Abstract
Objective:
We conducted a longitudinal study to explore the clinical and pathological correlates of cerebral microbleeds (CMBs) in institutionalized patients with dementia.
Methods:
Clinical and magnetic resonance imaging (MRI) data were extracted from 182 nursing home patients (mean age [standard deviation]: 81.3 [6.9], 78.0% female, and 83.4% moderate to severe dementia), which were divided according to the CMBs number and location. One-year follow-up data were obtained from 153 patients, and postmortem pathological diagnosis was available in 40 patients.
Results:
Cerebral microbleeds were observed in 42.9% of patients and were associated with MRI ischemic lesions (P < .0005). In the adjusted analysis, lobar CMB predicted worsening of parkinsonism (standardized β: 0.43) and gait (standardized β: 0.24). A pathological diagnosis of Alzheimer’s disease was less frequent in the brains of patients with lobar and deep CMB (33.3% vs 85.3%; P < .05).
Conclusion:
Cerebral microbleeds were linked to cerebrovascular disease and predicted motor deterioration in institutionalized people with advanced dementia.
Keywords: Alzheimer’s disease, amyloid angiopathy, cerebral microbleeds, cerebrovascular disease, dementia
Introduction
Cerebral microbleeds (CMBs) are hemosiderin-laden macrophages, which appear as small, round, hypointense lesions by T2* magnetic resonance imaging (MRI) acquisition techniques. 1,2 Cerebral microbleeds are strongly associated with aging 3 but may also be observed in patients with Alzheimer’s disease (AD) 4 and, most frequently, in patients with hypertension or cerebrovascular disease. 1 -3,5 Cerebral microbleeds are due to the rupture of small arteries or arterioles, which is mediated by lipohyalinosis (subcortical CMBs) or cerebral amyloid angiopathy (lobar CMBs). 6 Blood–brain barrier disruption and hemorrhagic infarction may be also involved in the pathogenesis of CMBs. 7
Although the pathological substrate and the clinical relevance of CMBs in terms of vascular disease and mortality have been extensively investigated, 5,8 -10 little is known about the clinical implications of CMBs in people with dementia. Recently, a key role of CMBs in the physiopathology of AD was proposed, 11 which has not been specifically investigated. Consistent with that hypothesis, CMBs should convey specific clinical characteristics, such as more severe course of disease or clinical signs congruent with CMBs location.
Several studies conducted in the last 2 decades displayed consistent association of CMBs and cognitive impairment, particularly regarding executive functions, even if potential confounders, such as ischemic lesions, were controlled. However, most studies were cross-sectional and were conducted in the general population 12,13 or in patients with cerebrovascular disease. 14,15 To date, few studies have addressed the clinical relevance of CMBs in patients with AD. 16 -18 This lack of empirical data is particularly evident regarding areas other than cognition, such as motor skills, functional capacities, mood, and behavior. 19,20 A hypothetical influence of CMBs in those areas could be especially relevant for the quality of life of people with advanced dementia. 21
The aim of this investigation was to explore the potential implications of CMBs in the domains of cognition, function, mood, and behavior of people with advanced dementia. In addition, the pathological correlates of CMBs were investigated in a subset of 40 patients. The present investigation augments the sample and expands the scope of analysis of a previous cross-sectional report of prevalence, topography, and risk factors of CMBs. 22
Materials and Methods
Setting
The database of the Alzheimer’s Center Reina Sofía Foundation-CIEN Foundation (ACRSF) was searched for the present investigation. The mission and the clinical protocol of the ACRSF was described previously. 23,24 Briefly, patients with neurodegenerative dementia are admitted to nursing home or day care center through the usual channels of the Community of Madrid Public System of Health. Upon admission, the families are invited to sign consent to participate in a research program that includes clinical and social assessment, blood determinations, MRI study, and brain donation. The informed consent form for the ACRSF research protocol was previously approved by the ACRSF ethics committee. Clinical assessments and blood extraction are performed every 6 months, whereas MRI is performed annually. All the patients who received the initial (ie, 2-6 weeks after admission) clinical visit and had also MRI study were included in the study.
Clinical Variables
The following variables were selected from the ACRSF protocol for the purposes of the present investigation:
Demographic variables: age, sex, and education.
Vascular risk factors and vascular diseases: hypertension, diabetes, dyslipidemia, tobacco use, ischemic cardiopathy, atrial fibrillation, and cerebrovascular episodes (including transient ischemic attacks). These variables were coded as positive if there was evidence of any of them, at any time of the patient’s life, after the interview with the relatives and the review of all available medical reports.
Medications: antiaggregant or anticoagulant agents, cholinesterase inhibitors, typical neuroleptics, and atypical neuroleptics. The medications were analyzed on a double basis, that is, life-time years of treatment and whether or not patient was treated at assessment date.
Cognitive variables: cognitive capacities were evaluated using the mini-mental state examination (MMSE), 25 the severe MMSE (SMMSE), 26 and the verbal fluency task (number of animals in 1 minute). 27
Functional variables: functional dependence was assessed with the index of activities of daily living (IADL) 28 and the functional assessment staging. 29
Neuropsychiatric variables: the neuropsychiatric inventory 30 was utilized for the evaluation of neuropsychiatric symptoms.
Motor variables: parkinsonism was evaluated using the motor evaluation scale of the Scales for Outcomes in Parkinson’s Disease (SCOPA-Motor), 31 and gait was assessed using the Rating Scale for Gait Evaluation in Cognitive Deterioration (RSGE-CD). 32
Other dementia-related variables: disease duration, memory failure, and falls at symptom onset were obtained from medical records and family interview. Severity of dementia was rated following the Global Deterioration Scale 33 and the Clinical Dementia Rating Scale, sum of boxes score. 34 Etiological diagnosis of dementia was established after consensus meeting, following standard criteria. 35 -37 The number of ε4 apolipoprotein E gen alleles was obtained using the real-time polymerase chain reaction technique. 38
Magnetic Resonance Imaging Study and CMB Assessment
Magnetic resonance images were acquired in a Signa HDxt (GEHC, Waukesha, Wisconsin) 3 T scanner at the Neuroimaging Department of the ACRSF. The scanner was equipped with an 8-channel phased array receive coil and a gradient system capable of producing a gradient strength of 50 mT/m. The MRI protocol included the following pulse sequences: (1) oblique reconstructions of a 3-dimensional fast-spoiled gradient recalled echo-based sequence (matrix: 288 × 288, field of view [FOV]: 24 × 24 cm, slice thickness: 1 mm, repetition time [TR]: 7.1 ms, echo time [TE]: 3.1 ms, 1 excitation); (2) axial 2-dimensional (2D) gradient-echo echo-planar imaging (matrix: 224 × 192, FOV: 24 × 24, slice thickness: 2.4 mm, TR: 2225 ms, TE: 11.5 ms, 2 excitations); (3) axial 2D fluid-attenuated inversion recovery (matrix: 256 × 192, FOV: 24 × 24 cm, slice thickness: 3.4 mm, TR: 9000 ms, TE: 124 ms, inversion time: 2500 ms), and (4) coronal 2D, T2-weighted fast-spin echo (matrix: 384 × 224, FOV: 22 × 22 cm, slice thickness: 3.4 mm, TR: 5340 ms, TE: 85 ms).
Cerebral microbleeds were systematically searched, counted, and located following a previously elaborated protocol. They were identified as very hypointense, intra-axial, and rounded lesions of less than 10 mm diameter. 39,40 The predefined locations of CMBs were as follows: cerebral lobes (with the distinction of frontal, temporal, parietal, and occipital lobes), periventricular and deep white matter (for CMBs that were in the intimate vicinity of, or less than 1 cm far from, the ventricular wall), basal ganglia (thalamus included), cerebellum, and brain stem. The protocol of evaluation of MRI results also included categorical ratings of global and hippocampal brain atrophy, using a scale from 0 (no atrophy) to 4 (maximal atrophy) 41 and white matter and subcortical small vessel disease, using a scale from 0 (no disease) to 3 (maximal disease). 42 The presence of either ischemic or hemorrhagic cortical and cerebellar infarction was also qualitatively evaluated. All the MRI assessments were performed by an experienced neuroradiologist (AR) who was unaware of all clinical data.
Pathological Study
The ACRSF procedure for brain extraction and pathological study was described in detail elsewhere. 43 Briefly, immediately after extraction, the brain is divided into 2 halves by means of a midsagittal incision. The right half of the brain is subsequently cut following the brain bank protocol and frozen in isopentane at −50°C. Before freezing, all tissue slices are examined, and gross focal or regional lesions are discarded. The left half of the brain, including left cerebellar hemisphere and left hemi-brainstem, is fixed in 4% buffered formaldehyde for 3 weeks. After fixation, 25 tissue blocks are obtained for routine paraffin processing, comprising all cortical and subcortical regions relevant for the assessment of neurodegenerative and vascular pathology. Paraffin sections of all tissue blocks are stained with hematoxylin-eosin; and specific sections, according to current consensus criteria, are immunostained for β-amyloid, τ, ubiquitin, α-synuclein, TAR DNA binding protein 43 (TDP-43), adaptor protein p62, and fused in sarcoma RNA-binding protein (FUS). The presence of amyloid angiopathy is also systematically sought, and its severity is graded on a 4-category scale. 43 Only Alzheimer’s, Lewy body’s, and vascular pathology was considered and analyzed in the present investigation. 44 -46
Statistical Analysis
Descriptive statistics, such as frequency, central tendency, and distribution methods, were utilized for the inspection of data quality and for the results presentation. Of special interest in the study of patients with advanced dementia, percentage of data lost was calculated for the different cognitive, functional, motor, and neuropsychiatric outcome measures. Acceptable limits for data lost and floor effect were set at 10% and 15%, respectively. 47,48
Patients were divided according to CMBs location as follows: no CMBs, strictly lobar CMBs, strictly deep CMBs, and lobar and deep CMBs. The clinical and paraclinical characteristics of the study groups at study inception were compared using 1-way analysis of variance for continuous variables and χ2test for categorical variables. The influence of CMBs in the clinical evolution at 1-year follow-up assessment was analyzed using regression analysis, which was adjusted for age, MRI ischemic lesions (sum of scores at the different regions), 42 and initial score in the dependent variable. Once the control variables were introduced, the effect of CMBs number and location (lobar CMBs or deep CMBs) was explored using a forward stepwise procedure (P-to-enter < .05 and P-to-leave > .10). 49
To more sensitively explore the potential effects of CMBs in the clinical evolution, the last available MRI of the ACRSF protocol was also evaluated and analyzed for each patient, along with the corresponding clinical outcomes at that visit. Bivariate correlations (ie, Spearman r coefficient) between change in number of CMBs (total CMBs, lobar CMBs, or deep CMBs) and change in clinical outcome were conducted for that analysis, which were interpreted as follows: negligible, <0.20; weak, 0.20-0.34; moderate, 0.35-0.50; and strong, >0.50. 50 All the statistical tests were 2 sided. Given the essentially exploratory nature of the study, multiple comparison adjustments were not planned. 51 The statistical analyses were conducted using the Statistical Package for Social Sciences software, version 15.0 (SPSS, Chicago, Illinois).
Results
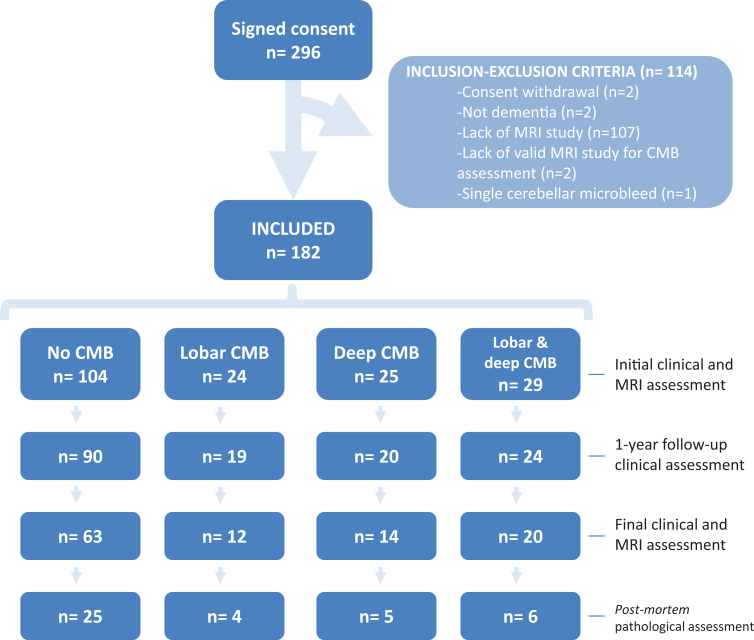
Consent for research was obtained for 296 patients from time of ACRSF inception (ie, June 2007) to time of data extraction for the present investigation (ie, June 2014). That proportion of consent approximately represented two thirds of the patients admitted to the ACRSF. Once consent was signed, 107 (36.1%) patients were excluded from the study due to lack of MRI study and 7 (2.4%) patients were excluded due to other reasons (Figure 1). The included patients had a lower prevalence of dyslipidemia and less exposure to antiaggregant medications and typical neuroleptics. The included patients also displayed better cognitive performance, exhibited less neuropsychiatric symptoms—particularly anxiety and agitation—had less severe dementia, received more frequently a clinical diagnosis of mixed dementia (ie, AD with cerebrovascular disease), and had less frequently pathological diagnosis of AD (P < .05, all other P values ≥ .05, data not shown).
Figure 1.
Patient inclusion and attrition throughout the 4 study assessments.
The study sample consisted of 182 people of advanced age (mean age: 81.3 and SD: 6.9) and predominantly female sex (142/182, 78.0%). Most patients (167/182, 91.8%) were in the moderate to severe stages of dementia and the most frequent etiological diagnosis of dementia, made on the basis of clinical, laboratory, and neuroimaging data, was AD (152/182, 83.5%).
The amount of missing data was superior to 10% of patients for the following variables: SMMSE (21/182 patients with missing data in the initial visit, 11.5%), IADL (29/182, 15.9%), and RSGE-CD (30/182, 16.5%). Floor effect was observed in the verbal fluency test (63/182 patients with the lowest score in the initial visit, 34.6%) and in the IADL (38/182, 20.9%).
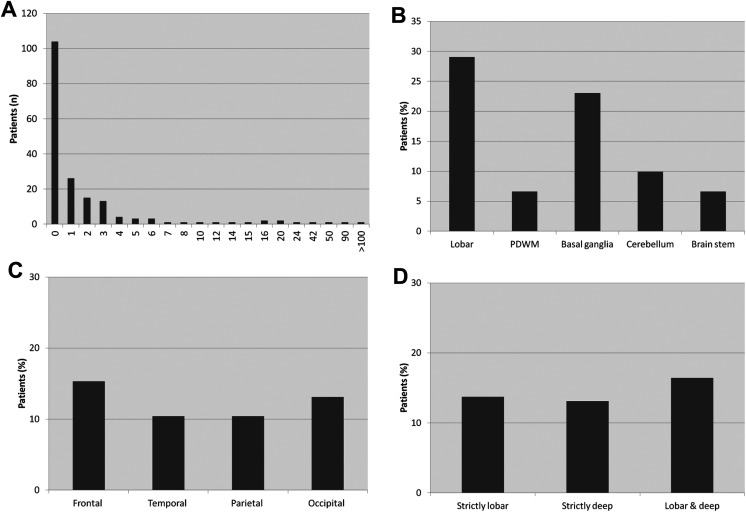
Most patients (104/182, 57.1%) did not display CMBs and only a minority of patients (15/182, 8.2%) presented 7 or more CMBs (Figure 2). The presence of CMBs was significantly related to age: mean (SD) age of patients with CMBs was 83.0 (6.0) years versus 80.1 (7.3) years for patients without CMBs (P < .01). Cerebral microbleeds were mostly located in the cerebral lobes (53 [29.1%] of 182 patients,) and in the basal ganglia (42 [23.1%] of 182 patients) and were uniformly distributed across the 4 cerebral lobes. Apart from the patients without CMBs, no other study group showed predominance in prevalence: 24 (13.2%) of 182 patients displayed strictly lobar CMBs, 25 (13.7%) of 182 strictly deep CMBs, and 29 (15.9%) of 182 lobar and deep CMBs.
Figure 2.
Number or frequency of patients according to CMBs number (A) and location (B-D; n = 182). CMBs indicates cerebral microbleeds; PDWM, periventricular and deep white matter.
The characteristics of the study groups are presented in Table 1. No differences were observed in symptom duration, initial clinical manifestations, vascular risk factors, or medications. There was consistent association between profound white matter lesions (WML), basal ganglia, thalamic, and brainstem ischemic lesions and deep CBM (all P values <.05) regardless of whether those CMBs were associated with or not with lobar CMBs. In addition, a trend of more frequent ischemic (P = .053) or hemorrhagic (P = .132) cortical infarction was detected in the groups of patients with CMBs. Severe or very severe hippocampal atrophy was less frequently observed in patients with lobar and deep CMBs (P < .05), and the diagnosis of probable AD tended to be more frequently given to those patients displaying no, or strictly lobar, CMBs (P = .089)
Table 1.
Clinical, Neuroimaging, and Pathological Characteristics of the Study Groups.a
| No CMBs (n = 104) | Strictly Lobar CMBs (n = 24) | Strictly Deep CMBs (n = 25) | Lobar and Deep CMB (n = 29) |
P | |
|---|---|---|---|---|---|
| Age | 80.1 (7.3) | 82.8 (7.4) | 83.4 (4.3) | 82.7 (6.0) | .052 |
| Sex, % female | 79.8 | 75.0 | 72.0 | 78.6 | .837 |
| Education, % | 0.616 | ||||
| Illiterate | 6.8 | 8.7 | 8.0 | 17.9 | |
| Incomplete primary education | 17.5 | 13.0 | 20.0 | 14.3 | |
| Primary education | 70.9 | 78.3 | 64.0 | 67.9 | |
| Secondary education | 4.9 | 0.0 | 8.0 | 0.0 | |
| Vascular risk factors (present or past), % | |||||
| Hypertension | 58.8 | 66.7 | 52.0 | 56.6 | .754 |
| Diabetes | 25.5 | 12.5 | 36.0 | 33.3 | .232 |
| Dyslipemia | 38.2 | 50.0 | 32.0 | 44.4 | .568 |
| Ischemic cardiopathy | 6.9 | 4.2 | 4.0 | 3.7 | .872 |
| Atrial fibrillation | 5.9 | 8.3 | 12.0 | 11.1 | .672 |
| Tobacco use | 10.8 | 12.5 | 12.0 | 7.4 | .933 |
| Cerebrovascular episode | 8.8 | 4.2 | 24.0 | 11.1 | .107 |
| Antiaggregant medication | |||||
| Present or past use, % | 24.5 | 20.8 | 44.0 | 37.0 | .146 |
| Years of use | 5.3 (8.6) | 3.2 (1.8) | 3.4 (2.0) | 4.4 (3.5) | .842 |
| Current use, % | 22.5 | 20.8 | 40.0 | 29.6 | .295 |
| Cholinesterase inhibitors | |||||
| Present or past use, % | 71.6 | 91.7 | 76.0 | 85.2 | .130 |
| Years of use | 2.0 (1.1) | 3.4 (2.6) | 3.7 (2.7) | 3.1 (2.4) | .480 |
| Current use, % | 56.4 | 54.2 | 56.0 | 66.7 | .692 |
| Typical neuroleptics | |||||
| Present or past use, % | 19.6 | 37.5 | 28.0 | 11.1 | .106 |
| Years of use | 2.4 (3.9) | 0.9 (0.5) | 0.9 (0.6) | 4.5 (5.0) | .236 |
| Current use, % | 10.8 | 12.5 | 4.0 | 7.4 | .692 |
| Atypical neuroleptics | |||||
| Present or past use, % | 38.2 | 16.7 | 40.0 | 22.2 | .106 |
| Years of use | 2.5 (2.2) | 2.4 (1.5) | 2.1 (1.1) | 2.4 (1.1) | .947 |
| Current use, % | 33.3 | 16.7 | 36.0 | 18.5 | .194 |
| Antidepressant medication | |||||
| Present or past use, % | 49.0 | 58.3 | 64.0 | 48.1 | .503 |
| Years of use | 4.5 (6.4) | 2.3 (1.4) | 3.1 (2.6) | 2.9 (2.2) | .457 |
| Current use, % | 42.0 | 41.7 | 60.0 | 44.4 | .432 |
| Symptom duration, years | 6.6 (2.8) | 6.8 (3.6) | 6.7 (2.8) | 7.0 (3.4) | .953 |
| Memory symptoms at onset, % | 74.5 | 78.3 | 79.2 | 81.5 | .866 |
| Falls at onset, % | 8.8 | 17.4 | 16.7 | 11.1 | .541 |
| Dementia severity (GDS), % | .414 | ||||
| GDS 3 | 0.0 | 4.2 | 0.0 | 0.0 | |
| GDS 4 | 7.7 | 8.3 | 4.0 | 10.7 | |
| GDS 5 | 22.1 | 20.8 | 20.0 | 32.1 | |
| GDS 6 | 47.1 | 50.0 | 64.0 | 46.4 | |
| GDS 7 | 23.1 | 16.7 | 12.0 | 10.7 | |
| MRI variables | |||||
| Global atrophy, % | .582 | ||||
| None | 1.9 | 4.2 | 0.0 | 3.6 | |
| Mild | 3.8 | 8.3 | 8.0 | 7.1 | |
| Moderate | 28.8 | 29.2 | 12.0 | 42.9 | |
| Severe | 48.1 | 41.7 | 64.0 | 39.3 | |
| Very severe | 17.3 | 16.7 | 16.0 | 7.1 | |
| Hippocampal atrophy, % | .048 | ||||
| None | 1.9 | 4.2 | 0.0 | 3.6 | |
| Mild | 2.9 | 0.0 | 12.0 | 7.1 | |
| Moderate | 23.1 | 16.7 | 8.0 | 42.9 | |
| Severe | 32.7 | 54.2 | 40.0 | 17.9 | |
| Very severe | 39.4 | 25.0 | 40.0 | 28.0 | |
| Periventricular WML, % | .416 | ||||
| No lesions | 18.3 | 16.7 | 8.0 | 6.9 | |
| Focal lesions | 35.6 | 29.2 | 24.0 | 34.5 | |
| Confluent lesions | 30.8 | 29.2 | 36.0 | 44.8 | |
| Diffuse lesions | 15.4 | 25.0 | 32.0 | 13.8 | |
| Profound WML, % | .001 | ||||
| No lesions | 16.3 | 12.5 | 12.0 | 3.4 | |
| Focal lesions | 40.4 | 54.2 | 8.0 | 20.7 | |
| Confluent lesions | 24.0 | 8.3 | 44.0 | 27.6 | |
| Diffuse lesions | 19.2 | 25.0 | 36.0 | 48.3 | |
| Basal ganglia ischemic lesions, % | .000 | ||||
| No lesions | 71.2 | 50.0 | 36.0 | 25.0 | |
| Focal lesion | 7.7 | 8.3 | 12.0 | 14.3 | |
| Focal lesions | 18.3 | 33.3 | 28.0 | 42.9 | |
| Confluent lesions | 2.9 | 8.3 | 24.0 | 17.9 | |
| Thalamic ischemic lesions, % | .039 | ||||
| No lesions | 76.9 | 75.0 | 54.2 | 60.7 | |
| Focal lesion | 14.4 | 16.7 | 16.7 | 14.3 | |
| Focal lesions | 8.7 | 8.3 | 20.8 | 14.3 | |
| Confluent lesions | 0.0 | 0.0 | 8.3 | 10.7 | |
| Brain stem ischemic lesions, % | .000 | ||||
| No lesions | 79.8 | 47.8 | 36.4 | 41.7 | |
| Focal lesion | 3.0 | 4.3 | 9.1 | 0.0 | |
| Focal lesions | 12.1 | 26.1 | 36.4 | 41.7 | |
| Confluent lesions | 5.1 | 21.7 | 18.2 | 16.7 | |
| Cortical infarct | |||||
| Ischemic infarct, % | 1.9 | 4.2 | 8.0 | 14.3 | .053 |
| Hemorrhagic infarct, % | 1.0 | 8.3 | 8.0 | 7.1 | .132 |
| Clinical diagnosis, % | .089 | ||||
| Probable AD | 55.8 | 60.9 | 40.0 | 39.3 | |
| Possible AD | 13.5 | 7.4 | 4.0 | 3.6 | |
| AD and cerebrovascular disease | 13.5 | 17.4 | 32.0 | 42.9 | |
| Lewy body dementia | 6.7 | 0.0 | 12.0 | 3.6 | |
| Parkinson’s disease with dementia | 3.8 | 0.0 | 4.0 | 0.0 | |
| Other dementia | 6.7 | 4.3 | 8.0 | 10.7 | |
| Pathological diagnosis, %b | |||||
| AD | 80.0 | 100.0 | 100.0 | 33.3 | .024 |
| Vascular dementia | 52.0 | 75.0 | 40.0 | 83.0 | .380 |
| Lewy body disease | 20.0 | 0.0 | 20.0 | 16.7 | .806 |
Abbreviations: AD, Alzheimer’s disease; CMBs, cerebral microbleeds; GDS, Global Deterioration Scale; MRI, magnetic resonance imaging; SD, standard deviation; WML, white matter lesions.
aValues represent mean (SD) unless % is indicated.
bn = 40 (pathological diagnosis).
One-year follow-up assessment was conducted in 153 (84.1%) of 182 patients. The presence of strictly lobar CMBs at the initial MRI was associated with worsening of parkinsonism and gait after 1 year (Table 2), and these results were confirmed in the multivariate analyses. The regression model for worsening of parkinsonism (change in the SCOPA-Motor score) depending on the initial clinical and MRI characteristics is shown in Table 3. The β-coefficient of 4.77 represents the change in the motor scale depending on whether there was or not lobar CMBs in the initial MRI. The corresponding β coefficient (95% confidence interval) for worsening of gait (change in the RSGE-CD score) was 4.98 (1.03-8.93, P = .01, corrected R2 = .04, model not shown). No other significant correlations were found between CMBs in the initial MRI and clinical outcomes at 1-year follow-up assessment.
Table 2.
Cognitive, Functional, Motor, and Neuropsychiatric Measures at the Initial Assessment and Change at 1-Year Follow-Up Assessment.a
| Outcome Domain Scale (Possible Score) | No CMBs (n = 90) | Strictly Lobar CMB (n = 19) | Strictly Deep CMB (n = 20) | Lobar and Deep CMB (n = 24) | P |
|---|---|---|---|---|---|
| Cognitive performance | |||||
| MMSE (0-30) | 8.1 (6.7) | 8.7 (6.2) | 8.2 (7.3) | 11.3 (6.8) | .188 |
| 1.1 (3.1) | −1.8 (3.3) | −1.0 (2.9) | −1.1 (3.8) | .873 | |
| SMMSE (0-30) | 17.6 (10.0) | 18.1 (9.8) | 17.0 (10.9) | 21.8 (8.6) | .256 |
| 3.2 (4.9) | −3.9 (5.0) | −0.9 (3.3) | −2.8 (3.9) | .251 | |
| Verbal fluency (≥0) | 2.8 (3.6) | 2.7 (2.7) | 2.5 (3.1) | 4.2 (5.7) | .314 |
| −0.7 (2.5) | −0.1 (2.2) | −0.3 (2.2) | −0.1 (2.7) | .586 | |
| Functional dependence | |||||
| IADL (0-6) | 2.3 (2.0) | 2.0 (1.8) | 1.9 (1.7) | 2.5 (1.7) | .635 |
| −0.4 (1.5) | −0.4 (1.2) | 0.2 (0.9) | −1.0 (1.1) | .106 | |
| FAST (1-16) | 8.6 (2.7) | 7.8 (2.7) | 8.4 (2.1) | 8.0 (2.3) | .458 |
| −0.1 (1.9) | 1.0 (2.2) | 0.4 (2.2) | 0.2 (3.0) | .337 | |
| CDRs (0-18) | 14.6 (3.6) | 13.9 (4.2) | 14.7 (3.5) | 13.3 (3.7) | .329 |
| 0.5 (2.3) | 0.6 (2.7) | 1.0 (2.4) | 1.0 (3.2) | .837 | |
| Motor deterioration | |||||
| SCOPA-Motor (0-30) | 9.7 (6.8) | 8.9 (7.4) | 8.9 (6.3) | 9.1 (5.1) | .933 |
| 1.1 (3.7)b | 4.8 (6.2)b | 0.5 (4.6) | 3.3 (6.7) | .007 | |
| RSGE-CD (0-48) | 19.6 (13.7) | 20.2 (14.8) | 20.8 (13.3) | 20.4 (11.1) | .978 |
| 5.1 (7.9) | 11.5 (12.3) | 5.1 (4.7) | 7.9 (12.2) | .060 | |
| Neuropsychiatric symptoms (NPI) | |||||
| NPI (0-144) | 19.7 (14.7) | 19.1 (12.5) | 17.4 (10.5) | 18.3 (10.2) | .845 |
| 3.1 (16.3) | −1.7 (13.6) | 3.6 (12.9) | −0.8 (20.3) | .529 |
Abbreviations: CDRs, Clinical Dementia Rating Scale, sum of boxes; CMBs, cerebral microbleeds; FAST, functional assessment staging; IADL, index of activities of daily living; MMSE, mini-mental state examination; NPI, neuropsychiatric inventory; RSGE-CD, Rating Scale for Gait Evaluation in Cognitive Deterioration; SCOPA-Motor, motor evaluation scale of the Scales for Outcomes in Parkinson’s Disease; SMMSE, severe mini-mental state examination.
aValues represent mean (SD) (initial value up, change at 1-year follow-up assessment down).
bP < .050 (post hoc between group comparisons, Scheffe test, for the groups with the same superscript letter).
Table 3.
Regression Model for Deterioration in Parkinsonism at 1-Year Follow-Up Assessment According to the Initial Characteristics.a
| β (95% CI) | P | R2 (corrected) | |
|---|---|---|---|
| Age | 0.00 (−0.12 to 0.13) | .943 | |
| Ischemic lesions | −0.07 (−0.31 to 0.17) | .579 | |
| SCOPA-Motor | −0.13 (−0.25 to 0.00) | .053 | 0.019 |
| Lobar CMBs (yes, no) | 4.77 (2.57 to 6.97) | .000 | 0.103 |
| CMB (total n) | −0.33 (−0.6 1 to −0.05) | .023 | 0.133 |
Abbreviations: CMBs, cerebral microbleeds; SCOPA-Motor, motor evaluation scale of the Scales for Outcomes in Parkinson’s Disease.
aVariables not included in the model: deep CMBs (yes, no).
Follow-up MRI study was obtained in 109 (59.9%) patients. The median time between initial and final MRI study was 18 months (range 6-77). In most cases (75 (68.8%) of 109 patients), the number of CMBs remained stable. Increase in CMBs was observed in 29 (26.6%) of 109 patients, whereas decrease in CMBs was detected in 5 (4.6%) of 109 patients. Increases in lobar and deep CMBs were related to each other (r = 0.30), but no significant correlation was observed between change in number of lobar, deep, or total CMBs and evolution in any of the cognitive, functional, motor, or neuropsychiatric measures (all r < .20, data not shown).
To understand the observed association between lobar CMBs and worsening of parkinsonism and gait, we explored post hoc the distribution of CMBs in the group of patients with strictly lobar CMBs. The frequency of patients with CMBs in the different cerebral lobes was as follows: 14 (58.3%) of 24 frontal, 8 (33.3%) of 24 temporal, 9 (37.5%) of 24 parietal, and 12 (50.0%) of 24 occipital CMBs, which was similar to the observed distribution of lobar CMBs in the total sample (Figure 1). We also analyzed the evolution of CMBs in the subgroup of patients with strictly lobar CMBs who had follow-up MRI study (n = 12). None of those patients displayed decrease in the number of CMBs, 4 patients had an increase in the number of lobar CMBs, 3 patients had new deep CMBs, and 5 patients remained unchanged. We repeated the regression analyses excluding the 3 patients with incident deep CMBs, and the predictive effect of lobar CMBs for motor deterioration was not significantly altered (β for parkinsonism deterioration: 4.85 [2.62-7.07], P < .0005, β for gait deterioration: 4.55 [0.48-8.63], P < .05, data not shown).
Postmortem brain pathological diagnosis was performed in 40 (62.5%) of 64 dead patients (Figure 1). The most frequent pathological diagnosis was AD (31 [77.5%] of 40), followed by vascular dementia (23 [57.5%] of 40) and diffuse Lewy body disease (7 [17.5%] of 40, 19 brains received a combination of more than 1 pathological diagnosis). The pathological diagnosis of AD was less frequent in patients with lobar and deep CMBs (33.3% vs 85.3% in the rest of the sample, P < .05; Table 1). Cerebral microbleeds in the initial MRI study were most frequently observed in patients with pathological diagnosis of vascular dementia (10 [43.5%] of 23), followed by pathological diagnoses of AD (11 [35.5%] of 23) and Lewy body (2 [28.6%] of 7). In fact, both brains with CMBs and Lewy body disease received additional pathological diagnoses (1 patient with AD, other patient with AD and vascular dementia).
Pathological data regarding amyloid angiopathy were available in 37 brains. Amyloid angiopathy was observed in 27 (72.9%) of those brains, which was of mild severity in 18 (48.6%) cases, moderate in 7 (18.9%) cases, and severe in 2 (5.4%) cases. Amyloid angiopathy was weakly associated with frontal (r = 0.25) and deep (r = −0.22) CMBs (all other bivariate correlations between amyloid angiopathy and CMBs were < 0.20, data not shown).
There were 4 patients with strictly lobar CMBs and postmortem study. All of them received a pathological diagnosis of AD, but in 3 of them, a concomitant diagnosis of vascular dementia was given. Amyloid angiopathy was observed in all of those patients. There were 5 patients with strictly deep CMBs and postmortem study. All of them received a pathological diagnosis of AD, which was given in combination with vascular dementia in 2 cases and in combination with Lewy body disease in 2 other cases. Amyloid angiopathy was observed only in 2 of those patients. There were 6 patients with both lobar and deep CMBs and pathological examination, which received the following diagnoses: vascular pathology (3 cases), Alzheimer pathology (1 case), Alzheimer and vascular pathology (1 case), and vascular and Lewy body pathology (1 case). All patients who had 4 or more CMBs and postmortem study (n = 5) received a pathological diagnosis of vascular pathology, either alone (2 cases) or in combination (2 cases AD, 1 case Lewy body disease).
Discussion
The correlates of CMBs were investigated in institutionalized people with advanced dementia, including clinical outcomes at 1-year follow-up assessment and postmortem pathological associations in a subsample of patients. Although several cross-sectional 16,18,52 and longitudinal 17 investigations described the prevalence and clinical implications of CMBs in people with dementia, the present investigation was novel in the sense that most patients presented advanced dementia and that clinical areas beyond cognition (ie, motor, function, and neuropsychiatric symptoms) were evaluated.
Considering the usually frail and difficult to collaborate target population, a notable sample size of 182 patients was achieved, along with acceptable rates of missing values and floor effects in the study variables. However, many residents could not be included in the study due to severe dementia and neuropsychiatric symptoms, which prevented MRI performance. Clearly, more efforts are needed to develop methods and instruments, which are comfortable and suitable for research involving this type of patients.
At least 1 CMBs was observed in 42.9% of the patients, which is higher than prevalence described in other studies of people with cognitive impairment or dementia. 4,16,18,52 Our high figure could be due to very old age of patients, advanced dementia, and sensible MRI acquisition protocol. Also in contrast with data from population-based studies, 3,53 we did not find significant associations between CMBs and vascular risk factors (Table 1). That discrepancy could be due to selective survival of patients free from severe vascular disease in our sample but also to more important role of amyloid angiopathy (vs lipohyalinosis) in the genesis of CMBs in old people with advanced dementia. In agreement with previous investigations, CMBs were associated with ischemic lesions, supporting the view of a common pathological substrate of lypohialinosis for isquemic lesions and CMBs. 6 Interestingly, profound but not periventricular WML were associated with CMBs, suggesting nonvascular origin of periventricular WML (eg, ependymal disruption). 54
The presence of CMBs was not associated with symptom duration or specific clinical manifestations at disease onset (Table 1). Those results should be regarded cautiously, since the initial symptoms of dementia were retrospectively collected. Nor the presence of CMBs was associated with more impairment in the cognitive, functional, neuropsychiatric, or motor domains at nursing home admission (Table 2). Certainly, these results do not give support to the hypothesis of key role of CMBs in the pathophysiology of AD. 11 Moreover, widespread (ie, lobar and deep) CMBs were associated with less severe hippocampal atrophy in MRI and less frequent pathological diagnosis of AD at postmortem examination (Table 1).
As a unique finding in the longitudinal assessment, worsening of parkinsonism and gait was documented in those patients who had strictly lobar CMBs in the MRI study at the initial visit (Table 2). These results are not easy to explain, given the prominent contribution of subcortical neural structures to gait performance 55 but were robust, since the results were confirmed in the adjusted models and remained unchanged when the patients with incident deep CMBs were eliminated from the analysis. In a previous cross-sectional investigation of 485 nondemented individuals with small vessel disease, the number of CMBs was related to less stride length and worse balance performance, as measured by the Tinetti and Timed-Up-and-Go tests, and those results were driven by frontal, occipital, basal ganglia, and thalamic CMBs. 56 Using positron emission tomography and functional MRI, occipital and frontal regions were demonstrated to be involved in gait performance in healthy people. 57 A salient role of lobar CMBs for predicting motor deterioration in people with advanced dementia could be explained in terms of greater pathophysiological weight of lobar (vs deep) CMBs, which could be related to amyloid angiopathy. In a postmortem study of brains with AD and amyloid angiopathy, cellular apoptosis and inflammatory reaction were described in the proximity of CMBs. 58
The present study had several limitations. First, our patients were not fully representative of institutionalized people with dementia. Compared with non-included patients, they displayed less severe dementia, less neuropsychiatric symptoms, and were less frequently exposed to potentially relevant medications. That limitation may have prevented the detection of potential associations between neuropsychiatric symptoms or antiaggregant medications and CMBs. Second, floor effect or lack of sensibility of some of the utilized measurements could have diminished the power for detecting some clinical correlates of CMBs. And third, some of the potential contributors to CBM, particularly blood pressure control, were not recorded. 59
In conclusion, CMBs were mostly linked to cerebrovascular disease, but only strictly lobar CMBs predicted motor deterioration. These results illustrate the complexity of the biological substrate of dementia, particularly in old people. The contribution of CMBs to the clinical manifestations of advanced dementia deserves further investigation, especially in the domains of motor capacities, functional dependence, and neuropsychiatric symptoms. This investigation could finally lead to the emergence of treatments that may contribute to improving the quality of life of the often research-neglected population of institutionalized people with advanced dementia.
Footnotes
Authors’ Note: The protocol and data of this study can be requested from the principal investigator.
The authors declared no potential conflicts of interest with respect to the research, authorship, and/or publication of this article.
Funding: The authors disclosed receipt of the following financial support for the research, authorship, and/or publication of this article: The present investigation was fully financed by the Reina Sofía Foundation and the Carlos III Institute of Health.
ORCID iD: Inmaculada Boyano  http://orcid.org/0000-0002-3641-8172
http://orcid.org/0000-0002-3641-8172
References
- 1. Offenbacher H, Fazekas F, Schmidt R, Koch M, Fazekas G, Kapeller P. MR of cerebral abnormalities concomitant with primary intracerebral hematomas. Am J Neuroradiol. 1996;17(3):573–578. [PMC free article] [PubMed] [Google Scholar]
- 2. Greenberg SM, Finklestein SP, Schaefer PW. Petechial hemorrhages accompanying lobar hemorrhage: detection by gradient-echo MRI. Neurology. 1996;46(6):1751–1754. [DOI] [PubMed] [Google Scholar]
- 3. Poels MMF, Vernooij MW, Ikram MA, et al. Prevalence and risk factors of cerebral microbleeds: an update of the Rotterdam scan study. Stroke. 2010;41(suppl 10):S103–S106. [DOI] [PubMed] [Google Scholar]
- 4. Cordonnier C, van der Flier WM, Sluimer JD, Leys D, Barkhof F, Scheltens P. Prevalence and severity of microbleeds in a memory clinic setting. Neurology. 2006;66(9):1356–1360. [DOI] [PubMed] [Google Scholar]
- 5. Song TJ, Kim J, Kim YD, et al. The distribution of cerebral microbleeds determines their association with arterial stiffness in non-cardioembolic acute stroke patients. Eur J Neurol. 2014;21(3):463–469. [DOI] [PubMed] [Google Scholar]
- 6. Fazekas F, Kleinert R, Roob G, et al. Histopathologic analysis of foci of signal loss on gradient-echo T2*-weighted MR images in patients with spontaneous intracerebral hemorrhage: evidence of microangiopathy-related microbleeds. Am J Neuroradiol. 1999;20(4):637–642. [PMC free article] [PubMed] [Google Scholar]
- 7. Fisher M. Cerebral microbleeds: where are we now? Neurology. 2014;83(15):1304–1305. [DOI] [PubMed] [Google Scholar]
- 8. Henneman WJ, Sluimer JD, Cordonnier C, et al. MRI biomarkers of vascular damage and atrophy predicting mortality in a memory clinic population. Stroke. 2009;40(2):492–498. [DOI] [PubMed] [Google Scholar]
- 9. Akoudad S, Ikram MA, Koudstaal PJ, Hofman A, van der Lugt A, Vernooij MW. Cerebral microbleeds and the risk of mortality in the general population. Eur J Epidemiol. 2013;28(10):815–821. [DOI] [PubMed] [Google Scholar]
- 10. Benedictus MR, Prins ND, Goos JD, Scheltens P, Barkhof F, van der Flier WM. Microbleeds, mortality, and stroke in Alzheimer disease: the MISTRAL Study. JAMA Neurol. 2015;72(5):539–545. [DOI] [PubMed] [Google Scholar]
- 11. Cordonnier C, van der Flier WM. Brain microbleeds and Alzheimer’s disease: innocent observation or key player? Brain. 2011;134(Pt 2):335–344. [DOI] [PubMed] [Google Scholar]
- 12. van Norden AG, van den Berg HA, de Laat KF, Gons RA, van Dijk EJ, de Leeuw FE. Frontal and temporal microbleeds are related to cognitive function: the Radboud University Nijmegen Diffusion Tensor and Magnetic Resonance Cohort (RUN DMC) Study. Stroke. 2011;42(12):3382–3386. [DOI] [PubMed] [Google Scholar]
- 13. Poels MM, Ikram MA, van der Lugt A, et al. Cerebral microbleeds are associated with worse cognitive function: the Rotterdam Scan Study. Neurology. 2012;78(5):326–333. [DOI] [PubMed] [Google Scholar]
- 14. Seo SW, Hwa Lee B, Kim EJ, et al. Clinical significance of microbleeds in subcortical vascular dementia. Stroke. 2007;38(6):1949–1951. [DOI] [PubMed] [Google Scholar]
- 15. Yamashiro K, Tanaka R, Okuma Y, et al. Cerebral microbleeds are associated with worse cognitive function in the nondemented elderly with small vessel disease. Cerebrovasc Dis Extra. 2014;4(3):212–220. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 16. Pettersen JA, Sathiyamoorthy G, Gao FQ, et al. Microbleed topography, leukoaraiosis, and cognition in probable Alzheimer disease from the Sunnybrook dementia study. Arch Neurol. 2008;65(6):790–795. [DOI] [PubMed] [Google Scholar]
- 17. van der Vlies AE, Goos JD, Barkhof F, Scheltens P, van der Flier WM. Microbleeds do not affect rate of cognitive decline in Alzheimer disease. Neurology. 2012;79(8):763–769. [DOI] [PubMed] [Google Scholar]
- 18. Heringa SM, Reijmer YD, Leemans A, et al. Multiple microbleeds are related to cerebral network disruptions in patients with early Alzheimer’s disease. J Alzheimers Dis. 2014;38(1):211–221. [DOI] [PubMed] [Google Scholar]
- 19. van Es AC, van der Grond J, de Craen AJ, et al. Cerebral microbleeds and cognitive functioning in the PROSPER study. Neurology. 2011;77(15):1446–1452. [DOI] [PubMed] [Google Scholar]
- 20. Kim TW, Lee SJ, Koo J, et al. Cerebral microbleeds and functional outcomes after ischemic stroke. Can J Neurol Sci. 2014;41(5):577–582. [DOI] [PubMed] [Google Scholar]
- 21. Sanders AE, Nininger J, Absher J, Bennett A, Shugarman S, Roca R. Quality improvement in neurology: Dementia management quality measurement set update. Neurology. 2017;88(20):1951–1957. [DOI] [PubMed] [Google Scholar]
- 22. Olazarán J, Ramos A, Boyano I, et al. Pattern of and risk factors for brain microbleeds in neurodegenerative dementia. Am J Alzheimers Dis Other Demen. 2014;29(3):263–269. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 23. Martínez-Martín P, Avila J; AD Research Unit Investigators. Alzheimer Center Reina Sofia Foundation: fighting the disease and providing overall solutions. J Alzheimers Dis. 2010;21(2):337–348. [DOI] [PubMed] [Google Scholar]
- 24. Olazarán J, Agüera-Ortiz L, Osorio RS, et al. Promoting research in advanced dementia: early clinical results of the Alzheimer Center Reina Sofía Foundation. J Alzheimers Dis. 2012;28(1):211–222. [DOI] [PubMed] [Google Scholar]
- 25. Folstein MF, Folstein SE, McHugh PR. "Mini-mental state". A practical method for grading the cognitive state of patients for the clinician. J Psychiatr Res. 1975;12(3):189–198. [DOI] [PubMed] [Google Scholar]
- 26. Harrell LE, Marson D, Chatterjee A, Parrish JA. The severe mini-mental state examination: a new neuropsychologic instrument for the bedside assessment of severely impaired patients with Alzheimer disease. Alzheimer Dis Assoc Disord. 2000;14(3):168–175. [DOI] [PubMed] [Google Scholar]
- 27. Monsch AU, Bondi MW, Butters N, Salmon DP, Katzman R, Thal LJ. Comparisons of verbal fluency tasks in the detection of dementia of the Alzheimer type. Arch Neurol. 1992;49(12):1253–1258. [DOI] [PubMed] [Google Scholar]
- 28. Katz S, Ford AB, Moskowitz RW, Jackson BA, Jaffe MW. Studies of illness in the aged. the index of ADL. a standardized measure of biological and psychosocial function. JAMA. 1963;185:914–919. [DOI] [PubMed] [Google Scholar]
- 29. Reisberg B. Functional assessment staging (FAST). Psychopharmacol Bull. 1988;24(4):653–659. [PubMed] [Google Scholar]
- 30. Cummings JL, Mega M, Gray K, Rosenberg-Thompson S, Carusi DA, Gornbein J. The neuropsychiatric inventory: comprehensive assessment of psychopathology in dementia. Neurology. 1994;44(12):2308–2314. [DOI] [PubMed] [Google Scholar]
- 31. Marinus J, Visser M, Stiggelbout AM, et al. A short scale for the assessment of motor impairments and disabilities in Parkinson’s disease: the SPES/SCOPA. J Neurol Neurosurg Psychiatry. 2004;75(3):388–395. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 32. Martínez-Martín P, Osa-Ruiz E, Gómez-Conesa A, Olazarán J; RSGE-CD Validation Group. A rating scale for gait evaluation in cognitive deterioration (RSGE-CD): validation study. J Alzheimers Dis. 2012;31(3):543–553. [DOI] [PubMed] [Google Scholar]
- 33. Reisberg B, Ferris SH, de Leon MJ, Crook T. The global deterioration scale for assessment of primary degenerative dementia. Am J Psychiatry. 1982;139:1136–1139. [DOI] [PubMed] [Google Scholar]
- 34. Hughes CP, Berg L, Danziger WL, Coben LA, Martin RL. A new clinical scale for the staging of dementia. Br J Psychiatry. 1982;140:566–572. [DOI] [PubMed] [Google Scholar]
- 35. Neary D, Snowden JS, Gustafson L, et al. Frontotemporal lobar degeneration: a consensus on clinical diagnostic criteria. Neurology. 1998;51(6):1546–1554. [DOI] [PubMed] [Google Scholar]
- 36. McKeith IG, Dickson DW, Lowe J, et al. ; Consortium on DLB. Diagnosis and management of dementia with Lewy bodies: third report of the DLB Consortium. Neurology. 2005;65(12):1863–1872. [DOI] [PubMed] [Google Scholar]
- 37. McKhann GM, Knopman DS, Chertkow H, et al. The diagnosis of dementia due to Alzheimer’s disease: recommendations from the National Institute on Aging-Alzheimer’s Association workgroups on diagnostic guidelines for Alzheimer’s disease. Alzheimers Dement. 2011;7(3):263–269. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 38. Calero O, Hortigüela R, Bullido MJ, Calero M. Apolipoprotein E genotyping method by real time PCR, a fast and cost-effective alternative to the TaqMan and FRET assays. J Neurosci Methods. 2009;183(2):238–240. [DOI] [PubMed] [Google Scholar]
- 39. Greenberg SM, Vernooij MW, Cordonnier C, et al. Cerebral microbleeds: a guide to detection and interpretation. Lancet Neurol. 2009;8(2):165–174. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 40. Wardlaw JM, Smith EE, Biessels GJ, et al. Neuroimaging standards for research into small vessel disease and its contribution to ageing and neurodegeneration. Lancet Neurol. 2013;12(8):822–838. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 41. Scheltens P, Leys D, Barkhof F, et al. Atrophy of medial temporal lobes on MRI in ‘probable’ Alzheimer’s disease and normal ageing: diagnostic value and neuropsychological correlates. J Neurol Neurosurg Psychiatry. 1992;55(10):967–972. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 42. Wahlund LO, Barkhof F, Fazekas F, et al. A new rating scale for age-related white matter changes applicable to MRI and CT. Stroke. 2001;32(6):1318–1322. [DOI] [PubMed] [Google Scholar]
- 43. Zea-Sevilla MA, Fernández-Blázquez MA, Calero M, Bermejo-Velasco P, Rábano A. Combined Alzheimer’s disease and cerebrovascular staging explains advanced dementia cognition. Alzheimers Dement. 2015;11(11):1358–1366. [DOI] [PubMed] [Google Scholar]
- 44. Montine TJ, Phelps CH, Beach TG, et al. National Institute on Aging-Alzheimer’s Association guidelines for the neuropathologic assessment of Alzheimer’s disease: a practical approach. Acta Neuropathol. 2012;123(1):1–11. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 45. Deramecourt V, Slade JY, Oakley AE, et al. Staging and natural history of cerebrovascular pathology in dementia. Neurology. 2012;78(14):1043–1050. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 46. Braak H, Del Tredici K, Rub U, de Vos RA, Jansen Steur EN, Braak E. Staging of brain pathology related to sporadic Parkinson’s disease. Neurobiol Aging. 2003;24(2):197–211. [DOI] [PubMed] [Google Scholar]
- 47. Hobart JC, Riazi A, Lamping DL, Fitzpatrick R, Thompson AJ. Improving the evaluation of therapeutic interventions in multiple sclerosis: development of a patient-based measure of outcome. Health Technol Assess. 2004;8(9):1–48. [DOI] [PubMed] [Google Scholar]
- 48. McHorney CA, Tarlov AR. Individual-patient monitoring in clinical practice: are available health status surveys adequate? Qual Life Res. 1995;4(4):293–307. [DOI] [PubMed] [Google Scholar]
- 49. Kleinbaum DG, Kupper LL, Muller KE. Applied Regression Analysis and Other Multivariable Methods, 2nd ed. Belmont, CA: Duxbury Press; 1988. [Google Scholar]
- 50. Juniper EF, Guyatt GH, Jaeschke R. How to develop and validate a new health-related quality of life instrument. In: Spilker B, ed. Quality of Life and Pharmacoeconomics in Clinical Trials. Philadelphia, PA: Lippincott-Raven Publishers; 1996:49–56. [Google Scholar]
- 51. Rothman KJ. No adjustments are needed for multiple comparisons. Epidemiology. 1990;1(1):43–46. [PubMed] [Google Scholar]
- 52. Hilal S, Saini M, Tan CS, et al. Cerebral microbleeds and cognition: the epidemiology of dementia in Singapore study. Alzheimer Dis Assoc Disord. 2014;28(2):106–112. [DOI] [PubMed] [Google Scholar]
- 53. Sveinbjornsdottir S, Sigurdsson S, Aspelund T, et al. Cerebral microbleeds in the population based AGES-Reykjavik study: prevalence and location. J Neurol Neurosurg Psychiatry. 2008;79(9):1002–1006. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 54. Schmidt R, Schmidt H, Haybaeck J, et al. Heterogeneity in age-related white matter changes. Acta Neuropathol. 2011;122(2):171–185. [DOI] [PubMed] [Google Scholar]
- 55. Fukuyama H, Ouchi Y, Matsuzaki S, et al. Brain functional activity during gait in normal subjects: a SPECT study. Neurosci Lett. 1997;228(3):183–186. [DOI] [PubMed] [Google Scholar]
- 56. de Laat KF, van den Berg HA, van Norden AG, Gons RA, Olde Rikkert MG, de Leeuw FE. Microbleeds are independently related to gait disturbances in elderly individuals with cerebral small vessel disease. Stroke. 2011;42(2):494–497. [DOI] [PubMed] [Google Scholar]
- 57. la Fougère C, Zwergal A, Rominger A, et al. Real versus imagined locomotion: a [18F]-FDG PET-fMRI comparison. Neuroimage. 2010;50(4):1589–1598. [DOI] [PubMed] [Google Scholar]
- 58. Schrag M, McAuley G, Pomakian J, et al. Correlation of hypointensities in susceptibility-weighted images to tissue histology in dementia patients with cerebral amyloid angiopathy: a postmortem MRI study. Acta Neuropathol. 2010;119(3):291–302. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 59. Uiterwijk R, Staals J, Huijts M, de Leeuw PW, Kroon AA, van Oostenbrugge RJ. Framingham Stroke Risk Profile is related to cerebral small vessel disease progression and lower cognitive performance in patients with hypertension. J Clin Hypertens (Greenwich). 2018;20(2):240–245. [DOI] [PMC free article] [PubMed] [Google Scholar]