Abstract
Background:
Exposure to per- and polyfluoroalkyl substances (PFAS) throughout gestation and childhood may impact cardiometabolic risk.
Methods:
In 179 HOME Study participants (Cincinnati, OH; recruited 2003–2006), we used latent profile analysis to identify two distinct patterns of PFAS exposure from serum concentrations of four PFAS measured at birth and ages 3, 8, and 12 years. We assessed the homeostatic model of insulin resistance, triglycerides-to-high-density lipoprotein cholesterol ratio, leptin-to-adiponectin ratio, systolic blood pressure, visceral fat, and hemoglobin A1c levels at age 12 years. We used multivariable linear regression to assess the association of membership in the longitudinal PFAS mixture exposure group with a summary measure of overall cardiometabolic risk and individual components.
Results:
One PFAS exposure profile (n = 66, 39%) had higher geometric means of all PFAS across all visits than the other. Although adjusted associations were null in the full sample, child sex modified the association of longitudinal PFAS mixture exposure group with overall cardiometabolic risk, leptin-to-adiponectin ratio, systolic blood pressure, and visceral fat (interaction term P values: 0.02–0.08). Females in the higher exposure group had higher cardiometabolic risk scores (ß = 0.43; 95% CI = −0.08, 0.94), systolic blood pressures (ß = 0.6; 95% CI = 0.1, 1.1), and visceral fat (ß = 0.44; 95% CI = −0.13, 1.01); males had lower cardiometabolic risk scores (ß = −0.52; 95% CI = −1.06, −0.06), leptin-to-adiponectin ratios (ß = −0.7; 95% CI = −1.29, −0.1), systolic blood pressures (ß = −0.14; 95% CI = −0.7, 0.41), and visceral fat (ß = −0.52; 95% CI = −0.84, −0.19).
Conclusions:
Exposure to this PFAS mixture throughout childhood may have sex-specific effects on adolescent cardiometabolic risk.
Keywords: Adolescents, Cardiometabolic risk, Chemical mixture, Latent profile analysis, polyfluoroalkyl substances
What this study adds
We used latent profile analysis to characterize exposure to a mixture of four polyfluoroalkyl substances over the first 12 years of life. Females in the higher exposure group had greater cardiometabolic risk, while the opposite was true for males. These findings add to a small but growing body of literature showing that cumulative exposure to polyfluoroalkyl substance mixtures may adversely affect adolescent health.
Introduction
Per- and polyfluoroalkyl substances (PFAS), chemicals characterized by fluorinated carbon chains, have been used extensively since the 1940s in fire-fighting foams, oil and water-repellant textiles, protective coatings, the electronics industry, and paints.1 PFAS are universally detected in the serum of adults and children in the United States.2,3 Children have higher PFAS body burdens than adults, likely due to hand-to-mouth behaviors, dust ingestion, larger body weight-adjusted food and water intake, placental transfer, and breastfeeding.4,5 Childhood PFAS exposure may adversely affect child neurodevelopment, immunity, thyroid function, renal function, and puberty onset.6
Exposure to PFAS during gestation and childhood may increase cardiometabolic risk, which includes impaired glucose metabolism, excess central adiposity, hypertension, elevated triglycerides, and decreased high-density lipoprotein (HDL) concentrations.7 Cross-sectional studies found that perfluorooctanoic acid (PFOA) and perfluorooctane sulfonic acid (PFOS) are positively associated with total cholesterol in children and adolescents.8–10 Some of these found a positive association between PFOA and triglyceride levels.9–11 Other studies found inconsistent associations between postnatal serum PFAS concentrations and body mass index (BMI).12–14 A previous Health Outcomes and Measures of the Environment (HOME) Study analysis that evaluated individual PFAS concentrations at single time points found that prenatal serum PFOA was positively associated with cardiometabolic risk, although the association at other time points varied.15 Of the studies that examined associations between PFAS and cardiometabolic risk in children, several found sex-specific associations; many of these reported more pronounced associations in females.12,16–18 The biological mechanism linking PFAS exposure to cardiometabolic risk in children is not fully understood. However, metabolomics studies have found an association of PFAS exposure with disrupted fatty acid oxidation, amino acid, and lipid metabolism in children and adolescents.19–21
Prior studies examining childhood PFAS exposure and cardiometabolic risk have some limitations. First, most were cross-sectional, limiting causal inference.8–11,22 Furthermore, few studies have assessed the effect of PFAS mixture exposure or only considered PFAS exposure at one time in childhood. PFAS exposure is dynamic during childhood and occurs as a mixture of PFAS instead of a single chemical.
To this end, latent profile analysis (LPA) has emerged as a technique to identify unobservable, latent subgroups of individuals based on chemical exposure biomarkers.23,24 Kuiper et al.25 applied a repeated measures LPA approach using the HOME Study data to characterize childhood PFAS mixture exposure patterns over the first 12 years of life. Thus, we took advantage of these advancements to address the previously mentioned gaps and prospectively investigate the relation of PFAS mixture exposure during gestation and throughout childhood with cardiometabolic risk in adolescence, hypothesizing that this relation is modified by child sex.
Materials and methods
Study participants
We used data from the HOME Study, a prospective pregnancy and birth cohort study. Participants were recruited from March 2003 to January 2006 from nine prenatal practices affiliated with three hospitals in Cincinnati, Ohio.26 Eligibility criteria for inclusion in the study were as follows: women at 16 ± 3 weeks gestation, aged 18 years or older, residing in a residence built in or before 1978, not living in mobile/trailer homes, no HIV infection history, no seizure or thyroid disorder medication use, planning to continue prenatal care at collaborating clinics, intending to deliver at collaborating hospitals, expecting to live in the greater Cincinnati area for the subsequent year, possessing English fluency, and having no diagnosis of diabetes, bipolar disorder, schizophrenia, or cancer requiring radiation or chemotherapy. Of the initial 468 enrolled women, 412 women and their children were deemed eligible for follow-up when their children reached age 12 years.27 This analysis includes 179 singleton children with at least one gestational or childhood serum PFAS concentration measurement (taken from the umbilical cord at birth, or at the 3-,8- or 12-year visit), complete data for the cardiometabolic risk factors at the 12-year visit (Supplemental Table S1; http://links.lww.com/EE/A258), and complete data for all relevant covariates.
The Institutional Review Boards (IRBs) of Cincinnati Children’s Hospital Medical Center (CCHMC) and all delivery hospitals approved the study protocol. The Centers for Disease Control and Prevention and Brown University deferred to the CCHMC IRB as the IRB of record. Women provided written informed consent for themselves and their children. Children provided written informed consent at the 12-year visit.
Postnatal PFAS exposure assessment
We measured four PFAS in serum, using blood samples that were collected from the umbilical cord at birth, and directly from the children themselves at ages 3, 8, and 12 years. Child blood samples were collected by venipuncture by trained phlebotomists. After separating the serum from the blood, samples were stored at −80 °C until Centers for Disease Control and Prevention laboratory staff quantified PFOA, PFOS, perfluorononanoic acid (PFNA), and perfluorohexane sulfonate (PFHxS) using online solid-phase extraction coupled with high-performance liquid chromatography-isotope dilution mass spectrometry.28 In each analytic batch, reagent blanks and low- and high-concentration quality control materials were incorporated and assessed using standard statistical probability rules. Repeated measures in the quality control materials had a coefficient of variation of 6%. At the 3-, 8- and 12-year visits, all samples were above the limit of detection (0.2 ng/mL for PFOS and 0.1 ng/mL for all others). One participant’s birth sample was below the limit of detection for PFOS and PFNA; for these values, we substituted the LOD/√2.
Children’s cardiometabolic risk markers
We measured cardiometabolic risk features when the children were 12 years old. Trained technicians used dual-energy X-ray absorptiometry to measure fat inside the abdominal cavity with a Hologic Horizon densitometer.29 Three sitting blood pressure measures were taken with a Dinamap Pro100; the first was discarded, and the average of the second and third measures was used in our analyses.30 Finally, insulin, glucose, triglycerides, HDLs, and hemoglobin A1C (HbA1c) concentrations were measured using valid immunoassays in overnight-fasting samples analyzed by trained laboratory technicians in the Clinical Translational Research Center Core Laboratory. We calculated the Homeostatic Model Assessment of Insulin Resistance (HOMA-IR) index as (fasting insulin [μU/mL] × fasting glucose [mg/dL])/405).
We developed a continuous measure of cardiometabolic risk described by Li et al.15 The five cardiometabolic risk score components were HOMA-IR, triglyceride-to-HDL ratio, leptin-to-adiponectin ratio, systolic blood pressure, and the cross-sectional area of fat inside the abdominal cavity. Except for systolic blood pressure, all individual cardiometabolic risk components were internally age- and sex-standardized via linear regression. The studentized residuals were then employed as the dependent variable.15 HbA1c was similarly standardized. Blood pressure was nationally age-, sex-, and height-standardized before internal standardization.31,32Additionally, since HOMA-IR, the triglycerides-to-HDL ratio, the leptin-to-adiponectin ratio, and the cross-sectional fat area inside the abdominal cavity were right-skewed, these were log2-transformed before standardization. The final cardiometabolic risk z-score is the standardized sum of the five component risk factor z-scores. Higher cardiometabolic risk z-scores indicate greater cardiometabolic risk.
Covariates and modifiers
Drawing on prior literature and employing a directed acyclic graph, we identified potential confounders that may be associated with both PFAS exposure and adolescent cardiometabolic risk while excluding causal intermediates and colliders from consideration (Supplemental Figure S1; http://links.lww.com/EE/A258).33 As we enrolled women mid-pregnancy, we used self-reported prepregnancy height and weight measurements to calculate maternal prepregnancy BMI to avoid measuring pregnancy-related weight gain. When necessary, we imputed maternal prepregnancy weight.34 We used the mean of log10-transformed serum cotinine concentrations at 16 and 26 weeks of gestation to assess gestational tobacco smoke exposure.35 Trained interviewers administered standardized questionnaires during pregnancy or after delivery to collect information on maternal age at delivery, household income, and child race. Similarly, breastfeeding duration data were gathered in interviews during the first 3 years of life. Child sex was abstracted from hospital medical charts.
At the 12-year visit, we collected data on the participant’s physical activity, dietary quality, and pubertal development. We assessed participants’ physical activity using the Physical Activity Questionnaire for Older Children (PAQ-C), a validated measure for general activity level during the school year, and we calculated a summary score for analysis. Trained research staff collected three 24-hour dietary recalls (2 weekdays and 1 weekend day). These recalls were analyzed using the Nutrition Data Systems for Research software and foods database.36 We calculated Healthy Eating Index (HEI) composite scores as a summary dietary quality measure, reflecting how closely an individual’s diet aligns with federal guidelines37; higher scores indicate greater adherence to dietary recommendations. Participants self-assessed their pubertal development using Tanner stages (I–V) based on pubic hair growth. They followed standardized instructions in a private room with a full-length mirror to evaluate their stage accurately.38
Statistical analyses
We used LPA to identify longitudinal PFAS mixture exposure profiles. LPA is a person-centered mixture modeling technique that is used to identify “hidden” groups in the population using measured continuous variables. In this study, LPA uses repeated measures of PFAS exposure biomarkers to identify participant subgroups with similar longitudinal exposure to the four PFAS chemicals. Thus, LPA can be used to identify higher-order interactions among multiple PFAS and characterize how these interact to form profiles that may be differentially associated with predictors and outcomes.39,40
We used the HOME Study cohort LPA profiles previously described by Kuiper et al.25 (Supplemental Methods; http://links.lww.com/EE/A258, Latent profile analysis). Briefly, since LPA requires nonmissing data at all timepoints, we imputed missing PFAS concentrations using multiple imputation by chained equations. We fit 1-, 2-, and 3-class solution LPA models to each imputed dataset using log2-transformed PFAS concentrations. Based on comparable sample-size-adjusted Bayesian Information Criterion, model entropy, and bootstrapped likelihood ratio tests comparing the 2- and 3-profile solutions, we selected the 2-profile solution to ensure adequate sample size in each profile (Supplemental Table S1; http://links.lww.com/EE/A258). Then, we pooled estimates across all the 2-profile, imputed LPA models and used these parameters within each imputed dataset to refit the LPA model and assign participants to their most likely latent profile. We used Stata v15.1 to perform multiple imputations (Stata Statistical Software: Release 15. College Station, TX: StataCorp LLC) and conducted LPA analyses using MPlus version 8.6 (Methuén & Methuén).
We examined the distribution (means/SDs or counts/proportions) of covariates by LPA profile assignment. After these exploratory data analyses, we used linear regression to evaluate the unadjusted and adjusted mean difference in cardiometabolic risk z-scores and individual risk components, comparing those in the higher longitudinal PFAS mixture exposure group to those in the lower longitudinal PFAS mixture exposure group (reference). In our primary analyses, we adjusted for breastfeeding duration (weeks), maternal age at delivery (years), maternal prepregnancy BMI (kg/m2), household income at baseline (dollars), child’s dietary fiber intake at the 12-year visit (g/day), maternal gestational log10-transformed serum cotinine (ng/mL), and child race. We conducted exploratory data analyses and created all models using R, version 4.1.1 (R Foundation for Statistical Computing, Vienna, Austria.).
To evaluate whether child sex- or physical activity modified our associations, we examined the association between longitudinal PFAS exposure group and cardiometabolic risk in sex- or physical-activity-stratified samples. We stratified participants according to whether their PAQ-C score fell above or below the median value in the data. Additionally, we estimated the P value of the interaction coefficient between the modifier and longitudinal PFAS exposure group in the full sample. Earlier research in the same cohort found that physical activity modified the association between gestational PFOA concentrations and adolescent cardiometabolic risk—as such, we evaluated whether physical activity modified the association between longitudinal exposure to a PFAS mixture and adolescent cardiometabolic risk.41
Finally, we performed sensitivity analyses to evaluate our results’ robustness wherein we separately added dietary quality scores and pubertal stage to the primary multivariable linear regression model. Moreover, the exposure groups were fit using postnatal serum biomarker concentrations, including those from the umbilical cord at birth, which correlate with maternal PFAS serum concentrations at 16 weeks of gestation.42 As such, these exposure groups may partially reflect prenatal exposures (Supplemental Table S2; http://links.lww.com/EE/A258). To further isolate lifetime PFAS exposure’s effect on adolescent cardiometabolic risk, we adjusted for prenatal PFOA and PFHxS levels in a sensitivity analysis as these have previously been most strongly associated with cardiometabolic risk in the HOME Study.15 Finally, to address potential selection bias due to baseline differences between included and excluded participants, we conducted an inverse probability-of-censoring weighted regression with a robust variance estimator for the primary multivariable linear regression model using SAS software (SAS Institute Inc., Cary, NC).
Results
A total of 179 children had complete data for this analysis. The mean age of the mothers at delivery was 29 years; 54% of the participants were assigned female at birth (Supplemental Table S3; http://links.lww.com/EE/A258). Compared with the children from the HOME Study who were not included, children in the analytic sample were more likely to be Black and were more likely to come from a household that earns <$20,000 a year (Supplemental Table S3; http://links.lww.com/EE/A258). Finally, the proportion of female participants was similar in both the included and excluded children.
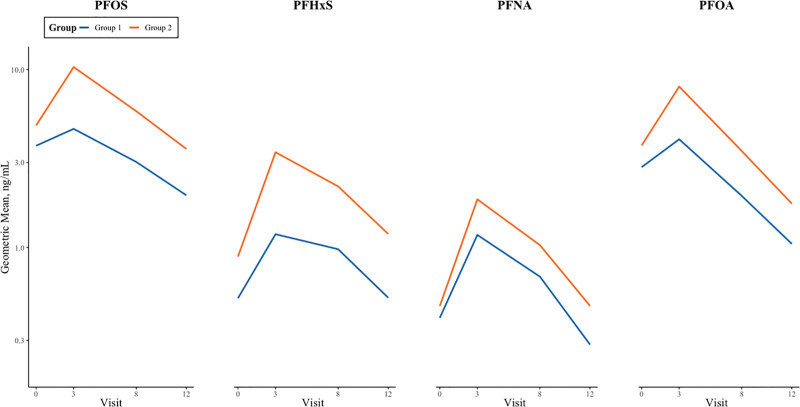
Group 1 (n = 113) was the lower longitudinal PFAS mixture exposure group, while Group 2 (n = 66) was the higher longitudinal PFAS mixture exposure group. Across all four time points, the geometric means of all four measured PFAS were higher among those assigned to the higher exposure group (Figure 1). Those in the higher exposure group had 25–183% higher geometric mean concentrations of the four PFAS across all four visits (Supplemental Table S4; http://links.lww.com/EE/A258). Participants in the higher exposure group were more likely to have mothers who were older at delivery, come from families with higher household income, be non-Hispanic White, and have mothers with lower prepregnancy BMIs than those in the lower longitudinal PFAS mixture exposure group. Additionally, those in the higher exposure group were more likely to have ever been breastfed relative to those with lower exposure (Table 1).
Figure 1.
Geometric mean PFAS biomarker concentration trajectories, stratified by longitudinal PFAS mixture exposure group. Geometric means of PFAS biomarker concentration at birth and ages 3, 8, and 12 years (ng/mL), stratified by longitudinal PFAS mixture exposure group (1 = low exposure, 2 = high exposure) in HOME Study children. PFHxS indicates perfluorohexane sulfonate; PFNA, perfluorononanoic acid; PFOA, Perfluorooctanoic acid; PFOS, perfluorooctane sulfonate.
Table 1.
Maternal and child covariates, and child cardiometabolic risk scores at age 12 years, stratified by longitudinal PFAS mixture exposure group. The HOME Study (n = 179)
| N (%) or mean (SD) | ||
|---|---|---|
| Lower PFAS exposure group n=113 |
Higher PFAS exposure group n=66 |
|
| Prepregnancy BMI (kg/m2) | 28.8 (7.3) | 25.8 (4.5) |
| Maternal age at delivery (years) | 28.0 (6.3) | 30.9 (4.7) |
| Annual household income ($) | ||
| >80,0000 | 20 (17.7) | 24 (36.4) |
| 40,000–80,000 | 34 (30.1) | 26 (39.4) |
| 20,000–40,000 | 18 (15.9) | 11 (16.7) |
| <20,000 | 41 (36.3) | 5 (7.5) |
| Gestational serum cotinine (ng/mL) | ||
| <0.015 (Unexposed) | 26 (23.0) | 23 (34.9) |
| 0.015–3 (Secondhand) | 71 (62.8) | 41 (62.1) |
| >3 (Active smoker) | 16 (14.2) | 2 (3.0) |
| Child race | ||
| Black, non-Hispanic | 63 (55.8) | 7 (10.6) |
| White, non-Hispanic | 43 (38.1) | 55 (83.3) |
| Other | 7 (6.1) | 4 (6.1) |
| Child sex | ||
| Female | 61 (54.0) | 36 (54.5) |
| Male | 52 (46.0) | 30 (45.5) |
| Any breastfeeding | 81 (71.7) | 60 (90.9) |
| Child pubertal stage | ||
| Stage 1 | 7 (6.2) | 9 (13.6) |
| Stage 2 | 27 (24.1) | 19 (28.8) |
| Stage 3 | 27 (24.1) | 24 (36.4) |
| Stage 4 | 30 (26.8) | 8 (12.1) |
| Stage 5 | 21 (18.8) | 6 (9.1) |
| Cardiometabolic risk score | 0.35 (3.33) | -0.40 (3.68) |
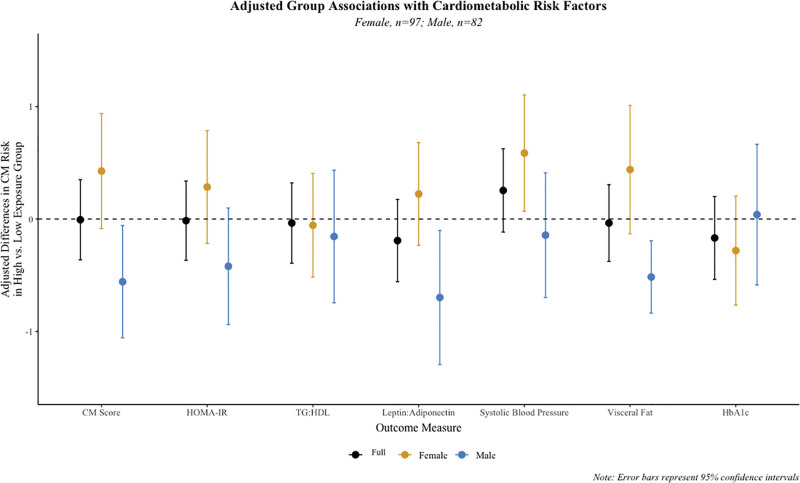
Before adjustment for covariates, children in the higher longitudinal PFAS mixture exposure group had an average cardiometabolic risk z-score 0.2 SDs lower compared to those in the reference group (95% confidence interval [CI] = −0.50, 0.11) (Supplemental Table S5; http://links.lww.com/EE/A258). Consistently, they had lower average HOMA-IR, leptin-to-adiponectin ratios, visceral fat, and hemoglobin A1c z-scores. However, after adjustment for covariates, most estimates were attenuated, all 95% CIs included the null (Figure 2, Supplemental Table S5; http://links.lww.com/EE/A258), and membership in the higher longitudinal PFAS mixture exposure group was not associated with the cardiometabolic risk z-score (β = 0.01; 95% CI = −0.36, 0.35).
Figure 2.
Adjusted mean difference in cardiometabolic risk and individual risk component z-scores at age 12 years between those in the higher and lower (reference) longitudinal PFAS mixture exposure group in the HOME Study: In the full and sex-stratified samples. Results are stratified by participant sex; yellow and blue circles represent adjusted estimates for females and males respectively. Black circles represent adjusted estimates for the full sample. All associations are adjusted for breastfeeding duration (weeks, continuous), maternal age at delivery (years, continuous), maternal prepregnancy BMI (kg/m2 continuous), household income at baseline (dollars, continuous), dietary fiber (g/day, continuous), log 10-trasformed gestational serum cotinine (ng/mL, continuous) and child race (White, non-Hispanic/Other). In each model, the outcomes are internally standardized values of individual cardiometabolic risk components or their combined total (composite risk score), which enables comparison across these components. Interaction term P values between LPA group and participant sex were 0.06, 0.2, 0.6, 0.08, 0.07, 0.02, and 0.9, respectively. The sample size for the HbA1c analysis was n = 175. CM Score indicates cardiometabolic risk score; HbA1C, hemoglobin A1c; HOMA-IR, Homeostatic Model Assessment for Insulin Resistance; Leptin: Adiponectin, leptin/adiponectin ratio; TG:HDL, triglyceride/high-density lipoprotein ratio.
Child sex modified the association between longitudinal PFAS mixture exposure group and some cardiometabolic risk components; we observed adverse associations in females but not males for cardiometabolic risk z-scores (P-interaction: 0.06), leptin-to-adiponectin ratios (P-interaction: 0.08), systolic blood pressures (P-interaction: 0.07), and visceral fat (P-interaction: 0.02). Females assigned to the higher exposure group had 0.6 SDs higher average systolic blood pressures (95% CI = 0.1, 1.1) than those in the lower exposure group (Figure 2, Supplemental Table S6; http://links.lww.com/EE/A258). Similarly, we observed a positive association with both the composite cardiometabolic risk measure and visceral fat, though the CIs included the null (ß = 0.43; 95% CI = −0.08, 0.94; and ß = 0.44; 95% CI = −0.13, 1.01, respectively) (Figure 2, Supplemental Table S6; http://links.lww.com/EE/A258).
Male participants assigned to the higher longitudinal PFAS mixture exposure group had, on average, 0.52 SDs lower cardiometabolic risk scores than males in the lower exposure group (95% CI = −1.06, −0.06). Males in the higher exposure group also had lower average leptin-to-adiponectin ratios, visceral fat z-scores, and HOMA-IR scores (ß = −0.7 to −0.42) (Figure 2, Supplemental Table S6; http://links.lww.com/EE/A258).
The median of children’s self-reported physical activity (PAQ-C) in the sample was 2.53. Children’s self-reported physical activity did not modify the association between the longitudinal PFAS mixture exposure group and cardiometabolic risk (interaction P values >0.2) (Supplemental Figure S2; http://links.lww.com/EE/A258).
In the full and sex-stratified samples, our findings were robust to additional adjustment for pubertal development and Healthy Eating Index scores (Supplemental Table S7; http://links.lww.com/EE/A258). Moreover, when further adjusting for prenatal PFOA and PFHxS, the effect estimates’ magnitude increased slightly in the full sample but continued to include the null (Supplemental Table S7; http://links.lww.com/EE/A258) and remained consistent in the sex-stratified samples (Supplemental Table S8; http://links.lww.com/EE/A258, Supplemental Table S9; http://links.lww.com/EE/A258). Similarly, the estimates and CIs from our primary multivariable linear regression model were consistent after applying an inverse probability-of-censoring weighted regression with a robust variance estimator (Supplemental Table S10; http://links.lww.com/EE/A258).
Discussion
We assessed the association of exposure to a mixture of PFOA, PFOS, PFNA, and PFHxS from birth to age 12 years with cardiometabolic risk at age 12 years in the HOME Study. Using LPA, we identified two longitudinal PFAS exposure patterns; one group had higher PFAS exposures across all four timepoints. In the entire sample, cardiometabolic risk was similar in the higher and lower longitudinal PFAS mixture exposure groups; however, sex-stratified analyses revealed that females in the higher exposure group had higher cardiometabolic risk, while males in the higher exposure group predominantly exhibited lower risk.
Participants in the higher longitudinal PFAS mixture exposure group were more likely to have older mothers, come from wealthier families, be non-Hispanic White, and have ever been breastfed. In the full sample, serum PFAS biomarker concentrations increased from birth to age 3 years and decreased thereafter. This trend, which is consistent with previous findings in the HOME Study and other cohorts, may be attributed to PFAS phase-outs, increased blood volume with age, and varying PFAS exposure from human milk during childhood.5,33,43–45
Our finding that child sex modified the association between longitudinal PFAS mixture exposure group and certain cardiometabolic risk factors, with adverse associations observed in females but not males, is consistent with previous studies. A cross-sectional study of highly-exposed Italian children (8–11 years old) found that the positive association of PFOS and PFHxS with total cholesterol and low-density lipoprotein cholesterol (LDL-C) was higher among females.12 In the Project Viva Cohort, a prospective cohort study of 665 mother-child pairs in Boston, the inverse association of PFAS exposure with HOMA-IR was more pronounced among female participants.16 In the same cohort, PFAS exposure was associated with detrimental changes in lipid profiles among females but not males.18 In the SOLAR study, a longitudinal study of overweight/obese adolescents in Southern California, serum PFHxS concentrations were associated with dysregulated glucose metabolism among females.17 Finally, in a Danish birth cohort, prenatal PFOA exposure was positively associated with waist circumference among female participants at age twenty.46 Although the PFAS type, exposure timing, and cardiometabolic outcome varies between studies, together these findings suggest that early-life PFAS exposure may have sex-specific effects on cardiometabolic risk, though exact mechanisms remain unclear.
The SOLAR Cohort study found that PFHxS correlated with worse beta-cell function in females.17 The authors suggest that PFAS might affect estrogen receptor-alpha (ER- ); since these receptors play a critical role in beta-cell function, ER- disruption could explain the observed association in females but not males.47 Furthermore, PFAS’ potential impact on sex-hormone production might account for the sexual dimorphism in the observed effect. In the HOME Study cohort, PFAS exposure at age 12 years was associated with decreased estradiol levels in females but was not associated with serum sex hormone levels in males.48 A cross-sectional study of 2,292 children (6–9 years old) from the C8 Health Project reported negative associations in males of PFOA with testosterone and PFOS with estradiol, testosterone, and IGF-1. Meanwhile, in females, while PFOS was negatively associated with testosterone and IGF-1, none of the PFAS were associated with estradiol.49 Finally, in vitro studies revealed that PFAS possess estrogenic and antiandrogenic effects, which could account for sex-specific effects.12
Few studies have investigated the link between PFAS exposure and a composite cardiometabolic risk measure in children. A cross-sectional study involving 474 adolescents (12–20 years old) from the 1999–2000 and 2003–2004 National Health and Nutrition Examination Surveys discovered an inverse correlation between serum PFNA concentrations and the prevalence of metabolic syndrome but did not evaluate effect modification by sex.50 In the INMA birth cohort study, researchers reported a positive association of prenatal PFOS, PFOA, and PFNA concentrations with cardiometabolic risk scores at age 4 and no evidence of sex-specific differences.51 The discrepancies might stem from the window of exposure (prenatal vs. postnatal) and the age of participants (4 years vs. 12 years). In another report from the HOME Study cohort that investigated exposure to individual PFAS at single timepoints in childhood, investigators found that at age 12 years, gestational PFOA and PFHxS exposure was associated with higher cardiometabolic risk, PFNA and PFOS exposure at age three was associated with lower cardiometabolic risk and there were no sex-specific patterns.15 Differences between our findings and these previous results could stem from categorizing participants based on longitudinal exposure to a PFAS mixture, especially considering that PFAS exposure occurs as a mixture.19
Most previous studies, albeit cross-sectional, consistently report positive associations between PFAS exposure and total cholesterol and LDL-C in children. A 2008 systematic review of PFAS exposure and health outcomes in children identified five cross-sectional studies that examined dyslipidemia.50 Since then, three additional cross-sectional studies and one matched case–control study have reported positive associations between PFAS, total cholesterol, and LDL-C.9,11,12,52 Only two of these identified a positive association between PFAS and triglycerides. While most research on this topic comes from cross-sectional studies, a consistent positive association has emerged between PFAS, total cholesterol, and LDL-C in children. We did not find an association between longitudinal exposure to a PFAS mixture and the ratio of triglycerides-to-HDL-C, which may align with some previous studies.8,11
The existing literature on the association between PFAS and other components of cardiometabolic risk in children remains inconclusive. One cross-sectional and one longitudinal study in European children found positive associations between PFAS exposure and blood pressure in children and adolescents22,52; meanwhile, the cross-sectional study of highly-exposed Italian children did not find an association.12 A longitudinal study of 444 European adolescents found that higher serum PFAS concentrations were associated with lower leptin in the entire sample and lower adiponectin in males.13 Similarly, in a study of 490 mother-child pairs in the Faroe Islands, PFAS biomarker concentrations at 18 months and 5 and 9 years were associated with decreased leptin at age nine.53
A unique strength of this study is that LPA enabled us to identify two distinct longitudinal PFAS exposure groups that differed by covariates such as breastfeeding, which may contribute to higher PFAS exposure in children.54–56 This has implications for policy-directed research: LPA may help researchers concisely characterize longitudinal exposure patterns, describe potential routes and related risk factors, and estimate the impact of longitudinal exposure to chemical mixtures on health. Additionally, we used a continuous cardiometabolic risk measure, which may better predict cardiovascular disease later in life.57–60 Furthermore, we adjusted for many potential confounders because the HOME Study collected information about a wide array of covariates.
Our study is not without limitations. Our sample size was modest, especially in sex-stratified analyses. Furthermore, LPA assumes a homogenous exposure pattern within a group, which may not hold in the real world since exposure pathways can be complex. Moreover, LPA does not capture windows of sensitivity or highlight the effect of individual PFAS. Additionally, children in the higher PFAS exposure group exhibited lower cardiometabolic risk in unadjusted analyses, but after adjustment, most of these associations were attenuated. The observed positive confounding could partly stem from adjusting for breastfeeding. Previous studies found a positive association between breastfeeding duration and PFAS levels.33 Breastfeeding is also positively associated with maternal education and socioeconomic status,61,62 which are, in turn, associated with children’s cardiometabolic health. If residual confounding is present, it may follow a similar pattern: the associations in females may be negatively confounded, whereas the inverse associations in males may be positively confounded. Finally, although we used birth serum PFAS measurements to create the exposure groups, PFAS concentrations obtained from umbilical cord serum are highly correlated with maternal PFAS serum concentrations at 16 weeks of gestation.41 Therefore, our exposure groups represent both gestational and postnatal exposures, complicating the interpretation of the specific impact of postnatal longitudinal exposure to a PFAS mixture.
Conclusions
We used LPA to characterize two distinct longitudinal PFAS mixture exposure groups and related these to a continuous adolescent cardiometabolic risk measure. In the full sample, we did not observe an association between higher exposure to a mixture of PFAS over the first 12 years of life and cardiometabolic risk. However, in stratified analyses, females in the higher exposure group had, for the most part, greater cardiometabolic risk, while the inverse was true in males. These results suggest that higher childhood exposure to PFAS may have sex-specific effects on adolescent cardiometabolic outcomes.
Conflicts of interest statement
J.B. and C.E. were financially compensated for their services as expert witnesses for plaintiffs in litigation related to PFAS-contaminated drinking water. Other authors declare that they have no conflicts of interest with regard to the content of this report.
Supplementary Material
Footnotes
Supported by National Institute of Environmental Health Sciences grants R01 ES032836, R01 ES025214, P01 ES011261, R01 ES014575, R01 ES020349, R01 ES027224, and R01 ES033252.
Authors are receiving funding to support open-access publishing.
Data are available upon reasonable request. The HOME Study Principal Investigators welcome new collaborations with other investigators and have actively engaged in collaborative data-sharing projects. Interested investigators should visit https://homestudy.research.cchmc.org/contact or contact J.M.B. (joseph_braun_1@brown.edu) and K.Y. (kimberly.yolton@cchmc.org) to obtain additional information about The HOME Study, discuss collaborative opportunities, and request a project proposal form. The HOME Study Protocol Review Committee reviews proposed research projects to ensure that they do not overlap with extant projects and are an efficient use of scarce resources (eg, biospecimens).
Code available upon request. Interested investigators should contact Elvira Fleury (elvira_fleury@brown.edu).
Supplemental digital content is available through direct URL citations in the HTML and PDF versions of this article (www.environepidem.com).
References
- 1.Glüge J, Scheringer M, Cousins IT, et al. An overview of the uses of per- and polyfluoroalkyl substances (PFAS). Environ Sci Processes Impacts. 2020;22:2345–2373. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 2.Kato K, Wong LY, Jia LT, Kuklenyik Z, Calafat AM. Trends in exposure to polyfluoroalkyl chemicals in the US population: 1999−2008. Environ Sci Technol. 2011;45:8037–8045. [DOI] [PubMed] [Google Scholar]
- 3.Ye X, Kato K, Wong LY, et al. Per- and polyfluoroalkyl substances in sera from children 3 to 11 years of age participating in the national health and nutrition examination survey 2013–2014. Int J Hyg Environ Health. 2018;221:9–16. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 4.Mondal D, Lopez-Espinosa MJ, Armstrong B, Stein CR, Fletcher T. Relationships of perfluorooctanoate and perfluorooctane sulfonate serum concentrations between mother–child pairs in a population with perfluorooctanoate exposure from drinking water. Environ Health Perspect. 2012;120:752–757. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 5.Wu XM, Bennett DH, Calafat AM, et al. Serum concentrations of perfluorinated compounds (PFC) among selected populations of children and adults in California. Environ Res. 2015;136:264–273. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 6.Rappazzo KM, Coffman E, Hines EP. Exposure to perfluorinated alkyl substances and health outcomes in children: a systematic review of the epidemiologic literature. Int J Environ Res Public Health. 2017;14:691. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 7.Isomaa B, Almgren P, Tuomi T, et al. Cardiovascular morbidity and mortality associated with the metabolic syndrome. Diabetes Care. 2001;24:683–689. [DOI] [PubMed] [Google Scholar]
- 8.Geiger SD, Xiao J, Ducatman A, Frisbee S, Innes K, Shankar A. The association between PFOA, PFOS and serum lipid levels in adolescents. Chemosphere. 2014;98:78–83. [DOI] [PubMed] [Google Scholar]
- 9.Koshy TT, Attina TM, Ghassabian A, et al. Serum perfluoroalkyl substances and cardiometabolic consequences in adolescents exposed to the World Trade Center disaster and a matched comparison group. Environ Int. 2017;109:128–135. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 10.Zeng XW, Qian Z, Emo B, et al. Association of polyfluoroalkyl chemical exposure with serum lipids in children. Sci Total Environ. 2015;512-513:364–370. [DOI] [PubMed] [Google Scholar]
- 11.Khalil N, Ebert JR, Honda M, et al. Perfluoroalkyl substances, bone density, and cardio-metabolic risk factors in obese 8–12 year old children: a pilot study. Environ Res. 2018;160:314–321. [DOI] [PubMed] [Google Scholar]
- 12.Canova C, Di Nisio A, Barbieri G, et al. PFAS concentrations and cardiometabolic traits in highly exposed children and adolescents. Int J Environ Res Public Health. 2021;18:12881. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 13.Domazet SL, Grøntved A, Timmermann AG, Nielsen F, Jensen TK. Longitudinal associations of exposure to perfluoroalkylated substances in childhood and adolescence and indicators of adiposity and glucose metabolism 6 and 12 years later: the European Youth Heart Study. Diabetes Care. 2016;39:1745–1751. [DOI] [PubMed] [Google Scholar]
- 14.Fassler CS, Pinney SE, Xi C, Biro FM, Pinney SM. Complex relationships between perfluorooctanoate, body mass index, insulin resistance and serum lipids in young girls. Environ Res. 2019;176:108558. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 15.Li N, Liu Y, Papandonatos GD, et al. Gestational and childhood exposure to per- and polyfluoroalkyl substances and cardiometabolic risk at age 12 years. Environ Int. 2021;147:106344. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 16.Fleisch AF, Rifas-Shiman SL, Mora AM, et al. Early-Life exposure to perfluoroalkyl substances and childhood metabolic function. Environ Health Perspect. 2017;125:481–487. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 17.Goodrich JA, Alderete TL, Baumert BO, et al. Exposure to perfluoroalkyl substances and glucose homeostasis in youth. Environ Health Perspect. 2021;129:97002. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 18.Mora AM, Fleisch AF, Rifas-Shiman SL, et al. Early life exposure to per- and polyfluoroalkyl substances and mid-childhood lipid and alanine aminotransferase levels. Environ Int. 2018;111:1–13. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 19.Goodrich JA, Walker DI, He J, et al. Metabolic signatures of youth exposure to mixtures of per- and polyfluoroalkyl substances: a multi-cohort study. Environ Health Perspect. 2023;131:27005. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 20.Chen Z, Yang T, Walker DI, et al. Dysregulated lipid and fatty acid metabolism link perfluoroalkyl substances exposure and impaired glucose metabolism in young adults. Environ Int. 2020;145:106091. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 21.Kingsley SL, Walker DI, Calafat AM, et al. Metabolomics of childhood exposure to perfluoroalkyl substances: a cross-sectional study. Metabolomics. 2019;15:95. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 22.Papadopoulou E, Stratakis N, Basagaña X, et al. Prenatal and postnatal exposure to PFAS and cardiometabolic factors and inflammation status in children from six European cohorts. Environ Int. 2021;157:106853. [DOI] [PubMed] [Google Scholar]
- 23.Kuiper J, Braun J, Liu S, et al. Latent profiles of early life perfluoroalkyl substances exposure and body composition at age 12 years: the Health Outcomes and Measures of the Environment (HOME) Study. ISEE Conference Abstracts. 2022;2022 [Google Scholar]
- 24.Yonkman AM, Alampi JD, Kaida A, et al. Using latent profile analysis to identify associations between gestational chemical mixtures and child neurodevelopment. Epidemiology. 2022;34:45–55. [DOI] [PubMed] [Google Scholar]
- 25.Kuiper JR, Liu S, Lanphear B, et al. Estimating effects of longitudinal and cumulative exposure to PFAS mixtures on early adolescent body composition. Am J Epidemiol. 2023. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 26.Braun JM, Kalloo G, Chen A, et al. Cohort profile: the Health Outcomes and Measures of the Environment (HOME) study. Int J Epidemiol. 2017;46:24. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 27.Braun JM, Buckley JP, Cecil KM, et al. Adolescent follow-up in the Health Outcomes and Measures of the Environment (HOME) study: cohort profile. BMJ Open. 2020;10:e034838. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 28.Kato K, Basden BJ, Needham LL, Calafat AM. Improved selectivity for the analysis of maternal serum and cord serum for polyfluoroalkyl chemicals. J Chromatogr A. 2011;1218:2133–2137. [DOI] [PubMed] [Google Scholar]
- 29.Wang Z, Heymsfield SB, Chen Z, Zhu S, Pierson RN. Estimation of percentage body fat by dual-energy x-ray absorptiometry: evaluation by in vivo human elemental composition. Phys Med Biol. 2010;55:2619–2635. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 30.Lacruz ME, Kluttig A, Kuss O, et al. Short-term blood pressure variability – variation between arm side, body position and successive measurements: a population-based cohort study. BMC Cardiovasc Disord. 2017;17:31. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 31.Expert panel on integrated guidelines for cardiovascular health and risk reduction in children and adolescents: summary report. Pediatrics. 2011;128(Suppl 5):S213–S256. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 32.National High Blood Pressure Education Program Working Group on High Blood Pressure in Children and Adolescents. The fourth report on the diagnosis, evaluation, and treatment of high blood pressure in children and adolescents. Pediatrics. 2004;114(Supplement_2):555–576. [PubMed] [Google Scholar]
- 33.Kingsley SL, Eliot MN, Kelsey KT, et al. Variability and predictors of serum perfluoroalkyl substance concentrations during pregnancy and early childhood. Environ Res. 2018;165:247–257. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 34.Braun JM, Eliot M, Papandonatos GD, et al. Gestational perfluoroalkyl substance exposure and body mass index trajectories over the first 12 years of life. Int J Obes (Lond). 2021;45:25–35. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 35.Braun JM, Daniels JL, Poole C, et al. A prospective cohort study of biomarkers of prenatal tobacco smoke exposure: the correlation between serum and meconium and their association with infant birth weight. Environ Health. 2010;9:53. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 36.Sievert YA, Schakel SF, Buzzard IM. Maintenance of a nutrient database for clinical trials. Control Clin Trials. 1989;10:416–425. [DOI] [PubMed] [Google Scholar]
- 37.Guenther PM, Casavale KO, Reedy J, et al. Update of the healthy eating index: HEI-2010. J Acad Nutr Diet. 2013;113:569–580. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 38.Jones NHY, Khoury JC, Xu Y, et al. Comparing adolescent self staging of pubertal development with hormone biomarkers. J Pediatr Endocrinol Metab. 2021;34:1531–1541. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 39.Hendryx M, Luo J. Latent class analysis to model multiple chemical exposures among children. Environ Res. 2018;160:115–120. [DOI] [PubMed] [Google Scholar]
- 40.Spurk D, Hirschi A, Wang M, Valero D, Kauffeld S. Latent profile analysis: a review and “how to” guide of its application within vocational behavior research. J Vocat Behav. 2020;120:103445. [Google Scholar]
- 41.Braun JM, Papandonatos GD, Li N, et al. Physical activity modifies the relation between gestational perfluorooctanoic acid exposure and adolescent cardiometabolic risk. Environ Res. 2022;214:114021. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 42.Kato K, Wong LY, Chen A, et al. Changes in serum concentrations of maternal poly- and perfluoroalkyl substances over the course of pregnancy and predictors of exposure in a multiethnic cohort of Cincinnati, Ohio pregnant women during 2003-2006. Environ Sci Technol. 2014;48:9600–9608. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 43.Mogensen UB, Grandjean P, Nielsen F, Weihe P, Budtz-Jørgensen E. Breastfeeding as an exposure pathway for perfluorinated alkylates. Environ Sci Technol. 2015;49:10466–10473. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 44.Papadopoulou E, Sabaredzovic A, Namork E, Nygaard UC, Granum B, Haug LS. Exposure of Norwegian toddlers to perfluoroalkyl substances (PFAS): the association with breastfeeding and maternal PFAS concentrations. Environ Int. 2016;94:687–694. [DOI] [PubMed] [Google Scholar]
- 45.Olsen GW, Lange CC, Ellefson ME, et al. Temporal trends of perfluoroalkyl concentrations in American Red Cross adult blood donors, 2000–2010. Environ Sci Technol. 2012;46:6330–6338. [DOI] [PubMed] [Google Scholar]
- 46.Halldorsson TI, Rytter D, Haug LS, et al. Prenatal exposure to perfluorooctanoate and risk of overweight at 20 years of age: a prospective cohort study. Environ Health Perspect. 2012;120:668–673. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 47.Gannon M, Kulkarni RN, Tse HM, Mauvais-Jarvis F. Sex differences underlying pancreatic islet biology and its dysfunction. Mol Metab. 2018;15:82–91. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 48.Liu Y, Calafat AM, Chen A, et al. Associations of prenatal and postnatal exposure to perfluoroalkyl substances with pubertal development and reproductive hormones in females and males: the HOME study. Sci Total Environ. 2023;890:164353. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 49.Lopez-Espinosa MJ, Mondal D, Armstrong BG, Eskenazi B, Fletcher T. Perfluoroalkyl substances, sex hormones, and insulin-like growth factor-1 at 6–9 years of age: a cross-sectional analysis within the C8 health project. Environ Health Perspect. 2016;124:1269–1275. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 50.Lin CY, Chen PC, Lin YC, Lin LY. Association among serum perfluoroalkyl chemicals, glucose homeostasis, and metabolic syndrome in adolescents and adults. Diabetes Care. 2008;32:702–707. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 51.Manzano-Salgado CB, Casas M, Lopez-Espinosa MJ, et al. Prenatal exposure to perfluoroalkyl substances and cardiometabolic risk in children from the Spanish INMA birth cohort study. Environ Health Perspect. 2017;125:097018. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 52.Averina M, Brox J, Huber S, Furberg AS. Exposure to perfluoroalkyl substances (PFAS) and dyslipidemia, hypertension and obesity in adolescents the fit futures study. Environ Res. 2021;195:110740. [DOI] [PubMed] [Google Scholar]
- 53.Shih YH, Blomberg AJ, Jørgensen LH, Weihe P, Grandjean P. Early-life exposure to perfluoroalkyl substances in relation to serum adipokines in a longitudinal birth cohort. Environ Res. 2022;204:111905. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 54.Fromme H, Mosch C, Morovitz M, et al. Pre- and postnatal exposure to perfluorinated compounds (PFCs). Environ Sci Technol. 2010;44:7123–7129. [DOI] [PubMed] [Google Scholar]
- 55.Haug LS, Huber S, Becher G, Thomsen C. Characterisation of human exposure pathways to perfluorinated compounds--comparing exposure estimates with biomarkers of exposure. Environ Int. 2011;37:687–693. [DOI] [PubMed] [Google Scholar]
- 56.Thomsen C, Haug LS, Stigum H, Frøshaug M, Broadwell SL, Becher G. Changes in concentrations of perfluorinated compounds, polybrominated diphenyl ethers, and polychlorinated biphenyls in Norwegian breast-milk during twelve months of lactation. Environ Sci Technol. 2010;44:9550–9556. [DOI] [PubMed] [Google Scholar]
- 57.Kamel M, Smith BT, Wahi G, Carsley S, Birken CS, Anderson LN. Continuous cardiometabolic risk score definitions in early childhood: a scoping review. Obes Rev. 2018;19:1688–1699. [DOI] [PubMed] [Google Scholar]
- 58.Kelly AS, Steinberger J, Jacobs DR, Hong CP, Moran A, Sinaiko AR. Predicting cardiovascular risk in young adulthood from the metabolic syndrome, its component risk factors, and a cluster score in childhood. Int J Pediatr Obes. 2011;6(sup3):e283–e289. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 59.Magge SN, Goodman E, Armstrong SC; COMMITTEE ON NUTRITION. The metabolic syndrome in children and adolescents: shifting the focus to cardiometabolic risk factor clustering. Pediatrics. 2017;140:e20171603. [DOI] [PubMed] [Google Scholar]
- 60.Sovio U, Skow A, Falconer C, Park MH, Viner RM, Kinra S. Improving prediction algorithms for cardiometabolic risk in children and adolescents. J Obes. 2013;2013:e684782. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 61.Flacking R, Nyqvist KH, Ewald U. Effects of socioeconomic status on breastfeeding duration in mothers of preterm and term infants. Eur J Public Health. 2007;17:579–584. [DOI] [PubMed] [Google Scholar]
- 62.Scott JA, Binns CW. Factors associated with the initiation and duration of breastfeeding: a review of the literature. Breastfeed Rev. 1999;7:5–16. [PubMed] [Google Scholar]