Abstract
BACKGROUND
Prostate cancer is regulated by steroid hormones, even in castration-resistant disease. ODM-208, a novel inhibitor of cytochrome P450 11A1 (which catalyzes the first step of steroid-hormone biosynthesis), was investigated in patients with heavily pretreated metastatic castration-resistant prostate cancer (mCRPC).
METHODS
CYPIDES is a first-in-human phase 1 (3 + 3 design) and phase 2 study. We administered ODM-208 twice daily with glucocorticoid/mineralocorticoid replacement and ongoing androgen deprivation therapy to adults with previously treated mCRPC, regardless of androgen receptor gene (AR) ligand-binding domain mutations (phase 1) and with activating AR ligand-binding domain mutations (ARmut; phase 2). Safety, pharmacokinetics, steroid-hormone pharmacodynamics, and preliminary efficacy were the key outcomes.
RESULTS
Ninety-two patients received one or more doses of ODM-208: 47 in phase 1 (20 [42.6%] with ARmut) and 45 in phase 2 (all ARmut). A dose of ODM-208 of 5 mg twice a day with dexamethasone 1 mg/fludrocortisone 0.1 mg provided a balance between decreased steroidogenesis and toxicity. Treatment-related adrenal insufficiency was the most common toxicity in phase 1 (n=17, 36.2%; necessitating ODM-208 discontinuation in one patient); this toxicity occurred in six patients (13.3%) at 5 mg twice a day in phase 2. Median circulating testosterone levels declined from 3.0 ng/dl (interquartile range, 1.3 to 6.2 ng/dl) at baseline to undetectable levels within the first week of ODM-208 5 mg twice a day treatment in 46 of 53 (87%) patients. A decrease in prostate-specific antigen levels of 50% or more occurred in 14 of 19 (73.7%) patients with ARmut and 2 of 23 (8.7%) patients with AR wild type in phase 1 and in 24 of 45 (53.3%) patients with ARmut in phase 2.
CONCLUSIONS
ODM-208 potently inhibited steroid-hormone biosynthesis with the expected toxicity of adrenal insufficiency. Evidence of antitumor activity was observed in this heavily pretreated mCRPC population, especially in those with ARmut. (Funded by Orion Pharma; Clinical-Trials.gov number, NCT03436485.)
Introduction
Despite initial generally favorable responses to androgen deprivation therapy (ADT),1–3 some patients with prostate cancer ultimately acquire resistance to treatment, resulting in disease recurrence and potential progression to lethal metastatic castration-resistant prostate cancer.4,5 A major contributor to ADT resistance is persistence or reactivation of androgen receptor (AR) signaling, rendering this pathway a rational treatment target.6 Although the cytochrome P450 17A1 (CYP17A1) inhibitor abiraterone acetate (referred to here as abiraterone) and the second-generation AR pathway inhibitors apalutamide, darolutamide, and enzalutamide confer a survival advantage in men with castration-resistant prostate cancer,7–10 they are not curative in the metastatic setting.1 In most patients, the AR axis remains activated, leading once again to treatment resistance.11,12 Potential underlying mechanisms include amplification of the AR gene (AR) or adaptive phenotypes and non-AR pathways, allowing residual androgen production.6,13,14
Activating AR mutations affecting the C-terminal ligand-binding domain (LBD; ARmut) occur rarely in treatment-naïve prostate cancer, but they are found in approximately 20% of patients with metastatic castration-resistant disease.15 Mutant AR (e.g., L702H, W742C, H875Y, F877L, and T878A) may have increased binding affinity and allow promiscuous AR signaling pathway activation by low-potency androgens, other steroid hormones (e.g., progesterone or cortisol), and AR antagonists, enabling cancer survival and promoting resistance to AR pathway inhibitors.15,16
ODM-208 (also called MK-5684) is a novel, oral, nonsteroidal, selective inhibitor of CYP11A1, a steroidogenic enzyme that catalyzes the first and rate-limiting step of androgen biosynthesis: conversion of cholesterol to pregnenolone.17 Preclinically, ODM-208 blocked the steroidogenic cascade beyond cholesterol, suppressing the production of all androgenic and nonandrogenic steroid hormones and precursors that may activate the AR signaling pathway.18 The CYPIDES study (NCT03436485) was conducted to evaluate the safety and preliminary efficacy of ODM-208 in pretreated patients with metastatic castration-resistant prostate cancer.
Methods
STUDY DESIGN AND OVERSIGHT
CYPIDES was an open-label, nonrandomized, single-arm, multicenter study in two phases. Phase 1 was dose escalation, and phase 2 was dose expansion. Primary objectives were to evaluate the safety and side effect profile of ODM-208 (phases 1 and 2), define the maximum tolerated dose and the dose that represented a balance between drug adverse effects and effects on steroidogenesis (recommended phase 2 dose), and, at that dose, assess the preliminary efficacy of ODM-208 in patients with ARmut metastatic castration-resistant prostate cancer with progression on AR pathway inhibitors and taxane-based therapy (phase 2). The study was conducted in accordance with the principles of the Declaration of Helsinki, the International Conference on Harmonisation Good Clinical Practice guidelines, and local regulatory guidelines. The protocol and any amendments (available with the full text of this article at https://evidence.nejm.org) were approved by the institutional review board or ethics committee at each site. All patients provided written informed consent to participate.
The study was designed by the authors in collaboration with Orion Pharma personnel, who gathered the data. The data were analyzed by the authors, who vouch for the data and the conformity of the analysis of the data to the Supplementary Protocol. The article was drafted by the authors and a medical writer paid for by Orion Corporation. The decision to publish was made by the authors. There were no agreements concerning confidentiality of the data between the sponsor and the authors or their institutions.
PATIENTS
Adults (≥18 years of age) with metastatic, histologically confirmed adenocarcinoma of the prostate (without neuroendocrine differentiation [phase 1 only] or small-cell features) with progression on one or more AR pathway inhibitors and one or more taxane-based chemotherapies were enrolled from sites across Finland, France, the United Kingdom, and the United States. Patients in phase 2 were required to harbor ARmut in plasma circulating tumor DNA (ctDNA), because we detected a stronger efficacy signal in phase 1 in the subset of patients with ARmut. Although testing was not mandatory in phase 1, ARmut status was known for some patients. All patient eligibility criteria are provided in the Supplementary Protocol.
INTERVENTIONS
In the phase 1 3 + 3 dose escalation study,19 ODM-208 was administered orally, initially escalating from a starting dose of 50 mg twice daily (with food) defined on the basis of preclinical data,18 with subsequent de-escalation because of maximum steroid suppression at 50 and 75 mg twice a day (Fig. S1A and S1B in the Supplementary Appendix). In phase 2, patients received ODM-208 at 5 mg twice a day. The Safety Monitoring Board determined the dose to be used (i.e., 5 mg twice a day) on the basis of review of all pharmacokinetic and pharmacodynamic (steroid hormone inhibition) findings (i.e., the lowest dose studied that efficiently inhibited steroid hormone production) (Fig. S1C). In both phases, ODM-208 dosing was accompanied by oral glucocorticoid and mineralocorticoid replacement to prevent adrenal insufficiency–like events and ADT. Treatment continued until the investigator considered it no longer beneficial to the patient or until the occurrence of death or intolerable toxicity. In the case of an adrenal insufficiency–like event, the decision to pause and subsequently restart ODM-208 dosing was at the investigator’s discretion. Adrenal recovery was monitored upon gradual glucocorticoid and mineralocorticoid replacement withdrawal during a 28-day posttreatment period (Supplementary Appendix Sections 1 and 2). An end-of-study visit was organized 28 days after the last date of ODM-208 administration.
In phase 1, replacement therapy comprised dexamethasone (starting dose, 1 mg/day), which was selected because, unlike other glucocorticoids, it does not activate the AR pathway,20 and fludrocortisone (starting dose, 0.05 mg), with dose adjustment as clinically indicated. Alternative corticosteroids and doses were also tested to inform the regimen (Supplementary Appendix Sections 1 and 2 and Table S2), which was used in phase 2: dexamethasone 1 mg and fludrocortisone 0.1 mg (both administered once daily).
ASSESSMENTS AND STUDY END POINTS
Safety and changes in laboratory variables were recorded from initiation of ODM-208 until the end of the study (28 days after the last dose of ODM-208), study discontinuation, or death, whichever occurred first. Safety end points included treatment-emergent and serious adverse events and dose-limiting toxicities. Dose-limiting toxicities were collected during the first 28 days of ODM-208 treatment. All adverse events were graded for severity according to the National Cancer Institute Common Terminology Criteria for Solid Tumors (version 4.03).21
Blood samples were collected to monitor safety, plasma pharmacokinetic (ODM-208) and pharmacodynamic (steroid hormones) variables (listed in Table S3), prostate-specific antigen (PSA), and tumor ARmut status (ctDNA). Chest, abdomen, and pelvic computed tomography or magnetic resonance imaging and radionuclide bone scanning were conducted at screening and regularly throughout the study. Efficacy end points included PSA response (PSA level reduction of ≥50% from baseline [PSA50]), soft-tissue response (per the Response Evaluation Criteria in Solid Tumors version 1.1 [RECIST version 1.1]22), and assessment of bone metastasis (per the Prostate Cancer Working Group 3 [PCWG3] criteria23). ctDNA analysis of somatic point mutations in AR LBD from a panel that included L702H, V716 M, W742C, W742L, H875Y, F877L, T878A, T878S, M896T, and M896V was performed centrally in phase 1 using the OncoBEAM test (digital polymerase chain reaction technology; Sysmex Inostics) and in phase 2 using the Guardant360 CDx test (next-generation sequencing technology; Guardant Health).
STATISTICAL ANALYSIS
There were no formal statistical hypotheses or sample size calculations. Descriptive statistics were used throughout. Efficacy variables were analyzed in the intention-to-treat population (all enrolled patients). Data were analyzed by dose and ARmut status (positive [ARmut] vs. negative [AR wild type; ARwt]) if available, with 95% confidence intervals and a two-sided type I error of 0.05 where possible in cases where appropriate median and interquartile ranges are reported. Time-to-event parameters were analyzed using descriptive and Kaplan–Meier statistics. Imaging-based progression-free survival (time from enrollment to imaging-based progression or death)24 and time to PSA progression (time from enrollment to date of PSA progression according to PCWG3 criteria or death) were also evaluated. Pharmacokinetic parameters were analyzed after logarithmic transformation. Safety data were analyzed for all patients in phase 1 and phase 2 (separately) who received one or more doses of ODM-208. The data cutoff date was January 23, 2023, when five patients had ODM-208 treatment ongoing.
Results
PATIENTS AND TREATMENTS
At data cutoff in phase 1, 47 patients (median age, 70.0 years) had received one or more doses of ODM-208 and were evaluable for safety. Of the 47 patients in this phase, 20 (42.6%) patients harbored ARmut (Table 1), most frequently T878A, and most patients (n=46, 97.9%) harbored three or fewer ARmut mutations (Table S4). Patients who participated in phase 1 had bone-only disease more often than patients who participated in phase 2 (44.7% vs. 23.3%, respectively), whereas patients in phase 2 more frequently had node-only cancer (2.1% vs. 23.3%, respectively) (Table S4). Forty-five (95.7%) patients discontinued the phase 1 part, primarily because of progressive disease (n=31, 68.9%). Treatment was ongoing for two (4.3%) patients (Fig. S2). Seven doses of ODM-208 were tested: 3, 5, 15, 25, 50, and 75 mg twice a day and 25 mg once daily. The alternative glucocorticoid agents tested did not appear more effective at preventing adrenal insufficiency–like events and were not evaluated further.
Table 1.
Patient Demographics and Baseline Characteristics.*
| Characteristic | Phase 1 (All Doses Combined; N=47) | Phase 2 (5 mg Twice a Day; N=45) |
|---|---|---|
| Age, median (IQR) — yr | 70.0 (61–73) | 69.0 (61–75) |
| Body mass index, median (IQR) — kg/m2 | 26.7 (24.8–28.6) | 25.8 (23.5–27.7) |
| ECOG performance status — no. (%) | ||
| 0 | 17 (36.2) | 10 (22.2) |
| 1 | 30 (63.8) | 35 (77.8) |
| PSA concentration, median (IQR) — μg/l | 126.0 (30.7–385.9) | 319.6 (137.9–768.5) |
| Gleason total score — no. (%) † | ||
| 7 | 14 (32.6) | 16 (38.1) |
| 8 | 13 (30.2) | 6 (14.3) |
| 9 | 14 (32.6) | 17 (40.5) |
| 10 | 0 | 3 (7.1) |
| Testosterone, median (IQR) — ng/dl | 3.8 (1.4–6.0) | 3.2 (1.1–6.5) |
| AR mutation — no. (%) | ||
| Yes | 20 (42.6) | 45 (100) |
| Prior lines of systemic therapy — no. (%) | ||
| 1 | 1 (2.1) | 0 |
| 2 | 2 (4.3) | 7 (15.6) |
| ≥3 | 44 (93.6) | 38 (84.4) |
| Select prior systemic therapies — no. (%) | ||
| Taxanes | ||
| Docetaxel | 44 (93.6) | 44 (97.8) |
| Cabazitaxel | 25 (53.2) | 31 (68.9) |
| Taxane naïve | 3 (6.4) | 0 |
| Abiraterone | 34 (72.3) | 38 (84.4) |
| Enzalutamide | 36 (76.6) | 31 (68.9) |
| Abiraterone and enzalutamide | 24 (51.1) | 25 (55.6) |
The full table can be found in Table S4. AR denotes androgen receptor gene; ECOG, Eastern Cooperative Oncology Group, with a score of 0 indicating fully active and a score of 1 restricted in strenuous activity; IQR, interquartile range; and PSA, prostate-specific antigen.
The percentage of patients with Gleason total score is calculated on the basis of patients with an available score (n=43 for phase 1 and n=42 for phase 2).
In phase 2, 45 distinct patients with heavily pretreated ARmut (median age 69.0 years) received one or more doses of ODM-208. All had received at least one AR pathway inhibitor and one taxane. Two thirds of the cohort (n=30, 66.7%) harbored a single AR mutation; the most commonly detected mutation was L702H (n=27, 40.9% of the 66 mutations detected) (Table S4). Forty-two (93.3%) patients discontinued the phase 2 part, primarily because of progressive disease (n=37, 88.1%). At data cutoff, median treatment and follow-up durations were 4.4 months (range, 1 to 18) and 5.8 months (range, 1 to 14), respectively; three (6.7%) patients were still on treatment. All 45 patients were evaluable for safety and PSA response (Fig. S2). Of the 92 patients enrolled in the study, 14 (15.2%) died during dosing or follow-up; all deaths were attributed to prostate cancer.
SAFETY AND ADVERSE EVENT PROFILE
A summary of all treatment-emergent adverse events occurring in greater than or equal to 15% of either cohort is provided in Tables 2 and 3; more comprehensive lists of adverse events are provided in Tables S5 and S6. In phase 1, there was one dose-limiting toxicity: grade 3 adrenal insufficiency during dosing with ODM-208 50 mg twice a day. The most frequently observed treatment-emergent adverse event was adrenal insufficiency, often manifesting with fever, asthenia/fatigue, nausea, vomiting, abdominal pain, and elevated serum C-reactive protein (n=17 patients, 36.2%) (Table 3 and Table S5; Table S6 lists by the dose group); 15 patients (31.9%) had grade 3 or higher adverse events, and all were considered related to ODM-208. In phase 1, the incidence of adrenal insufficiency appeared to be lower with ODM-208 daily doses less than or equal to 25 mg (29.4%, n=5 of 17) compared with daily doses greater than 25 mg (40.0%, n=12 of 30). At these lower doses, 23.5% of patients had grade 3 or higher toxicity compared with 36.7% at doses above 25 mg. At the lower doses, the mean time across both phases to the first serious adverse event of adrenal insufficiency ranged from 1.3 to 6.0 months versus from 0.4 to 1.5 months for high doses. Adrenal insufficiency led to temporary ODM-208 interruption in eight (17.0%) patients; seven patients responded rapidly to standard rescue therapy (brief hospitalization and/or intravenous hydrocortisone and fluids, n=7), and withdrawal of ODM-208 was required in one (2.1%) patient. Adverse events caused ODM-208 discontinuation in 20 (42.6%) patients; the most common adverse event was tumor pain (n=6, 12.8%), with two patients having disease progression.
Table 2.
Summary of Treatment-Emergent Adverse Events and a List of Adverse Events (Regardless of Causality) Occurring in Greater Than or Equal to 15% of Patients in Either the Phase 1 or Phase 2 Cohort by Preferred Term and Maximum National Cancer Institute Common Terminology Criteria for Adverse Events (Version 4.03) Grade.*
| Adverse Events by Preferred Term | Phase 1 (All Dose Cohorts Combined; N=47), n (%) | Phase 2 (5 mg Twice a Day; N=45), n (%) |
|---|---|---|
| All adverse events | 47 (100.0) | 45 (100) |
| Grade ≥3 | 33 (70.2) | 36 (80.0) |
| Related to ODM-208 | 44 (93.6) | 38 (84.4) |
| Grade ≥3 | 21 (44.7) | 10 (22.2) |
| Serious adverse events | 29 (61.7) | 29 (64.4) |
| Related to ODM-208 | 19 (40.4) | 6 (13.3) |
| Serious adrenal insufficiency-like events † | 16 (34.0) | 3 (6.7) |
| Adverse events leading to permanent discontinuation | 20 (42.6) | 10 (22.2) |
| Adverse events leading to ODM-208 interruption | 26 (55.3) | 20 (44.4) |
| No. of patients who died | 2 (4.3) | 10 (22.2) |
| Deaths related to ODM-208 | 0 | 0 |
The treatment relatedness of adverse events was determined by site investigators; it is possible that adverse events not considered treatment related were, in fact, so related.
Adrenal insufficiency–like events (i.e., adrenal insufficiency and glucocorticoid deficiency) were determined by the investigators on the basis of clinical symptoms and signs (such as fever, asthenia/fatigue, nausea, vomiting, anorexia, abdominal pain, weight loss, muscle cramps/pain, and orthostatic hypotension) and laboratory tests (such as C-reactive protein, lowered serum sodium, and elevated serum potassium).
Table 3.
Adverse Events Occurring in More Than 15% of Patients at Any Grade in Either the Phase 1 or Phase 2 Cohort by Preferred Term.*
| Phase 1, No. (%) |
Phase 2, No. (%) |
|||
|---|---|---|---|---|
| Adverse Events by Preferred Term | Any Grade | Grade ≥3 | Any Grade | Grade ≥3 |
| All | 47 (100.0) | 33 (70.2) | 45 (100) | 36 (80.0) |
| Adrenal insufficiency | 17 (36.2) | 15 (31.9) | 6 (13.3) | 3 (6.7) |
| Anemia | 16 (34.0) | 7 (14.9) | 17 (37.8) | 6 (13.3) |
| Asthenia | 14 (29.8) | 2 (4.3) | 13 (28.9) | 2 (4.4) |
| Fatigue | 14 (29.8) | 0 | 17 (37.8) | 3 (6.7) |
| Dyspnea | 6 (12.8) | 0 | 12 (26.7) | 2 (4.4) |
| Muscle spasms | 14 (29.8) | 0 | 8 (17.8) | 1 (2.2) |
| Hyponatremia | 15 (31.9) | 6 (12.8) | 10 (22.2) | 2 (4.4) |
| Hyperkalemia | 13 (27.7) | 1 (2.1) | 9 (20.0) | 1 (2.2) |
| Edema peripheral | 10 (21.3) | 0 | 12 (26.7) | 0 |
| Tumor pain | 10 (21.3) | 4 (8.5) | 11 (24.4) | 3 (6.7) |
| ALT increased | 10 (21.3) | 1 (2.1) | 7 (15.6) | 0 |
| Arthralgia | 5 (10.6) | 0 | 8 (17.8) | 1 (2.2) |
| Decreased appetite | 0 | 0 | 8 (17.8) | 1 (2.2) |
| Amylase increased | 9 (19.1) | 4 (8.5) | 0 | 1 (2.2) |
| AST increased | 8 (17.0) | 1 (2.1) | 9 (20.0) | 0 |
| Hypertension | 8 (17.0) | 5 (10.6) | 5 (11.1) | 3 (6.7) |
| Insomnia | 8 (17.0) | 0 | 9 (20.0) | 0 |
| Bone pain | 7 (14.9) | 1 (2.1) | 9 (20.0) | 2 (4.4) |
| Abdominal pain | 0 | 1 (2.1) | 7 (15.6) | 0 |
| Hypocalcemia | 0 | 0 | 7 (15.6) | 0 |
| Hypotension | 0 | 0 | 7 (15.6) | 2 (4.4) |
| Myalgia | 6 (12.8) | 0 | 7 (15.6) | 0 |
| Platelet count decreased | 0 | 0 | 7 (15.6) | 1 (2.2) |
| ALP increased | 6 (12.8) | 3 (6.4) | 7 (15.6) | 3 (6.7) |
| Nausea | 7 (14.9) | 0 | 10 (22.2) | 0 |
| Diarrhea | 1 (2.1) | 1 (2.1) | 9 (20.0) | 2 (4.4) |
ALP denotes alkaline phosphatase; ALT, alanine aminotransferase; and AST, aspartate aminotransferase.
In phase 2, the most common treatment-emergent adverse events were anemia (n=17 [37.8%]) and fatigue (n=17 [37.8%]) (Table 3). Adrenal insufficiency (all related to ODM-208) occurred in six (13.3%) patients; this was grade 1/2 in three (6.7%) patients and serious in three (6.7%) patients, and it led to temporary interruption of ODM-208 in three (6.7%) patients. Adverse events led to ODM-208 interruption and permanent discontinuation in 20 (44.4%) and 10 (22.2%) patients, respectively (Tables 2 and 3); tumor pain was the only event causing interruption and ODM-208 discontinuation in more than one patient (n=2 [4.4%]). Ten (22.2%) patients had died by the end of follow-up, the most common causes being progression of prostate cancer in four patients and sepsis in two patients. Although there were no deaths that were attributed by investigators to ODM-208, as it is a novel agent, it is impossible to clearly determine the role of treatment in all adverse events.
Serum thyroid-stimulating hormone (TSH) and bilirubin were examined in all patients, as these were increased in nonclinical studies in rats and dogs. Only one patient had elevated TSH (present already at baseline), and two patients had a transient increase in serum bilirubin. These events did not require adjustment of ODM-208 treatment (Table S7).
PHARMACOKINETICS
After a single dose of ODM-208, the median time to maximum plasma concentration was 2 hours (range, 0.5 to 6.0 hours), and the maximum plasma concentration and the mean terminal elimination half-life for all doses combined (±SD) were 258 ± 230.4 ng/ml and 2.7 ± 0.9 hours, respectively. After multiple doses, steady state was reached within 1 week. The median exposure (area under the plasma concentration–time curve from time zero to infinity) for all doses combined at day 8 was 1131.0 ng×hour/ml (range, 80.4 to 3427.8 ng×hour/ml), which increased almost dose proportionally. Exposure and maximum plasma concentration of ODM-208 plateaued after 7 days of repeated dosing.
PHARMACODYNAMICS
Serum levels of testosterone, androstenedione, dehydroepiandrosterone sulfate, aldosterone, cortisol, 11-ketotestosterone, 11b-hydroxyandrostenedione, and pregnenolone were undetectable in most patients by week 4 of ODM-208 treatment (Fig. 1, Fig. S3, and Table S8). At 5 mg twice a day, serum testosterone decreased by 51% and 72% from the pretreatment concentration within 6 and 24 hours, respectively (Fig. 1A and Fig. S3A) and to undetectable levels within the first week of treatment in 46 of 53 (87%) patients. The impact of ODM-208 on serum steroid levels upon discontinuation of ODM-208 was reversible. Although the results are scant, the measured hormone concentrations had typically substantially recovered at the end of the study visit (Table S8), and glucocorticoid/mineralocorticoid replacement therapy could be gradually withdrawn.
Figure 1. Plasma Levels of Androgenic and Nonandrogenic Steroid Hormones during Treatment with ODM-208 5 mg Twice a Day in Patients in Phase 2.
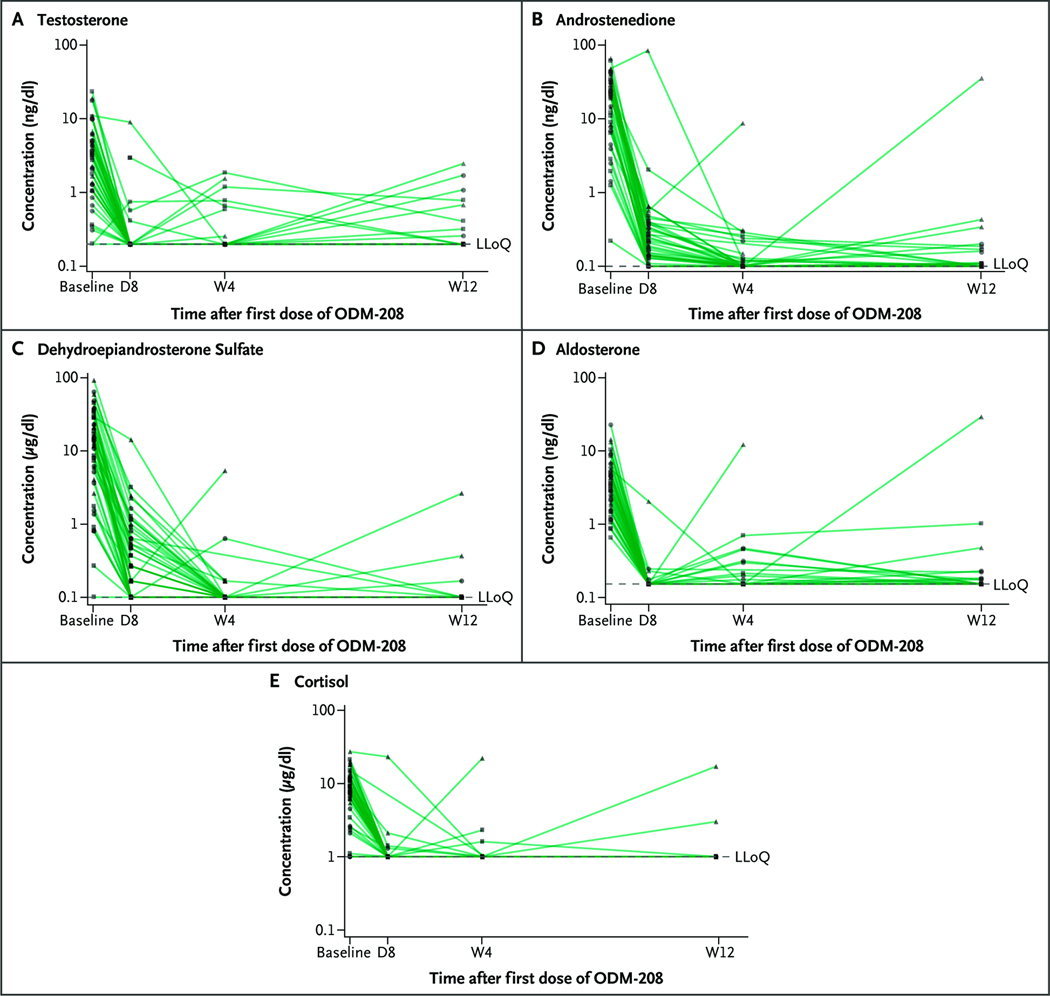
In all five panels, the y axis is a log scale. Most values above the lower limit of quantification (LLoQ) after day 1 were detected in patients in whom ODM-208 administration had been interrupted. Samples below the LLoQ were superimposed as half the LLoQ. Testosterone (Panel A). Androstenedione (Panel B). Dehydroepiandrosterone sulfate (Panel C). Aldosterone (Panel D). Cortisol (Panel E). D denotes day; and W, week.
EFFICACY
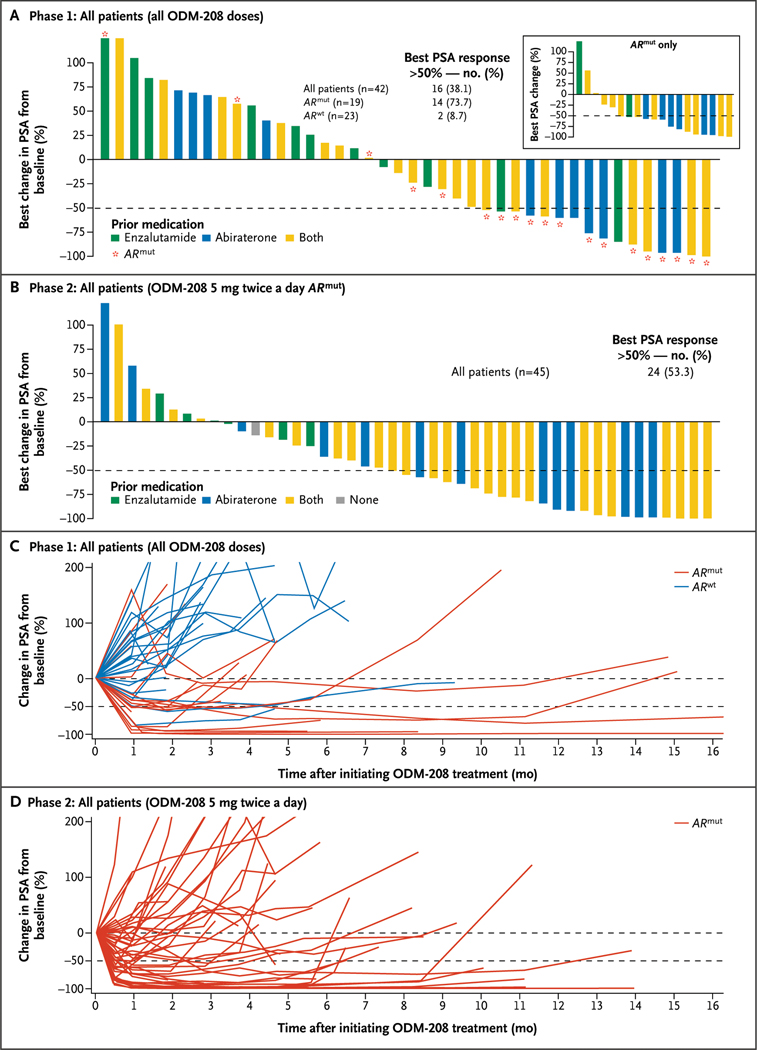
PSA Response
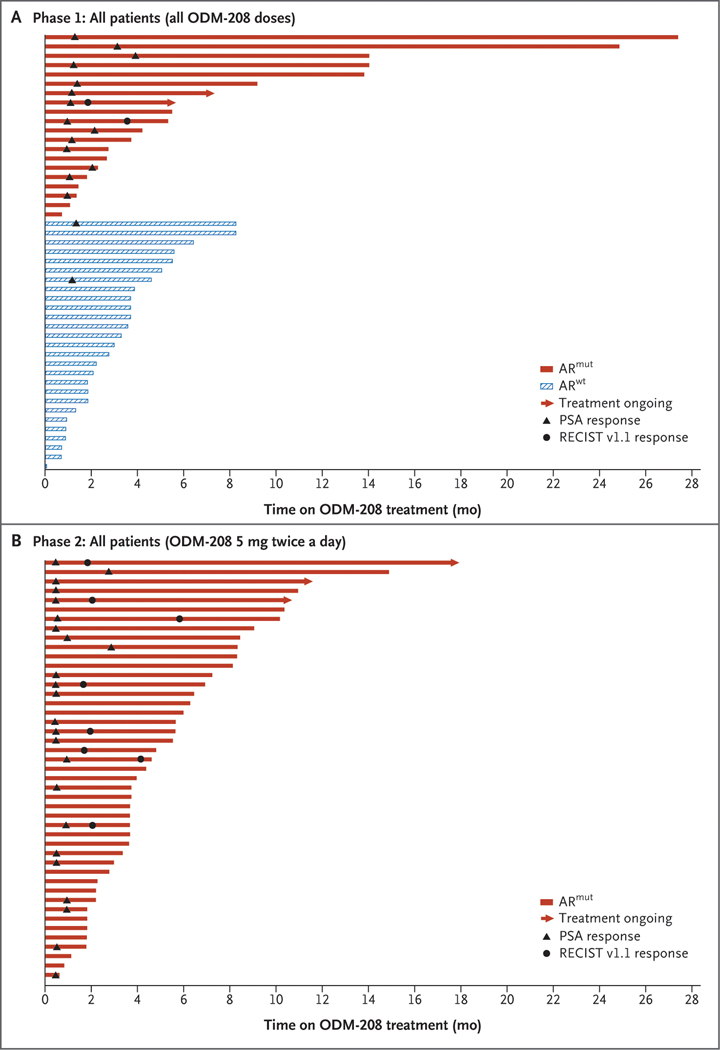
In phase 1, 16 of 42 (38.1%) patients had a PSA50 response (median follow-up duration, 4.5 months; interquartile range, 2 to 6 months). Among the patients harboring an ARmut, 14 of 19 (73.7%) achieved PSA50 compared with 2 of 23 (8.7%) of their ARwt counterparts (Fig. 2A and 2C). Median time in the entire cohort to PSA progression was 7 months (interquartile range, 4 to 15 months); it was 9 months (interquartile range, 5 to 25 months) in patients with ARmut and 6 months (interquartile range, 4 to 9 months) in those with ARwt (Table S9). The time on treatment for patients with ARmut compared with patients with ARwt is shown in Figure 3A.
Figure 2. Change in Prostate-Specific Antigen (PSA) after Treatment with ODM-208.
Best change (percentage) in PSA from baseline in the total phase 1 population (Panel A) and in the androgen receptor gene mutation (ARmut) subpopulation (inset) and in phase 2 (Panel B). Change in PSA from baseline over time in individual patients in phase 1 (Panel C) and phase 2 (Panel D) by androgen receptor gene mutation status. ARwt denotes androgen receptor gene wild type; and mo, months.
Figure 3. Swimmer Plots Showing Time on Treatment.
Panel A shows phase 1 and Panel B shows phase 2 by androgen receptor gene mutation status. ARmut denotes androgen receptor gene mutation; ARwt, androgen receptor gene wild type; mo, months; PSA, prostate-specific antigen; and RECIST v1.1, Response Evaluation Criteria in Solid Tumors version 1.1.
In phase 2, 24 of 45 (53.3%) patients achieved PSA50 (median follow-up duration, 5.8 months; interquartile range, 4 to 8 months); rates of PSA response were 8 of 13 (61.5%) in patients who had been pretreated with abiraterone and 16 of 25 (64.0%) in patients treated with both abiraterone and enzalutamide (Fig. 2B and 2D). Median time to PSA progression was 7 months (interquartile range, 4 months to not reached); median duration of PSA response was 2 months (interquartile range, 2 to 4 months) (Table S9). Among the patients treated in phase 2 of the study, 17 (37.8%) were able to continue treatment with ODM-208 for more than 6 months (Fig. 3B).
Objective Response per RECIST Version 1.1
Three of 18 (16.7%) evaluable patients in phase 1 achieved a partial response (all 3 patients were ARmut positive), and 7 (38.9%) patients had stable disease, for a disease control rate of 55.6%. In phase 2, 8 of 30 (26.7%) evaluable patients achieved a partial response, and 13 (43.3%) had stable disease; the disease control rate was 70.0% (Table S9).
Imaging-Based Progression-Free Survival
Median time to imaging-based progression in phase 1 was 5 months (interquartile range, 2 to 25 months) and was similar between patients with ARmut and patients with ARwt (Table S9). In phase 2, median time to imaging-based progression was 5 months (interquartile range, 2 to 8 months).
Discussion
In this phase 1/2 study, ODM-208 had a reasonable safety profile in men with metastatic castration-resistant prostate cancer who had progressed on second-generation AR pathway inhibitors and taxanes, particularly in those harboring ARmut. In line with preclinical findings,18 ODM-208 blocked production of all steroid hormones/precursors measured, with concomitant lowering of patient serum levels from baseline; clinical activity was also noted, although our study was not designed to measure this with accuracy.
Although ODM-208 appeared to demonstrate antitumor activity in patients with ARwt disease in phase 1, the benefit appears to be greater for those harboring ARmut (PSA50 rate, 8.7% vs. 73.7%), a finding that appears to have been confirmed in the ARmut phase 2 cohort (PSA50 rate, 53.3%). These findings may be explained by the binding of lower-affinity steroid hormones, such as progestins, to the mutated AR and subsequent activation of the AR pathway.18
ODM-208 blocked all steroidogenesis in some patients, and prophylactic replacement therapy did not always prevent the occurrence of adrenal insufficiency–like events. Adrenal insufficiency was the most frequently observed adverse event in phase 1, and it tended to occur less frequently at lower ODM-208 daily doses (29.4% [≤25 mg] vs. 40.0% [>25 mg]) and with milder severity and longer times to the first serious event. This finding was corroborated in phase 2 (ODM-208 5 mg twice daily), in which adrenal insufficiency occurred less frequently than in phase 1 (13.3% vs. 36.2%) and was generally milder. Treatment of adrenal insufficiency–like events was successful for all but one patient, who ultimately required discontinuation of ODM-208. From our data in phase 2, the 5 mg twice a day dose of ODM-208 maintained androgen hormone blockade while allowing reasonably successful prophylaxis with glucocorticoid/mineralocorticoid replacement. In our study, we used close monitoring of the sufficiency of the steroid replacement therapy and provided patient education about the prevention of acute adrenal insufficiency.
This study has limitations. ODM-208 was more potent than initially anticipated on the basis of preclinical data, requiring gradual dose de-escalations instead of the expected dose escalations. Most patients in phase 1 were, therefore, treated with a substantially higher dose of ODM-208 than in phase 2. Because ODM-208’s mechanism of action is novel, as a selective CYP11A1 inhibitor, it was not known exactly how to adjust glucocorticoid/mineralocorticoid replacement therapy. We found acceptable treatment doses for glucocorticoids and mineralocorticoids as the study progressed, leading to heterogeneity in administered replacement therapies. Finally, the high incidence of adrenal insufficiency and dose interruptions observed in phase 1 may have impacted the treatment response in this predominantly ARwt population.
In conclusion, the oral, selective CYP11A1 inhibitor ODM-208 blocked steroid biosynthesis in patients with metastatic castration-resistant prostate cancer without dose limiting side effects once a stable corticosteroid replacement regimen was determined. Our data are consistent with clinical activity in this heavily pretreated population, particularly in those harboring ARmut; further trials are needed.
Supplementary Material
Acknowledgments
Supported by Orion Pharma.
We thank all patients, caregivers, and their families as well as the investigators and research staff for their contribution to the ongoing CYPIDES study, including study teams at Orion Corporation and Parexel for running the study. Medical writing and editorial assistance were provided by Jacqueline Kolston, Ph.D. (Parexel), funded by Orion Pharma.
Footnotes
Disclosures
Author disclosures and other supplementary materials are available at evidence.nejm.org.
A data sharing statement provided by the authors is available with the full text of this article at NEJM.org.
References
- 1.National Comprehensive Cancer Network. NCCN clinical practice guidelines in oncology (NCCN guidelines): prostate cancer. January 2023. (https://www.nccn.org/professionals/physician_gls/pdf/prostate.pdf). [Google Scholar]
- 2.Mottet N, Cornford P, Van den Bergh RCN, et al. EAU–EANM– ESTRO–ESUR–ISUP–SIPG guidelines on prostate cancer. March 2022. (https://d56bochluxqnz.cloudfront.net/documents/full-guideline/EAU-EANM-ESTRO-ESUR-ISUP_SIOG-Guidelines-on-Prostate-Cancer-2022_2022-04-25-063938_yfos.pdf). [Google Scholar]
- 3.Parker C, Castro E, Fizazi K, et al. Prostate cancer: ESMO clinical practice guidelines for diagnosis, treatment and follow-up. Ann Oncol 2020;31:1119–1134. DOI: 10.1016/j.annonc.2020.06.011. [DOI] [PubMed] [Google Scholar]
- 4.Chen J, Zhang Y, Zhang X, et al. Comparison of systemic treatments for metastatic castration-resistant prostate cancer after docetaxel failure: a systematic review and network meta-analysis. Front Pharmacol 2022;12:789319. DOI: 10.3389/fphar.2021.789319. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 5.Sayegh N, Swami U, Agarwal N. Recent advances in the management of metastatic prostate cancer. JCO Oncol Pract 2022;18:45–55. DOI: 10.1200/OP.21.00206. [DOI] [PubMed] [Google Scholar]
- 6.Dai C, Heemers H, Sharifi N. Androgen signaling in prostate cancer. Cold Spring Harb Perspect Med 2017;7:a030452. DOI: 10.1101/cshperspect.a030452. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 7.Fizazi K, Scher HI, Molina A, et al. Abiraterone acetate for treatment of metastatic castration-resistant prostate cancer: final overall survival analysis of the COU-AA-301 randomised, double-blind, placebo-controlled phase 3 study. Lancet Oncol 2012;13:983–992. DOI: 10.1016/S1470-2045(12)70379-0. [DOI] [PubMed] [Google Scholar]
- 8.Smith MR, Saad F, Chowdhury S, et al. Apalutamide and overall survival in prostate cancer. Eur Urol 2021;79:150–158. DOI: 10.1016/j.eururo.2020.08.011. [DOI] [PubMed] [Google Scholar]
- 9.Fizazi K, Shore N, Tammela TL, et al. Nonmetastatic, castration-resistant prostate cancer and survival with darolutamide. N Engl J Med 2020;383:1040–1049. DOI: 10.1056/NEJMoa2001342. [DOI] [PubMed] [Google Scholar]
- 10.Scher HI, Fizazi K, Saad F, et al. Increased survival with enzalutamide in prostate cancer after chemotherapy. N Engl J Med 2012; 367:1187–1197. DOI: 10.1056/NEJMoa1207506. [DOI] [PubMed] [Google Scholar]
- 11.Feng Q, He B. Androgen receptor signaling in the development of castration-resistant prostate cancer. Front Oncol 2019;9:858. DOI: 10.3389/fonc.2019.00858. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 12.Pinto F, Dibitetto F, Ragonese M, Bassi P. Mechanisms of resistance to second-generation antiandrogen therapy for prostate cancer: actual knowledge and perspectives. Med Sci (Basel) 2022;10: 25. DOI: 10.3390/medsci10020025. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 13.Chandrasekar T, Yang JC, Gao AC, Evans CP. Mechanisms of resistance in castration-resistant prostate cancer (CRPC). Transl Androl Urol 2015;4:365–380. DOI: 10.3978/j.issn.2223-4683.2015.05.02. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 14.Bertaglia V, Tucci M, Vignani F, et al. An exploratory analysis of the association between levels of hormones implied in steroid biosynthesis and activity of abiraterone in patients with metastatic castration-resistant prostate cancer. Minerva Urol Nefrol 2017;69: 349–358. DOI: 10.23736/S0393-2249.16.02746-6. [DOI] [PubMed] [Google Scholar]
- 15.Jernberg E, Bergh A, Wikström P. Clinical relevance of androgen receptor alterations in prostate cancer. Endocr Connect 2017;6: R146–R161. DOI: 10.1530/EC-17-0118. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 16.Ai N, DeLisle RK, Yu SJ, Welsh WJ. Computational models for predicting the binding affinities of ligands for the wild-type androgen receptor and a mutated variant associated with human prostate cancer. Chem Res Toxicol 2003;16:1652–1660. DOI: 10.1021/tx034168k. [DOI] [PubMed] [Google Scholar]
- 17.Pippione AC, Boschi D, Pors K, et al. Androgen-AR axis in primary and metastatic prostate cancer: chasing steroidogenic enzymes for therapeutic intervention. J Cancer Metastasis Treat 2017;3:328–361. DOI: 10.20517/2394-4722.2017.44. [DOI] [Google Scholar]
- 18.Karimaa M, Riikonen R, Kettunen H, et al. First-in-class small molecule to inhibit CYP11A1 and steroid hormone biosynthesis. Mol Cancer Ther 2022;21:1765–1776. DOI: 10.1158/1535-7163.MCT-22-0115. [DOI] [PubMed] [Google Scholar]
- 19.Le Tourneau C, Lee JJ, Siu LL. Dose escalation methods in phase I cancer clinical trials. J Natl Cancer Inst 2009;101:708–720. DOI: 10.1093/jnci/djp079. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 20.Snaterse G, Mies R, van Weerden WM, et al. Androgen receptor mutations modulate activation by 11-oxygenated androgens and glucocorticoids. Prostate Cancer Prostatic Dis 2023;26:293–301. DOI: 10.1038/s41391-022-00491-z. [DOI] [PubMed] [Google Scholar]
- 21.U.S. Department of Health and Human Services, National Institutes of Health, National Cancer Institute. Common terminology criteria for adverse events (CTCAE), version 4.03. Washington, DC: U.S. Department of Health and Human Services, June 14, 2010 (https://evs.nci.nih.gov/ftp1/CTCAE/CTCAE_4.03/CTCAE_4.03_2010-06-14_QuickReference_8.5×11.pdf). [Google Scholar]
- 22.Eisenhauer EA, Therasse P, Bogaerts J, et al. New response evaluation criteria in solid tumours: revised RECIST guideline (version 1.1). Eur J Cancer 2009;45:228–247. DOI: 10.1016/j.ejca.2008.10.026. [DOI] [PubMed] [Google Scholar]
- 23.Scher HI, Morris MJ, Stadler WM, et al. Trial design and objectives for castration-resistant prostate cancer: updated recommendations from the Prostate Cancer Clinical Trials Working Group 3. J Clin Oncol 2016;34(12):1402–1418. DOI: 10.1200/JCO.2015.64.2702. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 24.Morris MJ, Molina A, Small EJ, et al. Radiographic progression-free survival as a response biomarker in metastatic castration-resistant prostate cancer: COU-AA-302 results. J Clin Oncol 2015;33:1356–1363. DOI: 10.1200/JCO.2014.55.3875. [DOI] [PMC free article] [PubMed] [Google Scholar]
Associated Data
This section collects any data citations, data availability statements, or supplementary materials included in this article.