Abstract
Helicobacter pylori infection is widespread in some breeding groups of a rhesus monkey colony (71% H. pylori positive by 1 year), and the rate of seroconversion is also high. As a result, these groups can be used to test the safety and efficacy of an anti-H. pylori vaccine. Nine-month-old female animals were randomized to receive either 8 mg of recombinant urease (rUre) plus 25 μg of Escherichia coli heat-labile enterotoxin (LT) (n = 26) or placebo plus LT (n = 29), given four times at 1-week intervals followed by a booster 1 month later. Ten months after the start of the immunization, the animals were subjected to endoscopy and biopsy samples were obtained. H. pylori negativity was defined as no H. pylori growth by culture and no H. pylori observed at histology. By this criterion, 2 (7%) of 29 animals receiving placebo and 8 (31%) of 26 immunized animals were H. pylori negative (P < 0.035). In addition, antral gastritis score was significantly less in H. pylori-negative immunized monkeys than in H. pylori-positive animals, whether they were given rUre plus LT or placebo plus LT (P < 0.02 or P < 0.01, respectively). Interestingly, antral gastritis was also significantly less in H. pylori-positive animals given rUre plus LT than in H. pylori-positive animals given placebo plus LT (P < 0.02). However, quantitative cultures did not demonstrate significant differences between the two latter groups. It is concluded that oral administration of rUre vaccine plus LT significantly protects nonhuman primates against H. pylori infection while not causing undesirable side effects.
Helicobacter pylori is present in the stomachs of most humans throughout the five continents. It causes chronic active gastritis, duodenal ulcer, and gastric ulcer disease, and it considerably increases the risk of gastric cancer (1, 2, 39). Many antimicrobials can prevent the growth of H. pylori in vitro, but eradication of the bacterium from the stomach is often difficult. This lack of in vivo efficacy may be due to the breakdown of the antibiotic by gastric acid and the fact that the bacterium resides in a layer of mucus in which the antibiotic cannot penetrate easily. A combination of at least two, and sometimes three, antimicrobials associated with an antisecretory agent (especially proton pump inhibitors) has been effective in 60 to 90% of the patients treated. However, H. pylori can become resistant to specific antibiotics such as metronidazole and, more recently, clarithromycin (32). Therefore, it is imperative to find new approaches for the treatment and prevention of this universal infection. One particularly attractive strategy is to develop a safe and effective vaccine against H. pylori.
The feasibility of prophylactic vaccination against Helicobacter infection was demonstrated by experiments in which administration of H. pylori sonicates, together with the mucosal adjuvant cholera toxin (CT), induced the production of specific serum and gastrointestinal immunoglobulin A (IgA) and IgG (7). Subsequent studies indeed demonstrated that administration of H. pylori or H. felis antigens given concurrently with CT or Escherichia coli heat-labile enterotoxin (LT) protected mice against experimental infection with H. felis (6, 19, 26, 28) and H. pylori (29). In addition, the feasibility of curative immunization against established H. felis infection in mice was demonstrated when bacterial sonicates, given together with CT, were found to eradicate the organism in 50% of animals experimentally infected with H. felis (10). The next step in the development of a prophylactic, and possibly curative, vaccine in the murine model was completed when it was demonstrated that (i) mice given recombinant enzymatically inactive urease B (but not urease A) were protected against experimental infection with H. felis (34) and (ii) the same procedure cured 50% of mice experimentally inoculated with H. felis (4).
Although the mouse model had to be used in these initial steps of the development of a vaccine, the mouse is not a natural host for either H. felis or H. pylori (25), establishment of a chronic infection requires selection of particular strains of H. pylori (27, 29) and/or mice (31), and the duration of observation is limited by the life span of the animal. In addition, the pattern of the colonization as well as the associated inflammatory response are different from those observed in H. pylori-infected humans (8). The observation that treatment with tinidazole alone results in 100% eradication of H. felis infection in this mouse model (23) exemplifies the relevance of these differences. Therefore, an animal that can be naturally infected by the cognate Helicobacter had to be used at the preclinical stage to evaluate the efficacy of a vaccine. Ferrets are naturally infected by H. mustelae (20), but there has been no previous study exploring the question of prophylactic vaccination in this model. In contrast, a recent curative immunization study has shown that 30% of ferrets naturally infected with H. mustelae were cured of this infection following administration of doses ranging from 0.1 to 10 mg of recombinant urease (rUre) plus CT, there being no dose-response effect (5). However, H. mustelae, like H. felis, is genetically and phenotypically different from H. pylori, and the pattern of colonization by this organism differs from that observed in H. pylori-infected humans (36). As in the case of the mouse model, the clinical relevance of these differences was recently demonstrated by the observation that, in contrast to the observations in mice and ferrets, none of the H. pylori-infected volunteers who received the same treatment (i.e., rUre plus LT) were cured at the end of the 2-month study period, although a decrease in colonization was observed in 5 of 14 subjects (35).
A most human-like model of H. pylori infection is provided by the rhesus monkey. This species can be naturally infected with H. pylori when socially housed (14, 15, 22), and gastric colonization by the organism is consistently associated with chronic active gastritis (16). In addition, rhesus monkeys can develop persistent infection following a single intragastric administration of H. pylori isolated from humans (12, 13). Finally, the classical triple therapy (metronidazole, amoxicillin, and bismuth subsalicylate twice a day) is only 60% curative (14), while the more recent clarithromycin-omeprazole-based treatment is more effective (11). Thus, although the use of monkeys is restricted due to the specialized care that is required, the involvement of a primate model appears to be an essential step in the development of anti-H. pylori vaccines (24). Therefore, the rhesus monkey model of naturally acquired H. pylori infection was selected to determine the safety and efficacy of a rUre vaccine.
(This report was presented in part at the American Gastroenterological Association’s Digestive Diseases Week, Washington, D.C., 11 to 14 May 1997.)
MATERIALS AND METHODS
Animals.
For this study, female rhesus monkeys (Macaca mulatta), 9 months of age and weighing 1.5 to 2.0 kg, were used. The animals were bred, reared, and socially housed in 60- by 100-ft outdoor corrals with grass and dirt substrate and a large arboreal jungle gym; they were provided with tap water ad libitum, commercial primate chow, and fruits. The mean group size was 30 animals. All animals tested negative following three intradermal tuberculin injections at 2-week intervals. All experiments were approved by an Institutional Animal Care and Use Committee as required by the Animal Use Welfare Act. This animal facility is approved by the American Association for Accreditation of Laboratory Animal Care. The experiments reported herein were conducted according to the principles set forth in reference 35a.
Selection and randomization.
Serum IgG levels were determined in 109 female rhesus monkeys by using an enzyme-linked immunosorbent assay (ELISA) against H. pylori sonicate antigen and H. pylori urease. Sixty of these animals with the lowest ELISA reactivities to the two antigens were selected and then randomized into two groups of 30 animals each (see below). IgG ELISA units were comparable in the two groups and were similar to those of rhesus monkeys that were H. pylori negative by culture (well below the levels observed in H. pylori-positive monkeys).
Immunization procedure.
All animals were captured and lightly anesthetized with ketamine HCl (10 mg/kg of body weight), and serum was obtained for IgG antibody determination. Ten to 15 days later, the animals were again captured and lightly anesthetized with ketamine and were given, in a blinded fashion, the contents of coded vials containing either lyophilized placebo or rUre reconstituted in water and admixed with LT. Group 1 received placebo plus 25 μg of LT, and group 2 received 8 mg of rUre plus 25 μg LT. The dose of urease selected was comparable to the body-weight-adjusted dose found to be effective in mice (28). An identical dose was sprayed on the back of the throat of each animal. The procedure was performed four times at 1-week intervals, with a fifth dose given 1 month later. Throughout the observation period, veterinary technicians and caretakers were instructed to report symptoms of anorexia, vomiting, withdrawal, and diarrhea.
Follow-up.
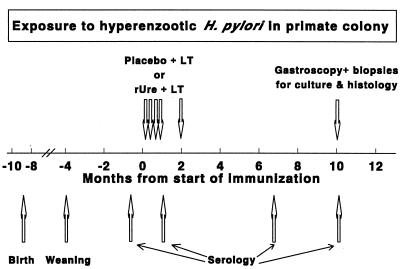
One week after the fourth dose and again 6 months later, serum was obtained for determination of serum anti-H. pylori IgG (Fig. 1). Three and a half months later (i.e., 10 months after the first immunization), 58 of the 60 animals were subjected to endoscopy under anesthesia to examine the macroscopic appearance of the stomach and to obtain gastric mucosal biopsy samples (see below). In each group, one animal could not be captured and was not available for endoscopy.
FIG. 1.
Time course of experiments. Arrows represent the time at which the corresponding event or intervention occurred. (n = 30 in each group before and after immunization; n = 29 in each group for endoscopy).
Endoscopic procedures and biopsies.
After an overnight fast with free access to water, the animals were anesthetized (atropine sulfate [0.02 mg/kg intramuscularly, followed by ketamine HCl [10 mg/kg] plus acepromazine [0.1 mg/kg] intramuscularly) and then underwent gastroscopy using a VB-1530T Video Bronchoscope (Pentax Instruments, Orangeburg, N.Y.) with an outer diameter of 5.0 mm. In each animal, pinch mucosal biopsy samples were taken from the gastric corpus (n = 4) and antrum (n = 5). Between each endoscopy, the equipment was flushed, rinsed, disinfected sequentially with water, a proteolytic solution (Metrizyme; Metrex Research Corp., Parker, Colo.), water, and activated dialdehyde solution of 2% glutaraldehyde (Cidex; Johnson & Johnson Medical, Inc., Arlington, Tex.), and then air dried.
Histologic examination.
Two biopsy samples each from the distal corpus and the distal antrum were fixed in neutral 10% buffered formalin and embedded in paraffin. Then 5-μm-thick sections were stained with hematoxylin and eosin and viewed under magnification of ×100 to ×1,000. Antral gastritis was scored on coded slides, using a scale of 0 to 3 modified from that of Marshall and Warren (30) (0, intact mucosal lining and essentially no infiltration of the lamina propria with monocytes; 1, mild increase of mononuclear infiltration, localized in the upper half of the mucosa; 2, mononuclear infiltration extending from the surface into the lamina propria resulting in atrophy; 3, marked mononuclear infiltration extending from the surface into the lamina propria and disrupting the structure of the glands and leading to marked atrophy, and/or polymorphonuclear leukocytes in glands and surface erosions). Slides also were scored for the presence or absence of H. pylori infection after Genta-Robason staining (21).
Microbiological methods.
Five other biopsy samples (two from the corpus and three from the antrum) were immediately inoculated onto Mueller-Hinton agar supplemented with 5% sheep erythrocytes and trimethoprim (5 μg/ml), vancomycin (10 μg/ml), amphotericin B (5 μg/ml), and polymyxin B (10 U/ml) (TVAP; Remel, Lenexa, Kans.) and placed in a plastic bag with an atmosphere of 90% N2, 5% O2, and 5% CO2 (CampyPak Plus; BBL Microbiological Systems, Becton Dickinson and Co., Cockeysville, Md.). The sealed bags were sent by overnight courier to the laboratory, where quantitative cultures were performed. Each biopsy sample was weighed and homogenized in 0.5 ml of Brucella broth supplemented with 5% calf serum and TVAP in a 0.5-ml Dounce homogenizer fitted with a loose pestle. Then 100-μl aliquots of 1:1, 1:10, 1:100, and 1:1,000 dilutions were plated on Mueller-Hinton agar supplemented with 5% sheep erythrocytes and TVPA. The plates were incubated for 5 days at 37°C in 90% N2–5% O2–5% CO2, pinhead-sized colonies were enumerated, and bacterial density was expressed as the total number of CFU for the five biopsy samples (20 mg of tissue). H. pylori-like organisms were confirmed by Gram stain, catalase, oxidase, and indoxyl-acetate tests.
Measurement of serum and salivary antibodies.
Five milliliters of blood was drawn from each monkey on four occasions (Fig. 1): (i) 10 to 15 days before the first administration of placebo plus LT or rUre plus LT; (ii) 1 week after the fourth immunization; (iii) 6 months after the boost; and (iv) at the time of the endoscopy (10 months after the first immunization). Blood was collected into 7-ml tubes and centrifuged, and the supernatant serum was frozen at −70°C. Antibodies were determined via ELISA (see above) by personnel blinded to the treatment code. Flat-bottomed 96-well plates were coated with either 1 μg of crude sonic extract of wild-type H. pylori (for initial screening) or 0.5 μg of purified native H. pylori urease holoenzyme (for testing seroconversion for urease and for subsequent serological analysis of exposure to H. pylori) in carbonate buffer and then blocked with phosphate-buffered saline-Tween containing 2.5% nonfat dry milk. Sera were tested at 1:100 dilution; saliva samples were tested at 1:5 dilution. The detecting antibodies were goat anti-rhesus IgG or IgA conjugated to horseradish peroxidase (Nordic Immunological Laboratories, The Netherlands). ELISAs were performed on all samples on the same day, and standardization of the assay was ensured by including positive and negative controls in each test. All assays were done in duplicate, and differences between duplicates were <1%. Seroconversion in IgG or IgA against urease was defined as an optical density (OD) of >0.2 combined with at least a twofold increase above baseline. The arbitrary cutoff of 0.2 absorbance unit was chosen to ensure that only animals with high values were scored as having seroconverted.
Data analysis and statistical analysis.
Animals were considered to be negative if both histology and culture failed to demonstrate the presence of H. pylori. The statistical significance of differences observed between the two groups was determined by Fisher’s exact test for nonparametric data or analysis of variance with repeated measured for parametric data.
RESULTS
H. pylori status at endoscopy.
To determine whether administration of rUre plus LT significantly protected against H. pylori infection, the animals were examined by endoscopy 10 months after the first immunization, and gastric mucosal biopsy samples were harvested. By histology, 25 of the 29 placebo animals and 15 of the 29 immunized animals were H. pylori positive. By culture, 27 (93%) of 29 placebo animals and 18 of 29 immunized animals were positive. In addition, one or both of the biopsy samples of three immunized animals that were H. pylori negative by histology were contaminated by the growth of other, unidentified bacteria and were therefore excluded from analysis. Thus, by the criterion outlined in Materials and Methods (i.e., H. pylori negativity requires no H. pylori growth by culture and no H. pylori observed at histology), 27 (93%) of 29 placebo animals and 18 (69%) of 26 immunized animals were H. pylori positive (P < 0.035) (Table 1). Quantitative cultures were highly variable between regions within animals, as expected in a focal infectious disease. In addition, the mean gastric bacterial densities were similar in immunized animals and in placebo-treated monkeys (means ± standard errors [SE], 1.9 × 104 ± 1.2 × 104 and 7.3 × 103 ± 3.0 × 103/5 biopsy samples [approximately 20 mg], respectively).
TABLE 1.
Percentage of animals with negative results by histology and culture
| Treatment | No. of animals with indicated resultsa
|
% Negative | |
|---|---|---|---|
| H. pylori negative | H. pylori positive | ||
| Placebo + LT | 2 | 27 | 7 |
| rUre + LT | 8 | 18 | 31 |
P value (Fisher’s exact test) of 0.035.
Histological inflammation at endoscopy.
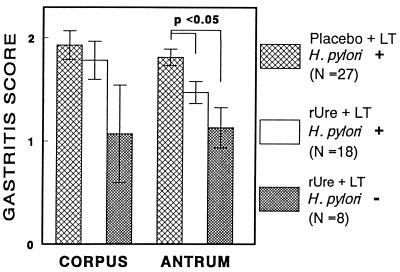
Histological examination of gastric mucosal biopsy samples was performed to determine the effect of immunization on gastric inflammation. Antral gastritis scores were significantly lower in H. pylori-negative immunized monkeys (Fig. 2A) than in H. pylori-positive animals, whether they were given rUre plus LT (Fig. 2B) or placebo plus LT (P < 0.02 and P < 0.01, respectively [Fig. 3]). Interestingly, antral gastritis was also significantly less in H. pylori-positive animals given rUre plus LT than in H. pylori-positive animals given placebo plus LT (P < 0.02 [Fig. 3]). Similar trends were observed in the corpus, but the differences did not reach statistical significance.
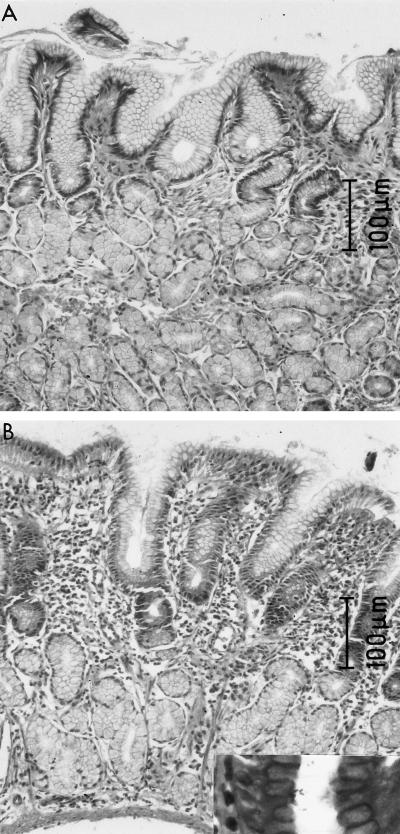
FIG. 2.
(A) H. pylori-negative animal. There are normal epithelial cells both on the surface and within the glands and only minimal mononuclear infiltration (gastritis grade of 0.5). (B) H. pylori-positive animal. Surface and glandular epithelial cells lack normal mucus; there is intense mononuclear infiltration especially on the surface but also through the lamina propria (gastritis grade of 2.0).
FIG. 3.
Effect of rUre plus LT or placebo plus LT and H. pylori infection status (indicated by + or −) on gastritis. Data for the two H. pylori-negative animals that had received placebo plus LT are not illustrated.
Serum and salivary antibody responses.
Serology was performed to determine the H. pylori infection status before immunization as well as the serum antibody response to immunization. Before administration of LT and placebo or rUre, all animals were seronegative by specific anti-H. pylori IgG, and values were similar in animals given rUre and those given placebo animals (mean OD ± SE, 0.167 ± 0.015 and 0.188 ± 0.013, respectively). In addition, the initial serum anti-H. pylori IgG titers were not significantly different in animals that were found to be H. pylori-negative or -positive by endoscopy at the end of the study (mean OD ± SE, 0.165 ± 0.031 versus 0.160 ± 0.018 [group 1] and 0.189 ± 0.014 versus 0.155 ± 0.037 [group 2]).
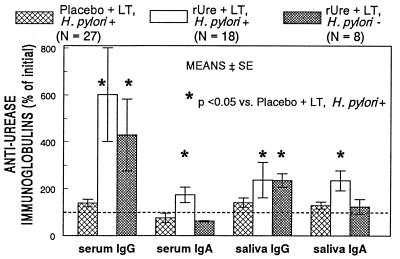
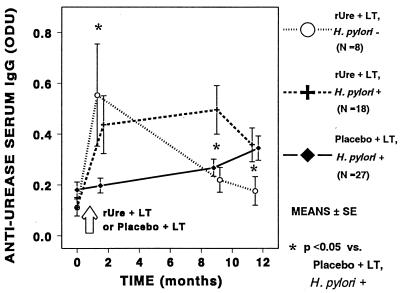
One week after the fourth immunization, specific antiurease IgG conversion was observed in the serum and saliva samples of animals given rUre plus LT but not in those receiving placebo plus LT (P < 0.05). Changes in serum IgG titers after immunization were not different between animals that were H. pylori positive or negative at endoscopy at 10 months after the first immunization (Fig. 4). We also observed conversion in serum and saliva IgA in animals treated with rUre plus LT (P < 0.05), but, interestingly, not in animals that were H. pylori negative at endoscopy.
FIG. 4.
Effect of administration of rUre plus LT or placebo plus LT on conversion in specific antiurease serum and saliva IgG and IgA at 1 week after the fourth immunization. The H. pylori + or − designation reflects the status at endoscopy 10 months after the first immunization. ODU, OD units.
During the subsequent months, specific serum antiurease IgG decreased in rUre-LT-treated animals that were H. pylori negative at endoscopy but did not change in those that were H. pylori positive at endoscopy (Fig. 5). Mean serum anti-H. pylori IgG increased progressively and significantly in placebo-treated animals that were H. pylori positive at endoscopy (Fig. 5). Serum antiurease IgG remained low in the two placebo-treated animals that were H. pylori negative, but this low number precludes statistical analysis.
FIG. 5.
Time course of H. pylori serum IgG response following administration of either rUre plus LT or placebo plus LT. Groups of animals are also separated based on their H. pylori infection status (indicated by + or −) at the time of the endoscopy. Values are means ± SE. Data for the two H. pylori-negative animals that had received placebo plus LT are not illustrated.
Adverse reactions.
To evaluate the safety of the regimen used in this study, veterinary technicians and caretakers were instructed to closely observe the animals and to report any unusual symptom, focusing especially on anorexia, vomiting, withdrawal, and diarrhea. Throughout the observation period, there was no serious adverse reaction attributable to the administration of LT and placebo or rUre. There was no unusual report of anorexia, vomiting, or withdrawal. No diarrhea was observed following administration of LT, and although diarrhea was occasionally observed in the corrals in subsequent months, it did not occur at a greater frequency in animals that received LT than in the rest of the breeding colony.
DISCUSSION
This study shows for the first time that oral immunization of nonhuman primates with rUre plus LT protected against naturally acquired H. pylori infection. Such a study is feasible only in the two species that have been documented to become naturally infected by this bacterium, i.e., the cat and the nonhuman primate. At this time, however, the rhesus monkey is the only animal in which the epizootiology of natural H. pylori infection is documented.
Most earlier studies of immunization against H. pylori were performed with mice, and they used either H. pylori whole-cell sonicates or urease, plus CT as an adjuvant. In this study, we used purified rUre in conjunction with LT (40, 43). This adjuvant has been shown to be more effective and less toxic than CT in primates (42). In addition, administration of LT in association with rUre was safe and produced a strong urease-specific IgG production in squirrel monkeys (40), although that study did not attempt to assess the protective effect of the response. LT was also shown to be a safe and effective adjuvant in another vaccine trial against Campylobacter jejuni in rhesus monkeys (3). Similarly, diarrhea was not observed after administration of either rUre plus LT or placebo plus LT during the present study, and no other side effects were noted. In contrast, in a recent clinical trial using LT as an adjuvant, diarrhea was observed in 66% of the volunteers, but the number of loose stools as well as the frequency of subjects experiencing diarrhea decreased over time (62, 50, 17, and 25% at the first, second, third, and fourth doses, respectively) (35). This decrease of the level of reactogenicity may reflect progressive immunization against LT, and the lack of diarrhea in monkeys may be explained by a similar immunization following natural exposure to E. coli toxin from an early age in the breeding colony. If this explanation is correct, diarrhea may be absent in trials to be performed in developing countries. However, data for monkeys suggest that such a preexisting immunity is not likely to have a detrimental effect on the adjuvant properties of LT (3).
Specific anti-H. pylori IgG levels at preimmunization suggest that all animals were H. pylori negative before immunization, but serum levels of IgG are known to rise only 2 to 3 months after experimental inoculation (13). Because technical limitations precluded determination of the H. pylori status at the time of immunization by other methods, we cannot exclude the possibility that a number of animals were already positive at that time, i.e., at 9 months. This time point was selected because it appeared to represent the age at which immunization would be given to humans. Although an earlier time point seems a posteriori to have been a better choice, monkeys and humans of any age placed in a high-risk environment may become infected during the 1- to 2-month period of immunization, and the study thus may reflect a real-life scenario.
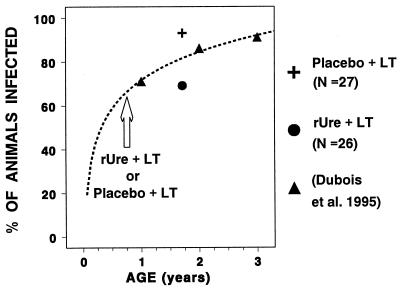
The protective effect observed in this study may reflect prophylactic and/or curative immunization. In an attempt to distinguish between these two possibilities, we performed a regression analysis of partly unpublished data that had been obtained previously for the same colony (15). Using a logarithmic fit of the seroepizootiology data (r = 0.99 [Fig. 6]), the expected rate of H. pylori infection in 20.5-month-old females was estimated at 82%. Thus, the 93 and 69% infection rates observed in placebo-treated and rUre-treated animals are 113 and 84%, respectively, of the expected rates. These historical controls can also be used to extrapolate the H. pylori prevalence at 9 months, indicating that approximately 34% of the animals (10 of 29 in the placebo group and 9 of 26 in the vaccinated group) were negative at the time of the immunization (Fig. 6). Using these estimates in conjunction with the data obtained in the present study, one can calculate that 8 of 10 of the placebo-plus-LT H. pylori-negative animals became infected, for an infection rate of 80%, whereas 1 of 9 rUre-plus-LT-treated H. pylori-negative animals became infected, for an infection rate of 11%. Thus, the vaccine efficacy [(percent infected in unvaccinated group − percent infected in vaccinated group)/percent infected in unvaccinated group] can be estimated at 69%. Since the percentage of vaccinated animals that were H. pylori positive at 20.5 months was slightly greater than the extrapolated frequency at 9 months, this protection appears to result from a prophylactic effect rather than from a curative effect. Furthermore, any significant curative effect would have to be associated with an increased rate of subsequent reinfection to explain the observed prevalence of H. pylori infection at the time of endoscopy. Finally, a curative effect of this immunization regimen was not detected in a recent study using H. pylori-positive humans (35). These observations are in agreement with the observation that urease-negative mutants can survive in the stomach without urease but cannot colonize the stomach when experimentally inoculated (17).
FIG. 6.
Prevalence of infection in animals treated with rUre plus LT or placebo plus LT compared to historical controls in the same colony.
The mechanism of the protection observed in immunized animals is unknown. The classical view is that mucosal defense in the intestines is principally mediated by secretory IgA (33). Studies using the mouse model have indicated that the same concept is valid for the stomach mucosa, and protection against experimental infection was achieved by intragastric administration of anti-H. felis monoclonal IgA at the time of the challenge. In addition, oral immunization with bacterial antigens resulted both in protection against infection and elevated anti-H. felis IgA, but also IgG, titers (6). Similarly, production of secretory IgA against urease was correlated with successful protection against challenge of mice with H. felis following administration of rUre vaccine (28, 37). In contrast, other studies have shown a lack of relationship between protection of mice following immunization and the levels of specific mucosal IgA levels in the blood, feces, and/or intestinal secretions (34). In the present study, only the monkeys that were H. pylori positive at endoscopy had converted in antiurease serum or saliva IgA at 1 week after administration of rUre plus LT (Fig. 4). This observation suggests that neither serum nor secretory IgA plays a protective role, which is also indicated by the observation that persistent infection in humans can occur despite increased production of IgA, and actual IgA coating of at least a fraction of H. pylori present in the gastric lumen has been observed (44). A recent study suggests that this apparent paradox can be explained by the observation that a large fraction of the bacteria present in persistently infected humans escape antibody deposition (9).
If IgA is not involved in the protection conferred by immunization, then it is worth considering the possible role of IgG, which is generally neglected in the case of mucosal immunity (38). Administration of IgG is sufficient to confer passive protection against most infections, including gastrointestinal infections (38), and it can opsonize H. pylori for destruction by mononuclear cells (41). In further support of the role of IgG in the case of Helicobacter infection is the recent finding that immunization against H. felis can induce proliferation of a large number of IgG-secreting cells in the protected animals, but no gastric anti-H. pylori IgA response, whereas colonization preferentially induces the recruitment of IgA-producing plasma cells (18). In the present study, we detected a rapid rise in specific antiurease IgG immediately following administration of rUre plus LT (whether the animals eventually became colonized by H. pylori or not) but not after administration of placebo plus LT. Although the levels of immunoglobulin in gastric secretion were not determined, the observed rise in serum anti-H. pylori IgG is probably associated with a concurrent rise of IgG in the stomach mucosa and in the gastrointestinal fluids (38). The possible role of IgG is also supported by the observation that administration of the GroES homolog of H. pylori, alone or in association with urease, increased the production of specific anti-H. pylori IgG1 in mice and concurrently protected against experimental colonization by H. felis (19). However, the situation appears to be very complex, as the present long-term study showed that serum antiurease IgG decreased in H. pylori-negative immunized animals despite their continuous exposure to hyperenzootic environment and despite the fact that they remained protected against H. pylori infection (Fig. 4). In contrast, continued antigen stimulation may explain why serum antiurease IgG increased over time in placebo-treated animals that had become H. pylori positive at endoscopy and why it remained elevated in the vaccinated animals that were not protected against H. pylori infection (Fig. 4). These findings suggest that the efficacy of immunization in the animals that were H. pylori negative prior to vaccination does not depend on the long-term persistence of elevated serum antiurease IgG.
Therefore, the continued protection observed in this study is probably dependent on some other, yet undefined factor(s) likely related to cellular immunity. Although we did not explore this possibility, our findings suggest that the vaccine may exert a therapeutic effect, as indicated by the observation that among H. pylori-positive animals, antral gastritis was significantly less severe in immunized than in nonimmunized animals. Interestingly, similar observations were also made in studies using mice (37) and ferrets (5). In addition, and as expected, the difference was even more pronounced between immunized H. pylori-negative animals and placebo-treated H. pylori-positive animals. Thus, immunization appears to reduce the cellular inflammatory response to a preexistent H. pylori infection, although it does not significantly modify the extent of colonization by this bacterium. This observation also suggests that the vaccine regimen used in the present study may increase the oral tolerance to H. pylori infection and/or have an anti-inflammatory effect that may be beneficial, although it is insufficient to eradicate established infection.
In conclusion, the present study demonstrates that rUre in combination with LT can significantly protect nonhuman primates against naturally acquired H. pylori infection. This finding is important, as it suggests that a similar immunoprophylaxis could be induced in humans. The population to which such a vaccination should be applied is not yet defined. However, it is tempting to propose that infants living in areas with high incidence and prevalence of H. pylori infection would benefit from such a vaccination. In addition, adults living in these regions could benefit from an immunization regimen administered concurrently with curative antibiotics.
ACKNOWLEDGMENTS
We gratefully acknowledge the excellent technical assistance of the staff at OraVax (Kathleen Georgakopoulos, Timothy Tibbitts, Jennifer Ingrassia, Heather Gray, and James Wiseman) as well as Georges Ward and Michael Henry. We also thank Gwen Myers (OraVax) for preparing and supervising the immunization procedure.
REFERENCES
- 1.Anonymous. Evaluation of carcinogenic risks to humans. Schistosomes, liver flukes and Helicobacter pylori. IARC monograph 61. Lyon, France: International Agency for Research on Cancer; 1994. [PMC free article] [PubMed] [Google Scholar]
- 2.Anonymous. Helicobacter pylori in peptic ulcer disease. JAMA. 1994;272:65–69. [PubMed] [Google Scholar]
- 3.Baqar S, Bourgeois A L, Schultheiss P J, Walker R I, Rollins D M, Haberberger R L, Pavlovskis O R. Safety and immunogenicity of a prototype oral whole-cell killed Campylobacter vaccine administered with a mucosal adjuvant in non-human primates. Vaccine. 1995;13:22–28. doi: 10.1016/0264-410x(95)80006-y. [DOI] [PubMed] [Google Scholar]
- 4.Corthesy-Theulaz I, Porta N, Glauser M, Saraga E, Vaney A, Haas R, Krahenbuhl J, Blum A L, Michetti P. Oral immunization with Helicobacter pylori urease B subunit as a treatment against Helicobacter infection in mice. Gastroenterology. 1995;109:115–121. doi: 10.1016/0016-5085(95)90275-9. [DOI] [PubMed] [Google Scholar]
- 5.Cuenca R, Blanchard T G, Czinn S J, Nedrud J G, Monath T P, Lee C K, Redline R W. Therapeutic immunization against Helicobacter mustelae in naturally infected ferrets. Gastroenterology. 1996;110:1170–1175. doi: 10.1053/gast.1996.v110.pm8964402. [DOI] [PubMed] [Google Scholar]
- 6.Czinn S J, Cai A, Nedrud J G. Protection of germ-free mice from infection by Helicobacter felis after active oral or passive IgA immunization. Vaccine. 1993;11:637–642. doi: 10.1016/0264-410x(93)90309-l. [DOI] [PubMed] [Google Scholar]
- 7.Czinn S J, Nedrud J G. Oral immunization against Helicobacter pylori. Infect Immun. 1991;59:2359–2363. doi: 10.1128/iai.59.7.2359-2363.1991. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 8.Danon S J, O’Rourke J L, Moss N D, Lee A. The importance of local acid production in the distribution of Helicobacter felis in the mouse stomach. Gastroenterology. 1995;108:1386–1395. doi: 10.1016/0016-5085(95)90686-x. [DOI] [PubMed] [Google Scholar]
- 9.Darwin P E, Sztein M B, Zheng Q X, James S P, Fantry G T. Immune evasion by Helicobacter pylori: gastric spiral bacteria lack surface immunoglobulin deposition and reactivity with homologous antibodies. Helicobacter. 1996;1:20–27. doi: 10.1111/j.1523-5378.1996.tb00004.x. [DOI] [PubMed] [Google Scholar]
- 10.Doidge C, Gust I, Lee A, Buck F, Hazell S, Manne U. Therapeutic immunization against Helicobacter infection. Lancet. 1994;343:914–915. doi: 10.1016/s0140-6736(94)90032-9. [DOI] [PubMed] [Google Scholar]
- 11.Dubois A, Berg D E, Fiala N, Heman-Ackah L M, Perez-Perez G I, Blaser M J. Cure of Helicobacter pylori infection by omeprazole-clarithromycin-based therapy in non-human primates. J Gastroenterol. 1998;33:18–22. doi: 10.1007/pl00009961. [DOI] [PubMed] [Google Scholar]
- 12.Dubois, A., D. E. Berg, E. T. Incecik, and N. Fiala. 1996. H. pylori infection of rhesus monkeys: strains differ in specificity for individual hosts. Gut 39(Suppl. 2):A74.
- 13.Dubois A, Berg D E, Incecik E T, Fiala N, Heman-Ackah L M, Perez-Perez G I, Blaser M J. Transient and persistent experimental infection of nonhuman primates with Helicobacter pylori: implications for human disease. Infect Immun. 1996;64:2885–2891. doi: 10.1128/iai.64.8.2885-2891.1996. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 14.Dubois A, Fiala N, Heman-Ackah L M, Drazek E S, Tarnawski A, Fishbein W N, Perez-Perez G L, Blaser M J. Natural gastric infection with Helicobacter pylori in monkeys. Gastroenterology. 1994;106:1405–1417. doi: 10.1016/0016-5085(94)90392-1. [DOI] [PubMed] [Google Scholar]
- 15.Dubois A, Fiala N, Weichbrod R H, Ward G S, Nix M, Mehlman P T, Taub D M, Perez-Perez G I, Blaser M J. Seroepizootiology of Helicobacter pylori gastric infection in socially housed rhesus monkeys. J Clin Microbiol. 1995;33:1492–1495. doi: 10.1128/jcm.33.6.1492-1495.1995. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 16.Dubois A, Tarnawski A, Newell D G, Fiala N, Dabros W, Stachura J, Krivan H, Heman-Ackah L M. Gastric injury and invasion of parietal cells by spiral bacteria in rhesus monkeys. Gastroenterology. 1991;100:884–891. doi: 10.1016/0016-5085(91)90260-r. [DOI] [PubMed] [Google Scholar]
- 17.Eaton K, Morgan D R, Krakowka S. Essential role of urease in pathogenesis of gastritis induced by Helicobacter pylori in gnotobiotic piglets. Infect Immun. 1991;59:2470–2475. doi: 10.1128/iai.59.7.2470-2475.1991. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 18.Ferrero R L, Thiberge J, Labigne A. Local immunoglobulin G antibodies in the stomach may contribute to immunity against Helicobacter infection in mice. Gasteroenterology. 1997;113:185–194. doi: 10.1016/s0016-5085(97)70094-5. [DOI] [PubMed] [Google Scholar]
- 19.Ferrero R L, Thiberge J M, Kansau I, Wuscher N, Huerre M, Labigne A. The GroES homolog of Helicobacter pylori confers protective immunity against mucosal infection in mice. Proc Natl Acad Sci USA. 1995;92:6499–6503. doi: 10.1073/pnas.92.14.6499. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 20.Fox J G. Gastric disease in ferrets: effects of Helicobacter mustelae, nitrosamines and reconstructive gastric surgery. Eur J Gastroenterol Hepatol. 1994;6:S57–S65. [PubMed] [Google Scholar]
- 21.Genta R M, Robason G O, Graham D Y. Simultaneous visualization of Helicobacter pylori and gastric morphology: a new stain. Hum Pathol. 1994;25:221–226. doi: 10.1016/0046-8177(94)90191-0. [DOI] [PubMed] [Google Scholar]
- 22.Handt L K, Fox J G, Yan L L, Shen Z, Pouch W J, Ngai D, Motzell S L, Nolan T E, Klein H J. Diagnosis of Helicobacter pylori infection in a colony of rhesus monkeys. J Clin Microbiol. 1997;35:165–168. doi: 10.1128/jcm.35.1.165-168.1997. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 23.Höök-Nikanne J, Aho P, Karkkainen P, Kosunen T U, Salaspuro M. The Helicobacter felis mouse model in assessing anti-Helicobacter therapies and gastric mucosal prostaglandin E2 levels. Scand J Gastroenterol. 1996;31:334–338. doi: 10.3109/00365529609006406. [DOI] [PubMed] [Google Scholar]
- 24.Lee A. Animal models and vaccine development. Baillière Clin Gastroenterol. 1995;9:615–632. doi: 10.1016/0950-3528(95)90051-9. [DOI] [PubMed] [Google Scholar]
- 25.Lee A. Therapeutic immunization against Helicobacter infection. Gastroenterology. 1996;110:2003–2006. doi: 10.1053/gast.1996.v110.agast962003. [DOI] [PubMed] [Google Scholar]
- 26.Lee A, Chen M. Successful immunization against gastric infection with Helicobacter species: use of a cholera toxin B-subunit-whole-cell vaccine. Infect Immun. 1994;62:3594–3597. doi: 10.1128/iai.62.8.3594-3597.1994. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 27.Lee A, O’Rourke J, Corazon de Ungria M, Robertson B, Daskapoulos G, Dixon M F. A standardized mouse model of Helicobacter pylori infection: introducing the Sidney strain. Gastroenterology. 1997;112:1386–1397. doi: 10.1016/s0016-5085(97)70155-0. [DOI] [PubMed] [Google Scholar]
- 28.Lee C K, Weltzin R, Thomas W D, Jr, Kleanthous H, Ermak T H, Soman G, Hill J E, Ackerman S K, Monath T P. Oral immunization with recombinant Helicobacter pylori urease induces secretory IgA antibodies and protects mice from challenge with Helicobacter felis. J Infect Dis. 1995;172:161–172. doi: 10.1093/infdis/172.1.161. [DOI] [PubMed] [Google Scholar]
- 29.Marchetti M, Arico B, Burroni D, Figura N, Rappuoli R, Ghiara P. Development of a mouse model of Helicobacter pylori infection that mimics human disease. Science. 1995;267:1655–1658. doi: 10.1126/science.7886456. [DOI] [PubMed] [Google Scholar]
- 30.Marshall B J, Warren J R. Unidentified curved bacilli in the stomach of patients with gastritis and peptic ulceration. Lancet. 1984;i:1311–1315. doi: 10.1016/s0140-6736(84)91816-6. [DOI] [PubMed] [Google Scholar]
- 31.McColm, A. A., J. A. Bagshaw, C. O’Malley, and A. McLaren. 1995. Development of a mouse model of gastric colonisation with Helicobacter pylori. Gut 37(Suppl. 1):A50.
- 32.Mégraud F. What is the clinical relevance of Helicobacter pylori resistance to antibiotics. In: Hunt R H, Tytgat G A T, editors. Basic mechanisms to clinical care. Amsterdam, The Netherlands: Kluwer; 1996. pp. 348–356. [Google Scholar]
- 33.Mestecky J, McGhee J R, Elson C O. Intestinal IgA system. Immunol Allergy Clin North Am. 1988;8:349–368. [Google Scholar]
- 34.Michetti P, Corthesy-Theulaz I, Davin C, Haas R, Vaney A, Heitz M, Bille J, Krahenbuhl J, Saraga E, Blum A L. Immunization of BALB/c mice against Helicobacter felis infection with Helicobacter pylori urease. Gastroenterology. 1994;107:1002–1011. doi: 10.1016/0016-5085(94)90224-0. [DOI] [PubMed] [Google Scholar]
- 35.Michetti P, Kreiss C, Kotloff K, Porta N, Blanco J L, Bachmann D, et al. Oral immunization of H. pylori-infected adults with recombinant urease and LT adjuvant. Gastroenterology. 1997;112:A1042. doi: 10.1016/s0016-5085(99)70063-6. [DOI] [PubMed] [Google Scholar]
- 35a.National Research Council, Institute of Laboratory Animal Resources. Guide for the care and use of laboratory animals. HHS/NIH publication no. 85-23. Washington, D.C: National Research Council; 1985. [Google Scholar]
- 36.O’Rourke J L, Lee A, Fox J G. An ultrastructural study of Helicobacter mustelae and evidence of a specific association with gastric mucosa. J Med Microbiol. 1992;36:420–427. doi: 10.1099/00222615-36-6-420. [DOI] [PubMed] [Google Scholar]
- 37.Pappo J, Thomas W D J, Kabok Z, Taylor N S, Murphy J C, Fox J G. Effect of oral immunization with recombinant urease on murine Helicobacter felis gastritis. Infect Immun. 1995;63:1246–1252. doi: 10.1128/iai.63.4.1246-1252.1995. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 38.Robbins J B, Schneerson R, Szu S C. Perspective: hypothesis: serum IgG antibody is sufficient to confer protection against infectious diseases by inactivating inoculum. J Infect Dis. 1995;171:1387–1398. doi: 10.1093/infdis/171.6.1387. [DOI] [PubMed] [Google Scholar]
- 39.Riegg S J, Dunn B E, Blaser M J. Microbiology and pathogenesis of Helicobacter pylori. In: Blaser M J, Smith P D, Radvin J I, Greenberg H B, Guerrant R L, editors. Infections of the gastrointestinal tract. New York, N.Y: Raven Press; 1995. pp. 535–550. [Google Scholar]
- 40.Stadtlander C T K H, Gangemi J D, Khanolkar S S, Kitsos C M, Farris H E, Jr, Fulton L K, Hill J E, Huntington F K, Lee C K, Monath T P. Immunogenicity and safety of recombinant Helicobacter pylori in a nonhuman primate. Digest Dis Sci. 1996;41:1853–1862. doi: 10.1007/BF02088757. [DOI] [PubMed] [Google Scholar]
- 41.Tosi M F, Czinn S J. Opsonic activity of specific human IgG against Helicobacter pylori. J Infect Dis. 1990;162:156–162. doi: 10.1093/infdis/162.1.156. [DOI] [PubMed] [Google Scholar]
- 42.Walker R I. New strategies for using mucosal vaccination to achieve more effective immunization. Vaccine. 1994;12:387–400. doi: 10.1016/0264-410x(94)90112-0. [DOI] [PubMed] [Google Scholar]
- 43.Walker R I, Clements J D. Use of the heat-labile toxin of enterotoxigenic Escherichia coli to facilitate mucosal immunization. Vaccine Res. 1993;2:1–10. [Google Scholar]
- 44.Wyatt J I, Rathbone B J, Heatley R V. Local immune response to gastric Campylobacter in non-ulcer dyspepsia. J Clin Pathol. 1986;39:863–870. doi: 10.1136/jcp.39.8.863. [DOI] [PMC free article] [PubMed] [Google Scholar]