Abstract
PURPOSE
High hyperdiploidy, the largest and favorable subtype of childhood ALL, exhibits significant biological and prognostic heterogeneity. However, factors contributing to the varied treatment response and the optimal definition of hyperdiploidy remain uncertain.
METHODS
We analyzed outcomes of patients treated on two consecutive frontline ALL protocols, using six different definitions of hyperdiploidy: chromosome number 51-67 (Chr51-67); DNA index (DI; DI1.16-1.6); United Kingdom ALL study group low-risk hyperdiploid, either trisomy of chromosomes 17 and 18 or +17 or +18 in the absence of +5 and +20; single trisomy of chromosome 18; double trisomy of chromosomes 4 and 10; and triple trisomy (TT) of chromosomes 4, 10, and 17. Additionally, we characterized ALL ex vivo pharmacotypes across eight main cytotoxic drugs.
RESULTS
Among 1,096 patients analyzed, 915 had B-ALL and 634 had pharmacotyping performed. In univariate analysis, TT emerged as the most favorable criterion for event-free survival (EFS; 10-year EFS, 97.3% v 86.8%; P = .0003) and cumulative incidence of relapse (CIR; 10-year CIR, 1.4% v 8.8%; P = .002) compared with the remaining B-ALL. In multivariable analysis, accounting for patient numbers using the akaike information criterion (AIC), DI1.16-1.6 was the most favorable criterion, exhibiting the best AIC for both EFS (hazard ratio [HR], 0.45; 95% CI, 0.23 to 0.88) and CIR (HR, 0.45; 95% CI, 0.21 to 0.99). Hyperdiploidy and subgroups with favorable prognoses exhibited notable sensitivities to asparaginase and mercaptopurine. Specifically, asparaginase sensitivity was associated with trisomy of chromosomes 16 and 17, whereas mercaptopurine sensitivity was linked to gains of chromosomes 14 and 17.
CONCLUSION
Among different definitions of hyperdiploid ALL, DI is optimal based on independent prognostic impact and also the large proportion of low-risk patients identified. Hyperdiploid ALL exhibited particular sensitivities to asparaginase and mercaptopurine, with chromosome-specific associations.
We assessed survival outcomes and drug sensitivities across various definitions of hyperdiploidy in childhood ALL.
INTRODUCTION
ALL, the most common childhood cancer, comprises a constellation of heterogeneous subtypes.1,2 High hyperdiploidy (typically defined as 51-65 or 67 chromosomes) is the largest subtype, accounting for approximately 30%-35% of B-ALL.3,4 Although generally associated with excellent survival with most patients treated on a low-intensity regimen,5 there is a wide heterogeneity of biology across hyperdiploidy, with disparate treatment response and outcomes among subgroups.6-10 Although higher modal numbers of chromosomes are generally associated with a better prognosis, the exact chromosomal modal group with the best prognosis remains unclear.3,9-11 Specific patterns of chromosomal gains, such as individual trisomies of +6,12 +17,13 or +18,6 were linked to better outcomes, and simultaneous double trisomies of +4 and +10 or triple trisomies of +4, +10, and +17 have been found to confer an exceptionally good prognosis.8,14-16 In fact, it has been posited that these specific trisomies, rather than increased chromosome number, are the main drivers of good outcome in hyperdiploidy.8,16 More recently, the United Kingdom ALL (UK-ALL) study group has defined a good risk subgroup comprising karyotypes with +17 and +18 or +17 or +18 in the absence of +5 and +20.7 Overall, the prognosis of various subgroups are not always consistently replicated across studies, and consequently, trial groups use different criteria to define this low-risk group for treatment deintensification.
CONTEXT
Key Objective
We sought to determine the optimal definition of hyperdiploidy in childhood ALL that identified patients with the best prognosis, to aid in treatment deintensification. Additionally, we investigated the relationship between hyperdiploidy and drug sensitivities, specifically if these associations occur in a chromosome-specific manner.
Knowledge Generated
DNA-index appears to be the most effective approach, identifying a substantial group of patients with excellent prognosis, maximizing the impact on patient outcomes by considering the balance between patient numbers and favorable treatment outcomes. Hyperdiploid ALL exhibits distinct sensitivities to asparaginase and mercaptopurine, with chromosome-specific associations observed.
Relevance (S. Bhatia)
-
Identifying subgroups of patients with excellent prognosis provides the evidence needed for treatment deintensification and reduction in long-term morbidity.*
*Relevance section written by JCO Associate Editor Smita Bhatia, MD, MPH, FASCO.
Additionally, the biological basis of these differences in prognosis remains enigmatic. The excellent prognosis of hyperdiploidy overall as a subtype has been attributed in part to its favorable drug response profile in comparison with nonhyperdiploidy,17-23 although nondrug-related mechanisms are also thought to be important, such as propensity for spontaneous apoptosis and decreased proliferative ability.24-26 However, the drug sensitivity profile of subgroups of hyperdiploidy, for example, triple trisomy (TT) or higher modal numbers, and their impact on treatment response or outcomes have not yet been characterized. The functional impact of individual or patterns of chromosomal gains remains poorly understood, particularly from a drug sensitivity standpoint.
Moreover, there is no clear agreement on the optimal method of defining high hyperdiploidy and its subgroups.27 Traditional definition of hyperdiploidy using chromosome number using conventional G-banded karyotypic profiling requires a high level of technical expertise, with varying degrees of availability across hospitals.11 DNA index (DI), which is a surrogate measure of the amount of DNA inside leukemic cells,28 can be performed more quickly and with greater technical ease29; however, it cannot identify individual chromosome gain or loss.27,30 Overall, the utility of these methods for the diagnosis of hyperdiploidy and its subgroups have not yet been directly compared. Therefore, the clinical question of which method is the most optimal for defining hyperdiploidy for low-risk stratification? remains unanswered.
To these ends, we conducted comprehensive comparison of the survival outcomes among various definitions of hyperdiploidy. Additionally, we aimed to determine the correlations of hyperdiploidy with drug sensitivity profiles to uncover pharmacological factors affecting prognosis. Here, we systematically examined a cohort of 1,096 children with newly diagnosed ALL treated under two consecutive Total Therapy trials from 2000 to 2017,31,32 of whom 634 children had pharmacotyping performed on primary ALL cells. We analyzed survival outcomes according to different hyperdiploid definitions and characterized their ex vivo pharmacotypes across eight main cytotoxic drugs used in ALL therapy.
METHODS
Patient and Clinical Treatment Protocols
The 1,096 children and adolescents (915 B-ALL; 181 T-ALL) were treated on two consecutive ALL Total Therapy protocols, Total XV31 (ClinicalTrials.gov identifier: NCT00137111) and XVI32 (ClinicalTrials.gov identifier: NCT00549848), at St Jude Children's Research Hospital. This study was approved by the institutional review board, and written informed consent was obtained from parents, guardians, and/or patients, as appropriate. Details of leukemia immunophenotyping, genomic profiling, and minimal residual disease (MRD) testing are provided in the Data Supplement (online only).
DI, Karyotyping, and Classification of Hyperdiploid Groups
Diagnostic pretreatment bone marrow and/or peripheral blood samples were analyzed by standard G-banded karyotyping methods. All karyotypes were curated to exclude masked hypodiploidy from the hyperdiploid cohort. Hyperdiploidy was defined as chromosome number 51-67 inclusive (Chr51-67), and was examined across two (Chr51-67 and remainder) or three (Chr51-55 [Chr51-55], 56-67 [Chr56-67], and remainder)10,33 predefined categories.
Flow cytometry was used to derive the DI, which represents the ratio of DNA content in leukemic G0/G1 cells compared with normal diploid cells, which was then used to distinguish prognostic categories, as previously described.34 Hyperdiploidy was defined as DI ≥1.16-1.6 (DI1.16-1.6),29 and we examined DI across two predefined categories (≥1.16 to 1.6 and remainder).
Only patients with B-ALL were included in a hyperdiploid criterion. Patients with concomitant BCR::ABL1, ETV6::RUNX1, or TCF3::PBX1 fusions or rearranged KMT2A were excluded from the hyperdiploid group, as they were regarded as the primary genetic abnormality with prognostic and therapeutic impact potentially overriding the beneficial effect of the high hyperdiploidy.6 Additionally, we classified patients into various hyperdiploid groups based on the following: (1) TT, defined by simultaneous trisomy of chromosomes 4, 10 and 17; (2) double trisomy (DT), by simultaneous trisomy of 4 and 10; (3) UK-ALL low-risk hyperdiploid (UK-ALL)7 by either simultaneous trisomy of 17 and 18 or +17 or +18 in the absence of +5 and +20. We also examined individual single chromosomal gains from +1 to +X.
RNA Sequencing and Subtype Calling
RNA sequencing and subtype calling were performed as previously described.5,35 Full details are described in the Data Supplement.
Pharmacotyping of Primary ALL Cells
Primary ALL cells from 634 patients underwent ex vivo pharmacotyping, 526 of whom had B-ALL.23 Details of the ex vivo drug panel (Data Supplement, Table S1) and assays are elaborated further in the Data Supplement.
Statistical Analysis
The association between drug LC50s and hyperdiploidy, along with its subgroups or clinical features, were assessed using the two-sided Mann-Whitney test or Kruskal-Wallis test. For categorical variables, the Chi-square or Fisher exact test were used. Treatment outcomes were examined as event-free survival (EFS) or cumulative incidence of relapse (CIR). We also examined overall survival (OS) as a secondary outcome. We evaluated associations between hyperdiploidy definitions or their subgroups and EFS or OS using the log-rank test and for CIR using the Gray test. Multivariable analysis of EFS or OS was performed with the Cox hazard regression model36 and for CIR using the Fine-Gray hazard rate regression model.37 Akaike information criteria (AIC) were applied to evaluate model fitness. Full details of statistical analysis across these survival end points are elaborated further in the Data Supplement.
All analyses were performed with R (version 3.6.3), SAS software (version 9.4; SAS Institute, Cary, NC), or GraphPad Prism (version 9.0.0). All statistical tests were two-sided, and P values < .05 were considered nominally significant.
RESULTS
Comparison of Different ALL Hyperdiploidy Definitions and Their Prognostic Impact in B-ALL
We first sought to compare six definitions of hyperdiploid subtype of ALL currently used by different cooperative study groups to determine if there was a criterion that would be the most optimal for identifying the patient group with the best prognosis. The analyses were performed among the 915 patients with B-lineage ALL treated on two consecutive frontline trials at St Jude Children's Research Hospital (Table 1). There was significant overlap between hyperdiploid cases defined by different criteria but with large variation in numbers, and the intersectionality between each criterion are shown in Data Supplement (Fig S1). Two hundred eighty-one patients (30.7%) had chromosome count of 51-67 (Chr51-67); 230 patients (25.3%) had DI1.16-1.6; 220 patients (24.0%) were classified UK-ALL low-risk hyperdiploidy (UK-ALL); 201 patients (22.0%) had trisomy 18; 176 patients (19.2%) had DT; and 148 patients (16.2%) had TT. Only 123 patients (13.4%) were classified as hyperdiploid by all six definitions. All hyperdiploid groups had a higher proportion of patients treated on the low-risk arm compared with the standard/high-risk arm (Data Supplement, Table S2).
TABLE 1.
Clinical Characteristics of 915 Patients With B-ALL in Total Therapy XV and XVI Cohorts

Since MRD is a key factor in risk stratification in ALL therapy, we first evaluated differences in MRD proportions at day 15 (mid-induction) and day 42 (end-of-induction [EOI]) by hyperdiploidy classifications (Data Supplement, Table S3). At day 15, DI1.16-1.6 and TT had the highest proportions of MRD negativity (42.2% and 42.6%, respectively). At day 42, TT had the highest proportion of MRD negativity (96.0%).
All definitions of hyperdiploidy demonstrated favorable, statistically significant survival rates for EFS and CIR, except Chr51-67 for only EFS, as compared with other B-ALL cases (Table 2; Data Supplement, Tables S4 and S5). TT patients had the best absolute EFS (10-year EFS, 97.3% v 86.8%; P = .0003), and Chr51-67 had the lowest (93.2% v 86.3%; P = .001). For CIR, TT patients again had the lowest relapse rate versus non-TT patients (10-year CIR, 1.4% v 8.8%; P = .002), and only Chr51-67 did not meet a significant difference for CIR (5.4% v 8.7%; P = .054). For both end points, Chr56-67 trended for better outcomes over Chr51-55, although this difference was not statistically significant likely due to small numbers of events within hyperdiploidy (Data Supplement, Table S6).
TABLE 2.
Ten-Year EFS and Relapse Probabilities by Hyperdiploid Criterion
We performed multivariable regression to account for clinical prognostic features of age at diagnosis, presenting leukocyte count, and EOI MRD. We evaluated AIC to determine the best model fit for our data (lower is better). The only factor changing among the multivariable models is the definition of hyperdiploidy; therefore, the lowest AIC helped to identify the best definition of hyperdiploidy in association with outcome (Table 3). For EFS, DI1.16-1.6 had the lowest (ie, best) AIC at 1,282 and remained significant after multivariable adjustment (hazard ratio [HR], 0.45; 95% CI, 0.23 to 0.88; P = .02). By contrast, Chr51-67 and trisomy 18 had the highest (ie, poorest) AIC (both at 1,300) and no prognostic significance (P = .17 and P = .258, respectively). For CIR, DI again had the lowest AIC of 863 with independent significance (HR, 0.45; 95% CI, 0.21 to 0.99; P = .046), and Chr51-67 had the highest AIC of 879 and no prognostic significance (P = .446). We also assessed OS as a secondary end point (Data Supplement, Tables S7 and S8) and obtained similar trends. Taken together, DI was consistently the most significant prognostic factor in multivariable analysis; by contrast, Chr51-67, trisomy 18, and UK-ALL low-risk hyperdiploid group did not remain statistically significant after adjusting for clinical prognostic factors.
TABLE 3.
Multivariable Analysis of EFS and Relapse Probabilities Across Hyperdiploid Criterion
In Total Therapy studies, although we performed karyotyping and identified patients with Chr51-67, we used DNA index (ie, DI1.16-1.6) for risk stratification because this measure is readily available in our center. Within this patient group classified as hyperdiploid (n = 295) using either criterion, the presence of DI ≥1.16, DT, or TT further stratified patients into subgroups with significantly better outcomes (Data Supplement, Table S9). Parsing individual chromosomal gains, the presence of +17 was associated with a better EFS (95.7% v 88.6%; P = .016) and CIR (2.9% v 10.3%; P = .005) compared with those without, and +10 was associated with a lower CIR (3.1% v 9.8%; P = .024; Data Supplement, Table S10). Therefore, within the hyperdiploid subtype, the presence of specific trisomies or individual chromosomal gains was able to further identify patients with even more favorable outcomes.
Leukemia Pharmacotypic Profiles Across Hyperdiploid ALL
Next, we sought to understand if differential sensitivities to cytotoxic drugs may explain the heterogeneous prognoses across hyperdiploidy. We chose to focus on the eight main cytotoxic drugs used in contemporary ALL protocols worldwide—asparaginase, cytarabine, daunorubicin, dexamethasone, mercaptopurine, prednisolone, thioguanine, and vincristine. Our pharmacotyping cohort consisted of 634 patients, of whom 524 had B-ALL and 140 had hyperdiploidy (26.7% of B-ALL). Other than a higher WBC count at diagnosis in the pharmacotyped cohort, there were no significant differences in clinical characteristics between the cohorts included or excluded in pharmacotyping (Data Supplement, Table S11).
Across the B-ALL cohort, patients with DI1.16-1.6 demonstrated significantly higher sensitivity to asparaginase (LC50 0.26 v 0.52; P = .025) and cytarabine (LC50 0.44 v 0.55; P = .008) compared with other patients (Data Supplement, Fig S2), plausibly contributing to their excellent prognosis. Since higher modal numbers trended for better prognosis in our cohort and in other studies,9-11 we also explored whether the subgroup with chromosome number 56-67 (Chr56-67) may demonstrate better drug sensitivity. Comparison of Chr51-55 versus Chr56-67 showed that the latter group demonstrated higher sensitivity to several drugs: asparaginase (LC50 0.48 v 0; P = .023), mercaptopurine (LC50 0.55 v 0.45; P = .025), cytarabine (LC50 0.55 v 0.41; P = .002), and thioguanine (LC50 0.35 v 0.24; P = .009; Fig 1). We also found similar differential drug sensitivities of hyperdiploidy with TT to these drugs (Data Supplement, Fig S3), also plausibly explaining this subgroup's outstanding prognosis. Taken overall, asparaginase, cytarabine, and thiopurines demonstrated differential sensitivity in hyperdiploid ALL and may contribute to the excellent outcomes in this group.
FIG 1.

Comparison of drug LC50s for Chr51-55 versus Chr56-67. Drug LC50s of all eight cytotoxic drugs are plotted for Chr51-55 (blue) versus Chr56-67 (red) in violin plots, with the median for each group indicated as a bold black horizontal line. Lower LC50s indicate higher drug sensitivity, and higher LC50s indicate higher drug resistance. P values are determined by the two-sided Mann-Whitney test and are indicated directly above each respective comparison.
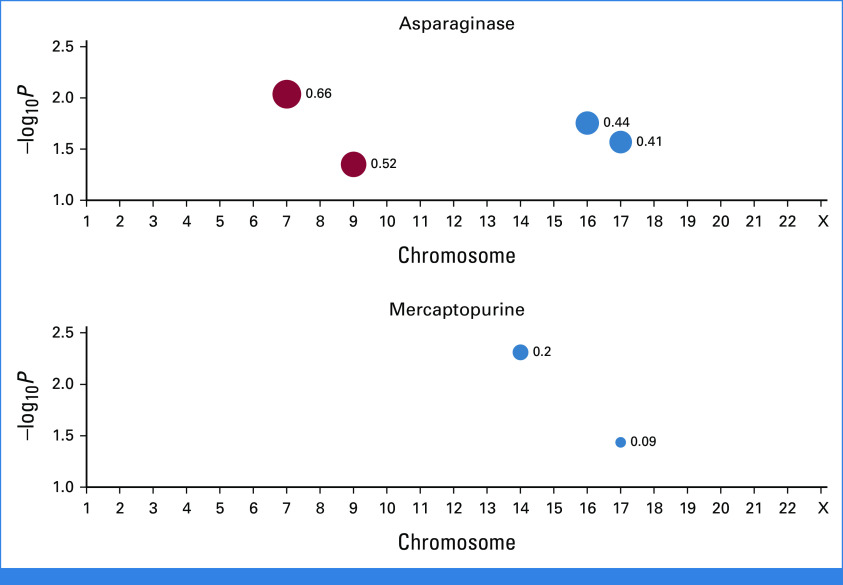
Finally, we sought to evaluate whether associations with sensitivities to these drugs arose in a chromosome-specific fashion. To this end, we examined drug sensitivities of asparaginase, cytarabine, mercaptopurine, and thioguanine across the entire range of trisomy from +1 to +X (Fig 2). Here, we compared the delta (δ) that is difference between median LC50s of patients with a given chromosome gain versus without. A positive delta reflects a higher median LC50 (ie, higher resistance) for patients with that specific chromosome gain while a negative delta indicates a lower median LC50 (increased sensitivity), with P values evaluated using the two-sided Mann-Whitney test. Chr 7 (δ, +0.66; P = .009) and Chr 9 (δ, +0.52; P = .045) were associated with asparaginase resistance while Chr 16 (δ, –0.44; P = .018) and Chr 17 (δ, –0.41; P = .027) with asparaginase sensitivity. Chr 14 (δ, –0.20; P = .005) and Chr 17 (δ, –0.09; P = .037) were associated with mercaptopurine sensitivity. Taken together, these reflected chromosome-specific associations in drug sensitivities in hyperdiploidy.
FIG 2.
Association of drug sensitivities of asparaginase and mercaptopurine with individual chromosome gain among hyperdiploid patients. Among hyperdiploid patients, the delta (δ) that is absolute difference of median LC50s for patients with a given chromosome gain versus the remainder of hyperdiploid patients is derived. A positive delta is shown as a red dot and reflects a higher median LC50 (ie, higher resistance) for patients with that specific chromosome gain while a negative delta is shown as a blue dot and indicates a lower median LC50 (increased sensitivity). The size of the dot is proportional to the magnitude of the delta, which is shown as a label for each respective dot. P values (indicated as –log10P on y-axis) are plotted against each respective chromosome on the x-axis. P values are evaluated by the two-sided Mann-Whitney test, and only associations with P < .05 are shown. For example, +7 is significantly associated with asparaginase resistance while +17 is significantly associated with asparaginase sensitivity.
DISCUSSION
Pediatric oncologists have long grappled with a practical question: Which method is optimal for defining hyperdiploidy? This is particularly relevant in resource-limited countries, where this patient group can be effectively treated with deintensified therapy, resulting in reduced toxicities and resource demands. However, no comprehensive comparative analysis has addressed this question so far. In our univariate analysis, all assessed definitions of hyperdiploidy demonstrated excellent outcomes, with only minor differences among them. This similarity can be attributed to significant overlap among these patient populations. In this regard, TT identified the population with the highest survival rates, but it only detected hyperdiploidy in 16.2% of patients, whereas the DI method encompassed 25.3% of patients. The classification criterion Chr51-67 identified the largest group of hyperdiploid patients, but it included a subset of patients with relatively poorer outcomes. Notably, patients who were Chr51-67 but did not meet the DI1.16-1.6 criteria did not exhibit significantly superior EFS compared with the rest of B-ALL group (Data Supplement, Table S12). Overall, our results suggest that DI may be the most optimal definition of hyperdiploidy because it identifies a sufficiently large group with excellent prognosis, achieving maximal impact by balancing patient numbers and favorable outcomes. Moreover, DI offers inherent technical advantages such as lower cost, ease of implementation, and relatively quick turnaround time. However, whenever possible, genomic testing methods that allow for identification of specific trisomies (eg, RNAseq, FISH, etc) should also be used. This approach should enable more precise treatment, such as the potential for therapy de-escalation on the basis of the presence of certain prognostic chromosomes (eg, +17) while excluding (+7 or +9), as demonstrated in our study.
Although we found that the more favorable outcomes of higher modal numbers (ie, Chr56-67 compared with Chr51-55) might be attributable to the good ex vivo drug sensitivities of this group to asparaginase and antimetabolites, pharmacotype does not appear to account for the good prognostic outcomes of several other criteria. For example, although the favorable drug sensitivities to asparaginase and thiopurines plausibly accord TT its excellent prognosis, there were no significant associations of drug sensitivities with trisomy 186 or the UK-ALL low-risk hyperdiploid group7 that might account for their reported better outcomes. Therefore, it is plausible that the excellent prognosis of these other criteria are more attributable to factors unrelated to drug response, such as decreased intrinsic proliferative ability,26 general cell instability, or increased propensity for spontaneous apoptosis.24,25 In the context of exploring chemotherapy deintensification in all these putatively lower-risk groups, it becomes crucial to understand the molecular effects of these chromosomal gains because this may affect the decisions of drug intensification (or deintensification). For example, the Children's Oncology Group AALL0331 found no additional benefit from intensifying polyethylene glycol-conjugated asparaginase in the lowest-risk groups comprising triple trisomies.15 In another analysis of a group of National Cancer Institute standard risk B-ALL with rapid early response, discontinuation of asparaginase because of hypersensitivity did not adversely affect the excellent outcome.38 Therefore, taken together, it could be postulated that dose reduction, rather than intensification, may be considered in patients with exquisite drug-sensitive profile. For this reason, understanding the true association of chromosomal gains with drug sensitivities thus becomes clinically relevant to plan such strategies.
Unbiased screening of each chromosome gain allowed us to delineate association between specific chromosomes and particular drug sensitivities or resistance. The biological mechanisms behinds these associations are uncertain, but additional specific chromosomes may influence sensitivity through varied expression of drug metabolism-associated genes.20 Although TT exhibited significant sensitivities to asparaginase, mercaptopurine, and thioguanine, it is surprising that only +17, and not +4 and +10, showed an association with mercaptopurine and asparaginase when each trisomy was analyzed separately. We postulate that this could be attributed to chromosome 17 being the primary driver of drug sensitivities in hyperdiploid ALL, whereas +4 and +10 contribute to favorable prognosis through separate mechanisms unrelated to drug sensitivity. Therefore, ex vivo pharmacotyping, in addition to genomic characterization of chromosomal gains, may prove valuable in informing personalized treatment approaches. The comprehension of the functional implications associated with specific trisomies or chromosome gains plays a pivotal role in the development of pharmacogenomic biomarkers, where drug therapy is optimized according to the presence or absence of certain chromosomes. Indeed, we found that hyperdiploid patients with gains of drug sensitivity–associated chromosomes (either 14, 16 or 17) exhibited better survival rates compared with those with gains of drug resistance–associated chromosomes (namely, 7 or 9; Data Supplement, Table S13). These findings highlight a prognostic impact that extends beyond the mere correlations with drug sensitivity. Furthermore, identification of certain prognostic fusions or epigenetic alterations such as SETD2 alterations39 is crucial for identifying hyperdiploid cases with potentially poorer outcome.
In conclusion, we have comprehensively evaluated the prognostic impact of different hyperdiploidy definitions and profiled drug sensitivity of its various subgroups. Our study unravels the pharmacological basis for heterogeneity within this largest subtype of childhood ALL. Our findings provide a practical foundation for determining the optimal definition of hyperdiploidy, assisting study groups in selecting diagnostic methods. Additionally, our study contributes to the growing body of evidence emphasizing the importance of identifying specific chromosomes associated with a poorer prognosis and drug resistance in this particular subtype.
ACKNOWLEDGMENT
The authors thank the patients and parents who participated in this study and the clinicians and research staff at participating institutions.
Emily Ashcraft
Employment: Cleveland Clinic, St Jude Children's Research Hospital
Kathryn G. Roberts
Stock and Other Ownership Interests: Amgen
Hiroto Inaba
Consulting or Advisory Role: Servier, Jazz Pharmaceuticals, Amgen
Research Funding: Servier
Seth E. Karol
Consulting or Advisory Role: Kura Oncology, Servier, Jazz Pharmaceuticals
Charles G. Mullighan
Stock and Other Ownership Interests: Amgen
Honoraria: Amgen, Illumina
Consulting or Advisory Role: Illumina, Faze Medicines, Beam Therapeutics
Speakers' Bureau: Amgen, Pfizer
Research Funding: Loxo, Pfizer, AbbVie
Patents, Royalties, Other Intellectual Property: Inventor on a pending patent application related to gene-expression signatures for detection of underlying Philadelphia chromosome-like events and therapeutic targeting in leukemia (PCT/US2012/069228), WO 2021/022076 A1. This patent highlight shows representative PROTAC compounds bound to JAK2, where ruxolitinib and baricitinib bind to the human JAK2 JH1. Furthermore, representative data illustrate protein degradation, cytotoxicity, and effect of the JAKSTAT signaling pathway of the PROTAC compounds in MHHCALL-4 cells, Marcus FISHER, Fatemeh KERAMATNIA, Kevin MCGOWAN, Jaeki MIN, Gisele A. NISHIGUCHI, Jeanine PRICE, Zoran RANKOVIC, Das SOURAV, Charles G. MULLIGHAN, Yunchao CHANG 2021 SUBSTITUTED N-(2-(2,6-DIOXOPIPERIDIN-3-YL)-1,3-DIOXOISOINDOLIN-5-YL)ARYLSULFONAMIDE ANALOGS AS MODULATORS OF CEREBLON PROTEIN, Application No: PCT/US2021/051648 Filed: September 23, 2021. Patent pending (Inst)
Travel, Accommodations, Expenses: Amgen, Illumina
Mary V. Relling
Stock and Other Ownership Interests: Bioskryb
Research Funding: Servier
William E. Evans
Stock and Other Ownership Interests: BioSkryb
Cheng Cheng
This author is a member of the Journal of Clinical Oncology Editorial Board. Journal policy recused the author from having any role in the peer review of this manuscript.
Jun J. Yang
Employment: St Jude Children's Research Hospital
Research Funding: Takeda (Inst), AstraZeneca (Inst)
Patents, Royalties, Other Intellectual Property: Compositions and methods comprising substituted kinase inhibitor PROTACs
Ching-Hon Pui
Leadership: Adaptive Biotechnologies
Honoraria: Novartis, Amgen
Consulting or Advisory Role: Adaptive Biotechnologies
Research Funding: National Cancer Institute
Travel, Accommodations, Expenses: Amgen
No other potential conflicts of interest were reported.
See accompanying Article, p. 5433
DISCLAIMER
The content is solely the responsibility of the authors and does not necessarily represent the official views of the National Institutes of Health. The funding agencies were not directly involved in the design of the study, gathering, analysis and interpretation of the data, writing of the manuscript, or decision to submit the manuscript for publication.
SUPPORT
Supported by the American Lebanese Syrian Associated Charities; Singapore National Medical Research Council Research Training Fellowship MOH-000302 (to S.H.R.L); and National Institutes of Health grant nos. R35 CA197695 (to C.G.M), R01 CA264837 (to J.J.Y), U01 CA264610 (to J.J.Y), R35 GM141947 (to J.J.Y), P30 CA021765 (St Jude Children's Research Hospital [SJCRH] Cancer Center Support Grant) and P50 GM115279 (Center for Precision Medicine of Leukemia Grant, to C.C., M.V.R., C.G.M., W.E.E. and J.J.Y). C.G.M. is the William E. Evans Endowed Chair, J.J.Y. is the Endowed Chair of Pharmacogenomics, and C.H.-P. is the Fahad Nassar Al-Rashid Chair of Leukemia Research at SJCRH.
J.J.Y. and C.-H.P. contributed equally to this work as senior authors.
DATA SHARING STATEMENT
RNAseq and pharmacotyping data are available as per detailed in our previous publication (SHR Lee et al Nat Med 2023). Measured drug sensitivities and clinical data are available in the Data Supplement (Table S1) of that paper. RNAseq data have been deposited in the European Genome-phenome Archive under accession nos. EGAS00001001952, EGAS00001001923, EGAS00001000447, EGAS00001000654, EGAS00001003266, EGAS00001004739, EGAS00001005084, and EGAS00001006336. Data are also available at St Jude Cloud Genomics Platform for the Pan-Acute Lymphoblastic Leukemia data set and the Real-time Clinical Genomics data set.
AUTHOR CONTRIBUTIONS
Conception and design: Shawn H.R. Lee, Yoshihiro Gocho, Sima Jeha, William E. Evans, Jun J. Yang, Ching-Hon Pui
Financial support: Ching-Hon Pui
Administrative support: Ching-Hon Pui
Provision of study materials or patients: Hiroto Inaba, Seth E. Karol, Sima Jeha, Kristine R. Crews, Charles G. Mullighan, Ching-Hon Pui
Collection and assembly of data: Shawn H.R. Lee, Emily Ashcraft, Wenjian Yang, Kathryn G. Roberts, Yoshihiro Gocho, Lauren Rowland, Hiroto Inaba, Seth E. Karol, Kristine R. Crews, Charles G. Mullighan, Mary V. Relling, Jun J. Yang, Ching-Hon Pui
Data analysis and interpretation: Shawn H.R. Lee, Emily Ashcraft, Wenjian Yang, Yoshihiro Gocho, Cheng Cheng, Jun J. Yang, Ching-Hon Pui
Manuscript writing: All authors
Final approval of manuscript: All authors
Accountable for all aspects of the work: All authors
AUTHORS' DISCLOSURES OF POTENTIAL CONFLICTS OF INTEREST
Prognostic and Pharmacotypic Heterogeneity of Hyperdiploidy in Childhood ALL
The following represents disclosure information provided by authors of this manuscript. All relationships are considered compensated unless otherwise noted. Relationships are self-held unless noted. I = Immediate Family Member, Inst = My Institution. Relationships may not relate to the subject matter of this manuscript. For more information about ASCO's conflict of interest policy, please refer to www.asco.org/rwc or ascopubs.org/jco/authors/author-center.
Open Payments is a public database containing information reported by companies about payments made to US-licensed physicians (Open Payments).
Emily Ashcraft
Employment: Cleveland Clinic, St Jude Children's Research Hospital
Kathryn G. Roberts
Stock and Other Ownership Interests: Amgen
Hiroto Inaba
Consulting or Advisory Role: Servier, Jazz Pharmaceuticals, Amgen
Research Funding: Servier
Seth E. Karol
Consulting or Advisory Role: Kura Oncology, Servier, Jazz Pharmaceuticals
Charles G. Mullighan
Stock and Other Ownership Interests: Amgen
Honoraria: Amgen, Illumina
Consulting or Advisory Role: Illumina, Faze Medicines, Beam Therapeutics
Speakers' Bureau: Amgen, Pfizer
Research Funding: Loxo, Pfizer, AbbVie
Patents, Royalties, Other Intellectual Property: Inventor on a pending patent application related to gene-expression signatures for detection of underlying Philadelphia chromosome-like events and therapeutic targeting in leukemia (PCT/US2012/069228), WO 2021/022076 A1. This patent highlight shows representative PROTAC compounds bound to JAK2, where ruxolitinib and baricitinib bind to the human JAK2 JH1. Furthermore, representative data illustrate protein degradation, cytotoxicity, and effect of the JAKSTAT signaling pathway of the PROTAC compounds in MHHCALL-4 cells, Marcus FISHER, Fatemeh KERAMATNIA, Kevin MCGOWAN, Jaeki MIN, Gisele A. NISHIGUCHI, Jeanine PRICE, Zoran RANKOVIC, Das SOURAV, Charles G. MULLIGHAN, Yunchao CHANG 2021 SUBSTITUTED N-(2-(2,6-DIOXOPIPERIDIN-3-YL)-1,3-DIOXOISOINDOLIN-5-YL)ARYLSULFONAMIDE ANALOGS AS MODULATORS OF CEREBLON PROTEIN, Application No: PCT/US2021/051648 Filed: September 23, 2021. Patent pending (Inst)
Travel, Accommodations, Expenses: Amgen, Illumina
Mary V. Relling
Stock and Other Ownership Interests: Bioskryb
Research Funding: Servier
William E. Evans
Stock and Other Ownership Interests: BioSkryb
Cheng Cheng
This author is a member of the Journal of Clinical Oncology Editorial Board. Journal policy recused the author from having any role in the peer review of this manuscript.
Jun J. Yang
Employment: St Jude Children's Research Hospital
Research Funding: Takeda (Inst), AstraZeneca (Inst)
Patents, Royalties, Other Intellectual Property: Compositions and methods comprising substituted kinase inhibitor PROTACs
Ching-Hon Pui
Leadership: Adaptive Biotechnologies
Honoraria: Novartis, Amgen
Consulting or Advisory Role: Adaptive Biotechnologies
Research Funding: National Cancer Institute
Travel, Accommodations, Expenses: Amgen
No other potential conflicts of interest were reported.
REFERENCES
- 1. Hunger SP, Mullighan CG. Acute lymphoblastic leukemia in children. N Engl J Med. 2015;373:1541–1552. doi: 10.1056/NEJMra1400972. [DOI] [PubMed] [Google Scholar]
- 2. Teachey DT, Pui C-H. Comparative features and outcomes between paediatric T-cell and B-cell acute lymphoblastic leukaemia. Lancet Oncol. 2019;20:e142–e154. doi: 10.1016/S1470-2045(19)30031-2. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 3. Paulsson K, Forestier E, Lilljebjorn H, et al. Genetic landscape of high hyperdiploid childhood acute lymphoblastic leukemia. Proc Natl Acad Sci U S A. 2010;107:21719–21724. doi: 10.1073/pnas.1006981107. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 4. Lee SHR, Li Z, Tai ST, et al. Genetic alterations in childhood acute lymphoblastic leukemia: Interactions with clinical features and treatment response. Cancers (Basel) 2021;13:4068. doi: 10.3390/cancers13164068. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 5. Jeha S, Choi J, Roberts KG, et al. Clinical significance of novel subtypes of acute lymphoblastic leukemia in the context of minimal residual disease-directed therapy. Blood Cancer Discov. 2021;2:326–337. doi: 10.1158/2643-3230.BCD-20-0229. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 6. Moorman AV, Richards SM, Martineau M, et al. Outcome heterogeneity in childhood high-hyperdiploid acute lymphoblastic leukemia. Blood. 2003;102:2756–2762. doi: 10.1182/blood-2003-04-1128. [DOI] [PubMed] [Google Scholar]
- 7. Enshaei A, Vora A, Harrison CJ, et al. Defining low-risk high hyperdiploidy in patients with paediatric acute lymphoblastic leukaemia: A retrospective analysis of data from the UKALL97/99 and UKALL2003 clinical trials. Lancet Haematol. 2021;8:e828–e839. doi: 10.1016/S2352-3026(21)00304-5. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 8. Harris MB, Shuster JJ, Carroll A, et al. Trisomy of leukemic cell chromosomes 4 and 10 identifies children with B-progenitor cell acute lymphoblastic leukemia with a very low risk of treatment failure: A Pediatric Oncology Group study. Blood. 1992;79:3316–3324. [PubMed] [Google Scholar]
- 9. Dastugue N, Suciu S, Plat G, et al. Hyperdiploidy with 58-66 chromosomes in childhood B-acute lymphoblastic leukemia is highly curable: 58951 CLG-EORTC results. Blood. 2013;121:2415–2423. doi: 10.1182/blood-2012-06-437681. [DOI] [PubMed] [Google Scholar]
- 10. Raimondi SC, Pui CH, Hancock ML, et al. Heterogeneity of hyperdiploid (51-67) childhood acute lymphoblastic leukemia. Leukemia. 1996;10:213–224. [PubMed] [Google Scholar]
- 11. Paulsson K, Johansson B. High hyperdiploid childhood acute lymphoblastic leukemia. Genes Chromosomes Cancer. 2009;48:637–660. doi: 10.1002/gcc.20671. [DOI] [PubMed] [Google Scholar]
- 12. Jackson JF, Boyett J, Pullen J, et al. Favorable prognosis associated with hyperdiploidy in children with acute lymphocytic leukemia correlates with extra chromosome 6. A Pediatric Oncology Group study. Cancer. 1990;66:1183–1189. doi: 10.1002/1097-0142(19900915)66:6<1183::aid-cncr2820660618>3.0.co;2-p. [DOI] [PubMed] [Google Scholar]
- 13. Heerema NA, Sather HN, Sensel MG, et al. Prognostic impact of trisomies of chromosomes 10, 17, and 5 among children with acute lymphoblastic leukemia and high hyperdiploidy (>50 chromosomes) J Clin Oncol. 2000;18:1876–1887. doi: 10.1200/JCO.2000.18.9.1876. [DOI] [PubMed] [Google Scholar]
- 14. Schultz KR, Pullen DJ, Sather HN, et al. Risk- and response-based classification of childhood B-precursor acute lymphoblastic leukemia: A combined analysis of prognostic markers from the Pediatric Oncology Group (POG) and Children's Cancer Group (CCG) Blood. 2007;109:926–935. doi: 10.1182/blood-2006-01-024729. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 15. Mattano LA, Jr, Devidas M, Maloney KW, et al. Favorable trisomies and ETV6-RUNX1 predict cure in low-risk B-cell acute lymphoblastic leukemia: Results from Children's Oncology Group trial AALL0331. J Clin Oncol. 2021;39:1540–1552. doi: 10.1200/JCO.20.02370. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 16. Sutcliffe MJ, Shuster JJ, Sather HN, et al. High concordance from independent studies by the Children's Cancer Group (CCG) and Pediatric Oncology Group (POG) associating favorable prognosis with combined trisomies 4, 10, and 17 in children with NCI Standard-Risk B-precursor Acute Lymphoblastic Leukemia: a Children's Oncology Group (COG) initiative. Leukemia. 2005;19:734–740. doi: 10.1038/sj.leu.2403673. [DOI] [PubMed] [Google Scholar]
- 17. Lopez-Lopez E, Autry RJ, Smith C, et al. Pharmacogenomics of intracellular methotrexate polyglutamates in patients' leukemia cells in vivo. J Clin Invest. 2020;130:6600–6615. doi: 10.1172/JCI140797. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 18. Whitehead VM, Vuchich MJ, Lauer SJ, et al. Accumulation of high levels of methotrexate polyglutamates in lymphoblasts from children with hyperdiploid (greater than 50 chromosomes) B-lineage acute lymphoblastic leukemia: A Pediatric Oncology Group study. Blood. 1992;80:1316–1323. [PubMed] [Google Scholar]
- 19. Belkov VM, Krynetski EY, Schuetz JD, et al. Reduced folate carrier expression in acute lymphoblastic leukemia: A mechanism for ploidy but not lineage differences in methotrexate accumulation. Blood. 1999;93:1643–1650. [PubMed] [Google Scholar]
- 20. Kaspers GJ, Smets LA, Pieters R, et al. Favorable prognosis of hyperdiploid common acute lymphoblastic leukemia may be explained by sensitivity to antimetabolites and other drugs: Results of an in vitro study. Blood. 1995;85:751–756. [PubMed] [Google Scholar]
- 21. Kaspers GJL, Veerman AJP, Pieters R, et al. Vitro cellular drug resistance and prognosis in newly diagnosed childhood acute lymphoblastic leukemia. Blood. 1997;90:2723–2729. [PubMed] [Google Scholar]
- 22. Synold TW, Relling MV, Boyett JM, et al. Blast cell methotrexate-polyglutamate accumulation in vivo differs by lineage, ploidy, and methotrexate dose in acute lymphoblastic leukemia. J Clin Invest. 1994;94:1996–2001. doi: 10.1172/JCI117552. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 23. Lee SHR, Yang W, Gocho Y, et al. Pharmacotypes across the genomic landscape of pediatric acute lymphoblastic leukemia and impact on treatment response. Nat Med. 2023;29:170–179. doi: 10.1038/s41591-022-02112-7. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 24. Ito C, Kumagai M, Manabe A, et al. Hyperdiploid acute lymphoblastic leukemia with 51 to 65 chromosomes: A distinct biological entity with a marked propensity to undergo apoptosis. Blood. 1999;93:315–320. [PubMed] [Google Scholar]
- 25. Flotho C, Coustan-Smith E, Pei D, et al. Genes contributing to minimal residual disease in childhood acute lymphoblastic leukemia: Prognostic significance of CASP8AP2. Blood. 2006;108:1050–1057. doi: 10.1182/blood-2006-01-0322. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 26. Uckun FM, Kersey JH, Gajl-Peczalska KJ, et al. Heterogeneity of cultured leukemic lymphoid progenitor cells from B cell precursor acute lymphoblastic leukemia (ALL) patients. J Clin Invest. 1987;80:639–646. doi: 10.1172/JCI113116. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 27. Haas OA, Borkhardt A. Hyperdiploidy: The longest known, most prevalent, and most enigmatic form of acute lymphoblastic leukemia in children. Leukemia. 2022;36:2769–2783. doi: 10.1038/s41375-022-01720-z. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 28. Rachieru-Sourisseau P, Baranger L, Dastugue N, et al. DNA index in childhood acute lymphoblastic leukaemia: A karyotypic method to validate the flow cytometric measurement. Int J Lab Hematol. 2010;32:288–298. doi: 10.1111/j.1751-553X.2009.01189.x. [DOI] [PubMed] [Google Scholar]
- 29. Arico M, Valsecchi MG, Rizzari C, et al. Long-term results of the AIEOP-ALL-95 trial for childhood acute lymphoblastic leukemia: Insight on the prognostic value of DNA index in the framework of Berlin-Frankfurt-Muenster based chemotherapy. J Clin Oncol. 2008;26:283–289. doi: 10.1200/JCO.2007.12.3927. [DOI] [PubMed] [Google Scholar]
- 30. Yu CH, Lin TK, Jou ST, et al. MLPA and DNA index improve the molecular diagnosis of childhood B-cell acute lymphoblastic leukemia. Sci Rep. 2020;10:11501. doi: 10.1038/s41598-020-68311-9. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 31. Pui CH, Campana D, Pei D, et al. Treating childhood acute lymphoblastic leukemia without cranial irradiation. N Engl J Med. 2009;360:2730–2741. doi: 10.1056/NEJMoa0900386. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 32. Jeha S, Pei D, Choi J, et al. Improved CNS control of childhood acute lymphoblastic leukemia without cranial irradiation: St Jude Total Therapy Study 16. J Clin Oncol. 2019;37:3377–3391. doi: 10.1200/JCO.19.01692. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 33. Paulsson K, Forestier E, Andersen MK, et al. High modal number and triple trisomies are highly correlated favorable factors in childhood B-cell precursor high hyperdiploid acute lymphoblastic leukemia treated according to the NOPHO ALL 1992/2000 protocols. Haematologica. 2013;98:1424–1432. doi: 10.3324/haematol.2013.085852. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 34. Trueworthy R, Shuster J, Look T, et al. Ploidy of lymphoblasts is the strongest predictor of treatment outcome in B-progenitor cell acute lymphoblastic leukemia of childhood: A Pediatric Oncology Group study. J Clin Oncol. 1992;10:606–613. doi: 10.1200/JCO.1992.10.4.606. [DOI] [PubMed] [Google Scholar]
- 35. Gu Z, Churchman ML, Roberts KG, et al. PAX5-driven subtypes of B-progenitor acute lymphoblastic leukemia. Nat Genet. 2019;51:296–307. doi: 10.1038/s41588-018-0315-5. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 36. Cox DR. Regression models and life-tables. J R Stat Soc Ser B (Methodol) 1972;34:187–202. [Google Scholar]
- 37. Fine JP, Gray RJ. A proportional hazards model for the subdistribution of a competing risk. J Am Stat Assoc. 1999;94:496–509. [Google Scholar]
- 38. Gupta S, Wang C, Raetz EA, et al. Impact of asparaginase discontinuation on outcome in childhood acute lymphoblastic leukemia: A report from the Children's Oncology Group. J Clin Oncol. 2020;38:1897–1905. doi: 10.1200/JCO.19.03024. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 39. Brady SW, Roberts KG, Gu Z, et al. The genomic landscape of pediatric acute lymphoblastic leukemia. Nat Genet. 2022;54:1376–1389. doi: 10.1038/s41588-022-01159-z. [DOI] [PMC free article] [PubMed] [Google Scholar]
Associated Data
This section collects any data citations, data availability statements, or supplementary materials included in this article.
Data Availability Statement
RNAseq and pharmacotyping data are available as per detailed in our previous publication (SHR Lee et al Nat Med 2023). Measured drug sensitivities and clinical data are available in the Data Supplement (Table S1) of that paper. RNAseq data have been deposited in the European Genome-phenome Archive under accession nos. EGAS00001001952, EGAS00001001923, EGAS00001000447, EGAS00001000654, EGAS00001003266, EGAS00001004739, EGAS00001005084, and EGAS00001006336. Data are also available at St Jude Cloud Genomics Platform for the Pan-Acute Lymphoblastic Leukemia data set and the Real-time Clinical Genomics data set.