Abstract
Coiling phagocytosis has previously been studied only with the bacteria Legionella pneumophila and Borrelia burgdorferi, and the results were inconsistent. To learn more about this unconventional phagocytic mechanism, the uptake of various eukaryotic microorganisms by human monocytes, murine macrophages, and murine dendritic cells was investigated in vitro by video and electron microscopy. Unconventional phagocytosis of Leishmania spp. promastigotes, Trypanosoma cruzi trypomastigotes, Candida albicans hyphae, and zymosan particles from Saccharomyces cerevisiae differed in (i) morphology (rotating unilateral pseudopods with the trypanosomatids, overlapping bilateral pseudopods with the fungi), (ii) frequency (high with Leishmania; occasional with the fungi; rare with T. cruzi), (iii) duration (rapid with zymosan; moderate with the trypanosomatids; slow with C. albicans), (iv) localization along the promastigotes (flagellum of Leishmania major and L. aethiopica; flagellum or posterior pole of L. donovani), and (v) dependence on complement (strong with L. major and L. donovani; moderate with the fungi; none with L. aethiopica). All of these various types of unconventional phagocytosis gave rise to similar pseudopod stacks which eventually transformed to a regular phagosome. Further video microscopic studies with L. major provided evidence for a cytosolic localization, synchronized replication, and exocytic release of the parasites, extending traditional concepts about leishmanial infection of host cells. It is concluded that coiling phagocytosis comprises phenotypically similar consequences of various disturbances in conventional phagocytosis rather than representing a single separate mechanism.
According to the generally accepted “zipper” hypothesis (18), phagocytosis is mediated by sequential, circumferential interactions between receptors and ligands on the surfaces of the phagocytes and the particles, respectively. Consequently, the engulfing pseudopods should strictly follow the outline of the attached particles, but several phagocytic events are not consistent with this model (discussed in reference 33). One of these exceptions is coiling phagocytosis (19), in which unilateral pseudopods of the phagocytes wrap around microorganisms in multiple turns, giving rise to largely self-apposed pseudopodal surfaces.
Such pseudopod whorls have randomly been observed during studies of phagocytosis of marine yeast (22), Trypanosoma brucei (32), Leishmania donovani promastigotes (9), Staphylococcus aureus (29), Legionella pneumophila (19), quartz crystals (4), Borrelia burgdorferi (35), Haemophilus influenzae (36), and Escherichia coli (25) or were unmentioned parts of electron micrographs showing phagocytosis of L. donovani amastigotes (8), Chlamydia psittaci (37), and Mycobacterium smegmatis (3). The number of reports may indicate that coiling phagocytosis is the most frequent of the unusual uptake mechanisms, but these random observations in general were neither pursued nor even reproduced, and therefore their significance remains uncertain. Only with Legionella and Borrelia was work continued, but these two bacterial models gave inconsistent results. Due to the limited information available so far and the disparity of methods applied to the different systems, it is not clear which of the reported results reflect general features of coiling phagocytosis or peculiarities of the experimental system being used.
To reach a broader understanding of coiling phagocytosis, the uptake of eukaryotic microorganisms by human and murine phagocytes was investigated by video and electron microscopy. Intrigued by the initial accidental findings of pseudopod whorls with marine yeast (22), T. brucei (32), and L. donovani (9), we studied the uptake of trypanosomatids and fungal cells. The results obtained strongly suggest that coiling phagocytosis reflects phenotypically similar consequences of heterogenous disturbances in the course of conventional phagocytosis rather than representing a mechanism on its own. The common denominator of the different disturbances obviously is the missing fusion of the engulfing pseudopods, which then slide along each other and give rise to transient pseudopod stacks.
MATERIALS AND METHODS
Phagocytes.
Human peripheral blood monocytes were isolated from leukocyte-rich plasma of healthy human blood donors (generously provided by the Department of Transfusion Medicine, Erlangen University Hospital) by buoyant density gradient centrifugation. A 1.068-g/ml Nycodenz solution (Nycoprep; provided by Life Technologies, Eggenstein, Germany) was used as the separation medium as specified in the manufacturer’s manual (24). CD2+ lymphocytes were removed from the mononuclear cell fraction by a rosetting step with neuraminidase-treated sheep erythrocytes. All preparative steps were performed in polypropylene tubes (Corning; provided by Dunn, Asbach, Germany) at room temperature unless otherwise indicated. Dulbecco’s Ca- and Mg-free phosphate-buffered saline (PBS) supplemented with 0.13% (wt/vol) sodium EDTA and 1% (vol/vol) fetal calf serum (FCS; all from BioConcept, Umkirch, Germany), which had been heat inactivated twice for 30 min at 56°C, was used for washings and dilutions.
Resident peritoneal macrophages were harvested from BALB/c and CD1 mice (Charles River, Sulzfeld, Germany) by flushing the peritoneal cavity twice with 8 to 10 ml of sterile PBS. From other mice, peritoneal exudate macrophages were harvested 4 days after intraperitoneal injection of 2 to 3 ml of sterile 4% (wt/vol) Brewer’s thioglycolate broth (Difco Laboratories, Detroit, Mich.). The dendritic cell line D2SC/1 from spleen cells of neonatal BALB/c mice (17) (generously provided by Paola Ricciardi-Castagnoli, Consiglio Nazionale delle Ricerche, Milan, Italy) were maintained in polystyrene cell culture flasks (Corning). RPMI 1640 supplemented with 2 mM l-glutamine and 10% (vol/vol) heat-inactivated FCS (hiFCS) (all from BioConcept) was used as the culture medium. The loosely adherent dendritic cells were detached by replacing the culture medium by ice-cold Dulbecco’s Ca- and Mg-free PBS supplemented with 0.13% (wt/vol) sodium EDTA and tapping the flasks vigorously.
Microbes.
The origin and propagation of Leishmania major MHOM/IL/81/FE/BNI has been described elsewhere (31). L. donovani IPB/399 was isolated from an Ethiopian patient suffering from kala-azar, and L. aethiopica was isolated from Ethiopian patients with either diffuse (strain Gere Gessie) or localized (strain 999/93) cutaneous leishmaniosis. These Ethiopian strains were established at the Armauer Hansen Research Institute in Addis Ababa, Ethiopia, characterized by isoenzyme analysis as reported previously (2), and kindly provided by Tamás Laskay, University of Lübeck, Lübeck, Germany. A Trypanosoma cruzi isolate from a Brazilian patient with Chagas’ disease was a kind gift from Paolo Andrade, Universidade Federal de Pernambuco, Recife, Brasil. The trypanosomatids were cultured in RPMI-hiFCS on NNN agar slants as described previously. In the phagocytosis assays, Leishmania promastigotes of the late logarithmic/early stationary phase and T. cruzi trypomastigotes were used. Candida albicans 3153A was routinely grown on YPD agar at 30°C and kept at stationary phase for 48 h prior to the phagocytosis experiments. To induce germ tube formation in liquid culture, cells were inoculated into modified Lee’s medium (30) containing 10% (vol/vol) hiFCS and were incubated on a rotary shaker at 120 rpm for 2 h at 37°C. Immediately prior to the phagocytosis assays, the germ tubes were pelleted, washed, counted, and aliquoted in incubation medium. Zymosan A particles prepared from the cell wall of Saccharomyces cerevisiae were obtained from Sigma (Deisenhofen, Germany).
Experimental conditions.
The phagocytes were washed, checked for viability by means of trypan blue exclusion, counted, resuspended in RPMI-hiFCS, and allowed to recover for 1 h at 4°C prior to incubation with the microbes in RPMI-FCS for periods of 20 min to 8 h. The supplemented serum used was either fresh FCS (fFCS) or hiFCS. In some experiments, the nucleophile sodium salicylhydroxamate (SSH; 1 mM) was added to the fFCS in order to block the alternative pathway of complement activation (6). Some monocytes were pretreated for 10 min with lipopolysaccharide (LPS; 1 μg/ml; Sigma), prepared by phenol extraction from E. coli serotype O111:B4, either alone or together with 10 phorbol 12-myristate 13-acetate (PMA; 10 ng/ml; Sigma). LPS and PMA remained in the incubation medium, and the concentration of each was readjusted to the final volume when the microorganisms were added. In some experiments, the microorganisms were killed prior to the incubation, either by exposing them to heat (80°C for 5 min) or by resuspending the pelleted pathogens in 2.5% (vol/vol) glutaraldehyde for 15 min. The chemically killed microorganisms were subsequently incubated with 1% (wt/vol) sodium borohydride to block reactive aldehyde groups (21) and then washed thoroughly.
Electron microscopy.
For electron microscopy, 2 × 106 phagocytes and 2 × 107 pathogens, each washed and resuspended in 0.5 ml of incubation medium, were mixed in 15-ml polypropylene tubes (Corning), giving a total incubation volume of 1.0 ml. Incubation took place at 37°C under 7% CO2 and was stopped by filling the tubes with cold 2.5% (vol/vol) glutaraldehyde. Following fixation for 4 h at 4°C, the specimens were prepared for electron microscopy according to standard protocols. Briefly, they were postfixed with reduced osmium, encapsulated in agar, stained en bloc with a combination of uranyl acetate and phosphotungstic acid, dehydrated in a series of graded ethanolic solutions ending with pure acetone, and finally embedded in Epon 812. Ultrathin sections were placed onto 200-mesh standard square copper grids, stained with uranyl acetate and lead citrate (all chemicals were from Roth, Karlsruhe, Germany), and evaluated with a Zeiss type 906 transmission electron microscope.
Video-enhanced phase-contrast microscopy.
For the light microscopic survey of Leishmania adhesion and uptake, phagocytes (106 per chamber in 2 ml of RPMI-hiFCS) were seeded onto two-chamber glass slides (LabTek; Nunc, Wiesbaden, Germany). After 60 min, the chambers were rinsed and Leishmania promastigotes (107 per chamber in 2.0 ml of incubation medium) were added to the adherent phagocytes. The slides were immediately transferred to a Zeiss Axiovert 135 TV invert microscope equipped with a custom-made incubation chamber adjusted to 37°C and 7% CO2 with saturated humidity. The interaction between the phagocytes and the promastigotes was recorded with an AVT-Horn video camera and a BTS Betacam Sp video set on Sony Betacam Sp video cassettes, using 40× to 100× phase-contrast lenses.
Evaluation of the results.
All experiments were performed in triplicate with phagocytes from different donors or animals. Two different ultrathin sections (or cell culture wells) from each experiment were scored in a blinded fashion by counting at least 100 events of phagocytosis in randomly chosen areas, which gave the relative frequencies of the phagocytic mechanisms. These values were normalized by calculating for each donor or animal the experimental values proportionally to the values of the control incubations, which were set as 100%. The differences were analyzed with the χ2 test and were considered to be significant for P < 0.05.
RESULTS
Patterns of coiling phagocytosis of opsonized promastigotes from various Leishmania spp. are similar in frequency and morphology but different in topology.
Human monocytes and murine macrophages were incubated with 10-fold the number of viable promastigotes from L. major, L. donovani, or L. aethiopica in culture medium containing fFCS for 30 to 120 min. The morphological patterns of phagocytosis were examined by transmission electron microscopy (Fig. 1).
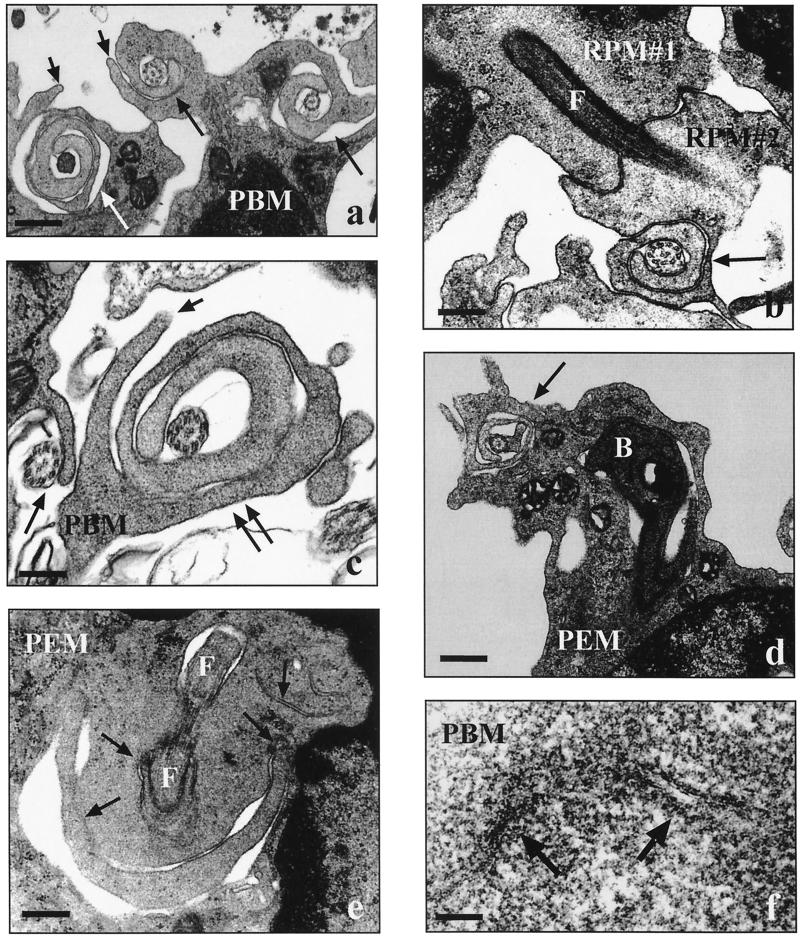
FIG. 1.
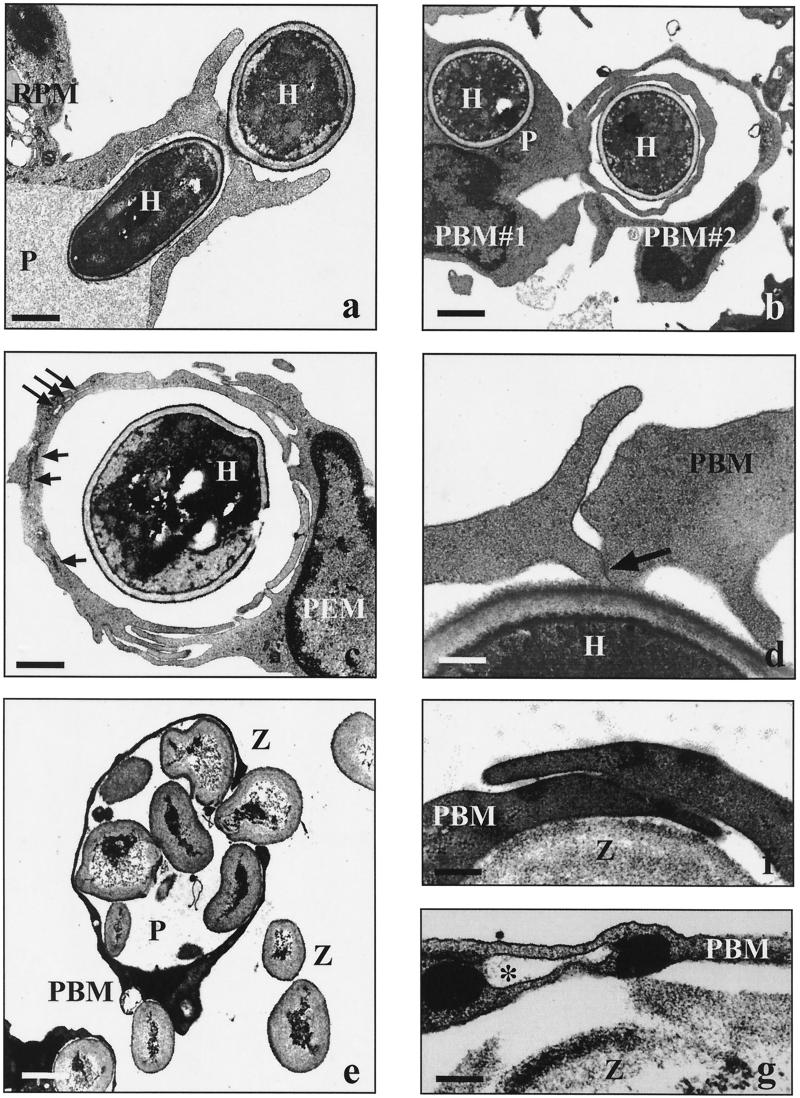
Electron micrographs showing the uptake of Leishmania spp. by human and murine phagocytes. Human peripheral blood monocytes (PBM) and murine resident (RPM) or thioglycolate-elicited (PEM) peritoneal macrophages (2 × 106 each) were incubated with live promastigotes (2 × 107 each) from three Leishmania spp. in the presence of fFCS for 30 min unless otherwise indicated. (a) Unilateral pseudopods (long arrows) rotating around flagella of heat-killed parasites (L. donovani; short arrows, additional contrarotating pseudopods; bar = 0.4 μm). (b) Two phagocytes (RPM#1 and RPM#2) simultaneously internalizing a parasite via conventional phagocytosis (L. major; F, flagellum; arrow, pseudopod coil; bar = 0.2 μm). (c) Beginning (single long arrow) and advanced (double long arrows) coiling phagocytosis of parasite flagella in presence of hiFCS (L. aethiopica; short arrow, additional contrarotating pseudopod; bar = 0.2 μm). (d) Parasite with its flagellum engulfed by a pseudopod coil (arrow) and its body (B) located in a regular phagosome (L. aethiopica; bar = 0.6 μm). (e) Contorted coils (arrows) of a pseudopod whorl starting to transform into a confluent phagosome wall (L. major; F, flagellum; bar = 0.2 μm). (f) Fused self-apposed membranes (arrows) in the transition zone of a transforming pseudopod coil (L. major; bar = 0.01 μm).
It became apparent that the promastigotes from L. major and L. aethiopica usually were taken up with the flagellum (anterior pole) first (Fig. 1a to c). If parasites were internalized by two phagocytes simultaneously (Fig. 1b), one phagocyte would engulf the flagellum and the other one would engulf the posterior pole. With most (73 to 85%) of the phagocytosed promastigotes, the flagellum was enclosed by a pseudopod coil (Fig. 1a to d), whereas the flagellum of the remaining promastigotes was enclosed by a tubular, funnel-like protrusion of the phagocytes (Fig. 1b). The pseudopod coils were formed by single pseudopods repetitively winding themselves along the flagella in a helical movement (Fig. 1a to d), displaying central pseudopod-microbial and peripheral pseudopod-pseudopodal membrane appositions. Occasionally, the pseudopod whorls were flanked by an additional contrarotating pseudopod (Fig. 1a, and c). Pseudopod coils were observed only along the length of the flagellum; posterior of the flagellar pocket, the parasite bodies were surrounded by circumferential phagocyte extensions (Fig. 1d). At the transition zone between these two features, the multilayered pseudopods became irregular and contorted (Fig. 1e) and eventually fused (Fig. 1f) to a confluent wall encircling the microbes. In contrast to the clear restriction of coiling phagocytosis to the anterior pole of L. major and L. aethiopica promastigotes, pseudopod coils were seen not only at the flagellum (Fig. 1a) but also at the posterior pole (data not shown) of L. donovani promastigotes.
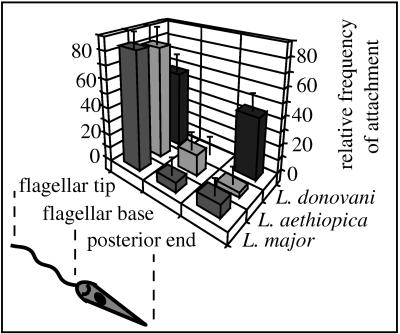
To understand the different topological patterns of coiling phagocytosis, the interaction of Leishmania promastigotes with their host cells was directly visualized by video-enhanced phase-contrast microscopy. For this purpose, we incubated adherent monocytes, macrophages, and dendritic cells with the parasites and monitored the events under cell culture conditions. It was seen that the promastigotes from all Leishmania spp. generally moved in the direction of the rotating flagellum. Although it was expected that the promastigotes would attach to the host cells with the flagellar tip, the parasites adhered to the phagocytes with different sites of the body (Fig. 2 shows mean relative frequencies ± standard error of the mean [SEM]): L. major promastigotes attached almost exclusively with the flagellar tip, occasionally with the flagellar base, and very rarely with the posterior pole of the body; L. aethiopica promastigotes also adhered preferentially with the flagellar tip but more often with the flagellar base than L. major and as rarely with the posterior pole; L. donovani promastigotes were affixed with either the flagellar tip or the posterior pole in about the same proportions but not with the flagellar base. Internalization of all attached promastigotes was achieved by funnel-like extensions of the phagocyte surface which advanced along the radiating part of the parasites (Fig. 3a and b). Unfortunately, the resolution achieved by the phase-contrast lenses did not allow the depiction of spiral movements of the engulfing pseudopods as revealed by electron microscopy.
FIG. 2.
Regional differences in the attachment of promastigotes from various Leishmania spp. to human monocytes. Human peripheral blood monocytes (106) were incubated with either L. major, L. donovani, or L. aethiopica promastigotes (107) in the presence of fFCS for 15 min. The bars represent the relative frequencies of the attachment sites observed with randomly chosen promastigotes, given as mean ± SEM of duplicate determinations. Essentially the same results were observed in two additional experiments using phagocytes from different donors. Promastigotes from L. major and L. aethiopica attach predominantly with the flagellar tip and only occasionally with the flagellar base or posterior pole, whereas L. donovani promastigotes attach with either the flagellar tip or the posterior pole in approximately equal proportions.
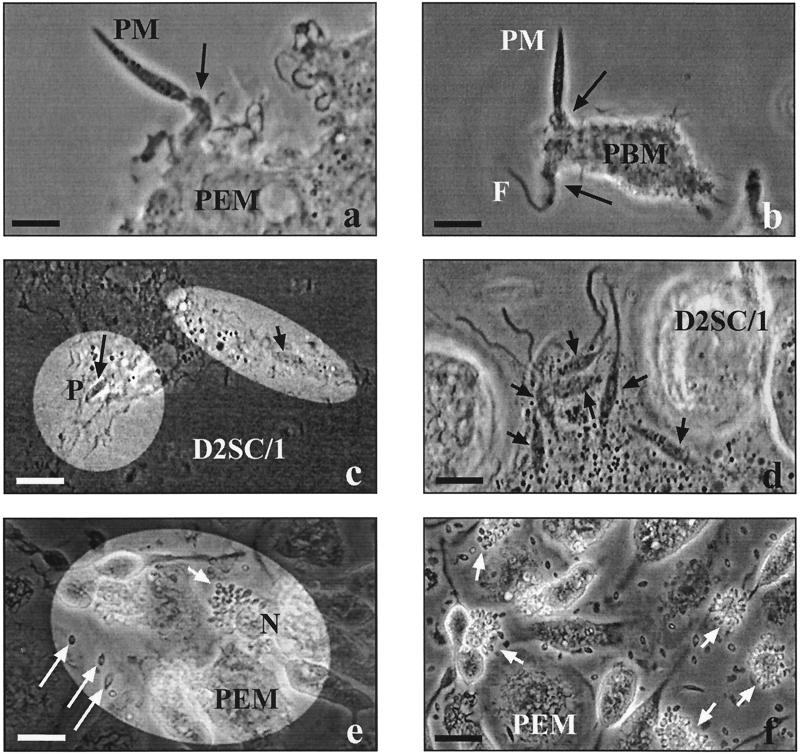
FIG. 3.
Video microscopic observations on the infection of various host cells by L. major. Human peripheral blood monocytes (PBM), murine peritoneal macrophages (PEM), or D2SC/1 cells (106 each) were pulsed with L. major promastigotes (PM; 107) in presence of fFCS for 30 min and subsequently chased by video-enhanced phase-contrast microscopy for several days. (a) Funnel-like extension (arrow) of a phagocyte moving along a radiating PM which has been attached with its flagellar tip (bar = 10 μm). (b) Funnel-like extensions (arrows) of a phagocyte moving along in either direction of a PM which has been attached with its flagellar base (F, flagellum; bar = 10 μm). (c) Two PM inside a host cell, one (long arrow) clearly located within a phagosome (P) and the other (short arrow) obviously not bounded by a host cell membrane (bar = 15 μm). (d) Multiple intracellular PM (arrows) obviously not bounded by host cell membranes (bar = 10 μm). (e) Asymmetrical accumulation of small vacuoles (arrow) at the periphery of the host cell (5 days postinfection; N, host cell nucleus; long arrows, released amastigotes; bar = 20 μm). (f) Numerous host cells (arrows) simultaneously releasing replicated parasites; the pericellular fluid is full of amastigotes (5 days postinfection; bar = 20 μm). Reprinted from “Macrophages: Infection with Leishmania major” (28a) with permission of the publisher.
L. major is seen in two different sites in the host cells and has synchronized replication and release.
For L. major, the subsequent course of infection was tracked via video microscopy. After a 30-min pulse with L. major promastigotes, the adherent phagocytes were washed vigorously to remove nonattached parasites and surveyed by time-lapse video microscopy (Fig. 3). Following their uptake via funnel-like pseudopods of the phagocytes (Fig. 3a and b), the majority of the internalized promastigotes were clearly localized inside phagosomes (Fig. 3c). However, some intracellular promastigotes were not surrounded by an apparent host cell membrane but appeared to lay in the cytosol (Fig. 3c and d). These possibly cytosolic parasites slowly forced their way through the cytoplasm of the phagocytes by pushing aside host cell organelles (event shown in the videotape). The infected cells eventually rounded, which unfortunately hindered further phase-contrast microscopic observations. After several uneventful days, small vacuoles suddenly accumulated asymmetrically at the periphery of the infected phagocytes (Fig. 3e and f). From these peripheral vacuoles, L. major amastigotes were constantly released over a period of several hours, leaving the somewhat shriveled remnants of their host cells; soon the pericellular space filled with replicated parasites (Fig. 3f).
Coiling phagocytosis of Leishmania promastigotes is influenced by neither microbial viability nor motility.
It has been assumed that the pseudopod whorls enwrapping T. brucei and L. donovani are caused by the vigorous movements of the trypanosomatids (9, 32). Indeed, pseudopod coils have been described for viable but not for glutaraldehyde-killed L. donovani promastigotes (9). To test this hypothesis, Leishmania promastigotes were killed either by a chemical (glutaraldehyde) or a physical (heat) method before being added to monocytes. No difference was found between the killed (nonmotile) and viable (motile) Leishmania promastigotes, either in terms of frequency or in terms of morphology of coiling phagocytosis (Fig. 1a). However, glutaraldehyde-killed promastigotes were not internalized unless they were subsequently treated with borohydride and thoroughly washed.
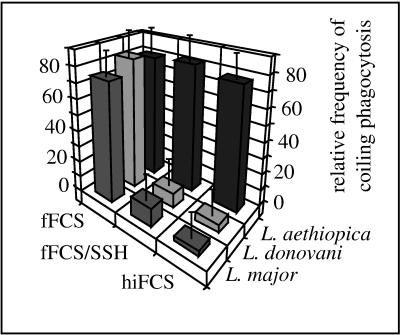
Coiling and conventional phagocytosis of L. major and L. donovani but not of L. aethiopica is strongly dependent on complement.
Attachment of different Leishmania spp. is known to differ with respect to the requirement of opsonization (reviewed in references 7 and 23), which may be associated with coiling phagocytosis. Therefore, the uptake of L. major, L. donovani, or L. aethiopica promastigotes was additionally investigated in the presence of hiFCS and fresh fFCS to which the nucleophile SSH had been added. It was found that inactivation of the complement system, either via heat inactivation of its heat-labile components or via SSH-mediated blockade of the pivotal component C3, almost completely abolished coiling and conventional phagocytosis of L. major and L. donovani promastigotes (Fig. 4). In contrast, phagocytosis of L. aethiopica promastigotes was not affected (Fig. 4 and 1c). This lack of difference toward complement (as well as the morphological features and the topological restriction of phagocytosis before) was true for two different L. aethiopica strains which were isolated from patients with the localized and the diffuse manifestation of disease (2).
FIG. 4.
Influence of complement on the frequency of coiling phagocytosis of promastigotes from various Leishmania spp. Human peripheral blood monocytes (2 × 106) were incubated with promastigotes from either L. major, L. donovani, or L. aethiopica (2 × 107 each) for 30 min in the presence of 10% (vol/vol) hiFCS or fFCS, the latter with or without 1 mM SSH. Phagocytosis was evaluated in a blinded fashion by electron microscopy of randomly chosen ultrathin sections. The bars represent the relative frequencies of coiling phagocytosis, given as mean ± SEM of duplicate determinations in a typical experiment. Essentially the same results were observed in additional two experiments using phagocytes from different donors. Coiling and conventional phagocytosis of L. major and L. donovani, but not of L. aethiopica, was strongly reduced in presence of heat-inactivated or SSH-neutralized serum.
Coiling phagocytosis of T. cruzi has a morphology similar to those of Leishmania spp. but a very low frequency.
In contrast to the predominance of coiling phagocytosis with Leishmania promastigotes, this uptake mechanism was very rare with T. cruzi trypomastigotes under identical experimental settings (presence of fresh serum, 30-min incubation period, 10-fold number of microorganisms). Virtually all trypomastigotes were internalized conventionally via phagocytic cups; coiling phagocytosis was observed only in a total of four of several hundred cases evaluated (Fig. 5). These four pseudopod whorls observed with T. cruzi trypomastigotes were morphologically similar to those found with Leishmania promastigotes, although the rotating pseudopods enclosed both the flagellum and the adjacent parasite body (data not shown).
FIG. 5.
Frequency of coiling phagocytosis observed with L. major, T. cruzi, and C. albicans. Human peripheral blood monocytes (PBM) and resident (RPM) or thioglycolate-elicited (PEM) macrophages from the peritoneal cavities of mice (2 × 106 each) were incubated with either L. major promastigotes, T. cruzi trypomastigotes, or C. albicans hyphae (2 × 107 each) in the presence of fFCS for 30 min. Phagocytosis was evaluated by electron microscopy of randomly chosen ultrathin sections. The bars represent the relative frequencies of coiling phagocytosis, given as mean ± SEM of duplicate determinations in a typical experiment. Essentially the same results were observed in additional two experiments using phagocytes from different donors and animals. The majority of the L. major promastigotes are engulfed via pseudopod coils by all phagocyte populations, whereas this is a very rare event with T. cruzi trypomastigotes. Coiling phagocytosis is only occasionally observed with C. albicans hyphae by PBM and RPM but not by PEM.
Coiling phagocytosis of C. albicans and zymosan particles is rare and morphologically distinct from the hitherto observed mechanisms.
Under the same experimental settings (presence of fresh serum, 30-min incubation period, 10-fold number of microorganisms), the uptake of C. albicans hyphae showed considerable differences in both the frequency (Fig. 5) and morphology (Fig. 6) of coiling phagocytosis compared to those of Leishmania; moreover, differences were found between the different phagocyte populations tested (Fig. 5).
FIG. 6.
Electron micrographs showing the uptake of C. albicans and zymosan particles by human and murine phagocytes. Human peripheral blood monocytes (PBM) and murine resident (RPM) or thioglycolate-elicited (PEM) peritoneal macrophages (2 × 106 each) were incubated with live C. albicans hyphae (H) or zymosan particles (Z) (2 × 107) in the presence of fFCS for incubation periods of 20 min to 8 h unless otherwise indicated. (a) Phagocytosis of C. albicans via a symmetrical phagocytic cup (30 min; P, phagosome; bar = 1.7 μm). (b) Two phagocytes (PBM#1 and PBM#2) simultaneously engulfing C. albicans via overlapping pseudopods (60 min; P, conventional phagosome; bar = 3.0 μm). (c) Various stages of fusion events along the overlapping pseudopods enclosing a heat-killed C. albicans cell (240 min; long arrows, remaining membrane fissures; short arrows, closed membrane fissures; bar = 4.0 μm). (d) Slightly overlapping pseudopods of a PBM in the presence of 1 μg of LPS per ml (30 min; arrow, starting membrane fusion; bar = 0.2 μm). (e) Giant phagosome (P) filled with several zymosan particles (20 min; bar = 2.5 μm). (f) Overlapping pseudopods along a zymosan particle in the absence of serum (20 min; bar = 0.5 μm). (g) Remaining membrane-bounded fissure (asterisk) in the phagosome wall enclosing a zymosan particle, indicative of incomplete fusion of overlapping pseudopods (20 min; bar = 0.1 μm).
Studying the morphological features of yeast uptake, we found that all attached C. albicans hyphae initially were engulfed via symmetrical pairs of pseudopods (Fig. 6a). However, in 0.5 to 2.5% of the phagocytosing monocytes and resident peritoneal macrophages, the approaching bilateral pseudopods did not close over the hyphae to form a conventional phagosome. Instead, they slid past each other and overlapped; additional pseudopods frequently lay on top of them from both sides (Fig. 6b and c). Stacks of countercurrent pseudopods were formed thereby, with the central pseudopods apposing the enclosed hyphae but the peripheral ones apposing each other. Occasionally, the engulfing pseudopods were part of two different phagocytes attacking the same C. albicans cell from opposite directions (Fig. 6b). As with the pseudopod whorls around Leishmania promastigotes, the formation of overlapping pseudopods around C. albicans hyphae was not reduced when killed hyphae were used (Fig. 6c). Instead, heat killing (but not glutaraldehyde killing) tended to increase phagocytosis (data not shown).
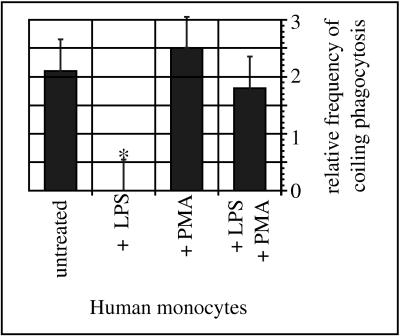
Thioglycolate-elicited peritoneal exudate macrophages did not display such pseudopod stacks but the rims of the phagocytic cups overlapped for a short distance instead, and membrane-bounded fissures were present in some of their phagosomal walls similar to those found in the transition zone of the Leishmania promastigotes. These findings suggested that the absence of pseudopod stacks was due to a rapid fusion of slightly overlapping pseudopods. To confirm this hypothesis, two additional sets of experiments were performed. First, monocytes were pretreated with LPS or PMA, either alone or in combination, for 10 min before addition of C. albicans hyphae for another 60 min. In these experiments, the LPS-treated monocytes showed the same features as described for the thioglycolate-elicited macrophages (Fig. 6d). This effect could be blocked by adding PMA in addition to LPS, whereas PMA alone did not affect uptake of the hyphae (Fig. 7). Second, monocytes were left untreated but were incubated with C. albicans hyphae for longer periods of up to 8 h. Beginning with an incubation period of 6 h, the multilayered pseudopods of the nonactivated monocytes started to fuse as well (data not shown), indicating that nonactivated phagocytes were slower than preactivated phagocytes but were still capable of fusing the overlapping pseudopods.
FIG. 7.
Influence of LPS and PMA on the frequency of coiling phagocytosis of C. albicans. Untreated human peripheral blood monocytes (PBM; 2 × 106) and PBM pretreated with LPS (1 μg/ml) and/or PMA (10 ng/ml) for 10 min were incubated with C. albicans hyphae (2 × 107) in the presence of fFCS for another 30 min. Phagocytosis was evaluated in a blinded fashion by electron microscopy of randomly chosen ultrathin sections. The bars represent the normalized frequencies of coiling phagocytosis, expressed as mean ± SEM of three separate experiments with monocytes from different donors. Pretreatment of PBM with LPS abolished coiling phagocytosis of C. albicans hyphae. The effect of LPS could be neutralized by additional treatment with PMA, whereas PMA alone did not affect the frequency of coiling phagocytosis to a significant extent (∗, P < 0.05 in the χ2 test).
Our finding of overlapping pseudopods with C. albicans is in contrast to the pseudopod whorls described for marine yeast (22). To provide an additional example of yeast phagocytosis, monocytes were incubated with zymosan particles prepared from S. cerevisiae. In the presence of fresh serum, the zymosan particles were internalized by the monocytes avidly up to their physical limits (Fig. 6e). In 1 to 2% of the phagocytic events, the engulfing bilateral pseudopods did not fuse immediately but overlapped for a short distance first (Fig. 6f), as indicated by transient membrane-bounded fissures in the confluent phagosomal walls (Fig. 6g). Overlapping pseudopods and fissures were even more frequent (up to 3.5%) when the serum additive either was heat inactivated or omitted.
DISCUSSION
This first systematic study on coiling phagocytosis of eukaryotic microorganisms yielded several important results leading to a new understanding of this poorly characterized mechanism. First and foremost, considerable differences in coiling phagocytosis of trypanosomatids and fungal cells became apparent with respect to frequency, morphology, duration, localization, and dependence on complement. These results strongly suggest that coiling phagocytosis represents neither a single nor a separate event. Instead, it most likely comprises various disturbances in conventional phagocytosis. Second, coiling unilateral pseudopods—although used to name this event—turned out to be a separate phenomenon; the missing fusion of the pseudopods and the pseudopod stacks are the actual hallmarks of coiling phagocytosis. Third, most likely classical phagocytosis-promoting receptors rather than a special receptor promote coiling phagocytosis, obviously involving both opsonic as well as nonopsonic binding and complement receptors (CRs) as well as non-CRs.
The different frequencies of coiling phagocytosis among the different microorganisms, the equal uptake of live and killed pathogens, and coiling phagocytosis of inanimate membrane preparations show that coiling phagocytosis is not a random trapping of microorganisms by spontaneously coiling pseudopods but a reaction of the phagocytes to the attachment of particles. These results also rule out a role of a specific microbial morphology, motility, and/or viability in this process. However, the shape of a particle may influence the phenotype of coiling phagocytosis, since the rotating type was found with more or less elongated microorganisms adhering end-on and the overlapping type was found with spherical ones.
Coiling phagocytosis by now is validated not only with gram-negative bacteria but also with protozoan parasites and fungal cells. It is unlikely that all of these different microorganisms have microbe-specific epitopes in common. Thus, it has to be assumed either that more than one coiling phagocytosis-promoting receptor exists or that these evolutionarily distant pathogens need the help of bridging opsonins to bind to a single coiling phagocytosis-promoting receptor. These possibilities may not be mutually exclusive; indeed, results from the two bacterial models (discussed in reference 28) as well as from the present study suggest that both opsonic attachment and nonopsonic attachment take place. Since coiling phagocytosis of L. major and L. donovani promastigotes, but not of L. aethiopica, was strongly dependent on complement opsonization, CRs may function as coiling phagocytosis-promoting receptors for some microorganisms.
However, CRs usually promote zipper-type phagocytosis, and therefore the deviating effect of a coiling-promoting factor has to be assumed. For L. major and L. donovani promastigotes, it has been suggested that CR1 mediates their initial attachment and CR3 mediates their actual engulfment (7, 23). If this CR1-CR3 interplay hinders the symmetrical clustering of CR3 at the microbial attachment site, coiling phagocytosis may result from the disturbed formation of a regular phagocytic cup. The transient formation of multireceptor complexes is known from other phagocytosis-promoting receptors as well (20), and therefore this asymmetry hypothesis may also apply to the complement-independent cases of coiling phagocytosis. An asymmetrical uptake may be linked to the polarized adhesion of the promastigotes, which obviously is a general but hitherto neglected feature of leishmanial infection. This feature was found not only in the present study with L. major, L. donovani, and L. aethiopica but also previously with L. mexicana, L. brasiliensis, L. tropica, and L. donovani (reviewed in reference 10). The major leishmanial adhesin, the complement-binding lipophosphoglycan, however, is the most abundant component of the promastigote surface (5), which makes a polar capping unlikely. Thus, the obviously nonrandom distribution of leishmanial adhesins remains to be elucidated.
On the other hand, asymmetry is unlikely to cause the overlapping type of coiling phagocytosis. Coiling phagocytosis of the fungal cells is indistinguishable from conventional phagocytosis apart from the missing fusion of the bilateral pseudopods. Fusion of membranes is not a spontaneous event but depends on fusion proteins which have to be reciprocally present at the approaching membrane areas (13). It is tempting to assume that the missing fusion of the bilateral overlapping pseudopods reflects the dysfunction of fusion factors, whereas the missing fusion of the unilateral pseudopod coils results simply from the absence of a fusion partner.
The pseudopod stacks around Leishmania and especially Candida frequently are flanked by additional pseudopods which contribute to the self-apposed pseudopod layers. This phenomenon has only recently been recognized with coiling phagocytosis of various spirochetes (28) but can retrospectively be seen with B. burgdorferi (25, 35), T. brucei (32), and L. pneumophila (19) as well, which makes it a general characteristic of coiling phagocytosis. Similar overshooting pseudopods are triggered by Salmonella typhimurium (14, 15) and some diffusely adherent E. coli strains (11, 38) in their host cells. Entry of Salmonella likely occurs via the lateral spreading of transducing signals following the ligation of various host cell receptors, including that for epidermal growth factor, which obviously trigger calcium fluxes as a common downstream effect (reviewed in reference 16). It will be interesting to determine the extent to which the overshooting pseudopods of the directed macropinocytosis (14) and the surplus pseudopods of coiling phagocytosis share receptors, effector molecules, and signal transduction pathways.
The phagocytes eventually overcome the disturbations in microbial uptake and transform the pseudopod stacks to a confluent phagosomal wall. The resulting delay in phagosome formation in combination with the metabolically stress imposed on the phagocytes may hamper the phagocytic clearance of invading pathogens. Once again the data obtained in the bacterial models differ: L. pneumophila cells end up in ribosome-studded, membrane-bound vacuoles (34), whereas the complete disintegration of the pseudopod membranes leaves B. burgdorferi and other spirochetes in the cytosol (25–28). Coiling phagocytosis of Leishmania promastigotes as well could account for the possibly cytosolic localization of some parasites, as seen with L. major in the present study and with L. donovani in a previous one (1). With the caveat that these video microscopic observations must be validated by additional studies, we can speculate that the cytosolic localization may lead to a major histocompatibility complex class I-restricted presentation of leishmanial antigens, as has already been reported for L. braziliensis (12). Further studies also are required to confirm the observation that the replicated L. major amastigotes accumulated at the periphery of the host cells before being released, leaving a shriveled but structurally intact phagocyte. This observation indicates a controlled exocytic release rather than the rupturing of the infected cells, as depicted in textbooks.
In conclusion, the present study provided important insights into the nature of coiling phagocytosis and clues for the assessment of different experimental models. It became clear that the missing fusion of the pseudopods and the formation of pseudopod stacks are the common denominator of the different types of coiling phagocytosis, whereas coiling unilateral pseudopods are a separate phenomenon. The mechanisms involved in the subsequent steps obviously can vary between the different experimental models and therefore need to be determined for each microorganism separately.
ACKNOWLEDGMENTS
This study was supported by grants from the Deutsche Forschungsgemeinschaft to K.S. (grant Schr450/2-1), W.S. (grant SFB263/A1), and C.B. (grant SFB263/A5).
We are indebted to Tamás Laskay and Paolo Andrade for providing the L. donovani, L. aethiopica, and T. cruzi strains, to Reinhold Eckstein for the buffy coats, and to Paola Ricciardi-Castagnoli for the D2SC/1 cells. The skillful technical assistance of Andrea Hilpert and Inge Zimmermann is appreciated. Christiane Wittek was particularly helpful with the electronic processing of the photographic material.
REFERENCES
- 1.Akiyama H J, Haight R D. Interaction of Leishmania donovani and hamster peritoneal macrophages. A phase-contrast microscopical study. Am J Trop Med Hyg. 1971;20:539–545. doi: 10.4269/ajtmh.1971.20.539. [DOI] [PubMed] [Google Scholar]
- 2.Akuffo H, Maasho K, Blostedt M, Höjeberg B, Britton S, Bakhiet M. Leishmania aethiopica derived from diffuse leishmaniosis patients preferentially induce mRNA for interleukin-12 while those from localized leishmaniosis patients induce interferon-γ. J Infect Dis. 1997;175:737–741. doi: 10.1093/infdis/175.3.737. [DOI] [PubMed] [Google Scholar]
- 3.Barker K, Fan H, Carroll C, Kaplan G, Barker J, Hellmann W, Cohn Z A. Nonadherent cultures of human monocytes kill Mycobacterium smegmatis, but adherent cultures do not. Infect Immun. 1996;64:428–433. doi: 10.1128/iai.64.2.428-433.1996. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 4.Behrendt H, Seemayer N H, Braumann A, Nissen M. Electron microscopy investigations of the effect of quartz dust DQ 12 on human monocytes/macrophages in vitro. Silikoserep Nordrhein-Westphalen. 1987;16:171–183. [Google Scholar]
- 5.Beverley S M, Turco S J. Lipophosphoglycan (LPG) and the identification of virulence genes in the protozoan parasite Leishmania. Trends Microbiol. 1998;6:35–40. doi: 10.1016/S0966-842X(97)01180-3. [DOI] [PubMed] [Google Scholar]
- 6.Blackwell J M, Ezekowitz A B, Roberts M B, Channon J Y, Sim R B, Gordon S. Macrophage complement and lectin-like receptors bind Leishmania in the absence of serum. J Exp Med. 1985;162:324–331. doi: 10.1084/jem.162.1.324. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 7.Brittingham A, Mosser D M. Exploitation of the complement system by Leishmania promastigotes. Parasitol Today. 1996;12:444–447. doi: 10.1016/0169-4758(96)10067-3. [DOI] [PubMed] [Google Scholar]
- 8.Chang K P, Dwyer D M. Leishmania donovani-hamster macrophage interactions in vitro: cell entry, intracellular survival, and multiplication of amastigotes. J Exp Med. 1978;147:515–530. doi: 10.1084/jem.147.2.515. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 9.Chang K P. Leishmania donovani: promastigote-macrophage surface interactions in vitro. Exp Parasitol. 1979;48:175–189. doi: 10.1016/0014-4894(79)90097-3. [DOI] [PubMed] [Google Scholar]
- 10.Chang K P. Cellular and molecular mechanisms of intracellular symbiosis in leishmaniasis. Int Rev Cytol Suppl. 1983;14:267–305. [PubMed] [Google Scholar]
- 11.Cookson S T, Nataro J P. Characterization of HEp-2 cell projection formation induced by diffusely adherent Escherichia coli. Microb Pathog. 1996;21:421–434. doi: 10.1006/mpat.1996.0073. [DOI] [PubMed] [Google Scholar]
- 12.Da-Cruz A M, Conceicao-Silve F, Bertho A L, Coutinho S G. Leishmania-reactive CD4+ and CD8+ T cells associated with cure of human cutaneous leishmaniasis. Infect Immun. 1994;62:2614–2618. doi: 10.1128/iai.62.6.2614-2618.1994. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 13.Düzgünes, N. Molecular mechanisms of membrane fusion, p. 97–129. In M. C. P. de Lima, N. Düzgünes, and D. Hoekstra (ed.), Trafficking of intracellular membranes. NATO ASI Series, vol. H91. Springer Verlag, Berlin, Germany.
- 14.Francis C L, Ryan T A, Jones B D, Smith S J, Falkow S. Ruffles induced by Salmonella and other stimuli direct macropinocytosis of bacteria. Nature. 1993;364:639–642. doi: 10.1038/364639a0. [DOI] [PubMed] [Google Scholar]
- 15.Galán J E, Pace J, Hayman M J. Involvement of the epidermal growth factor receptor in the invasion of cultured mammalian cells by Salmonella typhimurium. Nature. 1992;357:588–589. doi: 10.1038/357588a0. [DOI] [PubMed] [Google Scholar]
- 16.Galán J E. Molecular and cellular bases of Salmonella entry into host cells. Curr Top Microbiol Immunol. 1996;209:43–60. doi: 10.1007/978-3-642-85216-9_3. [DOI] [PubMed] [Google Scholar]
- 17.Granucci F, Girolomoni G, Lutz M B, Foti M, Marconi G, Gnocchi P, Nolli L, Ricciardi-Castagnoli P. Modulation of cytokine expression in mouse dendritic cell clones. Eur J Immunol. 1994;24:2522–2526. doi: 10.1002/eji.1830241039. [DOI] [PubMed] [Google Scholar]
- 18.Griffin F M, Griffin J A, Leider J E, Silverstein S C. Studies on the mechanism of phagocytosis. I. Requirements for circumferential attachment of particle-bound ligands to specific receptors on the macrophage plasma membrane. J Exp Med. 1975;142:1263–1282. doi: 10.1084/jem.142.5.1263. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 19.Horwitz M A. Phagocytosis of the Legionnaires’ disease bacterium (Legionella pneumophila) occurs by a novel mechanism: engulfment within a pseudopod coil. Cell. 1984;36:27–33. doi: 10.1016/0092-8674(84)90070-9. [DOI] [PubMed] [Google Scholar]
- 20.Krieger M, Herz J. Structures and functions of multiligand lipoprotein receptors: macrophage scavenger receptors and LDL receptor-related protein (LRP) Annu Rev Biochem. 1994;63:601–637. doi: 10.1146/annurev.bi.63.070194.003125. [DOI] [PubMed] [Google Scholar]
- 21.Lillie R D, Pizzolato P. Histochemical use of borohydrides as aldehyde blocking reagents. Stain Technol. 1972;47:13–16. doi: 10.3109/10520297209116528. [DOI] [PubMed] [Google Scholar]
- 22.McKinney E C, Smith S B, Haines H G, Sigel M M. Phagocytosis by fish cells. RES J Reticuloendothel Soc. 1977;21:89–95. [PubMed] [Google Scholar]
- 23.Mosser D M, Rosenthal L A. Leishmania-macrophage interactions: multiple receptors, multiple ligands and diverse cellular responses. Semin Cell Biol. 1993;4:315–322. doi: 10.1006/scel.1993.1038. [DOI] [PubMed] [Google Scholar]
- 24.Nycomed Pharma AS. Isolation of blood cells. 4th ed. Oslo, Norway: Nycomed Pharma AS; 1993. [Google Scholar]
- 25.Rittig M, Krause A, Häupl T, Schaible U E, Modolell M, Kramer M D, Lütjen-Drecoll E, Simon M M, Burmester G R. Coiling phagocytosis is the preferential phagocytic mechanism for Borrelia burgdorferi. Infect Immun. 1992;60:4205–4212. doi: 10.1128/iai.60.10.4205-4212.1992. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 26.Rittig M G, Häupl T, Krause A, Kressel M, Groscurth P, Burmester G R. Borrelia burgdorferi-induced ultrastructural alterations in human phagocytes: a clue to pathogenicity? J Pathol. 1994;173:269–282. doi: 10.1002/path.1711730311. [DOI] [PubMed] [Google Scholar]
- 27.Rittig M G, Kuhn K H, Dechant C A, Gauckler A, Modolell M, Ricciardi-Castagnoli P, Krause A, Burmester G R. Phagocytes from both vertebrate and invertebrate species use “coiling” phagocytosis. Dev Comp Immunol. 1996;20:393–406. doi: 10.1016/s0145-305x(96)00023-7. [DOI] [PubMed] [Google Scholar]
- 28.Rittig M G, Jagoda J C, Wilske B, Murgia R, Cinco M, Repp R, Burmester G R, Krause A. Coiling phagocytosis discriminates between different spirochetes and is enhanced by phorbol myristate acetate and granulocyte-macrophage colony-stimulating factor. Infect Immun. 1998;66:627–635. doi: 10.1128/iai.66.2.627-635.1998. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 28a.Rittig M G, Seack K-H, Sander U, Solbach W, Bogdan C. Macrophages: infection with Leishmania major. Göttingen, Germany: Istitut für den Wissenschaftlichen Film; 1998. . (videotape) [Google Scholar]
- 29.Sher R, Wadee A A. A scanning electron microscopy study of eosinophil phagocytosis. RES J Reticuloendothel Soc. 1980;28:179–189. [PubMed] [Google Scholar]
- 30.Soll D R, Bedell G W, Brummel M. Zinc and regulation of growth and phenotype in the infectious yeast Candida albicans. Infect Immun. 1981;32:1139–1147. doi: 10.1128/iai.32.3.1139-1147.1981. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 31.Stenger S, Donhauser N, Thüring H, Röllinghoff M, Bogdan C. Reactivation of latent leishmaniasis by inhibition of inducible nitric oxide synthase. J Exp Med. 1996;183:1501–1514. doi: 10.1084/jem.183.4.1501. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 32.Stevens D R, Moulton J E. Ultrastructural and immunological aspects of the phagocytosis of Trypanosoma brucei by mouse peritoneal macrophages. Infect Immun. 1978;19:972–982. doi: 10.1128/iai.19.3.972-982.1978. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 33.Swanson J A, Baer S C. Phagocytosis by zippers and triggers. Trends Cell Biol. 1995;5:89–93. doi: 10.1016/s0962-8924(00)88956-4. [DOI] [PubMed] [Google Scholar]
- 34.Swanson M S, Isberg R R. Association of Legionella pneumophila with the macrophage endoplasmic reticulum. Infect Immun. 1995;63:3609–3620. doi: 10.1128/iai.63.9.3609-3620.1995. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 35.Szczepanski A, Fleit H B. Interaction between Borrelia burgdorferi and polymorphonuclear leukocytes. Phagocytosis and the induction of the respiratory burst. Ann NY Acad Sci. 1988;539:425–428. [Google Scholar]
- 36.Virji M, Käyhty H, Ferguson D J P, Alexandrescu C, Moxon E R. Interactions of Haemophilus influenzae with cultured human endothelial cells. Microb Pathog. 1991;10:231–245. doi: 10.1016/0882-4010(91)90057-h. [DOI] [PubMed] [Google Scholar]
- 37.Wyrick P B, Brownridge E A. Growth of Chlamydia psittaci in macrophages. Infect Immun. 1978;19:1054–1060. doi: 10.1128/iai.19.3.1054-1060.1978. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 38.Yamamoto T, Koyama Y, Matsumoto M, Sonoda E, Nakayama S, Uchimura M, Paveenkittiporn W, Tamura K, Yokota T, Echeverria P. Localized, aggregative, and diffuse adherence to HeLa cells, plastic, and human small intestines by Escherichia coli isolated from patients with diarrhea. J Infect Dis. 1992;166:1295–1310. doi: 10.1093/infdis/166.6.1295. [DOI] [PubMed] [Google Scholar]