Abstract
Objectives:
These 2 studies evaluated technology-based behavioral interventions for promoting daily activities and supported ambulation in people with mild-to-moderate and moderate-to-severe Alzheimer’s disease, respectively.
Methods:
Study 1 included 8 participants who were taught to start and carry out daily activities on their own using a tablet or smartphone device, which provided activity reminders, step instructions, and praise. Study 2 included 9 participants who were taught to engage in brief periods of ambulation using a walker combined with a tilt microswitch and a notebook computer, which monitored step responses and provided stimulation and prompts.
Results:
The participants of study 1 succeeded in starting the activities independently and carrying them out accurately. The participants of study 2 largely increased their ambulation levels and also showed signs of positive involvement (eg, smiles and verbalizations).
Conclusion:
The aforementioned technology-based interventions may represent practical means for supporting people with Alzheimer’s disease.
Keywords: Alzheimer’s disease, daily activities, ambulation, technology-based programs, reminders, instructions
Introduction
Alzheimer’s disease is a neurodegenerative disorder that causes progressive decline in people’s condition. 1 -5 For example, during the mild and the high moderate stages of the disease, people are increasingly likely to show activity engagement problems, with a tendency to progressively become more passive and inaccurate. 6 -8 They do not seem to be fully aware of time and fail to start daily activities when these are due. 2,8 -10 Likewise, they do not seem to recall all activity steps, with an increasing deterioration of their performance. 11 -15
During the low moderate and severe stages of the disease, people tend to lose most of their adaptive and purposeful engagement and eventually their ambulation skills as well (ie, becoming uncertain in their ambulation, and thus at risk of falls, or unable to ambulate without support). 1,16 -18 Lack of ambulation skills restricts their opportunities to move (ie, carrying out basic, useful physical exercise, and attaining positive environmental stimulation for it) inside their daily context with negative implications for their physical and emotional conditions. 19,20
At present, there is no way to prevent or cure the disease. Yet, one can adopt various pharmacological and behavioral interventions to reduce the impact of the disease and/or slow down its progression. 21 -23 Pharmacological interventions (frequently involving the use of acetylcholinesterase inhibitors and memantine) are typically considered complementary to behavioral interventions. 21,24 -26 Behavioral interventions, which increasingly rely on the use of assistive technology to maximize their impact and reduce staff costs, are deemed essential for providing people extra opportunities of positive engagement capable of temporarily curbing the disease’s symptoms and degenerative process. 6,8,9,14,15,27,28 Technology-based behavioral interventions have varied widely regarding their aims (eg, from sustaining the performance of daily activities to promoting arm movements or supported ambulation), depending upon the participants’ level of functioning and environmental prospects. 6,12,15,16 One of the most recent studies concerning daily activities for people in the mild and high moderate stages of the disease targeted not only the independent and accurate performance of those activities but also their independent start. 29 The study relied on the use of a tablet, which served to provide the 8 participants with timely reminders about the daily activities to carry out, verbal instructions concerning the steps included in those activities as well as praise statements. Data showed that all participants managed to start the activities independently and at the appropriate times and carried them out with high levels of accuracy.
A recent, preliminary study concerning supported ambulation for persons in the advanced stages of the disease relied on the use of a 4-wheel walker combined with a tilt microswitch, and a notebook computer. 30 The microswitch monitored the step responses, while the computer delivered (a) brief periods of preferred stimulation contingent on those responses and (b) verbal prompts in case of immobility. Data showed that during the intervention, the 10 participants included in the study had an increase in ambulation and tended to display signs of positive involvement (eg, talking or singing).
The promising results obtained in the aforementioned studies underline the possibility of using technology-based behavioral interventions to reach relatively advanced objectives, such as the combination of independent start and accurate performance of multistep activities and the practice of supported (self-determined) ambulation. Although this new evidence can be considered very encouraging, and practically relevant for services directed at people with Alzheimer’s disease, caution is required in making general statements until the results of the aforementioned studies are successfully replicated with additional participants as well as across research groups. 31,32 The present 2 studies were designed as first-level replication efforts, carried out by the same research group involved in the original work. Indeed, the 2 studies were aimed at assessing the effectiveness of the interventions described above to promote (a) independent start and accurate performance of daily activities and (b) supported ambulation, 31 with the involvement of 8 and 9 new participants, respectively.
Study 1
Methods
Participants
The 8 participants included in the study are indicated here as participants 1 to 8. Two other persons, who had been recruited for the study, dropped out because of lack of interest in daily activities or poor health. The 8 participants were deemed to be in the mild or moderate stages of the disease, attended centers for people with Alzheimer’s disease and other dementias, and represented a convenience sample. 33 They ranged from 73 to 92 years of age and had scores varying between 16 and 24 on the Mini-Mental State Examination (see Table 1). 34 Their selection was based on 3 criteria. First, they were generally passive when left alone. Staff and caregivers, informally interviewed prior to the study, had indicated that the participants generally failed to remember the times at which daily activities were due and the steps involved in those activities. Second, the participants were capable of following simple verbal reminders about the activities to carry out and instructions concerning the steps of those activities. Third, staff and participants had verbalized interest in using a program, such as that adopted in this study, which had been illustrated to them. Notwithstanding the aforementioned interest, the participants were reported to have problems in reading/understanding and signing a consent form for the study. Thus, their families had signed such form on their behalf. The study complied with the 1964 Declaration of Helsinki and its later amendments and was approved by the ethics committee of the Alzheimer’s Association, Bari, Italy.
Table 1.
Participants’ Characteristics (Study 1).
| Participants | Sex | Age | MMSE |
|---|---|---|---|
| 1 | M | 82 | 19 |
| 2 | M | 89 | 18 |
| 3 | F | 82 | 21 |
| 4 | F | 89 | 18 |
| 5 | F | 81 | 16 |
| 6 | M | 73 | 24 |
| 7 | M | 90 | 16 |
| 8 | M | 92 | 17 |
Abbreviations: F, female; M, male; MMSE, Mini-Mental State Examination.
Setting, activities, technology, sessions, and data recording
The centers that the participants attended served as the setting for the study. For each participant, 8 or 9 daily activities were selected (eg, preparing coffee, setting the table, watering plants, making the bed, performing self-care routines, preparing a snack, and arranging the calendar page). The activities, which were adapted to the participants’ characteristics in terms of steps and complexity, included means of over 16 steps. Specific verbal instructions were recorded for the single activity steps and presented to the participants during the intervention with the technology-based program (see below). The technology included a tablet or smartphone device with Android operating system and the Talking Alarm Clock application as well as a wireless Bluetooth earpiece. The Talking Alarm Clock application was highly suitable to schedule the activities with related reminders, step instructions, and praise statements. In total, 5 or 6 activities were scheduled over 1.5- or 2-hour morning or afternoon periods representing the sessions of the study.
Scheduling an activity consisted of setting up the tablet or smartphone with files containing a time and a verbal reminder for the activity, specific instructions to guide the participant through the activity steps, and praise statements. When the time for an activity was reached, the participant was reminded to start that activity and thereafter he or she was presented with the step instructions for it. The instructions were presented individually or in strings of 2 to cover sets of 2 steps, such as, “take the container and fill it with water.” The instructions (or instruction strings) were separated by intervals (eg, 10-30 seconds), which varied across participants and activities based on preliminary observations of participants’ step performance. During some of the intervals, praise statements occurred. The participants received the reminders, instructions, and praise through the aforementioned Bluetooth earpiece. The earpiece was easy to wear and freed the participants from the need to carry the tablet or smartphone.
Data recording was conducted by research assistants in charge of the sessions and concerned the (a) number of activities the participants started independently (ie, without reminders from the research assistants and at the appropriate time) and (b) the number of activity steps the participants performed correctly. Inter-rater agreement was checked in about 30% of the sessions. Percentages of agreement were computed on single sessions for the first measure and single activities for the second measure by dividing activities or steps with agreement by the total number of activities or steps and multiplying by 100%. The percentages were in the 80 to 100 range, with means exceeding 95 on both measures for all participants.
Experimental conditions and data analysis
The study included 2 baseline phases and a technology-based intervention phase. The numbers of sessions the participants received within the 2 baseline phases varied according to an adapted nonconcurrent multiple baseline design across participants. 35 The number of sessions for the single participant was preset. Yet, sessions would be added if the participants’ percentages of activities started correctly or activity steps carried out correctly were above 40 and the value of the last session exceeded those of previous sessions. (This condition never applied.). The intervention sessions served to determine the effects of the technology-based intervention on each of the measures recorded. The baseline and intervention data for activities started independently and activity steps carried out correctly were summarized/graphed as means per session over blocks of sessions. The differences between baseline and intervention session values were analyzed for the single participants via the Kolmogorov-Smirnov test. 36
Baseline I and II
Each session of the first baseline started with a research assistant familiar to the participant reading a list of 5 or 6 activities and the times at which they were due and placing the list on the table in front of the participant. This phase served to determine how many of the scheduled activities the participant would start independently. During each session of the second baseline, the research assistant asked the participant to carry out 5 or 6 activities (ie, one at a time) to determine how many activity steps he or she carried out correctly. The research assistant intervened on an error only if this precluded the continuation of the activity. At the end of an activity (ie, when the participant said he or she had completed it or was unable to continue), the research assistant praised the participant for his or her efforts.
Intervention
At the beginning of each session of the intervention phase, the participant was provided with the wireless Bluetooth earpiece linked to the tablet or smartphone, which worked as described above to promote independent start and correct performance of the activities. The initial 3 or 4 sessions served as introductory/practice sessions, during which the participant received explanations and guidance from the research assistant on using the technology, at the beginning of the session and at each activity scheduled so that the activity was started and performed accurately. During the subsequent sessions, the participant received no specific help from the research assistant, who provided error corrections and praise as during the second baseline phase.
Results
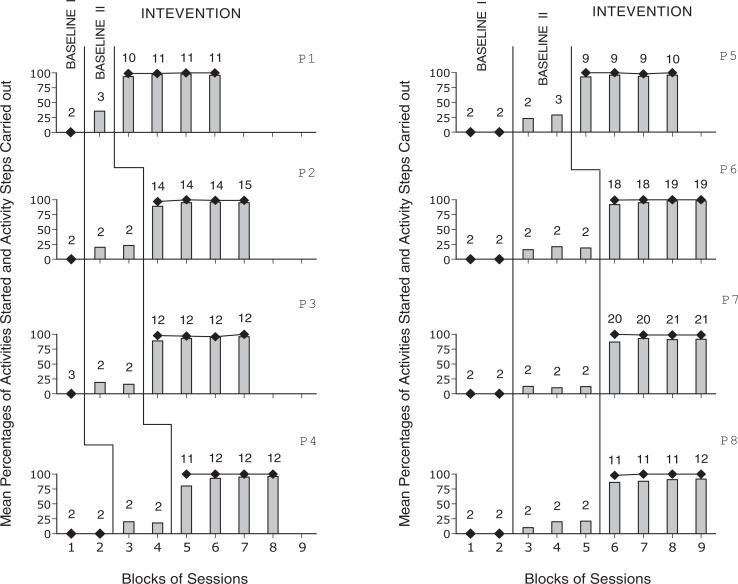
The 8 panels of Figure 1 report the participants’ mean percentages of activities started independently and of activity steps carried out correctly per session, across blocks of baseline and intervention sessions. During the first baseline phase, which included between 2 and 4 sessions per participant, the participants’ mean percentages of activities started independently were 0. During the second baseline phase, which included between 3 and 6 sessions per participant, the participants’ mean percentages of activity steps carried out correctly were always below 40. During the intervention phase, which included between 37 and 82 sessions (following the 3 or 4 introductory sessions), the participants’ mean percentages of activities started independently were (close to) 100. That is, the participants responded to all technology-regulated reminders or missed only very few of them. The participants’ overall mean percentages of correct steps were near or above 90. The Kolmogorov-Smirnov test showed that the differences between intervention and baseline session data on activities started independently, and correct activity steps were statistically significant for all participants (P < .01 or P < .05, if only 2 baseline sessions were available).
Figure 1.
Black diamonds and bars represent mean percentages of activities started independently and mean percentages of activity steps carried out correctly per session over blocks of sessions for each of the participants (ie, P1-P8). The number of sessions included in the blocks is indicated by the numerals above the diamonds, bars, or diamond-bar combinations.
Study 2
Methods
Participants
The study included 9 participants who are indicated here as participants 1 to 9. Four other persons, who had been recruited for the study, dropped out due to health or practical reasons. The 9 participants ranged from 70 to 92 years of age, represented a convenience sample, were considered to function at the lower half of the moderate or at the severe level of Alzheimer’s disease and had scores varying from < 6 to 14 on the Mini-Mental State Examination (see Table 2). The participants attended centers for people with Alzheimer’s disease and other dementias and were selected on the basis of 3 criteria. First, they were unable to ambulate independently and showed only minimal or moderate ambulation levels (ie, step frequencies) when provided with a walker device. Second, preliminary observations had shown that they enjoyed stimulation events, such as music and religious hymns, and started ambulating in response to verbal prompts (ie, encouragements) to do so. Third, staff and families considered ambulation relevant for the participants and favored the use of an intervention program such as that adopted in this study for supporting it. The participants were unable to provide consent to the study. Thus, the families had signed a consent form on the participants’ behalf. The study complied with the 1964 Declaration of Helsinki and its later amendments and was approved by the same ethics committee as study 1.
Table 2.
Participants’ Characteristics (Study 2).
| Participants | Sex | Age | MMSE |
|---|---|---|---|
| 1 | F | 70 | <6 |
| 2 | M | 82 | 8 |
| 3 | F | 86 | 12 |
| 4 | M | 84 | 12 |
| 5 | M | 72 | <6 |
| 6 | M | 83 | 12 |
| 7 | F | 92 | 14 |
| 8 | F | 75 | 9 |
| 9 | F | 72 | <6 |
Abbreviations: F, female; M, male; MMSE, Mini-Mental State Examination.
Setting, sessions, step responses, technology, and walker
The centers that the participants attended served as the setting for the study. Sessions lasted 3 minutes and typically occurred 3 to 7 times a day. Short sessions were decided on the assumption that they would not cause tiredness and discomfort. A step response consisted of the participant’s moving either foot forward. The technology included a tilt microswitch and a notebook computer with earpieces. The microswitch was fixed to the participant’s right foot, detected the step responses performed with that foot, and signaled them to the computer. The computer (a) recorded those step responses (whose final frequency was doubled to also account for the steps made with the left foot) during the baseline and intervention and (b) delivered and recorded stimulation events and verbal prompts during the intervention (see below). The technology was used together with a 4-wheel walker, which had a frame passing around the participant’s chest and under his or her arms and could also include a harness to avoid risks of falls.
Stimulation events and prompts
Stimulation events consisted of 5-s segments of old songs, religious hymns, and prayers, which were deemed preferred for the participants based on staff reports and direct screening. Screening involved at least 10 nonconsecutive presentations of each of several segments representing the aforementioned stimulus categories (ie, songs, hymns, and prayers). Stimuli were selected only if a research assistant and a staff member had judged the related segments effective in triggering positive reactions (eg, orienting, smiling, or verbalizations) in 60% or more of their presentations. 37 Prompts were utterances of 1 to 3 words and occurred after 10 to 15 seconds of no microswitch activation. Stimulation events and prompts were channeled through the participants’ earpieces.
Signs of positive involvement
Signs of positive involvement (ie, singing, praying, positive verbalizations, and smiles) were recorded for each participant during the baseline and intervention sessions. Recording was carried out by research assistants using a partial interval system, with 10-s observation intervals followed by 5-s scoring periods. 31 Inter-rater agreement was assessed in about 25% of the aforementioned sessions by having a research assistant and a reliability observer involved in the recording. The percentages of agreement (computed for the single sessions by dividing the intervals with matching scores by the total number of intervals and multiplying by 100%) were within the 80 to 100 range, with means exceeding 90 for all participants.
Experimental conditions and data analysis
A nonconcurrent multiple baseline design across participants 35 was used to assess the effects of the intervention program on ambulation (ie, step responses) and signs of positive involvement. 38 The baseline and intervention data on step responses and signs of positive involvement of each participant were summarized/graphed as means per session over blocks of sessions. The differences between baseline and intervention session values were analyzed for the single participants via the Kolmogorov-Smirnov test. 36
Baseline
The participants were provided with the walker and the technology but did not receive stimulation or prompts.
Intervention
During the intervention phase, the participants used the walker with the technology, which provided stimulation and prompts as described in the setting, sessions, step responses, technology, and walker section. Five introductory sessions were carried out to familiarize the participants with the intervention conditions. During these sessions, the research assistants used verbal and physical guidance to foster participants’ ambulation, experience of contingent stimulation, and responding to prompts.
Results
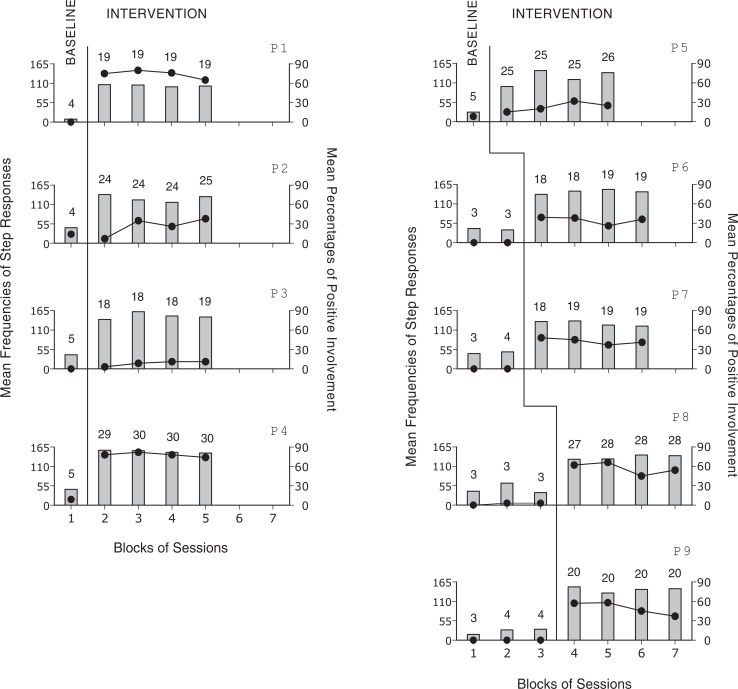
The 9 panels of Figure 2 report the participants’ mean frequencies of step responses and mean percentages of positive involvement per session over blocks of baseline and intervention sessions. During baseline, which included between 4 and 11 sessions, the participants had mean frequencies of step responses per session below 50 with no increasing trends. Their mean percentages of intervals with signs of positive involvement were between 0 and 8. During the intervention, which included between 73 and 119 sessions, the mean frequencies of step responses per session increased to between about 100 and over 150. The mean percentages of intervals with signs of positive involvement ranged from below 10 to near 80. The Kolmogorov-Smirnov test showed that the differences between baseline and intervention were statistically significant (P < .01) for all participants on step responses and for 6 participants (ie, participants 1, 4, 6, 7, 8, and 9) on signs of positive involvement. Computer prompts (not reported in the figure) were mostly sporadic with the overall mean frequency across participants well below 1 per session.
Figure 2.
Bars and black circles represent mean frequencies of step responses and mean percentages of positive involvement per session over blocks of baseline and intervention sessions for each of the participants (ie, P1-P9). The number of sessions included in the blocks is indicated by the numerals above the bar-circle combinations.
Discussion
The results of the 2 studies, in line with preliminary data in those areas, 29,30 emphasize the effectiveness of technology-based intervention programs to promote (a) independent start and accurate performance of daily activities in people at the early stages of the disease and (b) supported ambulation in people at advanced stages of the disease. In light of these results, a number of considerations may be put forward regarding the relevance of the intervention programs, their practical implications, and the technology solutions involved.
First, previous technology-based intervention programs with persons with mild or moderate Alzheimer’s disease had typically been aimed at either (a) reminding the persons of simple activities to perform (eg, taking medication) 39,40 or (b) providing the persons with instructions for complex activities (eg, preparing food recipes) started after staff/caregiver reminders. 6,8,14,15 The program used in this study combines the 2 different functions of the aforementioned types of programs previously applied in the area. In fact, the present program provides reminders as well as activity instructions to support both the independent and timely start and the accurate execution of daily activities. The availability of both functions within the same intervention program may be considered practically advantageous, allowing participants to be successfully engaged with minimal supervision and thus with potentially big savings in terms of staff time.
Second, enabling persons with mild or moderate Alzheimer’s disease to start and carry out daily activities independently, without specific demands on staff/caregiver, may be critical to allow them extended, beneficial activity engagement during the day. 29,41 -43 Extended activity engagement can have a positive impact in terms of cognitive stimulation (ie, by facilitating the practice of performance and communication functions), from a social standpoint (ie, by advancing the participants’ social status and fostering their relationship with families and staff), as well as in terms of physical exercise. 29,40,44 -47 With regard to the last point, it is important to note that daily activities, like those used in this study, involve motor responses, such as ambulating, body bending and balancing, arm and leg stretching, and arm lifting, deemed relevant for mild physical exercise. 48 -50
Third, the technology used to support independent and accurate activity is readily available, easy to use, and largely affordable. Indeed, tablets and smartphones are common (everyday) tools and can be purchased for relatively small costs (ie, from about US$250 to US$400). Those tools can be easily fitted with a number of audio files (ie, each representing one of the activities scheduled) and the number of files/activities included might be fairly large so as to provide participants with ample occupational opportunities. Each file would contain a verbal reminder occurring at the time when the related activity is due and the instructions for the activity steps. The instructions could be (a) interspersed with praise to motivate the participants to stay active and positively involved and (b) delivered via wireless Bluetooth earpieces that the participants wear during the sessions (thus avoiding to carry the smartphone or tablet). 29
Fourth, the ambulation increase shown by the participants of study 2 suggests that they were motivated to perform this skill, which is deemed to have multiple positive health implications, once the appropriate support (ie, walker device) was combined with preferred stimulation for step responses. Indeed, the large difference observed between the baseline period (in which the participants were provided with the walker device without stimulation) and the intervention phase seems to largely stress the importance of the stimulation available during this latter phase. The role of the automatically delivered prompts might have been negligible for most of the participants who received very sporadic prompt instances.
Fifth, the participants’ general increase in signs of positive involvement during the intervention phase can be taken as a convincing indication that the participants were comfortable with the response requirements and typically enjoyed the stimulation available during the ambulation sessions. 50 -53 This evidence allows one to claim that the intervention program not only had favorable implications for the participants’ physical engagement but also promoted their emotional well-being with potential benefits for their overall condition. 20,54,55 The same evidence might reassure staff and families about the positive effects of supported ambulation and eventually encourage them to adopt a program such as that reported within daily contexts. 41,44,46,47
Sixth, the technology employed for the ambulation program can be considered relatively simple and easy to access. Indeed, the specific technology components used in this program were a tilt microswitch for monitoring ambulation steps and a notebook computer providing stimulation for ambulation steps and prompts in case of passivity. Both components are commercially available and largely affordable in terms of cost (ie, with the total amount below US$500) and application requirements. Without these technology components, staff efforts to help the participants ambulate did not seem very effective, leaving the participants in an unfavorable condition. 17 -20
Seventh, several limitations of the studies need to be reviewed here. One limitation is the relatively small number of participants included in each of the studies. With regard to this issue, the following points could be made. The evidence provided by the studies can be considered an important addition to the early data available in the respective areas. In spite of this, additional participants need to be exposed to the programs, and different research groups need to be involved in carrying out the programs to definitely determine (a) the robustness of the present data and (b) the wisdom of recommending the programs’ use on a wider scale. 31,32 A second limitation is the absence of social validation assessment for the 2 programs (eg, absence of staff interviews about the benefits and applicability of those programs). 56,57 Positive social validation data might be relevant to predict the level of program acceptance and use within daily contexts. 58,59 A third limitation is the failure to carry out any specific interview or observation check with the participants of study 1 to gather evidence about their level of satisfaction with the program conditions and their activity performance.
In conclusion, the studies provide encouraging evidence on the suitability and effectiveness of the technology-based intervention programs used for promoting independent start and accurate performance of daily activities and supported ambulation, respectively. New research needs to address the aforementioned limitations of the studies to verify the strength of these findings and the overall level of acceptability of the programs within daily contexts. Research efforts may also be directed at (a) determining a wider range of activities that could match the needs and abilities of participants like those included in study 1 and (b) gathering additional evidence on signs of positive involvement with participants like those included in study 2. 29,30,60
Footnotes
Authors’ Note: Approval from a relevant ethics committee and informed consent were obtained for the studies. All procedures performed were in accordance with the ethical standards of the institutional and/or national research committee and with the 1964 Declaration of Helsinki and its later amendments or comparable ethical standards.
The authors declared no potential conflicts of interest with respect to the research, authorship, and/or publication of this article.
Funding: The authors received no financial support for the research, authorship, and/or publication of this article.
ORCID iD: Giulio E. Lancioni  http://orcid.org/0000-0002-6515-5690
http://orcid.org/0000-0002-6515-5690
References
- 1. Bernick C, Cummings J, Raman R, Sun X, Aisen P. Age and rate of cognitive decline in Alzheimer disease: implications for clinical trials. Arch Neurol. 2012;69(7):901–905. [DOI] [PubMed] [Google Scholar]
- 2. Brown PJ, Devanand DP, Liu X, Caccappolo E. Alzheimer’s disease neuroimaging initiative. Functional impairment in elderly patients with mild cognitive impairment and mild Alzheimer disease. Arch Gen Psychiatry. 2011;68(6):617–626. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 3. Kim S. Cognitive rehabilitation for elderly people with early-stage Alzheimer’s disease. J Phys Ther Sci. 2015;27(2):543–546. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 4. Sikkes SA, Pijnenburg YA, Knol DL, de Lange-de Klerk ES, Scheltens P, Uitdehaag BM. Assessment of instrumental activities of daily living in dementia: diagnostic value of the Amsterdam instrumental activities of daily living questionnaire. J Geriatr Psychiatry Neurol. 2013;26(4):244–250. [DOI] [PubMed] [Google Scholar]
- 5. Wilson RS, Segawa E, Boyle PA, Anagnos SE, Hizel LP, Bennett DA. The natural history of cognitive decline in Alzheimer’s disease. Psychol Aging. 2012;27(4):1008–1017. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 6. Boyd HC, Evans NM, Orpwood RD, Harris ND. Using simple technology to prompt multistep tasks in the home for people with dementia: an exploratory study comparing prompting formats. Dementia (London). 2017;16(4):424–442. doi: 10.1177/1471301215602417. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 7. Lancioni GE, Singh NN, O’Reilly MF, et al. Persons with moderate Alzheimer’s disease improve activities and mood via instruction technology. Am J Alzheimers Dis Other Demen. 2009;24(3):246–257. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 8. Perilli V, Lancioni GE, Hoogeveen F, et al. Video prompting versus other instruction strategies for persons with Alzheimer’s disease. Am J Alzheimers Dis Other Demen. 2013;28(4):393–402. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 9. Nijhof N, Van Gemert-Pijnen JEWC, Burns CM, Seydel ER. A personal assistant for dementia to stay at home safe at reduced cost. Gerontechnology. 2013;11(3):469–479. [Google Scholar]
- 10. Spíndola L, Dozzi Brucki SMD. Prospective memory in Alzheimer’s disease and mild cognitive impairment. Dement Neuropsychol. 2011;5(2):64–68. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 11. Campos C, Rocha NB, Vieira RT, et al. Treatment of cognitive deficits in Alzheimer’s disease: a psychopharmacological review. Psychiatr Danub. 2016;28(1):2–12. [PubMed] [Google Scholar]
- 12. Gibson G, Newton L, Pritchard G, Finch T, Brittain K, Robinson L. The provision of assistive technology products and services for people with dementia in the United Kingdom. Dementia (London). 2016;15(4):681–701. [DOI] [PubMed] [Google Scholar]
- 13. Khosravi P, Ghapanchi AH. Investigating the effectiveness of technologies applied to assist seniors: a systematic literature review. Int J Med Inform. 2016;85(1):17–26. [DOI] [PubMed] [Google Scholar]
- 14. Lancioni GE, Perilli V, Singh NN, et al. Technology-aided pictorial cues to support the performance of daily activities by persons with mild or moderate Alzheimer’s disease. Res Dev Disabil. 2012;33(1):265–273. [DOI] [PubMed] [Google Scholar]
- 15. Wang RH, Sudhama A, Begum M, Huq R, Mihailidis A. Robots to assist daily activities: views of older adults with Alzheimer’s disease and their caregivers. Int Psychogeriatr. 2017;29(1):67–79. [DOI] [PubMed] [Google Scholar]
- 16. Lancioni GE, Singh NN, O’Reilly MF, et al. Persons with advanced Alzheimer’s disease engage in mild leg exercise supported by technology-aided stimulation and prompts. Behav Modif. 2017;41(1):3–20. doi:10.1177/0145445516649581. [DOI] [PubMed] [Google Scholar]
- 17. Nishikata S, Yamakawa M, Shigenobu K, Suto S, Makimoto K. Degree of ambulation and factors associated with the median distance moved per day in Alzheimer’s disease patients. Int J Nurs Pract. 2013;19(suppl 3):56–63. [DOI] [PubMed] [Google Scholar]
- 18. Soto ME, Secher M, Gillette-Guyonnet S, et al. Weight loss and rapid cognitive decline in community-dwelling patients with Alzheimer’s disease. J Alzheimers Dis. 2012;28(3):647–654. [DOI] [PubMed] [Google Scholar]
- 19. Hernández SS, Sandreschi PF, da Silva FC, et al. What are the benefits of exercise for Alzheimer’s disease? A systematic review of the past 10 years. J Aging Phys Act. 2015;23(4):659–68. [DOI] [PubMed] [Google Scholar]
- 20. Trudeau SA. Enhanced ambulation and quality of life in advanced Alzheimer’s disease. J Am Geriatr Soc. 2003;51(3):429–431. [DOI] [PubMed] [Google Scholar]
- 21. Berk C, Paul G, Sabbagh M. Investigational drugs in Alzheimer’s disease: current progress. Expert Opin Investig Drugs. 2014;23(6):837–846. [DOI] [PubMed] [Google Scholar]
- 22. Staedtler AV, Nunez D. Nonpharmacological therapy for the management of neuropsychiatric symptoms of Alzheimer’s disease: linking evidence to practice. Worldviews Evid Based Nurs. 2015;12(2):108–115. [DOI] [PubMed] [Google Scholar]
- 23. Ashford JW. Treatment of Alzheimer’s disease: the legacy of the cholinergic hypothesis, neuroplasticity, and future directions. J Alzheimers Dis. 2015;47(1):149–156. [DOI] [PubMed] [Google Scholar]
- 24. Fisher A, Carney G, Bassett K, Dormuth CR. Tolerability of cholinesterase inhibitors: a population-based study of persistence, adherence, and switching. Drugs Aging. 2017;34(3):221–231. [DOI] [PubMed] [Google Scholar]
- 25. Owen RT. Memantine and donepezil: a fixed drug combination for the treatment of moderate to severe Alzheimer’s dementia. Drugs Today (Barc.). 2016;52(4):239–248. [DOI] [PubMed] [Google Scholar]
- 26. Yiannopoulou KG, Papageorgiu SG. Current and future treatments for Alzheimer’s disease. Ther Adv Neurol Disord. 2013;6(1):19–33. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 27. Jensen L, Padilla R. Effectiveness of environment-based interventions that address behavior, perception, and falls in people with Alzheimer’s disease and related major neurocognitive disorders: a systematic review. Am J Occup Ther. 2017;71(5):7105180030p1–7105180030p10. [DOI] [PubMed] [Google Scholar]
- 28. Pareja-Galeano H, Garatachea N, Lucia A. Exercise as a polypill for chronic diseases. Prog Mol Biol Transl Sci. 2015;135:497–526. [DOI] [PubMed] [Google Scholar]
- 29. Lancioni G, Singh N, O’Reilly M, et al. A technology-aided program for helping persons with Alzheimer’s disease perform daily activities. J Enabling Technol. 2017;11(3):85–91. [Google Scholar]
- 30. Lancioni GE, Singh NN, O’Reilly MF, et al. Promoting supported ambulation in persons with advanced Alzheimer’s disease: a pilot study. Disabil Rehabil: Assist Technol. 2018;13(1):101–106. doi.org/10.1080/17483107.2017.1297856 [DOI] [PubMed] [Google Scholar]
- 31. Kazdin AE. Single-Case Research Designs: Methods for Clinical and Applied Settings. 2nd ed. New York: Oxford University Press; 2011. [Google Scholar]
- 32. Makel MC, Plucker JA. Facts are more important than novelty: replication in the education sciences. Educ Res. 2014;43:304–316. [Google Scholar]
- 33. Pedhazur E, Schmelkin L. Measurement Design and Analysis: An Integrated Approach. New York: Psychology Press; 1991. [Google Scholar]
- 34. Folstein MF, Folstein SE, McHugh PR. “Mini-Mental State” a practical method for grading the cognitive state of patients for the clinician. J Psychiatr Res.1975;12(3):189–198. [DOI] [PubMed] [Google Scholar]
- 35. Barlow DH, Nock M, Hersen M. Single-Case Experimental Designs: Strategies for Studying Behavior Change. 3rd ed. New York: Allyn & Bacon; 2009. [Google Scholar]
- 36. Siegel S, Castellan NJ. Nonparametric Statistics for the Behavioral Sciences. 2nd ed . New York: McGraw-Hill; 1988. [Google Scholar]
- 37. Staal J, Pinkney L, Roane DM. Assessment of stimulus preferences in multisensory environment therapy for older people with dementia. Br J Occup Ther. 2003;66(12):542–550. [Google Scholar]
- 38. Lancioni GE, Singh NN, O’Reilly MF, et al. Technology-aided programs to support positive verbal and physical engagement in persons with moderate or severe Alzheimer’s disease. Front. Aging Neurosci. 2016;8:87. doi:10.3389/fnagi.2016.00087. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 39. Kamimura T, Ishiwata R, Inoue T. Medication reminder device for the elderly patients with mild cognitive impairment. Am J Alzheimers Dis Other Demen. 2012;27(4):238–242. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 40. Oriani M, Moniz-Cook E, Binetti G, et al. An electronic memory aid to support prospective memory in patients in the early stages of Alzheimer’s disease: a pilot study. Aging Ment Health. 2003;7(1):22–27. [DOI] [PubMed] [Google Scholar]
- 41. Christofoletti G, Oliani MM, Bucken-Gobbi LT, Gobbi S, Beinotti F, Stella F. Physical activity attenuates neuropsychiatric disturbances and caregiver burden in patients with dementia. Clinics (Sao Paolo). 2011;66(4):613–618. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 42. Imbeault H, Bier N, Pigot H, et al. Electronic organizer and Alzheimer’s disease: fact or fiction? Neuropsychol Rehabil. 2014;24(1):71–100. [DOI] [PubMed] [Google Scholar]
- 43. Kerkhof YJ, Graff MJ, Bergsma A, de Vocht HH, Dröes RM. Better self-management and meaningful activities thanks to tablets? Development of a person-centered program to support people with mild dementia and their carers through use of hand-held touch screen devices. Int Psychogeriatr. 2016;28(11):1917–1929. [DOI] [PubMed] [Google Scholar]
- 44. Brett L, Traynor V, Stapley P. Effects of physical exercise on health and well-being of individuals living with a dementia in nursing homes: a systematic review. J Am Med Dir Assoc. 2016;17(2):104–116. [DOI] [PubMed] [Google Scholar]
- 45. Eggermont LH, Gavett BE, Volkers KM, et al. Lower-extremity function in cognitively healthy aging, mild cognitive impairment, and Alzheimer’s disease. Arch Phys Med Rehabil. 2010;91(4):584–588. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 46. Hoffmann K, Frederiksen KS, Sobol NA, et al. Preserving cognition, quality of life, physical health and functional ability in Alzheimer’s disease: the effect of physical exercise (ADEX trial) – rationale and design. Neuroepidemiology. 2013;41(3-4):198–207. [DOI] [PubMed] [Google Scholar]
- 47. Letts L, Edwards M, Berenyi J, et al. Using occupations to improve quality of life, health and wellness, and client and caregiver satisfaction for people with Alzheimer’s disease and related dementias. Am J Occup Ther. 2011;65(5):497–504. [DOI] [PubMed] [Google Scholar]
- 48. Canonici AP, Andrade LP, Gobbi S, Santos-Galduroz RF, Gobbi LT, Stella F. Functional dependence and caregiver burden in Alzheimer’s disease: a controlled trial on the benefits of motor intervention. Psychogeriatrics. 2012;12(3):186–192. [DOI] [PubMed] [Google Scholar]
- 49. De Vreede PL, Samson MM, Van Meeteren NL, Duursma SA, Verhaar HJ. Functional task exercise versus resistance strength exercise to improve daily function in older women: a randomized, controlled trial. J Am Geriatr Soc. 2005;53(1):2–10. [DOI] [PubMed] [Google Scholar]
- 50. Maliszewska-Cyna E, Lynch M, Oone JJ, Nagy PM, Aubert I. The benefits of exercise and metabolic interventions for the prevention and early treatment of Alzheimer’s disease. Curr Alzheimer Res. 2017;14(1):47–60. [DOI] [PubMed] [Google Scholar]
- 51. Godwin KM, Mills WL, Anderson JA, Kunik ME. Technology-driven interventions for caregivers of persons with dementia: a systematic review. Am J Alzheimers Dis Other Demen. 2013;28(3):216–222. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 52. Scherer MJ, Craddock G, Mackeogh T. The relationship of personal factors and subjective well-being to the use of assistive technology devices. Disabil Rehabil. 2011;33(10):811–817. [DOI] [PubMed] [Google Scholar]
- 53. Pierce WD, Cheney CD. Behavior Analysis and Learning. 4th ed . New York: Psychology Press; 2008. [Google Scholar]
- 54. Moore K, Delaney JA, Dixon MR. Using indices of happiness to examine the influence of environmental enhancements for nursing home residents with Alzheimer’s disease. J Appl Behav Anal. 2007;40(3):541–544. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 55. Perales J, Cosco TD, Stephan BC, Haro JM, Brayne C. Health-related quality-of-life instruments for Alzheimer’s disease and mixed dementia. Int Psychogeriatr. 2013;25(5):691–706. [DOI] [PubMed] [Google Scholar]
- 56. Callahan K, Henson R, Cowan AK. Social validation of evidence-based practices in autism by parents, teachers, and administrators. J Autism Dev Disord. 2008;38(4):678–692. [DOI] [PubMed] [Google Scholar]
- 57. Luiselli JK, Bass JD, Whitcomb SA. Teaching applied behavior analysis knowledge competencies to direct-care service providers: outcome assessment and social validation of a training program. Beh Modif. 2010;34(5):403–414. [DOI] [PubMed] [Google Scholar]
- 58. Lenker JA, Harris F, Taugher M, Smith RO. Consumer perspectives on assistive technology outcomes. Disabil Rehabil: Assist Technol. 2013;8(5):373–380. [DOI] [PubMed] [Google Scholar]
- 59. Mao HF, Chang LH, Yao G, Chen WY, Huang WN. Indicators of perceived useful dementia care assistive technology: caregivers’ perspectives. Geriatr Gerontol Int. 2015;15(8):1049–1057. [DOI] [PubMed] [Google Scholar]
- 60. Levy CE, Silverman E, Jia H, Geiss M, Omura D. Effects of physical therapy delivery via home video telerehabilitation on functional and health-related quality of life outcomes. J Rehabil Res Dev. 2015;52(3):361–370. [DOI] [PubMed] [Google Scholar]