Abstract
Background:
In recent years, self-tanners have become a well-liked alternative to sun tanning and tanning bed usage, as strikingly similar results can be achieved without the harmful side effects of ultraviolet exposure.
Objective:
The aim of this study is to investigate the presence and prevalence of potential allergens in the most popular self-tanning products.
Methods:
Five major retailers in the United States were evaluated, from which 17 different brands and 44 unique self-tanning products were analyzed. The ingredients in each self-tanning product were compared with 80 and 36 notable contact allergens taken from the North American Contact Dermatitis Group and Food and Drug Administration–approved T.R.U.E (Thin-Layer Rapid Use Epicutaneous Patch Test), respectively.
Results:
We found that contact allergens are frequently present in self-tanning products; allergens especially common are propylene glycol, linalool, polysorbate, d-limonene, benzyl alcohol, tocopherol (vitamin E), fragrances, and other scented botanicals. On average, each self-tanner we analyzed contained 11.86 allergens.
Limitations:
The limitation is that commercial names could not be eliminated from the analysis, introducing potential bias.
Conclusion:
While self-tanning products are a safer alternative to tanning bed use or sunbathing, consumers and clinicians alike must be aware that they may cause an allergic reaction of the skin for some users.
Keywords: allergic contact dermatitis, contact dermatitis, cosmetics
What is known about this subject in regard to women and their families?
Topical self-tanning products are a popular method to realize tan skin without exposure to ultraviolet light.
What is new from this article as messages for women and their families?
Though topical self-tanners are inherently less detrimental to the skin than sunbathing or tanning beds, consumers and physicians should note that they may be a source of allergic contact dermatitis given that several contact allergens are common constituents of self-tanning products.
Introduction
The appearance of tanned skin became a symbol for wealth and beauty in 1928 as Coco Chanel returned to Paris from holiday after accidentally receiving a sunburn. Her golden-brown skin was envied by her peers and has continued to be idolized by multiple generations today.1 Numerous studies have shown that UVA/UVB emission from the sun and artificial ultraviolet ultraviolet (UV) radiation from tanning beds can predispose people to melanoma and nonmelanoma skin cancer.2,3 To avoid harmful effects of UVA/UVB emission, sunless tanners are topical products applied to the skin to give the appearance of tanned skin. These products have been the preferred alternative to achieve the equivalent cosmetic look without the UV radiation damage. A majority of tanners contain dihydroxyacetone (DHA), a sugar molecule derived by plants. When applied to the skin, DHA reacts with keratin proteins within the stratum corneum to produce polymer melanoidins that results in a bronze-colored pigment change.4 This reaction is known as the Maillard reaction.4,5 Once applied to the skin, it takes 2 to 4 hours for the tan to set in, and the tanned skin ultimately fades within 2 to 7 days due to normal skin exfoliation.5 The formulations of self-tanners available include foam/mousse, lotion, cream, serum, aerosol, gel, oil, and wipes and vary in color from mild (fair) to ultra dark. The product can be applied to all areas of the skin except for mucus membranes and around the eyes as safety data for these areas have not been evaluated by the Food and Drug Administration (FDA).6 Total body application can result in significant discomfort if someone is in fact allergic to one of the product constituents resulting in widespread dermatitis that can disrupt daily activity.
Since the days of Coco Chanel, the idea of having a tan has been favored by many individuals. Recent popular culture has created the perception that tanned skin symbolizes a healthier and more attractive appearance due to the darker skin tone being better to conceal minor skin imperfections such as birth marks, sunspots, spider veins, and cellulite, all while accenting muscle tone.7,8 The personal cosmetic benefits along with easy application of the products have led to increased popularity and have flooded the beauty industry. Although these products are a safer alternative to tanning beds, skin irritation can be present with the application of self-tanners, especially because of the fragrances and essential oils that are often incorporated to mask the odor of DHA. These additives can increase the risk of allergic contact dermatitis (ACD) among patients with sensitive or eczema-prone skin. Therefore, the aim of this study is to investigate the presence and prevalence of potential allergens in the most popular self-tanning products.
Methods
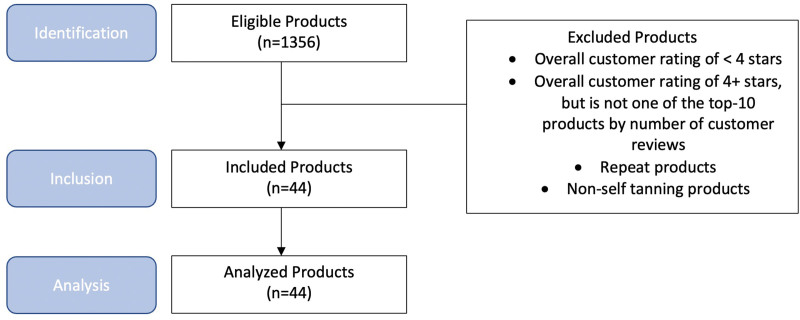
An internet-based search of the 5 major United States retailers, Walmart, Target, Amazon, Sephora, and Ulta, was performed to identify the current best-selling name brand and generic self-tanners marketed to consumers in the United States. In order to identify and select products, each retailer’s website was searched using the following key terms: self-tanner, self-tanning lotion, tanning oil, tanning foam, self-tanning lotion body, and self-tanning lotion face.
The top 10 most popular products on each retailer website were included for evaluation. Two factors were taken into consideration when rating popularity: total number of customer reviews and the star rating (consumers can assign a rating of 1–5 stars, with 5 stars being the best possible rating). Given the expansive nature of the selected retailers (particularly Amazon), products with at least a 4-star rating and those with the highest relative number of customer reviews among these products were chosen. Due to the fact that some products were popular among many different retailers, the products that were considered to be the top 10 among more than one retailer were only evaluated once. In total, 17 different brands yielding 44 unique self-tanning products were selected for review (Fig. 1). The manufacturer ingredient list for each unique self-tanner was analyzed and compared to the North American Contact Dermatitis Group 80 allergens and an additional 36 allergens from the FDA’s list of Common Allergens Found in Cosmetic Products (FDA-approved T.R.U.E test [Thin-Layer Rapid Use Epicutaneous Patch Test]), for a total of 114 total allergens.9,10 Of the 114 allergens, 21 of them overlapped between the 2 lists. These allergens were considered only once.
Fig. 1.
STROBE flow diagram for product selection.
Results
A total of 17 brands, resulting in 44 unique products, were reviewed (Fig. 1). The total number of allergens per product ranged from 1 to 24 allergens, with no products being completely allergen free (Fig. 2; Table 1). Overall, the mean number of allergens per product was 11.86, with a median of 12 allergens. Over half (56.8%) of the products contained 10 or more potential allergens, while 5 (11.4%) products contained 20 or more allergens.
Fig. 2.
Total number of allergens in reviewed self-tanning products.
Table 1.
Allergens identified in self-tanning products
| Product name | Ingredients identified as allergens |
|---|---|
| Bondi Sands Self Tanning Foam Dark | Tocopheryl acetate, parfum, benzyl alcohol, polysorbate 20, propylene glycol, scented botanical |
| Jergens Natural Glow Instant Sun Sunless Tanning Mousse Light Bronze | Hexyl cinnamal, methylparaben, parfum, hydroxyethyl acrylate, decyl glucoside, benzyl alcohol, dipropylene glycol, linalool |
| St. Tropez Self Tan Classic Bronzing Mousse Vegan Self Tanner | Hexyl cinnamal, tocopheryl, parfum, benzyl salicylate, propylene glycol, anisyl alcohol, citronellol, coumarin, geraniol, hydroxycitronellal, d-limonene, linalool, α-isomethyl ionone |
| Ed Hardy Coconut Kisses Golden Tanning Lotion | Diazolidinyl urea, tocopheryl acetate, paraben, propylparaben, parfum, benzyl salicylate, isopropyl myristate, polysorbate 80, propylene glycol, 14× scented botanicals, lilial, linalool |
| Beauty by Earth Self Tanner Tanning Lotion | 8× scented botanicals |
| Bondi Sands Self Tanning Foam Ultra Dark | Tocopheryl acetate, parfum, benzyl alcohol, polysorbate 20, propylene glycol, 2× scented botanicals, coumarin |
| Australian Gold Dark Tanning Accelerator Spray Gel | Tocopheryl acetate, methylparaben, parfum, triethanolamine, tea tree oil, acrylates/C10-30 alkyl acrylate cross polymer, polysorbate 20, propylene glycol, 3× scented botanicals, citrate, coumarin, d-limonene, linalool |
| Australian Gold Dark Tanning Exotic Oil | Tocopheryl acetate, parfum, tea tree oil, 6× scented botanicals, citrate, coumarin, d-limonene, linalool |
| Jergens Natural Glow 3 Day Self Tanner for Medium to Deep Skin Daily Moisturizer | Tocopheryl acetate, paraben mix (methylparaben, ethylparaben, propylparaben), parfum, hydroxyethyl acrylate/sodium acryloyldimethyl taurate copolymer, octyldodecyl myristate, polysorbate 60, 6× scented botanicals |
| D-Bronzi Anti-Pollution Sunshine Drops (by Drunk Elephant) | Tocopheryl, polysorbate, 6× botanicals |
| 2-HR Express Self Tanning Mousse Dark (by Loving Tan) | Propylene glycol, decyl glucoside, fragrance (unspecified), 2× botanicals |
| Self Tanning Foam (by Bondi Sands) | Propylene glycol, polysorbate, parfum, coumarin, tocopheryl acetate, benzyl alcohol, 2× botanicals |
| Sunny Honey Bali Bronzing Foam (by Coco & Eve) | Pentylene glycol, parfum, propylene glycol, 10× scented botanicals, benzyl alcohol, coumarin, limonene, benzyl salicylate |
| Self Tanning Mousse (by Bali Body) | 4× scented botanicals, parfum, benzyl alcohol, limonene, linalool, hexyl cinnamal, citronellol, geraniol |
| Instant Self Tanning Mousse (by St. Moriz) | Propylene glycol, polysorbate, 2-bromo-2-nitropropane-1,3-diol, 1× botanical |
| Everyday Gradual Tanning Milk (by Bondi Sands) | Paraben mix (methylparaben, propylparaben, butylparaben, ethylparaben), tocopheryl acetate, parfum, triethanolamine |
| Aero Self Tanning Foam (by Bondi Sands) | Propylene glycol, polysorbate, 1× botanical, parfum, tocopheryl acetate, coumarin |
| Face Tan Water (by Bali Body) | Polysorbate, 5× botanicals, tocopherol, parfum, benzyl alcohol, citronellol, geraniol, linalool |
| Jergens Natural Glow + Firming Self Tanner | Paraben mix (methylparaben, propylparaben, ethylparaben), fragrance mix, ethyl acrylate (hydroxyethyl acrylate/sodium acryloyldimethyl taurate copolymer), DMDM hydantoin, octyldodecyl myristate, polysorbate 60, ethylhexyl isononanoate, 5× scented botanicals |
| Malibu Tan Hemp Skin Firming and Bronzing Body | Diazolidinyl urea, tocopherol, paraben mix (methylparaben, propylparaben), fragrance mix, tea tree oil oxidized, isopropyl myristate, polysorbate 60, propylene glycol, 11× scented botanicals |
| Jergens Natural Glow Self Tanner Lotion Sunless Tanning Moisturizer | Fragrance mix, hydroxyethyl acrylate, octyldodecyl myristate, polysorbate 60, 7× scented botanicals |
| Bondi Sands Self Tanning Foam Dark for Face and Body | Tocopherol acetate, fragrance mix, benzyl alcohol, polysorbate 20, propylene glycol, scented botanical |
| Jergens Natural Glow Instant Sun Sunless Tanning Mousse | Cinnamal, methylparaben, fragrance mix, hydroxyethyl acetate, decyl glucoside, benzyl alcohol, polysorbate 60, propylene glycol, linalool |
| L’Oreal Paris Sublime Bronze Hydrating Self Tanning Water Mousse | Tocopherol, fragrance mix, butylated hydroxytoluene, benzyl salicylate, benzyl alcohol, cocamidopropyl betaine, scented botanical, citronellol, coumarin, geraniol, d-limonene, linalool |
| Neutrogena Build A Tan Gradual Sunless Tanning Lotion | Paraben mix (methylparaben, propylparaben, ethylparaben), fragrance mix, butylated hydroxytoluene, hydroxyethyl acrylate, polysorbate 20 and 60, ethylhexyl hydroxystearate benzoate |
| Australian Gold Bronzing Intensifier Dry Oil Spray | Tocopherol, fragrance mix, acrylic acid copolymer, triethanolamine, tea tree oil oxidized, polysorbate 20, propylene glycol, 7× scented botanicals, coumarin, d-limonene, linalool |
| L’Oreal Paris Natural Looking Self Tanning Wipes | Hexyl cinnamal, methylparaben, fragrance mix, propylene glycol, citral, citronellol, coumarin, d-limonene, linalool |
| Isle of Paradise Self Tanning Water | Fragrance mix, ethylhexylglycerin, propylene glycol, 10× scented botanicals |
| St. Tropez Tan Tonic Glow Drops | Hexyl cinnamal, tocopherol, fragrance mix, benzyl salicylate, 3× scented botanicals, coumarin, geraniol, d-limonene, linalool, α-isomethyl ionone |
| Isle of Paradise Self Tanning Natural Glow Face Drops | Tocopherol, fragrance mix, polysorbate 80, ethylhexylglycerin, propylene glycol, 10× scented botanicals |
| St. Tropez Self Tan Luxe Whipped Creme Mousse | Hexyl cinnamal, tocopherol, fragrance mix, benzyl salicylate, decyl glucoside, ethylhexylglycerin, 1× scented botanicals, benzyl benzoate, hydroxycitronellal, coumarin, geraniol, d-limonene, linalool, α-isomethyl ionone |
| Isle of Paradise Self Tanning Oil Mist | Fragrance mix, ethylhexylglycerin, propylene glycol, 15× scented botanicals |
| St. Tropez Gradual Tan Watermelon Daily Firming Lotion | Tocopherol, fragrance mix, hydroxyhydrocinnamate, 6× scented botanicals, linalool, α-isomethyl ionone |
| St. Tropez Self Tan Express Bronzing Mouse | Fragrance mix, decyl glucoside, ethylhexylglycerin, propylene glycol, 1× scented botanicals, linalool, α-isomethyl ionone |
| Isle of Paradise Even Skin Tone Self-Tanning Body Butter | Tocopherol, fragrance mix, benzophenone-4, isopropyl myristate, propylene glycol, 10× scented botanicals, linalool |
| St. Tropez Self Tan Purity Vitamins Bronzing Water Body Mist | Hexyl cinnamal, fragrance mix, polysorbate 20, ethylhexylglycerin, betaine, propylene glycol, 5× scented botanicals, citronellol, lilial, linalool |
| Isle of Paradise Glow Clear, Color Self Tanning Mousse | Fragrance mix, decyl glucoside, ethylhexylglycerin, propylene glycol, 10× scented botanicals |
| L’Oreal Paris Sublime Bronze Self Tanning Facial Drops Fragrance Free | Scented botanical |
| Tanologist Sunless Self Tanning Mousse | Unspecified fragrance, vitamin E, hexyl cinnamal, decyl glucoside, dipropylene glycol, hydroxycitronellal, geraniol, linalool, d-limonene, 8× scented botanicals |
| Jergens Natural Glow Ultra Deep Instant Moisturizing Self Tanner | Fragrance mix I, hexyl cinnamal, benzyl salicylate, decyl glucoside, hydroxyethyl acrylate/sodium, benzyl alcohol, citronellol, coumarin, geraniol, limonene, linalool, α-isomethyl ionone |
| St. Tropez Self Tan Bronzing Water Purity Face Mist | Hexyl cinnamal, unspecified fragrance, benzyl alcohol, 3× scented botanicals, citronellol, d-limonene, linalool |
| Bondi Sands Self Tanning Foam Light/Medium | Tocopherol acetate, unspecified fragrance, benzyl alcohol, polysorbate 80, propylene glycol, scented botanical |
| St. Tropez Self Tan Express Bronzing Mousse | α-isomethyl ionone, linalool, propylene glycol, scented botanical, unspecified fragrance, decyl glucoside |
| Bondi Sands Liquid Gold Self Tanning Foam | Tocopherol acetate, unspecified fragrance, polysorbate, propylene glycol, 3× scented botanicals, coumarin, farnesol |
DMDM, dimethyl-dimethyl.
The most commonly encountered allergen was fragrance (including mix I, mix II, unspecified), which was found in all but one product (Fig. 3). Most (84.1%) products contained other scented botanicals (essential oils, extracts, etc), especially common were linalool hydroperoxide (47.7%), coumarin (34.1%), d-limonene (27.3%), and citronellol (25%). One product contained as many as 15 unique scented botanicals. Other common allergens included propylene glycol (61.4%), tocopherol (also known as vitamin E) (59.1%), polysorbate 80 (47.7%), and benzyl alcohol (29.5%). One product contained butane.
Fig. 3.
Total number of self-tanning products containing each allergen.
The average number of allergens varied based on product formulation (Fig. 4). On average, foams/mousses (the most popular formulation) contained 10.1 allergens per product, while lotions, water-based sprays/liquids, oil-based sprays/liquids, and drops contained 13.5, 12.5, 17.7, and 10.5 allergens per product, respectively. However, a 1-way analysis of variance revealed there was not a statistically significant difference in the number of allergens based on product formulation (F(4) = 1.96, P = 0.121) (Table 2). One gel-based spray was analyzed, which contained 15 allergens. Similarly, a single product categorized as a tanning wipe was analyzed, which contained 10 allergens.
Fig. 4.
Average number of allergens per product by formulation.
Table 2.
Analysis of variation of allergens per product by formulation
| Source of variation | ANOVA | |||||
|---|---|---|---|---|---|---|
| SS | df | MS | F | P value | F critical | |
| Between groups | 203.282251 | 4 | 50.8205628 | 1.9603552 | 0.120928 | 2.62605228 |
| Within groups | 959.193939 | 37 | 25.9241605 | |||
| Total | 1162.47619 | 41 | ||||
ANOVA, analysis of variance; df, degrees of freedom; MS, mean squares; SS, sum of squares.
Discussion
Self-tanning products have become increasingly popular options to realize tan skin. Many users believe they appear more refreshed and younger when using these products, and that self-tanners can help hide skin blemishes and imperfections. While extremely popular among women, especially for beauty enhancement purposes, they are also used by men for the same reason. Additionally, men and women in the bodybuilding industry often use these products to enhance their appearance for competitions. In this sector, self-tanners are thought to make competitors appear taller, thinner, and more defined on stage. Another popular reason to use self-tanners is that they are inherently less harmful than sunbathing or tanning bed options. They are also often cheaper and more convenient than getting a spray tan.
Given the increasing popularity and widespread use of self-tanners, the present study sought to analyze a number of popular self-tanning products across multiple retailers for inclusion of known contact allergens in their ingredient list, given that these products may act as another source of skin allergy in the form of ACD. To our knowledge, no prior studies have evaluated the prevalence of known contact allergens in self-tanning products, nor does the literature contain reports of patient cases of ACD due to the use of these products.
ACD is a T-cell-mediated delayed hypersensitivity reaction (type IV hypersensitivity reaction) that occurs in 2 phases: sensitization and elicitation. Sensitization occurs when the skin is first exposed to the allergen. The allergen binds with a larger carrier protein within the skin and is later engulfed by dermal dendritic cells or Langerhans cells. The allergen-protein complex then migrates to the regional lymph nodes of the skin where they activate naive T-cells and lead to the proliferation of effector and memory T-cells that respond specifically to that allergen. During the elicitation phase, reexposure of the allergen to the skin triggers sensitized T-cells to initiate an inflammatory cascade that produces the symptoms of ACD. The response to the allergen is delayed and can sometimes take up to 72 hours before the skin reaction occurs.11,12 Acute ACD presents as pruritic erythematous papules and vesicles in the areas of skin exposed to the allergen. Bullae can also form in severe cases of acute ACD. More chronic exposure can lead to scaling, lichenification, and fissuring within these areas.11,13 Finally, subacute ACD displays a mixture of features from both acute and chronic ACD and thus can be more difficult to diagnose. In all 3 forms, skin inflammation occurs in areas that came into contact with the allergen, most commonly the hands, face, or a scattered/generalized pattern. A particularly relevant subtype of contact dermatitis includes photoallergic contact dermatitis. This occurs when a chemical product applied to the skin is exposed to electromagnetic radiation, such as UV light, causing an inflammatory reaction similar to the type IV hypersensitivity reaction described above.14 Common offending agents include, but are not limited to, 6-methylcoumarin, benzophenone, nonsteroidal anti-inflammatory drugs, promethazine, benzocaine, p-aminobenzoic acid, thiourea (thiocarbamide), oxybenzone (BZP-3), octyl dimethyl para-aminobenzoic acid, octisalate (octyl salicylate), butyl methoxydibenzoylmethane, ketoprofen, and 2-hydroxy-methoxy methyl.
Among the products analyzed in this study, fragrances and other scented botanicals were the most commonly encountered allergens. In fact, fragrance was present in all but one product. Fragrances are widely present in everyday personal care products, even among those labeled “fragrance free,” either as preservatives, masking perfumes, or in the form of botanicals. Fragrance allergies are relatively common, and after nickel, fragrance allergies are cited as the most common allergens found in offices that perform patch testing.15 Fragranced products contain rather potent chemicals that can induce skin sensitization and subsequent ACD.16 Consumers should be cautioned against trusting products that claim to be “fragrance free” or “unscented,” as many of these products still contain fragrances that act as a common source of allergy in the skin.
Other frequently encountered contact allergens in order of prevalence include propylene glycol, vitamin E, polysorbate 80, and benzyl alcohol. Propylene glycol is found in a number of personal care products, as it is commonly used as a preservative, solvent, and emollient.15 Vitamin E acts as a moisturizer, antioxidant, and antiaging agent by absorbing UV wavelengths to prevent oxidative stress; yet, it is also considered an allergen, especially at higher concentrations.15,17 Polysorbate 80 (also known as Tween 80) is an emulsifier and foaming agent used in many personal care products.15 Benzyl alcohol is found in many products as a preservative or antibacterial agent.15 It is found in many naturally occurring products like Balsam of Peru and is a component of many essential oils. Each of these products has been shown capable of triggering an allergic reaction in the form of ACD, though there are varying opinions regarding whether or not vitamin E is truly a source of contact allergy outside of rare instances.18 Additionally, some products contained photocontact allergens such as octyldodecyl myristate, thiourea (thiocarbamide), benzocaine, or benzophenone. Finally, there were a few products containing possible allergens or substances that were unexpected. Specifically, one product contained 2-hydroxyethyl methacrylate, an acrylate, not typical of sunless tanners, while another contained cocamidopropyl betaine, which tends to be in wash-off products.
Given that thousands of substances are capable of causing a skin reaction, patch testing can be a vital tool to better understand an individual’s susceptibility to certain allergenic substances common in everyday items. Patch testing is typically performed in a dermatology clinic. The provider will place a small amount of allergen on the skin and cover each with a patch. The patches remain on the skin for 48 hours, at which time a dermatologist will remove the patches in office and make note of any reactions on the skin. Two to five days later, a patient will once again return to the office to check for any additional or enhanced reaction sites. Once the dermatologist determines the cause(s) of the contact dermatitis, they can create a plan to help the patient avoid what is causing the rash. The American Contact Dermatology Society has created a Contact Allergy Management Program, which provides patients with a so-called “Safe List,” which is a detailed list of items that are safe for patients to use based on their unique patch test results. The goal is to aid patients in avoidance of products that can cause allergic reactions on their skin. Of note, not every individual with a contact allergy necessitates having patch testing done, but this should be decided on through shared decision making under the guidance of a dermatologist or other physician trained in patch testing.
As mentioned earlier, the most important step for the treatment of ACD is to avoid exposure to the offending allergen, which can be identified through patch testing. First-line treatment for acute ACD is mid- to high-potency topical corticosteroid.12 A 14-day course followed by a taper of systemic corticosteroids can also be considered if the dermatitis is severe.11,19
Recommended therapy for chronic ACD is low-potency topical corticosteroid and fragrance-free barrier creams/emollients to aid with dryness and pruritus of the affected area.11,20 Calcineurin inhibitors offer an alternative for ACD located on the face and eyelid, although they have not yet been approved for use in ACD.11,21
The main limitation of this study is that commercial names could not be eliminated from the analysis, introducing potential bias. Future research could investigate contact allergens in other personal care products, including makeup products such as liquid or powder foundation, body wash, and menstrual pads or tampons.
Conclusion
While self-tanning products are certainly a safer alternative to sunbathing or tanning beds for those desiring tan skin, consumers and clinicians alike must be aware of their potential to cause ACD. Every popular product reviewed here contained at least one potential contact allergen, with fragrances being overwhelmingly common, while propylene glycol, vitamin E, polysorbate, and benzyl alcohol were also frequently encountered. ACD is a delayed hypersensitivity reaction and may take up to 72 hours to present. It often manifests as itchy red papules and vesicles, and occasionally blisters can be seen in more severe instances. Consumers who experience any of these symptoms after applying a self-tanning product are advised to immediately discontinue application, rinse the skin thoroughly with water, and consider consulting a dermatologist. Dermatologists, allergists, and primary care providers should consider ACD in the differential in patients using self-tanning products that develop skin lesions consistent with contact dermatitis and counsel and treat patients accordingly.
Conflicts of interest
None.
Funding
None.
Study approval
N/A
Author contributions
JN and OO: Research design, performance of research, data analysis, and writing of the paper. RTP, ML, and MS: Performance of research, data analysis, and writing of the paper. MGB: Research design and writing of the paper.
References
- 1.Presley AB. Fifty years of change: societal attitudes and women’s fashions, 1900-1950. Historian 1998;60:307–24. [Google Scholar]
- 2.Narayanan DL, Saladi RN, Fox JL. Ultraviolet radiation and skin cancer. Int J Dermatol 2010;49:978–86. [DOI] [PubMed] [Google Scholar]
- 3.Young AR. Tanning devices—fast track to skin cancer? Pigment Cell Res 2004;17:2–9. [DOI] [PubMed] [Google Scholar]
- 4.Tamanna N, Mahmood N. Food processing and Maillard reaction products: effect on human health and nutrition. Int J Food Sci 2015;2015:526762. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 5.Garone M, Howard J, Fabrikant J. A review of common tanning methods. J Clin Aesthet Dermatol 2015;8:43–7. [PMC free article] [PubMed] [Google Scholar]
- 6.Center for Food Safety and Applied Nutrition. Sunless tanners & bronzers. U.S. Food and Drug Administration; n.d.. Available from: https://www.fda.gov/cosmetics/cosmetic-products/sunless-tanners-bronzers, accessed June 15, 2023. [Google Scholar]
- 7.Suppa M, Gandini S, Bulliard JL, et al. Who, why, where: an overview of determinants of sunbed use in Europe. J Eur Acad Dermatol Venereol 2019;33:6–12. [DOI] [PubMed] [Google Scholar]
- 8.Ajay SM. Why do bodybuilders tan? The tan factor: a game-changer! Dr Workout; 2023. Available from: https://www.drworkout.fitness/why-do-bodybuilders-tan/, accessed June 15, 2023. [Google Scholar]
- 9.DeKoven JG, Silverberg JI, Warshaw EM, et al. North American Contact Dermatitis Group patch test results: 2017–2018. Dermatitis 2021;32:111–23. [DOI] [PubMed] [Google Scholar]
- 10.U.S. Food & Drug Administration. Allergens in cosmetics. Updated February 2, 2022. Available from: https://www.fda.gov/cosmetics/cosmetic-ingredients/allergens-cosmetics, accessed June 15, 2023. [Google Scholar]
- 11.Nassau S, Fonacier L. Allergic contact dermatitis. Med Clin North Am 2020;104:61–76. [DOI] [PubMed] [Google Scholar]
- 12.Rustemeyer T, van Hoogstraten IM, von Blomberg BME, et al. Contact dermatitis - mechanisms of irritant and allergic contact dermatitis. In: Johansen JD, Frosch PJ, Lepoittevin JP, editors. Contact dermatitis. Berlin: Springer; 2011. p. 43–90. [Google Scholar]
- 13.DeKoven JG, Warshaw EM, Zug KA, et al. North American Contact Dermatitis Group patch test results: 2015–2016. Dermatitis 2018; 29:297–309. [DOI] [PubMed] [Google Scholar]
- 14.Kerr A, Ferguson J. Photoallergic contact dermatitis. Photodermatol Photoimmunol Photomed 2010;26:56–65. [DOI] [PubMed] [Google Scholar]
- 15.Contact Dermatitis Institute. Allergen Database. 2014. Available from: https://www.contactdermatitisinstitute.com/database.php, accessed June 15, 2023. [Google Scholar]
- 16.van Amerongen CCA, Ofenloch RF, Cazzaniga S, et al. Skin exposure to scented products used in daily life and fragrance contact allergy in the European general population - the EDEN Fragrance Study. Contact Dermatitis 2021;84:385–94. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 17.Kosari P, Alikhan A, Sockolov M, Feldman SR. Vitamin E and allergic contact dermatitis. Dermatitis 2010;21:148–53. [PubMed] [Google Scholar]
- 18.Teo CWL, Tay SHY, Tey HL, Ung YW, Yap WN. Vitamin E in atopic dermatitis: from preclinical to clinical studies. Dermatology 2021;237:553–64. [DOI] [PubMed] [Google Scholar]
- 19.Fisher A, Rietschel R, Fowler J. Fisher’s contact dermatitis. Marceline: Walsworth;2019. p. 76–9, 25–43, 323–6, 689–91. [Google Scholar]
- 20.Adelman DC, Thomas CJ. Manual of allergy and immunology. 5th ed. Philadelphia: Wolters Kluwer Health/Lippincott Williams & Wilkins; 2012. [Google Scholar]
- 21.Fonacier L, Noor I. Contact dermatitis and patch testing for the allergist. Ann Allergy Asthma Immunol 2018;120:592–8. [DOI] [PubMed] [Google Scholar]