Abstract
PURPOSE
Patients with locally advanced or metastatic urothelial cancer (la/mUC) who are ineligible for cisplatin-based therapy have limited first-line (1L) treatment options and significant need for improved therapies. Enfortumab vedotin (EV) and pembrolizumab (Pembro) individually have shown a survival benefit in urothelial cancer in second-line + la/mUC settings. Here, we present data from the pivotal trial of EV plus Pembro (EV + Pembro) in the 1L setting.
PATIENTS AND METHODS
In Cohort K of the EV-103 phase Ib/II study, cisplatin-ineligible patients with previously untreated la/mUC were randomly assigned 1:1 to receive EV as monotherapy or in combination with Pembro. The primary end point was confirmed objective response rate (cORR) per blinded independent central review. Secondary end points included duration of response (DOR) and safety. There were no formal statistical comparisons between treatment arms.
RESULTS
The cORR was 64.5% (95% CI, 52.7 to 75.1) and 45.2% (95% CI, 33.5 to 57.3) for patients treated with EV + Pembro (N = 76) and EV monotherapy (N = 73), respectively. The median DOR was not reached for the combination and was 13.2 months for monotherapy; 65.4% and 56.3% of patients who responded to the combination and monotherapy, respectively, maintained a response at 12 months. The most common grade 3 or higher treatment-related adverse events (TRAEs) in patients treated with the combination were maculopapular rash (17.1%), fatigue (9.2%), and neutropenia (9.2%). EV TRAEs of special interest (any grade) in the combination arm included skin reactions (67.1%) and peripheral neuropathy (60.5%).
CONCLUSION
EV + Pembro showed a high cORR with durable responses as 1L treatment in cisplatin-ineligible patients with la/mUC. Patients who received EV monotherapy had a response and safety profile consistent with previous studies. Adverse events for EV + Pembro were manageable, with no new safety signals observed.
INTRODUCTION
The occurrence of urothelial cancer is increasing worldwide, with locally advanced or metastatic urothelial cancer (la/mUC) demonstrating an especially poor prognosis.1-4 Cisplatin-based chemotherapy is the gold standard first-line (1L) treatment in patients with la/mUC, but approximately half of all patients are ineligible for 1L cisplatin-based chemotherapy because of impaired renal function, poor performance status, and other comorbidities.5-8 Carboplatin plus gemcitabine is a commonly used regimen for cisplatin-ineligible patients but has shown lower activity and poor tolerability.9-12 Other therapeutic options exist but are limited to subgroups of patients; avelumab maintenance therapy is approved for patients who have remained free from disease progression after 1L platinum-based treatment and has shown improved survival; however, only patients who do not progress after four to six cycles of 1L therapy are eligible.13,14 Single-agent PD-1/PD-L1 checkpoint inhibitors (CPIs) may be another 1L option for cisplatin-ineligible patients; however, they have become increasingly restricted to certain populations. In the United States currently, pembrolizumab (Pembro) is limited to 1L patients who are not eligible for any platinum-based chemotherapy.15-19 Additionally, approximately 60% of cisplatin-ineligible patients with la/mUC who receive 1L treatment do not receive second-line (2L) treatment.20 This underscores the need for efficacious and tolerable 1L therapies.
CONTEXT
Key Objective
To assess the efficacy, tolerability, and safety of enfortumab vedotin (EV) in combination with pembrolizumab (Pembro) in cisplatin-ineligible patients with previously untreated locally advanced or metastatic urothelial cancer.
Knowledge Generated
EV + Pembro showed a high confirmed objective response rate of 64.5%, and rapid and durable responses, with 65.4% of responders maintaining a response at 12 months (Kaplan-Meier estimate). The combination demonstrated a manageable safety profile.
Relevance (M.A. Carducci)
-
This report provides a strong foundation for the ongoing phase III studies of the EV and Pembro combination in muscle invasive bladder cancer. Toxicities although manageable are increased over EV alone and maturation of the survival data may also shed light on addressing toxicity, both clinical and financial, concerns as well as sequencing approaches.*
*Relevance section written by JCO Associate Editor Michael A. Carducci, MD.
Enfortumab vedotin (EV), an antibody-drug conjugate (ADC), comprises a fully human monoclonal antibody specific for nectin-4 and monomethyl auristatin E (MMAE). EV delivers MMAE to cells expressing nectin-4, leading to cell cycle arrest and cell death. Pembro is an anti–PD-1 antibody that uses the PD-1 receptor as a therapeutic target and has antitumor activity in multiple tumor types.16
EV and Pembro as individual agents have shown overall survival (OS) benefits compared with 2L or third-line treatment in patients with la/mUC.16,21,22 Preclinical data have shown that vedotin ADCs, including EV, in combination with PD-1/PD-L1 inhibitors such as Pembro, may enhance antitumor activity relative to their respective mechanisms of action and support complimentary efficacy.23-25
In the phase Ib/II study EV-103, results from the Dose Escalation/Cohort A demonstrated high antitumor activity and durable responses with encouraging survival and a manageable safety profile for EV plus Pembro (EV + Pembro), providing the rationale for further evaluation.26 Randomized Cohort K is intended to provide efficacy and safety data on the treatment combination. The EV monotherapy arm was included to better understand the safety and efficacy of EV monotherapy in 1L cisplatin-ineligible patients. No statistical comparison between treatment arms was performed.
Here, we present the efficacy and safety results of EV-103 randomized Cohort K for cisplatin-ineligible patients with la/mUC treated with EV + Pembro or EV monotherapy in the 1L setting.
PATIENTS AND METHODS
Trial Participants
In Cohort K of the EV-103 study, eligible patients were 18 years or older with histologically documented la/mUC, including squamous differentiation or mixed cell types. Patients had an Eastern Cooperative Oncology Group performance status (ECOG PS) of 2 or less and were eligible for Pembro therapy. Patients in Cohort K were deemed ineligible for cisplatin-based chemotherapy on the basis of at least one of the following: glomerular filtration rate (GFR) <60 mL/min and ≥30 mL/min, grade ≥ 2 hearing loss, ECOG PS of 2, or New York Heart Association (NYHA) Class III heart failure. Patients with an ECOG PS of 2 met additional criteria: hemoglobin ≥ 10 g/dL, GFR ≥ 50 mL/min, and no NYHA Class III heart failure. Patients were excluded from the trial if they had any of the following: previous systemic treatment for locally advanced or metastatic disease, including adjuvant/neoadjuvant platinum-based therapy within 12 months before random assignment, previous treatment with a PD-1, PD-L1, or PD-L2 inhibitor, or any previous treatment with stimulatory or coinhibitory T-cell receptor agents, previous treatment with EV or another MMAE-based ADC, ongoing grade 2 or higher sensory or motor neuropathy, or ongoing clinically significant toxic effects associated with previous treatment, active central nervous system metastases, or uncontrolled diabetes (defined as hemoglobin A1c [HbA1c] ≥ 8% or HbA1c 7% to <8% with associated diabetes symptoms [polyuria or polydipsia] that were not otherwise explained). Full eligibility criteria are provided in the study Protocol (online only).
Random Assignment and Treatment
Enrolled patients were randomly assigned 1:1 to receive EV alone or in combination with Pembro. Random assignment was stratified by ECOG PS (0 v 1 or 2) and liver metastasis (present v absent) at baseline. EV was administered at a dose of 1.25 mg per kilogram of body weight (maximum total dose of 125 mg) as a single intravenous infusion over 30 minutes on days 1 and 8 of a 3-week cycle. Pembro was administered at a dose of 200 mg as a single intravenous infusion over 30 minutes on day 1 of a 3-week cycle. Dose modifications and reductions were permitted to manage treatment-related adverse events (TRAEs) as described in the study Protocol.
Trial Oversight
The study was designed by the sponsors in collaboration with an advisory committee. The trial received approval from site independent institutional review boards or ethics committees and was conducted in accordance with the ethics principles of the Declaration of Helsinki and with Good Clinical Practice guidelines defined by the International Council for Harmonization. All patients provided written informed consent. The trial was sponsored by Seagen Inc (Bothell, WA), Astellas Pharma (Northbrook, IL), and Merck Sharp & Dohme LLC (Rahway, NJ). The authors vouch for the accuracy and completeness of the data and for the adherence of the trial to the Protocol.
End Points
The primary end point was confirmed objective response rate (cORR; proportion of patients with a complete or partial response) per RECIST version 1.1 by blinded independent central review (BICR). Secondary end points were cORR by investigator, disease control rate (DCR) by BICR and investigator assessment, duration of response (DOR) and progression-free survival (PFS) by BICR and investigator assessment, OS, and safety and tolerability of EV monotherapy and EV + Pembro. Exploratory end points included biomarkers of activity, including baseline PD-L1 status and nectin-4 expression.
Assessments
Investigators assessed and confirmed antitumor activity by reviewing computed tomography scans or magnetic resonance imaging with or without intravenous contrast of the chest, abdomen, and pelvis. Patients were evaluated for response assessments using the same imaging method throughout the study. Bone and brain scans were required at screening and repeated if positive at baseline and/or if clinically indicated. Objective responses were confirmed per RECIST version 1.1, with repeat scans 4-5 weeks after a first documented response. Subsequent response assessments were timed from cycle 1 day 1 and were performed every 9 weeks (±7 days) until 1 year after the first dose and then every 12 weeks (±7 days).
EV adverse events of special interest (AESIs) are medical concepts/composite terms used to characterize identified and potential risks for EV. AESI time-to-onset is calculated as time from the date of the first dose to the start date of the first treatment emergent event that meets the respective AESI search criteria. TRAEs were determined by the investigator and assessed for both arms. Pembro AESIs (AEOSIs), including immune-mediated adverse events (AEs) and infusion reactions, were evaluated using previously described criteria for Pembro monotherapy.16
Treatment discontinuations were summarized according to the percentage of patients who had TRAEs leading to discontinuation of either agent, including patients who had TRAEs leading to discontinuation of EV only, Pembro only, or both. Of note, discontinuations because of each agent alone, or both, are counted by patient and are not mutually exclusive.
Nectin-4 and PD-L1 expression were assessed centrally with archival or freshly obtained formalin-fixed, paraffin-embedded tumor tissue using analytically validated immunohistochemistry (IHC) assays. Nectin-4 expression was evaluated at Q2 Solutions as described previously, and stained slides were scored by a pathologist to generate an H-score (range, 0-300).27 PD-L1 expression was assessed using the Agilent PD-L1 IHC 22C3 pharmDx assay, with stained slides scored by a pathologist to generate a PD-L1 combined positive score (CPS; low, <10; high, ≥10).
Statistical Analysis
Efficacy and safety end points were assessed for all patients who received EV + Pembro or EV monotherapy. The sample size was based on the precision of the estimate for objective response rate (ORR) as characterized by 95% CIs. The cORR by BICR was summarized with two-sided 95% CI and calculated using the Clopper-Pearson method for each treatment arm. No statistical comparisons were made between treatment arms; EV monotherapy arm was included for isolation of the monotherapy contribution. Secondary end points, cORR by investigator assessment and DCR by BICR and investigator assessment, were summarized with two-sided 95% CIs using the Clopper-Pearson method. DOR and PFS by BICR and by investigator assessment and OS were analyzed using Kaplan-Meier methodology. ORR, DOR, and PFS are presented by BICR in results.
RESULTS
Patient Disposition and Baseline Characteristics
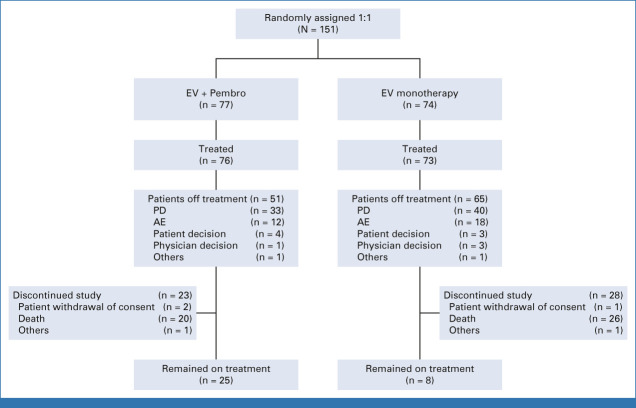
A total of 151 patients were randomly assigned and 149 received EV + Pembro (N = 76) or EV monotherapy (N = 73; Appendix Table A1, online only). Two patients, one in each arm, were randomly assigned but never received corresponding study treatment (Fig 1). At data cutoff (June 10, 2022), 29 patients in the combination arm had discontinued treatment but remained in the study in long-term follow-up. The median follow-up on study was 14.8 months (95% CI, 12.9 to 17.3) in the combination arm and 15.0 months (95% CI, 12.7 to 17.4) in the monotherapy arm. The median duration of treatment was 9.0 months (range, 0.6-26.1) in the combination arm with patients receiving a median of 11.0 cycles (range, 1-29). The median duration of treatment was 5.5 months (range, 0.5-26.9) in the monotherapy arm with patients receiving a median of 8.0 cycles (range, 1-33).
FIG 1.
CONSORT diagram. Screening, allocation, follow-up, and analyses. A patient is considered discontinued from the treatment only if both agents are discontinued, including patients who discontinued both agents because of AE(s) or discontinued the latter of the two agents because of an AE (the other agent may be discontinued because of a non-AE at an earlier time). AE, adverse event; EV, enfortumab vedotin; PD, progressive disease; Pembro, pembrolizumab.
In the combination arm, patients were predominately male (71.1%) and White (80.3%), with a median age of 71 years (range, 51-91 years). Most patients had an ECOG PS of 0 (43.4%) or 1 (43.4%). The primary disease location was the lower tract (60.5%). Visceral metastases were present in 84.2% of patients, including 17.1% with liver metastases. At baseline, 57.9% of patients had low PD-L1 expression (defined as CPS < 10) (Table 1). The median nectin-4 H-score at baseline was 262.5 (interquartile range [IQR], 200.0-297.0).
TABLE 1.
Patient Demographics and Disease Characteristics at Baseline
Baseline characteristics of patients in the monotherapy arm are summarized in Table 1. The median H-score at baseline was 284.0 (IQR, 240.0-298.0).
Efficacy
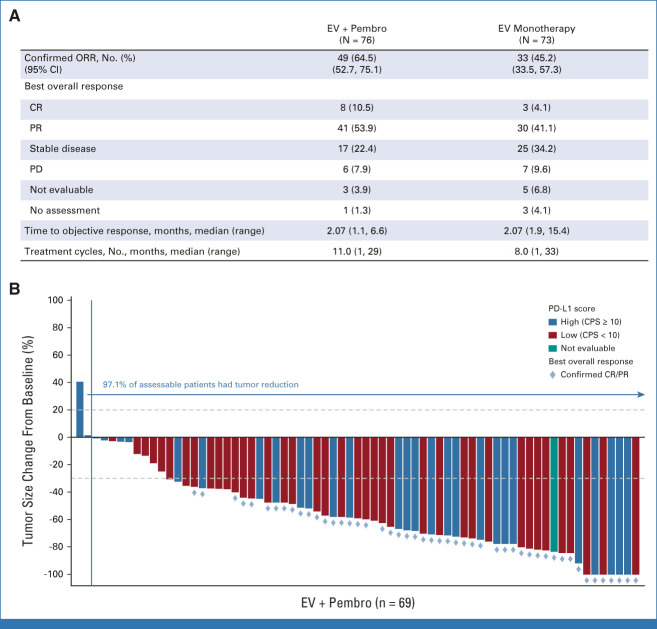
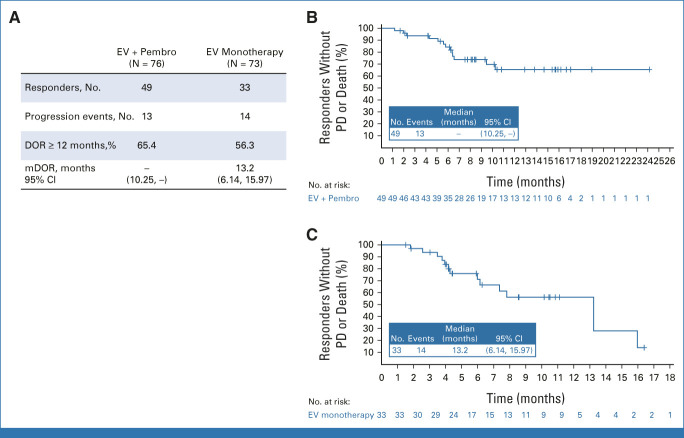
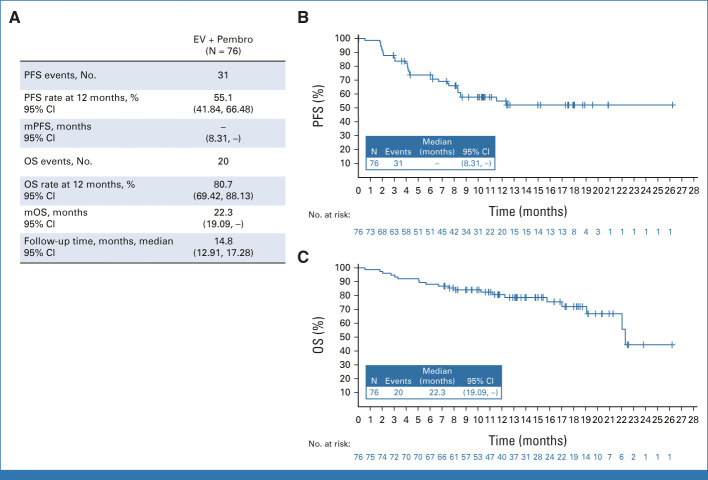
In patients treated with EV + Pembro (N = 76), the cORR by BICR was 64.5% (95% CI, 52.7 to 75.1; 49 of 76 patients). Eight patients (10.5%) had a complete response; 41 patients (53.9%) achieved a partial response; the median time to response was 2.1 months (range, 1.1-6.6; Fig 2). A total of 97.1% of assessable patients had a reduction in their target lesions per BICR (Fig 2). The median DOR per BICR has not yet been reached; 65.4% of responders maintained a response at 12 months (Fig 3). The DCR was 86.8% (95% CI, 77.1 to 93.5; 66 of 76 patients). The PFS rate per BICR at 6 and 12 months was 73.8% and 55.1%, respectively, and the OS rate at 6 and 12 months was 88.2% and 80.7%, respectively (Fig 4). The median OS was 22.3 months (95% CI, 19.09 to not achieved) with 54 (of 76) patients remaining on study for OS follow-up at the time of data cutoff (Appendix Fig A1, online only).
FIG 2.
(A) Best overall response by BICR. (B) Antitumor activity of EV + Pembro, waterfall plot of percentage reduction of tumor size from baseline of target lesions by BICR per RECIST v1.1. BICR, blinded independent central review; CPS, combined positive score; CR, complete response; EV, enfortumab vedotin; ORR, objective response rate; PD, progressive disease; Pembro, pembrolizumab; PR, partial response.
FIG 3.
(A) Durations of response per BICR by RECIST v1.1. (B) Kaplan-Meier estimate of durations of response per BICR, EV + Pembro treatment arm. (C) Kaplan-Meier estimate of durations of response per BICR, EV monotherapy treatment arm. This study was not designed with statistical comparison between the two treatment arms; direct comparisons should not be made. BICR, blinded independent central review; DOR, duration of response; EV, enfortumab vedotin; mDOR, median duration of response; mono, monotherapy; PD, progressive disease; Pembro, pembrolizumab; v1.1, version 1.1.
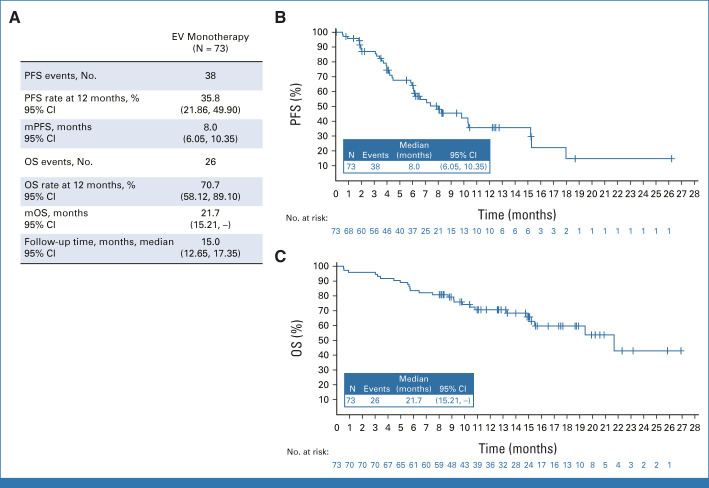
FIG 4.
EV + Pembro. (A) PFS per BICR, by RECIST v1.1, OS, and median follow-up time. (B) Kaplan-Meier estimate of PFS per BICR by RECIST v1.1. (C) Kaplan-Meier estimate of OS. Preliminary mPFS and mOS are reported here and are expected to evolve over time. At the time of data cutoff, 54 (of 76) patients remain on study for OS follow-up. BICR, blinded independent central review; EV, enfortumab vedotin; mOS, median overall survival; mPFS, median progression-free survival; OS, overall survival; Pembro, pembrolizumab; PFS, progression-free survival; v1.1, version 1.1.
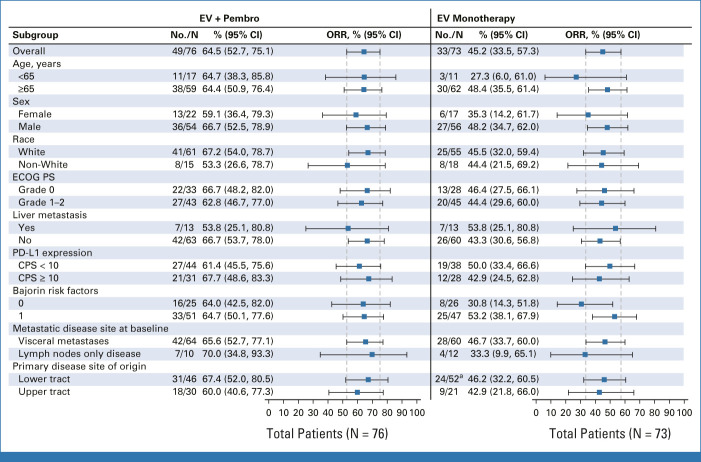
The cORR in prespecified subgroups in the combination arm (including PD-L1 expression, liver metastasis, ECOG PS, and primary disease site of origin) was consistent with overall cORR (Appendix Fig A2, online only).
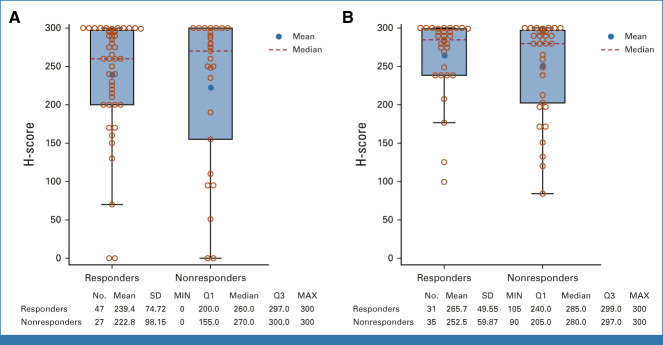
Nectin-4 expression was generally high as indicated by median H-score in the combination arm, and the distribution was similar between responders (median, 260.0; IQR, 200.0-297.0) and nonresponders (270.0; 155.0-300.0; Appendix Fig A3, online only).
In the monotherapy arm, the cORR per BICR was 45.2% (95% CI, 33.5 to 57.3; 33 of 73 patients; Fig 2). The DOR was 13.2 months (range, 6.14-15.97), and the median time to response was 2.1 months (range, 1.9-15.4). The DCR was 79.5% (95% CI, 68.4 to 88.0; 58 of 73 patients). The 12-month PFS and OS were 35.8% and 70.7%, respectively (Fig 5). The cORR per BICR for prespecified subgroups can be found in Appendix Figure A2. The nectin-4 H-score median and distribution were similar between responders and nonresponders (Appendix Fig A3).
FIG 5.
EV monotherapy. (A) PFS per BICR by RECIST v1.1, OS, and median follow-up time. (B) Kaplan-Meier estimates of PFS per BICR by RECIST v1.1. (C) Kaplan-Meier estimates of OS. Preliminary mPFS and mOS are reported here and are expected to evolve over time. At the time of data cutoff, 46 (of 73) patients remain on study for OS follow-up. BICR, blinded independent central review; EV, enfortumab vedotin; mOS, median overall survival; mPFS, median progression-free survival; OS, overall survival; PFS, progression-free survival; v1.1, version 1.1.
The concordance rate of best overall response per RECIST between BICR and investigator assessments was 86.7% in the combination arm and 85.5% in the monotherapy arm.
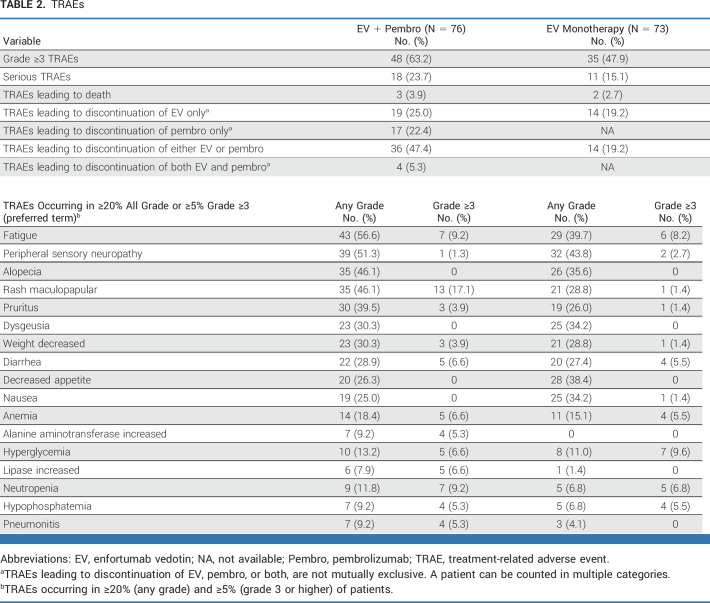
Safety
In patients treated with the combination, the most common TRAEs were fatigue, peripheral sensory neuropathy, alopecia, and maculopapular rash; the most common grade 3 or higher event was maculopapular rash (Table 2). Eighteen (23.7%) patients experienced a serious TRAE, and three (3.9%) patients died due to a TRAE (respiratory failure, pneumonitis, and sepsis). TRAEs leading to dose interruptions of either EV or Pembro occurred in 68.4% of patients. TRAEs leading to discontinuation of EV and/or Pembro are listed in Table 2. The most frequently occurring TRAEs leading to discontinuation of either agent was peripheral neuropathy (nine of 76 patients, 11.8%) and of EV only was peripheral neuropathy (nine of 76 patients, 11.8%) and Pembro only was pneumonitis (three of 76 patients, 3.9%). TRAEs leading to discontinuation of both agents were peripheral motor neuropathy, myasthenia gravis, pneumonitis, and sepsis (one patient [1.3%] each).
TABLE 2.
TRAEs
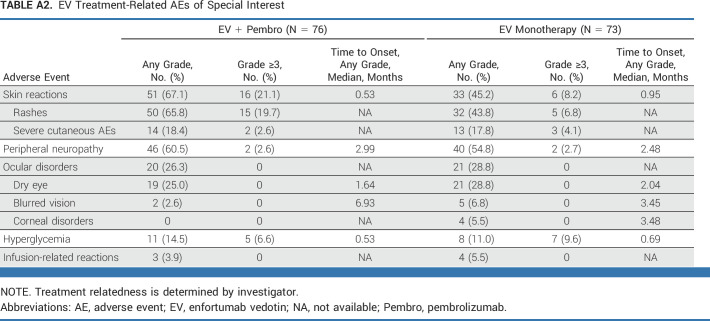
Treatment-related EV AESIs in the combination arm were peripheral neuropathy (46 of 76 patients, 60.5%), skin reactions (51 of 76 patients, 67.1%), hyperglycemia (11 of 76 patients, 14.5%), ocular events (20 of 76 patients, 26.3%), and infusion-related reactions (three of 76 patients, 3.9%; Appendix Table A2, online only). Grade 3 or higher skin reactions, peripheral neuropathy, and hyperglycemia in the combination arm occurred in 16 of 76 (21.1%), two of 76 (2.6%), and five of 76 (6.6%) patients, respectively. No serious skin reactions occurred, including no severe cutaneous adverse reaction events. One patient experienced serious peripheral neuropathy (1.3%).
The most frequent Pembro treatment-emergent AEOSIs in the combination arm were severe skin reactions (21 of 76 patients, 27.6%) and hypothyroidism (10 of 76 patients, 13.2%); the most common grade 3 or higher AEOSIs were severe skin reactions (15 of 76 patients, 19.7%) and pneumonitis (four of 76 patients, 5.3%; Appendix Table A3, online only). Pembro AEOSIs associated with the use of systemic steroids are reported in the Protocol.
TRAEs and deaths in patients treated with monotherapy are summarized in Table 2. The most frequent treatment-related EV AESIs reported in patients in the monotherapy arm were peripheral neuropathy and skin reactions (Appendix Table A2).
DISCUSSION
Approximately half of all patients with la/mUC are unfit for 1L cisplatin-based chemotherapy, and limited 1L therapeutic options exist for these patients.20,28 Carboplatin plus gemcitabine is currently a standard option for cisplatin-ineligible patients but is associated with low response rate (approximately 36%-42%), short DOR (6.3-7.1 months), and relatively poor tolerability.9,29,30 In this study of cisplatin-ineligible patients with la/mUC, EV in combination with Pembro showed a 64.5% overall response rate, with responses lasting beyond 1 year for 65.4% of responders, trending similarly with previously disclosed data from EV-103 Dose Escalation/Cohort A.26 Additionally, the median DOR has not yet been reached for the combination arm. These results stand out from historical data for gemcitabine and carboplatin chemotherapy and confirm the findings of EV-103 Dose Escalation/Cohort A. These data suggest that the EV plus Pembro combination represents a potential new therapeutic option as a 1L regimen for cisplatin-ineligible patients with la/mUC.
Pembro is an US Food and Drug Administration–approved option that has shown durable responses as monotherapy but is currently restricted in the 1L setting to platinum-ineligible patients.16,19,31 Avelumab maintenance therapy has shown a survival benefit compared with observation after platinum-based chemotherapy, but it is only an option for patients who remain free from disease progression after four to six cycles of 1L platinum-based therapy. In fact, modeling data suggest that approximately half of patients with la/mUC may not be eligible for maintenance therapy.14 Despite the availability of these therapies, approximately 60% of cisplatin-ineligible patients with la/mUC who receive 1L treatment never receive 2L treatment, likely because of early progression, tolerability, or short DORs.20 At the time of the primary analysis, the minority of patients in this study had data captured on subsequent therapies; thus this study required additional follow-up to determine whether there is any potential effect from subsequent treatments on patient outcomes.
A high unmet need remains for a highly efficacious 1L treatment option with rapid and durable responses and a manageable safety profile so that a larger proportion of the cisplatin-ineligible population with la/mUC receives more durable therapeutic benefit. In this study, the overall response rates reported for patients in Cohort K (from either arm) numerically exceeded those reported in trials for the current standard for cisplatin-ineligible patients, gemcitabine plus carboplatin, or for PD-1/PD-L1 CPIs as monotherapies.16,17,19,32,33
Although the primary objective was to evaluate the efficacy and safety of the combination, the monotherapy arm was studied to characterize the activity of EV alone in this patient population. The overall response rate of patients treated with EV in the monotherapy arm was 45.2%. The monotherapy arm demonstrated a manageable safety profile, consistent with previous EV monotherapy studies.22,26,34
The ORRs observed in this study were consistent across a range of prespecified subgroups, including patients with liver metastases, and antitumor activity was seen regardless of PD-L1 status. High nectin-4 expression was observed, and the median and distribution was similar between responders and nonresponders. The preliminary PFS and OS results reported here are promising, and data will evolve with additional follow-up, which are trending similarly to median OS found in previously disclosed Dose Escalation/Cohort A data. The safety profile of the combination was manageable and consistent with results from Cohort A of EV-103, with no new safety concerns emerging.26,35
Most skin reactions and peripheral neuropathy events were grade ≤ 2 in severity, consistent with observations in the previously reported results for EV-103 Dose Escalation/Cohort A. Skin reactions (including the Pembro AEOSI category of severe skin reactions) and pneumonitis are an identified part of the safety profile for both EV and Pembro monotherapy and were more frequently observed in the combination arm. Higher rates of skin reactions were managed with EV treatment interruption, dose reduction, treatment discontinuation (EV and/or Pembro), and/or corticosteroids, as previously described.36,37 The safety results highlight the importance of educating both health care practitioners and patients; early intervention for AEs are key components of successfully treating patients with the combination.
It is important to note that this study was not designed to make statistical comparisons between arms. Similarly, although the cORR found in the EV + Pembro cohort is numerically higher than contemporary studies, including even cisplatin-based chemotherapy arms, the study was not designed as a direct comparison with current standard-of-care regimens. This study builds on the promising results from Dose Escalation/Cohort A and further justifies the ongoing investigation of EV with Pembro in a randomized, phase III study compared with cisplatin or carboplatin plus gemcitabine in biomarker-unselected 1L patients (EV-302/KN-A39, ClinicalTrials.gov identifier: NCT04223856). EV + Pembro are also being evaluated in muscle invasive bladder cancer in randomized phase III trials (EV-303/KN-905, ClinicalTrials.gov identifier: NCT03924895 and EV-304/KNK-B15, ClinicalTrials.gov identifier: NCT04700124).
In conclusion, in the 1L cisplatin-ineligible la/mUC patient population with high unmet need, EV + Pembro had a manageable safety profile and resulted in a high ORR with durable responses and encouraging PFS and OS results, which will evolve with follow-up. These results from Cohort K add to the previously reported results from Dose Escalation/Cohort A of this study and indicate that this combination may represent a new 1L treatment option for a patient population with high unmet need.
ACKNOWLEDGMENT
We thank the patients who participated in this study, their families, the investigators, and staff at EV-103 clinical study sites, and the members of the safety monitoring committee. We acknowledge the medical monitoring of the study, the entire EV-103 study team, Ray Liao (Seagen Inc, Safety) and Leah Hogdal (Seagen Inc, Biomarkers). Hanna Thomsen, PhD, of Seagen Inc (Bothell, WA) provided medical writing and editorial support, in accordance with Good Publication Practice guidelines.
APPENDIX
TABLE A1.
Cisplatin-Ineligibility Criteria
TABLE A2.
EV Treatment-Related AEs of Special Interest
TABLE A3.
Pembro AEs of Special Interest
FIG A1.
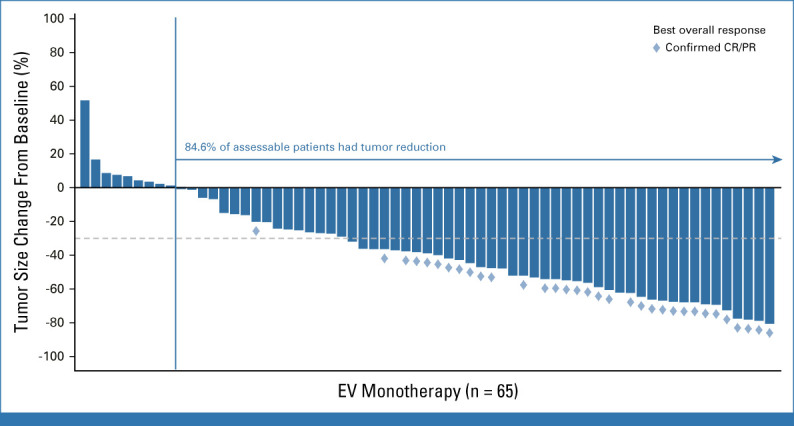
Antitumor activity of EV monotherapy. Waterfall plot of percentage reduction of tumor size from baseline of target lesions by blinded independent central review per RECIST version 1.1. CR, complete response; EV, enfortumab vedotin; PR, partial response.
FIG A2.
Subgroup analysis of ORR in patients treated with EV + Pembro and patients treated with EV monotherapy. aOne patient had primary disease at both bladder and ureter. CPS, combined positive score; ECOG PS, Eastern Cooperative Oncology Group performance status; EV, enfortumab vedotin; ORR, objective response rate; Pembro, pembrolizumab.
FIG A3.
H-score of nectin-4 expression at baseline by best overall response by blinded independent central review in responders and nonresponders. (A) EV + pembrolizumab. (B) EV monotherapy. EV, enfortumab vedotin; MAX, maximum; MIN, minimum; Q1, 1st quartile; Q3, 3rd quartile; SD, standard deviation.
Peter H. O'Donnell
Honoraria: Merck, Astellas Pharma, Pfizer, CLD Inc, Axiom Healthcare Strategies, EMD Serono, IntrinsiQ, ISMIE, NAMCP, Seagen, Curio Science, FirstWord, MedLearning Group, Research to Practice, Great Debates and Updates, MJH Life Sciences, Peerview, Vaniam Group, Institute for Enquiring Minds
Research Funding: Boehringer Ingelheim (Inst), Merck (Inst), Genentech/Roche (Inst), AstraZeneca/MedImmune (Inst), Acerta Pharma (Inst), Janssen (Inst), Seagen (Inst), Bristol Myers Squibb (Inst), Astellas Pharma (Inst)
Expert Testimony: Oregon Health & Science University (OHSU)
Travel, Accommodations, Expenses: Curio Science
Other Relationship: Janssen, Nektar, NIH, Dragonfly Therapeutics, G1 Therapeutics
Matthew I. Milowsky
Stock and Other Ownership Interests: Pfizer, Merck, Gilead Sciences
Consulting or Advisory Role: Loxo/Lilly
Research Funding: Merck (Inst), Roche/Genentech (Inst), Bristol Myers Squibb (Inst), Mirati Therapeutics (Inst), Incyte (Inst), Seagen (Inst), G1 Therapeutics (Inst), Alliance Foundation Trials (Inst), Alliance for Clinical Trials in Oncology (Inst), Clovis Oncology (Inst), Arvinas (Inst), ALX Oncology (Inst), Loxo (Inst), Hoosier Cancer Research Network (Inst)
Other Relationship: Elsevier, Medscape
Daniel P. Petrylak
Consulting or Advisory Role: Bayer, Exelixis, Pfizer, Roche, Astellas Pharma, AstraZeneca, Lilly, Amgen, Boehringer Ingelheim, Bristol Myers Squibb, Clovis Oncology, Incyte, Janssen, Pharmacyclics, Seagen, Urogen Pharma, Advanced Accelerator Applications, Ipsen, Bicycle Therapeutics, Mirati Therapeutics, Monopteros Therapeutics, Regeneron, Gilead Sciences
Research Funding: Progenics (Inst), Sanofi (Inst), Endocyte (Inst), Genentech (Inst), Merck (Inst), Astellas Medivation (Inst), Novartis (Inst), AstraZeneca (Inst), Bayer (Inst), Lilly (Inst), Innocrin Pharma (Inst), MedImmune (Inst), Pfizer (Inst), Roche (Inst), Seagen (Inst), Clovis Oncology (Inst), Bristol Myers Squibb (Inst), Advanced Accelerator Applications (Inst), Agensys (Inst), BioXCel Therapeutics (Inst), Eisai (Inst), Mirati Therapeutics (Inst), Replimune (Inst), Medivation (Inst), Gilead Sciences (Inst)
Expert Testimony: Celgene, Sanofi
Christopher J. Hoimes
Honoraria: Seagen
Consulting or Advisory Role: Bristol Myers Squibb, Eisai, Prometheus, Seagen, Genentech/Roche, Merck Sharp & Dohme, 2bPrecise
Speakers' Bureau: Bristol Myers Squibb, Genentech/Roche, Astellas Pharma, Seagen, Eisai
Research Funding: Merck Sharp & Dohme (Inst), Janssen Oncology (Inst), Novartis (Inst), Alkermes (Inst), Dynavax Technologies (Inst), Nektar (Inst), NanoCarrier (Inst), Seagen (Inst), Astellas Pharma (Inst), Bristol Myers Squibb Foundation (Inst), BioNTech SE (Inst), Crispr Therapeutics (Inst), NeoImmuneTech (Inst), Mirati Therapeutics (Inst)
Uncompensated Relationships: 2bPrecise (Inst)
Thomas W. Flaig
Leadership: Aurora Oncology, University of Colorado/UC Health
Stock and Other Ownership Interests: Aurora Oncology
Consulting or Advisory Role: Seagen, Janssen Oncology
Research Funding: Novartis, Bavarian Nordic, Dendreon, GTx, Janssen Oncology, Medivation, Sanofi, Pfizer, Bristol Myers Squibb, Roche/Genentech, Exelixis, Aragon Pharmaceuticals, Sotio, Tokai Pharmaceuticals, Astrazeneca/MedImmune, Lilly, Astellas Pharma, Agensys, Seagen, La Roche-Posay, Merck, Seagen, Myovant Sciences, Criterium
Patents, Royalties, Other Intellectual Property: The University of Colorado has filed 2 patents in which I am an inventor. These are related to early-stage bladder cancer treatment and detection. Neither is commercialized or in active clinical development right now (eg, neither are in clinical trials)
Nataliya Mar
Speakers' Bureau: Seagen, Astellas Pharma, Aveo, Eisai, Tempus
Helen H. Moon
Honoraria: EMD Serono, Pfizer/EMD Serono
Research Funding: Bristol Myers Squibb (Inst), Amgen (Inst), Prometheus (Inst), Genentech (Inst), Seagen (Inst), Arcus Biosciences (Inst), Apollomics (Inst), Nektar (Inst), Revimmune (Inst), HUYA Bioscience International (Inst), Aveo (Inst), Xenocor (Inst)
Travel, Accommodations, Expenses: Aveo, Seagen
Terence W. Friedlander
Leadership: Med BioGene
Honoraria: EMD Serono, AstraZeneca/MedImmune, Astellas Scientific and Medical Affairs Inc, Astellas Pharma
Consulting or Advisory Role: Dava Oncology, EMD Serono, Merck, Astellas Pharma, Foundation Medicine, Basilea, Taiho Oncology, Seagen, AADi
Research Funding: Seagen (Inst), Bristol Myers Squibb (Inst), Neon Therapeutics (Inst), Roche/Genentech (Inst)
Travel, Accommodations, Expenses: AstraZeneca/MedImmune, Genentech/Roche, Jounce Therapeutics, Astellas Pharma
Rana R. McKay
Consulting or Advisory Role: Janssen, Novartis, Tempus, Exelixis, Pfizer, Bristol Myers Squibb, Astellas Medivation, Astellas Medivation, Bayer, Sanofi, Merck, Vividion Therapeutics, Calithera Biosciences, AstraZeneca, Myovant Sciences, Caris Life Sciences, Sorrento Therapeutics, AVEO, Seagen, Telix Pharmaceuticals, Lilly
Research Funding: Pfizer (Inst), Bayer (Inst), Tempus (Inst)
Mehmet A. Bilen
Consulting or Advisory Role: Exelixis, Sanofi, Nektar, EMD Serono, Eisai, Janssen, Genomic Health, Pfizer, Bristol Myers Squibb, Bayer, Calithera Biosciences, AstraZeneca, Seagen
Research Funding: Bayer (Inst), Bristol Myers Squibb (Inst), Genentech/Roche (Inst), Incyte (Inst), Nektar (Inst), AstraZeneca (Inst), Tricon Pharmaceuticals (Inst), Pfizer (Inst), Seagen (Inst), Xencor (Inst), Exelixis (Inst), Advanced Accelerator Applications (Inst), Genome & Company (Inst), Peloton Therapeutics (Inst), Merck (Inst), NiKang Therapeutics (Inst)
Sandy Srinivas
Consulting or Advisory Role: Eisai, Bayer, Bristol Myers Squibb, Merck, AstraZeneca, Seagen, Janssen Oncology, Novartis
Research Funding: Bristol Myers Squibb (Inst), Genentech (Inst), Merck (Inst), Exelixis (Inst), Eisai (Inst), Bayer (Inst), AstraZeneca (Inst), Seattle Genetics/Astellas (Inst)
Other Relationship: Pfizer
Earle F. Burgess
Stock and Other Ownership Interests: Exelixis, Becton Dickinson, Calithera Biosciences, Gilead Sciences, Medtronic, Macrogenics, Arvinas
Honoraria: Exelixis, Janssen Oncology, Novartis, Pfizer, Merck
Consulting or Advisory Role: Johnson & Johnson
Speakers' Bureau: AstraZeneca, Exelixis
Research Funding: Pfizer, Astellas Pharma
Chethan Ramamurthy
Honoraria: Gilead Sciences
Consulting or Advisory Role: Seagen, Exelixis
Research Funding: Dispersol (Inst), Novartis (Inst), Seagen (Inst), Gilead Sciences (Inst), Mirati Therapeutics (Inst), Nuvation Bio (Inst)
Saby George
Consulting or Advisory Role: Bristol Myers Squibb, Bayer, Pfizer, Exelixis, Corvus Pharmaceuticals, Sanofi, EMD Serono, Seattle Genetics/Astellas, Eisai, Merck, Aveo, QED Therapeutics
Research Funding: Pfizer (Inst), Merck (Inst), Agensys (Inst), Novartis (Inst), Bristol Myers Squibb (Inst), Bayer (Inst), Eisai (Inst), Seattle Genetics/Astellas (Inst), Surface Oncology (Inst), Exelixis (Inst), Aravive (Inst), Aveo (Inst), Gilead Sciences (Inst)
Travel, Accommodations, Expenses: Bristol-Myers Squibb/Medarex, Sanofi
Daniel M. Geynisman
Consulting or Advisory Role: Pfizer, Exelixis, AstraZeneca, Seattle Genetics/Astellas, Merck, Myovant Sciences, Bristol Myers Squibb
Research Funding: Genentech (Inst), Merck (Inst), Calithera Biosciences (Inst), Astellas Pharma (Inst), Harpoon Therapeutics (Inst)
Sergio Bracarda
Travel, Accommodations, Expenses: MSD Oncology, Pfizer
Delphine Borchiellini
Consulting or Advisory Role: Astellas Pharma, AstraZeneca, Bristol Myers Squibb, Ipsen, Janssen-Cilag, MSD Oncology, Pfizer, Merck, Bayer, AAA/Endocyte/Novartis
Research Funding: Astellas Pharma (Inst), AstraZeneca (Inst), Bristol Myers Squibb (Inst), Exelixis (Inst), Infinity Pharmaceuticals (Inst), Janssen (Inst), MSD (Inst), Roche (Inst), Bayer (Inst), Taiho Oncology (Inst), AVEO (Inst)
Travel, Accommodations, Expenses: Bristol Myers Squibb, Janssen, Pfizer, Ipsen, MSD Oncology, Bayer,
Lionnel Geoffrois
Honoraria: Ipsen, Merck Serono, BMS, Pfizer, MSD, Ipsen, Merck Serono
Travel, Accommodations, Expenses: Ipsen, Janssen Oncology, MSD Oncology, Merck KGaA, Merck Serono
Jose Pablo Maroto Rey
Consulting or Advisory Role: Astellas Pharma, Ipsen, BMS, Merck/Pfizer, Bayer, Janssen
Travel, Accommodations, Expenses: Merck/Pfizer, Bayer
Christiano Ferrario
Honoraria: Pfizer, Bayer, Novartis, AstraZeneca, Merck, Astellas Pharma, Roche Canada, Knight Pharmaceuticals
Consulting or Advisory Role: Merck, AstraZeneca, Novartis, Roche
Speakers' Bureau: Merck, Knight Therapeutics, AstraZeneca, Novartis
Research Funding: Astellas Pharma (Inst), AstraZeneca (Inst), Bayer (Inst), Lilly (Inst), Merck (Inst), Novartis (Inst), Roche/Genentech (Inst), Sanofi (Inst), Pfizer (Inst), Janssen Oncology (Inst), Zymeworks (Inst), Seagen (Inst), Immunomedics (Inst), Bicycle Therapeutics (Inst), Sermonix Pharmaceuticals (Inst)
Expert Testimony: Seattle Genetics/Astellas
Anne-Sophie Carret
Employment: Seagen
Stock and Other Ownership Interests: Seagen
Honoraria: Seagen
Travel, Accommodations, Expenses: Seagen
Yao Yu
Employment: Seagen
Stock and Other Ownership Interests: Seagen
Maria Guseva
Employment: Astellas Pharma
Blanca Homet Moreno
Employment: Merck Sharp & Dohme
Stock and Other Ownership Interests: Merck Sharp & Dohme
Jonathan E. Rosenberg
Honoraria: UpToDate, Medscape, Peerview, Research To Practice, Clinical Care Options, Physicans' Education Resource, MJH Life Sciences, EMD Serono, Pfizer
Consulting or Advisory Role: Lilly, Merck, Roche/Genentech, AstraZeneca/MedImmune, Bristol Myers Squibb, Seagen, Bayer, BioClin Therapeutics, QED Therapeutics, Pharmacyclics, GlaxoSmithKline, Janssen Oncology, Astellas Pharma, Boehringer Ingelheim, Pfizer/EMD Serono, Mirati Therapeutics, Immunomedics, Tyra Biosciences, Infinity Pharmaceuticals, Gilead Sciences, Hengrui Pharmaceutical, Alligator Bioscience, Imvax
Research Funding: Genentech/Roche (Inst), Seagen (Inst), Bayer (Inst), AstraZeneca (Inst), QED Therapeutics (Inst), Astellas Pharma (Inst)
Patents, Royalties, Other Intellectual Property: Predictor of platinum sensitivity (Inst)
No other potential conflicts of interest were reported.
See accompanying Editorial, p. 4084
PRIOR PRESENTATION
Presented at the 2022 European Society for Medical Oncology Congress, Paris, France, September 12, 2022.
SUPPORT
Supported by Astellas Pharma US; Merck Sharp & Dohme Corp, a subsidiary of Merck & Co Inc, Kenilworth, NJ; and Seagen Inc, EV-103/KN-869. Supported by the National Cancer Institute of the National Institutes of Health under Award No. K12CA076917 (C.J.H.). Supported in part by National Cancer Institute Cancer Center Support Grant No. P30 CA008748.
CLINICAL TRIAL INFORAMATION
DATA SHARING STATEMENT
Deidentified patient-level trial data that underlie the results reported in this publication will be made available on a case-by-case basis to researchers who provide a methodologically sound proposal. Additional documentation may also be made available. Data availability will begin after approval of the qualified request and end 30 days after receipt of data sets. All requests can be submitted to CTDR@seagen.com and will be reviewed by an internal review committee. Please note that the data sharing policy of this clinical study's sponsor, Seagen Inc, requires all requests for clinical trial data be reviewed to determine the qualification of the specific request. This policy is available at https://www.seagen.com/healthcare-professionals/clinical-data-requests and is aligned with BIO's Principles on Clinical Trial Data Sharing (available at https://www.bio.org/blogs/principles-clinical-trial-data-sharing-reaffirm-commitment).
AUTHOR CONTRIBUTIONS
Conception and design: Daniel P. Petrylak, Christopher J. Hoimes, Terence W. Friedlander, Saby George, Anne-Sophie Carret, Yao Yu, Blanca Homet Moreno, Jonathan E. Rosenberg
Provision of study materials or patients: Peter H. O'Donnell, Matthew I. Milowsky, Thomas W. Flaig, Sandy Srinivas, Saby George, Sergio Bracarda, Jose Pablo Maroto Rey, Jonathan E. Rosenberg
Collection and assembly of data: Peter H. O'Donnell, Christopher J. Hoimes, Thomas W. Flaig, Nataliya Mar, Helen H. Moon, Terence W. Friedlander, Rana R. McKay, Sandy Srinivas, Chethan Ramamurthy, Saby George, Sergio Bracarda, Delphine Borchiellini, Jose Pablo Maroto Rey, Christiano Ferrario, Anne-Sophie Carret, Yao Yu, Maria Guseva, Jonathan E. Rosenberg
Data analysis and interpretation: Peter H. O'Donnell, Matthew I. Milowsky, Daniel P. Petrylak, Christopher J. Hoimes, Thomas W. Flaig, Nataliya Mar, Terence W. Friedlander, Rana R. McKay, Mehmet A. Bilen, Sandy Srinivas, Earle F. Burgess, Chethan Ramamurthy, Saby George, Daniel M. Geynisman, Sergio Bracarda, Lionnel Geoffrois, Christiano Ferrario, Anne-Sophie Carret, Yao Yu, Maria Guseva, Blanca Homet Moreno, Jonathan E. Rosenberg
Manuscript writing: All authors
Final approval of manuscript: All authors
Accountable for all aspects of the work: All authors
AUTHORS' DISCLOSURES OF POTENTIAL CONFLICTS OF INTEREST
Enfortumab Vedotin With or Without Pembrolizumab in Cisplatin-Ineligible Patients With Previously Untreated Locally Advanced or Metastatic Urothelial Cancer
The following represents disclosure information provided by authors of this manuscript. All relationships are considered compensated unless otherwise noted. Relationships are self-held unless noted. I = Immediate Family Member, Inst = My Institution. Relationships may not relate to the subject matter of this manuscript. For more information about ASCO's conflict of interest policy, please refer to www.asco.org/rwc or ascopubs.org/jco/authors/author-center.
Open Payments is a public database containing information reported by companies about payments made to US-licensed physicians (Open Payments).
Peter H. O'Donnell
Honoraria: Merck, Astellas Pharma, Pfizer, CLD Inc, Axiom Healthcare Strategies, EMD Serono, IntrinsiQ, ISMIE, NAMCP, Seagen, Curio Science, FirstWord, MedLearning Group, Research to Practice, Great Debates and Updates, MJH Life Sciences, Peerview, Vaniam Group, Institute for Enquiring Minds
Research Funding: Boehringer Ingelheim (Inst), Merck (Inst), Genentech/Roche (Inst), AstraZeneca/MedImmune (Inst), Acerta Pharma (Inst), Janssen (Inst), Seagen (Inst), Bristol Myers Squibb (Inst), Astellas Pharma (Inst)
Expert Testimony: Oregon Health & Science University (OHSU)
Travel, Accommodations, Expenses: Curio Science
Other Relationship: Janssen, Nektar, NIH, Dragonfly Therapeutics, G1 Therapeutics
Matthew I. Milowsky
Stock and Other Ownership Interests: Pfizer, Merck, Gilead Sciences
Consulting or Advisory Role: Loxo/Lilly
Research Funding: Merck (Inst), Roche/Genentech (Inst), Bristol Myers Squibb (Inst), Mirati Therapeutics (Inst), Incyte (Inst), Seagen (Inst), G1 Therapeutics (Inst), Alliance Foundation Trials (Inst), Alliance for Clinical Trials in Oncology (Inst), Clovis Oncology (Inst), Arvinas (Inst), ALX Oncology (Inst), Loxo (Inst), Hoosier Cancer Research Network (Inst)
Other Relationship: Elsevier, Medscape
Daniel P. Petrylak
Consulting or Advisory Role: Bayer, Exelixis, Pfizer, Roche, Astellas Pharma, AstraZeneca, Lilly, Amgen, Boehringer Ingelheim, Bristol Myers Squibb, Clovis Oncology, Incyte, Janssen, Pharmacyclics, Seagen, Urogen Pharma, Advanced Accelerator Applications, Ipsen, Bicycle Therapeutics, Mirati Therapeutics, Monopteros Therapeutics, Regeneron, Gilead Sciences
Research Funding: Progenics (Inst), Sanofi (Inst), Endocyte (Inst), Genentech (Inst), Merck (Inst), Astellas Medivation (Inst), Novartis (Inst), AstraZeneca (Inst), Bayer (Inst), Lilly (Inst), Innocrin Pharma (Inst), MedImmune (Inst), Pfizer (Inst), Roche (Inst), Seagen (Inst), Clovis Oncology (Inst), Bristol Myers Squibb (Inst), Advanced Accelerator Applications (Inst), Agensys (Inst), BioXCel Therapeutics (Inst), Eisai (Inst), Mirati Therapeutics (Inst), Replimune (Inst), Medivation (Inst), Gilead Sciences (Inst)
Expert Testimony: Celgene, Sanofi
Christopher J. Hoimes
Honoraria: Seagen
Consulting or Advisory Role: Bristol Myers Squibb, Eisai, Prometheus, Seagen, Genentech/Roche, Merck Sharp & Dohme, 2bPrecise
Speakers' Bureau: Bristol Myers Squibb, Genentech/Roche, Astellas Pharma, Seagen, Eisai
Research Funding: Merck Sharp & Dohme (Inst), Janssen Oncology (Inst), Novartis (Inst), Alkermes (Inst), Dynavax Technologies (Inst), Nektar (Inst), NanoCarrier (Inst), Seagen (Inst), Astellas Pharma (Inst), Bristol Myers Squibb Foundation (Inst), BioNTech SE (Inst), Crispr Therapeutics (Inst), NeoImmuneTech (Inst), Mirati Therapeutics (Inst)
Uncompensated Relationships: 2bPrecise (Inst)
Thomas W. Flaig
Leadership: Aurora Oncology, University of Colorado/UC Health
Stock and Other Ownership Interests: Aurora Oncology
Consulting or Advisory Role: Seagen, Janssen Oncology
Research Funding: Novartis, Bavarian Nordic, Dendreon, GTx, Janssen Oncology, Medivation, Sanofi, Pfizer, Bristol Myers Squibb, Roche/Genentech, Exelixis, Aragon Pharmaceuticals, Sotio, Tokai Pharmaceuticals, Astrazeneca/MedImmune, Lilly, Astellas Pharma, Agensys, Seagen, La Roche-Posay, Merck, Seagen, Myovant Sciences, Criterium
Patents, Royalties, Other Intellectual Property: The University of Colorado has filed 2 patents in which I am an inventor. These are related to early-stage bladder cancer treatment and detection. Neither is commercialized or in active clinical development right now (eg, neither are in clinical trials)
Nataliya Mar
Speakers' Bureau: Seagen, Astellas Pharma, Aveo, Eisai, Tempus
Helen H. Moon
Honoraria: EMD Serono, Pfizer/EMD Serono
Research Funding: Bristol Myers Squibb (Inst), Amgen (Inst), Prometheus (Inst), Genentech (Inst), Seagen (Inst), Arcus Biosciences (Inst), Apollomics (Inst), Nektar (Inst), Revimmune (Inst), HUYA Bioscience International (Inst), Aveo (Inst), Xenocor (Inst)
Travel, Accommodations, Expenses: Aveo, Seagen
Terence W. Friedlander
Leadership: Med BioGene
Honoraria: EMD Serono, AstraZeneca/MedImmune, Astellas Scientific and Medical Affairs Inc, Astellas Pharma
Consulting or Advisory Role: Dava Oncology, EMD Serono, Merck, Astellas Pharma, Foundation Medicine, Basilea, Taiho Oncology, Seagen, AADi
Research Funding: Seagen (Inst), Bristol Myers Squibb (Inst), Neon Therapeutics (Inst), Roche/Genentech (Inst)
Travel, Accommodations, Expenses: AstraZeneca/MedImmune, Genentech/Roche, Jounce Therapeutics, Astellas Pharma
Rana R. McKay
Consulting or Advisory Role: Janssen, Novartis, Tempus, Exelixis, Pfizer, Bristol Myers Squibb, Astellas Medivation, Astellas Medivation, Bayer, Sanofi, Merck, Vividion Therapeutics, Calithera Biosciences, AstraZeneca, Myovant Sciences, Caris Life Sciences, Sorrento Therapeutics, AVEO, Seagen, Telix Pharmaceuticals, Lilly
Research Funding: Pfizer (Inst), Bayer (Inst), Tempus (Inst)
Mehmet A. Bilen
Consulting or Advisory Role: Exelixis, Sanofi, Nektar, EMD Serono, Eisai, Janssen, Genomic Health, Pfizer, Bristol Myers Squibb, Bayer, Calithera Biosciences, AstraZeneca, Seagen
Research Funding: Bayer (Inst), Bristol Myers Squibb (Inst), Genentech/Roche (Inst), Incyte (Inst), Nektar (Inst), AstraZeneca (Inst), Tricon Pharmaceuticals (Inst), Pfizer (Inst), Seagen (Inst), Xencor (Inst), Exelixis (Inst), Advanced Accelerator Applications (Inst), Genome & Company (Inst), Peloton Therapeutics (Inst), Merck (Inst), NiKang Therapeutics (Inst)
Sandy Srinivas
Consulting or Advisory Role: Eisai, Bayer, Bristol Myers Squibb, Merck, AstraZeneca, Seagen, Janssen Oncology, Novartis
Research Funding: Bristol Myers Squibb (Inst), Genentech (Inst), Merck (Inst), Exelixis (Inst), Eisai (Inst), Bayer (Inst), AstraZeneca (Inst), Seattle Genetics/Astellas (Inst)
Other Relationship: Pfizer
Earle F. Burgess
Stock and Other Ownership Interests: Exelixis, Becton Dickinson, Calithera Biosciences, Gilead Sciences, Medtronic, Macrogenics, Arvinas
Honoraria: Exelixis, Janssen Oncology, Novartis, Pfizer, Merck
Consulting or Advisory Role: Johnson & Johnson
Speakers' Bureau: AstraZeneca, Exelixis
Research Funding: Pfizer, Astellas Pharma
Chethan Ramamurthy
Honoraria: Gilead Sciences
Consulting or Advisory Role: Seagen, Exelixis
Research Funding: Dispersol (Inst), Novartis (Inst), Seagen (Inst), Gilead Sciences (Inst), Mirati Therapeutics (Inst), Nuvation Bio (Inst)
Saby George
Consulting or Advisory Role: Bristol Myers Squibb, Bayer, Pfizer, Exelixis, Corvus Pharmaceuticals, Sanofi, EMD Serono, Seattle Genetics/Astellas, Eisai, Merck, Aveo, QED Therapeutics
Research Funding: Pfizer (Inst), Merck (Inst), Agensys (Inst), Novartis (Inst), Bristol Myers Squibb (Inst), Bayer (Inst), Eisai (Inst), Seattle Genetics/Astellas (Inst), Surface Oncology (Inst), Exelixis (Inst), Aravive (Inst), Aveo (Inst), Gilead Sciences (Inst)
Travel, Accommodations, Expenses: Bristol-Myers Squibb/Medarex, Sanofi
Daniel M. Geynisman
Consulting or Advisory Role: Pfizer, Exelixis, AstraZeneca, Seattle Genetics/Astellas, Merck, Myovant Sciences, Bristol Myers Squibb
Research Funding: Genentech (Inst), Merck (Inst), Calithera Biosciences (Inst), Astellas Pharma (Inst), Harpoon Therapeutics (Inst)
Sergio Bracarda
Travel, Accommodations, Expenses: MSD Oncology, Pfizer
Delphine Borchiellini
Consulting or Advisory Role: Astellas Pharma, AstraZeneca, Bristol Myers Squibb, Ipsen, Janssen-Cilag, MSD Oncology, Pfizer, Merck, Bayer, AAA/Endocyte/Novartis
Research Funding: Astellas Pharma (Inst), AstraZeneca (Inst), Bristol Myers Squibb (Inst), Exelixis (Inst), Infinity Pharmaceuticals (Inst), Janssen (Inst), MSD (Inst), Roche (Inst), Bayer (Inst), Taiho Oncology (Inst), AVEO (Inst)
Travel, Accommodations, Expenses: Bristol Myers Squibb, Janssen, Pfizer, Ipsen, MSD Oncology, Bayer,
Lionnel Geoffrois
Honoraria: Ipsen, Merck Serono, BMS, Pfizer, MSD, Ipsen, Merck Serono
Travel, Accommodations, Expenses: Ipsen, Janssen Oncology, MSD Oncology, Merck KGaA, Merck Serono
Jose Pablo Maroto Rey
Consulting or Advisory Role: Astellas Pharma, Ipsen, BMS, Merck/Pfizer, Bayer, Janssen
Travel, Accommodations, Expenses: Merck/Pfizer, Bayer
Christiano Ferrario
Honoraria: Pfizer, Bayer, Novartis, AstraZeneca, Merck, Astellas Pharma, Roche Canada, Knight Pharmaceuticals
Consulting or Advisory Role: Merck, AstraZeneca, Novartis, Roche
Speakers' Bureau: Merck, Knight Therapeutics, AstraZeneca, Novartis
Research Funding: Astellas Pharma (Inst), AstraZeneca (Inst), Bayer (Inst), Lilly (Inst), Merck (Inst), Novartis (Inst), Roche/Genentech (Inst), Sanofi (Inst), Pfizer (Inst), Janssen Oncology (Inst), Zymeworks (Inst), Seagen (Inst), Immunomedics (Inst), Bicycle Therapeutics (Inst), Sermonix Pharmaceuticals (Inst)
Expert Testimony: Seattle Genetics/Astellas
Anne-Sophie Carret
Employment: Seagen
Stock and Other Ownership Interests: Seagen
Honoraria: Seagen
Travel, Accommodations, Expenses: Seagen
Yao Yu
Employment: Seagen
Stock and Other Ownership Interests: Seagen
Maria Guseva
Employment: Astellas Pharma
Blanca Homet Moreno
Employment: Merck Sharp & Dohme
Stock and Other Ownership Interests: Merck Sharp & Dohme
Jonathan E. Rosenberg
Honoraria: UpToDate, Medscape, Peerview, Research To Practice, Clinical Care Options, Physicans' Education Resource, MJH Life Sciences, EMD Serono, Pfizer
Consulting or Advisory Role: Lilly, Merck, Roche/Genentech, AstraZeneca/MedImmune, Bristol Myers Squibb, Seagen, Bayer, BioClin Therapeutics, QED Therapeutics, Pharmacyclics, GlaxoSmithKline, Janssen Oncology, Astellas Pharma, Boehringer Ingelheim, Pfizer/EMD Serono, Mirati Therapeutics, Immunomedics, Tyra Biosciences, Infinity Pharmaceuticals, Gilead Sciences, Hengrui Pharmaceutical, Alligator Bioscience, Imvax
Research Funding: Genentech/Roche (Inst), Seagen (Inst), Bayer (Inst), AstraZeneca (Inst), QED Therapeutics (Inst), Astellas Pharma (Inst)
Patents, Royalties, Other Intellectual Property: Predictor of platinum sensitivity (Inst)
No other potential conflicts of interest were reported.
REFERENCES
- 1. Wong MCS, Fung FDH, Leung C, et al. The global epidemiology of bladder cancer: A joinpoint regression analysis of its incidence and mortality trends and projection. Sci Rep. 2018;8:1129. doi: 10.1038/s41598-018-19199-z. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 2. Richters A, Aben KKH, Kiemeney L. The global burden of urinary bladder cancer: An update. World J Urol. 2020;38:1895–1904. doi: 10.1007/s00345-019-02984-4. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 3. Ferlay J, Soerjomataram I, Dikshit R, et al. Cancer incidence and mortality worldwide: Sources, methods and major patterns in GLOBOCAN 2012. Int J Cancer. 2015;136:E359–E386. doi: 10.1002/ijc.29210. [DOI] [PubMed] [Google Scholar]
- 4. Shah MV, McGovern A, Hepp Z. Targeted literature review of the burden of illness in urothelial carcinoma. Value Health. 2018;21:S32–S33. [Google Scholar]
- 5. Dash A, Galsky MD, Vickers AJ, et al. Impact of renal impairment on eligibility for adjuvant cisplatin-based chemotherapy in patients with urothelial carcinoma of the bladder. Cancer. 2006;107:506–513. doi: 10.1002/cncr.22031. [DOI] [PubMed] [Google Scholar]
- 6. Galsky MD, Hahn NM, Rosenberg J, et al. Treatment of patients with metastatic urothelial cancer “unfit” for cisplatin-based chemotherapy. J Clin Oncol. 2011;29:2432–2438. doi: 10.1200/JCO.2011.34.8433. [DOI] [PubMed] [Google Scholar]
- 7. Galsky MD, Pal SK, Lin SW, et al. Real-world effectiveness of chemotherapy in elderly patients with metastatic bladder cancer in the United States. Bladder Cancer. 2018;4:227–238. doi: 10.3233/BLC-170149. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 8. Grivas P, Plimack E, Balar AV, et al. Pembrolizumab (pembro) as first-line therapy in cisplatin-ineligible advanced urothelial cancer (UC): Outcomes from KEYNOTE-052 in senior patients (pts) with poor performance status. Ann Oncol. 2017;28:v301. [Google Scholar]
- 9. De Santis M, Bellmunt J, Mead G, et al. Randomized phase II/III trial assessing gemcitabine/carboplatin and methotrexate/carboplatin/vinblastine in patients with advanced urothelial cancer who are unfit for cisplatin-based chemotherapy: EORTC study 30986. J Clin Oncol. 2012;30:191–199. doi: 10.1200/JCO.2011.37.3571. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 10. von der Maase H, Sengelov L, Roberts JT, et al. Long-term survival results of a randomized trial comparing gemcitabine plus cisplatin, with methotrexate, vinblastine, doxorubicin, plus cisplatin in patients with bladder cancer. J Clin Oncol. 2005;23:4602–4608. doi: 10.1200/JCO.2005.07.757. [DOI] [PubMed] [Google Scholar]
- 11. Bukhari N, Al-Shamsi HO, Azam F. Update on the treatment of metastatic urothelial carcinoma. Scientific World Journal. 2018;2018:5682078. doi: 10.1155/2018/5682078. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 12. Kim TJ, Cho KS, Koo KC. Current status and future perspectives of immunotherapy for locally advanced or metastatic urothelial carcinoma: A comprehensive review. Cancers (Basel) 2020;12:e192. doi: 10.3390/cancers12010192. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 13. Powles T, Park SH, Voog E, et al. Avelumab maintenance therapy for advanced or metastatic urothelial carcinoma. N Engl J Med. 2020;383:1218–1230. doi: 10.1056/NEJMoa2002788. [DOI] [PubMed] [Google Scholar]
- 14. Galsky MD, Wirtz HS, Bloudek B, et al. Benchmarking maintenance therapy survival in first-line advanced urothelial carcinoma using disease modeling. J Clin Oncol. 2022;40(16 suppl) doi: 10.2147/CLEP.S409791. abstr 4575. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 15. Stenehjem DD, Tran D, Nkrumah MA, et al. PD1/PDL1 inhibitors for the treatment of advanced urothelial bladder cancer. Onco Targets Ther. 2018;11:5973–5989. doi: 10.2147/OTT.S135157. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 16. Balar AV, Castellano D, O'Donnell PH, et al. First-line pembrolizumab in cisplatin-ineligible patients with locally advanced and unresectable or metastatic urothelial cancer (KEYNOTE-052): A multicentre, single-arm, phase 2 study. Lancet Oncol. 2017;18:1483–1492. doi: 10.1016/S1470-2045(17)30616-2. [DOI] [PubMed] [Google Scholar]
- 17. Galsky MD, Arija JÁA, Bamias A, et al. Atezolizumab with or without chemotherapy in metastatic urothelial cancer (IMvigor130): A multicentre, randomised, placebo-controlled phase 3 trial. Lancet. 2020;395:1547–1557. doi: 10.1016/S0140-6736(20)30230-0. [DOI] [PubMed] [Google Scholar]
- 18. Powles T, van der Heijden MS, Castellano D, et al. Durvalumab alone and durvalumab plus tremelimumab versus chemotherapy in previously untreated patients with unresectable, locally advanced or metastatic urothelial carcinoma (DANUBE): A randomised, open-label, multicentre, phase 3 trial. Lancet Oncol. 2020;21:1574–1588. doi: 10.1016/S1470-2045(20)30541-6. [DOI] [PubMed] [Google Scholar]
- 19. Powles T, Csoszi T, Ozguroglu M, et al. Pembrolizumab alone or combined with chemotherapy versus chemotherapy as first-line therapy for advanced urothelial carcinoma (KEYNOTE-361): A randomised, open-label, phase 3 trial. Lancet Oncol. 2021;22:931–945. doi: 10.1016/S1470-2045(21)00152-2. [DOI] [PubMed] [Google Scholar]
- 20. Sonpavde GP, Galsky MD, Wright P, et al. Real-world treatment patterns and clinical outcomes with first-line therapy in cisplatin-eligible and ineligible patients with advanced urothelial carcinoma. J Clin Oncol. 2022;40(16 suppl) abstr 4565. [Google Scholar]
- 21. Powles T, Rosenberg JE, Sonpavde GP, et al. Enfortumab vedotin in previously treated advanced urothelial carcinoma. N Engl J Med. 2021;384:1125–1135. doi: 10.1056/NEJMoa2035807. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 22. Yu EY, Petrylak DP, O'Donnell PH, et al. Enfortumab vedotin after PD-1 or PD-L1 inhibitors in cisplatin-ineligible patients with advanced urothelial carcinoma (EV201): A multicentre, single-arm, phase 2 trial. Lancet Oncol. 2021;22:872–882. doi: 10.1016/S1470-2045(21)00094-2. [DOI] [PubMed] [Google Scholar]
- 23. Krysko DV, Garg AD, Kaczmarek A, et al. Immunogenic cell death and DAMPs in cancer therapy. Nat Rev Cancer. 2012;12:860–875. doi: 10.1038/nrc3380. [DOI] [PubMed] [Google Scholar]
- 24. Doronina SO, Toki BE, Torgov MY, et al. Development of potent monoclonal antibody auristatin conjugates for cancer therapy. Nat Biotechnol. 2003;21:778–784. doi: 10.1038/nbt832. [DOI] [PubMed] [Google Scholar]
- 25. Challita-Eid PM, Satpayev D, Yang P, et al. Enfortumab vedotin antibody-drug conjugate targeting nectin-4 is a highly potent therapeutic agent in multiple preclinical cancer models. Cancer Res. 2016;76:3003–3013. doi: 10.1158/0008-5472.CAN-15-1313. [DOI] [PubMed] [Google Scholar]
- 26. Hoimes CJ, Flaig TW, Milowsky MI, et al. Enfortumab vedotin plus pembrolizumab in previously untreated advanced urothelial cancer. J Clin Oncol. 2023;41:22–31. doi: 10.1200/JCO.22.01643. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 27. Rosenberg J, Sridhar SS, Zhang J, et al. EV-101: a phase I study of single-agent enfortumab vedotin in patients with Nectin-4-positive solid tumors, including metastatic urothelial carcinoma. J Clin Oncol. 2020;38:1041–1049. doi: 10.1200/JCO.19.02044. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 28. Parikh RB, Feld EK, Galsky MD, et al. First-line immune checkpoint inhibitor use in cisplatin-eligible patients with advanced urothelial carcinoma: A secular trend analysis. Future Oncol. 2020;16:4341–4345. doi: 10.2217/fon-2019-0578. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 29. Galsky MD, Arranz JÁ, Grande E, et al. Atezolizumab (atezo) + platinum/gemcitabine (plt/gem) vs placebo + plt/gem in patients (pts) with previously untreated locally advanced or metastatic urothelial carcinoma (mUC): Updated overall survival (OS) from the randomized phase III study IMvigor130. Cancer Res. 2021;81 abstr CT042. [Google Scholar]
- 30. Powles T, Csoszi T, Ozguroglu M, et al. 1L pembrolizumab (pembro) versus chemotherapy (chemo) for choice-of-carboplatin patients with advanced urothelial carcinoma (UC) in KEYNOTE-361. J Clin Oncol. 2021;39(6 suppl) abstr 450. [Google Scholar]
- 31. Vuky J, Balar AV, Castellano D, et al. Long-term outcomes in KEYNOTE-052: Phase II study investigating first-line pembrolizumab in cisplatin-ineligible patients with locally advanced or metastatic urothelial cancer. J Clin Oncol. 2020;38:2658–2666. doi: 10.1200/JCO.19.01213. [DOI] [PubMed] [Google Scholar]
- 32. Balar AV, Galsky MD, Rosenberg JE, et al. Atezolizumab as first-line treatment in cisplatin-ineligible patients with locally advanced and metastatic urothelial carcinoma: A single-arm, multicentre, phase 2 trial. Lancet. 2017;389:67–76. doi: 10.1016/S0140-6736(16)32455-2. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 33. Galsky MD, Mortazavi A, Milowsky MI, et al. Randomized double-blind phase II study of maintenance pembrolizumab versus placebo after first-line chemotherapy in patients with metastatic urothelial cancer. J Clin Oncol. 2020;38:1797–1806. doi: 10.1200/JCO.19.03091. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 34. Rosenberg JE, O'Donnell PH, Balar AV, et al. Pivotal trial of enfortumab vedotin in urothelial carcinoma after platinum and anti-programmed death 1/programmed death ligand 1 therapy. J Clin Oncol. 2019;37:2592–2600. doi: 10.1200/JCO.19.01140. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 35. Pace A, Brower B, Conway D, et al. Enfortumab vedotin: Nursing perspectives on the management of adverse events in patients with locally advanced or metastatic urothelial carcinoma. Clin J Oncol Nurs. 2021;25:E1–E9. doi: 10.1188/21.CJON.E1-E9. [DOI] [PubMed] [Google Scholar]
- 36. Lacouture ME, Patel AB, Rosenberg JE, et al. Management of dermatologic events associated with the Nectin-4-directed antibody-drug conjugate enfortumab vedotin. Oncologist. 2022;27:e223–e232. doi: 10.1093/oncolo/oyac001. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 37. Belum VR, Benhuri B, Postow MA, et al. Characterisation and management of dermatologic adverse events to agents targeting the PD-1 receptor. Eur J Cancer. 2016;60:12–25. doi: 10.1016/j.ejca.2016.02.010. [DOI] [PMC free article] [PubMed] [Google Scholar]
Associated Data
This section collects any data citations, data availability statements, or supplementary materials included in this article.
Data Availability Statement
Deidentified patient-level trial data that underlie the results reported in this publication will be made available on a case-by-case basis to researchers who provide a methodologically sound proposal. Additional documentation may also be made available. Data availability will begin after approval of the qualified request and end 30 days after receipt of data sets. All requests can be submitted to CTDR@seagen.com and will be reviewed by an internal review committee. Please note that the data sharing policy of this clinical study's sponsor, Seagen Inc, requires all requests for clinical trial data be reviewed to determine the qualification of the specific request. This policy is available at https://www.seagen.com/healthcare-professionals/clinical-data-requests and is aligned with BIO's Principles on Clinical Trial Data Sharing (available at https://www.bio.org/blogs/principles-clinical-trial-data-sharing-reaffirm-commitment).