decrease in IL-8 production by PMN treated with (i) encapsulated cryptococci, (ii) acapsular cryptococci alone, or (iii) acapsular cryptococci in the presence of GXM. GXM-induced IL-8 secretion was absent if HIS was used in place of NHS. IL-8 levels comparable to background levels were observed when PMN were stimulated with C. neoformans or GXM in the absence of serum. However, under the latter condition, PMN secreted significant amounts of IL-8 in response to LPS. PMN viability under our experimental conditions was >98% after 18 h of incubation.
FIG. 1.
IL-8 release from human PMN exposed to LPS (10 μg/ml), GXM (250 μg/ml), encapsulated C. neoformans (6995), acapsular C. neoformans (7698), or acapsular C. neoformans (7698) plus GXM (250 μg/ml) (E:T, 1:5) in the presence of NHS or HIS or in the absence of serum. The results are given as the means ± standard errors of the means (error bars) of four separate experiments. ∗, P < 0.05 (treated versus untreated cells).
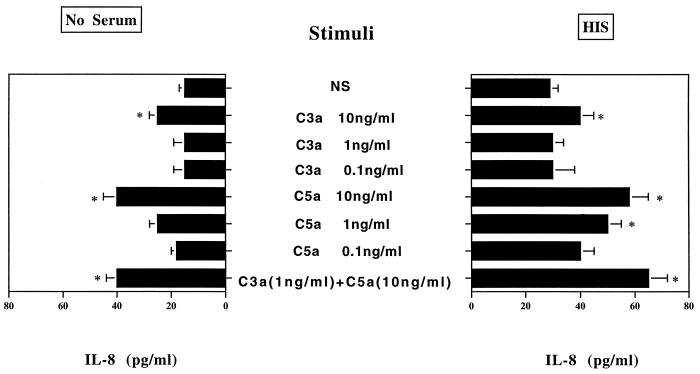
Data from Fig. 1 strongly suggest that both heat-sensitive and heat-resistant serum components play a crucial role in PMN activation in our experimental system. Natural antibodies to C. neoformans glucan (17) or GXM (9, 16) found in serum of normal subjects are possible candidates for IL-8 induction by PMN, which could activate phagocytic cells by Fc receptor stimulation (4). Alternatively, cleavage fragments of complement proteins (C3a and/or C5a) generated during complement system activation by C. neoformans and/or GXM may be responsible for IL-8 synthesis by PMN. C5a has been reported to stimulate PMN release of IL-8 (11). As an initial step in our examination of the role of C. neoformans-induced complement cleavage fragments in PMN release of IL-8, we determined the contribution of purified factors C3a and C5a to PMN-induced cytokine release in our experimental system. The results (Fig. 2) showed that C5a and C3a induced IL-8 secretion by PMN, and the association of these compounds did not result in a synergistic effect.
FIG. 2.
Effect of addition of various doses of C3a and/or C5a on IL-8 release by human PMN. The results are given as the means ± standard errors of the means (error bars) of three separate experiments. NS, no stimulus; ∗, P < 0.05 (treated versus untreated cells).
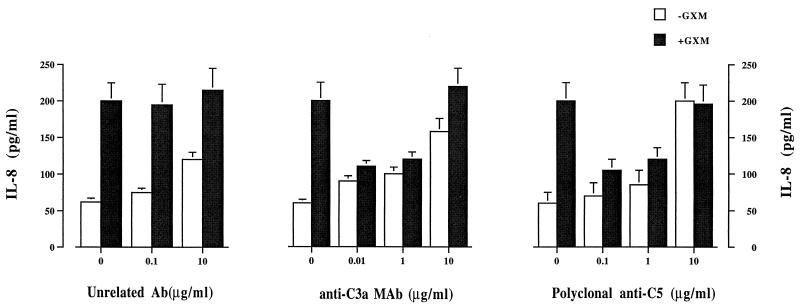
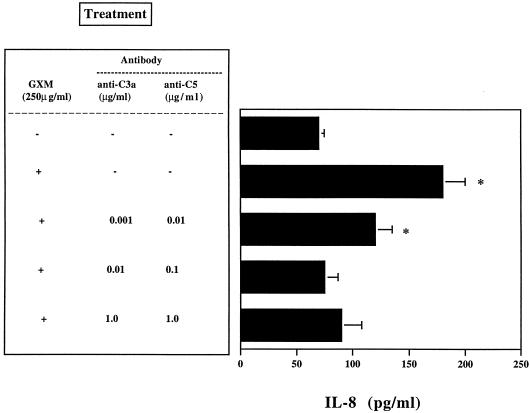
Having established that purified C3a and C5a induce IL-8 secretion in our experimental system, we next determined the effect of anti-C3a and anti-C5 antibodies on GXM-induced IL-8 secretion. The results showed that the optimal concentrations of neutralizing C3a and C5 were 0.01 and 0.1 μg/ml, respectively (Fig. 3). Lower concentrations were ineffective, and higher concentrations probably induced PMN activation. Neutralization of C3a and C5 resulted in a dramatic decrease in IL-8 levels after PMN were exposed to GXM. The combined use of anti-C3a and anti-C5 at optimal doses resulted in complete inhibition of GXM-induced IL-8 secretion (Fig. 4).
FIG. 3.
Dose-dependent effect of anti-C3a MAb or polyclonal anti-C5 on GXM (250 μg/ml)-induced (+GXM) and noninduced (−GXM) IL-8 release by human PMN. The results are given as the means ± standard errors of the means (error bars) of five separate experiments. Ab, antibody; ∗, P < 0.05 (GXM-plus-antibody-treated cells versus GXM-treated cells).
FIG. 4.
Effect of combined use of anti-C3a MAb and polyclonal anti-C5 on IL-8 release by PMN exposed (+) or not exposed (−) to GXM (250 μg/ml). The results are given as the means ± standard errors of the means (error bars) of four separate experiments. ∗, P < 0.05 (GXM-treated cells versus GXM-untreated cells).
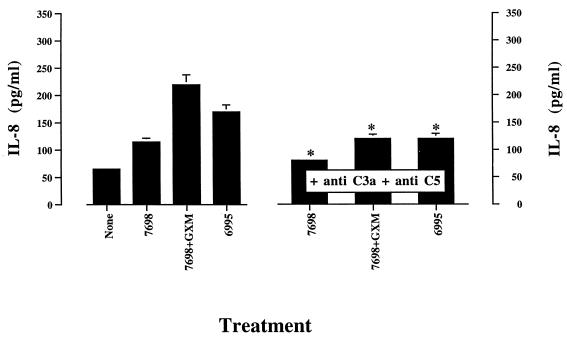
Encapsulated and acapsular strains of C. neoformans are powerful activators of the complement system via the alternative and classical pathways, respectively (20, 21, 40). Incorporation of anti-C3a and anti-C5 into the incubation medium was used to assess the contribution of C3a and C5a to IL-8 induction by PMN exposed to encapsulated or acapsular strains of C. neoformans. The results (Fig. 5) demonstrate that C3a and/or C5a play a significant role in C. neoformans-induced IL-8 secretion by PMN.
FIG. 5.
Effect of addition of anti-C3a (0.01 μg/ml) and anti-C5 (0.1 μg/ml) on IL-8 release by PMN exposed to indicated stimuli. Acapsular (7698) or encapsulated (6995) C. neoformans was added to PMN at an E:T of 1:5 in the presence of 10% NHS. GXM was added to PMN at a concentration of 250 μg/ml. IL-8 production by PMN stimulated with anti-C3a (0.1 μg/ml) and anti-C5 (0.1 μg/ml) was comparable to that produced by untreated cells (None). The results are given as the means ± standard errors of the means (error bars) of three separate experiments. ∗, P < 0.05 (C. neoformans-plus-antibody-treated cells versus C. neoformans-treated cells).
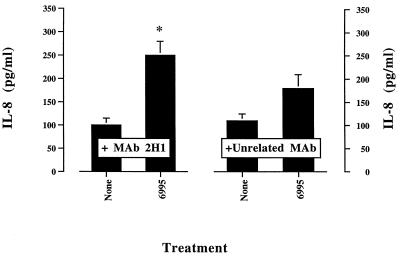
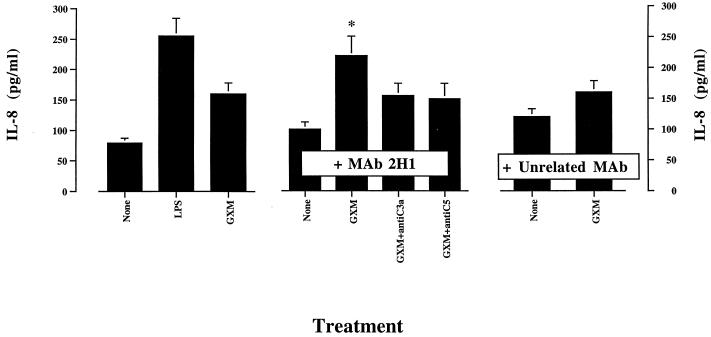
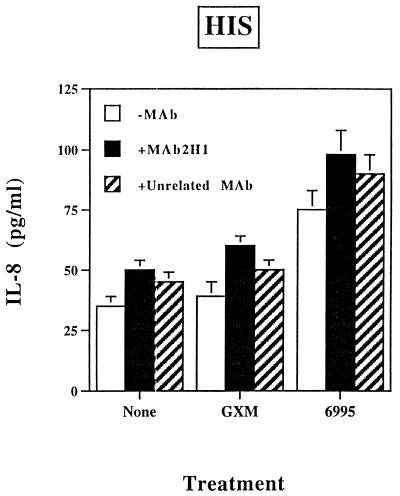
Passive immunization with MAbs to GXM can be protective in murine models of cryptococcosis (30, 31). The precise mechanism for MAb-mediated protection has not been determined. Contributing factors could include a shift toward activation of the classical pathway and modification of cytokine release. As a consequence, we examined the effect of the GXM-binding MAb 2H1 on 6995-induced IL-8 release by PMN. The results showed that addition of the anti-GXM MAb 2H1 enhanced IL-8 secretion induced by the encapsulated strain (6995) (Fig. 6). A potentiating effect of MAb 2H1 was also observed with soluble GXM as the stimulus (Fig. 7). Under these conditions, the addition of antibodies to C3a or C5 to neutralize these complement components results in a substantial reduction of the MAb 2H1-mediated effect (Fig. 7). The combination of GXM plus MAb 2H1 plus anti-C3a plus anti-C5 enhanced rather than suppressed IL-8 release by PMN relative to IL-8 secretion observed when the combination of GXM plus MAb 2H1 was used (data not shown), suggesting that the use of three different antibodies in our experimental system is not appropriate. MAb 2H1-mediated activation of PMN exposed to GXM could imply, at least in part, the activation of PMN via Fc receptor cross-linking. To test this hypothesis, the effects of MAb 2H1 on induction of IL-8 secretion by exposure of PMN to soluble GXM or whole cells of strain 6995 were tested in the presence of HIS. The results (Fig. 8) show the limited capability of MAb 2H1 to synergize with soluble GXM or whole cells of strain 6995 when HIS was used in place of NHS. These results strongly suggest that activation of the complement system is the primary mechanism by which MAb 2H1 facilitates IL-8 secretion.
FIG. 6.
Effect of addition of MAb 2H1 (10 μg/ml) on IL-8 release by PMN exposed to encapsulated C. neoformans (6995) (E:T, 1:5) in the presence of 10% NHS. The results are given as the means ± standard errors of the means (error bars) of three separate experiments. ∗, P < 0.05 (MAb 2H1-treated cells versus unrelated MAb-treated cells).
FIG. 7.
Effect of addition of MAb 2H1 (10 μg/ml) on IL-8 release by PMN exposed to GXM (250 μg/ml) in the presence or absence of anti-C3a (0.01 μg/ml) or anti-C5 (0.1 μg/ml). The results are given as the means ± standard errors of the means (error bars) of three separate experiments. ∗, P < 0.05 (GXM-plus-MAb 2H1-treated cells versus GXM-plus-unrelated-MAb-treated cells).
FIG. 8.
Effect of addition of MAb 2H1 (+MAb 2H1) on IL-8 secretion by PMN exposed to GXM (250 μg/ml) or encapsulated C. neoformans (E:T, 1:5) in the presence of HIS. The results are given as the means ± standard errors of the means (error bars) of three separate experiments. −MAb, without MAb, +Unrelated MAb, with unrelated MAb.
DISCUSSION
We previously demonstrated that PMN release proinflammatory cytokines in response to C. neoformans. The magnitude of the cytokine response correlated with the presence and size of the capsule. The major component of the capsule, GXM, was directly involved in the observed induction. In this study we examined the mechanism involved in IL-8 release by PMN exposed to C. neoformans or GXM. Our results show that heat labile components of NHS are required and that human C3a and C5a are the principal candidates for induction of IL-8 secretion by PMN. Neutralization of C3a and C5 with specific antibody resulted in complete inhibition of GXM-induced IL-8 in serum. The potential for involvement of C3a and C5a in cytokine release by PMN was confirmed by addition of purified C3a and C5a to PMN, showing that IL-8 is secreted in response to both stimuli. It has been recently reported that GXM induces IL-8 release from human microglia and that GXM is able to inhibit PMN migration toward IL-8 (26). As a consequence, the presence of IL-8 may not imply chemotactic activity. Hence, the availability of complement could be essential for cell migration towards the inflammatory sites.
GXM-binding MAb, in combination with GXM or with the encapsulated strain, further increased IL-8 secretion beyond levels observed in the absence of the MAb. Fc receptor cross-linking can activate phagocytic cells to release cytokines (4). However, in our experimental system Fc-mediated activation appears to play a modest role, because there was little specific enhancement of IL-8 secretion in the presence of HIS (Fig. 8). These results suggest that the principal mechanism for the potentiating effect induced by MAb 2H1 occurs through the generation of active complement fragments. C5 complement-deficient mice intravenously infected with C. neoformans die of acute pneumonia characterized by massive edema, which can be prevented by administration of capsule-specific IgG1 (10). The mechanism for this effect is not understood, but phagocytic cell recruitment is believed to be important for an effective host response in the lung. Our results demonstrate that the interaction among PMN, specific antibody, complement, and cryptococcus enhances the secretion of a proinflammatory cytokine, suggesting the need for detailed studies of cytokine expression in antibody-treated mice.
Both encapsulated and acapsular C. neoformans strains are powerful activators of the complement system via the alternative and classical pathways, respectively. In contrast, fluid-phase GXM has been described as a relatively weak activator of the complement system (5, 24). These earlier studies of complement activation by purified GXM used consumption of late complement components (5) or generation of chemotactic cleavage fragments (24) as indicators of complement activation. As a consequence, generation of biologically active complement fragments by GXM in the present study was somewhat unexpected. It is possible that stimulation of IL-8 release by PMN is a more sensitive measure of complement activation by GXM than endpoints used in previous studies.
The inability of GXM to stimulate IL-8 secretion in the presence of HIS demonstrates the essential role of the complement system in stimulation of IL-8 secretion by GXM alone. In contrast, appreciable levels of IL-8 were observed when PMN were stimulated in the presence of HIS by (i) encapsulated cryptococci, (ii) acapsular yeast alone, or (iii) acapsular cryptococci exposed to GXM. PMN could be activated by compounds of C. neoformans envelope other than GXM such as glucan, mannoprotein, or galactoxylomanna. Alternatively, GXM as it is displayed on the surface of the capsule could directly stimulate PMN in a manner that does not occur with purified GXM alone. Such stimulation could occur through PMN receptors such as CD-18 (8). It is known that C5a is able to induce IL-8 release from PMN (1). However, our data are novel in that we describe C3a-mediated induction of IL-8 and we explain a new mechanism by which GXM could affect cellular immune function.
A previous study has shown that immune complexes induce IL-8 production in human monocytes but not PMN (27). Our results showing that MAb 2H1 alone did not induce IL-8 production are consistent with this report. However, 2H1 did enhance IL-8 production in the presence of NHS to a level greater than that observed with NHS alone. Since anti-GXM antibodies to the capsule can promote rapid classical pathway activation, we interpret this result as consistent with increased C3a and C5a fragments in the presence of specific antibody.
Taken together, our results indicate that (i) heat-labile components of human serum are required for GXM-mediated IL-8 secretion by PMN, (ii) this phenomenon is dramatically reduced using appropriate doses of anti-C3a and anti-C5, (iii) the combination of anti-C3a and anti-C5 completely abrogates the effect, (iv) active C3a and C5a fragments directly induce IL-8 secretion by PMN, and (v) the use of anti-GXM MAb in combination with GXM or with the encapsulated strain (6995) improves IL-8 release. The capacity of GXM to induce PMN activation via generation of biologically active complement fragments could be a positive signal that contributes to recruitment and activation of PMN in the inflammation sites during cryptococcosis.
ACKNOWLEDGMENTS
We are grateful to Eileen Zannetti for excellent editorial and secretarial assistance.
This study was supported by the National AIDS Research Program “Opportunistic Infections and Tuberculosis,” contract 50A.0.35, Italy.
REFERENCES
- 1.Cassatella M A, Bazzoni F, Ceska M, Ferro I, Baggiolini M, Berton G. IL-8 production by human polymorphonuclear leukocytes. The chemoattractant formyl-methionyl-leucyl-phenylamine induces the gene expression and release of IL-8 through a pertussis toxin-sensitive pathway. J Immunol. 1992;148:3216–3220. [PubMed] [Google Scholar]
- 2.Cherniak R, Reiss E, Slodki M E, Plattner R D, Blumer S O. Structure and antigenic activity of the capsular polysaccharide to Cryptococcus neoformans. Mol Immunol. 1980;17:1025–1032. doi: 10.1016/0161-5890(80)90096-6. [DOI] [PubMed] [Google Scholar]
- 3.Davies S P, Clifford D P, Hoidal J R, Repine J E. Opsonic requirements for the uptake of Cryptococcus neoformans by human polymorphonuclear leukocytes and monocytes. J Infect Dis. 1982;145:870–874. doi: 10.1093/infdis/145.6.870. [DOI] [PubMed] [Google Scholar]
- 4.Debets J M, Van Der Linder J, Dieteren I E M, Leewenberg J F M, Bourman W A. Fc receptor cross-linking induces rapid secretion of tumor necrosis factor (cachectin) by human peripheral blood monocytes. J Immunol. 1988;4:197–201. [PubMed] [Google Scholar]
- 5.Diamond R D, May J E, Kane M A, Frank M M, Bennett J E. The role of the classical and alternative complement pathways in host defenses against Cryptococcus neoformans infection. J Immunol. 1974;112:2260–2270. [PubMed] [Google Scholar]
- 6.Diamond R D, Root R K, Bennett J E. Factors influencing killing of Cryptococcus neoformans by human leukocytes in vitro. J Infect Dis. 1972;125:367–376. doi: 10.1093/infdis/125.4.367. [DOI] [PubMed] [Google Scholar]
- 7.Dong Z M, Murphy J W. Cryptococcal polysaccharides induce L-selectin shedding and tumor necrosis factor receptor loss from the surface of human neutrophils. J Clin Invest. 1996;97:689–698. doi: 10.1172/JCI118466. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 8.Dong Z M, Murphy J W. Cryptococcal polysaccharides bind to CD-18 on human neutrophils. Infect Immun. 1997;65:557–563. doi: 10.1128/iai.65.2.557-563.1997. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 9.Dromer F, Aucouturier P, Clauvel J-P, Saimot G, Yeni P. Cryptococcus neoformans antibody levels in patients with AIDS. Scand J Infect Dis. 1988;20:283–285. doi: 10.3109/00365548809032452. [DOI] [PubMed] [Google Scholar]
- 10.Dromer F, Perronne C, Barge J, Vilde J L, Yeni P. Role of IgG and complement component C5 in the initial course of experimental cryptococcosis. Clin Exp Immunol. 1989;78:412–417. [PMC free article] [PubMed] [Google Scholar]
- 11.Ember J A, Sanderson S D, Hugli T E, Morgan E L. Induction of interleukin-8 synthesis from monocytes by human C5a anaphylotoxin. Am J Pathol. 1994;146:393–403. [PMC free article] [PubMed] [Google Scholar]
- 12.Fazekas G, Schwarz J. Histology of experimental murine cryptococcosis. Am J Pathol. 1958;34:517–529. [PMC free article] [PubMed] [Google Scholar]
- 13.Good C B, Coax W A. Cryptococcal infection in patients with AIDS. N Engl J Med. 1990;322:701–702. doi: 10.1056/nejm199003083221017. [DOI] [PubMed] [Google Scholar]
- 14.Graybill J R, Bocanegra R, Lambros C, Luther M F. Granulocyte colony stimulating factor therapy of experimental cryptococcal meningitis. J Med Vet Mycol. 1997;35:243–247. doi: 10.1080/02681219780001221. [DOI] [PubMed] [Google Scholar]
- 15.Graybill J R, Mitchell L. Cyclophosphamide effects on murine cryptococcosis. Infect Immun. 1978;21:674–677. doi: 10.1128/iai.21.2.674-677.1978. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 16.Houpt D C, Pfrommer G T, Young B J, Larson T A, Kozel T R. Occurrences, immunoglobulin classes, and biological activities of antibodies in normal human serum that are reactive with Cryptococcus neoformans glucuronoxylomannan. Infect Immun. 1994;62:2857–2864. doi: 10.1128/iai.62.7.2857-2864.1994. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 17.Keller R G, Pfrommer G S T, Kozel T R. Occurrences, specificities, and functions of ubiquitous antibodies in human serum that are reactive with the Cryptococcus neoformans cell wall. Infect Immun. 1994;62:215–220. doi: 10.1128/iai.62.1.215-220.1994. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 18.Kozel T R, Gotschlich E C. The capsule of Cryptococcus neoformans passively inhibits phagocytosis of the yeast macrophages. J Immunol. 1982;129:1675–1680. [PubMed] [Google Scholar]
- 19.Kozel T R, Mastroianni R P. Inhibition of phagocytosis by cryptococcal polysaccharide: dissociation of the attachment and ingestion phases of phagocytosis. Infect Immun. 1976;14:62–67. doi: 10.1128/iai.14.1.62-67.1976. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 20.Kozel T R, Wilson M A, Murphy J W. Early events in initiation of alternative complement pathway activation by the capsule of Cryptococcus neoformans. Infect Immun. 1991;59:3101–3110. doi: 10.1128/iai.59.9.3101-3110.1991. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 21.Kozel T R, Wilson M A, Pfrommer G S T, Schlageter A M. Activation and binding of opsonic fragments of C3 on encapsulated Cryptococcus neoformans by using an alternative complement pathway reconstituted from six isolated proteins. Infect Immun. 1989;57:1922–1927. doi: 10.1128/iai.57.7.1922-1927.1989. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 22.Kozel T R, Wilson M A, Welch W H. Kinetic analysis of the amplification phase for activation and binding of C3 to encapsulated and nonencapsulated Cryptococcus neoformans. Infect Immun. 1992;60:3122–3127. doi: 10.1128/iai.60.8.3122-3127.1992. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 23.Kwong-Chung K J, Bennett J E. Medical mycology. Baltimore, Md: Williams and Wilkins; 1992. pp. 397–446. [Google Scholar]
- 24.Laxalt K A, Kozel T R. Chemotaxigenesis and activation of the alternative complement pathway by encapsulated and non-encapsulated Cryptococcus neoformans. Infect Immun. 1979;26:435–440. doi: 10.1128/iai.26.2.435-440.1979. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 25.Levine S, Zimmerman H M, Scorza A. Experimental cryptococcosis (torulosis) Am J Pathol. 1957;33:385–409. [PMC free article] [PubMed] [Google Scholar]
- 26.Lipovsky M M, Gekker G, Hu S, Ehrlich L C, Hoepelman A I, Peterson P K. Cryptococcal glucuronoxylomannan induces interleukin (IL)-8 production by human microglia but inhibits neutrophil migration toward IL-8. J Infect Dis. 1998;177:260–263. doi: 10.1086/517368. [DOI] [PubMed] [Google Scholar]
- 27.Marsch C B, Gadek J E, Kindt G C, Moore S A, Wewers M D. Monocyte Fc-γ receptor cross-linking induces IL-8 production. J Immunol. 1995;155:3161–3167. [PubMed] [Google Scholar]
- 28.Miller G P G, Kohl S. Antibody-dependent leucocyte killing of Cryptococcus neoformans. J Immunol. 1983;131:1455–1459. [PubMed] [Google Scholar]
- 29.Miller M F, Mitchell T G. Killing of Cryptococcus neoformans strains by human neutrophils and monocytes. Infect Immun. 1991;59:24–28. doi: 10.1128/iai.59.1.24-28.1991. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 30.Mukherjee J, Scharff M D, Casadevall A. Protective murine monoclonal antibodies to Cryptococcus neoformans. Infect Immun. 1992;60:4534–4541. doi: 10.1128/iai.60.11.4534-4541.1992. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 31.Mukherjee S, Lee S, Mukherjee J, Scharff M D, Casadevall A. Monoclonal antibodies to Cryptococcus neoformans capsular polysaccharide modify the course of intravenous infection in mice. Infect Immun. 1994;62:1079–1088. doi: 10.1128/iai.62.3.1079-1088.1994. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 32.Murphy J W, Cozad G C. Immunological unresponsiveness induced by cryptococcal capsular polysaccharide assayed by the hemolytic plaque technique. Infect Immun. 1972;5:896–901. doi: 10.1128/iai.5.6.896-901.1972. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 33.Quidel . Technical bulletin, goat anti-C5. San Diego, Calif: Quidel; 1994. [Google Scholar]
- 34.Retini C, Vecchiarelli A, Monari C, Tascini C, Bistoni F, Kozel T R. Capsular polysaccharide of Cryptococcus neoformans induces proinflammatory cytokine release by human neutrophils. Infect Immun. 1996;64:2897–2903. doi: 10.1128/iai.64.8.2897-2903.1996. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 35.Tacker J B, Farhi F, Bulmer G S. Intracellular fate of Cryptococcus neoformans. Infect Immun. 1972;6:162–167. doi: 10.1128/iai.6.2.162-167.1972. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 36.Vecchiarelli A, Pietrella D, Dottorini M, Monari C, Retini C, Todisco T. Encapsulation of Cryptococcus neoformans regulates fungicidal activity and the antigen presentation process in human alveolar macrophages. Clin Exp Immunol. 1994;98:217–223. doi: 10.1111/j.1365-2249.1994.tb06128.x. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 37.Vecchiarelli A, Retini C, Monari C, Tascini C, Bistoni F, Kozel T R. Purified capsular polysaccharide of Cryptococcus neoformans induces interleukin-10 secretion by human monocytes. Infect Immun. 1996;64:2846–2849. doi: 10.1128/iai.64.7.2846-2849.1996. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 38.Vecchiarelli A, Retini C, Pietrella D, Monari C, Tascini C, Beccari T, Kozel T R. Downregulation by cryptococcal polysaccharide of tumor necrosis factor alpha and interleukin-1β secretion from human monocytes. Infect Immun. 1995;63:2919–2923. doi: 10.1128/iai.63.8.2919-2923.1995. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 39.Weiss C, Perry I H, Shevky M C. Infection of the human eye with Cryptococcus neoformans (Torula histolytica; Cryptococcus hominis) Arch Ophthalmol. 1948;39:739–751. doi: 10.1001/archopht.1948.00900020749003. [DOI] [PubMed] [Google Scholar]
- 40.Wilson M A, Kozel T R. Contribution of antibody in normal human serum to early deposition of C3 onto encapsulated and nonencapsulated Cryptococcus neoformans. Infect Immun. 1992;60:754–761. doi: 10.1128/iai.60.3.754-761.1992. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 41.Young B I, Kozel T R. Effects of strain variation, serotype, and structural modification on kinetics for activation and binding of C3 to Cryptococcus neoformans. Infect Immun. 1993;61:2966–2972. doi: 10.1128/iai.61.7.2966-2972.1993. [DOI] [PMC free article] [PubMed] [Google Scholar]