Abstract
Background:
Fine particulate matter (PM2.5) exposure has been linked to anxiety and depression in adults; however, there is limited research in the younger populations, in which symptoms often first arise.
Methods:
We examined the association between early-life PM2.5 exposure and symptoms of anxiety and depression in a cohort of 8–11-year-olds in Mexico City. Anxiety and depressive symptoms were assessed using the Spanish versions of the Revised Children’s Manifest Anxiety Scale and Children’s Depression Inventory. Daily PM2.5 was estimated using a satellite-based exposure model and averaged over several early and recent exposure windows. Linear and logistic regression models were used to estimate the change in symptoms with each 5-µg/m3 increase in PM2.5. Models were adjusted for child’s age, child’s sex, maternal age, maternal socioeconomic status, season of conception, and temperature.
Results:
Average anxiety and depressive symptom T-scores were 51.0 (range 33–73) and 53.4 (range 44–90), respectively. We observed consistent findings for exposures around the fourth year of life, as this was present for both continuous and dichotomized anxiety symptoms, in both independent exposure models and distributed lag modeling approaches. This window was also observed for elevated depressive symptoms. An additional consistent finding was for PM2.5 exposure during early pregnancy in relation to both clinically elevated anxiety and depressive symptoms, this was seen in both traditional and distributed lag modeling approaches.
Conclusion:
Both early life and recent PM2.5 exposure were associated with higher mental health symptoms in the child highlighting the role of PM2.5 in the etiology of these conditions.
Keywords: Air pollution, Mental health, Pediatric health, Fine particulate matter
What this study adds
Fine particulate matter (PM2.5) has been linked to anxiety and depression in adults; however, there is limited research in the younger populations in which symptoms often first arise. We evaluated the association between early-life PM2.5 and anxiety and depressive symptoms in a cohort of children living in Mexico City. We utilized both independent exposure models and distributed lag models to address the correlated structure of the data. Our study addresses this question in an area with high PM2.5 levels and identifies several critical windows for PM2.5 to contribute to increases in both overall symptoms and clinically elevated odds of symptoms.
Introduction
Anxiety and depression are the most common mental health conditions1 and are a particular issue for children and adolescents, as the prevalence of both disorders in this age group has rapidly increased over the past decade.2,3 Clinical and subclinical symptoms of anxiety and depression appear as early as the preschool years4—with half of all mental health conditions starting by age 14,5 and an average age of onset of 11 for anxiety.6 Given the early age of onset of symptoms and considerable burden, the late childhood/preadolescent period may represent a critical time period for early detection of anxiety and depressive symptoms.7 Identification of modifiable risk factors during these younger life stages could also prevent future mental and physical health burdens later in life.
Though genetics plays a role in psychiatric disorders and symptoms, it cannot explain the entire variability or rise in prevalence.8 There is a growing body of literature to support the importance of the environment in the etiology of these conditions.9 There is additionally a growing consensus that where you live can shape your mental health across the life course, with early and late childhood hypothesized to be significant time periods of vulnerability to poor neighborhood conditions.10,11 Effects from the urban environment are of increasing concern, as it is estimated that half of the global population lives in an urban setting; a proportion that is expected to reach 68% by 2050.12 The prevalence of mental health conditions is even higher for individuals living in urban areas,13,14 in part related to social and economic factors, but environmental factors that disproportionately impact urban populations, such as air pollution, may also play a role.15
Air pollution, including particulate matter less than 2.5 micrometers (PM2.5), is a ubiquitous exposure that is highest in urban settings.16 An accumulating body of literature links short and long-term PM2.5 exposure to psychiatric disorders in adults,17 including depression18–20 and anxiety.21,22 While early life PM2.5 has been extensively linked with alterations in brain development, including cognitive deficits23 and autism,24–27 there is limited research relating PM2.5 to psychiatric symptoms in the late childhood/preadolescent period. A growing number of studies are starting to address this research question with the majority in higher income areas including Australia,28 Europe,29–32 and the United States (US).33–35 Few have addressed this research question in a low- and middle-income country such as Mexico, where air pollution exposure levels are considerably higher.36
The most biologically relevant exposure window for air pollution to impact anxiety and depressive symptoms is currently unknown. Understanding the critical exposure window would add more effective and efficient public health interventions as the target population would be better identified. We are a priori interested in the in utero, early life, and preadolescent developmental windows given the heightened sensitivity of these periods for toxicant exposure. The brain is rapidly developing during early life and several critical processes occur during these time points.37 Disruptions of these critical processes secondary to toxic exposures during vulnerable periods alter normal brain development, potentially increasing the risk for mental health conditions.38,39 It is also plausible that short-term PM2.5 exposure could trigger an existing susceptibility to anxiety or depressive symptoms through short-term changes in oxidative stress.40
The purpose of the present study was to leverage highly temporally resolved PM2.5 estimates and apply distributed lag modeling (DLM) approaches to identify potentially critical windows for the association between early-life and recent PM2.5 exposure and preadolescent anxiety and depressive symptoms. Given that the most biologically relevant period of exposure is currently unknown, we focus on several potential critical windows including (1) prenatal, (2) postnatal through the eighth year of life, and (3) recent short-term exposures. We focus on symptoms of anxiety and depression during the preadolescent period because mental disorders vary along a continuum and first appear as subclinical symptoms.41 We further build upon the literature by assessing sex-specific effects and addressing this research question in a particularly vulnerable and understudied geographic area with high levels of chemical and social stressors.36,42
Materials and methods
Study population
This study takes place among mothers and their children enrolled in the Programming Research in Obesity, Growth, Environment and Social Stressors (PROGRESS) study, a prospective birth cohort study in Mexico City. Briefly, pregnant women were recruited between 2007 and 2011 at 12–24 weeks’ gestation in primary care clinics of the Mexican Social Security Institute. Women were eligible if they were ≥18 years old, planned to live in Mexico City, <20 weeks’ gestation, had no medical history of heart or kidney disease, and did not consume alcohol daily.43 In total, 948 women enrolled in the second trimester and delivered a live child who was then followed longitudinally. For this analysis, we used data from 517 mother-child pairs with complete exposure, outcome, and covariate information. Participant characteristics did not differ for those included and those in the original sample (Table S1; http://links.lww.com/EE/A253). Protocols were approved by the institutional review boards at the Icahn School of Medicine at Mount Sinai, Harvard School of Public Health, and Mexican National Institute of Public Health. All women provided informed consent and children provided assent.
Symptoms of anxiety and depression
The Children’s Depression Inventory 2 (CDI-2) is a validated44 self-reported instrument developed to evaluate symptoms of depression in 7–17-year-old children and adolescents.45 We used the short form of the children’s version of the CDI-2 in the current study, which contains 12 items. The short form of the CDI-2 is a validated screening tool for youth at risk for depression.45,46 Individual items on the CDI-2 short form were summed to create a total raw score (ranging from 0–21) and then raw scores were converted to age-standardized T-scores based on normative samples.47 We analyzed CDI-2 T-scores as continuous measures, with higher scores indicating more severe depressive symptoms. We additionally dichotomized scores using cutoff values recommended by the CDI-2 manual (≥65). These dichotomized scores represent those with clinically elevated depressive symptoms.
The Revised Children’s Manifest Anxiety Scale 2 (RCMAS-2) is a validated48 self-report screening instrument developed to measure the nature and degree of anxiety symptoms in 6–19-year-old children and adolescents.49 The RCMAS-2 short form is a validated screening tool for youth at risk for anxiety and has 12 items rated as yes/no.50 Higher scores on the RCMAS-2 indicate more severe anxiety symptoms. Individual items on the RCMAS-2 were summed to create a total raw score ranging from 0 to 10. Raw total anxiety scores were then converted to age-standardized T-scores based on normative samples.49 This allows the scores to be compared with other children their age. We analyzed scores as continuous and dichotomized scores based on the RCMAS-2 manual (≥60).49 These dichotomized scores represent those children with clinically elevated anxiety symptoms.
Air pollution exposure assessment
Daily average PM2.5 exposure at each participant’s residence was estimated using a previously developed hybrid satellite land use regression model at a 1 × 1 km spatial resolution.51 This model uses Extreme Gradient Boosting with inverse-distance weighted surfaces and several different meteorological and land use variables. Briefly, predictors include longitude and latitude, date, the density of roadways from OpenStreetMap, the aerosol optical depth from NASA’s Terra and Aqua satellites, daily mean predicted PM2.5 from the NASA MERRA-2 GMI atmospheric simulation, daily mean of the height of the atmospheric mixing layer from the European Center for Medium-Range Weather Forecasts global climate dataset, temperature, dewpoint temperature, and the daily sum of precipitation. Models were evaluated using leave-one-station-out cross-validation. Models for mean PM2.5 exhibited good performance, with an overall cross-validated mean absolute error of 3.68 µg/m3. The R2 varied year to year, ranging from 0.64 to 0.86.51
Participants were matched to the centroid of the nearest grid cell based on GPS coordinates collected at their residential address by study personnel. Residential addresses were updated throughout the study period, from birth to the most recent study visit. Thus, if an individual moved, we were able to link exposures to their updated address based on the length of residence. Gestational age was used to link the air pollution exposures on time. Gestational age was based on the last menstrual period, as reported by the mother, and by a standardized physical examination to determine gestational age at birth. Average levels of PM2.5 were calculated for the entire pregnancy period and each year of life up until 8 years by averaging the daily PM2.5 levels across these time periods. For recent exposures, we assigned these daily values based on the date of the RCMAS-2 and CDI-2 assessment visit. We then calculated exposure averages for the 12 weeks before the study visit.
Covariates
Covariate information was obtained from standardized questionnaires administered to mothers at baseline and follow-up study visits. Questionnaires collected sociodemographic information such as maternal age, years of education, and socioeconomic status (SES). Thirteen variables (i.e., level of education, number of bedrooms, access to internet, etc.) derived from questionnaire results were used to classify study participants into six levels based on the SES index created by the Asociación Mexicana de Agencias de Investigación de Mercados y Opinión Pública.52 We further collapsed these six levels into low (lower two levels), medium (middle two levels), and high (top two levels) SES based on the distribution in our study population.
The program DAGitty was used to construct our directed acyclic graph to identify the minimally sufficient adjustment set. All models were adjusted for child’s age at assessment (years), child’s sex, maternal age at assessment (years), and maternal SES (low, medium, high). Prenatal exposure models additionally adjusted for season of conception. Postnatal and recent exposure models additionally adjusted for season of study visit. Season was categorized as cold-dry (November–February), warm-dry (March–April), and rainy (May–October). Daily temperature was derived using a model that calibrated satellite surface temperature measurements to air temperature monitors by using land use regression methods.53
Statistical analyses
We used two approaches to assess the association between time-specific PM2.5 exposure and mental health symptoms in preadolescents in our study population. First, we assessed associations between early life and recent PM2.5 exposures in independent linear regression models. We report beta coefficients and 95% confidence intervals (CI) for each 5-μg/m3 increase in PM2.5. For early-life exposures, we used average exposures for the entire pregnancy period and then each year of life after birth through 8 years of age. We also assessed trimester-specific exposures; in these models, we mutually adjusted for exposures during the other trimesters.54 Next, we assessed the impacts of recent PM2.5 exposures with daily exposures averaged over 1 and 3 months before the study visit. We used separate models to examine associations with each exposure window and outcome, including self-reported anxiety and depressive symptoms.
We then estimated the time-varying association between PM2.5 and symptoms of anxiety and depression using distributed lag models. DLMs are an approach to include exposure data from multiple times in the same health effects model, which is of use in air pollution epidemiology since the risk of an event can be a result of exposure at time t, but also a combination of that exposure plus other time periods from the recent past.55 The approach has been adapted for identifying critical exposure windows.54 Using this approach, we assessed independent associations (in separate models) for prenatal, childhood, and recent PM2.5 exposures. For prenatal exposures we used weekly PM2.5 averages during pregnancy; for childhood, we used monthly PM2.5 exposure averages from birth through the eighth year of life. Finally, for recent exposures, we used weekly PM2.5 averages for the 12 weeks before RCMAS-2 and CDI-2 assessments.
In the presence of correlated exposure terms in the model, the DLM allows for the control of other periods of exposure when modeling the relation between PM2.5 and mental health symptoms for any given week (or month) and allows these associations to vary in a nonlinear fashion over time. Natural cubic splines were used to model the smoothed lag-response curve. The degrees of freedom for the natural spline models were chosen based on the lowest Akaike information criterion value. The exposure-response at each timepoint was modeled with a linear function. A sensitive window was identified when the pointwise 95% confidence bands did not contain zero. DLM models were adjusted for child age, child sex, maternal age, maternal SES, and season. We additionally controlled for temperature by creating additional crossbasis terms for mean temperature over the same lags as PM2.5.
We conducted several additional analyses. Previous studies have found differences in the effects of PM2.5 exposure on health outcomes for males and females.56,57 Therefore, we assessed if child sex modified PM2.5-mental health associations. Next, we considered mental health symptoms as binary rather than continuous measures. Logistic regression was used to assess associations between PM2.5 and odds of clinically elevated anxiety and depressive T-scores. For logistic models, we report odds ratios (OR) and 95% CIs per 5-μg/m3 increase in PM2.5.
Data management and statistical analyses were conducted using the R statistical software (version 4.1). DLM analyses were implemented using the dlnm package.55
Results
Study population characteristics and distribution of PM 2.5
Table 1 shows descriptive characteristics of the 517 mother-child pairs included in the current analysis. Mothers in the study population were on average of lower SES and had less than a high school education at study enrollment; the mean maternal age at birth was 28 years. There was an equal distribution of male and female children in the study population (52% males). Children were on average 9.7 years old at the anxiety and depressive symptom assessment study visit. Finally, children had a mean anxiety symptom T-score of 51.0 (standard deviation [SD] = 7.6) and a mean depressive symptom T-score of 53.4 (SD = 9.0). About 12% and 10% of participants scored above the clinical cutoff for anxiety and depressive symptoms, respectively (Table 1).
Table 1.
Characteristics of the mother-child pairs in the Programming Research in Obesity, Growth, Environment and Social Stressors study
| No. (%) or mean ± SD | |
|---|---|
| Number of participants (n) | 517 |
| Child sex, n (%) | |
| Male | 267 (52) |
| Female | 250 (48) |
| Maternal age at study enrollment (years) | 28.1 ± 5.6 |
| Maternal education, n (%) | |
| Less than high school | 214 (41) |
| High school | 188 (36) |
| More than high school | 115 (22) |
| Maternal socioeconomic status, n (%) | |
| Low | 274 (53) |
| Medium | 188 (36) |
| High | 55 (11) |
| Child age at assessment (years) | 9.65 ± 0.7 |
| Season of 8–11-year study visit, n (%) | |
| Cold-dry, November–February | 112 (23) |
| Warm-dry, March–April | 47 (10) |
| Rainy, May–October | 329 (67) |
| Child anxiety and depressive symptoms | |
| Anxiety symptom T-score | 51.0 ± 7.6 |
| Depressive symptoms T-score | 53.4 ± 9.0 |
| Clinically elevated anxiety symptoms, n (%) | 64 (12) |
| Clinically elevated depressive symptoms, n (%) | 51 (10) |
SD indicates standard deviation.
Average PM2.5 concentrations for participants in our study population were on average lower during the eighth year of life (20.8 μg/m3, SD: 1.9) compared with the pregnancy period (22.8 μg/m3, SD = 2.9) (Table 2). Overall, the levels in our study population were much higher compared with other parts of the world and exceeded the WHO Air Quality Guideline for PM2.5.58
Table 2.
Descriptive statistics for average PM2.5 (µg/m3) exposures for participants for the pregnancy period and years 1–8
| Average PM2.5 | Mean | SD | Minimum | Median | Maximum | IQR |
|---|---|---|---|---|---|---|
| Pregnancy | 22.8 | 2.9 | 16.4 | 22.9 | 30.2 | 4.6 |
| Year 1 | 22.6 | 2.5 | 17.6 | 23.4 | 26.7 | 4.3 |
| Year 2 | 22.2 | 2.3 | 17.8 | 22.0 | 29.3 | 3.6 |
| Year 3 | 23.0 | 1.8 | 18.7 | 22.8 | 28.2 | 2.9 |
| Year 4 | 22.2 | 1.9 | 18.0 | 22.1 | 27.8 | 2.6 |
| Year 5 | 21.6 | 2.1 | 17.3 | 21.5 | 31.7 | 2.8 |
| Year 6 | 21.2 | 1.9 | 16.1 | 21.2 | 27.9 | 3.0 |
| Year 7 | 20.8 | 1.8 | 17.3 | 20.6 | 26.8 | 2.6 |
| Year 8 | 20.8 | 1.9 | 17.0 | 20.5 | 28.0 | 2.8 |
IQR indicates interquartile range; PM2.5, fine particulate matter; SD, standard deviation.
Early life PM2.5 and anxiety and depression symptoms
First, we assessed associations between prenatal and childhood PM2.5 exposure and anxiety and depressive symptoms at ages 8–11 using separate models for each exposure window (Table 3). Overall, we observed associations between average PM2.5 exposures during the second (ß = 1.98; 95% CI = 0.22, 3.75) and fourth year of life (ß = 3.85; 95% CI = 1.34, 6.36) and increased anxiety symptoms. We additionally observed associations between PM2.5 exposure during the second year of life and increased depressive symptoms in the child (ß = 2.24; 95% CI = 0.10, 4.39). In these separate exposure window analyses, we initially identified an association between first-trimester PM2.5 exposure and increased depressive symptoms (ß = 1.05; 95% CI = −0.01, 2.11), though we note that the 95% CI includes the null value (Table S2; http://links.lww.com/EE/A253).
Table 3.
Associations between PM2.5 concentrations and continuous measures of anxiety and depressive symptoms in children, overall and by sex
| Anxiety symptoms (RCMAS-2) Betas (95% CI) |
Depressive symptoms (CDI-2) Betas (95% CI) |
|||||
|---|---|---|---|---|---|---|
| Overall | Females | Males | Overall | Females | Males | |
| Early life | ||||||
| Pregnancy | −0.58 (−1.82, 0.66) | −1.41 (−3.16, 0.33) | 0.22 (−1.52, 1.96) | −0.03 (−1.5, 1.45) | −0.52 (−2.66, 1.62) | 0.51 (−1.42, 2.64) |
| Year 1 | −1.20 (−2.80, 0.40) | −1.02 (−3.51, 1.47) | −1.38 (−3.50, 0.74) | −1.28 (−3.22, 0.66) | −0.56 (−3.58, 2.46) | −1.95 (−4.50, 0.60) |
| Year 2 | 1.98 (0.22, 3.75) | 1.96 (−0.78, 4.70) | 2.04 (−0.27, 4.36) | 2.24 (0.10, 4.39) | 1.45 (−1.87, 4.78) | 2.82 (0.01, 5.62) |
| Year 3 | −1.35 (−3.50, 0.81) | −2.73 (−6.05, 0.60) | −0.34 (−3.10, 2.43) | −1.89 (−4.44, 0.66) | −2.70 (−6.73, 1.33) | −1.58 (−4.89, 1.73) |
| Year 4 | 3.85 (1.34, 6.36) | 4.25 (0.63, 7.86) | 3.17 (−0.13, 6.46) | 2.19 (−0.80, 5.17) | 1.15 (−3.34, 5.64) | 3.17 (−0.85, 7.20) |
| Year 5 | −0.78 (−3.11, 1.55) | −1.49 (−4.56, 1.57) | 0.55 (−2.65, 3.75) | −1.96 (−4.59, 0.67) | −3.71 (−7.39, −0.04) | −0.67 (−4.49, 3.15) |
| Year 6 | 1.85 (−0.44, 4.13) | 2.54 (−0.81, 5.88) | 1.57 (−1.46, 4.59) | −0.70 (−3.39, 1.98) | 1.79 (−2.29, 5.87) | −2.89 (−6.54, 0.76) |
| Year 7 | 0.11 (−2.32, 2.54) | −0.81 (−4.32, 2.70) | 1.13 (−2.12, 4.38) | −0.94 (−3.78, 1.91) | −0.69 (−4.98, 3.60) | −1.85 (−5.70, 2.00) |
| Year 8 | −0.22 (−2.05, 1.60) | −1.04 (−4.81, 2.74) | 0.39 (−2.79, 3.56) | −1.47 (−4.42, 1.48) | −0.85 (−5.51, 3.70) | −2.29 (−6.19, 1.60) |
| Recent | ||||||
| 1-month | 0.71 (0.01, 1.40) | 1.07 (0.05, 2.09) | 0.41 (−0.57, 1.38) | 0.19 (−0.65, 1.03) | 0.27 (−0.97, 1.50) | 0.05 (−1.11, 1.21) |
| 3-month | 0.11 (−0.86, 1.07) | −0.11 (−1.45, 1.24) | 0.29 (−0.98, 1.56) | 0.34 (−0.75, 1.43) | 0.77 (−0.84, 2.37) | −0.10 (−1.62, 1.41) |
Results are reported per 5-μg/m3 increase in PM2.5.
CDI-2 indicates Children’s Depression Inventory (short form); CI, confidence interval; PM2.5, fine particulate matter; RCMAS-2, Revised Children’s Manifest Anxiety Scale (short form).
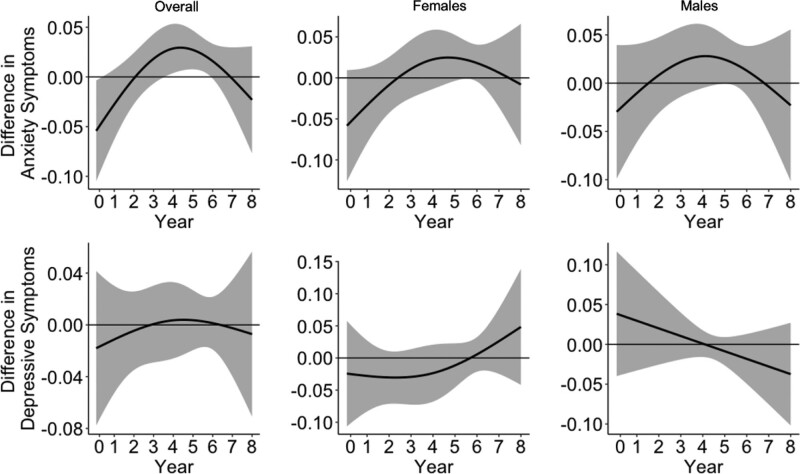
In addition to separate models for each exposure window, we also used DLMs to simultaneously control for the other exposure periods. Figure 1 shows the association between PM2.5 exposure from birth through the eighth year of life in relation to both anxiety and depressive symptoms, overall and stratified by sex. Monthly PM2.5 was positively associated with increased continuous anxiety symptoms—we identified a critical window during around the fourth–sixth year of life. No differences in effects by child sex were identified. We did not identify a sensitive window for depressive symptoms.
Figure 1.
Associations between monthly PM2.5 levels from birth through the eighth year of life and anxiety and depressive symptoms in the preadolescent period (ages 8–11). Results are shown overall (N = 517) and for males (N = 267) and females (N = 250) separately. Solid lines show the difference in anxiety and depressive symptoms per 5-μg/m3 increase in PM2.5. Gray areas indicate 95% confidence intervals. Models are adjusted for child’s age at assessment, child’s sex, maternal age, maternal SES, temperature, and season of study visit.
Recent PM2.5 and anxiety and depression symptoms
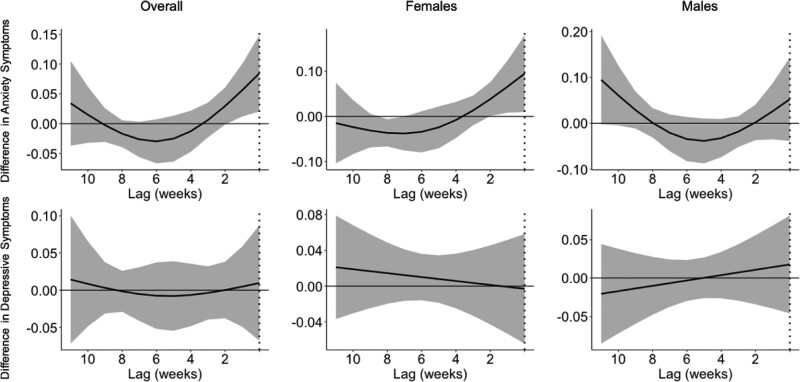
Next, we assessed associations between recent (or short-term) PM2.5 exposure and anxiety and depressive symptoms. In independent exposure models, we observed an association between the previous month average PM2.5 exposure and increased anxiety symptoms (ß = 0.71; 95% CI = 0.01, 1.40), particularly for females (ß = 1.07; 95% CI = 0.05, 2.09) (Table 2). We did not observe any association for 3-month average exposures, nor did we observe any associations with depressive symptoms for recent exposures (Table 3). In DLM analyses, weekly recent PM2.5 exposure was positively associated with anxiety symptoms, with a critical window identified for the 2 weeks before the study visit (Figure 2). This critical window was observed in females only (Figure 2). We did not observe any critical windows for depressive symptoms.
Figure 2.
Associations between weekly PM2.5 levels for the prior 12 weeks and anxiety and depressive symptoms in the preadolescent period (ages 8–11). Results are shown overall (N = 517) and for males (N = 267) and females (N = 250) separately. Solid lines show the difference in anxiety and depressive symptoms per 5-μg/m3 increase in PM2.5. Gray areas indicate 95% confidence intervals. Models are adjusted for child’s age at assessment, child’s sex, maternal age, maternal SES, temperature, and season of study visit.
Clinically elevated anxiety and depressive symptoms
We additionally investigated associations between PM2.5 exposure and odds of clinically elevated anxiety and depressive symptoms in dichotomized outcome models. In these analyses, we observed similar results for childhood and recent PM2.5 exposures (Table S3; http://links.lww.com/EE/A253). For trimester-specific mutually adjusted models, we observed associations between first-trimester PM2.5 exposure and increased odds of having clinically elevated anxiety (OR = 1.49; 95% CI = 1.03, 2.14) and depressive symptoms (OR = 1.54; 95% CI = 1.04, 2.28) (Table S2; http://links.lww.com/EE/A253).
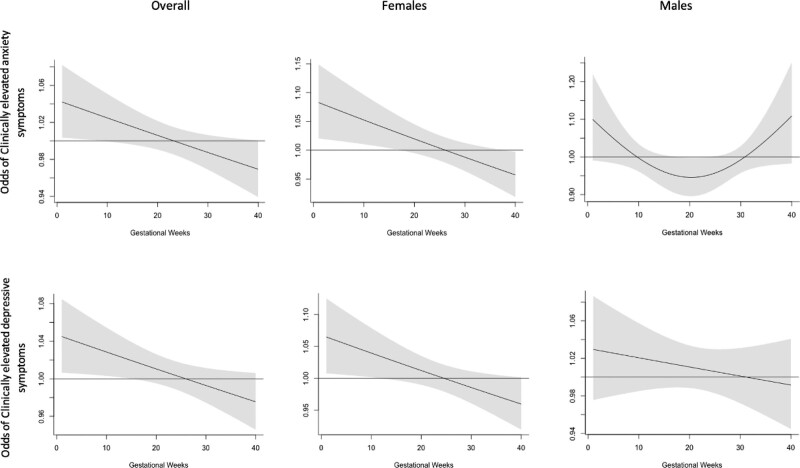
We additionally used DLM approaches and observed a critical window with PM2.5 exposure during the early pregnancy period. Specifically, for anxiety symptoms we observed associations particularly for exposures during around the first 15 weeks of gestation and for depressive symptoms we identified a critical window during around the first 10 weeks of pregnancy (Figure 3). These results and identified critical window are similar to our findings in trimester-specific models, where we observed associations during the first-trimester (or weeks 1–13) of pregnancy (Table S2; http://links.lww.com/EE/A253). These identified associations were seen in females but not males.
Figure 3.
Associations between weekly PM2.5 during the pregnancy period and clinically elevated anxiety and depressive symptoms in preadolescence (ages 8–11). Results are shown overall (N = 517) and for males (N = 267) and females (N = 250) separately. Solid lines show the odds of anxiety and depressive symptoms per 5-μg/m3 increase in PM2.5. Gray areas indicate 95% confidence intervals. Models were adjusted for child’s age at assessment, child’s sex, maternal age, maternal SES, season of conception, and temperature.
Discussion
We assessed the association between early-life PM2.5 exposure at multiple time points in relation to anxiety and depressive symptoms in preadolescents from a longitudinal cohort in Mexico City. In this study, both childhood and recent PM2.5 exposure were associated with higher self-reported continuous mental health symptoms in the child. Specifically, we observed associations between higher PM2.5 exposure during early childhood in relation to both continuous anxiety and depressive symptoms in the child. In recent (or short-term) exposure models, we observed associations between higher PM2.5 exposure in the 2 weeks before the study visit and higher continuous anxiety symptoms in the child. Finally, we observed associations between prenatal PM2.5 exposure early in pregnancy and higher odds of clinically elevated depressive and anxiety symptoms.
While early life PM2.5 has been extensively linked with alterations in brain development, research has often focused on cognitive deficits23 and autism,24,25,27 with limited research relating PM2.5 to psychiatric symptoms in the late childhood/ preadolescent period. One US-based study35 examined associations between both prenatal and childhood air pollution exposure and anxiety and depressive symptoms in 12-year-olds and found impacts for both prenatal and lifetime exposure, but not concurrent exposures. A UK-based study found associations for air pollution exposure at age 12 in relation to higher depressive symptoms at age 18.29 Another European-based study assessed impacts between air pollution and dispensed medication for psychiatric disorders in children. Overall, they found that those children living in neighborhoods with higher air pollution exposure were more likely to have a medication for a psychiatric disorder.30
Few epidemiologic studies have examined the association of short-term air pollution exposure with anxiety and depressive symptoms in children and adolescents. One recent study assessed impacts of recent weekly ultrafine particle exposure and mental and physical stress in adolescents and found associations with physical stress symptoms.59 Several studies have additionally assessed short-term impacts of air pollution exposure in older, adult populations.17 For example, Power et al.21 found associations between higher PM2.5 exposure in the prior 1-month and increased anxiety symptoms in adult women in the Nurses’ Health Study. The authors concluded that short-term averaging periods were more important than the longer averaging periods. Another US-based study found associations with PM2.5 exposure averaged over the prior 6 months in relation to anxiety symptoms and associations with prior month exposures with depressive symptoms.22 Another recent study found impacts of exposures in the prior 1 and 4 weeks and increases in psychiatric symptoms in older adults.60
We hypothesized that early-life air pollution would contribute to mental health symptoms in children during pre- and postnatal time windows, and early childhood. Given that the critical window remains unknown, the life stage at which these effects are most prominent could be at any point in pregnancy, infancy, or childhood. In addition to these multiple windows explored, we used two main approaches to assess associations, including independent exposure models, and DLM approaches. Given the multiple windows and modeling approaches, we emphasized results that were robust across several different modeling approaches. Overall, the most consistent window was seen around the fourth year of life, as this was present for both continuous and dichotomized anxiety symptoms, in both independent exposure models and DLM approaches. This critical window was also observed for elevated depressive symptoms. An additional finding was for PM2.5 exposure during early pregnancy in relation to both clinically elevated anxiety and depressive symptoms, this was seen in both independent and DLM approaches. This finding was not as apparent with continuous symptoms—it could be that prenatal air pollution exposure is associated with more severe psychiatric symptoms in this population and not overall continuous anxiety and depressive symptoms. We note that overall findings were more apparent and consistent for anxiety symptoms, particularly when using DLM approaches. It is unclear why similar results were not seen when using DLM approaches with depressive symptoms, though we note that neither modeling approach is perfect and these sensitive windows should be explored further in additional studies.
There are several plausible biologic mechanisms linking air pollution exposure to clinical and subclinical psychiatric symptoms. The brain is rapidly developing during early life and several critical processes occur during these time points, including neuron formation and migration, synapse formation and pruning, generation of glial cells, and myelination.37 These processes continue through childhood and adolescence, with synaptic density peaking around age 2, when the process of synaptic pruning dramatically increases until around age 10.61 Disruptions of these critical processes secondary to toxic exposures such as air pollution during vulnerable periods alter normal brain development, potentially increasing the risk for mental health conditions.38,39 Given the peak growth of the amygdala during preadolescence, this period may be a particularly vulnerable time window for stressors that impact development of mental health outcomes, particularly emotional disorders including anxiety and depression. Short-term recent air pollution exposure could additionally trigger an existing susceptibility for anxiety or depressive symptoms through short-term changes in oxidative stress.40
One potentially important pathway includes activation of the hypothalamic pituitary adrenal (HPA) axis and its role in the body’s response to stress. Once activated, the HPA axis releases stress hormones (i.e., cortisol), which are known to have an important role in the regulation of mood.62 PM2.5 exposure, particularly during critical windows of susceptibility, may act as a stressor on the body, activating the HPA axis,63 resulting in the release of stress hormones that impact the central nervous system. Animal studies support this theory, with the finding that air pollution can activate stress centers in the brain and increase corticosterone levels in rats.64–66 A recent epidemiologic study found that chronic exposure to nitrogen dioxide was associated with a flattened diurnal cortisol slope in adolescents,67 while another study found a relation between long-term air pollution and changes in salivary cortisol.68 Future studies in the PROGRESS cohort will assess mediating pathways between PM2.5 and mental health outcomes in children, including with child temperament and cortisol levels.
A major strength of our analyses was the use of a life course analytical approach to tease out the potential critical window.42,43 Most studies addressing these exposures, even for other similar outcomes, typically only assess the exposures during one-time point—that is either during pregnancy or early childhood. We leveraged data from a longitudinal cohort study with exposure, outcome, and covariate data measured at several different points in time. This allowed us to identify the role of timing of exposure in determining childhood mental health profiles. Additionally, our team recently developed an approach using the distributed lag model to identify susceptible periods of exposure.44 The use of the distributed lag model in these analyses allowed us to model air pollution exposures simultaneously while taking other time points into account.45 The focus on continuous symptoms of anxiety and depression in our analyses was an additional strength because mental disorders vary along a continuum and first appear as subclinical symptoms. Thus, using strict diagnostic criteria may have missed more mild symptoms. Nonetheless, we also dichotomized outcomes using clinically relevant cutpoints and assessed associations with clinically elevated symptoms.
Our study additionally has limitations. As in most other air pollution epidemiology studies, we used exposure data from an area-level satellite-based air pollution model. Using an area-level estimate may induce exposure misclassification, though we note that this is often nondifferential and underestimates the true effects of air pollution.69 Personal air sampling may provide a more individualized exposure but is more susceptible to confounding by individual-level behaviors.70 Nonetheless, we will explore personalized sampling in future studies looking at recent weekly personal air pollution exposure and mental health symptoms and biologic markers of stress including cortisol. We adjusted for several key covariates, though we did not have information on other urban correlates such as environmental noise exposure. Future studies will consider additional measures of other urban correlates including noise exposure.
In summary, our results suggest that both early life and recent PM2.5 exposure is associated with mental health symptoms in preadolescents, highlighting the role of air pollution in the etiology of these conditions. Future studies should expand on these identified windows and examine mediating pathways from air pollution to mental health outcomes. PROGRESS is continuing to follow-up children over time and an important next step will be to continue to assess impacts with longitudinal measures of mental health outcomes throughout the adolescent years.
Supplementary Material
Footnotes
The authors declare that they have no conflicts of interest with regard to the content of this report.
This work was supported by the NIH (grant numbers R00ES032480, R01ES013744, R01ES021357, P30ES023515, R00ES027496, and R24ES028522).
As data used in this study contain personally identifiable information, these data will not be made available. Analytic code may be requested from the corresponding author.
Supplemental digital content is available through direct URL citations in the HTML and PDF versions of this article (www.environepidem.com).
References
- 1.Vigo D, Thornicroft G, Atun R. Estimating the true global burden of mental illness. Lancet Psychiatry. 2016;3:171–178. [DOI] [PubMed] [Google Scholar]
- 2.Bitsko RH, Holbrook JR, Ghandour RM, et al. Epidemiology and impact of health care provider-diagnosed anxiety and depression among US children. J Dev Behav Pediatr. 2018;39:395–403. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 3.Twenge JM, Cooper AB, Joiner TE, Duffy ME, Binau SG. Age, period, and cohort trends in mood disorder indicators and suicide-related outcomes in a nationally representative dataset, 2005-2017. J Abnorm Psychol. 2019;128:185–199. [DOI] [PubMed] [Google Scholar]
- 4.Whalen DJ, Sylvester CM, Luby JL. Depression and anxiety in preschoolers: a review of the past 7 years. Child Adolesc Psychiatr Clin N Am. 2017;26:503–522. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 5.WHO. Mental health of adolescents. 2018. Available at: https://www.who.int/news-room/fact-sheets/detail/adolescent-mental-health.
- 6.Bandelow B, Michaelis S. Epidemiology of anxiety disorders in the 21st century. Dialogues Clin Neurosci. 2015;17:327–335. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 7.Fuhrmann D, Knoll LJ, Blakemore SJ. Adolescence as a sensitive period of brain development. Trends Cogn Sci. 2015;19:558–566. [DOI] [PubMed] [Google Scholar]
- 8.Taylor MJ, Martin J, Lu Y, et al. Association of genetic risk factors for psychiatric disorders and traits of these disorders in a Swedish population twin sample. JAMA Psychiatry. 2019;76:280–289. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 9.Schmidt CW. Environmental connections: a deeper look into mental illness. Environ Health Perspect. 2007;115:A406–A410. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 10.Anderson S, Johnston W, Leventhal T. When neighborhoods matter: developmental timing and youth reading achievement and problem behaviors. Soc Sci Res. 2019;81:1–11. [DOI] [PubMed] [Google Scholar]
- 11.Gruebner O, Rapp MA, Adli M, Kluge U, Galea S, Heinz A. Cities and mental health. Dtsch Arztebl Int. 2017;114:121–127. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 12.United Nations. World Urbanization Prospects: The 2018 Revision. 2022. Available at: https://population.un.org/wup/Publications/Files/WUP2018-Report.pdf. [Google Scholar]
- 13.Lambert KG, Nelson RJ, Jovanovic T, Cerda M. Brains in the city: neurobiological effects of urbanization. Neurosci Biobehav Rev. 2015;58:107–122. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 14.Peen J, Schoevers RA, Beekman AT, Dekker J. The current status of urban-rural differences in psychiatric disorders. Acta Psychiatr Scand. 2010;121:84–93. [DOI] [PubMed] [Google Scholar]
- 15.Kioumourtzoglou MA. Identifying modifiable risk factors of mental health disorders-the importance of urban environmental exposures. JAMA Psychiatry. 2019;76:569–570. Available at: https://www.ncbi.nlm.nih.gov/pubmed/30916721 [DOI] [PubMed] [Google Scholar]
- 16.McGuinn LA, Ward-Caviness C, Neas LM, et al. Fine particulate matter and cardiovascular disease: comparison of assessment methods for long-term exposure. Environ Res. 2017;159:16–23. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 17.Braithwaite I, Zhang S, Kirkbride JB, Osborn DPJ, Hayes JF. Air pollution (particulate matter) exposure and associations with depression, anxiety, bipolar, psychosis and suicide risk: a systematic review and meta-analysis. Environ Health Perspect. 2019;127:126002. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 18.Sheffield PE, Speranza R, Chiu YHM, et al. Association between particulate air pollution exposure during pregnancy and postpartum maternal psychological functioning. PLoS One. 2018;13:e0195267. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 19.Niedzwiecki MM, Rosa MJ, Solano-González M, et al. Particulate air pollution exposure during pregnancy and postpartum depression symptoms in women in Mexico City. Environ Int. 2020;134:105325. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 20.Kioumourtzoglou MA, Power MC, Hart JE, et al. The association between air pollution and onset of depression among middle-aged and older women. Am J Epidemiol. 2017;185:801–809. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 21.Power MC, Kioumourtzoglou MA, Hart JE, Okereke OI, Laden F, Weisskopf MG. The relation between past exposure to fine particulate air pollution and prevalent anxiety: observational cohort study. BMJ. 2015;350:h1111. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 22.Pun VC, Manjourides J, Suh H. Association of ambient air pollution with depressive and anxiety symptoms in older adults: results from the NSHAP study. Environ Health Perspect. 2017;125:342–348. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 23.Perera FP, Li Z, Whyatt R, et al. Prenatal airborne polycyclic aromatic hydrocarbon exposure and child IQ at age 5 years. Pediatrics. 2009;124:e195–e202. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 24.McGuinn LA, Windham GC, Kalkbrenner AE, et al. Early life exposure to air pollution and autism spectrum disorder: findings from a multisite case-control study. Epidemiology. 2020;31:103–114. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 25.Kalkbrenner AE, Windham GC, Serre ML, et al. Particulate matter exposure, prenatal and postnatal windows of susceptibility, and autism spectrum disorders. Epidemiology. 2015;26:30–42. [DOI] [PubMed] [Google Scholar]
- 26.Raz R, Levine H, Pinto O, Broday DM, Yuval, Weisskopf MG. Traffic-related air pollution and autism spectrum disorder: a population-based nested case-control study in Israel. Am J Epidemiol. 2018;187:717–725. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 27.Volk HE, Lurmann F, Penfold B, Hertz-Picciotto I, McConnell R. Traffic-related air pollution, particulate matter, and autism. JAMA Psychiatry. 2013;70:71–77. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 28.Ahmed SM, Mishra GD, Moss KM, Yang IA, Lycett K, Knibbs LD. Maternal and childhood ambient air pollution exposure and mental health symptoms and psychomotor development in children: an Australian population-based longitudinal study. Environ Int. 2022;158:107003. [DOI] [PubMed] [Google Scholar]
- 29.Roberts S, Arseneault L, Barratt B, et al. Exploration of NO2 and PM25 air pollution and mental health problems using high-resolution data in London-based children from a UK longitudinal cohort study. Psychiatry Res. 2019;272:8–17. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 30.Oudin A, Braback L, Astrom DO, Stromgren M, Forsberg B. Association between neighbourhood air pollution concentrations and dispensed medication for psychiatric disorders in a large longitudinal cohort of Swedish children and adolescents. BMJ Open. 2016;6:e010004. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 31.Kusters MSW, Essers E, Muetzel R, Ambrós A, Tiemeier H, Guxens M. Air pollution exposure during pregnancy and childhood, cognitive function, and emotional and behavioral problems in adolescents. Environ Res. 2022;214:113891. [DOI] [PubMed] [Google Scholar]
- 32.Latham RM, Kieling C, Arseneault L, et al. Childhood exposure to ambient air pollution and predicting individual risk of depression onset in UK adolescents. J Psychiatr Res. 2021;138:60–67. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 33.Brokamp C, Strawn JR, Beck AF, Ryan P. Pediatric psychiatric emergency department utilization and fine particulate matter: a case-crossover study. Environ Health Perspect. 2019;127:97006. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 34.Vancil A, Strawn JR, Rasnick E, et al. Pediatric anxiety and daily fine particulate matter: a longitudinal study. Psychiatry Res Commun. 2022;2:100077. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 35.Yolton K, Khoury JC, Burkle J, LeMasters G, Cecil K, Ryan P. lifetime exposure to traffic-related air pollution and symptoms of depression and anxiety at age 12 years. Environ Res. 2019;173:199–206. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 36.Calderon-Garciduenas L, Kulesza RJ, Doty RL, D’Angiulli A, Torres-Jardon R. Megacities air pollution problems: Mexico City Metropolitan area critical issues on the central nervous system pediatric impact. Environ Res. 2015;137:157–169. [DOI] [PubMed] [Google Scholar]
- 37.Rice D, Barone S. Critical periods of vulnerability for the developing nervous system: evidence from humans and animal models. Environ Health Perspect. 2000;108(Suppl 3):511–533. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 38.Marco EM, Macrì S, Laviola G. Critical age windows for neurodevelopmental psychiatric disorders: evidence from animal models. Neurotox Res. 2011;19:286–307. [DOI] [PubMed] [Google Scholar]
- 39.Paus T, Keshavan M, Giedd JN. Why do many psychiatric disorders emerge during adolescence? Nat Rev Neurosci. 2008;9:947–957. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 40.Li W, Wilker EH, Dorans KS, et al. Short-term exposure to air pollution and biomarkers of oxidative stress: the framingham heart study. J Am Heart Assoc. 2016;5:e002742. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 41.Sagiv SK, Kalkbrenner AE, Bellinger DC. Of decrements and disorders: assessing impairments in neurodevelopment in prospective studies of environmental toxicant exposures. Environ Health. 2015;14:8. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 42.Calderon-Garciduenas L, Torres-Jardon R. Air pollution, socioeconomic status, and children’s cognition in megacities: the Mexico City scenario. Front Psychol. 2012;3:217. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 43.Braun JM, Wright RJ, Just AC, et al. Relationships between lead biomarkers and diurnal salivary cortisol indices in pregnant women from Mexico City: a cross-sectional study. Environ Health. 2014;13:50. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 44.Davanzo P, Kerwin L, Nikore V, Esparza C, Forness S, Murrelle L. Spanish translation and reliability testing of the child depression inventory. Child Psychiatry Hum Dev. 2004;35:75–92. [DOI] [PubMed] [Google Scholar]
- 45.Kovacs M. The Children’s Depression, Inventory (CDI). Psychopharmacol Bull. 1985;21:995–998. [PubMed] [Google Scholar]
- 46.Caqueo-Urízar A, Urzúa A, De Munter K. Mental health of indigenous school children in Northern Chile. BMC Psychiatry. 2014;14:11. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 47.Kovacs M; MHS Staff. Children’s Depression Inventory 2nd Edition (CDI 2) Technical Manual. Toronto, Canada: Multi-Health Systems; 2011. [Google Scholar]
- 48.Ferrando PJ. Factorial structure of the revised children manifest anxiety scale in a Spanish sample: relations with eysenck personality dimensions. Pers Individ Dif. 1994;16:693–699. [Google Scholar]
- 49.Reynolds CR, Richmond BO. Revised Children’s Manifest Anxiety Scale. 1978. Available at: http://doi.apa.org/getdoi.cfm?doi=10.1037/t00514-000. [DOI] [PubMed] [Google Scholar]
- 50.Lowe PA. The revised children’s manifest anxiety scale–second edition short form: examination of the psychometric properties of a brief measure of general anxiety in a sample of children and adolescents. J Psychoeduc Assess. 2015;33:719–730. [Google Scholar]
- 51.Gutiérrez-Avila I, Arfer KB, Carrión D, et al. Prediction of daily mean and one-hour maximum PM25 concentrations and applications in Central Mexico using satellite-based machine-learning models. J Expo Sci Environ Epidemiol. 2022;32:917–925. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 52.Carrasco AJE. The Amai System of Classifying Households by Socio-economic Level: the Experience of Mexico and Its Comparison with Brazil and Argentina 2002. 2002. Available at: www.esomar.org [Google Scholar]
- 53.Gutiérrez-Avila I, Arfer KB, Wong S, Rush J, Kloog I, Just AC. A spatiotemporal reconstruction of daily ambient temperature using satellite data in the Megalopolis of Central Mexico from 2003 to 2019. Int J Climatol. 2021;41:4095–4111. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 54.Wilson A, Chiu YM, Hsu HL, Wright RO, Wright RJ, Coull BA. Potential for bias when estimating critical windows for air pollution in children’s health. Am J Epidemiol. 2017;186:1281–1289. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 55.Gasparrini A. Modeling exposure-lag-response associations with distributed lag non-linear models. Stat Med. 2014;33:881–899. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 56.Chiu YHM, Coull BA, Sternthal MJ, et al. Effects of prenatal community violence and ambient air pollution on childhood wheeze in an urban population. J Allergy Clin Immunol. 2014;133:713–22.e4. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 57.Lee A, Leon Hsu HH, Mathilda Chiu YH, et al. Prenatal fine particulate exposure and early childhood asthma: effect of maternal stress and fetal sex. J Allergy Clin Immunol. 2018;141:1880–1886. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 58.WHO. WHO Global Air Quality Guidelines. 2022. Available at: https://www.who.int/publications/i/item/9789240034228 [Google Scholar]
- 59.Turner AL, Brokamp C, Wolfe C, Reponen T, Brunst KJ, Ryan PH. Mental and physical stress responses to personal ultrafine particle exposure in adolescents. Int J Environ Res Public Health. 2022;19:7509. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 60.Qiu X, Danesh-Yazdi M, Weisskopf M, et al. Associations between air pollution and psychiatric symptoms in the normative aging study. Environ Res Lett. 2022;17:034004. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 61.Paolicelli RC, Bolasco G, Pagani F, et al. Synaptic pruning by microglia is necessary for normal brain development. Science. 2011;333:1456–1458. [DOI] [PubMed] [Google Scholar]
- 62.Ardayfio P, Kim KS. Anxiogenic-like effect of chronic corticosterone in the light-dark emergence task in mice. Behav Neurosci. 2006;120:249–256. [DOI] [PubMed] [Google Scholar]
- 63.Henriquez AR, Snow SJ, Schladweiler MC, et al. Adrenergic and glucocorticoid receptor antagonists reduce ozone-induced lung injury and inflammation. Toxicol Appl Pharmacol. 2018;339:161–171. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 64.Thomson EM. Air pollution, stress, and allostatic load: linking systemic and central nervous system impacts. J Alzheimers Dis. 2019;69:597–614. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 65.Thomson EM, Filiatreault A, Guénette J. Stress hormones as potential mediators of air pollutant effects on the brain: rapid induction of glucocorticoid-responsive genes. Environ Res. 2019;178:108717. [DOI] [PubMed] [Google Scholar]
- 66.Sirivelu MP, MohanKumar SMJ, Wagner JG, Harkema JR, MohanKumar PS. Activation of the stress axis and neurochemical alterations in specific brain areas by concentrated ambient particle exposure with concomitant allergic airway disease. Environ Health Perspect. 2006;114:870–874. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 67.Wing SE, Bandoli G, Telesca D, Su JG, Ritz B. Chronic exposure to inhaled, traffic-related nitrogen dioxide and a blunted cortisol response in adolescents. Environ Res. 2018;163:201–207. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 68.Hajat A, Hazlehurst MF, Golden SH, et al. The cross-sectional and longitudinal association between air pollution and salivary cortisol: evidence from the multi-ethnic study of atherosclerosis. Environ Int. 2019;131:105062. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 69.Zeger SL, Thomas D, Dominici F, et al. Exposure measurement error in time-series studies of air pollution: concepts and consequences. Environ Health Perspect. 2000;108:419–426. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 70.Weisskopf MG, Webster TF. Trade-offs of personal versus more proxy exposure measures in environmental epidemiology. Epidemiology. 2017;28:635–643. [DOI] [PMC free article] [PubMed] [Google Scholar]