Abstract
A concise linear synthesis of hypoxia inducible factor-2α (HIF-2α) inhibitor, belzutifan was achieved by reproducing key components of previous synthetic approaches to this molecule as described in several publications and patents. Belzutifan is an orally bioavailable small-molecule (HIF-2α) inhibitor for the treatment of von Hippel-Lindau (VHL) disease-associated renal cell carcinoma (RCC) that received FDA approval in 2021. Herein, we report a 13-step synthesis of PT2977 that proceeded in good overall yield with high diastereoselectivity. Separation of diastereomeric mixtures at two different stages of the synthesis proved advantageous in ease of separation. The X-ray structure of belzutifan was determined.
Keywords: Concise Synthesis of PT2977, Belzutifan, MK-6482, Welireg™, Hypoxia Inducible Factor (HIF) inhibitor, Single Crystal X-ray Structure of PT2977
Introduction
The 2019 Nobel Prize in physiology and medicine was granted for the elucidation of cellular responses to oxygenation.1 The function of hypoxia inducible factor (HIF) is essential in the regulation of oxygen homeostasis through pro-angiogenic transcription activity.2 The pro-angiogenic effect of HIFs allows for oxygenation of tissues and growth of new vasculature in response to hypoxia. Acting as oxygen sensors, prolyl hydroxylase domain (PHD) proteins oxidize proline and asparagine residues on HIF generating new active sites for proteins like the von Hippel-Lindau tumor suppressor protein (pVHL). pVHL interaction marks HIFs for ubiquitination and subsequent proteasomal degradation, allowing regions of sufficient oxygenation to regulate growth.3,4
Pro-angiogenic functions can lead to unchecked proliferation of vasculature and ultimately to tumorigenesis and metastasis. This is most clearly seen in the case of clear cell renal cell carcinoma (ccRCC), wherein 90% of patients exhibit a deficiency in pVHL and HIF-2α acts as the primary driver of tumorigenesis.5 The cells are unable to effectively clear the cytoplasm of HIF causing crossover of these factors into the nucleus regardless of oxygenation. Dimerization of HIF-2α with aryl hydrocarbon receptor nuclear translocator (ARNT), also known as HIF-1β, forms the transcription factor complex, which expresses numerous genes that regulate processes such as angiogenesis and cell proliferation.3–4 Many small-molecules have been found to modulate the activity of HIF transcriptional activity, several are highlighted in Figure 1 and further detailed in a variety of reviews.6–12
Figure 1.

Small-Molecules Capable of Influencing HIF Transcriptional Activity.
The key role that HIF-2α plays in tumor growth prompted synthetic-medicinal chemistry campaigns focused on the discovery of small-molecule agonists capable of disrupting formation of the transcription factor complex.13–15 To this end, Bruick and Gardner13 investigated potential sites for small-molecule binding, and ultimately demonstrated the existence of a small hydrophobic pocket capable of retaining water and appropriate small-molecules in the PAS-B domain of the protein. Further, small-molecules bound to this region were found to distort the wider protein structure, thus preventing the formation of the HIF-ARNT transcription factor complex. Beginning with the initial lead molecules developed by Bruick and Gardner, Peloton Therapeutics embarked on an extended structure-activity relationship (SAR) discovery program to optimize an ideal balance of binding affinity for the PAS-B domain, degree of allosteric effect, and lipophilicity.16,17 The study initiated with nitrobenzoxadiazole, 1; and resulted in the discovery and development of an advanced lead molecule PT2385, 2; and ultimately PT2977, 3 after pharmacokinetic optimization (Fig. 2).16,18
Figure 2.

Evolution of Belzutifan from Lead Molecule.
PT2977 gained the additional name MK-6482, and later belzutifan, following Merck’s acquisition of Peloton Therapeutics, and is now marketed by Merck as Welireg™ PT2977 showed excellent efficacy in treating VHL disease-associated ccRCC, and was afforded FDA approval in early 2021 as the first HIF-2α inhibitor to receive this designation.19 The novel mechanism of action and antiangiogenic effect prompted our interest in obtaining a sample of PT2977 for collaborative in vivo studies in combination with a promising vascular disrupting agent (VDA) OXi8007,20–24 previously discovered and advanced in our laboratory. At the time of the 2019 publication of PT2977, and the commencement in 2020 of our funded NIH R01 study (project number R01CA244579), the commercial availability of the agent was extremely limited, with even analytical samples being quite costly for purchase. Accordingly, we embarked on a synthetic campaign towards PT2977 to achieve a reliable, high purity supply to utilize in our studies. This effort required us to piece together, from various publications and patents along with our own insights, a robust synthetic pathway to the novel pharmaceutical agent PT2977 that had not been previously described as a single linear synthesis housed within any single publication.
While our synthetic effort ultimately culminated in utilization of reaction steps that mirror two publications16,18 and two patents,25,26 some historical perspective is relevant in this regard. Initially following guidance from a 2018 publication in the Journal of Medicinal Chemistry,16 we unfortunately reached an impasse that required exploration of alternative approaches. In brief, while initially successful in the synthesis of a key mid-stage intermediate 4, continued efforts to replicate this step were unsuccessful in our hands. While investigating alternative approaches, two patents25,26 from 2016 and 2017 were identified that provided an alternative synthetic route to this key intermediate, and this chemistry proved successful in our hands as well. With a synthetic route to intermediate 4 established, guidance from a 2019 publication in the Journal of Medicinal Chemistry18 facilitated successful completion of the synthesis of PT2977. Improvements in this route included our observation that separation of diastereomeric mixtures at two different points of the synthesis resulted in enhanced efficiency and ease of separation. Single crystal X-ray structures were obtained for both intermediate 26b and PT2977, and the optical rotation for PT2977 was obtained. To the best of our knowledge, this represents the first report of these two X-ray structures and the optical rotation of PT2977. During the course of our studies, Merck published six contiguous papers in Organic Process Research and Development (OPRD)27–32 outlining their scale-up approach to belzutifan for commercialization. In a separate study (see Supporting Information) we demonstrated the feasibility of this route on small (laboratory) scale. Validation of the biological activity of our synthetic sample of PT2977 was obtained through a RT-PCR experiment demonstrating inhibition of VEGFA mRNA expression in the 786-O renal cancer cell line.
Results and discussion
The synthesis of PT2977 can be effectively divided into two parts, initially involving generation of the diaryl ether indanone core bearing a methyl sulfone functionality, followed by elaboration of the all-cis stereotriad present at the indanol. Therefore, strategies used to synthesize PT2977 advanced through key intermediate 4. Three methods for the synthesis of intermediate 4 were identified in the literature (Fig. 3); the first was provided in a publication describing an early development molecule 2 (PT2385).16 A second route toward intermediate 4 was described in two patents for PT2385,25,26 and a third route was provided by Merck in their synthesis of belzutifan for commercial distribution.27 This key sulfone intermediate 4 may be advanced by one of two routes (Fig. 3) to access PT2977; one described in a publication outlining the pharmacokinetic optimization of PT2385 that led to PT2977,18 and a second in the process optimization published by Merck.28–32
Figure 3.

Summary of Reported Approaches to Key Sulfone Intermediate 4 and PT2977 (Belzutifan) 3.
Synthetic efforts toward advanced intermediate 4 in our laboratory began with the route outlined in Peloton’s initial publication (Scheme 1).16 The starting material used by Peloton was indanone 7 (4-fluoro-7-hydroxyindanone) which they purchased from a commercial supplier for their analogue development.16 The high cost of this starting material ($605/g Aldrich, $986.96/g Fisher, March 2023) encouraged preparation in our own laboratory, utilizing a 2-step acylation-Fries rearrangement methodology.33,34 Acylation of 4-fluorophenol 5 with 3-chloropropionyl chloride resulted in phenoxy ester 6 with 90% yield, which was then transformed to 4-fluoro-7-hydroxyindanone 7 through a Fries rearrangement in 70% yield. Upon reaction of intermediate 7 with N,N-dimethylthiocarbamoyl chloride, O-arylcarbamate 8 was obtained in 87% yield, and subsequent heating induced an aryl shift from oxygen to sulfur through a Newman-Kwart rearrangement to provide (quantitatively) the masked thiophenol 9, which underwent base mediated hydrolysis followed by methylation to afford compound 10 in 80% yield. Oxidation of intermediate 10 with Oxone established sulfone 11 in excellent yield (95%) as the desired SNAr precursor. Forging the second half of the desired aromatic substitution reaction from 3,5-difluorobenzonitrile 12 was accomplished through displacement of an aryl fluoride using sodium methoxide to afford intermediate 13 in 93% yield. Subsequent demethylation produced 3-fluoro-5-hydroxybenzonitrile 14 in 84% yield. The K2CO3-mediated coupling reaction between sulfone 11 and phenol 14 was achieved on one occasion in 41% yield, however, subsequent attempts to reproduce these results were unfortunately unsuccessful in our hands, yielding only intractable decomposition products.
Scheme 1.

Initial Approach to the Synthesis of Key Intermediate 4.
Consulting Peloton’s initial work,16 the authors hypothesized that in many analogues a charge transfer complex (CTC) formed between the various phenoxides and the keto sulfone compete with substitution and lead ultimately to decomposition. To mitigate formation of the CTC, the ketone moiety of the sulfone may be protected as a ketal (Scheme 2) as described in a 2017 patent.26 This was achieved through a reaction of sulfone 11 with ethylene glycol heated at reflux in benzene in the presence of catalytic para-toluenesulfonic acid to afford ketal 15. A Cs2CO3-mediated coupling reaction of ketal 15 with phenol 14 was unsuccessful in our hands.25,26 Additionally, one might invert the polarity of the substitution reaction by employing ketal phenol 16, prepared from the ketal 15, with nitrile 12 functioning as the electrophile.26 An efficient conversion of ketal 15 to ketal phenol 16 was achieved in the presence of KOH. Ketal phenol 16 was then successfully reacted with 3,5-difluorobenzonitrile 12 to synthesize the desired diaryl ether backbone 17. Deprotection of ketal 17 using pyridinium p-toluenesulfonate revealed ketone 4.26
Scheme 2.

Alternative Approach to Key Intermediate 4.
While this approach to diaryl ether 4 was successful, some challenges were encountered associated with by-products and potential scalability in the conversion of aryl fluoride 15 to its corresponding phenolic analogue 16. Searching for an alternative approach, we located a strategy for forming the diaryl ether prior to formation of the indanone (Scheme 3) in a patent.25 Commercially available 4-(methylthio)phenol 18 was heated with excess paraformaldehyde to obtain aldehyde 19 by selective ortho-formylation in 85% yield. The subsequent condensation reaction of aldehyde 19 with Meldrum’s acid formed coumarin 20 in high yield (88%). Heating intermediate 20 with a mixture of formic acid and triethylamine in DMF induced ring opening and decarboxylation to afford propionic acid intermediate 21 in 87% isolated yield. A Cs2CO3-mediated nucleophilic aromatic substitution reaction occurred upon treatment of intermediate 21 with 3,5-difluorobenzonitrile to form diaryl ether 22 in 91% yield. Ring closing Friedel-Crafts acylation was carried out by initial conversion of 22 to its corresponding acid chloride in situ followed by exposure to Lewis acid (AlCl3) to afford the desired indanone 23 in excellent yield (97%). Facile oxidation of indanone 23 with Oxone cleanly produced the key sulfone intermediate 4 in 84% yield. Thus, this alternative approach25 afforded key intermediate 4 in overall high yield.
Scheme 3.

Alternative Route to Key Intermediate 4.
With advanced key intermediate 4 in hand, we proceeded to establish the stereotriad utilizing the reported synthetic route (Scheme 4).18 A mixture of diaryl sulfone 4 and Selectfluor in methanol was refluxed to afford α-fluoro ketone 24 in 86% isolated yield. The subsequent Noyori asymmetric transfer hydrogenation produced the desired cis-diastereomer 25 preferentially due to a dynamic kinetic resolution caused by rapid epimerization of the α-carbonyl position. The indanol moiety in compound 25 was then protected as its corresponding acetate to afford 26a (trans-diastereomer) and 26b (cis-diastereomer) in 81% yield. These cis- and trans-diastereomers were separated at this point by chromatography on silica gel, which revealed a cis to trans ratio (3:1) and proved to be a more efficient method for separation and purification of these diastereomers in comparison to separation of cis/trans diastereomers 25 as described in the literature.16 Single crystals of cis-diastereomer 26b were obtained by slow evaporation from a hexanes/CH2Cl2 solution (ratio approximately 1/9, respectively) and X-ray crystallography further confirmed the cis-stereochemistry (see Supporting Information). Benzylic bromination of cis-diastereomer 26b with N-bromosuccinimide (NBS) catalyzed by 2,2′-azobis(2-methylpropionitrile) (AIBN) afforded bromo-compound 27 in 88% yield. Subsequent treatment of bromo-compound 27 with AgClO4 in 1,2-dimethoxyethane and water provided alcohol 28 in a 61% yield. Deoxyfluorination of alcohol 28 with N,N-diethylaminosulfur trifluoride (DAST) afforded difluoro compounds 29a (cis-diastereomer) and 29b (trans-diastereomer) in 76% combined yield. The mixture of diastereomers 29a/29b was readily separated by chromatography on silica gel, which in our hands was efficacious in comparison to separation of diastereomers 28, as described in the literature.18 Deprotection of trans-difluoroacetate 29b under mild conditions (0.5 N LiOH solution) afforded trans diastereomer 30 in 46% yield, and under similar conditions cis-difluoroacetate 29a delivered desired belzutifan (PT2977) 3 in 39% yield. Single crystals of PT2977 (3) suitable for X-ray crystallographic analysis were obtained via slow evaporation of a CH2Cl2/hexanes solution (see Supporting Information). To the best of our knowledge, this represents the first report of the X-ray structure of PT2977 (belzutifan).
Scheme 4.

Synthesis of Belzutifan, 3.
Following the acquisition of Peloton Therapeutics by Merck in 2019 the process route used by Merck to access belzutifan for commercial sales was divulged in six back-to-back publications in Organic Process Research and Development (OPRD) in early 2022.27–32 In a subsequent study, we utilized this scale-up route and demonstrated its feasibility on a much smaller scale in order to further streamline our internal supply route toward PT2977 for ongoing biological studies. Experimental details and reaction schemes are provided in the Supporting Information.
In addition, efforts were made to further optimize specific reaction steps in the Merck synthetic route to PT2977. In one example, we attempted to improve the regioselectivity of the final deoxyfluorination step by carrying out this reaction prior to unveiling the potentially reactive alcohol moiety (see Supporting Information Scheme S3). In a separate effort to potentially improve overall efficiency, we determined the diastereomeric ratio produced in the fluorination of a late-stage chiral intermediate associated with the Merck route, wherein we postulated that if this reaction proceeded with high diastereoselectivity, then it might obviate a later ruthenium mediated dynamic kinetic resolution of the fluoride (see Supporting Information, Scheme S4). Although these alternative approaches did not lead to improvements in the synthetic route, they provided further insights regarding the chemistry associated with these intermediates and might prove advantageous in future synthetic efforts directed towards related analogues.
Biological Evaluation
Confirmation of the requisite biological efficacy of PT2977 synthesized in this study was achieved through a RT-PCR experiment that demonstrated a significant decrease in vascular endothelial growth factor (VEGFA) mRNA levels in 786-O cells (human renal adenocarcinoma) treated with PT2977 in comparison to vehicle control (Fig. 4). VEGFA is a HIF target gene and downregulation of the VEGFA mRNA level after drug treatment validates the efficacy of the drug. This decrease in the VEGFA mRNA level is indicative of the anticipated decrease in DNA-binding activity of HIF-2α in 786-O cells treated with PT2977. An earlier development compound (PT2399)14 was used as a positive control. VEGFA mRNA levels were not diminished to an appreciable extent in RENCA cells (mouse renal adenocarcinoma) upon treatment with either PT2977 or PT2399, which is potentially due to a low level of HIF-2α expression in RENCA cells with wildtype von Hippel-Lindau tumor suppressor protein (pVHL),35 although it is also possible that RENCA cells are resistant to PT2977 and PT2399 treatment for reasons yet to be determined.
Figure 4.
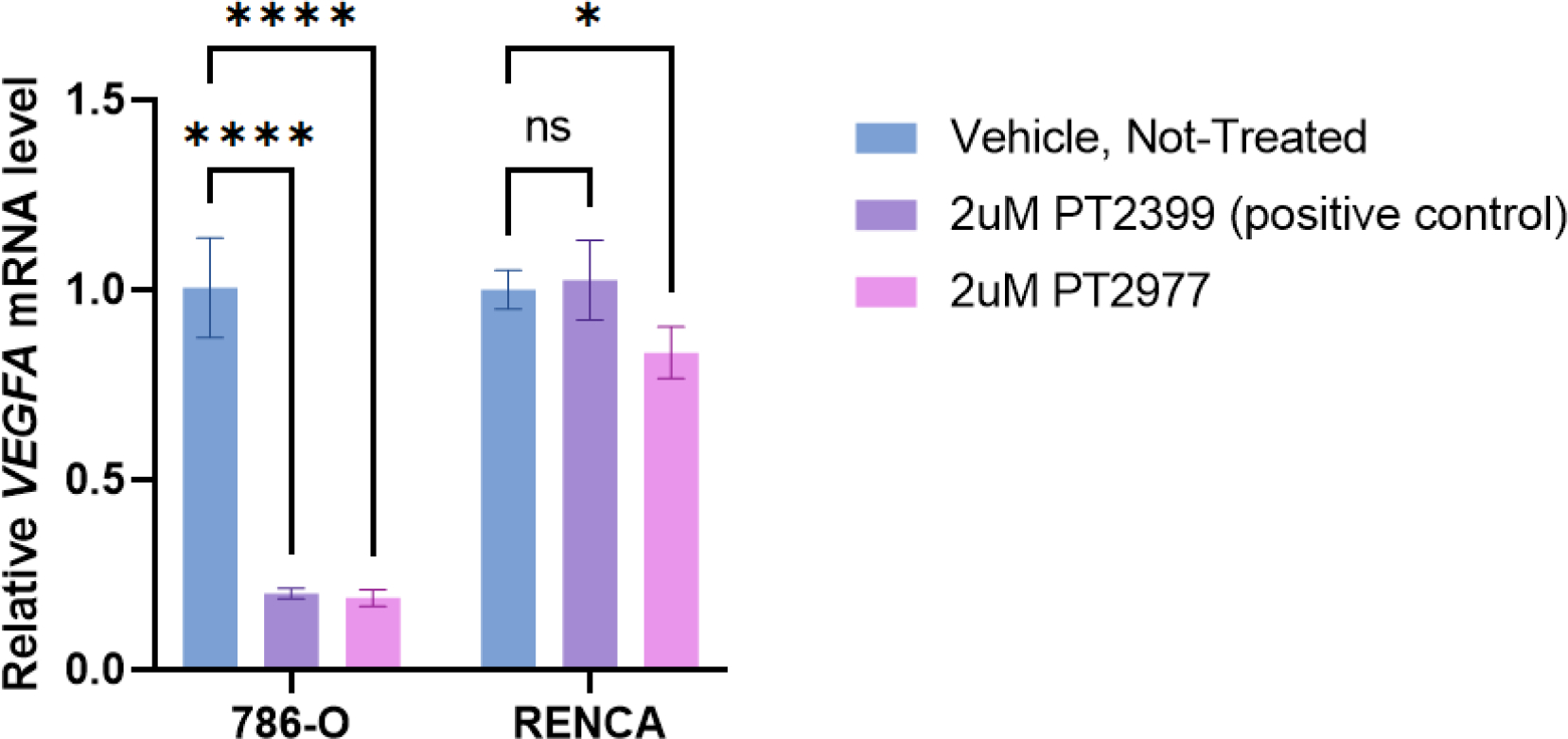
Efficacy of synthesized PT2977 was validated by downregulation of HIF-2α target gene VEGFA. RT-PCR experiments showed downregulation of VEGFA mRNA in the 786-O cell line after synthesized PT2977 treatment or commercial PT2399 treatment (as positive control). The RENCA cell line was not as sensitive to either drug treatment. Statistical significance was determined by two-way ANOVA, ****p<0.0001, *p<0.05, ns=nonsignificant.
PT2977 incorporates a bi-aryl ether motif that bears some level of structural resemblance to various bi-aryl analogues36 of the natural product combretastatin-A4 (CA4), which functions as a potent inhibitor of tubulin polymerization.37,38 Accordingly, we evaluated our synthetic sample of PT2977 for inhibition of tubulin polymerization and found it to be inactive with IC50 > 20 micromolar. PT2977 was also evaluated for its cytotoxicity against the human breast cancer cell line MCF-7 and found to have limited activity (IC50 > 5 micromolar), which is consistent with the demonstrated mechanism of action for PT2977.
Conclusion
Motivated by the need for a robust supply of PT2977 (belzutifan) for use in combination studies against renal cell carcinoma (in vivo mouse models), we successfully utilized synthetic guidance provided in several publications and patents along with our own initiatives for improvement to reproduce the synthesis of PT2977. Difficulties encountered in the formation of diaryl ether 4 from intermediates 11 and 14 prompted an investigation into alternative methods.25,26 Synthesis of the key intermediate 4 was achieved reliably by initial formation of the diaryl ether 22 from propionic acid 21 and aryl fluoride 12 followed by cyclization/oxidation.25,26 Advancement of intermediate 4 to the all-cis stereotriad PT2977 (3) was achieved by successful reproduction of the literature route.18 The crystal structure and optical rotation of PT2977 were obtained, and biological efficacy of PT2977 synthesized in this study was verified through a RT-PCR experiment that demonstrated inhibition of VEGFA mRNA expression upon treatment of 786-O cells (renal cell carcinoma) with PT2977.
Supplementary Material
Acknowledgements
The authors are grateful for financial support of this project provided, in part, by the National Cancer Institute (NCI) of the National Institutes of Health (NIH) under award number R01CA244579 and the Office of the Vice Provost for Research (Baylor University, Postdoctoral Hiring Program). The content is solely the responsibility of the authors and does not necessarily represent the official views of the National Institutes of Health. Special thanks to Professor Ralph P. Mason (University of Texas Southwestern Medical Center) for insight regarding combination studies of PT2977 with OXi8007 (VDA from the Pinney Laboratory) that provided inspiration for us to pursue this synthesis of PT2977. Appreciation is extended to Drs. Ernest Hamel and Ruoli Bai (National Cancer Institute, Frederick National Laboratory for Cancer Research, National Institutes of Health) for carrying out the inhibition of tubulin polymerization and MCF-7 cancer cell line studies and to Dr. Li Liu (University of Texas Southwestern Medical Center) for assistance with facilitating the RT-PCR study.
Footnotes
Declaration of competing interest
The authors declare that they have no known competing financial interests or personal relationships that could have appeared to influence the work reported in this paper.
Special Issue Honoring Professor John L. Wood
It is our privilege and joy to dedicate this manuscript to Professor John L. Wood to honor his transformational contributions to the field of synthetic organic chemistry and to celebrate his steadfast leadership and guidance to Tetrahedron Letters through his distinguished service as Editor over many years.
Appendix A. Supplementary data
Supplementary data (experimental synthetic procedures, biological assay procedures, 1H, 13C, 19F NMR of synthesized intermediates, HIRESMS spectra, HPLC trace of PT2977 (3), and X-ray crystallography for compounds 3 and 26b) to this article can be found online at https://doi.org/10.1016/j.tetlet.2023.154691
Data availability
The manuscript and associated Supporting Information contains the majority of the data. Additional data will be made available upon request.
References
- (1).Nobel Prize in Physiology or Medicine 2019. https://www.nobelprize.org/prizes/medicine/2019/press-release/ (accessed 2022-11-02).
- (2).Kaelin WG; Ratcliffe PJ Oxygen Sensing by Metazoans: The Central Role of the HIF Hydroxylase Pathway. Mol. Cell 2008, 30 (4), 393–402. 10.1016/j.molcel.2008.04.009. [DOI] [PubMed] [Google Scholar]
- (3).Keith B; Johnson RS; Simon MC HIF1α and HIF2α: Sibling Rivalry in Hypoxic Tumour Growth and Progression. Nat. Rev. Cancer 2012, 12 (1), 9–22. 10.1038/nrc3183. [DOI] [PMC free article] [PubMed] [Google Scholar]
- (4).Fong G-H; Takeda K Role and Regulation of Prolyl Hydroxylase Domain Proteins. Cell Death Differ. 2008, 15 (4), 635–641. 10.1038/cdd.2008.10. [DOI] [PubMed] [Google Scholar]
- (5).Sato Y; Yoshizato T; Shiraishi Y; Maekawa S; Okuno Y; Kamura T; Shimamura T; Sato-Otsubo A; Nagae G; Suzuki H; Nagata Y; Yoshida K; Kon A; Suzuki Y; Chiba K; Tanaka H; Niida A; Fujimoto A; Tsunoda T; Morikawa T; Maeda D; Kume H; Sugano S; Fukayama M; Aburatani H; Sanada M; Miyano S; Homma Y; Ogawa S Integrated Molecular Analysis of Clear-Cell Renal Cell Carcinoma. Nat. Genet. 2013, 45 (8), 860–867. 10.1038/ng.2699. [DOI] [PubMed] [Google Scholar]
- (6).Zhang P-C; Liu X; Li M-M; Ma Y-Y; Sun H-T; Tian X-Y; Wang Y; Liu M; Fu L-S; Wang Y-F; Chen H-Y; Liu Z AT-533, a Novel Hsp90 Inhibitor, Inhibits Breast Cancer Growth and HIF-1α/VEGF/VEGFR-2-Mediated Angiogenesis in Vitro and in Vivo. Biochem. Pharmacol. 2020, 172, 113771. 10.1016/j.bcp.2019.113771. [DOI] [PubMed] [Google Scholar]
- (7).Fallah J; Rini BI HIF Inhibitors: Status of Current Clinical Development. Curr. Oncol. Rep. 2019, 21 (1), 6. 10.1007/s11912-019-0752-z. [DOI] [PubMed] [Google Scholar]
- (8).Sapra P; Zhao H; Mehlig M; Malaby J; Kraft P; Longley C; Greenberger LM; Horak ID Novel Delivery of SN38 Markedly Inhibits Tumor Growth in Xenografts, Including a Camptothecin-11-Refractory Model. Clin. Cancer Res. Off. J. Am. Assoc. Cancer Res. 2008, 14 (6), 1888–1896. 10.1158/1078-0432.CCR-07-4456. [DOI] [PubMed] [Google Scholar]
- (9).Zhang C; Yang C; Feldman MJ; Wang H; Pang Y; Maggio DM; Zhu D; Nesvick CL; Dmitriev P; Bullova P; Chittiboina P; Brady RO; Pacak K; Zhuang Z Vorinostat Suppresses Hypoxia Signaling by Modulating Nuclear Translocation of Hypoxia Inducible Factor 1 Alpha. Oncotarget 2017, 8 (34), 56110–56125. 10.18632/oncotarget.18125. [DOI] [PMC free article] [PubMed] [Google Scholar]
- (10).Schönberger T; Fandrey J; Prost-Fingerle K Ways into Understanding HIF Inhibition. Cancers 2021, 13 (1), 159. 10.3390/cancers13010159. [DOI] [PMC free article] [PubMed] [Google Scholar]
- (11).Ricker JL; Chen Z; Yang XP; Pribluda VS; Swartz GM; Van Waes C 2-Methoxyestradiol Inhibits Hypoxia-Inducible Factor 1alpha, Tumor Growth, and Angiogenesis and Augments Paclitaxel Efficacy in Head and Neck Squamous Cell Carcinoma. Clin. Cancer Res. Off. J. Am. Assoc. Cancer Res. 2004, 10 (24), 8665–8673. 10.1158/1078-0432.CCR-04-1393. [DOI] [PubMed] [Google Scholar]
- (12).Choi S-H; Hong Z-Y; Nam J-K; Lee H-J; Jang J; Yoo RJ; Lee YJ; Lee CY; Kim KH; Park S; Ji YH; Lee Y-S; Cho J; Lee Y-J A Hypoxia-Induced Vascular Endothelial-to-Mesenchymal Transition in Development of Radiation-Induced Pulmonary Fibrosis. Clin. Cancer Res. 2015, 21 (16), 3716–3726. 10.1158/1078-0432.CCR-14-3193. [DOI] [PubMed] [Google Scholar]
- (13).Scheuermann TH; Tomchick DR; Machius M; Guo Y; Bruick RK; Gardner KH Artificial Ligand Binding within the HIF2α PAS-B Domain of the HIF2 Transcription Factor. Proc. Natl. Acad. Sci. 2009, 106 (2), 450–455. 10.1073/pnas.0808092106. [DOI] [PMC free article] [PubMed] [Google Scholar]
- (14).Cho H; Du X; Rizzi JP; Liberzon E; Chakraborty AA; Gao W; Carvo I; Signoretti S; Bruick RK; Josey JA; Wallace EM; Kaelin WG On-Target Efficacy of a HIF-2α Antagonist in Preclinical Kidney Cancer Models. Nature 2016, 539 (7627), 107–111. 10.1038/nature19795. [DOI] [PMC free article] [PubMed] [Google Scholar]
- (15).Chen W; Hill H; Christie A; Kim MS; Holloman E; Pavia-Jimenez A; Homayoun F; Ma Y; Patel N; Yell P; Hao G; Yousuf Q; Joyce A; Pedrosa I; Geiger H; Zhang H; Chang J; Gardner KH; Bruick RK; Reeves C; Hwang TH; Courtney K; Frenkel E; Sun X; Zojwalla N; Wong T; Rizzi JP; Wallace EM; Josey JA; Xie Y; Xie X-J; Kapur P; McKay RM; Brugarolas J Targeting Renal Cell Carcinoma with a HIF-2 Antagonist. Nature 2016, 539 (7627), 112–117. 10.1038/nature19796. [DOI] [PMC free article] [PubMed] [Google Scholar]
- (16).Wehn PM; Rizzi JP; Dixon DD; Grina JA; Schlachter ST; Wang B; Xu R; Yang H; Du X; Han G; Wang K; Cao Z; Cheng T; Czerwinski RM; Goggin BS; Huang H; Halfmann MM; Maddie MA; Morton EL; Olive SR; Tan H; Xie S; Wong T; Josey JA; Wallace EM Design and Activity of Specific Hypoxia-Inducible Factor-2α (HIF-2α) Inhibitors for the Treatment of Clear Cell Renal Cell Carcinoma: Discovery of Clinical Candidate ( S )-3-((2,2-Difluoro-1-Hydroxy-7-(Methylsulfonyl)-2,3-Dihydro-1 H -Inden-4-Yl)Oxy)-5-Fluorobenzonitrile (PT2385). J. Med. Chem. 2018, 61 (21), 9691–9721. 10.1021/acs.jmedchem.8b01196. [DOI] [PubMed] [Google Scholar]
- (17).Wehn PM; Yang S; Grina JA; Rizzi JP; Schlachter ST; Wang B; Xu R; Yang H; Du X; Han G; Wang K; Czerwinski RM; Ged EL; Huang H; Halfmann MM; Maddie MA; Morton ER; Olive SR; Tan H; Xie S; Josey JA; Wallace EM Lead Change of a HIF-2α Antagonist Guided by Multiparameter Optimization and Utilization of an Olp→π*Ar Interaction. Med. Chem. Res. 2023. 10.1007/s00044-023-03088-w. [DOI] [Google Scholar]
- (18).Xu R; Wang K; Rizzi JP; Huang H; Grina JA; Schlachter ST; Wang B; Wehn PM; Yang H; Dixon DD; Czerwinski RM; Du X; Ged EL; Han G; Tan H; Wong T; Xie S; Josey JA; Wallace EM 3-[(1 S, 2 S, 3 R )-2,3-Difluoro-1-Hydroxy-7-Methylsulfonylindan-4-Yl]Oxy-5-Fluorobenzonitrile (PT2977), a Hypoxia-Inducible Factor 2α (HIF-2α) Inhibitor for the Treatment of Clear Cell Renal Cell Carcinoma. J. Med. Chem. 2019, 62 (15), 6876–6893. 10.1021/acs.jmedchem.9b00719. [DOI] [PubMed] [Google Scholar]
- (19).FDA Approves Merck’s Hypoxia-Inducible Factor-2 Alpha (HIF-2α) Inhibitor WELIREGTM (belzutifan) for the Treatment of Patients With Certain Types of Von Hippel-Lindau (VHL) Disease-Associated Tumors. Merck.com. https://www.merck.com/news/fda-approves-mercks-hypoxia-inducible-factor-2-alpha-hif-2α-inhibitor-welireg-belzutifan-for-the-treatment-of-patients-with-certain-types-of-von-hippel-lindau-vhl-disease/ (accessed 2023-04-07).
- (20).Hadimani MB; MacDonough MT; Ghatak A; Strecker TE; Lopez R; Sriram M; Nguyen BL; Hall JJ; Kessler RJ; Shirali AR; Liu L; Garner CM; Pettit GR; Hamel E; Chaplin DJ; Mason RP; Trawick ML; Pinney KG Synthesis of a 2-Aryl-3-Aroyl Indole Salt (OXi8007) Resembling Combretastatin A-4 with Application as a Vascular Disrupting Agent. J. Nat. Prod. 2013, 76 (9), 1668–1678. 10.1021/np400374w. [DOI] [PMC free article] [PubMed] [Google Scholar]
- (21).Pinney KG Molecular Recognition of the Colchicine Binding Site as a Design Paradigm for the Discovery and Development of Vascular Disrupting Agents. In Vascular-Targeted Therapies in Oncology; John Wiley & Sons, Ltd, 2006; pp 95–121. 10.1002/0470035439.ch6. [DOI] [Google Scholar]
- (22).Pinney K; Wang F; Hadimani M Indole-Containing and Combretastatin-Related Anti-Mitotic and Anti-Tubulin Polymerization Agents. US6849656B1, February 1, 2005. https://patents.google.com/patent/US6849656B1/en (accessed 2023-01-25). [Google Scholar]
- (23).Strecker TE; Odutola SO; Lopez R; Cooper MS; Tidmore JK; Charlton-Sevcik AK; Li L; MacDonough MT; Hadimani MB; Ghatak A; Liu L; Chaplin DJ; Mason RP; Pinney KG; Trawick ML The Vascular Disrupting Activity of OXi8006 in Endothelial Cells and Its Phosphate Prodrug OXi8007 in Breast Tumor Xenografts. Cancer Lett. 2015, 369 (1), 229–241. 10.1016/j.canlet.2015.08.021. [DOI] [PMC free article] [PubMed] [Google Scholar]
- (24).Zhou H; Hallac RR; Lopez R; Denney R; MacDonough MT; Li L; Liu L; Graves EE; Trawick ML; Pinney KG; Mason RP Evaluation of Tumor Ischemia in Response to an Indole-Based Vascular Disrupting Agent Using BLI and 19F MRI. Am. J. Nucl. Med. Mol. Imaging 2015, 5 (2), 143–153. [PMC free article] [PubMed] [Google Scholar]
- (25).Dixon DD; Stengel PJ; Wang B Hif-2-Alpha Inhibitor Polymorphs. WO2016145236A1, September 15, 2016. [Google Scholar]
- (26).Bruick RK; Chen Y; Ruiz JCF HIF-2alpha INHIBITORS FOR TREATING IRON OVERLOAD DISORDERS. 20170304300, October 26, 2017. https://www.freepatentsonline.com/y2017/0304300.html (accessed 2023-03-01).
- (27).Peng F; Tan L; Chen L; Dalby SM; DiRocco DA; Duan J; Feng M; Gong G; Guo H; Hethcox JC; Jin L; Johnson HC; Kim J; Le D; Lin Y; Liu W; Shen J; Wan Y; Xiao C; Xiang B; Xiang Q; Xu J; Yan L; Yang W; Ye H; Yu Y; Zhang J Manufacturing Process Development for Belzutifan, Part 1: A Concise Synthesis of the Indanone Starting Material. Org. Process Res. Dev. 2022, 26 (3), 508–515. 10.1021/acs.oprd.1c00236. [DOI] [Google Scholar]
- (28).Bottecchia C; Lévesque F; McMullen JP; Ji Y; Reibarkh M; Peng F; Tan L; Spencer G; Nappi J; Lehnherr D; Narsimhan K; Wismer MK; Chen L; Lin Y; Dalby SM Manufacturing Process Development for Belzutifan, Part 2: A Continuous Flow Visible-Light-Induced Benzylic Bromination. Org. Process Res. Dev. 2022, 26 (3), 516–524. 10.1021/acs.oprd.1c00240. [DOI] [Google Scholar]
- (29).Chen Z; Salehi Marzijarani N; Quirie S; Pirrone GF; Dalby SM; Wang T; Kim J; Peng F; Fine AJ Manufacturing Process Development for Belzutifan, Part 3: Completing a Streamlined Through-Process with a Safe and Scalable Oxidation. Org. Process Res. Dev. 2022, 26 (3), 525–532. 10.1021/acs.oprd.1c00232. [DOI] [Google Scholar]
- (30).Salehi Marzijarani N; Fine AJ; Dalby SM; Gangam R; Poudyal S; Behre T; Ekkati AR; Armstrong BM; Shultz CS; Dance ZEX; Stone K Manufacturing Process Development for Belzutifan, Part 4: Nitrogen Flow Criticality for Transfer Hydrogenation Control. Org. Process Res. Dev. 2022, 26 (3), 533–542. 10.1021/acs.oprd.1c00231. [DOI] [Google Scholar]
- (31).Wang T; Phillips EM; Dalby SM; Sirota E; Axnanda S; Shultz CS; Patel P; Waldman JH; Alwedi E; Wang X; Zawatzky K; Chow M; Padivitage N; Weisel M; Whittington M; Duan J; Lu T Manufacturing Process Development for Belzutifan, Part 5: A Streamlined Fluorination–Dynamic Kinetic Resolution Process. Org. Process Res. Dev. 2022, 26 (3), 543–550. 10.1021/acs.oprd.1c00242. [DOI] [Google Scholar]
- (32).Pirnot M; Stone K; Wright TJ; Lamberto DJ; Schoell J; Lam Y; Zawatzky K; Wang X; Dalby SM; Fine AJ; McMullen JP Manufacturing Process Development for Belzutifan, Part 6: Ensuring Scalability for a Deoxyfluorination Reaction. Org. Process Res. Dev. 2022, 26 (3), 551–559. 10.1021/acs.oprd.1c00239. [DOI] [Google Scholar]
- (33).Hillver S-E; Björk L; Höök BB; Cortizo L; Nordvall G; Johansson AM; Ertan A; Csöregh I; Johansson L; Lewander T; Hacksell U Synthesis and Pharmacology of the Enantiomers of UH301: Opposing Interactions with 5-HT1A Receptors. Chirality 1996, 8 (8), 531–544. . [DOI] [PubMed] [Google Scholar]
- (34).Tsukada T; Takahashi M; Takemoto T; Kanno O; Yamane T; Kawamura S; Nishi T Structure-Based Drug Design of Tricyclic 8H-Indeno[1,2-d][1,3]Thiazoles as Potent FBPase Inhibitors. Bioorg. Med. Chem. Lett. 2010, 20 (3), 1004–1007. 10.1016/j.bmcl.2009.12.056. [DOI] [PubMed] [Google Scholar]
- (35).Schokrpur S; Hu J; Moughon DL; Liu P; Lin LC; Hermann K; Mangul S; Guan W; Pellegrini M; Xu H; Wu L CRISPR-Mediated VHL Knockout Generates an Improved Model for Metastatic Renal Cell Carcinoma. Sci. Rep. 2016, 6, 29032. 10.1038/srep29032. [DOI] [PMC free article] [PubMed] [Google Scholar]
- (36).Lawrence NJ; Rennison D; Woo M; McGown AT; Hadfield JA Antimitotic and Cell Growth Inhibitory Properties of Combretastatin A-4-like Ethers. Bioorg. Med. Chem. Lett. 2001, 11 (1), 51–54. 10.1016/S0960-894X(00)00596-5. [DOI] [PubMed] [Google Scholar]
- (37).Pettit GR; Singh SB; Niven ML; Hamel E; Schmidt JM Isolation, Structure, and Synthesis of Combretastatins A-1 and B-1, Potent New Inhibitors of Microtubule Assembly, Derived from Combretum Caffrum. J. Nat. Prod. 1987, 50 (1), 119–131. 10.1021/np50049a016. [DOI] [PubMed] [Google Scholar]
- (38).Dark GG; Hill SA; Prise VE; Tozer GM; Pettit GR; Chaplin DJ Combretastatin A-4, an Agent That Displays Potent and Selective Toxicity toward Tumor Vasculature. Cancer Res. 1997, 57 (10), 1829–1834. [PubMed] [Google Scholar]
Associated Data
This section collects any data citations, data availability statements, or supplementary materials included in this article.
Supplementary Materials
Data Availability Statement
The manuscript and associated Supporting Information contains the majority of the data. Additional data will be made available upon request.


