Abstract
Introduction:
The effects of mental, physical, and combination of these two trainings were investigated on cognitive performance, serum level of brain derived neurotrophic factor (BDNF), and irisin in women diagnosed with mild cognitive impairment (MCI).
Material and Methods:
Forty-four participants were randomized into 4 groups: physical training (PH; 8 weeks’ aerobic training, n = 11), mental training (ME; special computer gaming, n = 11), combined (PH + ME; n = 13), and control group (CO; n = 9).
Results:
Analysis of variance with Tukey post hoc test revealed a significant increase in working memory (P = .012) and BDNF (P = 0.024) in the ME compared with the CO group. Also the ME group in comparison with the PH group demonstrated better working memory (P = .014) and processing speed (P = .024).
Conclusion:
Positive effect of mental training on the cognitive parameters, parallel with BDNF elevation, suggests that mental training is a more useful, safe, and persistent strategy to attenuate the progression of MCI probably via BDNF elevation, but the effect size is relatively small elevation.
Keywords: cognitive performance, computer Stroop task, MCI, Alzheimer, BDNF, irisin
Introduction
Alzheimer’s disease (AD) is a major medical concern with significant social and economic costs. 1 To find appropriate interventions to prevent cognitive decline in predementia stages has great importance for health-care organizations.
Mild cognitive impairment (MCI) is a pre-AD condition state in which cognitive decline is greater than the level expected for an individual’s normal age and education level, with no notable interference in daily life. 2,3 Based on the epidemiological studies, there is a rate of progression of almost 10% to 15% from MCI to AD. 4
Current pharmacological interventions have limited effects of improving cognition, despite different side effects which have been reported in the literatures. 5,6 Therefore, finding nonpharmacological preventive solutions for progression of MCI to AD has great importance. Physical activity has been known to exert beneficial effects on cognitive functions. 7 -9 However, some studies reported contradictory results. 10,11 Moreover, elderly people usually have less motivation for physical activities due to aging problems. Therefore, finding new strategies with combination of low-intensive physical activities with different mental interventions might be logical approach to boost brain activity. 12 Mental activity has the potential to increase motivation of individuals with MCI who are not physically active 13 and has been known to improve working memory and verbal episodic memory. 14,15
Although promising, there are a few published data on whether combination of physical and mental training is reliably associated with enhanced cognitive performance with contradictory results. 16 -19
On the other hand, cellular mechanisms underlying mental and physical training effects on cognitive performance have not been understood well. Some studies believe that neurotrophic factors, especially brain derived neurotrophic factor (BDNF), elevate after neural and physical activities. 14,20 The BDNF is a protein expressed in the central nervous system and plays pivotal role in neural survival, 19 synaptic plasticity, and neurogenesis 21 in an activity-dependent manner. 22 The BDNF has the potency to be elevated in elderly people after both mental 14,23 and physical activities. 7,8,24
The cellular and molecular mechanisms of BDNF elevation after mental and physical challenging have not been understood yet. One of the latest explanations is the induction of a special protein “irisin” in skeletal muscles during physical exercise, which consequently induces BDNF release from neurons. 25 However, there are studies which seriously criticize the methodological assays measuring circulating irisin in human. 26,27 For example, Albrecht et al in their recent study reported that evidence for irisin circulating in blood is largely based on commercial enzyme linked immunosorbent assay (ELISA) kits which are based on polyclonal antibodies against C terminus of irisin and have not been tested for cross-reacting serum proteins. 27 In addition, there are contradictory findings in literatures regarding exercise-induced irisin elevation. Bostrom et al showed an increase in plasma irisin only after 10 weeks of training in humans, 28 but Timmons et al and Norheim et al expressed doubts about the response of fibronectin type III domain-containing protein 5 (FNDC5) messenger RNA in human muscle to exercise. 29,30
Here, we hypothesized that exercise-induced improvement in cognitive function and BDNF elevation in our previous studies 24,31 and also others 8,9 might not be mediated only by irisin signaling.
The goal of this study was to evaluate the effect of combination of physical and mental training on cognitive performance, serum BDNF, and irisin levels in patients with MCI.
Methods
Participants
At the start of the experiment, 101 elderly women from Yas adult center (nonresidential female adult daycare center, Aliabad-e Katul, Golestan Province, Iran) received information about procedures and signed consent voluntarily. The center that we had access to only, was taking care of old single women. Also pointing to the fact that gender difference influences on brain functions, the present experiment was limited at first step on female individuals and then on male ones at future studies.
Then the participants were matched according to education level and general medical examinations results. All participants were examined for a series of neurocognitive and psychological tasks considering the following inclusion criteria: (1) ages 60 to 85 years, (2) ability to read and write, (3) Global Deterioration Scale–Short Form (GDS-SF) level 0 to 5, 32,33 (4) normal visual and auditorial system, (5) no muscular disorders, (6) no medications for depression and AD, and (7) no history of regular exercise over the past 2 years.
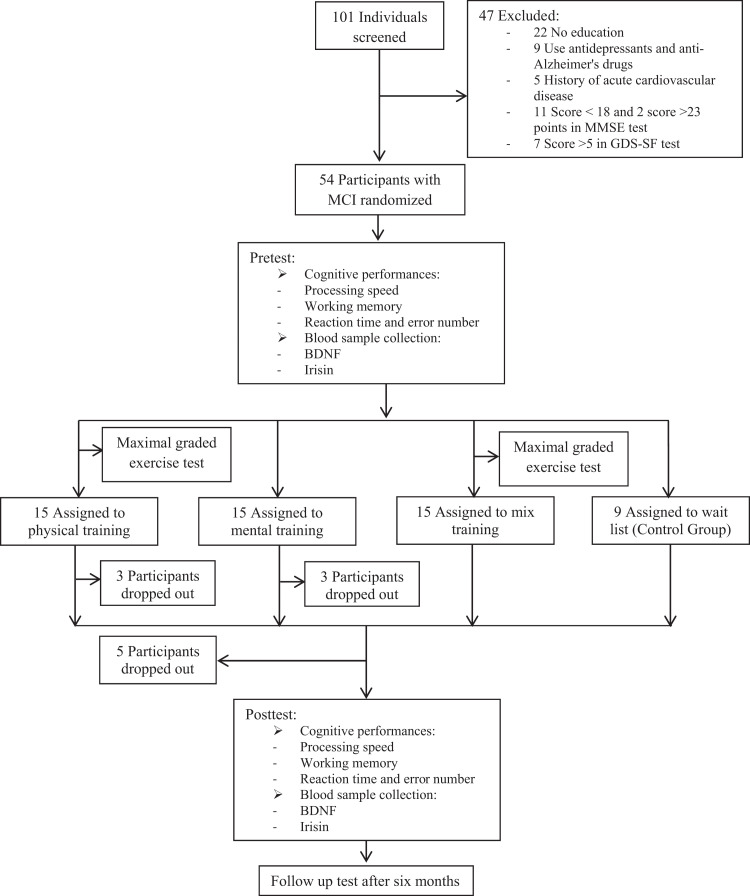
Finally, a total of 54 sedentary old women were randomized into 4 groups: physical training (PH; n = 15), mental training (ME; n = 15), PH + ME (n = 15), and control group (CO; n = 9), and 44 completed the protocol accurately (Figure 1) till the end of the experiment and 10 left the study.
Figure 1.
Subject flow diagram from the initial contact through the study completion.
Sample size was based on a power analysis with effect sizes from 2 cognitive studies. 34,35 Effect sizes between d = 0.26 and 0.7 were measured for these interventions, and authors assumed 80% power to detect a difference using one-way analysis of variance (ANOVA) at α = .05.
Study was conducted in accordance with The Code of Ethics of the World Medical Association (Declaration of Helsinki) and approved by the ethics and research committee of the Guilan University of Medical Sciences (code: IR.GUMS.REC.1394.447).
In order to diagnose MCI, Mini-Mental State Examination (MMSE), with extensive usages in both clinical practice and research, was used here. 2,36,37 The MMSE included a 30-point validated questionnaire pointing to arithmetic, memory, and orientation of samples, which was done under supervision of a neurologist in 10 minutes. Any score greater than or equal to 24 points (out of 30) indicates a normal cognition. Between the scores of 18-23, indicate mild, and 0-18 sever cognitive impairment.
The GDS-SF, a 15-item self-report assessment, was also used to identify depression. All questions were answered “yes” or “no.” Any score greater than 5 suggested depression. 32
Cognitive Assessments
Cognitive assessments included 4 tests: (1) working memory (composite score of the computer digit span forward subtests of the Wechsler Adult Intelligence Scale-Third Edition) [WAIS-III]), (2) processing speed (composite score of the computer digit-symbol coding subtest of the WAIS-III), (3) reaction time, and (4) error number of the computer modified Stroop color-word test. 38
Forward Digit Span test measures the capacity to hold numbers in working memory and the ability to work with them. More specifically, the repetition of the digits demands concurrent mental operations such as attention, allocation of multiple mental resources operations, and active control of conscious attention. 39 Participants were presented with a series of digits on computer monitor (eg, “8, 2, 4”) and their task was to repeat them immediately. Each digit was displayed 1 second with interval of 0.5 second. 24
Correct performers received a longer list of digits (eg, “9, 2, 4, 1”). The length of the list a person could remember was that person’s digit span.
Digit Symbol Coding test assesses key neurocognitive functions that underlie many substitution tasks, including attention, visual scanning, and motor speed. The brevity and ease of administration, and its unambiguous scoring, contribute to its widespread use. 40 It consists of (eg, 9) digit symbol pairs (eg, 1/-, 2/⊥…7/Λ, 8/X, 9/=) followed by a list of digits. Under each digit the participant should write down the related symbol as fast as possible in 90 seconds cut off. 41
Stroop test assesses cognitive processes presenting color words (eg, RED) displayed in either congruent (eg, red) or incongruent (eg, green) colors and control trials in which only 1 dimension of the stimulus is present (ie, presenting all words in black colors or presenting color patches but without words). 42 In the present study, we used modified versions of the simple Stroop test product (Sina behavioral and cognitive science) to measure error number and reaction time.
All cognitive measurements were carried out 2 days before and after the related interventions. Also, cognitive tests were repeated 6 months after the last training for testing the maintenance of behavior.
Sample Collection
Serum BDNF and irisin were measured before and after the ending of experiments (2 times). Blood samples were collected by Vacutest Kima (Arzergrande, Italy), after 10-hour fasting between 8 am and 10 am to minimize possible effects of circadian rhythms. The diameter of needle was 22 G. Briefly, blood was collected into EDTA tubes, then centrifuged for 20 minutes at 3000 rpm by Behdad Class 1/Type B Refrigerated Centrifuge (Tehran, Iran), and finally serums were placed in freezer (Nüve DF490, Ankara, Turkey) at −80°C until further analysis. The BDNF level was measured using a commercial ELISA kit (R&D, Minneapolis, USA, cat#: DBD00, with a detection range from 7.8 to 500 pg/mL and intra- and interassay variations of ±4.66% and ±9%). Irisin was measured using a commercial ELISA kit (ZellBio GmbH, Ulm, Germany, cat#: ZB-3253-H9648, with a detection range from 0.05 to 15 µg/mL, intra- and interassay variations of <10% and <12%).
Mental training
Participants from the ME group started exercising with a special program entitled “Modified My Better Mind” under the supervision of physiotherapist for 30 minutes on the first day and then was doubled at the seventh and eighth weeks. The program consisted of 4 different games: (1) Photo flaw: The task was to find small differences between a pair of identical pictures. This test engages visual attention, visual working memory, and speed of processing. (2) Headline Clues focused on anecdotal knowledge, engaging verbal memory, and reasoning, in a way that participants had to solve proverbs with missing words and letters. (3) Sokoban game that exercises strategic planning and utilizes both spatial executive processing and visual-spatial skills. The task was to move lenses through a maze onto targets in the least of moves. (4) Keep It In Mind, is a game for assessing working memory (both visuospatial and verbal). 43 Participants looked and memorized lists of faces and objects and then they had to recognize the correct items as fast as possible.
Physical training
The physical training consisted of 2 sections: 5-minute warm-up followed by 6-minute walking with 55% heart rate reserve in the first session, incrementally reaching to 20 minutes with 75% heart rate reserve at the eighth week; and muscular strength, range of movement (MSROM) Silver Sneakers. The participants were being monitored throughout the session by a heart rate meter to guarantee the proper intervention zone. Heart rate reserve was calculated using the Karvonen method 44 based on the resting and maximum heart rates achieved during the baseline maximal graded exercise test. 45
The MSROM Silver Sneakers section also involved a complex of body movements in a group fitness setting, following a similar 5-minute stretching protocol. Equipment used here consisted of chair, ball, 2 to 3 lb hand weights, and a resistance band with handles. Participants should mimic the motions of an instructor and complete muscular range of motion exercises coupled with light cardio that produces rating of perceived exertion within the desired 13 to 15 range for 25 minutes. 46
Combined training
Participants of this group participated in both physical and mental training for 8 weeks same as two other groups, performing the cognitive training prior to the exercise training. Duration of mental activity was 30 minutes/first week and reached to 60 minutes/seventh week. Total duration of physical activity was the same as mental activity 3 d/wk for 8 weeks. Participants of the third group with an interval of 45 minutes between physical and mental activities completed their tasks same as the first and second groups.
Statistical Analyses
Statistical analysis was performed using the SPSS 23 software packages. The differences between variables were compared using paired t test and also ANOVA followed by Tukey post hoc test. Data were presented as means (standard deviation) with significance set at P < .05. Association of BDNF, irisin levels, and improvement of cognitive factors was calculated with Pearson correlation coefficient (P < .05).
Results
Changes in Cognitive Performances
Descriptive characteristics of participants has been shown in Table 1. Table 2 shows the statistics of the neurological variables before and after the interventions. One-way ANOVA indicated no significant difference in the pretest between groups in terms of working memory (F 3, 40 = 0.658, P = .583), processing speed (F 3, 40 = 0.816, P = .493), reaction time (F 3, 40 = 2.217, P = .089), and error number (F 3, 40 = 0.97, P = .446; Table 2).
Table 1.
Descriptive characteristics of participants.
| Characteristics | PH (n = 11) | ME (n = 11) | PH + ME (n = 13) | CO (n = 9) |
|---|---|---|---|---|
| Age (years) | 68.81 (3.68) | 67.90 (3.75) | 67.76 (4.69) | 69.11 (4.93) |
| Education (years) | 3.45 (1.03) | 3.54 (1.29) | 2.76 (0.92) | 3.22 (1.20) |
| BMI (kg/m2) | 26.46 (3.18) | 28.40 (6.35) | 31.16 (4.92) | 27.68 (4.50) |
| WHR (%) | 90.00 (5.60) | 80.54 (9.07) | 87.92 (5.26) | 79.00 (4.60) |
| RHR (bpm) | 68.36 (6.05) | 70.54 (8.81) | 70.46 (6.00) | 96.77 (7.51) |
| HRmax (bpm) | 126.81 (10.10) | - | 127.53 (9.17) | - |
| MAP (mm Hg) | 98.88 (6.28) | 99.64 (7.37) | 101.97 (10.91) | 101.31 (7.36) |
| MMSE | 23.18 (2.18) | 23.81 (2.04) | 23.30 (1.84) | 23.44 (2.06) |
| GDS-SF | 1.63 (1.02) | 1.72 (1.42) | 1.46 (1.05) | 2.00 (1.58) |
Abbreviations: BMI, body mass index; CO, control group; GDS-SF, Global Deterioration Scale–Short Form; MAP, mean arterial pressure; ME, mental training; MMSE, Mini-Mental State Examination; PH, physical training; RHR, resting heart rate; WHR, waist hip ratio.
Table 2.
Cognitive parameters, serum level of BDNF and irisin before and after eight weeks.
| Characteristics | PH (n = 11) | ME (n = 11) | PH + ME (n = 13) | CO (n = 9) | ||||
|---|---|---|---|---|---|---|---|---|
| Pre | Post | Pre | Post | Pre | Post | Pre | Post | |
| WM (N) | 7.90 (2.34) | 7.81 (2.08) | 7.90 (2.47) | 9.45 (2.65)a,b,c | 7.30 (2.01) | 8.37 (2.93)a | 6.66 (2.44) | 6.44 (2.24) |
| PS (cn/90s) | 16.45 (5.57) | 16.32 (2.25) | 17.45 (5.41) | 19.45 (7.20)a,b,c | 14.30 (4.19) | 15.23 (4.71) | 15.77 (5.40) | 15.66 (4.64) |
| RT (ms) | 2911.81 (282.50) | 2715.81 (445.16) | 2519.63 (306.52) | 2229.81 (312.96)a | 2489.92 (527.58) | 2386.38 (444.94) | 3115.33 (393.21) | 2831.44 (306.30) |
| EN (N) | 7.63 (1.96) | 7.45 (2.25) | 8.27 (1.67) | 6.54 (1.29)a,c | 7.00 (1.94) | 5.84 (1.67) | 7.66 (2.00) | 7.88 (1.67) |
| Irisin (ng/ml) | 11.23 (2.77) | 11.47 (3.08) | 10.57 (1.99) | 9.92 (1.66) | 10.38 (1.02) | 11.33 (2.04)a,c,d | 13.67 (5.23) | 12.87 (4.95) |
| BDNF (pg/mL) | 1167.46 (473.91) | 1122.41 (542.66) | 1328.45 (282.10) | 1678.72 (356.56)a,c | 1326.84 (278.34) | 1516.76 (307.67)a | 1507.10 (427.46) | 1317.40 (506.83) |
Abbreviations: BDNF, brain derived neurotrophic factor; CO, control group; EN, error number; ME, mental training; PH, physical training; PS, processing speed; RT, reaction time; WM, working memory.
aSignificant differences between the pretest and posttest values in ME group (P = .004) and ME+PH (P = .020), between the b ME and CO groups (P = .012), c ME and PH groups (P = .014) in score of working memory.
a Significant differences between the pretest and posttest values in the ME group (P = .011), between the b ME and CO groups (P = .024), c ME and PH groups (P = .012) in processing speed. a Significant differences between the pretest and posttest values in the ME group (P = .001) in reaction time. a Significant differences between the pretest and posttest values in the ME group (P = .002), between the c ME and CO groups (P = .019) in error number.
a Significant differences between the pretest and posttest values in the ME+PH group (P = .020), between the c ME+PH and ME groups (P = .027), d ME+PH and CO groups (P = .021) in irisin levels. a Significant differences between the pretest and posttest values in the ME group (P = .013) and ME+PH group (P = .008), between the c ME and CO groups (P = .024) in BDNF (Brain Derived Neurotrophic Factor) levels.
Paired samples t test revealed a significant increase in working memory from pretest to posttraining in ME, t(10) = −3.74, P = .004, and PH + ME, t(12) = −2.69, P = .02 (Figure 2). One-way ANOVA revealed significant differences between groups in working memory (F 3, 40 = 5.55, P =.003). Changes in working memory was significantly higher in ME (21.64%) compared with PH (−0.20%, P = .014) and CO (−1.16%, P = .012; Table 2, Figure 2).
Figure 2.
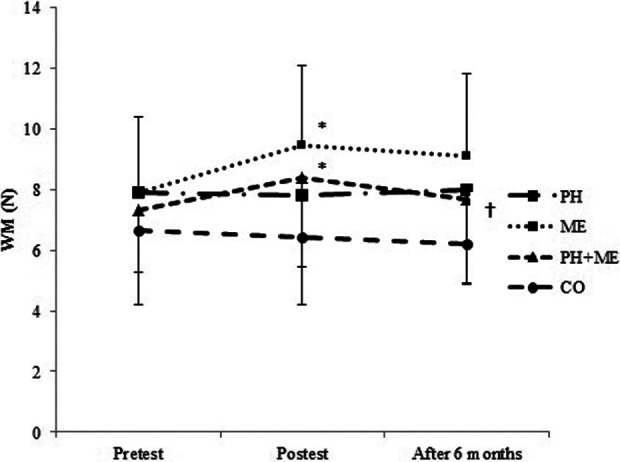
Mean and standard deviation of WM in pretest, posttest, and after 6 months. *P = .004: Significant differences between the pretest and posttest values in ME group.*P = .020: Significant differences between the pretest and posttest values in PH + ME group. †P = .044: Significant differences between the posttest and after 6 months values in the PH + ME group. The level of significant was set at 5% (P < .05). ME indicates mental training; N, number; PH, physical training; WM, working memory.
Similarly, significant improvements in the posttraining scores of processing speed, (t(10) = −3.13, P = .011, and reaction time, t(10) = 4.45, P = .001, were observed only in the ME group compared with pretest values (Table 2, Figures 3 and 4). One-way ANOVA showed significant differences between groups in processing speed (F 3, 40 = 4.47, P = .008), while the difference between groups in reaction time was not statistically significant (F 3, 40 = 2.38, P = .084; Table 2). Tukey post hoc test revealed that changes in processing speed was significantly higher in ME (10.24%) compared with PH (−0.42%, P = .012) and CO (−0.65%, P = .024; Table 2).
Figure 3.
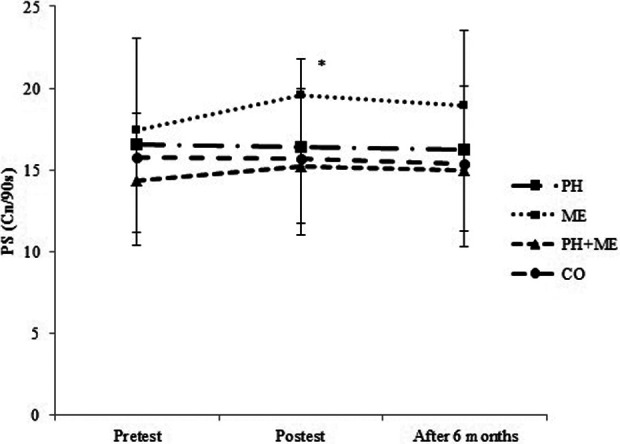
Mean and standard deviation of PS in pretest, posttest, and after 6 months. *P = .011: Significant differences between the pretest and posttest values in the ME group. The level of significant was set at 5% (P < .05). Cn/90s indicates correct number in 90 seconds; ME, mental training; PS, processing speed.
Figure 4.
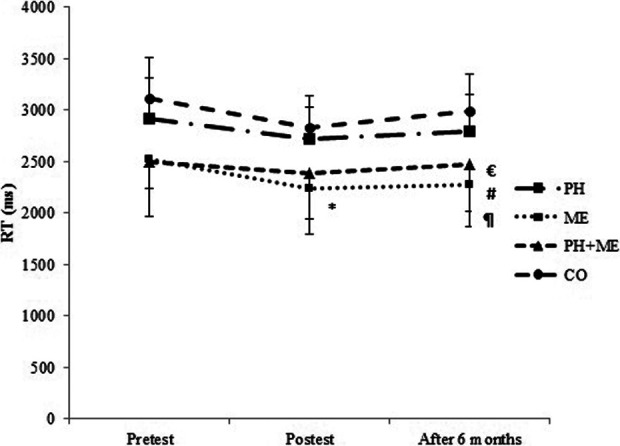
Mean and standard deviation of RT in pretest, posttest, and after 6 months. *P = .001: Significant differences between the pretest and posttest values in the ME group. #P = .006: Significant differences between the ME and PH groups; ¶P = .001: Significant differences between the ME and CO groups; €P = .008: Significant differences between the PH + ME and CO groups after 6 months. The level of significant was set at 5% (P < .05). CO indicates control group; ME, mental training; PH, physical training; RT, reaction time.
Posttraining score of error number in ME was significantly decreased compared with baseline, t(10) = 4.03, P = .002 (Table 2, Figure 5). Also, significant difference between groups changes was found in the score of error number by one-way ANOVA (F 3, 40 = 4.16, P = .012), indicating higher improvement in ME (19.36%) compared with CO (−6.86%, P = .019).
Figure 5.
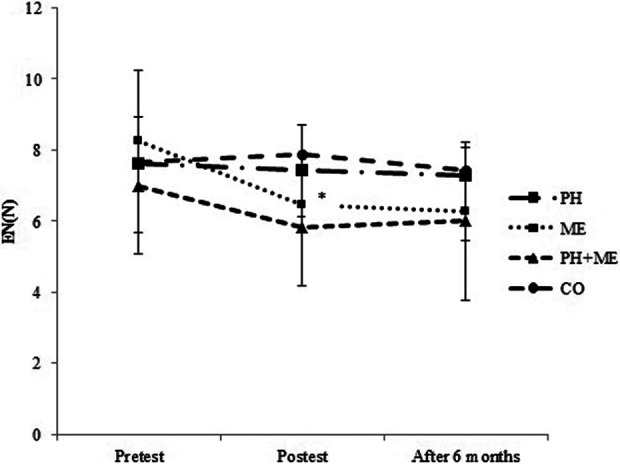
Mean and standard deviation of EN in pretest, posttest, and after 6 months. *P = .002: Significant differences between the pretest and posttest values in ME group. The level of significant was set at 5% (P < .05). EN indicates error number; ME, mental training; N, number.
Improvements in working memory, t(10) = 1.78, P = .104 (Figure 2), processing speed, t(10) = 2.05, P = .067 (Figure 3), reaction time, t(10) = −0.59, P = .576 (Figure 4), and error number, t(10) = −0.896, P = .391 (Figure 5) were preserved after 6 months of detraining in ME. Working memory, which was the only improved index in PH + ME, decreased significantly after 6 months of detraining compared with posttraining, t(12) = 1.83, P = .046 (Figure 2).
After 6 months, one-way ANOVA revealed no significant difference between groups changes in working memory (F 3, 40 = 2.50, P = .073; Figure 2), processing speed (F 3, 40 = 1.18, P = .329; Figure 3), and error number (F 3, 40 = 1.24, P = .307; Figure 5), while there was a significant difference between groups changes in reaction time (F 3, 40 = 8.56, P = .001; Figure 4). Tukey post hoc test revealed that changes in reaction time were significantly lower in ME compared with PH (P = .006) and CO (P = 0.001; Figure 4).
Changes in BDNF and Irisin
The BDNF increased significantly in ME, t(10) = −2.99, P = .013, and PH + ME, t(12) = −3.17, P = .008, 1 − β = .829; however, irisin elevated significantly only in PH + ME, t(12) = −2.29, P = .040, compared with baseline. The PH showed insignificant reduction in BDNF, t(10) = 0.266, P = .795 (Table 2, Figures 6 and 7).
Figure 6.
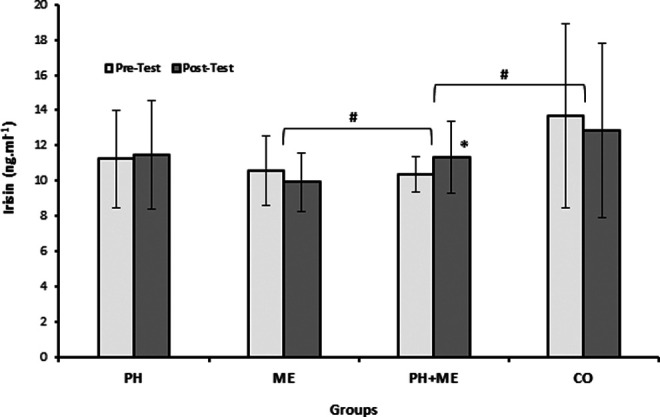
Pretest and posttest levels of irisin in the groups. *Significant difference compared to pretest (P < .05). #Significant differences between groups in irisin changes (P < .05).
Figure 7.
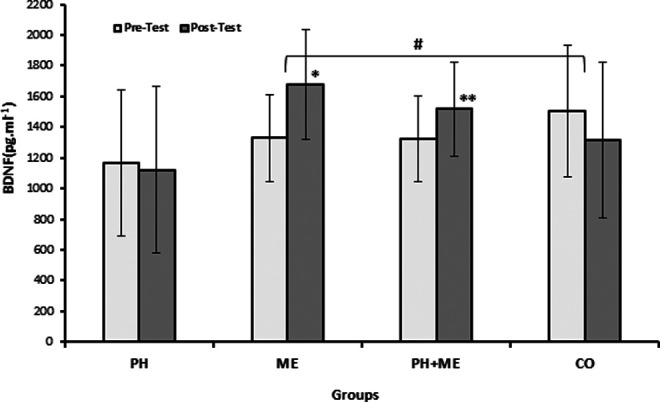
Pretest and posttest levels of brain derived neurotrophic factor (BDNF) in the groups. *Significant difference compared to pretest (P < .05). **Significant difference compared to pretest (P < .01). #Significant differences between groups in BDNF (P < .05).
Significant differences were found between groups changes in BDNF (F 3, 40 = 3.667, P = .020; Table 2, Figure 7) and irisin (F 3, 40 = 4.288, P = .01; Table 2, Figure 6) by one-way ANOVA. Tukey post hoc test revealed significantly higher BDNF changes in ME (26.36%, P = .024) and irisin changes in PH + ME (14.31%, P = .021) compared with CO (−12.58%; Table 2, Figures 6 and 7).
The BDNF level was positively correlated only with processing speed in ME (r = .697, P = .037; Table 3). No significant correlation was found between BDNF and irisin in ME (P = .284), PH (P = .067), PH + ME (P = .80), or CO (P = .788; Table 3).
Table 3.
Association between changes of selected parameters measured.
| PH | ME | PH + ME | CO | |||||
|---|---|---|---|---|---|---|---|---|
| r | P | r | P | r | P | r | P | |
| Change of working memory score | ||||||||
| Change of irisin | −.201 | .605 | .239 | .536 | −.121 | .723 | −.453 | .307 |
| Change of BDNF | −.102 | .795 | .426 | .253 | −.087 | .800 | −.476 | .280 |
| Change of processing speed score | ||||||||
| Change of irisin | −.059 | .879 | −.085 | .827 | .272 | .419 | −.497 | .256 |
| Change of BDNF | −.077 | .844 | .697a | .037a | −.400 | .223 | −.148 | .751 |
| Change of reaction time score | ||||||||
| Change of irisin | −.098 | .803 | −.197 | .612 | −.256 | .448 | −.036 | .939 |
| Change of BDNF | −.036 | .927 | .355 | .284 | −.344 | .300 | −.746 | .054 |
| Change of error number score | ||||||||
| Change of irisin | −.405 | .280 | .042 | .914 | −.071 | .835 | −.007 | .886 |
| Change of BDNF | −.323 | .396 | .425 | .254 | −.084 | .807 | .653 | .112 |
| Change of irisin | ||||||||
| Change of BDNF | −.570 | .067 | .355 | .284 | .078 | .800 | .110 | .778 |
Abbreviations: BDNF, brain derived neurotrophic factor; CO, control group; ME, mental training; PH, physical training.
aSignificant association between parameters.
Discussion
The present study showed an improvement in cognitive functions and serum BDNF level following 24 sessions of computerized mental training in elderly women with MCI. Since, temporal, parietal, and frontal cortex, are responsible for working memory, 47 and sensorimotor cortex, 48 for reaction time, and signal processing. Therefore, these findings similar to the previous ones 49,50 indicate beneficial effects of mental challenging in attenuating the progression of MCI to AD.
In addition, mental gaming group displayed a significantly greater increase in BDNF rather than physical exercise, suggesting the involvement of BDNF, at least in part for better cognitive performance. The BDNF as a neurotrophin family member was found to secrete from the brain 20 and induces a cascade of molecular signaling through tyrosine kinase receptor and leads to long-term potentiation and long-term memory. 51 There are evidences in aged rodents and primates, showing that BDNF administration reverses neuronal atrophy, 52 increases the synapses number, 53 , 54 and also leads to neurogenesis. 55
Interestingly, the group that experienced physical activity showed no significant change in cognitive function, BDNF, and irisin, in contradictory with our previous report 24 and similar studies 56,57 in elderly participants with metabolic syndrome. One possible explanation might be the intensity of physical activity, which was much lower than the intensities used in the studies with positive results. Other explanation could be the fact that irisin is increased after acute high-intensity exercise, 58 but not chronic one, 30 which was the protocol used in our experiment. In line with our findings, two other studies 26,30 reported no significant effects of endurance and resistance training on circulating irisin.
In addition, we cannot rule out serious concerns raised from two recent studies regarding methodological assays. 26,27 Albrecht et al 27 reported that immunoassay techniques used in most literatures might be reporting cross-reacting serum proteins, and not irisin itself. Also Raschke et al believe that the beneficial effect of irisin might not be translated from mice to humans, 26 since the human FNDC5 gene might be a transcribed pseudogene that unlikely is translated into the full-length FNDC5 protein and irisin. Considering our results and other previous studies, 26,29 the debate over irisin potential role in connecting skeletal muscles and brain to boost cognitive performance seems to be a myth.
On the other hand, combined physical and mental training showed significant changes in working memory and BDNF. This finding reflects either the potency of mental training or synergistic effect of physical and mental training on boosting cognitive performance. In other words, mind–body exercise with integrated cognitive and motor coordination may be useful for cognitive decline via stimulating cellular mechanisms underlying neurotrophins, more notably BDNF secretion compared with physical activity per se. It has been known that mental challenging leads to the activation of neurons and release of glutamate from presynaptic terminals and then increase in intracellular Na+ and Ca2+. Then Ca2+ influx through the N-methyl-d-aspartate receptors in dendrite activates the production of BDNF. Also cAMP responsive element binding protein and X-box binding protein 1 are two other pathways increasing BDNF from brain during mental challenging. 59,60
Interestingly, mental gaming induced improvement in cognitive performance preserved after 6 months, indicating capability and plasticity of brain in elderly individuals for learning and memory. Considering the fact that BDNF was increased in the group challenged mentally, the persistence of cognitive performance for at least 6 months could be justified at least in part via elevation in BDNF and further synaptic plasticity. Despite possible risk of falling for elderly passive people engaging physical activity, a slight insignificant impairment in cognitive performance after physical exercise detraining will question the efficacy of this activity compared with mental one. Finally, our findings suggest that engaging in mental activity is a useful way to protect neural processing in elderly women with low level of education.
One of the limitations of our study was the small sample size, due to different limitations such as inclusion and exclusion criteria and also number of participants who dropped out during the experiment. For future studies, a greater sample size, considering variety of BDNF genotypes such as Val66Met polymorphism also other technical assessment rather than ELISA, is suggested.
Conclusion
In conclusion, our findings indicate that mental training has beneficial effects across old ages as a useful, safe strategy to prevent cognitive decline via BDNF elevation.
Acknowledgments
The authors thank all authorities, staff, and participants from Yas adult day center in Aliabad-e Katul for their kind cooperation
Footnotes
The authors declared no potential conflicts of interest with respect to the research, authorship, and/or publication of this article.
Funding: The authors received no financial support for the research, authorship, and/or publication of this article.
References
- 1. Van der Elst W, Van Boxtel MP, Van Breukelen GJ, Jolles J. The Stroop color-word test: influence of age, sex, and education; and normative data for a large sample across the adult age range. Assessment. 2006;13(1):62–79. [DOI] [PubMed] [Google Scholar]
- 2. Petersen R. Mild cognitive impairment as a diagnostic entity. J Intern Med. 2004;256(3):183–194. [DOI] [PubMed] [Google Scholar]
- 3. Panegyres PK, Berry R, Burchell J. Early dementia screening. Diagnostics. 2016;6(1):6. doi:10.3390/diagnostics6010006. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 4. Farias ST, Mungas D, Reed BR, Harvey D, De Carli C. Progression of mild cognitive impairment to dementia in clinic vs community-based cohorts. Arch Neurol. 2009;66(9):1151–1157. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 5. Birks J. Cholinesterase inhibitors for Alzheimer’s disease. Cochrane Database Syst Rev. 2006;(1):CD005593. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 6. Cooper C, Mukadam N, Katona C, et al. Systematic review of the effectiveness of pharmacologic interventions to improve quality of life and well-being in people with dementia. Am J Geriatr Psychiatry. 2013;21(2):173–183. [DOI] [PubMed] [Google Scholar]
- 7. Byun JE, Kang EB. The effects of senior brain health exercise program on basic physical fitness, cognitive function and BDNF of elderly women—a feasibility study. J Exerc Nutrition Biochem. 2016;20(2):8–18. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 8. Nascimento CM, Pereira JR, Pires de Andrade L, et al. Physical exercise improves peripheral BDNF levels and cognitive functions in mild cognitive impairment elderly with different BDNF Val66Met genotypes. J Alzheimers Dis. 2015;43(1):81–91. [DOI] [PubMed] [Google Scholar]
- 9. Prakash RS, Voss MW, Erickson KI, Kramer AF. Physical activity and cognitive vitality. Annu Rev Psychol. 2015;66:769–797. [DOI] [PubMed] [Google Scholar]
- 10. Kelly ME, Loughrey D, Lawlor BA, Robertson IH, Walsh C, Brennan S. The impact of exercise on the cognitive functioning of healthy older adults: a systematic review and meta-analysis. Ageing Res Rev. 2014;16:12–31. [DOI] [PubMed] [Google Scholar]
- 11. Lautenschlager NT, Cox KL, Flicker L, et al. Effect of physical activity on cognitive function in older adults at risk for Alzheimer disease: a randomized trial. JAMA. 2008;300(9):1027–1037. [DOI] [PubMed] [Google Scholar]
- 12. Lam LCW, Chan RCM, Womg BML, et al. A 1-year randomized controlled trial comparing mind body exercise (Tai Chi) with stretching and toning exercise on cognitive function in older Chinese adults at risk of cognitive decline. J Med Dir Assoc. 2012;13(6):568.e15-e20. [DOI] [PubMed] [Google Scholar]
- 13. Jean L, Bergeron ME, Thivierge S, Simard M. Cognitive intervention programs for individuals with mild cognitive impairment: systematic review of the literature. Am J Geriatr Psychiatry. 2010;18(4):281–296. [DOI] [PubMed] [Google Scholar]
- 14. Pressler S, Titler M, Koelling T, et al. Nurse-enhanced computerized cognitive training increases serum brain derived neurotrophic factor levels and improves working memory in heart failure. J Card Fail. 2015;21(8):630–641. [DOI] [PubMed] [Google Scholar]
- 15. Willis SL, Tennstedt SL, Marsiske M, et al. Long-term effects of cognitive training on everyday functional outcomes in older adults. JAMA. 2006;296(23):2805–2814. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 16. Shatil E. Does combined cognitive training and physical activity training enhance cognitive abilities more than either alone? A four-condition randomized controlled trial among healthy older adults. Front Aging Neurosci. 2013;5:8. doi:10.3389/fnagi.2013.00008. eCollection 2013. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 17. Fabre C, Chamari K, Mucci P, Massé-Biron J, Préfaut C. Improvement of cognitive function via mental and/or individualised aerobic training in healthy elderly subject. Int J Sports Med. 2002;23(6):415–421. [DOI] [PubMed] [Google Scholar]
- 18. Oswald W, Gunzelmann T, Rupprecht R, Hagen B. Differential effects of single versus combined cognitive and physical training with older adults: the SIMA study in a 5-year perspective. Ageing. 2006;3(4):179–192. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 19. Marie-Ludivine CD, Papouin G, Saint-Val P, Lopez A. Effect of adapted karate training on quality of life and body balance in 50-year-old men. Open Access J Sports Med. 2010;1:143–150. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 20. Mattson M, Maudley S, Martin B. BDNF and 5-HT: a dynamic duo in age-related neuronal plasticity and neurodegenerative disorders. Trends Neurosci. 2004;27(10):589–594. [DOI] [PubMed] [Google Scholar]
- 21. Bath K, Lee F. Variant BDNF (Val66Met) impact on brain structure and function. Cogn Affect Behav Neurosci. 2006;6(1):79–85. [DOI] [PubMed] [Google Scholar]
- 22. Ganguly K, Poo M. Activity-dependent neural plasticity from bench to bedside. Neuron. 2013;80(30):729–741. [DOI] [PubMed] [Google Scholar]
- 23. Vazquez-Sanroman D, Sanchis-Segura C, Toledo R, Hernandez ME, Manzo J, Miquel M. The effects of enriched environment on BDNF expression in the mouse cerebellum depending on the length of exposure. Behav Brain Res. 2013;243:118–128. [DOI] [PubMed] [Google Scholar]
- 24. Babaei P, Azali Alamdari K, Soltani Tehrani B, Damirchi A. Effect of six weeks of endurance exercise and following detraining on serum BDNF and memory performance in middle aged males with metabolic syndrome. J Sports Med Phys Fitness. 2013;53(4):437–443. [PubMed] [Google Scholar]
- 25. Wrann CD, White JP, Salogiannnis J, et al. BM. Exercise induces hippocampal BDNF through a PGC-1a/FNDC5 pathway. Cell Metab. 2013;18(5):649–459. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 26. Raschke S, Elsen M, Gassenhuber H, et al. Evidence against a beneficial effect of irisin in humans. PLoS One. 2013;8(9):e73680. doi:10.1371/journal.pone.0073680. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 27. Albrecht E, Norheim F, Thiede B, et al. Irisin—a myth rather than an exercise-inducible myokine. Sci Rep. 2015;5:8889. doi:1038/srepo8889. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 28. Bostrom P, Wu J, Jedrychowski MP, et al. A PGC-1a-dependent myokine that drives brown-fat-like development of white fat and thermogenesis. Nature. 2012;481(7382):463–468. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 29. Timmons JA, Baar K, Davidsen PK, Atherton PJ. Is irisin a human exercise gene? Nature. 2012;488(7413):E9–E10. [DOI] [PubMed] [Google Scholar]
- 30. Norheim F, Langleite TM, Hjorth M, et al. The effects of acute and chronic exercise on PGC-1a, irisin and browning of subcutaneous adipose tissue in humans. FEBS J. 2014;281(3):739–749. [DOI] [PubMed] [Google Scholar]
- 31. Babaei P, Damirchi A, Mehdipoor M, Tehrani BS. Long term habitual exercise is associated with lower resting level of serum BDNF. Neurosci Lett. 2014;566:304–308. [DOI] [PubMed] [Google Scholar]
- 32. Aldwin CM, Gilmer DF. Health, Illness, and Optimal Aging. New York, NY: Springer; 2004. [Google Scholar]
- 33. Shen J, Anderson MC, Arehart KH, Souza PE. Using cognitive screening tests in audiology. Am J Audiol. 2016;25(4):319–331. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 34. Lucas SJ, Ainslie PN, Murrell CJ, Thomas KN, Franz EA, Cotter JD. Effect of age on exercise-induced alterations in cognitive executive function: relationship to cerebral perfusion. Exp Gerontol. 2012;47(8):541–551. [DOI] [PubMed] [Google Scholar]
- 35. Strenziok M, Parasuraman R, Clarke E, Cisler DS, Thompson JC, Greenwood PM. Neurocognitive enhancement in older adults: comparison of three cognitive training tasks to test a hypothesis of training transfer in brain connectivity. Neuroimage. 2014;85(3):1027–1039. [DOI] [PubMed] [Google Scholar]
- 36. Folstein MF, Folstein SE, McHugh PR. Mini mental state: a practical method for grading the cognitive state of patients for the clinician. J Psychiatr Res. 1975;12(3):189–198. [DOI] [PubMed] [Google Scholar]
- 37. Wei XH, Ji LL. Effect of handball training on cognitive ability in elderly with mild cognitive impairment. Neurosci Lett. 2014;566:98–101. [DOI] [PubMed] [Google Scholar]
- 38. Langlois F, Vu TM, Chass EK, Dupuis G, Kergoat MJ, Bherer L. Benefits of physical exercise training on cognition and quality of life in frail older adults. J Gerontol B Psychol Sci Soc Sci. 2012;68(3):400–404. [DOI] [PubMed] [Google Scholar]
- 39. Pisoni DB, Kronenberger WG, Roman AS, Geers AE. Measures of digit span and verbal rehearsal speed in deaf children after more than 10 years of cochlear implantation. Ear Hear. 2011;32(1 suppl):60–74. doi:10.1097/AUD.0b013e3181ffd58e. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 40. Lezak MD, Howieson DB, Bigler ED, Tranel D. Neuropsychological Assessment. New York, NY: Oxford University Press; 2004. [Google Scholar]
- 41. Brukner P, Khan K, Kron J. The Encyclopedia of Exercise, Sport and Health. Crows Nest, Australia: Allen & Unwin; 2004. [Google Scholar]
- 42. Stroop JR. Studies of interference in serial verbal reactions. Exp Psychol. 1935;18(6):643–662. [Google Scholar]
- 43. Bozoki A, Radovanovic M, Winn B, Heeter C. Effects of a computer-based cognitive exercise program on age-related cognitive decline. Arch Gerontol Geriatr. 2013;57(1):1–7. [DOI] [PubMed] [Google Scholar]
- 44. Strath SJ, Swartz AM, Bassett DR, O’Brien WL, King GA, Ainsworth BE. Evaluation of heart rate as a method for assessing moderate intensity physical activity. Med Sci Sports Exerc. 2000;3(9 suppl):465–470. [DOI] [PubMed] [Google Scholar]
- 45. Voss MW, Prakash RS, Erickson KI, et al. Plasticity of brain networks in a randomized intervention trial of exercise training in older adults. Front Aging Neurosci. 2010;2(32):1–17. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 46. Pennington R, Hanna S. The acute effects of exercise on cognitive performances of older adults. Ark Acad Sci. 2013;67(19):109–114. [Google Scholar]
- 47. Nadkarni NK, Nunley KA, Aizenstein H, et al. Association between cerebellar gray matter volumes, gait speed, and information-processing ability in older adults enrolled in the Health ABC study. J Gerontol A Biol Sci Med Sci. 2014;69(8):996–1003. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 48. Williamson JW, McColl R, Mathews D. Evidence for central command activation of the human insular cortex during exercise. J Appl Physiol. 2003;94(5):1726–1734. [DOI] [PubMed] [Google Scholar]
- 49. Li T, Yao Y, Xu B, et al. Cognitive training can reduce the rate of cognitive aging: a neuroimaging cohort study. BMC Geriatr. 2016;16:12. doi:10.1186/s12877-016-0194-5. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 50. Jeong JH, Na HR, Choi SH, et al. Group- and home-based cognitive intervention for patients with mild cognitive impairment: a randomized controlled trial. Psychother Psychosom. 2016;84(4):198–207. [DOI] [PubMed] [Google Scholar]
- 51. Bekinschtein P, Cammarota M, Katche C, et al. BDNF is essential to promote persistence of long-term memory storage. Proc Natl Acad Sci USA. 2008;105(7):2711–2716. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 52. Nagahara AH, Merrill DA, Coppola G, et al. Neuroprotective effects of brain-derived neurotrophic factor in rodent and primate models of Alzheimer’s disease. Nat Med. 2009;15(3):331–317. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 53. Tyler WJ, Pozzo-Miller LD. BDNF enhances quantal neurotransmitter release and increases the number of docked vesicles at the active zones of hippocampal excitatory synapses. J Neurosci. 2001;21(12):4249–4258. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 54. Lu Y, Christian K, Lu B. BDNF: a key regulator for protein synthesis-dependent LTP and long-term memory? Neurobiol Learn Mem. 2008;89(3):312–323. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 55. Bergami M, Rimondini R, Santi S, Blum R, Gotz M, Canossa M. Deletion of TrkB in adult progenitors alters newborn neuron integration into hippocampal circuits and increases anxiety-like behavior. Proc Natl Acad Sci. 2008;105(40):15570–15575. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 56. Baker LD, Frank LL, Foster-Schubert K, et al. Effects of aerobic exercise on mild cognitive impairment: a controlled trial. Arch Neurol. 2010;67(1):71–79. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 57. Eggermont LH, Swaab DF, Hol EM, Scherder EJ. Walking the line: a randomised trial on the effects of a short term walking programme on cognition in dementia. J Neurol Neurosurg Psychiatry. 2009;80(7):802–804. [DOI] [PubMed] [Google Scholar]
- 58. Huh JY, Mougios V, Kabasakalis A, et al. Exercise-induced irisin secretion is independent of age or fitness level and increased irisin may directly modulate muscle metabolism through AMPK activation. J Clin Endocrinol Metab. 2014;99(11):2154–2161. [DOI] [PubMed] [Google Scholar]
- 59. Alboni S, Tascedda F, Corsini D, et al. Stress induces altered CRE/CREB pathway activity and BDNF expression in the hippocampus of glucocorticoid receptor-impaired mice. Neuropharmacology. 2011;60(7-8):1337–1346. [DOI] [PubMed] [Google Scholar]
- 60. Martínez G, Vidal RL, Mardones P, et al. Regulation of memory formation by the transcription factor XBP1. Cell Rep. 2016;14(6):1382–1394. [DOI] [PubMed] [Google Scholar]