Abstract
Patients with Parkinson disease dementia (PDD) have deficits resulting mainly from frontostriatal dysfunction. The aim of this study was to assess the effectiveness of reality orientation therapy (ROT) combined with drug therapy (acetylcholinesterase inhibitors) in PDD treatment and compare it with that of drug therapy alone. Patients (n = 12) with a diagnosis of PDD were divided into 2 groups: group A—drug therapy and ROT; group B—drug therapy alone. Reality orientation therapy was applied weekly for 6 months, and patients were assessed during the same period. Significant improvements in frontostriatal deficits were observed in the group that received the combined therapy, as shown mainly by the scores in verbal fluency in the Consortium to Establish a Registry for Alzheimer’s Disease (CERAD) battery (P = .02) and in attention in Scales for outcomes of Parkinson’s Disease–Cognition (P = .021) and Clock Drawing Test (P = .037). Patients who received only medication had worse results in constructional praxis recall in the CERAD battery (P = .037). Our results indicate that ROT may help in the treatment of frontostriatal cognitive deficits and can potentially be used to complement drug therapy.
Keywords: Parkinson disease, dementia, reality orientation therapy (ROT)
Introduction
Dementia is a syndrome characterized by a range of cognitive deficits of sufficient intensity to interfere with an individual’s professional and social performance. The condition has many causes, and a specific diagnosis depends on a knowledge of the different clinical manifestations. In Parkinson disease (PD), cognitive deficits are found more frequently in patients in the advanced stages of the disease. However, cognitive deficits can also be present in about 20% to 30% of patients who have just been diagnosed or are in the early stages of the disease. 1,2 Patients with Parkinson disease dementia (PDD) present with cognitive deficits resulting mainly from frontostriatal dysfunction, such as frontal executive dysfunction, reduced problem-solving and planning ability, a deficit in organization of goal-directed behaviors, changes in cognitive flexibility, visuospatial dysfunction, and deficits in verbal fluency and inhibitory control, in addition to impaired working memory, learning, and memorization. 2 –4
Abnormalities in multineurotransmitter systems, including the acetylcholine system, contribute to cognitive impairment in patients with PDD. Thus, it is believed that the pace of cognitive deterioration can be delayed with acetylcholinesterase inhibitors, as these improve cognitive function in patients with PDD by increasing acetylcholine levels. Acetylcholinesterase inhibitors are therefore one of the pillars of dementia treatment. 5 Nonpharmacological cognitive enhancement strategies can also be used to improve cognitive function in patients with dementia. One such strategy is reality orientation therapy (ROT), a cognitive stimulation method used in combination with pharmacological therapy in patients with Alzheimer’s disease (AD). 6
Reality orientation therapy was described for the first time in 1966 as a rehabilitation therapy for confused elderly people. 7 It is one of the most widely used cognitive enhancement techniques, 8 and numerous procedures have been described. The therapy essentially involves presenting continuous orientation and memory information related to time, place, and person so that the individual may gain a greater understanding of his or her surroundings. 9,10 During ROT sessions, the patient is stimulated to discuss several subjects related to recent events of his or her daily routine. Stimulation of social engagement, particularly based on the individual’s personal interests, and planning and organization of his or her activities are also an important part of the therapy. 10,11
The present study sought to analyze the combined effect of ROT and acetylcholinesterase inhibitors on PDD patients’ responses to cognitive stimulation and compare their responses with those of patients treated with acetylcholinesterase inhibitors alone.
Methods
Seventeen patients with PDD who had been seen at the Campos Gerais Regional University Hospital (HURCG) Neurology Service and INOVARE Serviços de Saúde Ltda and agreed to take part in the study were selected according to the Parkinson’s Disease Society Brain Bank diagnostic criteria to confirm the diagnosis of PD. 12 Parkinson disease dementia was diagnosed using the International Parkinson and Movement Disorder Society criteria. 13 All participants confirmed that they had been under regular treatment with donepezil (10 mg once daily) or rivastigmine (6 mg/d twice daily or 10 cm2 patch) for at least 3 months and signed a voluntary informed consent form. Exclusion criteria, which were intended to exclude patients whose signs and symptoms made it impossible to perform a cognitive assessment or apply the proposed tests, were as follows: (1) advanced stage PD with severe motor or sensory impairment, (2) severe psychotic symptoms, (3) another dementia not associated with PD, and (4) cognitive changes that had started at least 1 year before motor symptoms (possible dementia with Lewy bodies). 13 The study was approved by the State University of Ponta Grossa Research Ethics Committee (reference no. 1.026.961 FA).
All the patients were assessed during the ON-phase of levodopa therapy, preferably 2 hours after medication had been administered. A team trained in movement disorders carried out the clinical assessment. A semistructured questionnaire was applied to collect epidemiologic data and data about disease progression and previous and current treatment. The Hoehn and Yahr Scale 14 and Unified Parkinson Disease Rating Scale III 15 were used to classify patients according to their motor symptoms.
Cognitive Assessment
During the cognitive assessment, a trained team applied the following cognitive tests:
The Consortium to Establish a Registry for Alzheimer’s Disease (CERAD) neuropsychological battery. 16 This consists of 7 subtests that together give a maximum score of 100 broken down as follows: (1) verbal fluency (maximum score 13), (2) modified Boston naming (maximum score 15), (3) word list learning (maximum score 30), (4) constructional praxis (maximum score 11), (5) word list recall (maximum score 10), (6) word list recognition (maximum score 10), and (7) constructional praxis recall (maximum score 10).
Scales for Outcomes of Parkinson’s Disease–Cognition (SCOPA-Cog), 17 consisting of 10 subtests with a maximum score of 43. Verbal and nonverbal memory and learning are assessed by means of a cube test (in which the patient copies the order in which 4 cubes are pointed to) and by reading/recalling 10 words. Attention is assessed by the patient saying the months of the year in reverse order and performing serial 3 subtractions. Aspects of measured executive functions include complex motor planning, working memory, and verbal fluency. A figure assembly task to evaluate visuospatial function (patients are asked to determine which shapes are needed to construct another figure) is the last subtest in the scale.
The Clock Drawing Test (CDT), ranging from 1 to 10 points. 18
Therapy Application and Description
Participants were divided into 2 groups, A and B. Participants in group A received ROT in addition to conventional treatment (rivastigmine or donepezil), while those in group B received only conventional (pharmacological) therapy, that is, no cognitive intervention.
Reality orientation therapy was applied to the patients in group A in weekly individual sessions lasting 30 to 60 minutes. Each session consisted of continuous exposure of the individual to memory-and-orientation-related information based on several approaches.
First, to stimulate personal orientation, patients had to fill out a form with their personal information (name, date and place of birth, marital status, occupation, religion, address, and number of children and their respective names). The answers to the questions in the form were obtained by constructive examiner-led questioning.
The next step included temporal and spatial orientation. Participants were asked to fill out another form with information about the current weather and approximate time, day of the week, and date using the same process adopted in the first stage. They then had to walk around the room where the sessions took place and attempt to recognize and assimilate, among other features of the room, the layout of the furniture, the number of doors and windows, and in particular, 5 reference objects positioned in the room by the examiner.
The following stage was based on an intensive dialog in which patient and examiner discussed casual topics and personal interests, such as the latest news and important social events, for example, imminent meetings, birthdays, holidays, and other occasions that acted as references. Participants were always encouraged to express their own opinions and to participate actively in the conversations.
Finally, intense social engagement was stimulated to reestablish the patient’s social ties and habits prior to disease onset, always based on their personal situation and interests. Guidance to caregivers was also included in the routine so that patients could be constantly stimulated. The therapy was thus tailored to the patients’ personal characteristics and preferences, and the benefits extended beyond the weekly meetings.
Assessment of Outcomes
Both groups had cognitive assessments during the initial patient selection process and at bimonthly intervals after the beginning of ROT sessions for 6 months (25.7 weeks). The CERAD neuropsychological battery and CDT were administered following a previous study in which ROT was assessed. 6 The SCOPA-Cog, a more specific battery for PD, 18 was applied during the patient selection process and in the last session at the end of the 6-month assessment period as a control for the CERAD battery.
Statistics
All data were tested for normality with the Shapiro-Wilk test. Statistical differences between the means of the groups were determined using the 2-tailed Student t test for normal distributions and the Mann-Whitney test for non-normal distributions. For the differences between the expected and observed values, Fisher exact test was used. Results are given as mean (standard deviation). Differences were considered significant when P < .05. All the statistical analysis was performed with Statistic for Windows (ver. 99) and Microsoft Excel 2010.
Results
Of the 17 patients selected, 3 had to be excluded at the very beginning of the study because they had to discontinue treatment with acetylcholinesterase inhibitors as a result of behavioral or gastrointestinal adverse effects. During the assessment process, 2 other patients developed clinical and neurological complications and had to be admitted to hospital. As this could have led to a change in the normal evolution of the disease and prevented these individuals completing the cognitive assessments within the study period, both patients (1 in each group) were also excluded. Exclusion of these patients did not change the balance between the groups, and the study continued with 6 patients in each group (Table 1). Mean patient age was 72.08 (13.19) years, 6 patients were male and 6 female, that is, a ratio of 1:1, and mean age of onset of PD symptoms was 59.75 (13.19) years.
Table 1.
Clinical and Epidemiologic Characteristics of Patients With PD.
| Total, N = 12 | Group A (ROT + Drug Therapy), n = 6 | Group B (Drug Therapy), n = 6 | P a | |
|---|---|---|---|---|
| Variablesb | ||||
| Genderc | 12 (100%) | 6 (50%) | 6 (50%) | |
| Female | 5 (41.67%) | 3 (50%) | 2 (33.33%) | 1 |
| Male | 7 (58.33%) | 3 (50%) | 4 (66.67%) | |
| Age | 72.08 ± 13.53 | 77 ± 9.34 | 67.16 ± 17.15 | .122 |
| Age at onset of symptoms | 59.75 ± 13.19 | 64.34 ± 8.14 | 57.85 ± 16.50 | .123 |
| Disease duration | 12 ± 5.78 | 12.33 ± 7.06 | 11.66 ± 4.84 | .426 |
| Hoehn and Yahr | 2.41 ± 1.35 | 2.75 ± 1.83 | 2.41 ± 1.46 | .367 |
| UPDRS–III | 23.75 ± 13.93 | 23 ± 16.23 | 24.5 ± 14.17 | .434 |
| Schooling | 6.41 ± 3.52 | 7 ± 4.39 | 5.83 ± 2.19 | .303 |
| Drug therapyc | 12 (100%) | 6 (50%) | 6 (50%) | |
| Rivastigmine (6 mg/d bid or 10 cm2 patch) | 6 (50%) | 3 (50%) | 3 (50%) | 1 |
| Donepezil (10 mg) | 6 (50%) | 3 (50%) | 3 (50%) | |
| First assessment | ||||
| Scopa-Cog | 11 ± 6.09 | 9.83 ± 5.78 | 12.16 ± 6.17 | .275 |
| CERAD | 69.41 ± 17.95 | 65.83 ± 19.40 | 73 ± 15.56 | .341 |
| CDT | 4.08 ± 2.84 | 3.16 ± 3.06 | 5 ± 2.82 | .153 |
Abbreviations: CDT, Clock Drawing Test; CERAD, Consortium to Establish a Registry for Alzheimer’s Disease; PD, Parkinson’s disease; ROT, reality orientation therapy; UPDRS, Unified Parkinson Disease Rating Scale.
aStatistically significant differences between groups A and B.
bAge, schooling, disease duration, and onset of symptoms are given in years. Student t test or Mann-Whitney test.
cFisher exact test.
Weekly ROT activities and follow-up were completed without complications by all 12 patients. Follow-up lasted 6 months for all the patients. At the initial evaluation, there were no statistically significant cognitive differences in total scores between the groups with any of the 3 instruments (CERAD battery, SCOPA-Cog or CDT; Table 1), and there were no differences between the groups in the CERAD or SCOPA-Cog subtests.
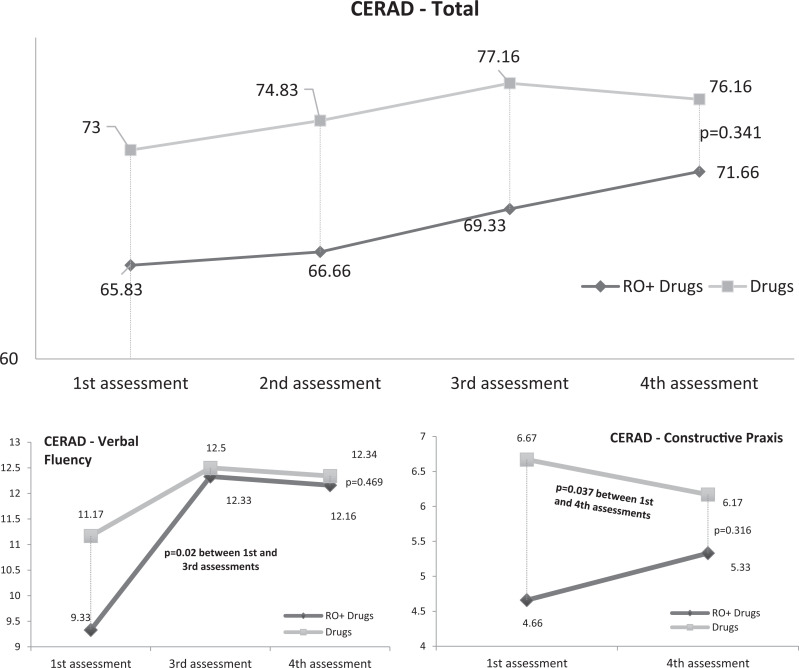
At the end of the study period, however, the results of some of the subtests indicated that ROT yielded cognitive benefits that complemented drug therapy, but no statistical differences were observed between the 2 groups (P = .341) in the CERAD battery. Although there were no statistically significant differences between the first and the second evaluations for patients in group A (P = .182) and those in group B (P = .145), when the CERAD subtests were evaluated separately, an improvement could be observed in verbal fluency in the patients in group A between the first and the third evaluations (P = .02). These patients maintained the same level of verbal fluency until the end of the study. When the results of constructional praxis recall were analyzed, a significant decline was observed in this function among patients in group B (P = .037), while patients in group A were stable (P = .222). Nevertheless, despite these positive results, there were no statistically significant differences when both groups were compared directly (Figure 1). There were no significant changes in the scores for the subtests that assess memory function apart from an improvement in verbal memory recall in the third and fourth evaluations in relation to the first evaluation (P = .018 and P = .046) in group B (medication only).
Figure 1.
Consortium to Establish a Registry for Alzheimer’s Disease (CERAD) results for both groups.
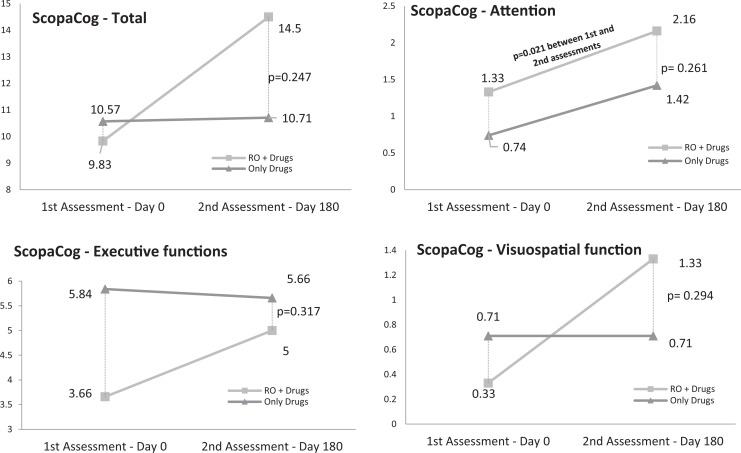
At the end of the evaluation period, there were no significant differences between the 2 groups (Figure 2) in SCOPA-Cog. Nevertheless, there was a trend toward an improvement in patients in group A and stabilization in group B, both overall and in memory and learning, attention, executive functions, and visuospatial function. The differences, however, were only statistically significant in tests related to attention in group A (P = .021; Figure 2).
Figure 2.
Scales for Outcomes in Parkinson’s Disease–Cognition (SCOPA-Cog) results for both groups.
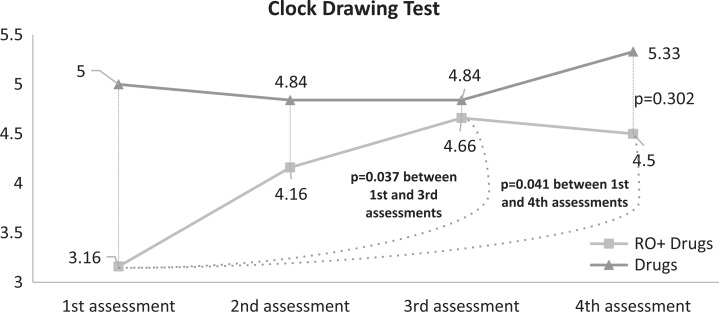
Statistically significant differences in the CDT were observed only between the first and third and third and fourth evaluations in group A (P = .037 and P = .041). There were no statistically significant differences between the 2 groups (Figure 3).
Figure 3.
Clock Drawing Test (CDT) results for both groups.
Discussion
This study has shown some improvements in cognitive functions in patients with PDD participating in weekly individual ROT sessions over 6 months. The sessions increased the efficacy of conventional pharmacological treatment, providing further evidence of the beneficial effects of cognitive stimulation therapy for patients with PD. The medications normally prescribed for these patients have been shown to have only limited benefit in the treatment of cognitive impairment. Hence, nonpharmacological interventions are of interest not only to improve cognition but also because most patients with advanced PD are already burdened with complex polypharmacy. 2 While our findings are promising and statistically significant, a study with a larger sample might have yielded more positive, consistent results. The main limitation of this study was the difficulty recruiting and maintaining patients with PDD who had been using acetylcholinesterase inhibitors and were prepared to receive one of the researchers in their homes for ROT sessions. Another limitation was that our sample consisted mainly of elderly patients with long disease durations and important motor complications and did not include younger patients with short disease durations, who may also present with cognitive changes. 1,3
A previous study by our group in which ROT was administered to 14 patients with AD suffered from the same limitations 6 but produced significant results and was the inspiration for the present study. Although an improvement in memory was expected in patients with AD receiving ROT, improvements were observed in constructional praxis recall, the Boston naming test, verbal fluency, and the CDT. These tests evaluate mainly planning, executive, and visuospatial functions, which are more affected in PDD than in AD. 1 –3,16 –19 The present study was, therefore, designed along similar lines to the earlier study, and the same evaluation tool, the CERAD neuropsychological battery, was used so that we could compare both studies and because we expected improvements in frontal cognitive functions in patients with PDD receiving ROT. While the results of previous studies validate the CERAD battery for cognitive assessment of patients with PD, 20,21 this instrument has limited sensitivity when used to assess cognitive functions of the prefrontal cortex. We, therefore, chose to use the CDT and include SCOPA-Cog in the final and initial assessments.
Despite the few statistically significant positive outcomes for the combined therapy group, an improvement was observed, especially when the results were compared with those for group B, for which the results stabilized or worsened. This improvement was more visible when SCOPA-Cog scores were analyzed. As expected, because there are more items related to frontal functions in SCOPA-Cog than in CERAD, 16 –19 the results for SCOPA-Cog were significantly different for patients receiving ROT, particularly in subtests related to attention and executive and visuospatial functions. In the CERAD battery, the verbal fluency subtest showed an improvement for patients receiving ROT, but there were no statistically significant differences in the results for constructional praxis recall between the groups. These CERAD results confirm the SCOPA-Cog findings that ROT is more effective for cognitive impairment resulting from frontostriatal dysfunction. Patients receiving ROT also had better scores in the CDT, which can be used to assess working memory, executive functions, and visuospatial function. 18
Although previous studies have shown that acetylcholinesterase inhibitors act on cholinergic pathways, 5 PDD is mainly associated with dopamine loss in the prefrontal cortex due to a decrease in the supply of this neurotransmitter from the pathways that originate in the pars compacta of the substantia nigra and in the ventral tegmental area. The appearance of Lewy bodies, which are normally observed in the advanced stages of PD, contributes to the development of severe PDD. 22 There has been shown to be an association between dopaminergic hypofunction in the pathways involving the dorsal caudate nucleus and orbitofrontal cortex (including connections with the amygdala), the same circuits that have been associated with the early stages of PD, when PDD is less prevalent. 23 Imaging studies have shown that as the disease progresses, hypofunction in the circuit involving the anterior cingulate cortex and nucleus accumbens (including connections with the amygdala) occurs in patients with PDD, followed by changes in the ventral caudate nucleus and orbitofrontal cortex. 22 –25 In the advanced stages of PD, dopamine depletion occurs in the corpus striatum (putamen, globus pallidus, and caudate nucleus) and frontal cortex, increasing the prevalence and the rate of progression of dementia and apathy as Lewy bodies develop. 22,25 Cholinergic drug therapy, together with possible dopaminergic activation by ROT, is a possible explanation for the better outcome in the group that received the combined therapy.
Previous studies have also suggested the importance of cognitive therapies in PD and have yielded results similar to those reported here. 2 A meta-analysis by Leung et al 2 of 7 studies of cognitive therapies indicated cognitive improvements (g = 0.23, 95% confidence interval [CI]: .014-0.44, P = .037) in patients receiving this type of treatment. Improvements were observed in executive functions (g = 0.30, 95% CI: 0.01-0.58, P = .042), processing speed (g = 0.31, 95% CI: 0.01-0.61, P = .04), and primarily, working memory (g = 0.74, 95% CI: 0.32-1.17, P = .001). However, as in our study, their results were not statistically significant (g = 0.13, 95% CI: −0.29 to 0.55, P = .55). Monticone et al 26 showed that a full multidisciplinary approach with motor, transfer, balance, gait, and cognitive training may result in motor improvements and improvements in quality of life, activities of daily living, and cognition in patients with PD. A rehabilitation program of this kind provided by the Italian national health-care system costs approximately €20 000 and is therefore reserved for individuals with mild to moderate disability. The high cost of the therapy may prevent its use in other countries, such as the United States, where inpatient rehabilitation is not usually considered for patients with PD unless an acute event has occurred. 26
A simpler and less expensive cognitive rehabilitation program than that provided by the Italian health-care system, ROT is easy to apply, has good replicability, 6,11 and can be used to train caregivers so that they can continue the treatment. Continuing ROT with the same researcher who administered the weekly sessions can also provide continuity and methodological uniformity, as well as patient fidelity, as observed here.
In conclusion, our findings suggest that ROT can be an effective and low-cost complementary intervention in PDD that helps to improve cognitive functions and complements the traditional pharmacological approach. The combination of drug therapy and ROT may result in an improvement in cognitive functions, mainly by acting in areas responsible for attention and executive functions, whereas the use of medications alone tends to offer only stabilization and may even allow the dementia signs to worsen. Further studies assessing more precisely the benefits of ROT and similar cognitive approaches are required to confirm the findings of this study.
Footnotes
The authors declared no potential conflicts of interest with respect to the research, authorship, and/or publication of this article.
Funding: The authors received no financial support for the research, authorship, and/or publication of this article.
ORCID iD: Carlos Henrique Ferreira Camargo  http://orcid.org/0000-0002-3533-0347
http://orcid.org/0000-0002-3533-0347
References
- 1. Goldman JG, Litvan I. Mild cognitive impairment in Parkinson’s disease. Minerva Med. 2011;102(6):441–459. [PMC free article] [PubMed] [Google Scholar]
- 2. Leung IHK, Walton CC, Hallock H, Lewis SJG, Valenzuela M, Lampit A. Cognitive training in Parkinson disease: a systematic review and meta-analysis. Neurology. 2015;85(21):1843–1851. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 3. Salat D, Noyce AJ, Schrag A, Tolosa E. Challenges of modifying disease progression in prediagnostic Parkinson’s disease. Lancet Neurol. 2016;15(6):1–12. doi:10.1016/S1474-4422(16)00060-0. [DOI] [PubMed] [Google Scholar]
- 4. Poewe W. Non-motor symptoms in Parkinson’s disease. Eur J Neurol. 2008;15(Suppl 1):14–20. doi:10.1111/j.1468-1331.2008.02056.x. [DOI] [PubMed] [Google Scholar]
- 5. Almaraz AC, Driver-Dunckley ED, Woodruff BK, et al. Efficacy of rivastigmine for cognitive symptoms in Parkinson disease with dementia. Neurologist. 2009;15(4):234–237. [DOI] [PubMed] [Google Scholar]
- 6. Camargo CH, Justus FF, Retzlaff G. The effectiveness of reality orientation in the treatment of Alzheimer’s disease. Am J Alzheimers Dis Other Demen. 2015;30(5):527–532. doi:10.1177/1533317514568004. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 7. Taulbee LR, Folsom JC. Reality orientation for geriatric patients. Hosp Community Psychiatry. 1966;17(5):133–135. [DOI] [PubMed] [Google Scholar]
- 8. Choi J, Twamley EW. Cognitive rehabilitation therapies for Alzheimer’s disease: a review of methods to improve treatment engagement and self-efficacy. Neuropsychol Rev. 2013;23(1):48–62. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 9. Aguirre E, Woods RT, Spector A, Orrell M. Cognitive stimulation for dementia: a systematic review of the evidence of effectiveness from randomized controlled trials. Ageing Res Rev. 2013;12(1):253–262. [DOI] [PubMed] [Google Scholar]
- 10. Spector A, Orrell M, Woods B. Cognitive stimulation therapy (CST): effects on different areas of cognitive function for people with dementia. Int J Geriatr Psychiatry. 2010;25(12):1253–1258. [DOI] [PubMed] [Google Scholar]
- 11. Spector A, Davies S, Woods B, Orrell M. Reality orientation for dementia: a systematic review of the evidence of effectiveness from randomized controlled trials. Gerontologist. 2000;40(2):206–212. [DOI] [PubMed] [Google Scholar]
- 12. Daniel SE, Lees AJ. Parkinson’s Disease Society Brain Bank, London: overview and research. J Neural Transm Supp. 1993;39:165–172. [PubMed] [Google Scholar]
- 13. Emre M, Aarsland D, Brown R, et al. Clinical diagnostic criteria for dementia associated with Parkinson’s disease. Mov Disord. 2007;22(12):1689–1707. [DOI] [PubMed] [Google Scholar]
- 14. Hoehn MM, Yahr MD. Parkinsonism: onset, progression and mortality. Neurology. 1967;17(5):427–442. [DOI] [PubMed] [Google Scholar]
- 15. Goetz CG, Tilley BC, Shaftman SR, et al. ; Movement disorder society UPDRS revision task force. Movement Disorder Society-sponsored revision of the Unified Parkinson’s Disease Rating Scale (MDS-UPDRS): scale presentation and clinimetric testing results. Mov Disord. 2008;23(15):2129–2170. [DOI] [PubMed] [Google Scholar]
- 16. Bertolucci PH, Okamoto IH, Brucki SM, et al. Applicability of the CERAD neuropsychological battery to Brazilian elderly. Arq Neuro-Psiquiatr. 2001;59(3A):532–536. doi:org/10.1590/S0004-282X2001000400009. [DOI] [PubMed] [Google Scholar]
- 17. Verbaan D, Jeukens-Visser M, Van Laar T, et al. SCOPA-cognition cutoff value for detection of Parkinson’s disease dementia. Mov Disord. 2011;26(10):1881–1886. [DOI] [PubMed] [Google Scholar]
- 18. Sunderland T, Hill JL, Mellow AM, et al. Clock drawing in Alzheimer’s disease: a novel measure of dementia severity. J Am Geriatr Soc. 1989;37(8):725–729. [DOI] [PubMed] [Google Scholar]
- 19. Rossetti HC, Cullum CM, Hynan LS, Lacritz LH. The CERAD neuropsychologic battery total score and the progression of Alzheimer disease. Alzheimer Dis Assoc Disord. 2010;4(2):138–142. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 20. Tedrus GM, Fonseca LC, Letro GH, Bossoni AS, Samara AB. Dementia and mild cognitive impairment in patients with Parkinson’s disease. Arq Neuropsiquiatr. 2009;67(2B):423–427. [DOI] [PubMed] [Google Scholar]
- 21. Karrasch M, Laatu S, Martikainen K, Marttila R. CERAD test performance and cognitive impairment in Parkinson’s disease. Acta Neurol Scand. 2013;128(6):409–413. [DOI] [PubMed] [Google Scholar]
- 22. De la Fuente-Fernández R. Frontostriatal cognitive staging in Parkinson’s disease. Parkinsons Dis. 2012;2012:561046. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 23. Remy P, Doder M, Lees A, Turjanski N, Brooks D. Depression in Parkinson’s disease: loss of dopamine and noradrenaline innervation in the limbic system. Brain. 2005;128(6):1314–1322. [DOI] [PubMed] [Google Scholar]
- 24. Reijnders JS, Scholtissen B, Weber WE, Aalten P, Verhey FR, Leentjens AF. Neuroanatomical correlates of apathy in Parkinson’s disease: a magnetic resonance imaging study using voxel-based morphometry. Mov Disord. 2010;25(14):2318–2325. [DOI] [PubMed] [Google Scholar]
- 25. Monchi O, Petrides M, Mejia-Constain B, Strafella AP. Cortical activity in Parkinson’s disease during executive processing depends on striatal involvement. Brain. 2007;130(1):233–244. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 26. Monticone M, Ambrosini E, Laurini A, Rocca B, Foti C. In-patient multidisciplinary rehabilitation for Parkinson’s disease: a randomized controlled trial. Mov Disord. 2015;30(8):1050–1058. [DOI] [PubMed] [Google Scholar]