Abstract
Alzheimer’s disease (AD), a neurological disorder, is as a complex chronic disease of brain cell death that usher to cognitive decline and loss of memory. Its prevalence differs according to risk factors associated with it and necropsy performs vital role in its definite diagnosis. The stages of AD vary from preclinical to severe that proceeds to death of patient with no availability of treatment. Biomarker may be a biochemical change that can be recognized by different emerging technologies such as proteomics and metabolomics. Plasma biomarkers, 5-protein classifiers, are readily being used for the diagnosis of AD and can also predict its progression with a great accuracy, specificity, and sensitivity. In this review, upregulation or downregulation of few plasma proteins in patients with AD has also been discussed, when juxtaposed with control, and thus serves as potent biomarker in the diagnosis of AD.
Keywords: Alzheimer’s disease, brain cell death, plasma biomarkers, diagnosis
Introduction
Alzheimer’s disease (AD) is the most familiar form of dementia. Dementia and AD affect the brain in similar way. It is a neurological disorder responsible for mortality of brain cells, thus cognitive decline and memory loss predicted. 1 It is a cumulative disorder of brain that is more prevalent among human beings. Alzheimer’s disease is a dynamic kind of deteriorating brain disorder. 2 Patients slowly but definitely lose their memory as well as their intellectual skill. It also has an impact on person’s capacity to carry out functions. These transformations occur due to the continuous dysfunction, resulting in destruction of nerve cells that deals with the processing of information and its storage.
From 2000 to 2013, AD prevalence enhanced to 71%, making it sixth well-known cause in United States. In 2015, approximately 18.1 billion hours are being served on patients suffering from AD by 15 million caretakers and family members. In 2016, approximately 700 000 Americans with age ≥65 years died with AD and its complications. Total deposit for health management was $236 billion. Alzheimer’s disease progresses every 66 seconds and is expected to grow every 33 seconds in 2050, emerging in 1 million new cases per year. 3
Prediction of AD at early stages is of tremendous importance, especially as a result of tactical treatment directing at the disease variation and also pinpoint symptoms that are merely slight. Thus, biomarkers are crucially required as they can be administered in global screening programs to precisely detect people at early stage of AD or older adults with amnesia who have a great threat of perspective deadening and future-associated disability. The impact of the disease is also a growing interest for the governments of developing communities, in particular due to huge number of elderly citizens being in danger. More than 35 million people worldwide are influenced by AD. 4
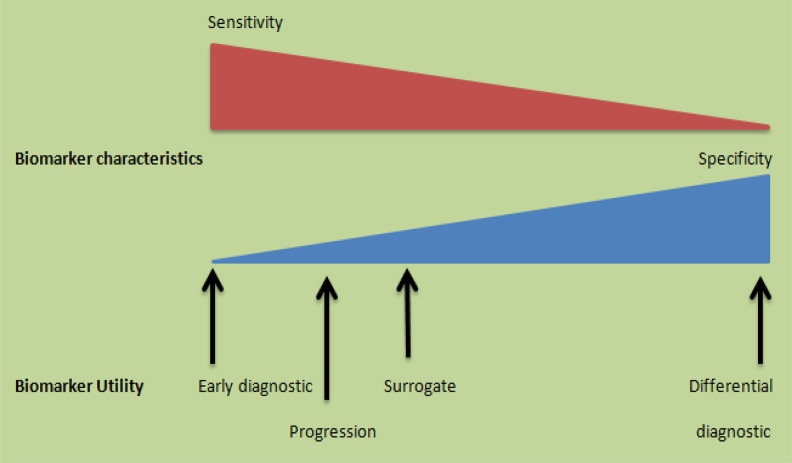
Therefore, it is an essential task to identify the AD biomarkers in order to decrease the budget of clinical tests, which will ultimately aid in developing different therapies for better diagnostic purpose. Due to elevated use of biomarkers in diagnostic area, extra efforts are needed to broaden and refine its research. More precisely, different biomarkers are required for different purposes, including sensitive biomarkers for early diagnosis and ideally before the onset of different symptoms, specific biomarkers for distinguishing the different diseases leading to dementia, and surrogate biomarker that can measure the progression of disease directly or indirectly during the course of clinical tests. Moreover, a biomarker should fulfill all the aspects of an ideal biomarker, that is, it should be applicable to all ages, gender, and phenotype, easily accessible, noninvasive, and reproducible, showing slight variability in healthy people and minute differences observed as a result of unrelated group of diseases (Figure 1).
Figure 1.
Different biomarkers needed to support clinical tests: The biomarkers for early diagnosis should be very accurate to recognize a patient population before the advent of cognitive symptoms. In addition, markers for differential diagnostic purposes should be very exceptional as they function to describe the different diseases that contribute to dementia. On the other hand, markers required to study disease development should be much sensitive in order to follow delicate cognitive variations during the course of clinical trials. Surrogate markers that measure the pathology of a disease should have characteristics that are the delicate balance of specificity and sensitivity.
There is great interest in plasma markers of AD since blood extraction is far less invasive. Additionally, the plasma biomarkers can be measured at relatively low cost, once a standard measuring system is established. 5 Plasma consists of significant undiscovered information. Alterations in plasma proteins, plasma metabolites, or even the microRNA levels can mirror either the already known mechanisms of AD pathology, including neuronal cell death, inflammation, oxidative mechanisms, and the complement cascade activation, or can give valuable insights into the novel pathways concerning the disease etiology. 6
This review is not a methodical review of all facts relating to the development of blood-based biomarkers, but in fact an effort to envisage particular plasma biomarkers, as benchmark for the development of AD. This review aims to scrutinize the discovery of different plasma biomarkers using proteomics, metabolomics, and emerging technologies. This review also discussed the crucial role of the early diagnosis and prognosis of AD in order to treat it. Then proceeds toward the identification and validation of a 5-protein classifier that acts as an indicator of AD. It also investigates upregulation of complement 4a protein and fibrinogen γ and downregulation of apolipoprotein A-1, α-2-HS-glycoprotein (AHSG), and afaminas an indication of AD.
Discovery of Different Plasma Biomarkers Using Proteomics, Metabolomics, and Emerging Technologies
Biomarker may be a protein or metabolite or may be a genetic trait that can be detected by different emerging technologies. As there is no such treatment of AD, there is a huge demand for biomarkers to diagnose the disease and to detect progression.
The ideal biomarker in accordance to AD is that which is present in patients with AD and absent in healthy people or those with alternative pathological disorders. Their levels would rise or fall when the disease become worse or improves, respectively. In brief, the biomarker should be measurable, should have a scientific rationale, should be reproducible, should be specific for AD, and also must change with the progression of disease in longitudinal observational studies.
The ideal biomarker ought to provide clinicians no other alternative than to depend on the clinical diagnosis. The category of plasma biomarkers for AD diagnosis ought to be intensely associated with the onset of AD and diagnose AD presymptomatically with no false-positive or false-negative results. Presently, for the diagnosis of AD with high accuracy, a biomarker should be able to fulfill the required standards of an ideal biomarker (Table 1). 7
Table 1.
Criteria for an Ideal Biomarker.a
| Biomarkers | Criteria |
|---|---|
| Diagnostic biomarkers | C > a > b > e > d > f |
| Disease activity biomarkers | B > f > a > e > d |
| Treat response biomarkers | F > b > a > e > d |
aa denotes a high specificity; b, a reliable sensitivity; c, detectable in AD early; d, inexpensive and user friendly; e, noninvasive or well accessible; and f, available in monitoring the progression.
A demand of biomarkers for diagnostic purpose increases at large scale. Hence there is a need of cost-effective biomarkers; therefore, trend shifted from cerebrospinal fluid (CSF) to plasma biomarker. 8 In making a clinical diagnosis, neuropsychological test is generally used. It predicts an upcoming disease development and its curative response. It also allows for a less intrusive and more authentic diagnosis by the identification of AD. 9 Hence, biomarkers will increase the capability to better recognize the disease, especially in its early stages. 10 They will also provide more authentic management of disease in its clinical practice. Also, they contribute in detecting different stages of AD. 9
With the aid of biomarkers, one can monitor the response of new drugs and therapeutic approaches. Proteomics have been widely used in the analysis of neurodegenerative diseases. The goal is to detect the diagnostic markers and novel drug targets. It can predict gene products involved in different pathways. 11 A proteomic analysis consists of 2 steps: (i) separated proteins evaluated by analytical methods, primarily by mass spectrometry (MS). 12 (ii) Protein mixture can be separated in order to allow the accurate detection of particular proteins. 13
In the study of AD, proteomics has been principally used for the analysis of human brain. 14 Metabolomics is the scientific study of small molecules called metabolites. It has wide range of applications, including drug target identification and biomarkers. Several studies determine the action of lipids and sterols in AD pathology. 15 Metabolomics research mainly targets the alterations in polar, nonpolar behavior, and other metabolites that are related to the disease. By the study of metabolome, new pathways regarding AD will be discovered. 16 The biochemical pathways following metabolite alterations could be identified, which corresponds to the cognitive impairment and memory loss. 6
Emerging Technologies for the Diagnosis of AD
Mass spectrometry investigations of amyloid β (Aβ) from human brains (patients with AD) showed a wide variety of Aβ peptides, Aβ peptides may change fundamentally in physical–synthetic properties. Therefore, MS examination and information handling are expected to dissect the whole scope of Aβ species. A few such methodological MS-based strategies has been made for this reason, such as immunoprecipitation (IP) combined with matrix-assisted laser desorption ionization (MALDI) time of flight (Tof) MS (IP-MALDI-ToF-MS), IP-assisted liquid chromatography–MS (IP-LC–MS/MS), and solid phase extraction–LC tandem MS (SPE–LC–MS/MS). 17 Likewise, in order to explore and precisely recognize large size Aβ peptides, with promising post-translational Aβ modifications, high-quality MS instruments are required along with approaches utilized in top-down proteomics, including complementary fragmentation methods (collision-induced dissociation, electron capture dissociation, and infrared multiphoton dissociation). 18
While trying to assess if MS investigation may give an increasingly exact measurement of Aβ peptides in plasma, an IP MS selected reaction monitoring method was developed for evaluation of Aβ42 and Aβ40. In this method, an isotope labeled with Aβ peptides was added to the test sample prior to examination (and thus examined and processed all together with Aβ peptides of endogenous nature), and octyl-glucopyranoside was used as a deterrent to disturb network between Aβ and plasma proteins, for example, albumin. 19 In a pilot clinical investigation, dependent on clinically analyzed cases, no huge change was observed, regardless of whether there was a clear pattern for a decrease in both plasma Aβ42 and the Aβ42/40 proportion in AD. Interestingly, utilizing a comparative IP-MS technique, involving LysN for digestion of Aβ peptides prior to analysis, fundamentally bring down Aβ42 concentration and Aβ42/Aβ40 ratio was observed in amyloid of the patients examined through positive emission tomography (PET-positive) compared with PET-negative ones. The Aβ42/Aβ40 proportion was lesser in the amyloid PET-positive group. 20 These exceptionally encouraging outcomes call for further examinations to assess plasma Aβ as a screening tool for AD, including large-scale clinical studies and comparisons of various diagnostic methods for estimation of results. 21
With delicate and explicit enzyme-linked immunosorbent assay (ELISA), broad investigations of plasma A1β42 level and Aβ142/Aβ140 proportion were accounted during the improvement of plasma biomarkers. High proportions of plasma A1β42 expanded danger of AD. 22 Most of the investigations also showed that elevated level of plasma A1β42 was found before or at the beginning of the AD progression and that the level of Aβ142 reduced as disease further progressed; the least A1β42/Aβ140 proportion reported was related with creating dementia. However, a few examinations indicated conflicting outcomes. Reliable strategies to find out concentration of τ and phosphorylated τ in the blood of patients with AD are yet being investigated. 23 Besides ELISA, studies have been carried out utilizing Luminex xMAP method to quantify the levels of Aβ in plasma. Luminex xMap method is a bead-based multiplexing test system in a microplate setup. 24 This method carries out distinct assays on the microspheres’ surface, and results are read by the compact analyzer. In one examination, plasma was gathered at baseline from 2 individual cohorts of mild cognitive impairment (MCI) and normal controls. Plasma concentrations of Aβ isoforms disclosed no prominent difference among the MCI group, cognitive healthy group, and patients with MCI to AD. These conflicting discoveries may be due to the usage of different technologies. 25
Plasma contains much lower levels of Aβ species and τ compared to their levels in CSF. Besides, plasma contains high concentration of assay-interfering factors, bringing about challenges in the ordinarily utilized singulex or multiplex ELISA methods. 26 To conquer the difficulties of detection experienced utilizing conventional ELISA platforms, new methodologies and advances are being developed with the possibility to give better specificity and sensitivity for determining Aβ and τ in blood tests. 27 For instance, another methodology that utilized IP to pull down different Aβ pieces in plasma tests pursued by MS examination has prompted disclosure of another marker, APP669-APP711, whose proportion to Aβ142 showed 96% specificity and 93% sensitivity to separate Pittsburgh compound B (PIB)-positive patients from PIB-negative patients. 28
Among the various exogenous and endogenous effectors of AD development, Aβ neurotoxic oligomers have been considered the potent agents, which elicit the development of Aβ plaques in the brain and results in neuron devastation. 29 In recent times, an immunoinfrared sensor (WO 2015121339 A1) that checked the changes in the secondary structure of Aβ peptides has been created. The sensor is an antibody (Ab) immunoglobulin-based technique to remove all Aβ peptides from the blood or CSF and spectroscopically senses the dispersion of the secondary structure of separated solvent Aβ species in the infrared. 30 The immunoinfrared sensor at the same time checks the secondary structure dispersion of all soluble peptides in plasma captured by monoclonal antibodies (mAbs) covalently connected to the surface of sensor. In case if the marker band (amide I) is ruled by scattered or α-helical monomeric isoforms, the patients would be analyzed as non-AD. If β-sheet isoforms are enhanced, the amide I signal is moved below the threshold level (1642/cm), demonstrating AD. 31
Immunoaffinity-based latest assays have also been applied in analyzing potent markers of AD in biofluids. Two of these are immunomagnetic reduction (IMR) and single-molecule array (SIMOA) for the study of plasma samples. 32 Measurement with both these assays depends on immunoreactivity between particular Abs and analytes. Though, the design and principle of detection used by both these assays is different. In IMR method, detection is carried out by alternating-current magnetic susceptibility by a device called SQUID, that is, superconducting quantum interference device, on the other hand, in SIMOA technology detection is carried out by recognizing the existence of antigen (Ag) by a fluorescence imaging of single enzyme-marked immunocomplexes reacting with the resorufin β-d-galactopyranoside, that is, fluorogenic substrate. 33 The IMR assays carried out in Taiwanese patients have discovered increased Aβ42 levels, decreased or no change in Aβ40 concentrations, and elevated τ levels in patients with AD observed when compared to normal people. Plasma τ investigations using SIMOA technology have demonstrated critical increments in τ in patients with AD and elevations or no changes in patients with MCI in comparison with normal controls. 34 When plasma concentrations of Aβ42 and Aβ40 were measured by SIMOA, considerable reduction was observed between patients with MCI and those with AD. 35,26 In this way, IMR and SIMOA tests were created to examine plasma concentrations of Aβ and τ in humans. 29
Early Diagnosis and Prognosis of AD
In the concern of tactical treatment that are directing at AD alteration, symptoms regarding AD are subsidiary. Clinical diagnosis of AD at its early stage is very enticing. Hence, in order to identify AD at early stages via extensive screening programs, there is an urgent need of biomarkers. But as there is a need for biomarkers for identifying AD at large scale, the biomarkers should be noninvasive and cost-effective.
The current study aimed at establishing a panel of biomarkers from plasma for distinguishing early AD from human aging (a physiological process) and comparing the results with previous reports. As AD is a subtype of dementia and for this reason, a particular indication of AD can only be possible with the aid of a suitable set of plasma biomarkers (Table 2).
Table 2.
Basic Differences Between AD and Dementia and the Mild, Moderate, and Severe Stages of AD.
| Basic differences between AD and dementia | |||
|---|---|---|---|
| Dementia | Alzheimer’s disease | ||
| General definition | It is a brain-linked disorder caused due to diseases and other severe conditions. | It is the subtype of dementia. | |
| Causes | Many causes including tumors and thyroid issues, vitamin deficiencies, AD, stroke, and reactions to different medicines. | Actual cause is unknown, but amyloid cascade hypothesis is the most putative one. | |
| Duration | It causes a permanent damage but at different stages. | It lasts for 8 to 20 years. | |
| Typical age of onset | 65 years and older | It can occur as early as at age 30. | |
| Symptoms | Different symptoms include issues related to memory, reasoning, judgment, visual perception, and many more. | Symptoms include mood swings, behavioral changes, difficulty in remembering new information, disorientation, and so on. | |
| Mild, moderate, and severe stages of AD | |||
| Mild | Moderate | Severe | |
| Activities of daily living | Problems with routine tasks | Needs help with basic ADL (eg, feeding, dressing, bathing) | Progresses to total dependence on caregiver (eg, feeding, toileting) |
| Behavior | Changes in personality | Anxiety, suspicion, pacing, insomnia, agitation, wandering | Crying, screaming, groaning |
| Cognition | Confusion and memory loss: Misplacing objects Forgetting names Disorientation |
Difficulty in recognizing family and friends Chronic loss of recent memory |
Loss of speech Misidentifies or is unable to recognize familiar people |
Abbreviations: AD, Alzheimer’s disease; ADL, activities of daily living.
Symptoms of the disease may vary from person to person and becomes poor over time, as there are difficulty in making decisions, loss of memory, alterations in the mood and personality, decreases or poor perception, confusion, and anxiety. Stages of AD vary from mild, moderate, to severe along with some advanced stages, that is, subjective cognitive decline, prodromal AD, and mild cognitive decline (MCI), and symptoms vary from preclinical to the severe stage (Table 2). 36
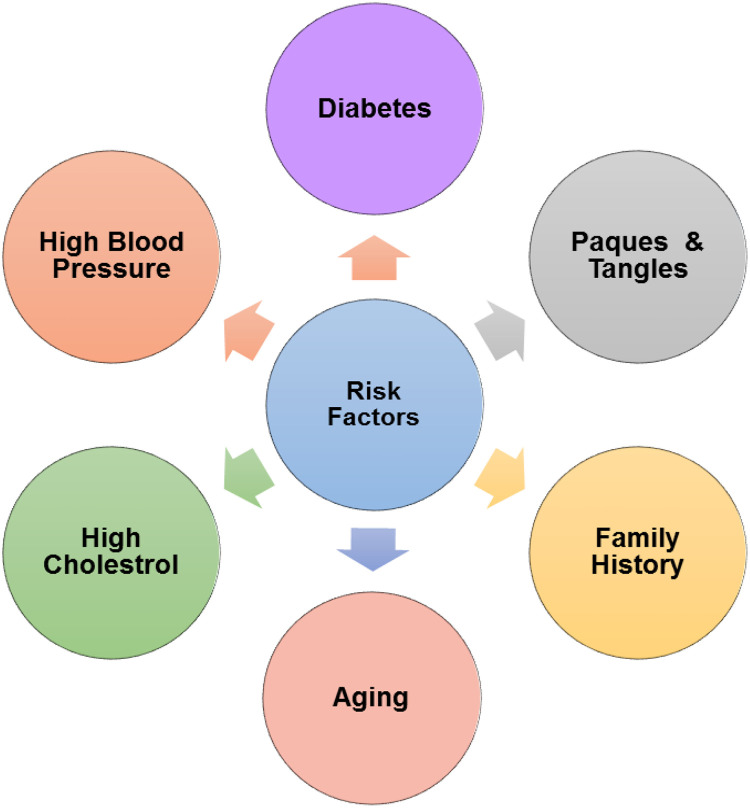
Alzheimer’s disease can be caused by genetic, physical, and environmental factors. There are many risk factors associated with AD (Figure 2). Some of them are discussed here, such as a person is at a higher risk if he or she belongs to a family that have the disease. 12 By autopsy, microscopic inclusions seen in the nerve tissue are known as plaques and tangles. The aggregation of protein fragment β-amyloid called β-amyloid plaques in the brain, outside neurons, and tangled strands of the protein τ, within neurons developed. These transformations are due to the death of neurons. Ratio of women who are affected is more than men. 37 Having certain genes, for example, apolipoprotein E (APOE) e4 gene, gives a model for a protein that helps in transporting cholesterol. The e4 form is a second most simple form, having this form elevated the possibility of developing AD at a younger age. 38 People with diabetes and high blood pressure also pose at high risk of AD.
Figure 2.
Risk factors associated with Alzheimer’s disease.
In 40% training and 60% test sample, 58 controls and 109 patients with AD were split. In the training sample that was in the large-scale panel of 107 plasma proteins, a significant plasma protein that distinguishes AD from other controls was identified while the accuracy of distinguishing between these 2 sets was evaluated in this test sample. A set of 5 plasma proteins that distinguishes controls from AD with 79.17% specificity and 89.36% sensitivity were identified. It is predicted that 4 of 5 plasma proteins associated with a shared network with τ and APP were revealed in a biological pathway analysis.
The proteomic study revealed that the significant protein in previous and current situation is APOE and most accepted diagnostic biomarker Aβ. Furthermore, the biomarkers fibrinogen γ and AHSG are general markers for cognitive decline and thus not specific for AD. This proves the feasibility of the plasma biomarker tactic for AD diagnosis, but problem of replication is still encountered; so there is a need to do further research to overcome the problem. This study also highlights an urgent need for replicating the findings of discovery phase. 39
Using AD prognosis as a variable of a measurable phenotype with the presumption that expeditious prognosis of AD is a result of rapid substantial pathology is an alternative approach to AD identification. With this tactic, using the frequency of the perceptive deadening as the appraisal of prognosis, it was observed that clusterin (also known as apolipoprotein J, a ubiquitous multifunctional glycoprotein capable of interacting with a broad spectrum of molecules encoded by CLU gene) levels were associated with the severity of AD prognosis and pathology. 40 Further, in patients with MCI, clusterin levels in a correlation with longitudinal brain atrophy were demonstrated. 41 These results proposed that the clusterin protein of plasma exists as a biomarker for pathology of central nervous system. In a genome-wide association study, it was observed that polymorphism of a single nucleotide in clusterin gene was crucially related to increased risk of AD in humans. 42
Further, in the asymptomatic bearers of the risk variants of the CLU gene, the hippocampal function was modified. 43 Other usual and rare nonsynonymous (amino acid replacement) switches in the CLU gene have been revealed and are associated with augmented threat of AD. 44 The impact of those alternates on the clusterin levels and functions is yet to be explained. For the biomarkers of prognosis in AD, a variant of study design is a quest for biomarkers that alternates from the predementia syndromes, for example, from MCI to the full dementia syndromes. One such review of this study design was the study by Ray et al, even though the plasma proteins in this study that play a role as biomarkers have not been widely replicated.
In AD and in our study, the presence of an inflammatory signature in body fluid (the plasma) has been confirmed. This inflammatory signature consists of a set of related molecules and cytokines predicting accurately alteration from the MCI once merged with the imaging. 45
Using proteomic approach in a characteristic or erudite study designs, a signature of AD in plasma was observed. However, the discovery of this biomarker depends on its efficacy to be turned into an authenticated biomarker for the medical trials and this task has yet to face many ongoing challenges in order to be completed. Therefore on the basis of all the abovementioned studies, there is a demand of research on plasma biomarkers for AD diagnosis at early stages and its progression. 46
Identification and Validation of 5-Protein Classifier as an Indicator of AD
Alzheimer’s disease is presently identified by biomarkers such as CSF biomarkers and neurobiomarkers, but these biomarkers involve either minimally invasive procedures or are expensive to measure; therefore instead of these biomarkers, the use of biomarkers from congenial body fluids, that is, plasma, is more alluring. Using proteomic technology, about 1129 plasma biomarkers for AD have been profiled. With further research, a 5-protein biomarker for AD diagnosis has also been introduced.
It is predicted that a 5-protein classifier was constructed for AD diagnosis. This 5-protein biomarker is a molecular signature, which constitutes abundant of S100A9, ESAM, ALF1, CD84, and CD226 proteins. S100A9 is the only protein of the 5-protein classifier that directly relates with AD. This unique 5-protein biomarker performs outstandingly with 10-fold cross-validation, that is, it comprises of 90.1% sensitivity, 87.9% precision, and 84.2% specificity, while in an independent validation study it performs with 100% sensitivity, 90% precision, and 80% specificity (outperforming the CSF biomarkers and neurobiomarkers).
Moreover, the 5-protein biomarker can even predict a mild perceptive impairment and can diagnose AD at an early stage with about 96.7% sensitivity, 92.5% precision, and 80% specificity. Thus, these studies reveal that plasma proteins are effective biomarkers compared to other traditional biomarkers and that this 5-protein biomarker diagnoses AD efficiently and also aids in differentiating of AD from other diseases. In spite of the fact that many additional and various cohorts are required to further validate the robustness of the 5-protein classifier, it is considered a significant blood test for AD diagnosis.
After the 5-protein biomarker robustness is confirmed, an assay for detection of 5-protein biomarker’s proteins in plasma is carried out via conventional immunoassays. For cost-effective and noninvasive screening of patients with AD, it would be conceivable to analyze the plasma samples of patients in the clinic by implementing the immunoassay-based tests. 47 There is an additional biomarker called as 5-protein biomarker, which is a subclass of 18 protein biomarker and has same overall performance as of 18-protein biomarker. This 5-protein biomarker consists of abundances of proteins, that is, tumor necrosis factor α (TNF-α), granulocyte colony-stimulating factor, epidermal growth factor, interleukin (IL)-1α, and IL-3, and it predicts AD with about 96% accuracy. When examined on 92 samples of patient with AD, it predicts AD with 100% success. In a Weka Software, out of 20 different classifiers, this 5-protein classifier, on an average, has only a small prediction error. This indicates the robustness and independence of this 5-protein biomarker. Thus, this 5-protein classifier turns out to be the potent source to diagnosis AD at early stages. 48
Using a Weka software, a 24 classifier set was selected and used to achieve all the reported outcome. Its purpose is to select various algorithmic procedures in modern practice. Four discrete results along with identical preparation set required by classifiers. To reassure consistency of stated methodology, no criteria were altered from the default site of classifier. Moreover, better results obtained by modifying the structure of each classifier, considering merely the samples of the preparation set.
Though by exhibiting the individuality of the signature enactment from the elected classifier, our aim is to spectacle the stoutness of our techniques with these tests to identify the biomarkers. It is very enticing to annotate that the algorithms and meticulous model that is being utilized indicated IL-6 and added this protein in 10-protein signature. There is a well-known fact that IL-6 along with some other cytokines exists as a center of interest in most of the studies of AD biomarkers. 49 Using an integrative bioinformatics approach, consideration was drawn to a smaller signature. By analyzing the protein-related graph, the 6-protein signature was then obtained. For the sake of another experiment, IL-6 is excluded in the 5-protein signature; thus, finally the 5-proteins become a suitable subclass of the original 18-protein signature (Table 3).
Table 3.
Average Results for Each Signature Over 24 Classifiers Revealing 5-Protein Classifiers.
| Overall (“AD” + “MCI”) | Test Set “AD” | Test Set “MCI” | ||||||
|---|---|---|---|---|---|---|---|---|
| Signature | Size | Overall | AD Er | NAD Er | AD Er | NAD Er | AD Er | NAD Er |
| 139 | 64 | 75 | 42 | 50 | 22 | 25 | ||
| 18-Protein signature | Error average | 24.34 | 9.02 | 15.33 | 3.66 | 4.79 | 5.36 | 10.53 |
| Agr % | 82 | 86 | 80 | 91 | 90 | 76 | 58 | |
| 82 | 91 | 66 | ||||||
| 10-Protein signature | Error average | 25.98 | 7.83 | 18.15 | 2.45 | 7.64 | 5.38 | 10.52 |
| Agr % | 81 | 88 | 76 | 94 | 85 | 76 | 58 | |
| 81 | 89 | 66 | ||||||
| 6-Protein signature | Error average | 24.44 | 8.60 | 15.84 | 2.02 | 5.70 | 6.58 | 10.14 |
| Agr % | 82 | 87 | 79 | 95 | 89 | 70 | 59 | |
| 82 | 92 | 64 | ||||||
| 5-Protein signature | Error average | 23.20 | 7.71 | 15.49 | 1.78 | 4.75 | 5.93 | 10.73 |
| Agr % | 83 | 88 | 79 | 96 | 90 | 73 | 57 | |
| 83 | 93 | 65 | ||||||
Abbreviations: AD, Alzheimer disease; MCI, mild cognitive impairment.
Note. This is a five-protein classifier where approximately ninety-seven percent efficacy was detected to predict AD using this data and each highlighted value showed the efficacy to predict AD in each class.
It is obvious that the trials performed by Ray et al provided an enormously operative data sets for discovering different biomarkers for the diagnosis of AD. Thus, a 5-protein classifier was detected having approximately 97% efficacy to predict AD using these data.
Upregulation of Fibrinogen γ as General Biomarker of Cognitive Decline
Upregulation of Fibrinogen γ
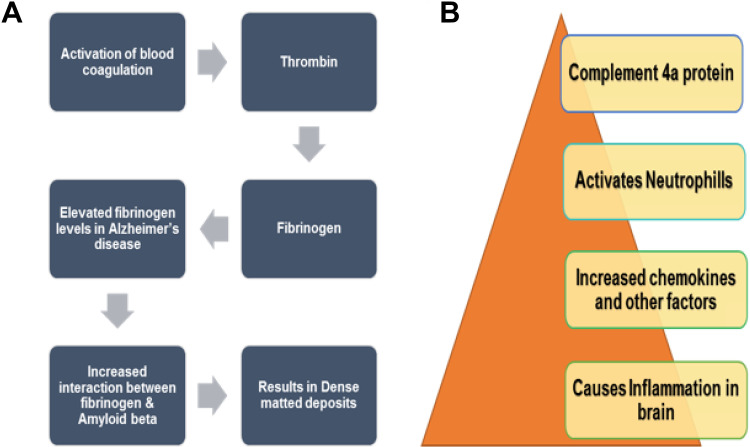
Fibrinogen is one of the principal protein constituent of blood clot. Its structure comprises of 3 sets of polypeptide chains, namely, α, β, and γ, which are linked by disulfide bonds. As a result of damage to blood–brain barrier that acts as a semipermeable membrane, fibrinogen gains entry to the brain. 50 In the normal coagulation, when plasma concentration of fibrinogen γ is normal, thrombin acts on fibrinogen and forms polymer fibers of fibrin, which dissociate easily under fibrinolytic situation and thus remove all the blood clots. 51 But with an elevated concentration of fibrinogen, as seen in the case of patients with AD, abnormal clotting occurs, resulting in the formation of a compact matted deposit, which is not removed by typical fibrinolysis. This is basically due to the interactions between Aβ and fibrin in patients with AD that may speed up neurovascular damage and make the condition more severe (Figure 3A). 52 Through different studies in patients with AD, the core part of Aβ42 is found to be the most crucial region for the interaction. Through x-ray crystallographic studies, it is revealed that Aβ42 binds to fragment of fibrinogen, that is, D fragment. This results in alteration in structure of the C-terminal region of the fibrinogen that belongs to β chain. Moreover, an additional site for binding of Aβ is present inside the aC part of fibrinogen. Ab binding to this aC region inhibits plasmin-facilitated fibrin removal at this site; thus, increased level of plasmin-resistant fibrin exists in AD. 53 In addition, tPA that stands for tissue plasminogen activator is greatly influenced in AD because the activity of plasmin is decreased due to the upregulation of one of the inhibitor of plasmin named as PAI-1. 50 Thus, as a result of enhanced permeability of the blood–brain barrier and reduction in activity of components of fibrinolysis pathway, the brain of patients with AD tends to mount up fibrin. 54
Figure 3.
A, Upregulation of fibrinogen γ as an indication of AD: Higher concentrations of fibrinogen leads to increased interactions between fibrinogen and amyloid β that cause abnormal clotting and dense-matted deposits resistant to typical fibrinolysis. 2 B, Upregulation of C4a protein as a biomarkers of AD: Upregulation of C4a results in inflammation in brain as a result of released chemokines and other factors. This inflammation acts as an indication of AD. 13 AD indicates Alzheimer’s disease.
Furthermore, studies revealed that Gelsolin is a protein associated with cytoskeleton. It also binds with Aβ and prevents the fibrillation of Aβ. In the patients with AD, this protein concentration increased, resulting in mounting up of fibrin. 55 Higher concentration of iron causes iron-induced fibrin to trap RBCs and thus hinders oxygen supply to the brain, which may contribute to AD. 56 Because of all these abovementioned reasons, upregulation of fibrinogen γ may be allocated as a potential biomarker of early AD.
Upregulation of C4a Protein
The complement system consists of a list of proteins specialized to cause destruction and removal of different foreign particles that gain entry within the human body. The system consists of almost 20 or more proteins that flow in the bloodstream and are stimulated in response to infection. Study revealed that in patient with AD, alteration in C4a protein observed among 11 plasma proteins. Comparison among patients with AD and control individuals occurs by techniques such as LC–MS/MS. It acts as a biomarker by generating signals, thereby creating inflammatory responses (Figure 3B). 57
Present studies proposed that components of classical pathway may be significantly elevated in AD because the levels of messenger RNAs for these proteins are amplified in AD brain. C4a is known as anaphylatoxin and is a component of the classical pathway of complement system. Carboxypeptidase N enzyme when act upon it, it splinters rapidly to its constituent C4a-desArg within the plasma.
Downregulation of Apolipoproteins
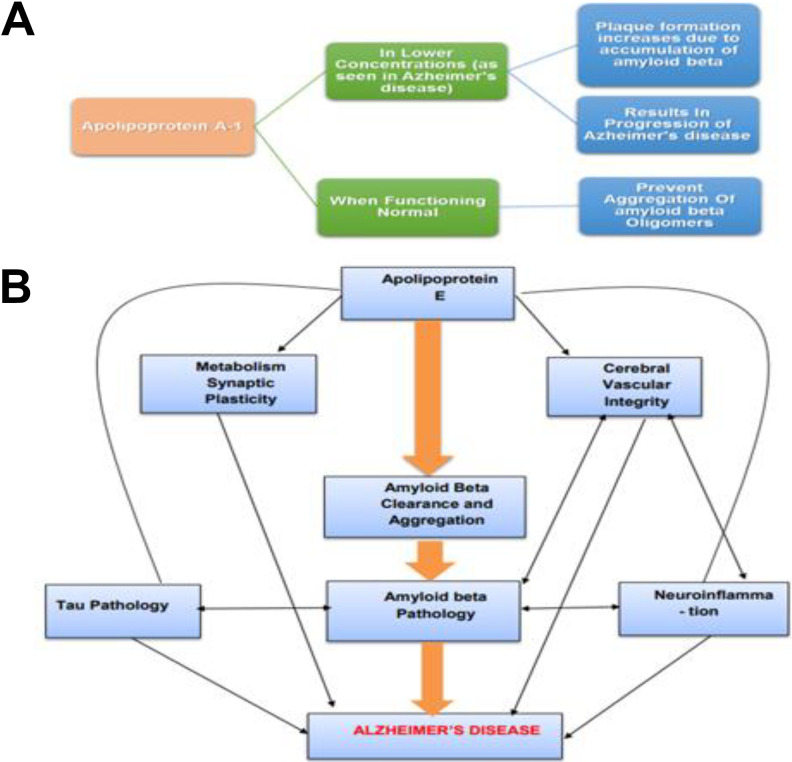
Apolipoproteins are a collection of proteins associated with metabolism of cholesterol and lipids. Current verdicts show that apolipoproteins most probably are also related to neurodegenerative processes. 58 There are different types of apolipoproteins; for example, Apo A-1 has seen to be involved in the process that prevents the aggregation of Aβ oligomers and lessens their accumulation (Figure 4A). 59
Figure 4.
A, Downregulation of apolipoproteins as an indication of AD: Apolipoprotein A-1 when functioning normal prevents aggregation of amyloid β, but lower concentration of apolipoprotein A-1 results in accumulation of amyloid β and thus increased plaque formation results in progression of AD. In this way, change in concentration of Apo A-1 acts as a biomarker of AD. 38 B, Apolipoprotein E facilitated pathways, resulting in AD: Apolipoprotein E by affecting amyloid β pathology mainly affects the pathogenesis of AD. Neuroinflammation is triggered by the accumulation of amyloid β in parenchymal plaques. Moreover, apolipoprotein E may have direct influence on neuroinflammation, metabolism, cerebral vascular integrity, synaptic plasticity as well as transcription using amyloid β–independent mechanisms. The pathways may be differently affected depending on the stage, isoform, and ApoElipidation level. 45 AD indicates Alzheimer’s disease.
In a study, it is revealed that downregulation of Apo A-1 was related to an increased risk of dementia and, in turn, progression of AD. 60 Experiments revealed that Apo A-1 has considerably reduced expression levels in the plasma of patients with mild AD compared with controls. These results also support the previous reports of reduced Apo A-1 (CSF) in neuropathologically confirmed AD. In addition to plasma and CSF, Apo A-1 concentrations were also reduced in serum of patients with AD as stated by Liu et al; therefore, Apo A-1 can be considered a susceptible marker of AD. 61
Most of AD cases are late onset, that is, LOAD; APOE, another type of apolipoprotein, has thought to be a risk factor, having semi-dominant inheritance in the late-onset cases; therefore, current data propose that APOE acts as a contributing factor in AD pathogenesis through both Aβ-dependent and Aβ-independent pathways ( Figure 4B ). 62 Therefore, change in plasma concentrations of APOE can also act as an indicator of AD. 63
Similarly, Apo A-4 is also found to be involved in AD progression, studies on mouse model has suggested that removal of Apo A-4 favors amyloid formation and also assists in Aβ clearance, but its pathologic function in AD is still yet to discover. 64 Studies also suggest the varied concentrations of Apo A-4 in patients with AD compared with controls. Thus, it also serves as a potent AD biomarker. 65
Downregulation of AHSG as General Biomarker of Cognitive Decline
Downregulation of AHSG
Alpha-2-HS-glycoprotein is commonly called as fetuin-A. This protein is quite abundant in plasma and released by hepatic cells; therefore, any change in the plasma concentration of fetuin-A protein can be detected easily. The normal function of this protein is in generating neuroprotective and anti-inflammatory effects. 66 Through different studies, it is revealed that AHSG levels in the plasma with mild AD symptoms were considerably lesser when compared with controls. Recent developments in this area revealed that plasma concentrations of AHSG are directly related to cognitive impairment. 66
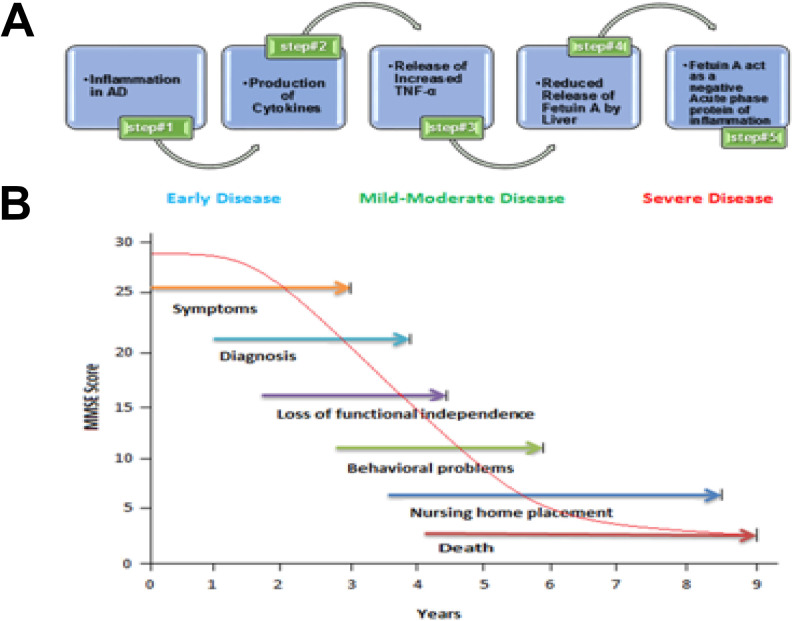
The downregulation of fetuin-A in plasma of patients with AD is explained as a result of subclinical inflammation. It has been revealed that due to higher concentrations of proinflammatory cytokines, the synthesis of fetuin-A by hepatocytes is suppressed in AD; thus, fetuin-A act as a negative acute-phase protein (Figure 5A). 67 Depending on this study, considerably higher levels of TNF-α were also found in the AD group, which found to be inversely linked with concentrations of fetuin-A. 66
Figure 5.
A, Process showing α-2-HS-glycoprotein as biomarker of AD: Inflammation in AD causes increased cytokine production, mainly TNF-α, resulting in decreased production of fetuin-A level by hepatocytes as fetuin-A acts as a negative acute-phase protein of inflammation and thus act as a biomarker of AD. 16 B, Effect of MMSE on AD: Evolution of mean patients’ decline on MMSE score cognitive function (with plus without treatment), that is, with or without consistent amateur caregivers, the patients who are at early stage of AD are in general under aegis of family. Though, the prerequisite for enduring care ALF or nursing upsurges vividly as AD grows to moderate and then goes to severe stages. AD indicates Alzheimer’s disease; ALF, assisted living facilities; MMSE, Mini-Mental State Examination.
Moreover, fetuin-A level also associated with the Mini-Mental State Examination score, which may consider as a factor contributing to severity as well as progression of AD (Figure 5B ). Different results from different findings proposed that abridged plasma measure of fetuin-A may possibly a potential AD biomarker for its early diagnosis. 68
Downregulation of Afamin
Afamin is recognized as a binding protein specific for vitamin E. It plays a significant role in the transport of vitamin E across the blood–brain barrier. Afamin belongs to the gene family of albumin that also consists of albumin, vitamin D–binding protein, and α-fetoprotein. 69 Western blotting and analysis from Isobaric tags for relative and absolute quantitation (iTRAQ) revealed that afamin is downregulated in MCI and AD plasma. It is also suggested by recent studies that afamin is produced in blood–brain barrier in endothelial cells. Vitamin E is a member of nonenzymatic fat-soluble antioxidants. 70 Through different studies, it is testified that afamin and vitamin E possess neuroprotective activity against oxidative destruction caused by H2O2 or Aβ. Oxidative damage has been found as a consequence of inflammatory reactions observed in the brain of patients with AD. 71
It has been found through recent gene chip technology that deficiency of vitamin E can have resilient effects on gene expression in brain region (hippocampus), which is the primary brain region to be affected in AD. 72 Therefore, decrease in the plasma levels of afamin results in reduced transport of vitamin E and thus greater oxidative stress; in this way, it acts as a biomarker. Moreover, studies supported by Chicago Health and Ageing Project and Rotterdam Study suggest delayed AD progression by the intake of vitamin E. 53
Future Perspective
The future prospect in the field of the AD requires a more delicate knowledge of not only the present scientific methods but also the linguistic and traditional perspectives of preclinical and experimental research and different policy conducts. The diagnostic challenges of AD or dementia are quite large and growing, but only a few steps apart from proper scientific, social, and health concerns. 73
Indication of different processes associated with the beginning of disease, phases of disease, and responses to sanative medications employs biomarkers as indicators. 74 During different developmental stages of the disease, the concentration of each specific biomarker becomes raised at different intervals of time. Therefore, it is critical to determine at which stage of disease specific biomarker is to be measured, it is essential because this will help us in future to provide therapeutic interventions at proper time to cure disease or to stop its progression.
Due to cost-effectiveness and ease of collection, blood testing is considered a widely accepted and easy way of measuring biomarkers. Furthermore, the escalating availability of sets of large samples obtained from the use of various technologies may help in predicting, analyzing, and observing the progression of AD. A reduction in the fragmentation and variability of data can be achieved if sample collection standardization, standardized operating procedures, management of comprehensive data, and scientific outcome altercation are acclaimed and if the concerted studies pursue development. Most probably, we will see biomarkers present in plasma as the most suitable benchmarks for the diagnosis of AD. For the sake of this rapidly growing crisis of health care, plasma biomarkers for the diagnosis of disease, subclass (ie, intermediate phenotype), and treatment reaction are essential to improve anticipatory and therapeutic prospects. 5
Conclusion
From all the above discussion, we can conclude that for a neurological disorder such as AD having varied symptoms and risk factors, different plasma biomarkers are required that can be discovered using proteomics and metabolomics. Plasma biomarkers have been proved as an emerging and an incredible source of identifying early stages and progression of AD. A plasma biomarker, 5-protein classifier, is identified, validated, and used for the diagnosis of AD. Through different experiments, it is concluded that plasma proteins such as apolipoproteins, AHSG, fibrinogen γ chain, C4a protein, and afamin showed significantly different expression levels in plasma of patients with AD compared to healthy individuals. For diagnostic purposes, lumber puncture is considered difficult and also invasive; therefore, plasma proteins could act as potential biomarkers for the diagnosis of AD at an early stage. In future, methods are required that can measure the specific biomarker for specific stage of AD to stop its progression and cure it on time. Thus, using various technologies, large samples sets may contribute in predicting, diagnosing, and monitoring prognosis of AD.
Footnotes
The authors declared no potential conflicts of interest with respect to the research, authorship, and/or publication of this article.
Funding: The authors received no financial support for the research, authorship, and/or publication of this article.
ORCID iD: Muhammad Naveed  https://orcid.org/0000-0002-4333-8226
https://orcid.org/0000-0002-4333-8226
References
- 1. Alzheimer’s Association. 2010 Alzheimer’s disease facts and figures. Alzheimer Dementia. 2010;6(2):158–194. [DOI] [PubMed] [Google Scholar]
- 2. Bateman RJ, Xiong C, Benzinger TL, et al. Clinical and biomarker changes in dominantly inherited Alzheimer’s disease. New Engl J Med. 2012;367(9):795–804. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 3. Unzeta M, Esteban G, Bolea I, et al. Multi-target directed Donepezil-like ligands for Alzheimer’s disease. Front Neurosci. 2016;10:205. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 4. Cai Z, Ratka A. Opioid system and Alzheimer’s disease. Neuromol Med. 2012;14(2):91–111. [DOI] [PubMed] [Google Scholar]
- 5. Sohma H, Kokai Y. Plasma biomarkers in Alzheimer’s disease. In: Moretti D, ed. Update on Dementia. InTech. https://www.intechopen.com/books/update-on-dementia/plasma-biomarkers-in-alzheimer-s-disease. 2016. Accessed May 15, 2018. [Google Scholar]
- 6. Bazenet C, Lovestone S. Plasma biomarkers for Alzheimer’s disease: much needed but tough to find. Biomark Med. 2012;6(4):441–454. [DOI] [PubMed] [Google Scholar]
- 7. Lu H, Zhu XC, Jiang T, Yu JT, Tan L. Body fluid biomarkers in Alzheimer’s disease. Ann Transl Med. 2015;3(5):70. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 8. La Mendola D, Pietropaolo A, Pappalardo G, Zannoni C, Rizzarelli E. Prion proteins leading to neurodegeneration. Curr Alzheimer Res. 2008;5(6):579–590. [DOI] [PubMed] [Google Scholar]
- 9. Craig-Schapiro R, Fagan AM, Holtzman DM. Biomarkers of Alzheimer’s disease. Neurobiol Dis. 2009;35(2):128–140. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 10. Hye A, Lynham S, Thambisetty M, et al. Proteome-based plasma biomarkers for Alzheimer’s disease. Brain. 2006;129(11):3042–3050. [DOI] [PubMed] [Google Scholar]
- 11. Hanash S. Disease proteomics. Nature. 2003;422(6928):226–232. [DOI] [PubMed] [Google Scholar]
- 12. Green RC, Cupples LA, Go R, et al. Risk of dementia among white and African American relatives of patients with Alzheimer disease. JAMA. 2002;287(3):329–336. [DOI] [PubMed] [Google Scholar]
- 13. Papassotiropoulos A, Fountoulakis M, Dunckley T, Stephan DA, Reiman EM. Genetics, transcriptomics and proteomics of Alzheimer’s disease. J Clin Psychiatry. 2006;67(4):652–670. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 14. Caudle WM, Pan S, Shi M, et al. Proteomic identification of proteins in the human brain: towards a more comprehensive understanding of neurodegenerative disease. Proteomics Clin Appl. 2008;2(10-11):1484–1497. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 15. Serkova NJ, Standiford TJ, Stringer KA. The emerging field of quantitative blood metabolomics for biomarker discovery in critical illnesses. Am J Respir Crit Care Med. 2011;184(6):647–655. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 16. Barba I, Fernandez-Montesinos R, Garcia-Dorado D, Pozo D. Alzheimer’s disease beyond the genomic era: nuclear magnetic resonance (NMR) spectroscopy-based metabolomics. J Cell Mol Med. 2008;12(5a):1477–1485. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 17. Galozzi S, Marcus K, Barkovits K. Amyloid-β as a biomarker for Alzheimer’s disease: quantification methods in body fluids. Expert Rev Proteomics. 2015;12(4):343–354. [DOI] [PubMed] [Google Scholar]
- 18. Nakamura A, Kaneko N, Villemagne VL, et al. High performance plasma amyloid-β biomarkers for Alzheimer’s disease. Nature. 2018;554(7691):249–254. [DOI] [PubMed] [Google Scholar]
- 19. Pannee J, Törnqvist U, Westerlund A, et al. The amyloid-β degradation pattern in plasma—a possible tool for clinical trials in Alzheimer’s disease. Neurosci Lett. 2014;573:7–12. [DOI] [PubMed] [Google Scholar]
- 20. Ovod V, Ramsey KN, Mawuenyega KG, et al. Amyloid β concentrations and stable isotope labeling kinetics of human plasma specific to central nervous system amyloidosis. Alzheimers Dement. 2017;13(8):841–849. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 21. Zakharova NV, Bugrova AE, Kononikhin AS, et al. Mass spectrometry analysis of the diversity of Aβ peptides: difficulties and future perspectives for AD biomarker discovery. Expert Rev Proteomics. 2018;15(10):773–775. Taylor & Francis. [DOI] [PubMed] [Google Scholar]
- 22. Blennow K, Mattsson N, Schöll M, Hansson O, Zetterberg H. Amyloid biomarkers in Alzheimer’s disease. Trends Pharmacol Sci. 2015;36(5):297–309. [DOI] [PubMed] [Google Scholar]
- 23. Wang T, Xiao S, Liu Y, et al. The efficacy of plasma biomarkers in early diagnosis of Alzheimer’s disease. Int J Geriatr Psychiatry. 2014;29(7):713–719. [DOI] [PubMed] [Google Scholar]
- 24. Baker HN, Murphy R, Lopez E, Garcia C. Conversion of a capture ELISA to a Luminex xMAP assay using a multiplex antibody screening method. J Vis Exp. 2012;6(65): 4084. doi:10.3791/4084. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 25. Pickering JW, Hill HR. Measurement of antibodies to pneumococcal polysaccharides with Luminex xMAP microsphere-based liquid arrays. Methods Mol Biol. 2012;808:361–375. [DOI] [PubMed] [Google Scholar]
- 26. Janelidze S, Stomrud E, Palmqvist S, et al. Plasma β-amyloid in Alzheimer’s disease and vascular disease. Sci Rep. 2016;6:26801. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 27. Zetterberg H, Wilson D, Andreasson U, et al. Plasma tau levels in Alzheimer’s disease. Alzheimers Res Ther. 2013;5(2):9. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 28. Kaneko N, Nakamura A, Washimi Y, et al. Novel plasma biomarker surrogating cerebral amyloid deposition. Proc Jpn Acad Ser B Phys Biol Sci. 2014;90(9):353–364. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 29. Lee SJC, Nam E, Lee HJ, Savelieff MG, Lim MH. Towards an understanding of amyloid-β oligomers: characterization, toxicity mechanisms, and inhibitors. Chem Soc Rev. 2017;46(2):310–323. [DOI] [PubMed] [Google Scholar]
- 30. Nabers A, Ollesch J, Schartner J, et al. Amyloid-β-secondary structure distribution in cerebrospinal fluid and blood measured by an immuno-infrared-sensor: a biomarker candidate for Alzheimer’s disease. Anal Chem. 2016;88(5):2755–2762. [DOI] [PubMed] [Google Scholar]
- 31. Nabers A, Perna L, Lange J, et al. Amyloid blood biomarker detects Alzheimer’s disease. EMBO Mol Med. 2018;10(5):e8763. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 32. Regeniter A, Kuhle J, Baumann T, et al. Biomarkers of dementia: comparison of electrochemiluminescence results and reference ranges with conventional ELISA. Methods 2012;56(4):494–499. [DOI] [PubMed] [Google Scholar]
- 33. Chiu MJ, Yang SY, Horng HE, et al. Combined plasma biomarkers for diagnosing mild cognition impairment and Alzheimer’s disease. ACS Chem Neurosci. 2013;4(12):1530–1536. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 34. Chiu MJ, Chen YF, Chen TF, et al. Plasma tau as a window to the brain—negative associations with brain volume and memory function in mild cognitive impairment and early Alzheimer’s disease. Hum Brain Mapp. 2014;35(7):3132–3142. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 35. Dage JL, Wennberg AM, Airey DC, et al. Levels of tau protein in plasma are associated with neurodegeneration and cognitive function in a population-based elderly cohort. Alzheimers Dement. 2016;12(12):1226–1234. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 36. Greenberg N, Grassano A, Thambisetty M, Lovestone S, Legido-Quigley C. A proposed metabolic strategy for monitoring disease progression in Alzheimer’s disease. Electrophoresis 2009;30(7):1235–1239. [DOI] [PubMed] [Google Scholar]
- 37. Hebert LE, Weuve J, Scherr PA, Evans DA. Alzheimer disease in the United States (2010–2050) estimated using the 2010 census. Neurology. 2013;80(19):1778–1783. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 38. Mahley RW, Rall SC, Jr. Apolipoprotein E: far more than a lipid transport protein. Annu Rev Genomics Hum Genet. 2000;1(1):507–537. [DOI] [PubMed] [Google Scholar]
- 39. Guo L-H, Alexopoulos P, Wagenpfeil S, Kurz A, Perneczky R.; Alzheimer’s Disease Neuroimaging Initiative, Plasma proteomics for the identification of Alzheimer’s disease. Alzheimer Dis Assoc Disord. 2013;27(4):337–342. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 40. Thambisetty M, Simmons A, Velayudhan L, et al. Association of plasma clusterin concentration with severity, pathology, and progression in Alzheimer disease. Arch Gen Psychiatry. 2010;67(7):739–748. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 41. Thambisetty M, An Y, Kinsey A, et al. Plasma clusterin concentration is associated with longitudinal brain atrophy in mild cognitive impairment. Neuroimage. 2012;59(1):212–217. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 42. Harold D, Abraham R, Hollingworth P, et al. Genome-wide association study identifies variants at CLU and PICALM associated with Alzheimer’s disease. Nat Genet. 2009;41(10):1088. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 43. Erk S, Meyer-Lindenberg A, von Boberfeld CO, et al. Hippocampal function in healthy carriers of the CLU Alzheimer’s disease risk variant. J Neurosci. 2011;31(49):18180–18184. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 44. Bettens K, Brouwers N, Engelborghs S, et al. Both common variations and rare non-synonymous substitutions and small insertion/deletions in CLU are associated with increased Alzheimer risk. Mol Neurodegener. 2012;7(1):3. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 45. Furney SJ, Kronenberg D, Simmons A, et al. Combinatorial markers of mild cognitive impairment conversion to Alzheimer’s disease-cytokines and MRI measures together predict disease progression. J Alzheimers Dis. 2011;26(s3):395–405. [DOI] [PubMed] [Google Scholar]
- 46. Kiddle SJ, Sattlecker M, Proitsi P, et al. Candidate blood proteome markers of Alzheimer’s disease onset and progression: a systematic review and replication study. J Alzheimers Dis. 2014;38(3):515–531. [DOI] [PubMed] [Google Scholar]
- 47. Zhao X, Lejnine S, Spond J, et al. A candidate plasma protein classifier to identify Alzheimer’s disease. J Alzheimers Dis. 2015;43(2):549–563. [DOI] [PubMed] [Google Scholar]
- 48. Ravetti MG, Moscato P. Identification of a 5-protein biomarker molecular signature for predicting Alzheimer’s disease. PLoS One. 2008;3(9):e3111. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 49. Bruunsgaard H, Andersen-Ranberg K, Jeune B, et al. A high plasma concentration of TNF-α is associated with dementia in centenarians. J Gerontol A Biol Sci Med Sci. 1999;54(7):M357–M364. [DOI] [PubMed] [Google Scholar]
- 50. Cortes-Canteli M, Strickland S. Fibrinogen, a possible key player in Alzheimer’s disease. J Thromb Haemost. 2009;7(suppl 1):146–150. [DOI] [PubMed] [Google Scholar]
- 51. Song F, Poljak A, Kochan NA, et al. Plasma protein profiling of mild cognitive impairment and Alzheimer’s disease using iTRAQ quantitative proteomics. Proteome Sci. 2014;12(1):5. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 52. Pretorius E, Kell DB. Diagnostic morphology: biophysical indicators for iron-driven inflammatory diseases. Integr Biol (Camb). 2014;6(5):486–510. [DOI] [PubMed] [Google Scholar]
- 53. Muenchhoff J, Poljak A, Song F, et al. Plasma protein profiling of mild cognitive impairment and Alzheimer’s disease across two independent cohorts. J Alzheimers Dis. 2015;43(4):1355–1373. [DOI] [PubMed] [Google Scholar]
- 54. van Oijen M, Witteman JC, Hofman A, Koudstaal PJ, Breteler MM. Fibrinogen is associated with an increased risk of Alzheimer disease and vascular dementia. Stroke. 2005;36(12):2637–2641. [DOI] [PubMed] [Google Scholar]
- 55. Zamolodchikov D, Berk-Rauch HE, Oren DA, et al. Biochemical and structural analysis of the interaction between β-amyloid and fibrinogen. Blood. 2016;128(8):1144–1151 [DOI] [PMC free article] [PubMed] [Google Scholar]
- 56. Pretorius E, Lipinski B. The role of iron-induced fibrin in the pathogenesis of Alzheimer’s disease and the protective role of magnesium. Front Hum Neurosci. 2013;7:735. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 57. Bennett S, Grant M, Creese AJ, et al. Plasma levels of complement 4a protein are increased in Alzheimer’s disease. Alzheimer Dis Assoc Disord. 2012;26(4):329–334. [DOI] [PubMed] [Google Scholar]
- 58. Merched A, Xia Y, Visvikis S, Serot J, Siest G. Decreased high-density lipoprotein cholesterol and serum apolipoprotein AI concentrations are highly correlated with the severity of Alzheimer’s disease. Neurobiol Aging. 2000;21(1):27–30. [DOI] [PubMed] [Google Scholar]
- 59. Koldamova RP, Lefterov IM, Lefterova MI, Lazo JS. Apolipoprotein AI directly interacts with amyloid precursor protein and inhibits Aβ aggregation and toxicity. Biochemistry. 2001;40(12):3553–3560. [DOI] [PubMed] [Google Scholar]
- 60. Song F, Poljak A, Smythe GA, Sachdev P. Plasma biomarkers for mild cognitive impairment and Alzheimer’s disease. Brain Res Rev. 2009;61(2):69–80. [DOI] [PubMed] [Google Scholar]
- 61. Castaño EM, Roher AE, Esh CL, Kokjohn TA, Beach T. Comparative proteomics of cerebrospinal fluid in neuropathologically-confirmed Alzheimer’s disease and non-demented elderly subjects. Neurol Res. 2006;28(2):155–163. [DOI] [PubMed] [Google Scholar]
- 62. Liao F, Yoon H, Kim J. Apolipoprotein E metabolism and functions in brain and its role in Alzheimer’s disease. Curr Opin Lipidol. 2017;28(1):60–67. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 63. Yu J-T, Tan L, Hardy J. Apolipoprotein E in Alzheimer’s disease: an update. Annu Rev Neurosci. 2014;37:79–100. [DOI] [PubMed] [Google Scholar]
- 64. Cui Y, Huang M, He Y, Zhang S, Luo Y. Genetic ablation of apolipoprotein A-IV accelerates Alzheimer’s disease pathogenesis in a mouse model. Am J Pathol. 2011;178(3):1298–1308. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 65. Liu HC, Hu CJ, Chang JG, et al. Proteomic identification of lower apolipoprotein AI in Alzheimer’s disease. Dement Geriatr Cogn Disord. 2006;21(3):155–161. [DOI] [PubMed] [Google Scholar]
- 66. Smith ER, Nilforooshan R, Weaving G, Tabet N. Plasma fetuin-A is associated with the severity of cognitive impairment in mild-to-moderate Alzheimer’s disease. J Alzheimers Dis. 2011;24(2):327–333. [DOI] [PubMed] [Google Scholar]
- 67. Mukhopadhyay S, Mondal S, Kumar M, Dutta D. Proinflammatory and antiinflammatory attributes of fetuin-A: a novel hepatokine modulating cardiovascular and glycemic outcomes in metabolic syndrome. Endocr Pract. 2014;20(12):1345–1351. [DOI] [PubMed] [Google Scholar]
- 68. Puchades M, Hansson SF, Nilsson CL, et al. Proteomic studies of potential cerebrospinal fluid protein markers for Alzheimer’s disease. Brain Res Mol Brain Res. 2003;118(1-2):140–146. [DOI] [PubMed] [Google Scholar]
- 69. Yang H, Lyutvinskiy Y, Herukka SK, et al. Prognostic polypeptide blood plasma biomarkers of Alzheimer’s disease progression. J Alzheimers Dis. 2014;40(3):659–666. [DOI] [PubMed] [Google Scholar]
- 70. Kratzer I, Bernhart E, Wintersperger A, et al. Afamin is synthesized by cerebrovascular endothelial cells and mediates α-tocopherol transport across an in vitro model of the blood–brain barrier. J Neurochem. 2009;108(3):707–718. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 71. Heiser M, Hutter-Paier B, Jerkovic L, et al. Vitamin E binding protein afamin protects neuronal cells in vitro. In: Heiser M1, Hutter-Paier B, Jerkovic L, Pfragner R, Windisch M, Becker-André M, Dieplinger H, eds. Ageing and Dementia Current and Future Concepts. Berlin, Germany: Springer; 2002:337–345. [DOI] [PubMed] [Google Scholar]
- 72. Ringman JM, Schulman H, Becker C, et al. Proteomic changes in cerebrospinal fluid of presymptomatic and affected persons carrying familial Alzheimer disease mutations. Arch Neurol. 2012;69(1):96–104. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 73. Jagtap A, Gawande S, Sharma S. Biomarkers in vascular dementia: a recent update. Biomark Genomic Med. 2015;7(2):43–56. [Google Scholar]
- 74. Wright C, Hall A, Matthews F, Brayne C. Biomarkers, dementia, and public health. Ann N Y Acad Sci. 2009;1180(1):11–19. [DOI] [PubMed] [Google Scholar]