Abstract
PURPOSE
Uterine leiomyosarcoma (uLMS) is an aggressive subtype of soft-tissue sarcoma with frequent metastatic relapse after curative surgery. Chemotherapy provides limited benefit for advanced disease. Multiomics profiling studies have identified homologous recombination deficiency in uLMS. In preclinical studies where olaparib and temozolomide provided modest activity, the combination was highly effective for inhibiting uLMS tumor growth.
PATIENTS AND METHODS
NCI Protocol 10250 is a single-arm, open-label, multicenter, phase II study evaluating olaparib and temozolomide in advanced uLMS. Patients with progression on ≥1 prior line received temozolomide 75 mg/m2 orally once daily with olaparib 200 mg orally twice a day both on days 1-7 in 21-day cycles. The primary end point was the best objective response rate (ORR) within 6 months. A one-stage binomial design was used. If ≥5 of 22 responded, the treatment would be considered promising (93% power; α = .06). All patients underwent paired biopsies that were evaluated with whole-exome sequencing (WES)/RNAseq and a RAD51 foci formation assay.
RESULTS
Twenty-two patients were evaluable. The median age was 55 years, and 59% had received three or more prior lines. Best ORR within 6 months was 23% (5 of 22). The overall ORR was 27% (6 of 22). The median progression-free survival (mPFS) was 6.9 months (95% CI, 5.4 months to not estimable). Hematologic toxicity was common (grade 3/4 neutropenia: 75%; thrombocytopenia: 32%) but manageable with dose modification. Five of 16 (31%) of tumors contained a deleterious homologous recombination gene alteration by WES, and 9 of 18 (50%) were homologous recombination-deficient by the RAD51 assay. In an exploratory analysis, mPFS was prolonged for patients with homologous recombination-deficient versus homologous recombination-proficient tumors (11.2 v 5.4 months, P = .05) by RAD51.
CONCLUSION
Olaparib and temozolomide met the prespecified primary end point and provided meaningful clinical benefit in patients with advanced, pretreated uLMS.
INTRODUCTION
Soft-tissue sarcoma (STS) is a heterogeneous malignancy of mesenchymal origin with more than 150 clinically and biologically distinct subtypes. Leiomyosarcoma (LMS), a sarcoma of smooth muscle lineage, accounts for approximately 20% of STS. In women, the uterus is the most common primary site. After total abdominal hysterectomy for high-grade LMS confined to the uterus, metastatic relapse occurs in 50%-70% of patients.1 Advanced disease is incurable and treated with palliative chemotherapy, but outcomes remain poor. Gemcitabine plus docetaxel and doxorubicin-based regimens used for initial treatment of unresectable or metastatic uterine leiomyosarcoma (uLMS) provide objective response rates (ORRs) of 14%-36% and a median progression-free survival (mPFS) of 4.4-6.7 months.2-4 Trabectedin and pazopanib are approved for later-line treatment (ORR, 11%; mPFS of 3.0-4.0 months).5,6 Efforts to advance the treatment of uLMS with immunotherapy and targeted therapy have resulted in limited success. uLMS is associated with a low tumor mutational burden and is enriched in immunosuppressive macrophages. In a phase II trial of nivolumab, none of 12 patients achieved an objective response, and the mPFS was 1.8 months.7 Recent studies of targeted agents also failed to demonstrate clinical activity.8-10
CONTEXT
Key Objective
Does the combination of olaparib and temozolomide show evidence of preliminary activity in advanced uterine leiomyosarcoma (uLMS) that has progressed on chemotherapy?
Knowledge Generated
In this phase II study, olaparib and temozolomide showed encouraging activity with an objective response rate of 27%, median progression-free survival of 6.9 months, and median duration of response of 12.0 months. Thirty-one percent of patients had a genomic alteration in a homologous recombination repair gene, and 50% were homologous recombination-deficient by the RAD51 foci formation assay.
Relevance (R.G. Maki)
-
In this small phase II trial, promising activity of the combination of a poly-ADP ribose polymerase inhibitor and temozolomide was observed in patients with advanced uLMS. These sarcomas also had a relatively high frequency of Homologous recombination DNA-repair deficiency, which was not previously appreciated.*
*Relevance section written by JCO Associate Editor Robert G. Maki, MD, PhD, FACP, FASCO.
uLMS is characterized by highly complex karyotypes, frequent copy number alterations, replicative stress, chromothripsis, whole-genome duplication and alternative lengthening of the telomeres but lacks recurrent actionable genomic alterations.11,12 Recently, several groups have identified frequent defects in the homologous recombination DNA repair pathway in uLMS, which may represent a novel therapeutic vulnerability.11,13-15 The homologous recombination pathway is responsible for high-fidelity repair of double-stranded DNA breaks (DSBs) and restarting stalled replication forks. In cancer cells with defective homologous recombination resulting from alterations in BRCA1/2 or related homologous recombination genes, these lesions are resolved through error-prone DNA repair mechanisms, resulting in progressive genomic instability and cell death. Cancer cells with defective HR repair are vulnerable to targeted therapy with poly-ADP ribose polymerase (PARP) inhibitors. PARP mediates repair of single-stranded DNA breaks. PARP inhibitors, such as olaparib, trap PARP at sites of DNA damage, resulting in replication fork collapse and DSBs that cannot be effectively repaired in homologous recombination-deficient cells. Alkylating agents, such as temozolomide, may potentiate PARP trapping by inducing DNA damage.16 PARP inhibition in the context of HR deficiency, as initially defined by germline BRCA1/BRCA2 loss and later by other germline and somatic alterations rendering homologous recombination functionality defective (BRCAness), exemplifies the concept of synthetic lethality, as demonstrated clinically for a subset of ovarian, breast, pancreatic, and prostate cancers.
These observations prompted evaluation of PARP inhibitors as monotherapy and with chemotherapy in preclinical uLMS models. The combination of olaparib with temozolomide at clinically achievable doses appeared highly effective and significantly more active than either agent alone, prompting this phase II clinical trial.17
PATIENTS AND METHODS
Patients
NCI Protocol 10250 (online only) enrolled female patients age 18 years or older with locally advanced and unresectable or metastatic uLMS. Patients had an Eastern Cooperative Oncology Group (ECOG) performance status ≤2 and progression after at least one prior line of systemic treatment for advanced disease. Adjuvant chemotherapy and endocrine therapy did not qualify as prior treatment. There was no upper limit on prior therapy; however, prior PARP inhibitors, temozolomide and dacarbazine, were not permitted. Patients were required to have measurable disease, a tumor lesion accessible for biopsy, and adequate bone marrow and organ function.
Study Design and Treatment
In this single-arm, open-label, multicenter phase II clinical trial, all patients received temozolomide 75 mg/m2 orally once daily in combination with olaparib capsules 200 mg orally twice daily both administered days 1-7 of continuous 21-day cycles. The dose and schedule were adapted from a phase I study in small-cell lung cancer.18 A maximum of two dose reductions were permitted for temozolomide (50, 25 mg/m2 once daily) and olaparib (150, 100 mg twice daily). Treatment could be interrupted for up to 3 weeks. Imaging to evaluate disease status was performed every 6 weeks. NCI Protocol 10250 was developed in collaboration with the National Cancer Institute (NCI) Cancer Therapy Evaluation Program, was approved by the NCI Central Institutional Review Board, and conducted in accordance with an assurance approved by the Department of Health and Human Services. All patients provided written informed consent.
Correlative Assays
Paired tumor biopsies were collected at baseline and on-treatment (cycle 2, days 3-5). Tissue was evaluated with whole-exome and RNA sequencing (Molecular Characterization Laboratory, Frederick National Laboratory for Cancer Research, Frederick, MD) and a RAD51 foci formation assay (Center for DNA Damage and Repair, Dana-Farber Cancer Institute, Boston, MA).
Whole-Exome Sequencing/RNAseq and Bioinformatics
Whole-exome sequencing (WES), RNA-seq, and bioinformatics were performed as previously described.19 Copy number data were inferred from WES using the Sequenza algorithm. Tumors were classified as homologous recombination-deficient if homozygous deletion or deleterious mutation was identified in any of the following genes: BRCA1, BRCA2, ATM, ATR, ATRX, CHK1, CHK2, RAD51, PALB2, FANCA, and NBS1. Homologous recombination deficiency (HRD) scores were calculated by scarHRD using Sequenza output.20 The RSEM pipeline using STAR aligner was implemented to process RNA-seq data. The results were further processed using the DESeq2 package to quantify gene expression as normalized counts.
RAD51 Assay
Serial sections from tumor biopsies were independently stained using antibodies to RAD51 and geminin as previously described.21 The RAD51 assay was developed using a training set of 14 high-grade serous ovarian patient-derived xenografts where genomic profiling and olaparib response were known.22 Formalin-fixed paraffin-embedded sections of a tissue microarray containing irradiated and unirradiated MDA-MB-436 and MDA-MB-468 breast cancer cell lines cultured as organoids in a 3D matrix and a BRCA2-null uLMS were used as technical controls. Samples were classified as homologous recombination-proficient if more than three RAD51 foci were present in a minimum of one cell in at least three independent 40× fields. If RAD51 foci were absent, the sample was classified as homologous recombination-deficient if >3% of tumor cells were geminin-positive within a continuous region of tumor circumscribing a minimum of 500 cells. If there were no RAD51 foci and <3% of the cells were geminin-positive, the proliferation rate of the tumor was classified as low, and homologous recombination status was indeterminate. Geminin positivity was evaluated in >500 cells in regions of the tumor with the greatest number of geminin-positive cells. To assign homologous recombination status, stained slides were independently scored by two blinded evaluators.
End Points and Statistical Analysis
The primary end point was the best ORR per RECIST criteria within 6 months of initiating treatment. A one-stage binomial design was used to evaluate for an improvement in best ORR rate from at most 10% to at least 35%. The design called for 22 eligible and evaluable patients. If five or more patients responded, the treatment would be considered promising. The design provided 93% power with a one-sided type 1 error of 6%. Secondary end points included overall ORR, mPFS, median OS (mOS), and safety. PFS was defined as the time from enrollment to the earlier of disease progression or death from any cause. Patients who were alive and progression-free were censored at the time of their last tumor assessment. OS was defined as the time from enrollment to death from any cause. The Kaplan-Meier method was used to evaluate time-to-event end points. Adverse events were recorded at each visit and categorized according to NCI Common Terminology Criteria for Adverse Events version 5.0. Tumors were evaluated for HR deficiency by (1) genomic alterations in the prespecified homologous recombination gene panel and (2) RAD51 foci formation. The presence of HRD was correlated with PFS. In an exploratory analysis, we measured Schlafen family member number 11 (SLFN11) and O6-methylguanine-DNA methyltransferase (MGMT) RNA expression and evaluated for any relationship with clinical benefit.
RESULTS
Patient Characteristics and Demographics
A total of 24 patients were enrolled from 11 Experimental Therapeutics Clinical Trials Network institutions between August 2019 and February 2020. One patient did not start treatment because of progressive anemia and another ended treatment on the first day for ongoing colitis from treatment with pembrolizumab and was considered ineligible, resulting in 22 eligible and evaluable patients. Patient characteristics are shown in Table 1. The median age was 55 years (range, 39-71), all were female, and 17 (77%) had metastatic disease. ECOG performance status was 0 for seven patients (32%) and 1 for the remaining patients. Seventeen patients (77%) were White, three (14%) were Black or African American, one was American Indian/Alaskan Native, and one had unknown race. The median number of prior treatment lines was three, and thirteen patients (59%) had received three or more systemic regimens, most commonly gemcitabine/docetaxel (86%), doxorubicin combinations (41%), and doxorubicin monotherapy (36%).
TABLE 1.
Patient Demographics

Efficacy
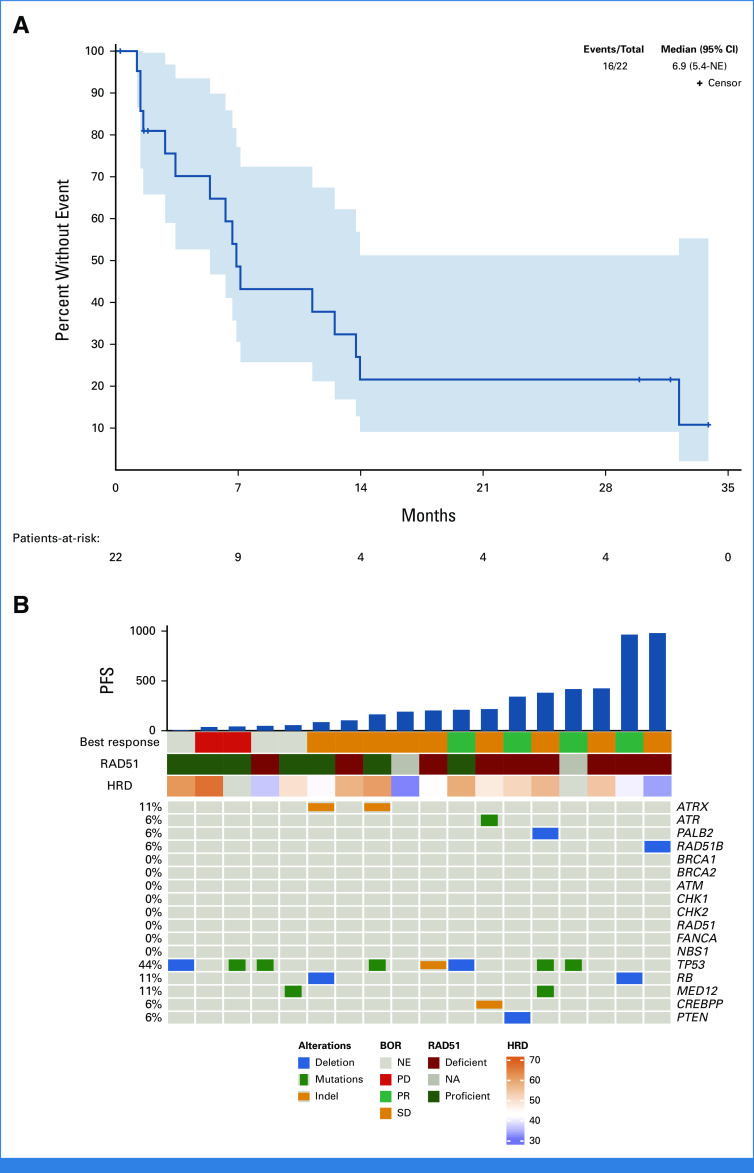
At the primary data analysis performed in September 2022, the median follow-up was 22 months. Three patients remained on treatment, and 19 had discontinued (16 for progressive disease [PD] and three for toxicity). Five of 22 patients (23%) achieved an objective response within 6 months, and the study met the primary end point. One patient responded after 11.5 months of treatment. Therefore, the overall ORR rate was six of 22 (27%). Best objective response, shown in Appendix Table A1 (online only), was partial response (PR) for six patients (27%), stable disease (SD) for nine (41%), PD for four (18%), and not evaluated for three (14%, all of whom ended treatment for toxicity before the first protocol-specified scan). The waterfall plot (Fig 1) shows that approximately 60% of patients achieved some reduction in the sum of target lesions. The spider plot (Fig 2) shows that PRs occurred early in treatment and several patients had prolonged SD. Among the six patients achieving PR, the median duration of response was 12.0 months (95% CI, 9.5 months to not estimable [NE]). Three patients remain on active treatment with a PFS of 30, 32, and 34 months. The mPFS was 6.9 months (95% CI, 5.4 months to NE, Fig 3A). PFS rates at 6, 12, and 24 months were 65%, 38%, and 22%, respectively. mOS has not been reached.
FIG 1.
Waterfall plot showing maximum reduction in the sum of longest diameters of target tumor lesions per RECIST version 1.1. Three patients who ended treatment for toxicity before the first imaging assessment was performed are not shown on the waterfall plot. aPatients progressed because of nonmeasureable lesions. PD, progressive disease; PR, partial response; SD, stable disease.
FIG 2.
Spider plot showing the change in the sum of target tumor measurements over time per patient. PD, progressive disease; PR, partial response; SD, stable disease.
FIG 3.
(A) Kaplan-Meir plot of PFS per RECIST criteria. All 22 patients are shown. (B) Co-Mut plot showing the correlation between genomic mutations in homologous recombination pathway and other genes by WES, HRD scores (HRD), RAD51 foci formation (RAD51), and clinical outcomes: BOR and PFS. Patients who did not undergo WES because of lack of available tumor tissue are not shown in the Co-Mut plot. BOR, best overall response; HRD, homologous recombination deficiency; NA, not available; NE, not estimable; PD, progressive disease; PFS, progression-free survival; PR, partial response; SD, stable disease; WES, whole-exome sequencing.
Toxicity
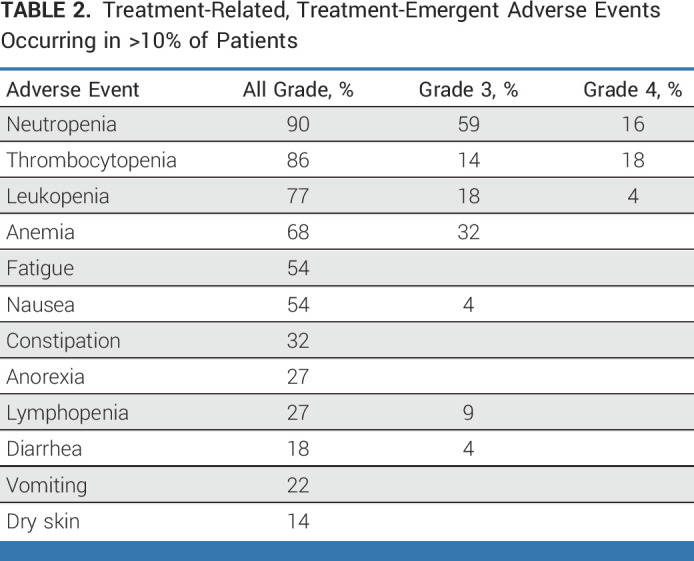
Treatment-related toxicity is summarized in Table 2. Hematologic toxicity was common, and all-grade hematologic adverse events included neutropenia (90%), thrombocytopenia (86%), leukopenia (77%), and anemia (68%). Grade 3 neutropenia, thrombocytopenia, and anemia were observed in 59%, 14%, and 32% of patients, respectively. Grade 4 neutropenia and thrombocytopenia each occurred in 18%. There were no events of neutropenic fever. Nonhematologic adverse events were less common and included fatigue (54%), nausea (54%), constipation (32%), anorexia (27%), diarrhea (18%), vomiting (22%), and dry skin (14%). Only three grade 3 nonhematologic adverse events occurred: nausea, rash, and diarrhea in one patient each.
TABLE 2.
Treatment-Related, Treatment-Emergent Adverse Events Occurring in >10% of Patients
For olaparib, eight patients (36%) required one dose reduction and two patients (9%) required two dose reductions. For temozolomide, 10 patients (45%) required one dose reduction and two patients (9%) required two dose reductions. Dose reductions occurred early in treatment and did not appear to effect efficacy. For example, each of the three patients who remain on active treatment underwent at least one dose reduction of temozolomide, and two underwent one dose reduction of olaparib. Three patients discontinued study treatment for toxicity (one each for neutropenia, thrombocytopenia, and rash).
Correlative Assays
WES was performed in tumor biopsy specimens from 16 of 22 (73%) participants. WES could not be performed for the remaining six patients because of lack of tumor tissue or insufficient DNA. Deleterious genomic alterations in the predefined homologous recombination panel were observed in 5 of 16 (31%) tumors and included one RAD51B and one PALB2 homozygous deletion, one ATR mutation, and two ATRX mutations. Patients with RAD51B, PALB2, and ATR alterations achieved best response of SD and PFS of 31.5, 27.3, and 7.1 months, respectively, before progression. The two patients with ATRX mutations had lesser benefit. Both had best response of SD and PFS of 5.4 and 2.7 months, respectively, before progression. Of the six patients achieving PR, two did not have WES performed, and the remaining four did not have any detectable alteration in the homologous recombination panel. Other genomic findings observed in this cohort included homozygous deletion or mutation in TP53, RB, PTEN, MED12, and CREBPP. In an exploratory analysis, we calculated HRD scores, representing the unweighted sum of telomeric allelic imbalance, loss of heterozygosity, and large-scale state transitions, from WES data using the scarHRD algorithm. HRD scores (mean, 51; range, 36-66) appeared elevated when compared with previously published pan-cancer analysis; however, there is no established HRD cutoff for uLMS, and comparisons must be made with caution considering differences in analytical methods.23 An overview of the genomic findings from WES and relationship to clinical outcomes is presented in Figure 3B.
The RAD51 foci formation assay, a functional assessment of homologous recombination pathway status, was performed using pretreatment biopsies from 18 of 22 (82%) of participants. The remaining four patients had insufficient tissue. Representative images showing the presence and absence of RAD51 foci are shown in Appendix Figure A1 (online only). Overall, 9 of 18 (50%) tumors were homologous recombination-deficient, 7 of 18 (39%) were homologous recombination-proficient, and 2 of 18 (11%) were indeterminate. The assay confirmed HR deficiency in the tumors with PALB2, RAD51B, and ATR alterations. However, both tumors with ATRX mutations were homologous recombination-proficient. The assay detected HRD in five patients with no identifiable homologous recombination genomic alteration. Excluding one patient who discontinued treatment for toxicity, each of these patients had prolonged benefit and two achieved PR. Of the six patients with objective responses, one was homologous recombination-proficient, two were homologous recombination-deficient, one was indeterminate, and two had insufficient tissue for testing. A swimmer's plot showing the relationship between WES and RAD51 results is shown in Figure 4. In an exploratory Kaplan-Meier analysis including 16 patients with RAD51 results (excluding three patients who ended treatment for toxicity), mPFS for patients with homologous recombination-deficient tumors was longer than those with homologous recombination-proficient tumors (11.2 v 5.4 months, P = .05, Appendix Figs A2 and Figs A3 [online only]). Integrated clinical and correlative data for the seven patients who received treatment for more than 12 months is shown in Appendix Table A2 (online only).
FIG 4.

Swimmer plot showing treatment duration for each patient coded by RAD51 foci result (bar color) and annotated by whole-exome sequencing result (text appearing to the right of each patient bar). PFS, progression-free survival.
Gene expression profiling was performed in paired pretreatment and on-treatment tumor biopsy specimens. SLFN11 has been identified as a determinant of response to DNA damaging agents and response to replication stress with higher levels associated with increased benefit from platinum and PARP inhibitors.24-29 MGMT repairs DNA damage induced by temozolomide and the absence of MGMT predicts responsiveness to temozolomide in some cancers. Using a Cox proportional hazards model, we found no correlation between PFS and pretreatment levels of SLFN11 (hazard ratio [HR], 0.98 [95% CI, 0.52 to 1.83]; P = .90) or MGMT (HR, 1.12 [95% CI, 0.65 to 1.92]; P = .70) RNA (Appendix Fig A4 [online only]). Immunohistochemical evaluation is ongoing.
Differential analysis on normalized RNAseq count data between pretreatment and on-treatment samples identified only two genes significantly overexpressed in the on-treatment samples: CXCL10 and PCDH15 (adjusted P < .05, |Fold Change| > 1.5). Detailed analysis of gene expression data and correlation with clinical outcomes will be presented separately.
DISCUSSION
This phase II clinical trial evaluated the combination of olaparib, a PARP inhibitor, and temozolomide, an alkylating agent, in advanced uLMS motivated by supportive preclinical evidence and observations that uLMS harbors homologous recombination defects (BRCAness). Olaparib and temozolomide met the prespecified primary efficacy end point and provided a best ORR rate of 27% among patients with advanced pretreated uLMS. Myelosuppression, although common and more significant than observed in monotherapy studies with olaparib or temozolomide, was manageable with dose modification and did not result in clinically significant adverse events.30,31 Only 8% of patients experienced grade 3 or higher nonhematologic toxicity. Considering the limited efficacy of later-line treatment options for uLMS (trabectedin: ORR, 11%; mPFS, 4 months; pazopanib: ORR, 8%; mPFS, 3 months), further study of this approach appears warranted while acknowledging the limitations of this small, single-arm study and cross-trial comparisons.5,6
There is increasing emphasis on developing subtype-specific therapeutic approaches in sarcoma which requires an understanding of the cancer biology of each histology. The most common genomic alterations in uLMS involve near universal inactivation of the tumor suppressors RB1 and TP53 through a variety of genetic mechanisms, but these events remain beyond the reach of currently available targeted therapy.12 The observation of HRD in uLMS is notable because this finding may have therapeutic implications. Homologous recombination defects appear enriched in uLMS as compared with LMS arising from other sites. In various retrospective uLMS cohorts, BRCA2 deletion is observed in approximately 10%, alterations in the homologous recombination pathway in 18%-23%, and HRD signatures in more than 50%.11-14 In a pan-cancer analysis of germline and somatic BRCA alterations and their relevance to initiation and progression of cancer, uterine sarcoma harbored the highest rate of somatic homozygous BRCA deletion and was hypothesized to represent a previously unrecognized homologous recombination-deficient cancer type.32
We evaluated several assays for identifying HRD and predicting clinical benefit from olaparib and temozolomide. Consistent with the retrospective studies described above, a minority of tumors (31%) contained deleterious alterations in homologous recombination genes, but this appeared to incompletely identify patients who derived clinical benefit from olaparib and temozolomide. As expected, patients with RAD51B and PALB2 homozygous deletions and ATR mutation had prolonged PFS. Tumors from two patients with ATRX mutation were homologous recombination-proficient by the RAD51 assay, and these patients derived limited clinical benefit. ATRX mutations have been associated with increased replication stress and responsiveness to PARP inhibition in preclinical models of glioblastoma, but our findings suggest that ATRX may not predict benefit from olaparib and temozolomide in uLMS.33 Importantly, the RAD51 foci formation assay classified five additional patients as homologous recombination-deficient despite the lack of identifiable genomic homologous recombination alterations. The biologic mechanism imparting HRD in these cases remains unclear and might involve epigenetic changes, alterations in genes not included in the panel or another mechanism. Altogether, these findings suggest that a functional assay providing real-time assessment of homologous recombination status might best identify tumors with BRCAness; however, further prospective evaluation and efforts to optimize the reliability and reproducibility of the assay are needed.
Olaparib and temozolomide was chosen for clinical evaluation on the basis of preclinical observations suggesting that the combination was more active than monotherapy. Our single-arm study is unable to discern the relative efficacy of each agent. Case reports describe activity for olaparib alone in patients with uLMS and BRCA2 homozygous deletions.13,15,34 Notably, in a prospective study of rucaparib and nivolumab in LMS, mPFS was 1.8 months, and one patient (6%) with BRCA2 deletion achieved an objective response, suggesting that PARP inhibitor monotherapy has limited activity in unselected LMS populations.35 Several studies evaluating temozolomide in sarcoma were published between 1998 and 2005; however, interpretation is complicated by varying doses and schedules, lack of data specific to uLMS, and use of WHO response criteria. In the first study, 30 patients with STS (10 LMS) received temozolomide 150-200 mg/m2 once daily (5 days of a 28-day cycle), and one response occurred in retroperitoneal LMS.36 A subsequent phase II study among 39 patients with STS (10 LMS) evaluated temozolomide 85 mg/m2 once daily (21 days on, 7 days off) and observed one response in pelvic LMS.37 In another study, temozolomide was administered with a 200 mg/m2 bolus once on day 1, followed by 90 mg/m2 twice daily (5 days of a 28-day cycle).30 Among 11 patients with LMS, two responded, and the mPFS was 3.9 months. In 2005, Garcia del Muro et al38 reported results with temozolomide 75-100 mg/m2 once daily (6 weeks on, 3 weeks off) and found an ORR of 14% and median time to progression of 2.2 months among 45 patients with STS. Responses were observed in five of 11 patients with gynecologic LMS. mPFS in LMS was not reported, and this protracted dosing regimen may not be tolerable in more heavily pretreated patients. The observation that clinical activity with temozolomide outside of the Garcia del Muro study was modest, mPFS from dacarbazine (the intravenous analog of temozolomide) was 1.5 months in the uLMS subset of a more recent, large randomized study, and that activity in our study appeared to correlate with HRD, suggest that the combination of olaparib and temozolomide underlies the observed efficacy, although this likely depends on the biologic context of a particular tumor.5
Our understanding of DNA damage repair defects and associated therapeutic vulnerabilities in sarcoma, including LMS, remains at an early stage. Newer PARP inhibitors with enhanced PARP trapping capabilities, such as talazoparib, appear active as monotherapy in uLMS models, although clinically achievable concentrations of stronger PARP trappers are limited by hematologic toxicity.39 PARP1-specific inhibitors may enhance the therapeutic index of these agents and allow for more tolerable combinations.40 Cell cycle checkpoints, including CHK1 and PLK1, are overexpressed in uLMS representing an adaptive response to forestall cell cycle progression in the setting of intolerable replicative stress. Accordingly, uLMS preclinical models are sensitive to inhibitors of CHK1, PLK1, and WEE1.39,41 HRD also invokes dependency on alternative DNA repair mechanisms, including non-homologous end-joining (NHEJ), and inhibition of NHEJ effectors, such as DNA-PK, is an elegant therapeutic possibility.39 Frequent alternative lengthening of the telomeres observed in LMS may impart sensitivity to ATR inhibitors.11 We observed upregulation of CXCL10 in on-treatment tumor biopsies suggesting that olaparib and temozolomide might impart favorable changes in the tumor immune microenvironment, thus implicating a role for combinations with immunotherapy.
In summary, the combination of olaparib and temozolomide provided encouraging activity in pretreated uLMS, an aggressive sarcoma subtype without an effective targeted therapy. A subset of uLMS harbor HRD as defined by genomic alterations or functional assessment of homologous recombination status. A randomized phase II/III clinical trial evaluating olaparib and temozolomide versus investigator's choice of trabectedin or pazopanib is underway (ClinicalTrials.gov identifier: NCT05633381).
ACKNOWLEDGMENT
The authors would like to acknowledge Carrie Strand, statistical analyst at Mayo Clinic, and Emily Mulvany of the ETCTN Biorepository for their contributions to the study.
APPENDIX
TABLE A1.
Best Objective Response per RECIST Version 1.1. Criteria

TABLE A2.
Summary Characteristics of Patients With PFS >12 Months
FIG A1.
Representative homologous recombination-proficient (left) and homologous recombination-deficient (right) tumors from patients treated on this study as assessed by a RAD51 foci formation assay. RAD51 foci in the homologous recombination-proficient biopsy are identified with red circles.
FIG A2.
Kaplan-Meir estimates of PFS subset by homologous recombination pathway status as determined by the RAD51 foci formation assay. Three patients who ended the study because of adverse events are included in this analysis. The median PFS for homologous recombination-deficient patients was 342 days and for homologous recombination-proficient patients was 86 days (log-rank P = .077). PFS, progression-free survival.
FIG A3.
Kaplan-Meir estimates of PFS subset by homologous recombination pathway status as determined by the RAD51 foci formation assay. Three patients who ended the study for adverse events were excluded from this analysis. The median PFS for homologous recombination-deficient patients was 342 days and for homologous recombination-proficient patients was 164 days (log-rank P = .05). PFS, progression-free survival.
FIG A4.
Correlation of (A) pre-treatment SLFN11 and (B) MGMT RNA expression with PFS. Using a Cox proportional hazards model, no association was observed between either SLFN11 (HR, 0.98 [95% CI, 0.52 to 1.83]; P = .90) or MGMT (HR, 1.12 [95% CI, 0.65 to 1.92]; P = .70) RNA expression at the pretreatment time point and PFS. The 15 patients with RNAseq results from the pretreatment time point are included. Three patients who ended treatment for toxicity were censored at the time of treatment discontinuation and one patient who remains on active treatment was censored at the time of last follow-up. HR, hazard ratio; MGMT, O6-methylguanine-DNA methyltransferase; PFS, progression-free survival; SLFN11, Schlafen family member number 11.
Matthew Ingham
Employment: Regeneron
Stock and Other Ownership Interests: Regeneron
Consulting or Advisory Role: Daiichi Sankyo, Xencor, Apexigen, Epizyme, Caris Life Sciences
Research Funding: Apexigen (Inst), Mirati Therapeutics (Inst), PTC Therapeutics (Inst), APICES, Intensity (Inst), Boehringer Ingelheim (Inst), Bioatla (Inst), Merck (Inst), Astellas Pharma (Inst), AstraZeneca (Inst)
Li Chen
Research Funding: Illumina (Inst)
Expert Testimony: Illumina (Inst)
Biswajit Das
Research Funding: Illumina (Inst)
Bose Kochupurakkal
Employment: Dana-Farber Cancer Institute, Evelo Biosciences (I)
Stock and Other Ownership Interests: Evelo Biosciences (I), Amgen (I)
Research Funding: Tango Therapeutics (Inst), Merck Serono (Inst), Bristol Myers Squibb (Inst), Moderna Therapeutics (Inst)
Suzanne George
Stock and Other Ownership Interests: Abbott Laboratories
Honoraria: CStone Pharmaceuticals
Consulting or Advisory Role: Blueprint Medicines, Deciphera, Bayer, Lilly, UpToDate, Research to Practice, MORE Health, Daiichi, Kayothera, Immunicum, BioAtla
Research Funding: Blueprint Medicines (Inst), Deciphera (Inst), Daiichi Sankyo RD Novare (Inst), Merck (Inst), Eisai (Inst), SpringWorks Therapeutics (Inst), TRACON Pharma (Inst), Theseus Pharmaceuticals (Inst), BioAtla, IDRx (Inst)
Patents, Royalties, Other Intellectual Property: UptoDate
Expert Testimony: Bayer
Other Relationship: Research to Practice, WCG
Steven Attia
Research Funding: AB Science (Inst), TRACON Pharma (Inst), Bayer (Inst), Novartis (Inst), Lilly (Inst), Karyopharm Therapeutics (Inst), Epizyme (Inst), Blueprint Medicines (Inst), Genmab (Inst), CBA Pharma (Inst), Desmoid Tumor Research Foundation, Merck (Inst), Philogen (Inst), Gradalis (Inst), Deciphera (Inst), Takeda (Inst), Incyte (Inst), SpringWorks Therapeutics (Inst), Adaptimmune (Inst), Advenchen Laboratories (Inst), Bavarian Nordic (Inst), BTG (Inst), PTC Therapeutics (Inst), GlaxoSmithKline (Inst), FORMA Therapeutics (Inst), Ayala Pharmaceuticals (Inst), Trillium Therapeutics (Inst), Boehringer Ingelheim (Inst), Salarius Pharmaceuticals (Inst), Theseus Pharmaceuticals (Inst), Monopar Therapeutics (Inst), C4 Therapeutics (Inst), InhibRx (Inst), Noxopharm (Inst), Rain Therapeutics (Inst)
Melissa A. Burgess
Consulting or Advisory Role: Rain Therapeutics, SpringWorks Therapeutics
Research Funding: Merck
Mahesh Seetharam
Honoraria: Horizon CME, Daiichi Sankyo, Deciphera, AADi
Travel, Accommodations, Expenses: Horizon CME
Sosipatros A. Boikos
Consulting or Advisory Role: Caris Life Sciences
Nam Bui
Consulting or Advisory Role: Rain Therapeutics, SpringWorks Therapeutics
James L. Chen
Employment: Tempus
Consulting or Advisory Role: Tempus
Research Funding: Eisai
Patents, Royalties, Other Intellectual Property: MatchTX
Gregory M. Cote
Consulting or Advisory Role: C4 Therapeutics, Ikena Oncology, Foghorn Therapeutics, Eisai, BioAtla, Daiichi Sankyo/UCB Japan
Research Funding: Macrogenics (Inst), PharmaMar (Inst), Epizyme (Inst), Agios (Inst), Eisai (Inst), Merck KGaA (Inst), CBA Research (Inst), Bavarian Nordic, Bayer (Inst), Springworks Therapeutics (Inst), Sumitomo Dainippon Pharma Oncology (Inst), Foghorn Therapeutics (Inst), Repare Therapeutics (Inst), Jazz Pharmaceuticals (Inst), C4 Therapeutics (Inst), Servier (Inst), Rain Therapeutics (Inst), BioAtla (Inst), Ikena Oncology (Inst), Kronos Bio (Inst)
Premal H. Thaker
Stock and Other Ownership Interests: Celsion
Consulting or Advisory Role: Iovance Biotherapeutics, Celsion, Novocure, GlaxoSmithKline, Eisai, Seagen, Merck, AstraZeneca, Immunogen, Clovis Oncology, AADi, R-Pharm, Zentalis
Research Funding: Merck (Inst), GlaxoSmithKline (Inst)
Alan D'Andrea
Stock and Other Ownership Interests: Cyteir, IMPAC Medical Systems, PrimeFour Therapeutics
Consulting or Advisory Role: Merck Serono, Cyteir, AstraZeneca, Bayer, IMPAC Medical Systems, Pfizer, Tango Therapeutics, Blacksmith/Lightstone Ventures, Bristol Myers Squibb, PrimeFour Therapeutics, Zentalis Pharmaceuticals/Zeno Management
Research Funding: Merck Serono, Bristol Myers Squibb, Moderna Therapeutics, Tango Therapeutics
Travel, Accommodations, Expenses: IDEAYA Biosciences
Geoffrey I. Shapiro
Consulting or Advisory Role: Lilly, Pfizer, Merck Serono, Cybrexa Therapeutics, Bayer, Fusion Pharmaceuticals, Bicycle Therapeutics, Artios, Boehringer Ingelheim, Concarlo, Atrin Pharmaceuticals, Syros Pharmaceuticals, Zentalis, CytomX Therapeutics, Blueprint Medicines, Kymera, Janssen Oncology, Xinthera, ImmunoMet
Research Funding: Pfizer (Inst), Bayer (Inst), Puma Biotechnology (Inst), Novartis (Inst), Cellceutix (Inst), Sanofi (Inst), Cyclacel (Inst), Mirati Therapeutics (Inst), AstraZeneca (Inst), GlaxoSmithKline (Inst), Lilly (Inst), Roche (Inst), CanBas (Inst), Merck Serono (Inst), Sierra Oncology (Inst), Syros Pharmaceuticals (Inst), Merck (Inst), Array BioPharma (Inst), Seagen (Inst), Clovis Oncology (Inst), Exelixis (Inst), Boehringer Ingelheim (Inst), Esperas Pharma (Inst), Amgen (Inst), Bristol Myers Squibb (Inst), AstraZeneca (Inst), Tango Therapeutics (Inst), Bristol Myers Squibb/Medarex (Inst), Senhwa Biosciences (Inst), Biosplice (Inst), Cyteir (Inst), AbbVie (Inst)
Patents, Royalties, Other Intellectual Property: Patent #: 9872874 Title: Dosage regimen for sapacitabine and seliciclib Issue Date: January 23, 2018, Provisional Patent #:62/538,319 Title: Compositions and methods for predicting response and resistance to CDK4/6 inhibition Filed: July 28, 2017
Gary K. Schwartz
This author is an Associate Editor for Journal of Clinical Oncology. Journal policy recused the author from having any role in the peer review of this manuscript.
Stock and Other Ownership Interests: GenCirq, Bionaut Labs, January Therapeutics
Consulting or Advisory Role: Bionaut Labs, Ellipses Pharma, Gencirq, Epizyme, Array BioPharma, Apexigen, Oncogenuity, OnCusp Therapeutics, Concarlo, Shanghai Pharma, Astex Pharmaceuticals, January Therapeutics, Sellas Life Sciences, PureTech, AADi, Kirilys Therapeutics, Agenus, Boehringer Ingelheim, Ipsen
Research Funding: Astex Pharmaceuticals (Inst), Incyte (Inst), Calithera Biosciences (Inst), Lilly (Inst), Daiichi Sankyo (Inst), Fortress Biotech (Inst), Karyopharm Therapeutics (Inst), Oxford BioTherapeutics (Inst), TopAlliance BioSciences Inc (Inst), Adaptimmune (Inst), SpringWorks Therapeutics (Inst), TRACON Pharma (Inst)
Patents, Royalties, Other Intellectual Property: Companion diagnostics for CD4 inhibitors (Inst), patent granted to develop a new technology called PNAs for cancer therapy
Travel, Accommodations, Expenses: Array BioPharma, Epizyme, Boehringer Ingelheim
No other potential conflicts of interest were reported.
PRIOR PRESENTATION
Presented in part at the 2021 ASCO annual meeting, virtual, June 4-8, 2021 and the 2022 ASCO annual meeting, Chicago, IL, June 3-7, 2022.
SUPPORT
CLINICAL TRIAL INFORMATION
AUTHOR CONTRIBUTIONS
Conception and design: Matthew Ingham, Jacob B. Allred, S. Percy Ivy, Geoffrey I. Shapiro, Gary K. Schwartz
Financial support: Matthew Ingham, Geoffrey I. Shapiro
Administrative support: Matthew Ingham, Katherine Gano
Provision of study materials or patients: Matthew Ingham, Suzanne George, Steven Attia, Melissa A. Burgess, Mahesh Seetharam, Sosipatros A. Boikos, Nam Bui, James L. Chen, Gregory M. Cote, Premal H. Thaker, Gary K. Schwartz
Collection and assembly of data: All authors
Data analysis and interpretation: All authors
Manuscript writing: All authors
Final approval of manuscript: All authors
Accountable for all aspects of the work: All authors
AUTHORS' DISCLOSURES OF POTENTIAL CONFLICTS OF INTEREST
Phase II Study of Olaparib and Temozolomide for Advanced Uterine Leiomyosarcoma (NCI Protocol 10250)
The following represents disclosure information provided by authors of this manuscript. All relationships are considered compensated unless otherwise noted. Relationships are self-held unless noted. I = Immediate Family Member, Inst = My Institution. Relationships may not relate to the subject matter of this manuscript. For more information about ASCO's conflict of interest policy, please refer to www.asco.org/rwc or ascopubs.org/jco/authors/author-center.
Open Payments is a public database containing information reported by companies about payments made to US-licensed physicians (Open Payments).
Matthew Ingham
Employment: Regeneron
Stock and Other Ownership Interests: Regeneron
Consulting or Advisory Role: Daiichi Sankyo, Xencor, Apexigen, Epizyme, Caris Life Sciences
Research Funding: Apexigen (Inst), Mirati Therapeutics (Inst), PTC Therapeutics (Inst), APICES, Intensity (Inst), Boehringer Ingelheim (Inst), Bioatla (Inst), Merck (Inst), Astellas Pharma (Inst), AstraZeneca (Inst)
Li Chen
Research Funding: Illumina (Inst)
Expert Testimony: Illumina (Inst)
Biswajit Das
Research Funding: Illumina (Inst)
Bose Kochupurakkal
Employment: Dana-Farber Cancer Institute, Evelo Biosciences (I)
Stock and Other Ownership Interests: Evelo Biosciences (I), Amgen (I)
Research Funding: Tango Therapeutics (Inst), Merck Serono (Inst), Bristol Myers Squibb (Inst), Moderna Therapeutics (Inst)
Suzanne George
Stock and Other Ownership Interests: Abbott Laboratories
Honoraria: CStone Pharmaceuticals
Consulting or Advisory Role: Blueprint Medicines, Deciphera, Bayer, Lilly, UpToDate, Research to Practice, MORE Health, Daiichi, Kayothera, Immunicum, BioAtla
Research Funding: Blueprint Medicines (Inst), Deciphera (Inst), Daiichi Sankyo RD Novare (Inst), Merck (Inst), Eisai (Inst), SpringWorks Therapeutics (Inst), TRACON Pharma (Inst), Theseus Pharmaceuticals (Inst), BioAtla, IDRx (Inst)
Patents, Royalties, Other Intellectual Property: UptoDate
Expert Testimony: Bayer
Other Relationship: Research to Practice, WCG
Steven Attia
Research Funding: AB Science (Inst), TRACON Pharma (Inst), Bayer (Inst), Novartis (Inst), Lilly (Inst), Karyopharm Therapeutics (Inst), Epizyme (Inst), Blueprint Medicines (Inst), Genmab (Inst), CBA Pharma (Inst), Desmoid Tumor Research Foundation, Merck (Inst), Philogen (Inst), Gradalis (Inst), Deciphera (Inst), Takeda (Inst), Incyte (Inst), SpringWorks Therapeutics (Inst), Adaptimmune (Inst), Advenchen Laboratories (Inst), Bavarian Nordic (Inst), BTG (Inst), PTC Therapeutics (Inst), GlaxoSmithKline (Inst), FORMA Therapeutics (Inst), Ayala Pharmaceuticals (Inst), Trillium Therapeutics (Inst), Boehringer Ingelheim (Inst), Salarius Pharmaceuticals (Inst), Theseus Pharmaceuticals (Inst), Monopar Therapeutics (Inst), C4 Therapeutics (Inst), InhibRx (Inst), Noxopharm (Inst), Rain Therapeutics (Inst)
Melissa A. Burgess
Consulting or Advisory Role: Rain Therapeutics, SpringWorks Therapeutics
Research Funding: Merck
Mahesh Seetharam
Honoraria: Horizon CME, Daiichi Sankyo, Deciphera, AADi
Travel, Accommodations, Expenses: Horizon CME
Sosipatros A. Boikos
Consulting or Advisory Role: Caris Life Sciences
Nam Bui
Consulting or Advisory Role: Rain Therapeutics, SpringWorks Therapeutics
James L. Chen
Employment: Tempus
Consulting or Advisory Role: Tempus
Research Funding: Eisai
Patents, Royalties, Other Intellectual Property: MatchTX
Gregory M. Cote
Consulting or Advisory Role: C4 Therapeutics, Ikena Oncology, Foghorn Therapeutics, Eisai, BioAtla, Daiichi Sankyo/UCB Japan
Research Funding: Macrogenics (Inst), PharmaMar (Inst), Epizyme (Inst), Agios (Inst), Eisai (Inst), Merck KGaA (Inst), CBA Research (Inst), Bavarian Nordic, Bayer (Inst), Springworks Therapeutics (Inst), Sumitomo Dainippon Pharma Oncology (Inst), Foghorn Therapeutics (Inst), Repare Therapeutics (Inst), Jazz Pharmaceuticals (Inst), C4 Therapeutics (Inst), Servier (Inst), Rain Therapeutics (Inst), BioAtla (Inst), Ikena Oncology (Inst), Kronos Bio (Inst)
Premal H. Thaker
Stock and Other Ownership Interests: Celsion
Consulting or Advisory Role: Iovance Biotherapeutics, Celsion, Novocure, GlaxoSmithKline, Eisai, Seagen, Merck, AstraZeneca, Immunogen, Clovis Oncology, AADi, R-Pharm, Zentalis
Research Funding: Merck (Inst), GlaxoSmithKline (Inst)
Alan D'Andrea
Stock and Other Ownership Interests: Cyteir, IMPAC Medical Systems, PrimeFour Therapeutics
Consulting or Advisory Role: Merck Serono, Cyteir, AstraZeneca, Bayer, IMPAC Medical Systems, Pfizer, Tango Therapeutics, Blacksmith/Lightstone Ventures, Bristol Myers Squibb, PrimeFour Therapeutics, Zentalis Pharmaceuticals/Zeno Management
Research Funding: Merck Serono, Bristol Myers Squibb, Moderna Therapeutics, Tango Therapeutics
Travel, Accommodations, Expenses: IDEAYA Biosciences
Geoffrey I. Shapiro
Consulting or Advisory Role: Lilly, Pfizer, Merck Serono, Cybrexa Therapeutics, Bayer, Fusion Pharmaceuticals, Bicycle Therapeutics, Artios, Boehringer Ingelheim, Concarlo, Atrin Pharmaceuticals, Syros Pharmaceuticals, Zentalis, CytomX Therapeutics, Blueprint Medicines, Kymera, Janssen Oncology, Xinthera, ImmunoMet
Research Funding: Pfizer (Inst), Bayer (Inst), Puma Biotechnology (Inst), Novartis (Inst), Cellceutix (Inst), Sanofi (Inst), Cyclacel (Inst), Mirati Therapeutics (Inst), AstraZeneca (Inst), GlaxoSmithKline (Inst), Lilly (Inst), Roche (Inst), CanBas (Inst), Merck Serono (Inst), Sierra Oncology (Inst), Syros Pharmaceuticals (Inst), Merck (Inst), Array BioPharma (Inst), Seagen (Inst), Clovis Oncology (Inst), Exelixis (Inst), Boehringer Ingelheim (Inst), Esperas Pharma (Inst), Amgen (Inst), Bristol Myers Squibb (Inst), AstraZeneca (Inst), Tango Therapeutics (Inst), Bristol Myers Squibb/Medarex (Inst), Senhwa Biosciences (Inst), Biosplice (Inst), Cyteir (Inst), AbbVie (Inst)
Patents, Royalties, Other Intellectual Property: Patent #: 9872874 Title: Dosage regimen for sapacitabine and seliciclib Issue Date: January 23, 2018, Provisional Patent #:62/538,319 Title: Compositions and methods for predicting response and resistance to CDK4/6 inhibition Filed: July 28, 2017
Gary K. Schwartz
This author is an Associate Editor for Journal of Clinical Oncology. Journal policy recused the author from having any role in the peer review of this manuscript.
Stock and Other Ownership Interests: GenCirq, Bionaut Labs, January Therapeutics
Consulting or Advisory Role: Bionaut Labs, Ellipses Pharma, Gencirq, Epizyme, Array BioPharma, Apexigen, Oncogenuity, OnCusp Therapeutics, Concarlo, Shanghai Pharma, Astex Pharmaceuticals, January Therapeutics, Sellas Life Sciences, PureTech, AADi, Kirilys Therapeutics, Agenus, Boehringer Ingelheim, Ipsen
Research Funding: Astex Pharmaceuticals (Inst), Incyte (Inst), Calithera Biosciences (Inst), Lilly (Inst), Daiichi Sankyo (Inst), Fortress Biotech (Inst), Karyopharm Therapeutics (Inst), Oxford BioTherapeutics (Inst), TopAlliance BioSciences Inc (Inst), Adaptimmune (Inst), SpringWorks Therapeutics (Inst), TRACON Pharma (Inst)
Patents, Royalties, Other Intellectual Property: Companion diagnostics for CD4 inhibitors (Inst), patent granted to develop a new technology called PNAs for cancer therapy
Travel, Accommodations, Expenses: Array BioPharma, Epizyme, Boehringer Ingelheim
No other potential conflicts of interest were reported.
REFERENCES
- 1. Gadducci A, Landoni F, Sartori E, et al. Uterine leiomyosarcoma: Analysis of treatment failures and survival. Gynecol Oncol. 1996;62:25–32. doi: 10.1006/gyno.1996.0185. [DOI] [PubMed] [Google Scholar]
- 2. Hensley ML, Blessing JA, Mannel R, et al. Fixed-dose rate gemcitabine plus docetaxel as first-line therapy for metastatic uterine leiomyosarcoma: A Gynecologic Oncology Group phase II trial. Gynecol Oncol. 2008;109:329–334. doi: 10.1016/j.ygyno.2008.03.010. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 3. Hensley ML, Blessing JA, Degeest K, et al. Fixed-dose rate gemcitabine plus docetaxel as second-line therapy for metastatic uterine leiomyosarcoma: A Gynecologic Oncology Group phase II study. Gynecol Oncol. 2008;109:323–328. doi: 10.1016/j.ygyno.2008.02.024. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 4. Seddon B, Strauss SJ, Whelan J, et al. Gemcitabine and docetaxel versus doxorubicin as first-line treatment in previously untreated advanced unresectable or metastatic soft-tissue sarcomas (GeDDiS): A randomised controlled phase 3 trial. Lancet Oncol. 2017;18:1397–1410. doi: 10.1016/S1470-2045(17)30622-8. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 5. Hensley ML, Patel SR, von Mehren M, et al. Efficacy and safety of trabectedin or dacarbazine in patients with advanced uterine leiomyosarcoma after failure of anthracycline-based chemotherapy: Subgroup analysis of a phase 3, randomized clinical trial. Gynecol Oncol. 2017;146:531–537. doi: 10.1016/j.ygyno.2017.06.018. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 6. Benson C, Ray-Coquard I, Sleijfer S, et al. Outcome of uterine sarcoma patients treated with pazopanib: A retrospective analysis based on two European Organisation for Research and Treatment of Cancer (EORTC) Soft Tissue and Bone Sarcoma Group (STBSG) clinical trials 62043 and 62072. Gynecol Oncol. 2016;142:89–94. doi: 10.1016/j.ygyno.2016.03.024. [DOI] [PubMed] [Google Scholar]
- 7. Ben-Ami E, Barysauskas CM, Solomon S, et al. Immunotherapy with single agent nivolumab for advanced leiomyosarcoma of the uterus: Results of a phase 2 study. Cancer. 2017;123:3285–3290. doi: 10.1002/cncr.30738. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 8. Hensley ML, Miller A, O'Malley DM, et al. Randomized phase III trial of gemcitabine plus docetaxel plus bevacizumab or placebo as first-line treatment for metastatic uterine leiomyosarcoma: An NRG Oncology/Gynecologic Oncology Group study. J Clin Oncol. 2015;33:1180–1185. doi: 10.1200/JCO.2014.58.3781. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 9. Hyman DM, Sill MW, Lankes HA, et al. A phase 2 study of alisertib (MLN8237) in recurrent or persistent uterine leiomyosarcoma: An NRG Oncology/Gynecologic Oncology Group study 0231D. Gynecol Oncol. 2017;144:96–100. doi: 10.1016/j.ygyno.2016.10.036. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 10. Duska LR, Blessing JA, Rotmensch J, et al. A phase II evaluation of ixabepilone (IND #59699, NSC #710428) in the treatment of recurrent or persistent leiomyosarcoma of the uterus: An NRG Oncology/Gynecologic Oncology Group Study. Gynecol Oncol. 2014;135:44–48. doi: 10.1016/j.ygyno.2014.07.101. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 11. Chudasama P, Mughal SS, Sanders MA, et al. Integrative genomic and transcriptomic analysis of leiomyosarcoma. Nat Commun. 2018;9:144. doi: 10.1038/s41467-017-02602-0. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 12. Choi J, Manzano A, Dong W, et al. Integrated mutational landscape analysis of uterine leiomyosarcomas. Proc Natl Acad Sci USA. 2021;118:e2025182118. doi: 10.1073/pnas.2025182118. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 13. Seligson ND, Kautto EA, Passen EN, et al. BRCA1/2 functional loss defines a targetable subset in leiomyosarcoma. Oncologist. 2019;24:973–979. doi: 10.1634/theoncologist.2018-0448. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 14. Rosenbaum E, Jonsson P, Seier K, et al. DNA damage response pathway alterations and clinical outcome in leiomyosarcoma. J Clin Oncol. 2019;37(suppl 15) abstr 11048. [Google Scholar]
- 15. Hensley ML, Chavan SS, Solit DB, et al. Genomic landscape of uterine sarcomas defined through prospective clinical sequencing. Clin Cancer Res. 2020;26:3881–3888. doi: 10.1158/1078-0432.CCR-19-3959. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 16. Lord CJ, Ashworth A. PARP inhibitors: Synthetic lethality in the clinic. Science. 2017;355:1152–1158. doi: 10.1126/science.aam7344. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 17. Oza J, Doshi SD, Hao L, et al. Homologous recombination repair deficiency as a therapeutic target in sarcoma. Semin Oncol. 2020;47:380–389. doi: 10.1053/j.seminoncol.2020.10.002. [DOI] [PubMed] [Google Scholar]
- 18. Farago AF, Yeap BY, Stanzione M, et al. Combination olaparib and temozolomide in relapsed small-cell lung cancer. Cancer Discov. 2019;9:1372–1387. doi: 10.1158/2159-8290.CD-19-0582. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 19. Takebe N, Naqash AR, O'Sullivan Coyne G, et al. Safety, antitumor activity, and biomarker analysis in a phase I trial of the once-daily Wee1 inhibitor adavosertib (AZD1775) in patients with advanced solid tumors. Clin Cancer Res. 2021;27:3834–3844. doi: 10.1158/1078-0432.CCR-21-0329. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 20. Sztupinszki Z, Diossy M, Krzystanek M, et al. Migrating the SNP array-based homologous recombination deficiency measures to next generation sequencing data of breast cancer. NPJ Breast Cancer. 2018;4:16. doi: 10.1038/s41523-018-0066-6. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 21. Kochupurakkal B, Parmar K, Lazaro JB, et al. Abstract 2796: Development of a RAD51-based assay for determining homologous recombination proficiency and PARP inhibitor sensitivity. Clin Cancer Res. 2017;77:2796. [Google Scholar]
- 22. Parmar K, Kochupurakkal BS, Lazaro JB, et al. The CHK1 inhibitor prexasertib exhibits monotherapy activity in high-grade serous ovarian cancer models and sensitizes to PARP inhibition. Clin Cancer Res. 2019;25:6127–6140. doi: 10.1158/1078-0432.CCR-19-0448. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 23. Knijnenburg TA, Wang L, Zimmermann MT, et al. Genomic and molecular landscape of DNA damage repair deficiency across the cancer genome atlas. Cell Rep. 2018;23:239–254.e6. doi: 10.1016/j.celrep.2018.03.076. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 24. Amuzu S, Carmona E, Mes-Masson AM, et al. Candidate markers of olaparib response from genomic data analyses of human cancer cell lines. Cancers (Basel) 2021;13:1296. doi: 10.3390/cancers13061296. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 25. Murai J, Thomas A, Miettinen M, et al. Schlafen 11 (SLFN11), a restriction factor for replicative stress induced by DNA-targeting anti-cancer therapies. Pharmacol Ther. 2019;201:94–102. doi: 10.1016/j.pharmthera.2019.05.009. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 26. Willis SE, Winkler C, Roudier MP, et al. Retrospective analysis of Schlafen11 (SLFN11) to predict the outcomes to therapies affecting the DNA damage response. Br J Cancer. 2021;125:1666–1676. doi: 10.1038/s41416-021-01560-1. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 27. Zhang B, Ramkumar K, Cardnell RJ, et al. A wake-up call for cancer DNA damage: The role of Schlafen 11 (SLFN11) across multiple cancers. Br J Cancer. 2021;125:1333–1340. doi: 10.1038/s41416-021-01476-w. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 28. Lok BH, Gardner EE, Schneeberger VE, et al. PARP inhibitor activity correlates with SLFN11 expression and demonstrates synergy with temozolomide in small cell lung cancer. Clin Cancer Res. 2017;23:523–535. doi: 10.1158/1078-0432.CCR-16-1040. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 29. Pietanza MC, Waqar SN, Krug LM, et al. Randomized, double-blind, phase II study of temozolomide in combination with either veliparib or placebo in patients with relapsed-sensitive or refractory small-cell lung cancer. J Clin Oncol. 2018;36:2386–2394. doi: 10.1200/JCO.2018.77.7672. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 30. Talbot SM, Keohan ML, Hesdorffer M, et al. A phase II trial of temozolomide in patients with unresectable or metastatic soft tissue sarcoma. Cancer. 2003;98:1942–1946. doi: 10.1002/cncr.11730. [DOI] [PubMed] [Google Scholar]
- 31. Tutt ANJ, Garber JE, Kaufman B, et al. Adjuvant olaparib for patients with BRCA1- or BRCA2-mutated breast cancer. N Engl J Med. 2021;384:2394–2405. doi: 10.1056/NEJMoa2105215. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 32. Jonsson P, Bandlamudi C, Cheng ML, et al. Tumour lineage shapes BRCA-mediated phenotypes. Nature. 2019;571:576–579. doi: 10.1038/s41586-019-1382-1. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 33. Garbarino J, Eckroate J, Sundaram RK, et al. Loss of ATRX confers DNA repair defects and PARP inhibitor sensitivity. Transl Oncol. 2021;14:101147. doi: 10.1016/j.tranon.2021.101147. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 34. Shammas N, Yang T, Abidi A, et al. Clinical use of PARP inhibitor in recurrent uterine leiomyosarcoma with presence of a somatic BRCA2 mutation. Gynecol Oncol Rep. 2022;42:101044. doi: 10.1016/j.gore.2022.101044. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 35.Movva S, Rosenbaum E, Kelly CM, et al. Phase II study of rucaparib and nivolumab in patients with leiomyosarcoma. Presented at CTOS annual meeting, Vancouver, Canada, November 16-19, 2022.
- 36. Woll PJ, Judson I, Lee SM, et al. Temozolomide in adult patients with advanced soft tissue sarcoma: A phase II study of the EORTC Soft Tissue and Bone Sarcoma Group. Eur J Cancer. 1999;35:410–412. doi: 10.1016/s0959-8049(98)00403-1. [DOI] [PubMed] [Google Scholar]
- 37. Trent JC, Beach J, Burgess MA, et al. A two-arm phase II study of temozolomide in patients with advanced gastrointestinal stromal tumors and other soft tissue sarcomas. Cancer. 2003;98:2693–2699. doi: 10.1002/cncr.11875. [DOI] [PubMed] [Google Scholar]
- 38. Garcia del Muro X, Lopez-Pousa A, Martin J, et al. A phase II trial of temozolomide as a 6-week, continuous, oral schedule in patients with advanced soft tissue sarcoma: A study by the Spanish Group for Research on Sarcomas. Cancer. 2005;104:1706–1712. doi: 10.1002/cncr.21384. [DOI] [PubMed] [Google Scholar]
- 39. Anderson ND, Babichev Y, Fuligni F, et al. Lineage-defined leiomyosarcoma subtypes emerge years before diagnosis and determine patient survival. Nat Commun. 2021;12:4496. doi: 10.1038/s41467-021-24677-6. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 40.Yap T, Im S, Schram A, et al. CT007—PETRA: First in class, first in human trial of the next generation PARP1-selective inhibitor AZD5305 in patients (pts) with BRCA1/2, PALB2 or RAD51C/D mutations. Presented at AACR annual meeting, New Orleans, LA, April 8-13, 2022.
- 41. Yoshida K, Yokoi A, Yamamoto T, et al. Aberrant activation of cell-cycle-related kinases and the potential therapeutic impact of PLK1 or CHEK1 inhibition in uterine leiomyosarcoma. Clin Cancer Res. 2022;28:2147–2159. doi: 10.1158/1078-0432.CCR-22-0100. [DOI] [PMC free article] [PubMed] [Google Scholar]