Abstract
People with a first-degree family history of Alzheimer’s disease are at an increased risk of developing dementia. Subjective memory impairment among individuals with no measurable cognitive deficits may also indicate elevated dementia risk. It remains unclear whether nondemented people with a positive family history of Alzheimer’s disease are more likely to experience cognitive deficits and whether such an association reflects underlying neuropathology. We therefore investigated subjective memory impairment and hippocampal cortical thickness in 40 healthy older adults and 35 patients with amnestic mild cognitive impairment. We found greater subjective memory impairment and left hemispheric hippocampal cortical thinning associated with a first-degree family history of Alzheimer’s disease in healthy older adults. This suggests that subjective memory impairment could reflect preclinical stage neurodegeneration among individuals with the family history risk factor.
Keywords: family history of Alzheimer’s disease, mild cognitive impairment, MCI, subjective memory impairment, hippocampal thickness
Introduction
Subjective memory impairment is a frequent phenomenon among people presenting to memory clinics or other institutions that provide diagnostic assessments for cognitive impairment. For clinicians, it is important to evaluate these subjective changes since they could indicate a risk of subsequent mild cognitive impairment (MCI) 1 and dementia. 2 Studies highlight how brain structure and function changes in people with subjective memory impairment may already suggest early neurodegeneration. 3,4 However, memory complaints could also reflect individual differences in perceived changes of performance during normal aging, and examining their predictive value has produced variable findings. 5 -7
Kryscio and colleagues 5 show that people with subjective memory impairment that also carry the e4-allele of the apolipoprotein E, the most important genetic risk factor for sporadic Alzheimer’s disease, have an even higher risk of future cognitive decline. More surprisingly, not only this risk status but also the knowledge of one’s own genetic risk is associated with subjective memory impairment in otherwise cognitively healthy people. 8 Although there is ongoing debate about whether disclosing genetic dementia risk information has negative psychological consequences, 9 dementia worries, mood changes, or a first-degree family history of Alzheimer’s disease are far more obvious in a clinical setting than genetic variants. These factors are frequently discussed during diagnostic assessments, but in contrast to depressive symptoms, 10 the impact of family history risk on subjective memory performance has been rarely studied. Whereas depression reflects a possible clinical feature of prodromal dementia rather than elevated dementia risk, 11 a family history of Alzheimer’s disease is associated with an increased risk of developing the disease, 12 but also with worrying about dementia. 13
The first-degree family history of Alzheimer’s disease can be utilized as a composite risk factor in dementia research 14 as it reflects known and yet undiscovered genetic and nongenetic risks associated with structural and functional brain changes. 15 -17 We previously demonstrated reduced hippocampal cortical thickness 15 and reduced memory performance 16 in healthy people with the family history risk factor when compared with healthy people at no familial risk for Alzheimer’s disease. Apolipoprotein E-e4 carrier status is also associated with brain structure and neurocognitive changes 15,18 as well as with subjective memory impairment. 8 However, an association between subjective memory impairment and family history risk has not yet been investigated. In the continuum from healthy cognitive aging to Alzheimer’s dementia, the transitional MCI stage is of particular interest. Patients with the amnestic MCI subtype have subjective and objective memory impairment, but they are not demented. 19 For clinicians, it is important to know among which individuals in the predementia spectrum an association between a risk factor, brain structure, and subjective function would yield additional information. We therefore investigated healthy older adults and patients with amnestic MCI and hypothesized that a family history of Alzheimer’s disease would be associated with subjective memory impairment and reduced hippocampal cortical thickness in healthy older individuals. We did not expect to find these associations among patients with MCI. In contrast to cognitively healthy people carrying the family history risk factor, the more extensive neurodegeneration may prevent the detection of the unique family history risk factor–associated brain morphology changes in patients with MCI. Furthermore, the clinical syndrome of amnestic MCI implies that all these patients have objective as well as subjective memory deficits. It is unlikely that the additional impact of a family history of Alzheimer’s disease on subjective memory impairment could be demonstrated in this population because we expected it to be subtler than the influence of the objective deficits.
Methods
Participants and Procedures
Seventy-five individuals were recruited through advertisements and through our memory clinic (university hospital and outpatient service). They were identified from a population of 180 participants varying in cognitive and affective health, participating in research aimed at investigating brain structure changes and behavioral characteristics associated with cognitive impairment and early-stage neurodegeneration. We obtained written informed consent, and our ethics committee approved the study. Among the participants were 40 healthy older adults with no measurable cognitive impairment (mean age: 66.2 years ± 7.5 years) and 35 patients with MCI (mean age: 70.3 years ± 6.5 years). Patients with MCI met standard diagnostic criteria for the amnestic subtype, 19 meaning that these patients show an objective memory deficit, but no impairment in any other cognitive domain. Amnestic MCI subtype patients are not demented and do not report any difficulties in daily routines. All study participants underwent clinical evaluation and detailed neuropsychological testing prior to study inclusion. The neuropsychological examination included several tests to investigate the individual performance across various cognitive domains, such as language, attention/executive function, visuospatial skills, or processing speed. The study participants did not have any neuropsychiatric disease possibly affecting cognitive performance other than MCI. Further exclusion criteria were a school education of less than 8 years and psychotropic medication. The family history risk factor represented at least 1 first-degree relative diagnosed with Alzheimer’s disease. We utilized a 7-item Likert scale to measure the subjective memory impairment (“Do you experience subjective memory impairment?”, 1 = severe to 7 = none). Although there are more comprehensive assessments for subjective cognitive impairment in general, with respect to memory, Likert scales have been used to investigate subjective changes. 20 It has been demonstrated that a greater number of items on a Likert scale enhances the diagnostic accuracy. 21 We also obtained laboratory and magnetic resonance imaging (MRI) data in order to rule out specific or systemic conditions, such as thyroid dysfunction or vascular brain disease that would explain cognitive impairment otherwise. In addition to our memory clinic’s standard diagnostic protocol, we obtained a high-resolution oblique coronal T2-weighted fast-spin echo sequence in 69 right-handed participants on a GE Signa HDxt 3-Tesla MRI scanner (General Electric Health Care, Waukesha, Wisconsin) to investigate hippocampal cortical thickness (repetition time: 5200 milliseconds; echo time: 105 milliseconds; slice thickness: 3 mm; spacing: 0 mm; 19 slices; inplane voxel size: 0.39 × 0.39 mm; field of view: 200 mm). With a semi-automated brain segmentation protocol and cortical unfolding data analysis, we were able to restrict our analyses to the region of interest without covering extrahippocampal cortices. This technique aims at enhancing the visibility of the convoluted hippocampal region by flattening its gray matter into 2-dimensional space. After segmenting gray matter, white matter, and cerebrospinal fluid using the high-resolution MRI data and mrGray software, 22 the MRI sequence is interpolated to achieve nearly isotropic voxels of approximately 0.4 mm3. Gray matter is then grown out in connected layers using a region-expansion algorithm and flattened based on metric multidimensional scaling. For each gray matter voxel in 2-dimensional space, the maximum distance value of the corresponding 3-dimensional voxels across all layers is taken and multiplied by 2. Additional details on cortical unfolding are reported elsewhere. 23 -26 According to the established protocols and in line with our previous investigations utilizing the method, 15,16,27 -30 we report raw data (eg, no intracranial volume correction), which are appropriate when investigating cortical thickness in contrast to volume. 31 Excellent inter-rater and retest reliability results have been demonstrated previously. 26,27,32
Statistical Analyses
We investigated a possible association between family history risk and subjective memory impairment using a univariate mixed general linear model with subjective memory impairment as the dependent variable, group (healthy/MCI) and family history (yes = FH+/no = FH−) as between-group factors, and age as a covariate. We also investigated the interaction between group and family history on subjective memory impairment. We only conducted post hoc univariate tests to investigate the influence of family history risk on subjective memory impairment, after we established significance with the multivariate F test. We further examined the association between family history risk and hippocampal cortical thickness using another multivariate mixed general linear model with left and right hippocampal cortical thickness as the dependent variables, group (healthy/MCI) and family history (FH+/FH−) as between-group factors, and age as a covariate. Due to sample size limitations, we did not additionally model subjective memory impairment as a between-group factor when investigating cortical thickness. However, we performed exploratory participant subgroup pairwise cortical thickness comparisons and correlation analyses. We investigated the effect sizes of group differences using Cohen d. Gender and family history distributions were compared with χ2 tests, and we used a significance level of P < .05 for all statistical analyses.
Results
Patients with MCI and healthy individuals did not differ in educational status, gender, and family history risk distribution (Table 1). Among patients with MCI, 31% carried the family history risk factor, whereas 25% of the healthy older adults carried the family history risk factor. The patients with MCI were significantly older than our healthy participants (P = .02); therefore, we modeled age as a covariate in all analyses.
Table 1.
Demographic and Clinical Characteristics.
| Characteristics and Measures | CTL | SD | MCI | SD | Significance (P Value)a |
|---|---|---|---|---|---|
| N | 40 | 35 | |||
| Age (years) | 66.2 | ±7.5 | 70.3 | ±6.5 | .02 |
| Female sex (%) | 62.5 | 45.7 | .15 | ||
| Education (years) | 14.1 | ±2.7 | 13.9 | ±2.5 | .82 |
| FH + (%) | 25.0 | 31.4 | .54 | ||
| MMSE (score range 0-30) | 29.4 | ±0.8 | 28.2 | ±1.3 | <.001 |
| Subjective memory impairment (score range: 7 = none to 1 = severe) | 4.7 | ±1.0 | 3.9 | ±1.2 | .003 |
Abbreviations: CTL, control participants, FH +, first-degree family history of Alzheimer’s disease, MCI, mild cognitive impairment, MMSE, Mini-Mental State Examination, SD, standard deviation.
a χ2 tests for gender and family history risk distribution.
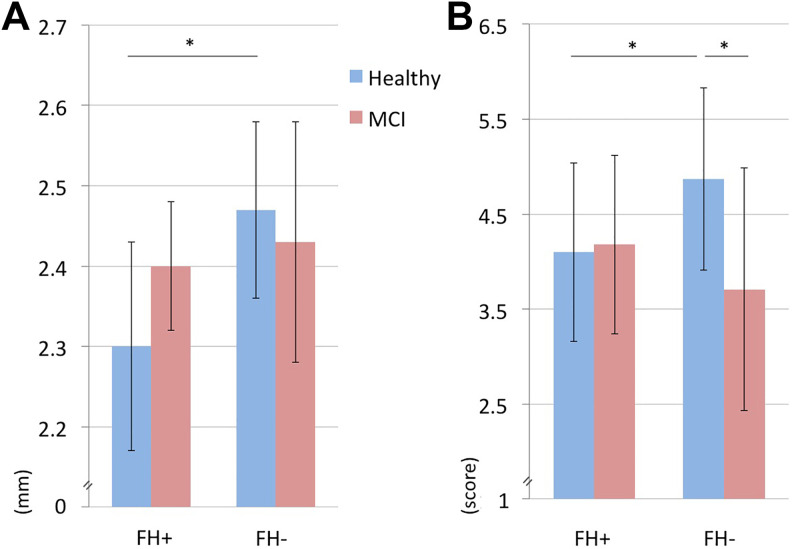
The mixed general linear model revealed a significant interaction between group (healthy/MCI) and family history risk (F = 4.6, P = .035; Figure 1). Patients with MCI reported greater subjective memory impairment than healthy older adults. However, subgroup analyses showed that this effect was due to people without family history risk (MCI FH−: 3,75 ± 1.26, healthy FH−: 4,87 ± 0.97, P < .001, d = 1), whereas, among the individuals with a family history of Alzheimer’s disease, there was no such difference (MCI FH+: 4,18 ± 0.98, healthy FH+: 4,1 ± 0.99, P = .85). Healthy older adults with the family history risk factor reported greater subjective memory impairment than healthy older adults without this risk (P = .019, d = 0.8). Patients with MCI did not differ in subjective memory impairment when considering family history risk status (P = .16).
Figure 1.
Subjective memory impairment and cortical thickness. Patients with amnestic mild cognitive impairment (MCI) report greater subjective memory impairment (B) than cognitively healthy older adults (Healthy), but this effect is only detectable among individuals without a first-degree family history of Alzheimer’s disease (FH−). Healthy older adults with the family history risk factor (FH+) show greater subjective memory impairment (B) and a thinner left hippocampus (A) when compared with healthy older adults without this risk factor.
Investigating hippocampal cortical thickness, the multivariate mixed general linear model yielded a significant effect for family history risk (F = 3.96, P = .027; Figure 1). Only the left hemisphere contributed to this finding (left hemisphere: F = 8.05, P = .007, right hemisphere: F = 1.37, P = .25). The left hemispheric cortical thickness reduction associated with family history risk was detectable only among healthy participants (FH+: 2,3 mm ± 0.13 mm, FH−: 2,47 mm ± 0.11 mm, P = .006, d = 1.5), whereas patients with MCI did not demonstrate this effect (FH+: 2,4 mm ± 0.08 mm, FH−: 2,43 mm ± 0.15 mm, P = .27). Additional exploratory analyses showed a positive correlation (Pearson r = 0.67, P = .04) between subjective memory impairment and left hemispheric hippocampal cortical thinning only among patients with MCI with the family history risk factor.
Discussion
In this study, we show that healthy older adults report greater subjective memory impairment when they have a positive first-degree family history of Alzheimer’s disease. These individuals also exhibit a thinner left hippocampus when compared to people without family history risk. Among patients with MCI, there was neither a robust association between the family history of Alzheimer’s disease with subjective memory impairment nor with hippocampal cortical thickness.
The few existing data on how a first-degree family history of Alzheimer’s disease may influence subjective memory performance are heterogeneous. 33,34 Tsai and colleagues 33 show that first-degree relatives of patients with Alzheimer’s disease report subjective memory impairment more frequently than the spouses of these patients. The authors conclude that this may either reflect the disease’s heritability and early-stage neurodegeneration, 33 or it could be a sign of depression, which is associated with preclinical dementia itself. 11 In contrast, Heun and colleagues 34 report no difference in the prevalence of subjective memory impairment among individuals with and without the family history risk factor. They did not find support for the hypothesis that psychosocial factors such as an increased awareness due to affected family members would influence memory complaints. 34 However, relatively large participant samples 33,34 also limit how objective cognition could be measured among individuals with the family history risk factor. Using a telephone-administered screening test 33 or a structured interview 34 to investigate cognition could make it more difficult to distinguish cognitively healthy older adults from patients with MCI. Therefore, prior to study inclusion, our participants received a detailed neuropsychological assessment of various cognitive domains including memory, visuospatial skills, attention/executive function, processing speed, and language.
Vannini and colleagues highlight the importance of neuropathology for self-reported memory impairment. 35 We therefore additionally investigated hippocampal cortical thickness, and the results are in line with our previous results. 15 A family history of Alzheimer’s disease was associated with left hemispheric hippocampal cortical thickness reduction in healthy older adults, but not in patients with MCI. This is congruent with the assumption that, with greater neurodegeneration, the unique impact of the family history risk factor on brain structure is no longer detectable. Although direct pairwise cortical thickness comparisons between the participant subgroups did not reveal significant results, we found a positive correlation between subjective memory impairment and left hemispheric hippocampal cortical thinning among patients with MCI with the family history risk factor. Hippocampal atrophy is one of the best parameters indicating imminent conversion to Alzheimer’s disease among patients with MCI 36 ; however, larger participant samples should be utilized to investigate how family history risk and subjective memory impairment interact in determining cortical thickness and to reveal the trajectories of cognitive decline in the presence of specific risk factor patterns.
It is a limitation that the genetic contributions to the family history of Alzheimer’s disease could vary between patients with MCI and healthy older adults. Future longitudinal investigations may contribute to reveal the predictive value of family history risk-associated brain structure and function changes. In contrast to single genetic variants, 8 it is difficult to investigate whether the family history risk factor itself or the psychological distress due to the knowledge of being at increased risk contributes to the subjective memory impairment. Although our study participants did not have anxiety or depression, we did not utilize psychometric scales to measure possible subthreshold symptoms. Specifically, with respect to depression or subclinical mood changes, future research is needed to better understand the mechanisms associated with the relationship between familial risk and declines in brain health. Although our examination of the subjective impairment is less susceptible to the effects of hyper- or anosognosia of memory decline 35,37 when compared with a dichotomous assessment, 21 multi-item scales would allow further differentiation of subjective cognitive deficits beyond memory.
Despite these limitations, our behavioral and brain imaging data suggest that subjective memory impairment in healthy older adults with a first-degree family history of Alzheimer’s disease should be acknowledged in clinical evaluations of cognitive performance. Clinicians should recommend detailed neuropsychological testing and regular follow-up assessments for these individuals at risk for future cognitive decline.
Footnotes
The authors declared no potential conflicts of interest with respect to the research, authorship, and/or publication of this article.
Funding: The authors disclosed receipt of the following financial support for the research, authorship, and/or publication of this article: Dr Linn has received research support from B. Braun Stiftung, Friedrich-Baur-Stiftung, and the Förderprogram für Forschung und Lehre and received travel expenses and/or honoraria for educational lectures from Bayer Healthcare, Phillips Healthcare, and Bracco. Dr Donix has received research support from the Roland Ernst Stiftung and a lecture fee from Mundipharma. The study was funded by a Grant (MeDDrive 60.338) of the TU Dresden to Markus Donix.
ORCID iD: Markus Donix  http://orcid.org/0000-0001-8947-0428
http://orcid.org/0000-0001-8947-0428
References
- 1. Luck T, Riedel-Heller SG, Luppa M, et al. Risk factors for incident mild cognitive impairment—results from the German Study on Ageing, Cognition and Dementia in Primary Care Patients (AgeCoDe). Acta Psychiatr Scand. 2010;121(4):260–272. [DOI] [PubMed] [Google Scholar]
- 2. Jessen F, Wiese B, Bachmann C, et al. ; German Study on Aging, Cognition and Dementia in Primary Care Patients Study Group. Prediction of dementia by subjective memory impairment: effects of severity and temporal association with cognitive impairment. Arch Gen Psychiatry. 2010;67(4):414–422. [DOI] [PubMed] [Google Scholar]
- 3. Ryu SY, Lim EY, Na S, et al. Hippocampal and entorhinal structures in subjective memory impairment: a combined MRI volumetric and DTI study. Int Psychogeriatr. 2017;29(5):785–792. [DOI] [PubMed] [Google Scholar]
- 4. Visser PJ, Verhey F, Knol DL, et al. Prevalence and prognostic value of CSF markers of Alzheimer’s disease pathology in patients with subjective cognitive impairment or mild cognitive impairment in the DESCRIPA study: a prospective cohort study. Lancet Neurol. 2009;8(7):619–627. [DOI] [PubMed] [Google Scholar]
- 5. Kryscio RJ, Abner EL, Cooper GE, et al. Self-reported memory complaints: implications from a longitudinal cohort with autopsies. Neurology. 2014;83(15):1359–1365. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 6. Mitchell AJ. The clinical significance of subjective memory complaints in the diagnosis of mild cognitive impairment and dementia: a meta-analysis. Int J Geriatr Psychiatry. 2008;23(11):1191–1202. [DOI] [PubMed] [Google Scholar]
- 7. Purser JL, Fillenbaum GG, Wallace RB. Memory complaint is not necessary for diagnosis of mild cognitive impairment and does not predict 10-year trajectories of functional disability, word recall, or short portable mental status questionnaire limitations. J Am Geriatr Soc. 2006;54(2):335–338. [DOI] [PubMed] [Google Scholar]
- 8. Lineweaver TT, Bondi MW, Galasko D, Salmon DP. Effect of knowledge of APOE genotype on subjective and objective memory performance in healthy older adults. Am J Psychiatry. 2014;171(2):201–208. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 9. Haussmann R, Donix M. Disclosing pleiotropic effects during genetic risk assessment for Alzheimer disease. Ann Intern Med. 2016;165(9):673. [DOI] [PubMed] [Google Scholar]
- 10. Rotenberg Shpigelman S, Sternberg S, Maeir A. Beyond memory problems: multiple obstacles to health and quality of life in older people seeking help for subjective memory complaints. Disabil Rehabil. 2017:1–7. [DOI] [PubMed] [Google Scholar]
- 11. Singh-Manoux A, Dugravot A, Fournier A, et al. Trajectories of depressive symptoms before diagnosis of dementia: a 28-year follow-up study. JAMA Psychiatry. 2017;74(7):712–718. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 12. van Duijn CM, Clayton D, Chandra V, et al. Familial aggregation of Alzheimer’s disease and related disorders: a collaborative re-analysis of case-control studies. Int J Epidemiol. 1991;20(suppl 2):S13–S20. [DOI] [PubMed] [Google Scholar]
- 13. Cutler SJ. Worries about getting Alzheimer’s: who’s concerned? Am J Alzheimers Dis Other Demen. 2015;30(6):591–598. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 14. Donix M, Small GW, Bookheimer SY. Family history and APOE-4 genetic risk in Alzheimer’s disease. Neuropsychol Rev. 2012;22(3):298–309. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 15. Donix M, Burggren A, Suthana N, et al. Family history of alzheimer’s disease and hippocampal structure in healthy people. Am J Psychiatry. 2010;167(11):1399–1406 [DOI] [PMC free article] [PubMed] [Google Scholar]
- 16. Donix M, Ercoli LM, Siddarth P, et al. Influence of Alzheimer disease family history and genetic risk on cognitive performance in healthy middle-aged and older people. Am J Geriatr Psychiatry. 2012;20(7):565–573. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 17. Mosconi L, Brys M, Switalski R, et al. Maternal family history of Alzheimer’s disease predisposes to reduced brain glucose metabolism. Proc Natl Acad Sci U S A. 2007;104(48):19067–19072. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 18. Wisdom NM, Callahan JL, Hawkins KA. The effects of apolipoprotein E on non-impaired cognitive functioning: A meta-analysis. Neurobiol Aging. 2011;32(1):63–74. [DOI] [PubMed] [Google Scholar]
- 19. Petersen RC. Clinical practice. Mild cognitive impairment. N Engl J Med. 2011;364(23):2227–2234. [DOI] [PubMed] [Google Scholar]
- 20. Paradise MB, Glozier NS, Naismith SL, Davenport TA, Hickie IB. Subjective memory complaints, vascular risk factors and psychological distress in the middle-aged: a cross-sectional study. BMC Psychiatry. 2011;11:108. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 21. Aji BM, Larner AJ. Screening for dementia: single yes/no question or Likert scale? Clin Med (Lond). 2017;17(1):93–94. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 22. Teo PC, Sapiro G, Wandell BA. Creating connected representations of cortical gray matter for functional MRI visualization. IEEE Trans Med Imaging. 1997;16(6):852–863. [DOI] [PubMed] [Google Scholar]
- 23. Zeineh MM, Engel SA, Bookheimer SY. Application of cortical unfolding techniques to functional MRI of the human hippocampal region. Neuroimage. 2000;11(6 pt 1):668–683. [DOI] [PubMed] [Google Scholar]
- 24. Zeineh MM, Engel SA, Thompson PM, Bookheimer SY. Unfolding the human hippocampus with high resolution structural and functional MRI. Anat Rec. 2001;265(2):111–120. [DOI] [PubMed] [Google Scholar]
- 25. Zeineh MM, Engel SA, Thompson PM, Bookheimer SY. Dynamics of the hippocampus during encoding and retrieval of face-name pairs. Science. 2003;299(5606):577–580. [DOI] [PubMed] [Google Scholar]
- 26. Ekstrom AD, Bazih AJ, Suthana NA, et al. Advances in high-resolution imaging and computational unfolding of the human hippocampus. Neuroimage. 2009;47(1):42–49. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 27. Donix M, Burggren AC, Suthana NA, et al. Longitudinal changes in medial temporal cortical thickness in normal subjects with the APOE-4 polymorphism. Neuroimage. 2010;53(1):37–43. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 28. Donix M, Scharf M, Marschner K, et al. Cardiovascular risk and hippocampal thickness in Alzheimer’s disease. Int J Alzheimers Dis. 2013;2013:108021. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 29. Baumgaertel J, Haussmann R, Gruschwitz A, et al. Education and genetic risk modulate hippocampal structure in Alzheimer’s disease. Aging Dis. 2016;7(5):553–560. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 30. Suthana NA, Donix M, Wozny DR, et al. High-resolution 7 T fMRI of human hippocampal subfields during associative learning. J Cogn Neurosci. 2015;27(6):1194–1206. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 31. Westman E, Aguilar C, Muehlboeck JS, Simmons A. Regional magnetic resonance imaging measures for multivariate analysis in Alzheimer’s disease and mild cognitive impairment. Brain Topogr. 2013;26(1):9–23. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 32. Burggren AC, Zeineh MM, Ekstrom AD, et al. Reduced cortical thickness in hippocampal subregions among cognitively normal apolipoprotein E e4 carriers. Neuroimage. 2008;41(4):1177–1183. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 33. Tsai DH, Green RC, Benke KS, Silliman RA, Farrer LA. Predictors of subjective memory complaint in cognitively normal relatives of patients with Alzheimer’s disease. J Neuropsychiatry Clin Neurosci. 2006;18(3):384–388. [DOI] [PubMed] [Google Scholar]
- 34. Heun R, Kockler M, Ptok U. Subjective memory complaints of family members of patients with Alzheimer’s disease and depression. Dement Geriatr Cogn Disord. 2003;16(2):78–83. [DOI] [PubMed] [Google Scholar]
- 35. Vannini P, Amariglio R, Hanseeuw B, et al. Memory self-awareness in the preclinical and prodromal stages of Alzheimer’s disease. Neuropsychologia. 2017;99:343–349. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 36. Risacher SL, Saykin AJ, West JD, Shen L, Firpi HA, McDonald BC. Baseline MRI predictors of conversion from MCI to probable AD in the ADNI cohort. Curr Alzheimer Res. 2009;6(4):347–361. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 37. Fragkiadaki S, Kontaxopoulou D, Beratis IN, et al. Self-awareness of cognitive efficiency: differences between healthy elderly and patients with mild cognitive impairment (MCI). J Clin Exp Neuropsychol. 2016;38(10):1144–1157. [DOI] [PubMed] [Google Scholar]