Abstract
Double sleeve lung resections are complex surgical procedures that require specialized surgical expertise and careful patient selection. These procedures allow for the preservation of lung tissue while still achieving complete tumor resection for central tumors. Although initially considered high-risk operations, double sleeve lung resections have become a viable option for central tumors. Recent studies have shown that double sleeve lung resections are associated with lower morbidity and mortality rates than pneumonectomy. Furthermore, double sleeve lung resections may be associated with similar or even better long-term oncological outcomes compared to pneumonectomy, with the added benefit of preserving lung parenchyma and reducing the incidence of postoperative complications.
Keywords: Double sleeve, extended, lung resections, parenchyma sparing
Definition and prevalence
A double sleeve lung resection, also known as a bronchovascular sleeve lung resection, is a surgical procedure that involves the resection of the lung, along with the resection and anastomosis of the associated bronchus and pulmonary artery (PA). The procedure is typically performed to resect centrally located lung cancer, as well as some benign diseases.
Thomas[1] reported the first bronchial sleeve resection for a carcinoid tumor in 1945. In 1952, Allison[2] performed the first tangential resection with a direct suture of the PA and, in 1959, he also performed the first arterial sleeve resection. During the 1970s and 1980s, only a few groups gained significant experience with these procedures. One of the most notable studies on this topic was published by Vogt-Moykopf et al.[3] in 1986, which included a series of 37 arterial sleeve resections.
Pneumonectomy was the choice of surgical procedure for central non-small cell lung cancer (NSCLC) with bronchial or vascular invasion. Sleeve resection surgery was suggested for patients who were unable to tolerate pneumonectomy due to comorbidities or compromised pulmonary function. However, the paradigm has been shifted to perform lung-sparing resections in patients who are able to tolerate pneumonectomy. This paradigm shift allows for the preservation of healthy lung tissue and is no longer limited to only those who are considered compromised or at higher risk for complications.[4,5] Currently, sleeve resection is performed in approximately 6 to 8% of resections for primary lung cancer. Double sleeve lobectomy is indeed more common on the left side due to the proximity of the left upper lobe and the first branches of the left PA. In 80% of cases, it is performed in the left upper lobe.
Benefits
Sleeve resections create an advantage compared to pneumonectomy by decreasing the loss of pulmonary reserve. There is some evidence to suggest that sleeve lobectomy may be associated with similar or even better survival and lower recurrence rates compared to pneumonectomy.[6-10]
Better overall survival (OS) may be associated with that sleeve lobectomy that allows the patient to retain a portion of their lung, improving respiratory function and reducing the risk of complications. Pneumonectomy always carries a higher risk of pulmonary edema, acute respiratory distress syndrome (ARDS) post-pneumonectomy syndrome, and bronchopleural fistula than lesser resections.[11,12]
Double sleeve lobectomy requires a high level of skill and expertise of surgeons to perform successfully. Specific outcomes of these procedures vary depending on the surgical skill and experience of the surgeon, individual patient characteristics, and the specifics of the case.
Indications and contraindications
Sleeve resection is indicated for the patient with primary tumors or N1 nodes which infiltrate the origin of the lobar branches of the PA, but not to the extent that a pneumonectomy is necessary. It is not always possible to identify definitively prior to surgery whether a reconstructive procedure of the PA would be required.
It should be kept in mind that any potential candidate for double sleeve lobectomy may ultimately require pneumonectomy according to surgical findings. Therefore, each patient should be evaluated, as if they are scheduled to undergo pneumonectomy. It is important to differentiate between an “obligated” double sleeve lobectomy, which is necessary for a patient who are unable to tolerate pneumonectomy and carries a higher risk than a “preferred” double sleeve lobectomy.
Double sleeve lobectomy is a complex procedure that requires careful planning and should only be performed by experienced surgeons in specialized centers. As with any lung resection, the complexity of surgery can lead to a longer operation time. It is of utmost importance to carefully evaluate the patient’s cardiovascular and pulmonary function before surgery. It is also critical to consider the possibility that the procedure may need to be converted to a pneumonectomy. In these cases, it is advisable to use advanced cardiac and pulmonary function tests to ensure the safety of the patients.
Preoperative evaluation
Preoperative evaluations before double sleeve lobectomies are similar to regular lobectomies; however, additional measures need to be taken.
Contrast-enhanced cross-sectional thoracic computed tomography (CT) and CT angiography before the operation should be performed for all resections. Various imaging post-processing techniques added to CT can be utilized to analyze and identify the extent of tumor invasion into the PA and bronchus. The multiplanar reconstruction (MPR), maximum intensity projection (MIP), and volume rendering (VR) techniques can be applied to reconstruct blood vessel images of the pulmonary trunk, apical, posterior, and anterior segmental artery of the upper lobe, while the minimum intensity projection image (MinIP) and VR techniques can be used to reconstruct images of the lobar and segmental bronchi. The results show that CT post-processing techniques and surgical pathological findings demonstrate consistent and high diagnostic accuracy, which can likely compensate for the limitations of routine cross-sectional CT.[13]
Magnetic resonance imaging can provide additional information about the extent of invasion to the PA. However, it may not be always clearly defined preoperatively, and the final decision may be made during surgery.
Operative procedures
Flexible bronchoscope should be performed before surgery to identify the extent of the tumor and aspirate retained secretions.
The patient should be intubated with a double-lumen endotracheal tube or endobronchial blocker and placed in a lateral decubitus position. In open surgery, an incision is typically made at the fourth or fifth intercostal space in the anterior axillary line. The same intercostal space can be used for uniportal video-assisted thoracoscopic surgery (VATS) surgery. In biportal VATS surgery, it is possible to add a camera port through the seventh or eighth intercostal space in the posterior axillary line.
Releasing the inferior pulmonary ligament can help to provide sufficient mobility during surgery and protect the anastomosis from tension. If there is high tension on the anastomosis, hilar and pericardial release may be performed.[14]
The recurrent laryngeal and vagus nerves are routinely identified during surgery and preserved, if these nerves are not invaded by the tumor.
From an oncological standpoint, dividing the pulmonary vein as the initial step of the resection may help prevent the spread of potential circulating tumor cells. For central tumors that also invade the superior pulmonary vein, it is possible to divide the vein through an intrapericardial approach. The dissection of the interlobar fissure is particularly important for the reconstruction phase.
A vascular clamp is placed at least 5 mm away from the proximal and distal end of the PA to ensure proper suture placement. This distance is necessary to provide sufficient space for the surgeon to properly suture the two ends of the PA.
The surgeon would consider as acceptable a vascular margin of 1.0 mm for cancer and 0.5 mm for a low-grade malignancy. The arterial anastomosis is performed, after the bronchial anastomosis is completed to minimize retraction and handling of the vascular anastomosis. The transected artery provides exposure to bronchial anastomosis. Before clamping the PA, heparin sodium is injected intravenously to prevent clotting. The main PA is clamped by a Satinsky clamp. Either the distal end of the PA or the vein of the residual lobe can be clamped to prevent backflow. Clamping the vein has the advantage of keeping vascular clamps out of the surgeon’s view and minimizing traction on the PA.
The corresponding lobe should be resected simultaneously. The resected bronchial and arterial stumps should be examined in the frozen-section pathology to determine whether the margin is clear of the tumor.
Different types of pulmonary arterial resections and reconstructions
Sleeve resection of the PA is usually performed for upper lobe tumors that invade the right or left main artery or its branches. When the artery is invaded, options for reconstruction are tangential resection of the PA, angioplasty, end-to-end anastomosis, and graft interposition.
There are various approaches to heparin dosage during surgery. One common method is to administer 5,000 units for a completely resected PA and 2,500 units for tangential clamping and resection. In the past, doses of 3,000 to 5,000 units were frequently used, but in recent years, some authors have reported that a lower dose of 1,500 to 2,000 units (25 U/kg) has been preferred to reduce the risk of postoperative bleeding and oozing, particularly from lymphadenectomy sites. These authors have advocated that this lower dose is effective in preventing thrombosis and that heparin does not need to be reversed with protamine after declamping.[15,16]
Tangential resection
Tangential resection of the PA is performed, if the tumor only invades less than one-third of the main circumference of the PA. To perform this procedure, the main artery and the distal trunk are first heparinized and clamped. Then, a tangential incision is made in the PA and it is stitched with running non-absorbable sutures, preferably prolene 5-0 suture. The distal clamp is removed first to allow any air to escape and to check that the artery is fully reperfused before the suture is tied.
Another approach for tangential resections is placing a vascular side clamp to PA and resection and primary repair of it. Placing a vascular stapler is also a fast and easy method for tangential resection of PA in selected cases. Another approach for tangential resections is placing a vascular side clamp to PA and resection and primary repair of it. The vascular stapler is also a fast and easy method for tangential resection of PA in selected cases.[17]
Pulmonary patchplasty
If primary repair carries a risk of narrowing the PA, it is usually more advisable to perform patch angioplasty using a piece of the pericardium or bovine pericardium to maintain lumen patency. Another option is to create a patch using the opposite wall of the PA, if it is free of tumor. An autologous pericardium is a preferred choice for reconstruction. Autologous pericardium is the preferred choice for the reconstruction of the PA which was first reported by Rendina et al.[18] in 1994.
The pericardium should be harvested anterior to the phrenic nerve, allowing for the preparation of large patches. The defect in the pericardium usually does not require closure. However, the autologous pericardium tends to shrink and curl during suturing. To improve its stiffness and facilitate manipulation during suturing, it can be soaked in a solution of two drops of 20% glutaraldehyde in 50 mL saline for a few seconds. An alternative option is to use the resected stump of the pulmonary vein as a patch, although it is typically quite small (Figures 1 and 2).[19,20]
Figure 1. Pulmonary artery invasion on thoracic computed tomography of a patient who underwent left upper bronchial sleeve lobectomy and pulmonary artery patchplasty with autologous pericardial graft.

Figure 2. Intraoperative view of pulmonary artery patchplasty with autologous pericardial graft.

End-to-end anastomosis
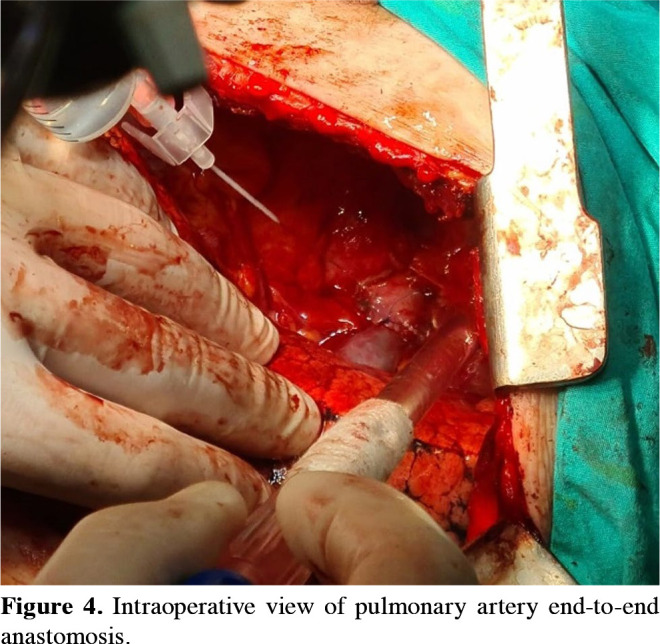
End-to-end anastomosis is advised for the invasion greater than the one-third of PA circumference. To avoid kinking, narrowing, or stenosis of the artery at the anastomosis site, it is important to carefully adjust the diameters of both ends. This can be done using traction sutures, which are placed on the anterior and posterior sides and gently pulling on the vessel ends to adjust their diameters. Once the diameters of the vessel ends have been adjusted, the anastomosis can be completed by using a running suture to join the artery walls together. The running suture should be placed in a back-to-front direction on the posterior wall and a front-to-back direction on the anterior wall. Once the suture is complete, it should be tied, and the proximal clamp opened to confirm proper blood flow and deairing through the reconstructed section of the artery (Figures 3 and 4).
Figure 3. Pulmonary artery invasion on computer tomography of a patient who underwent left upper lobe double sleeve lobectomy and end-to-end anastomosis.
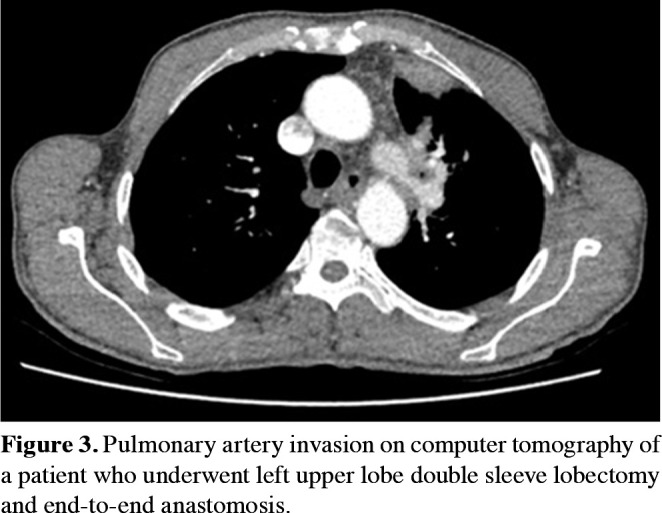
Figure 4. Intraoperative view of pulmonary artery end-to-end anastomosis.
If there is a large size mismatch between the two arteries being anastomosed, it can potentially lead to difficulties during surgery and potential complications afterward. The greater the size mismatch between the proximal and distal ends of the arterial resection, the more difficult the anastomosis and the more common the complications. Inappropriate anastomosis increases the risk of stenosis and thrombosis. To mitigate these risks, surgeons may use a variety of techniques to ensure proper blood flow through the anastomosis.
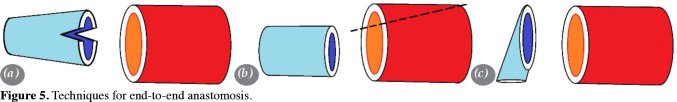
Fish mouth: This technique involves creating a “fish mouth”-shaped opening in the smaller artery and suturing the larger artery to it. This allows for a more gradual transition between the two arteries, reducing the risk of stenosis or thrombosis (Figure 5a).
Wedge resection: This technique involves removing a wedge-shaped piece of the larger artery and suturing the smaller artery in its place. This can help to even out the size difference between the two ends (Figure 5b).
Oblique section: This technique involves making an oblique (angled) cut at the end of the smaller artery, creating an artificial increase in diameter, and suturing the larger artery to this enlarged end. This can help to evenly distribute the blood flow between the two ends of anastomosis, reducing the risk of turbulent flow (Figure 5c).
Figure 5. Techniques for end-to-end anastomosis.
Pulmonary artery resections and graft interposition
It may be necessary to utilize a graft for patients whose resected segment of the PA is too long to allow for end-to-end anastomosis, even with the implementation of releasing techniques. Autologous pericardial grafts, pulmonary vein, bovine pericardium, saphenous vein autografts, and polytetrafluoroethylene (PTFE) grafts can be utilized.[18,21,22]
The end-to-end anastomosis of the tubular graft to the PA with 5/0 prolene sutures is the most common graft interposition technique. Rectangular grafts may be tabularized using a syringe or a segment of the thoracic tube. During tabularization, the free edge of the conduit can be sutured with 5/0 or 6/0 non-absorbable sutures or can also be stapled.[23,24]
An alternative approach for end-to-end anastomosis of the graft and PA using a rectangular graft without tubularization. Some authors argue that this technique, which involves anastomosing the two opposite edges of the graft to the vessel and closing the free edges according to the size of the pulmonary ends, better replicates the decrease in lumen diameter.
Regardless of the type of reconstruction method used, it is important to flush the anastomosis with heparinized saline and deairing it by removing the distal clamp first to ensure proper closure.
b. Supporting the anastomosis
The anastomotic side at the junction of the bronchus and PA has historically been considered at risk for complications, particularly dehiscence and erosion in the adjacent PA and bronchovascular fistula. To avoid complications in the anastomosis during double sleeve lobectomy, various methods can be used to support the anastomosis from dehiscence and prevent the anastomosis from potential intrathoracic infections and bronchial arterial fistulas. These methods include the use of pleural flaps, pericardial flaps, pericardial fat pad grafts, pedicled pericardiophrenic grafts, and omentum. These techniques help to protect the anastomosis and maintain its integrity, ultimately improving patient outcomes.[25-28]
Anticoagulant and antiaggregant therapy after double sleeve lobectomy
Currently, there is no consensus regarding the optimal type and duration of anticoagulant and antiplatelet therapy after double sleeve lobectomy. While much of the experience is inherited from distal arterial repair, it is important to note that the flow and pressure dynamics in the PA differ from those in distal arteries.[29] As a result, the approach to antiplatelet therapy after double sleeve lobectomy may vary between medical institutions.
A commonly utilized approach after double sleeve lobectomy is to administer unfractionated heparin[30] or low-molecular weight heparin[31,32] following the surgical procedure. The aim of this practice is to ensure the patency of the repaired section of the PA during the early postoperative period. After primary repair, some authors oppose to long-term treatment, while some others recommend administering acetylsalicylic acid (ASA) for six months to one year.
A prevailing belief is that autologous grafts become epithelized after six months, thereby reducing the likelihood of thrombus formation in the repaired area. However, turbulent flow may still contribute to the development of thrombus, despite the epithelialization of the graft. While certain approaches suggest that ASA be administered for a duration of six months to one year in patients undergoing autologous graft repair, an alternative approach advocates for lifelong ASA use in patients at high risk for thrombosis.
The use of prosthetic grafts poses the highest risk of graft thrombosis, necessitating lifelong oral anticoagulant therapy. For patients taking warfarin, maintaining an international normalized ratio (INR) between 1.5 and 2.5 is recommended. Of note, new generation oral anticoagulants offer a more convenient option, as they do not require routine INR monitoring.
Minimally invasive double sleeve resections
Double sleeve lobectomy is a complex procedure. Traditionally, this surgery was performed through thoracotomy. However, in recent years, some pioneer surgeons have begun to perform this procedure using minimally invasive techniques, such as VATS and uniportal VATS.
Minimally invasive surgery has several advantages over traditional thoracotomy. It involves smaller incisions, which can result in less pain and more comfort. It also has a shorter recovery time, hospital stay, lesser drainage, and can lead to fewer complications. Video-assisted thoracoscopic surgery has a lower level of acute phase reaction and slighter suppression of the patient̓s immune reaction.[33]
Robotic surgery, also known as robot-assisted thoracic surgery (RATS) can allow for even smaller incisions and can provide the surgeon with greater precision and control during the procedure. However, minimally invasive surgery and robotic surgery have their own set of potential disadvantages. They may not be suitable for all patients and require specialized equipment and training.
As with any surgical procedure, VATS sleeve lobectomy has a learning curve for both the surgeon and the assistant. Based on my personal experience, it is recommended to perform at least 200 VATS lobectomy cases and 10 to 30 open sleeve procedures to establish a solid foundation in anatomy and operative technique before attempting a double sleeve procedure.[34]
Some studies have reported that the traditional method of performing multi-port VATS involves creating a new visual plane that can be difficult to view on two-dimensional monitors due to the angle of the instruments. On the other hand, the uniportal VATS technique involves inserting instruments in a similar way to the movements used in open surgery, which makes it easier to perform complex procedures such as double bronchial and vascular anastomosis. This unique approach offers several advantages, including the ability to mimic open surgery and make it easier to perform complex procedures.
Compared to thoracotomy, uniportal VATS sleeve lobectomy and angioplasty yield similar results in oncological outcomes for OS and relapse-free survival. The perioperative outcome is favorable for uniportal VATS in terms of short hospital stay, less drainage volume overall and in the first 24 h, low incidence of prolonged air leak, and low need for transfusion.[35] In the uniportal VATS procedure, the total lymph node station and the total number of lymph nodes resected are comparable to those in thoracotomy.[36]
The operation time is longer for uniportal VATS compared to thoracotomy. As uniportal VATS double sleeve lobectomy is a more intricate procedure, the longer duration should be considered acceptable considering possible better outcomes in the postoperative period, including less complication and hospital stay.[37]
Triple sleeve resection
Several cases have been reported that vena cava superior resections and reconstruction along with the double sleeve lobectomy can be a feasible surgical procedure for the central tumors invading both the PA and vena cava superior. Although the long OS that the reports claim even for N1 and N2 disease, the oncological outcome is unknown due to the limited number of patients included in those studies.[38-42]
Double sleeve lobectomy after neoadjuvant therapy
Double sleeve resection can be safely and effectively performed following neoadjuvant therapy, without an increase in complications. It is possible that residual tumor or scar tissue may affect the bronchus and/or PA to varying degrees. Adhesions and fibrosis can make the procedure more challenging.
Double sleeve lobectomy following induction therapy has been shown to have similar morbidity and mortality rates as those performed upfront. However, a recent study has shown that hospital stay and drainage time may be longer after induction therapy.[43,44]
There is reliable evidence that concurrent chemoradiation (CRT) therapy combined with pneumonectomy may have high morbidity and mortality. Sleeve lobectomy is seen as an effective way to reduce morbidity and mortality in high-risk patients with central tumors after chemoradiotherapy (CRT). However, the safety of sleeve resection in the irradiated tumor site is uncertain for several reasons: there is limited information available, it can take a long time to achieve sufficient sleeve resection data after induction CRT, and treatment schemes and radiotherapy technology may vary during this period. Additionally, it can be difficult to distinguish fibrosis and inflammation caused by CRT from residual tumor infiltration, even for pathologists evaluating frozen sections. Furthermore, dissection of the vascular and bronchial structures in these treated areas may be challenging in some cases, and radiotherapy may also impair the healing of bronchial arterial anastomosis.
Multiple studies have demonstrated the safety of double sleeve resections after neoadjuvant chemotherapy.[45,46]
Procedure-specific complications
It is recommended to perform repeated bronchoscopies after undergoing a bronchial reconstruction, including at the end of surgery, before discharge, and during the follow-up period, typically at one, six, and 12 months.
Obstruction of the arterial anastomosis is a potential complication specific to a double sleeve lobectomy. Factors that may contribute to this obstruction include inadequate anticoagulant treatment, kinking of anastomosis, and anatomical changes occurring after the affected lung is resected and other lobes of the lung expanded, or the patient is placed supine position.
If the resected segments of the bronchial resection are longer than those of the resected PA in double sleeve lobectomy, or if a long bronchial segment resection is needed in bronchial sleeve surgery, there is a tendency for the PA to kink. As such, surgeons should exercise caution in ensuring the appropriate length and tension of both the artery and bronchus anastomoses.[47]
There are several treatment options for complications related to anastomosis obstruction, which include conservative or medical management, catheter-based interventions, and surgical revision. Technical failure is a common cause of early complications in these cases, and surgical revision may be necessary to re-anastomosis the PA avoiding kinking and interrupted flow. Thrombosis (blood clot formation) at the anastomosis site is a late complication that is more commonly seen after double sleeve resection. In these cases, treatment options may include embolectomy or stenting the affected side, which have also been reported to be possible.[48]
Bronchoarterial fistula is a serious complication that can occur after double sleeve resections. It is characterized by a connection between the bronchus and the PA, which can result in massive bleeding and hemoptysis. In such cases, urgent revision surgery is often necessary to address the issue. In some cases, the fistula may be small and may be able to be treated with medical management, but it is important to closely monitor the patient for any signs of worsening or progression of the fistula. If left untreated, bronchoarterial fistulas can be fatal.
Pulmonary artery pseudoaneurysm is a rare complication that can occur after a double sleeve lobectomy. A PA pseudoaneurysm can rupture, if left untreated, and cause life-threatening bleeding. Doyle and Mhandu[49] reported that treatment with an endovascular stent (a small metal mesh tube) could be used to repair the damaged arterial wall and prevent bleeding.
Another rare complication of double sleeve lobectomy is the development of heterotopic calcification in the muscular flap, which can lead to severe bronchial stenosis as described in some studies.[28]
Literature review
The survival rate of patients undergoing PA reconstruction is similar to patients undergoing standard major lung resections. Additionally, combined bronchial and vascular reconstructions improve survival compared to pneumonectomy. These findings suggest that even complex lung-sparing procedures, such as double sleeve lobectomy, can be performed with the intention of curing the patient, if a complete anatomical resection is achieved.[15,20,45,50]
Double sleeve lobectomy has been shown to have similar rates of distant and local recurrence compared to standard major lung resections, regardless of whether the neoadjuvant treatment is administered. This suggests that multimodality treatment, which is typically necessary to achieve locoregional control in advanced-stage tumors, should not necessarily exclude the use of reconstructive procedures. Sleeve lobectomy has consistently been demonstrated to have lower short-term mortality and morbidity, while maintaining equivalent long-term oncological outcomes. Additionally, previous studies have demonstrated that preserving lung parenchyma can improve postoperative quality of life, including a superior cardiopulmonary reserve, less pulmonary edema, and reduced right ventricular dysfunction resulting from lower pulmonary vascular resistance and higher postoperative forced expiratory volume in 1 sec (FEV1) (Table 1).[16,45,46,51-57]
Table 1. Intraoperative and postoperative outcomes of double sleeve resections.
| Year | Number of patients |
PA thrombosis | Tangential resection |
End-to-end anastomosis |
Patch | Conduit | Complication rate |
Mortality | 60-month survival | ||
| n | n | % | n | n | n | n | % | % | % | ||
| Rendina et al.,[30] | 1999 | 52 | 1 | 1.9 | 0 | 15 | 34 | 3 | 13.4 | 0 | 38.3 |
| Shrager et al.,[58] | 2000 | 33 | 0 | 0 | 19 | 3 | 11 | 0 | 45.1 | 0 | 46.7 |
| (48 months) | |||||||||||
| Lausberg et al.,[59] | 2005 | 67 | 0 | 0 | 0 | 39 | 28 | 0 | 34 | 15 | 42.9 |
| Nagayasu et al.,[50] | 2006 | 29 | 1 | 3.4 | 0 | 17 | 12 | 0 | 27.6 | 172 | 24.2 |
| Cerfolio et al.,[60] | 2007 | 42 | 0 | 0 | 31 | 4 | 7 | 0 | 38.1 | 23 | 60.0 |
| Alifano et al.,[32] | 2009 | 93 | 0 | 0 | 88 | 3 | 2 | 0 | 29.0 | 54 | 39.4 |
| Venuta et al.,[21] | 2009 | 105 | 1 | 0.9 | 0 | 47 | 55 | 3 | 28.5 | 95 | 44.0 |
| Barthet et al.,[31] | 2013 | 32 | 1 | 10 | 0 | 20 | 2 | 10 | 40* | 0 | 66.7 |
| D’Andrilli et al.,[20] | 2014 | 9 | 0 | 0 | 0 | 0 | 0 | 9 | 33.3 | 0 | Median survival |
| 38 month | |||||||||||
| D’Andrilli et al.,[15] | 2018 | 24 | 0 | 0 | 0 | 0 | 4 | 20 | 29.1 | 0 | 69.9 |
| Xie et al.,[35] | 2021 | 133 | 1 | 1.3 | - | - | - | - | 25.6 | 0 | - |
| Bao et al.,[43] | 2022 | 110 | 2 | 2.7 | - | - | - | - | 24.5 | 0 | - |
| Watanabe et al.,[16] | 2022 | 130 | 2 | 1.5 | 56 | 32 | 26 | 16 | 57.7 | 15 | 49.2 |
| Yang et al.,[61] | 2022 | 139 | 0 | 0 | - | - | - | - | 28.1 | 0 | 73.5 |
| (36 months) | |||||||||||
| Hattori et al.,[57] | 2022 | 17 | 1 | 5.9 | 4 | 8 | 1 | 4 | 58.8 | 23 | 29.4 |
| (36 months) | |||||||||||
| PA: Pulmonary artery. | |||||||||||
In conclusion, pulmonary artery reconstruction in combination with lobectomy is a safe and effective way to treat lung cancer, while preserving parenchymal tissue and results in improved long-term survival, lower rates of perioperative morbidity and mortality, and functional benefits compared to pneumonectomy.
Footnotes
Conflict of Interest: The authors declared no conflicts of interest with respect to the authorship and/or publication of this article.
Author Contributions: Idea/concept, supervision, design and critical review: B.O.; Data collection and processing: S.D., E.E.; Writing the article: S.D.; Analysis, literatüre review, references and materials: E.E.<br><br><b>Data Sharing Statement:</b><br> The data that support the findings of this study are available from the corresponding author upon reasonable request.
Financial Disclosure: The authors received no financial support for the research and/or authorship of this article.
References
- 1.Thomas CP. Conservative resection of the bronchial tree. J R Coll Surg Edinb. 1956;1:169–186. [PubMed] [Google Scholar]
- 2.Allison P. Course of thoracic surgery in Groningen. Ann R Coll Surg. 1954;25:20–22. [Google Scholar]
- 3.Vogt-Moykopf I, Fritz T, Meyer G, Bülzerbruck H, Daskos G. Bronchoplastic and angioplastic operation in bronchial carcinoma: Long-term results of a retrospective analysis from 1973 to 1983. Int Surg. 1986;71:211–220. [PubMed] [Google Scholar]
- 4.Deslauriers J, Grégoire J, Jacques LF, Piraux M, Guojin L, Lacasse Y. Sleeve lobectomy versus pneumonectomy for lung cancer: A comparative analysis of survival and sites or recurrences. Ann Thorac Surg. 2004;77:1152–1156. doi: 10.1016/j.athoracsur.2003.07.040. [DOI] [PubMed] [Google Scholar]
- 5.Gaissert HA, Mathisen DJ, Moncure AC, Hilgenberg AD, Grillo HC, Wain JC. Survival and function after sleeve lobectomy for lung cancer. J Thorac Cardiovasc Surg. 1996;111:948–953. doi: 10.1016/s0022-5223(96)70369-0. [DOI] [PubMed] [Google Scholar]
- 6.Pagès PB, Mordant P, Renaud S, Brouchet L, Thomas PA, Dahan M, et al. Sleeve lobectomy may provide better outcomes than pneumonectomy for non-small cell lung cancer. A decade in a nationwide study. J Thorac Cardiovasc Surg. 2017;153:184–195. doi: 10.1016/j.jtcvs.2016.09.060. [DOI] [PubMed] [Google Scholar]
- 7.Okada M, Yamagishi H, Satake S, Matsuoka H, Miyamoto Y, Yoshimura M, et al. Survival related to lymph node involvement in lung cancer after sleeve lobectomy compared with pneumonectomy. J Thorac Cardiovasc Surg. 2000;119:814–819. doi: 10.1016/S0022-5223(00)70018-3. [DOI] [PubMed] [Google Scholar]
- 8.Fadel E, Yildizeli B, Chapelier AR, Dicenta I, Mussot S, Dartevelle PG. Sleeve lobectomy for bronchogenic cancers: Factors affecting survival. Ann Thorac Surg. 2002;74:851–859. doi: 10.1016/s0003-4975(02)03792-x. [DOI] [PubMed] [Google Scholar]
- 9.Stallard J, Loberg A, Dunning J, Dark J. Is a sleeve lobectomy significantly better than a pneumonectomy. Interact Cardiovasc Thorac Surg. 2010;11:660–666. doi: 10.1510/icvts.2010.245506. [DOI] [PubMed] [Google Scholar]
- 10.Wang L, Pei Y, Li S, Zhang S, Yang Y. Left sleeve lobectomy versus left pneumonectomy for the management of patients with non-small cell lung cancer. Thorac Cancer. 2018;9:348–352. doi: 10.1111/1759-7714.12583. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 11.Ferguson MK, Karrison T. Does pneumonectomy for lung cancer adversely influence long-term survival. J Thorac Cardiovasc Surg. 2000;119:440–448. doi: 10.1016/s0022-5223(00)70121-8. [DOI] [PubMed] [Google Scholar]
- 12.Mizushima Y, Sugiyama S, Noto H, Kusajima Y, Yamashita R, Sassa K, et al. Prognosis for patients with pneumonectomy or lesser resections for non-small cell lung cancer based on histologic cell type. Oncol Rep. 1998;5:689–692. doi: 10.3892/or.5.3.689. [DOI] [PubMed] [Google Scholar]
- 13.Guan Y, Huang J, Xia T, You X, He J, He J. Preoperative evaluation of stage T3, central-type non-small cell lung cancer with double sleeve lobectomy under complete video-assisted thoracoscopic surgery using spiral computed tomography post-processing techniques. J Thorac Dis. 2016;8:1738–1746. doi: 10.21037/jtd.2016.05.93. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 14.He J, Zhong Y, Suen HC, Sengupta A, Murthy RA, Stoelben E, et al. The procedure and effectiveness of release maneuvers in tracheobronchial resection and reconstruction. Transl Lung Cancer Res. 2022;11:1154–1164. doi: 10.21037/tlcr-22-385. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 15.D’Andrilli A, Maurizi G, Ciccone AM, Andreetti C, Ibrahim M, Menna C, et al. Long-segment pulmonary artery resection to avoid pneumonectomy: Long-term results after prosthetic replacement. Eur J Cardiothorac Surg. 2018;53:331–335. doi: 10.1093/ejcts/ezx353. [DOI] [PubMed] [Google Scholar]
- 16.Watanabe I, Hattori A, Fukui M, Matsunaga T, Takamochi K, Suzuki K. Pulmonary artery reconstruction for nonsmall cell lung cancer: Surgical management and long-term outcomes. J Thorac Cardiovasc Surg. 2022;164:1200–1207. doi: 10.1016/j.jtcvs.2022.01.017. [DOI] [PubMed] [Google Scholar]
- 17.Rendina EA, Venuta F, Ciriaco P, Ricci C. Bronchovascular sleeve resection. Technique, perioperative management, prevention, and treatment of complications. J Thorac Cardiovasc Surg. 1993;106:73–79. [PubMed] [Google Scholar]
- 18.Rendina EA, Venuta F, De Giacomo T, Vizza DC, Ricci C. Reconstruction of the pulmonary artery by a conduit of autologous pericardium. J Thorac Cardiovasc Surg. 1995;110:867–868. doi: 10.1016/S0022-5223(95)70128-1. [DOI] [PubMed] [Google Scholar]
- 19.D’Andrilli A, Ibrahim M, Venuta F, De Giacomo T, Coloni GF, Rendina EA. Glutaraldehyde preserved autologous pericardium for patch reconstruction of the pulmonary artery and superior vena cava. Ann Thorac Surg. 2005;80:357–358. doi: 10.1016/j.athoracsur.2004.02.012. [DOI] [PubMed] [Google Scholar]
- 20.D’Andrilli A, Maurizi G, Andreetti C, Ciccone AM, Ibrahim M, Poggi C, et al. Pulmonary artery reconstruction with pulmonary vein conduit for lung cancer: Medium-term results. Ann Thorac Surg. 2014;98:990–995. doi: 10.1016/j.athoracsur.2014.04.110. [DOI] [PubMed] [Google Scholar]
- 21.Venuta F, Ciccone AM, Anile M, Ibrahim M, De Giacomo T, Coloni GF, et al. Reconstruction of the pulmonary artery for lung cancer: Long-term results. J Thorac Cardiovasc Surg. 2009;138:1185–1191. doi: 10.1016/j.jtcvs.2009.07.043. [DOI] [PubMed] [Google Scholar]
- 22.Yoshimi F, Amemiya R, Asato Y, Koizumi S, Hasegawa H, Matsueda K, et al. Pulmonary artery reconstruction using a great saphenous vein autograft in the treatment of bronchogenic cancer. Surg Today. 1994;24:570–573. doi: 10.1007/BF01884583. [DOI] [PubMed] [Google Scholar]
- 23.Ciccone AM, Venuta F, D’Andrilli A, Andreetti C, Ibrahim M, De Giacomo T, et al. Long-term patency of the stapled bovine pericardial conduit for replacement of the superior vena cava. Eur J Cardiothorac Surg. 2011;40:1487–1491. doi: 10.1016/j.ejcts.2011.03.008. [DOI] [PubMed] [Google Scholar]
- 24.D’Andrilli A, Ciccone AM, Ibrahim M, Venuta F, Rendina EA. A new technique for prosthetic reconstruction of the superior vena cava. J Thorac Cardiovasc Surg. 2006;132:192–194. doi: 10.1016/j.jtcvs.2006.03.021. [DOI] [PubMed] [Google Scholar]
- 25.Venuta F, Diso D, Anile M, Rendina EA. Techniques of protection and revascularization of the bronchial anastomosis. S181-5J Thorac Dis. 2016;8(Suppl 2) doi: 10.3978/j.issn.2072-1439.2016.01.68. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 26.Rendina EA, Venuta F, Ricci P, Fadda GF, Bognolo DA, Ricci C, et al. Protection and revascularization of bronchial anastomoses by the intercostal pedicle flap. J Thorac Cardiovasc Surg. 1994;107:1251–1254. [PubMed] [Google Scholar]
- 27.D’Andrilli A, Ibrahim M, Andreetti C, Ciccone AM, Venuta F, Rendina EA. Transdiaphragmatic harvesting of the omentum through thoracotomy for bronchial stump reinforcement. Ann Thorac Surg. 2009;88:212–215. doi: 10.1016/j.athoracsur.2009.04.025. [DOI] [PubMed] [Google Scholar]
- 28.Ciccone AM, Ibrahim M, D’Andrilli A, Vismara LG. Ossification of the intercostal muscle around the bronchial anastomosis does not jeopardize airway patency. Eur J Cardiothorac Surg. 2006;29:602–603. doi: 10.1016/j.ejcts.2005.12.056. [DOI] [PubMed] [Google Scholar]
- 29.Zavgorodnyaya D, Knight TB, Daley MJ, Teixeira PG. Antithrombotic therapy for postinterventional management of peripheral arterial disease. Am J Health Syst Pharm. 2020;77:269–276. doi: 10.1093/ajhp/zxz315. [DOI] [PubMed] [Google Scholar]
- 30.Rendina EA, Venuta F, De Giacomo T, Ciccone AM, Moretti M, Ruvolo G, et al. Sleeve resection and prosthetic reconstruction of the pulmonary artery for lung cancer. Ann Thorac Surg. 1999;68:995–1002. doi: 10.1016/s0003-4975(99)00738-9. [DOI] [PubMed] [Google Scholar]
- 31.Berthet JP, Boada M, Paradela M, Molins L, Matecki S, Marty-Ané CH, et al. Pulmonary sleeve resection in locally advanced lung cancer using cryopreserved allograft for pulmonary artery replacement. J Thorac Cardiovasc Surg. 2013;146:1191–1197. doi: 10.1016/j.jtcvs.2013.07.003. [DOI] [PubMed] [Google Scholar]
- 32.Alifano M, Cusumano G, Strano S, Magdeleinat P, Bobbio A, Giraud F, et al. Lobectomy with pulmonary artery resection: Morbidity, mortality, and long-term survival. J Thorac Cardiovasc Surg. 2009;137:1400–1405. doi: 10.1016/j.jtcvs.2008.11.002. [DOI] [PubMed] [Google Scholar]
- 33.Zhang LB, Wang B, Wang XY, Zhang L. Influence of video-assisted thoracoscopic lobectomy on immunological functions in non-small cell lung cancer patients. Med Oncol. 2015;32:201–201. doi: 10.1007/s12032-015-0639-2. [DOI] [PubMed] [Google Scholar]
- 34.Han Y, Zhou S, Yu D, Song X, Liu Z. Video-assisted thoracic surgery (VATS) left upper sleeve lobectomy with partial pulmonary artery resection. S301-3J Thorac Dis. 2013;5 Suppl 3 doi: 10.3978/j.issn.2072-1439.2013.07.16. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 35.Xie D, Zhong Y, Deng J, She Y, Zhang L, Fan J, et al. Comparison of uniportal video-assisted thoracoscopic versus thoracotomy bronchial sleeve lobectomy with pulmonary arterioplasty for centrally located non-small-cell lung cancer. Eur J Cardiothorac Surg. 2021;59:978–986. doi: 10.1093/ejcts/ezaa404. [DOI] [PubMed] [Google Scholar]
- 36.Zhou S, Pei G, Han Y, Yu D, Song X, Li Y, et al. Sleeve lobectomy by video-assisted thoracic surgery versus thoracotomy for non-small cell lung cancer. J Cardiothorac Surg. 2015;10:116–116. doi: 10.1186/s13019-015-0318-6. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 37.Wu L, Wang H, Cai H, Fan J, Jiang G, He Y, et al. Comparison of double sleeve lobectomy by uniportal video-assisted thoracic surgery (VATS) and thoracotomy for NSCLC treatment. Cancer Manag Res. 2019;11:10167–10174. doi: 10.2147/CMAR.S226459. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 38.Solli P, Spaggiari L, Grasso F, Pastorino U. Double prosthetic replacement of pulmonary artery and superior vena cava and sleeve lobectomy for lung cancer. Eur J Cardiothorac Surg. 2001;20:1045–1048. doi: 10.1016/s1010-7940(01)00908-3. [DOI] [PubMed] [Google Scholar]
- 39.Sekine Y, Yasufuku K, Motohashi S, Fujisawa T. Triple reconstruction of pulmonary artery, superior vena cava and bronchus for lung cancer. Interact Cardiovasc Thorac Surg. 2006;5:509–510. doi: 10.1510/icvts.2005.127746. [DOI] [PubMed] [Google Scholar]
- 40.Pochesci I, Ibrahim M, Vismara LG, Rendina EA. Superior vena cava replacement by the stapled pericardial conduit associated with double sleeve resection of the bronchus and pulmonary artery. Eur J Cardiothorac Surg. 2008;34:673–673. doi: 10.1016/j.ejcts.2008.05.039. [DOI] [PubMed] [Google Scholar]
- 41.Zhu D, Qiu X, Zhou Q. Combined double sleeve lobectomy and superior vena cava resection for non-small cell lung cancer with persistent left superior vena cava. Zhongguo Fei Ai Za Zhi. 2015;18:718–720. doi: 10.3779/j.issn.1009-3419.2015.11.10. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 42.Sun Y, Zheng H, Chen Q, Bao M, Jiang G, Chen C, et al. Triple plasty of bronchus, pulmonary artery, and superior vena cava for non-small cell lung cancer. Ann Thorac Surg. 2013;95:420–424. doi: 10.1016/j.athoracsur.2012.10.036. [DOI] [PubMed] [Google Scholar]
- 43.Bao Y, Jiang C, Wan Z, Wang Y, Zhong Y, Deng J, et al. Feasibility of double sleeve lobectomy after neoadjuvant chemotherapy in patients with non-small-cell lung cancer. ivac103Interact Cardiovasc Thorac Surg. 2022;35 doi: 10.1093/icvts/ivac103. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 44.D’Andrilli A, Venuta F, Maurizi G, Rendina EA. Bronchial and arterial sleeve resection after induction therapy for lung cancer. Thorac Surg Clin. 2014;24:411–421. doi: 10.1016/j.thorsurg.2014.07.006. [DOI] [PubMed] [Google Scholar]
- 45.Maurizi G, D’Andrilli A, Anile M, Ciccone AM, Ibrahim M, Venuta F, et al. Sleeve lobectomy compared with pneumonectomy after induction therapy for non-small-cell lung cancer. J Thorac Oncol. 2013;8:637–643. doi: 10.1097/JTO.0b013e318286d145. [DOI] [PubMed] [Google Scholar]
- 46.Gómez-Caro A, Boada M, Reguart N, Viñolas N, Casas F, Molins L. Sleeve lobectomy after induction chemoradiotherapy. Eur J Cardiothorac Surg. 2012;41:1052–1058. doi: 10.1093/ejcts/ezr184. [DOI] [PubMed] [Google Scholar]
- 47.Nakajima D, Oda H, Chen-Yoshikawa TF, Date H. Emergent surgical treatment for acute thrombosis caused by pulmonary artery kinking after left upper sleeve lobectomy. Interact Cardiovasc Thorac Surg. 2019;29:481–483. doi: 10.1093/icvts/ivz110. [DOI] [PubMed] [Google Scholar]
- 48.Tsuchiya T, Matsumoto K, Miyazaki T, Doi R, Tomoshige K, Watanabe H, et al. Successful pulmonary artery stenting for occlusion at a constructed pericardial conduit after right upper double sleeve lobectomy. Gen Thorac Cardiovasc Surg. 2022;70:402–405. doi: 10.1007/s11748-022-01770-1. [DOI] [PubMed] [Google Scholar]
- 49.Doyle J, Mhandu P. Late presentation of pulmonary artery pseudoaneurysm following bronchovascular sleeve resection of lung. e243294BMJ Case Rep. 2021;14 doi: 10.1136/bcr2021-243294. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 50.Nagayasu T, Matsumoto K, Tagawa T, Nakamura A, Yamasaki N, Nanashima A. Factors affecting survival after bronchoplasty and broncho-angioplasty for lung cancer: Single institutional review of 147 patients. Eur J Cardiothorac Surg. 2006;29:585–590. doi: 10.1016/j.ejcts.2005.12.044. [DOI] [PubMed] [Google Scholar]
- 51.Rendina EA, Venuta F, De Giacomo T, Flaishman I, Fazi P, Ricci C. Safety and efficacy of bronchovascular reconstruction after induction chemotherapy for lung cancer. J Thorac Cardiovasc Surg. 1997;114:830–837. doi: 10.1016/S0022-5223(97)70088-6. [DOI] [PubMed] [Google Scholar]
- 52.Parissis H, Leotsinidis M, Hughes A, McGovern E, Luke D, Young V. Comparative analysis and outcomes of sleeve resection versus pneumonectomy. Asian Cardiovasc Thorac Ann. 2009;17:175–182. doi: 10.1177/0218492309103309. [DOI] [PubMed] [Google Scholar]
- 53.Kalaycı G, Dilege Ş, Toker A, Tanju S, Ziyade S, Bayrak Y, et al. Sleeve rezeksiyonları: 77 olgunun değerlendirilmesi. Turk Gogus Kalp Dama. 2005;13:397–402. [Google Scholar]
- 54.Menna C, Rendina EA, D’Andrilli A. Parenchymal sparing surgery for lung cancer: Focus on pulmonary artery reconstruction. Cancers (Basel) 2022;14:4782–4782. doi: 10.3390/cancers14194782. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 55.Sayan M, Bas A, Gokce A, Dikmen AU, Celik A, Kurul IC, et al. Surgical outcomes of sleeve resections performed for non-small cell lung cancer; a single center experience. Niger J Clin Pract. 2020;23:829–834. doi: 10.4103/njcp.njcp_603_18. [DOI] [PubMed] [Google Scholar]
- 56.Hattori A, Matsunaga T, Fukui M, Takamochi K, Oh S, Suzuki K. Surgical outcome after extended sleeve lobectomy in centrally located non-small cell lung cancer. Ann Thorac Surg. 2022;114:1853–1862. doi: 10.1016/j.athoracsur.2022.02.082. [DOI] [PubMed] [Google Scholar]
- 57.Schiavon M, Comacchio GM, Mammana M, Faccioli E, Stocca F, Gregori D, et al. Lobectomy with artery reconstruction and pneumonectomy for non-small cell lung cancer: A propensity score weighting study. Ann Thorac Surg. 2021;112:1805–1813. doi: 10.1016/j.athoracsur.2020.12.029. [DOI] [PubMed] [Google Scholar]
- 58.Shrager JB, Lambright ES, McGrath CM, Wahl PM, Deeb ME, Friedberg JS, et al. Lobectomy with tangential pulmonary artery resection without regard to pulmonary function. Ann Thorac Surg. 2000;70:234–239. doi: 10.1016/s0003-4975(00)01492-2. [DOI] [PubMed] [Google Scholar]
- 59.Lausberg HF, Graeter TP, Tscholl D, Wendler O, Schäfers HJ. Bronchovascular versus bronchial sleeve resection for central lung tumors. Ann Thorac Surg. 2005;79:1147–1152. doi: 10.1016/j.athoracsur.2004.09.009. [DOI] [PubMed] [Google Scholar]
- 60.Cerfolio RJ, Bryant AS. Surgical techniques and results for partial or circumferential sleeve resection of the pulmonary artery for patients with non-small cell lung cancer. Ann Thorac Surg. 2007;83:1971–1977. doi: 10.1016/j.athoracsur.2007.01.048. [DOI] [PubMed] [Google Scholar]
- 61.Yang M, Zhong Y, Deng J, She Y, Zhang L, Wang Y, et al. Comparison of bronchial sleeve lobectomy with pulmonary arterioplasty versus pneumonectomy. Ann Thorac Surg. 2022;113:934–941. doi: 10.1016/j.athoracsur.2021.04.007. [DOI] [PubMed] [Google Scholar]