Abstract
Background:
The aim of this study was to investigate the frequency and severity of neuropsychiatric symptoms (NPS) in patients with early onset Alzheimer’s disease (EAOD) and late onset AD (LOAD).
Methods:
Patients were selected from a specialized memory outpatient clinic. The Mini-Mental State Examination, the Neuropsychiatric Inventory (NPI), and the Global Deterioration Scale results were analyzed.
Results:
By comparing EOAD (n = 35) and LOAD (n = 35) patients, no significant differences were found in clinical or demographic variables, matched for sex, education, and disease severity. There were no differences between groups in total NPI frequency or severity scores. The most common NPS were irritability, apathy, anxiety, and depression. We found an association of NPI scores with disease severity and duration, which was more specific in patients with LOAD and was also associated with the presence of delusions and hallucinations.
Conclusion:
Despite subtle differences, NPS is considered important in the assessment of patients with AD, regardless of the age of onset.
Keywords: early onset dementia, neuropsychiatric symptoms, Alzheimer’s disease, late onset dementia
Introduction
Alzheimer’s disease (AD), as the most common form of dementia, is characterized by a progressive increase in its prevalence with age 1 and is clinically categorized as either early onset or late onset, with the cutoff traditionally set at 65 years of age. Early onset AD (EOAD) is rare but still accounting for 30% to 40% of all early onset dementia cases, 2 with only a small proportion of EOAD cases associated with autosomal dominant mutations in 1 of 3 genes: the amyloid precursor protein gene on chromosome 21 (APP), the presenilin 1 gene on chromosome 14 (PSEN1), or the presenilin 2 gene on chromosome 1 (PSEN2). 3 Late onset AD (LOAD) accounts for most of dementia cases and is associated with several neuropathological processes related to inadequate amyloid β (Aβ) clearance leading to increased protein aggregation and accumulation. While deterministic genetic mutations for sporadic AD have not been found, the ∊4 allele of the apolipoprotein E (APOE) gene is the major known genetic risk factor. 3
Although patients with AD share clinical and histological features regardless of age of onset, there has been an increasing interest in the study of early onset forms of the disease. 4 In fact, if established that EOAD constitutes a distinct subgroup, there could be important implications for clinical practice. In this context, several studies have attempted to establish comparisons between EOAD and LOAD, particularly in pathophysiology, genetics, cognitive function, and psychiatric symptoms. Considering symptom presentation, the first symptom in LOAD is usually episodic memory dysfunction, followed by deficits in other cognitive domains, 5 whereas presentation in EOAD may be nonamnesic in about one-third of cases. 2 In patients with EOAD, there may be a more prominent posterior brain dysfunction, with more severe cortical atrophy, cerebral hypoperfusion, and glucose hypometabolism, particularly in parietal and lateral temporal cortices, 6 which may in turn lead to frequent focal cortical symptoms such as aphasia, apraxia, and agnosia, preceding memory disturbances, in about 30% of patients. 5 In patients with LOAD, functional neuroimaging studies have demonstrated a more localized network dysfunction confined to the limbic structures, in medial temporal and hippocampal regions, 7 associated with the typical memory presentation.
Despite evidence suggesting differences between EOAD and LOAD regarding rate of disease progression, cognitive profile, 8 perfusion and metabolic deficits in the temporal and parietal lobes, grade and distribution of gray matter atrophy, and prevalence of the allele ApoE ∊4, previous investigations on neuropsychiatric symptoms (NPS) are not so ubiquitous regarding differences in type and frequency of symptoms.
NPS refer to behavioral and psychological symptoms, affecting nearly 75% of individuals with dementia 9 and include a large phenomenological spectrum ranging from delusions, apathy, irritability, hallucinations, aggressiveness, depression, anxiety, aberrant motor behavior, to sleep disorders and eating problems. 10 Although NPS are widely recognized as the most stressful and challenging manifestations of AD, they are frequently overlooked in comparison to other research and therapeutic targets, such as cognitive and functional decline. 9 Importance of NPS has also been highlighted by recent studies suggesting that NPS, similarly to traditional cognitive symptoms, also confer a greater risk of conversion to dementia in patients with mild cognitive impairment (MCI), 11 converging on the proposal of a new diagnostic construct—the mild behavioral impairment—as a late life transitional state, where the presence of NPS in the absence of cognitive symptoms confers an increased risk of incident MCI and dementia. 11,12
Given that the expression of NPS can be attributed to a complex interaction between underlying pathology, preexisting morbidity, and premorbid psychosocial characteristics and exposures, 10 differences in NPS frequency and severity in patients with EOAD and LOAD are plausible. NPS are quite pervasive in EOAD like LOAD, but the differences in symptoms and underlying neural correlates have been proposed, 13,14 with salience of apathy and affective symptoms counting for the majority of reported behavioral disturbances. Particularly in the case of early onset cases, the presence of NPS may delay diagnosis, as symptoms are often misinterpreted as primary psychiatric syndrome, rather than associated with a neurodegenerative disorder. 15
We aimed to clarify NPS frequency and pattern in EOAD and LOAD and hypothesized differences symptoms rather than frequency, controlling for known confounders such as sociodemographic variables and disease severity.
Materials and Methods
Participants and Procedures
Patients were retrospectively selected from a specialized memory outpatient clinic. Diagnosis of AD was based on standard diagnostic procedures, according to national and international diagnostic guidelines for probable AD, 16 and included the clinical history, neurological examination, routine blood tests, clinical imaging of the brain (computed tomography or magnetic resonance), and a comprehensive neuropsychological assessment. A proportion of patients also had information available on cerebrospinal fluid biomarkers of AD (Aβ, total tau, and phospho-tau, Aβ/tau ratio) and/or functional imaging (fludeoxyglucose F18 positron emission tomography or single-photon emission computed tomography).
We identified patients younger than 65 years of age at disease onset with a diagnosis of AD, and these constituted the EOAD group. We then matched the EOAD group to a same-sized LOAD group (older than 65 years of age patients with AD), controlling for demographic variables (sex and education) and disease severity.
Clinical data were obtained from patient records. For this study, we specifically collected data on demographics—besides age, sex, educational level, marital status, previous professional occupation, clinical history—symptom onset features, previous medical history, family history of dementia, psychiatric, and AD-specific medication intake as well as scores for global cognitive performance (using the Mini-Mental State Examination [MMSE] 17 ) and disease severity (Global Deterioration Score [GDS] 18 ). The MMSE total score was obtained from the neuropsychological assessment records by a board-certified neuropsychologist or supervised student, using the standard procedure of the validated Portuguese version. The GDS score was recorded by the attending neurologist, based on clinical symptoms. Our primary measure was the presence and severity of NPS, as assessed by the Neuropsychiatric Inventory (NPI). 19 The NPI, based on the report from the primary caregiver, measures the frequency and severity of 12 psychiatric and behavioral symptoms/domains (delusions, hallucinations, agitation/aggression, depression/dysphoria, anxiety, elation/euphoria, apathy/indifference, disinhibition, irritability/lability, aberrant motor behavior, nighttime behavior, and appetite/eating) in the previous 4 weeks, in comparison to the premorbid status. If a symptom is reported to be present in the last month, the informant then rates both the frequency of the symptoms on a 5-point scale and its severity on a 3-point scale. Additionally, the NPI also includes a Caregiver Distress Scale, for evaluating the psychological impact of NPS reported to be present, which was not included in our study design. For all participants, NPI was conducted at part of the neuropsychological assessment by a board-certified neuropsychologist. We also collected data on the NPI informant characteristics, namely, the relationship with the patient (eg, spouse), gender, and habitation status (cohabitating or not).
Statistical Analysis
Descriptive analyses include mean with standard deviation and median with interquartile range, depending on variable characteristics. Group difference analyses were based on 2-tailed nonparametric procedures (χ2 and Mann-Whitney U test), with Bonferroni correction for multiple comparisons. To measure the degree of association between variables, Spearman rank correlation coefficient (rs) or Kendall rank correlation coefficient (T) was applied, depending on the type of variables. The significance level was set at .05 for all analyses. All statistical analyses were carried out with Statistical Package for the Social Sciences (SPSS) version 22.
Results
The total study sample consisted of 70 patients, 35 with EOAD and 35 with LOAD. As shown in Table 1, there were no significant group differences regarding demographic or clinical characteristics. Besides age, significant differences were found only for disease duration. There were also no statistically significant differences between EOAD and LOAD in psychiatric or AD-specific medication intake. In both groups, most patients were treated with psychiatric and/or AD-specific medications (77.1% and 71.4% for EOAD and 68.6% and 77.1% for LOAD). Antidepressants were the most common psychiatric medications in both groups (57.1% and 48.2%). Regarding AD-specific medications, higher frequencies were found for acetylcholinesterase inhibitors (48.6% for EOAD and 68.5% for LOAD).
Table 1.
Demographic, Clinical, and Caregiver Characteristics of Patients With EAOD and LOAD.
| Characteristics | EOAD (n = 35) | LOAD (n = 35) | P Value |
|---|---|---|---|
| Age, in years, mean (SD) | 64.5 (6.5) | 76.0 (3.5) | <.001 |
| Male, n (%) | 25 (71.4) | 25 (71.4) | >.999 |
| Education, in years, median (max-min) | 4 (17-0) | 3 (12-0) | .032 |
| Handedness, right-handed, n (%) | 35 (100) | 35 (100) | |
| Disease duration, mean (SD), years | 5.7 (2.3) | 4.0 (1.5) | .001 |
| Positive family history of dementia, n (%) | 12 (34.3) | 10 (28.6) | .607 |
| MMSE total score, mean (SD) | 16.2 (8.1) | 16.8 (6.0) | .737 |
| NPI total score, mean (SD) | 80.1 (77.0) | 76.4 (81.8) | .702 |
| Psychoactive medication, n (%) | 27 (77.1) | 24 (68.6) | .420 |
| Antipsychotics | 7 (20) | 5 (14.3) | |
| Antidepressants | 20 (57.1) | 17 (48.6) | |
| Benzodiazepines | 19 (54.3) | 6 (17.1) | |
| AD-specific medication (%) | 25 (71.4) | 27 (77.1) | .584 |
| Acetylcholinesterase inhibitors | 17 (48.6) | 24 (68.5) | |
| NMDA receptor antagonists | 13 (37.1) | 7 (20) | |
| GDS score, n (%) | .794 | ||
| Mild (4) | 24 (68.6) | 25 (71.4) | |
| Moderate (5)–severe (6-7) | 11 (31.4) | 10 (28.6) | |
| Caregiver characteristics | |||
| Caregiver sex: men, n (%) | 18 (51.4) | 11 (31.4) | .089 |
| Relationship with patient, n (%) | |||
| Spouse | 21 (60) | 17 (48.6) | |
| Child | 6 (17.1) | 12 (34.3) | |
| Friend | 1 (2.9) | 1 (2.9) | |
| Cohabitating, n (%) | 23 (65.7) | 21 (60) | .621 |
Abbreviations: AD, Alzheimer’s disease; EOAD, early onset AD; GDS, Global Deterioration Scale; LOAD, late onset AD; MMSE, Mini-Mental State Examination; NPI, Neuropsychiatric Inventory; NMDA, N-methyl-D-aspartate; SD, standard deviation.
Regarding the NPI informants’ characteristics, there were no significant differences in relation to gender and habitation status (cohabitating or not) when comparing EOAD and LOAD. In both groups, most informants were the patients’ spouses (60.0% in EOAD and 48.6% in LOAD).
The pattern of frequency and severity of NPS for each group is presented in Tables 2 and 3. The total NPI score did not differ significantly between patients with EOAD and LOAD. There were also no significant group differences for NPI frequency total score, nor NPI severity total score. Even controlling for disease duration and psychiatric or AD-specific medication usage, we found no differences between groups in the total NPI score, nor in frequency or severity scores.
Table 2.
Neuropsychiatric Inventory Frequency Per Symptom in Patients With EOAD and LOAD.
| Domain | Frequency, n (%) | P Value | |||||||||
|---|---|---|---|---|---|---|---|---|---|---|---|
| EOAD, n = 35 | LOAD, n = 35 | ||||||||||
| Not present | Occasionally | Often | Frequently | Very frequently | Not present | Occasionally | Often | Frequently | Very frequently | ||
| Delusions | 33 (94.3) | 0 | 1 (2.9) | 1 (2.9) | 0 | 31 (88.6) | 1 (2.9) | 1 (2.9) | 1 (2.9) | 1 (2.9) | .397 |
| Hallucinations | 35 (100) | 0 | 0 | 0 | 0 | 33 (94.3) | 1 (2.9) | 0 | 1 (2.9) | 0 | .154 |
| Agitation/aggression | 26 (74.3) | 3 (8.6) | 4 (11.4) | 1 (2.9) | 1 (2.9) | 22 (62.9) | 5 (14.3) | 5 (14.3) | 1 (2.9) | 2 (5.7) | .327 |
| Depression/dysphoria | 21 (60) | 3 (8.6) | 6 (17.1) | 2 (5.7) | 3 (8.6) | 17 (48.6) | 3 (8.6) | 5 (14.3) | 7 (20) | 3 (8.6) | .268 |
| Anxiety | 18 (51.4) | 0 | 4 (11.4) | 9 (25.7) | 4 (11.4) | 16 (45.7) | 1 (2.9) | 10 (28.6) | 6 (17.1) | 2 (5.7) | .748 |
| Elation/euphoria | 34 (97.1) | 0 | 1 (2.9) | 0 | 0 | 34 (97.1) | 0 | 0 | 0 | 1 (2.9) | .984 |
| Apathy/indifference | 14 (40) | 3 (8.6) | 4 (11.4) | 4 (11.4) | 10 (28.6) | 18 (51.4) | 0 | 4 (11.4) | 7 (20) | 6 (17.1) | .386 |
| Disinhibition | 31 (88.6) | 1 (2.9) | 1 (2.9) | 2 (5.7) | 0 | 31 (88.6) | 0 | 1 (2.9) | 0 | 3 (8.6) | .907 |
| Irritability/lability | 11 (31.4) | 4 (11.4) | 12 (32.3) | 7 (20) | 1 (2.9) | 15 (42.9) | 1 (2.9) | 7 (20) | 8 (22.9) | 4 (11.4) | .883 |
| Aberrant motor behavior | 26 (74.3) | 2 (5.7) | 0 | 3 (8.6) | 4 (11.4) | 29 (82.9) | 0 | 3 (8.6) | 2 (5.7) | 1 (2.9) | .330 |
| Nighttime behaviors | 24 (68.6) | 2 (5.7) | 4 (11.4) | 2 (5.7) | 3 (8.6) | 26 (74.3) | 0 | 3 (8.6) | 5 (14.3) | 1 (2.9) | .679 |
| Appetite/eating | 23 (65.7) | 2 (5.7) | 4 (11.4) | 1 (2.9) | 5 (14.3) | 27 (77.1) | 1 (2.9) | 5 (14.3) | 1 (2.9) | 1 (2.9) | .232 |
| NPI total frequency, mean (SD) | 9.2 (5.2) | 9.1 (6.0) | .966 | ||||||||
Abbreviations: EOAD, early onset Alzheimer’s disease; LOAD, late onset Alzheimer’s disease; NPI, Neuropsychiatric Inventory; SD, standard deviation.
Table 3.
The NPI Severity Per Domain in Patients With EOAD and LOAD.
| Domain | Severity n (%) | P Value | |||||
|---|---|---|---|---|---|---|---|
| EOAD (n = 35) | LOAD (n = 35) | ||||||
| Mild | Moderate | Severe | Mild | Moderate | Severe | ||
| Delusions | 1 (2.9) | 1 (2.9) | 0 | 2 (5.7) | 1 (2.9) | 1 (2.9) | .390 |
| Hallucinations | 0 | 0 | 0 | 1 (2.9) | 1 (2.9) | 0 | .154 |
| Agitation/aggression | 6 (17.1) | 2 (5.7) | 1 (2.9) | 8 (22.9) | 4 (11.4) | 1 (2.9) | .301 |
| Depression/dysphoria | 5 (14.3) | 6 (17.1) | 3 (8.6) | 7 (20) | 9 (25.7) | 2 (5.7) | .449 |
| Anxiety | 3 (8.6) | 7 (20) | 7 (20) | 7 (20) | 2 (22.9) | 4 (11.4) | .900 |
| Elation/euphoria | 1 (2.9) | 0 | 0 | 0 | 0 | 1 (2.9) | .984 |
| Apathy/indifference | 5 (14.3) | 7 (20) | 9 (25.7) | 8 (22.9) | 5 (14.3) | 4 (11.4) | .134 |
| Disinhibition | 1 (2.9) | 3 (8.6) | 0 | 1 (2.9) | 1 (2.9) | 2 (5.7) | .949 |
| Irritability/lability | 9 (25.7) | 14 (40) | 1 (2.9) | 9 (25.7) | 10 (28.6) | 1 (2.9) | .285 |
| Aberrant motor behavior | 4 (11.4) | 3 (8.6) | 2 (5.7) | 0 | 4 (11.4) | 2 (5.7) | .486 |
| Nighttime behaviors | 5 (14.3) | 3 (8.6) | 3 (8.6) | 3 (8.6) | 6 (17.1) | 0 | .574 |
| Appetite/eating | 6 (17.1) | 3 (8.6) | 3 (8.6) | 3 (8.6) | 5 (14.3) | 0 | .291 |
| NPI total severity score, mean (SD) | 6.6 (4.1) | 6 (4.0) | .475 | ||||
Abbreviations: EOAD, early onset Alzheimer’s disease; LOAD, late onset Alzheimer’s disease; NPI, Neuropsychiatric Inventory; SD, standard deviation.
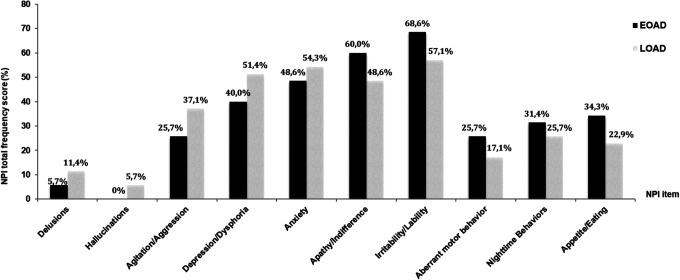
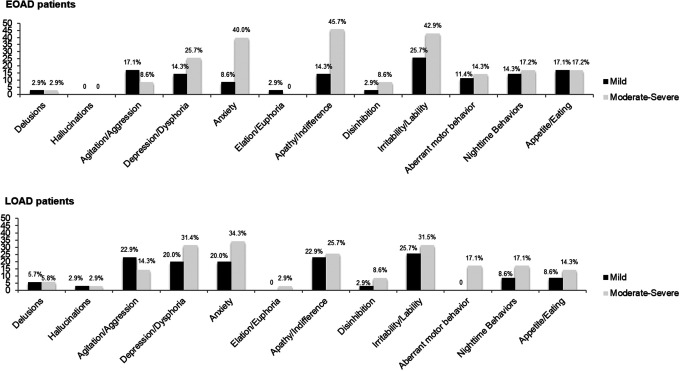
In both groups, the most common neuropsychiatric domain was irritability/lability, whereas hallucinations and euphoria were the least common symptoms. Patterns of symptom frequency are slightly different between early and late onset groups. As shown in Figure 1, in patients with EOAD, the most common neuropsychiatric domains were irritability/lability (68.6%), followed by apathy/indifference (60.0%), anxiety (48.6%), and depression/dysphoria (40.0%). In this group, patients with moderate/severe forms of disease presented higher frequency of apathy/indifference (cf Figure 2; 45.7%).
Figure 1.
Differences in NPI total frequency between patients with EOAD and LOAD. EOAD indicates early onset Alzheimer’s disease; LOAD, late onset Alzheimer’s disease; NPI, Neuropsychiatric Inventory.
Figure 2.
Frequency in NPI domain in patients with EOAD and LOAD per disease severity. EOAD indicates early onset Alzheimer’s disease; LOAD, late onset Alzheimer’s disease; NPI, Neuropsychiatric Inventory.
In the early onset group, disease duration and severity, as assessed through the GDS score, were positively associated with the NPI total score (rs = 0.34, P < .05 and T = 0.28, P < .05) and with NPI frequency total score (rs = 0.35, P < .05 and T = 0.31, P < .05).
The most commonly reported neuropsychiatric domains in patients with LOAD (cf Figure 1) were the same as in those with EOAD but in a different order: the most frequent symptom was irritability/lability (57.1%), followed by anxiety (54.3%), depression/dysphoria (51.4%), and then apathy/indifference (48.6%).
In this group (cf Figure 2), patients with moderate/severe disease stages presented a higher frequency of anxiety (34.3%). In the late onset group, the disease severity was associated with frequency and severity of delusions (T = 0.38, P < .05 and T = 0.37, P < .05) and with frequency and severity of hallucinations (T = 0.39, P < .05 and T = 0.39, P < .05).
Discussion
Our study suggests that the neuropsychiatric profiles between patients with EOAD and LOAD are globally comparable in total frequency and severity of symptoms, although subtle qualitative differences between profiles appear to exist, generally in accordance with previous research and derived hypothesis.
Despite NPS being commonly observed over the course of AD as well as being a significant contributor to caregiver burden and the main cause of institutionalization, 20 there has been a reduced effort to examine the profiles of NPS in patients with EOAD and LOAD. There are also inconsistencies between previous studies that may be related to the heterogeneity in methodologies and sample clinical characteristics, 21 making direct comparison more difficult. Similar to our results, 1 study 22 found differences in the pattern of symptoms frequency when comparing EOAD and LOAD cases, finding no differences in the prevalence of depression and anxiety symptoms, but fewer and less severe delusions, hallucinations, agitation, disinhibition, aberrant motor behavior, and total problem behavior in EOAD than in LOAD. A 2-year longitudinal study 20 also compared the frequency of NPS using the NPI and reported that the incidence, prevalence, and persistence of NPS were generally lower in EOAD than in LOAD, specifically for delusions, agitation, depression, anxiety, apathy, irritability, and aberrant motor behavior. In contrast, other authors 23 have found a higher frequency of inappropriate behavior in EOAD compared to LOAD and that patients with EOAD had significantly more anxiety symptoms than patients with LOAD, which was associated with male gender, higher MMSE score, and being separated from caregivers. 5
In our sample, euphoria and hallucinations were the least common NPS, which is consistent with previous studies. 13
Many authors 24,25 have reported that apathy, characterized by social withdrawal and loss of goal-oriented behavior, interest, and motivation, is the most common NPS associated with AD, based on NPI ratings. In our sample, nevertheless, irritability was the most common NPS, in both subgroups. Possible explanations for this finding are our clinical study setting, long disease duration, and higher patients’age 13 ; given that irritability can be associated early in the disease course, as a reaction to the cognitive decline and caregiver’s attempt to help, 26 and increases significantly and consistently over the time, as suggested by a 3-year longitudinal study. 27 The higher frequency of irritability has implications in disease management, that is, due to increasing caregiver burden, our patients may be at risk of institutionalization.
Comparability between studies may be hindered by several factors, which may include sociodemographic and cultural characteristics as well as access to health and diagnostic services, with repercussions in diagnosis time. In our sample, the low mean MMSE score is not only a reflection of cognitive impairment severity but also a low educational obtainment and premorbid characteristics, which are also known to be associated with NPS prevalence.
Regarding clinical implications, the lack of significant differences in terms of total frequency or severity of NPS, despite subtle differences in symptom pattern, implies that clinicians should give importance to specific symptoms considering the age of onset. Such findings have practical implications in clinical context, namely, at the level of differential diagnosis and increase diagnostic accuracy, particularly in younger patients. In these patients, approximately one-third present with an atypical nonmemory phenotype, 2 and NPS are frequently confounding factors, given that at younger ages there is a greater likelihood of a psychiatric than a neurodegenerative disorder diagnosis, which may translate into delayed diagnosis.
Additionally, in the EOAD group, a significant association was found between NPI total and disease severity and duration. In contrast, a previous study 28 reported that only particular domains, namely, the severity of apathy, agitation, disinhibition, irritability, and aberrant motor behavior were significantly worse with AD severity.
Given that patients with EOAD progress more rapidly in terms of cognition and brain atrophy, 29 we could assume the same pattern for NPS, which should be studied in future longitudinal investigations.
In patients with LOAD, we found a specific association between disease severity and duration and the presence of delusions and hallucinations. Based on previous studies, 13 the prevalence of psychotic symptoms (delusions and hallucinations) in AD ranged from 6% to 59%, and others have shown that cognitive impairment predicts an increased risk of psychosis onset, 21 particularly misidentification delusions, 30 and the presence of psychotic symptoms at onset is associated with worse prognosis. 31 Recently, 32,33 structural imaging correlates of psychosis have been suggested, particularly microstructural alterations within the left hemisphere associated with degeneration of major neurotransmitter pathways and reduced volume in the parahippocampal gyrus.
Other interesting finding of our study was that in both groups most patients were treated with psychiatric medications, particularly antidepressants. They do not appear to be a significant mediator, as we found no differences in frequency nor severity of NPI scores, even considering duration of disease and psychiatric medication. An explanation for the high percentage of psychiatric medication in our groups might be related to the specialized clinical context where the study was conducted, in which presumably health-care professionals are aware of AD-related NPS and their negative prognostic impact, which may differ in other clinical and research contexts.
This study has potential limitations. Firstly, considering the characteristics of patients with EOAD, with a mean age of 64.5 years, as well as a longer disease duration, one might consider these to be “older” EOAD patients who were further along in their natural disease course and therefore limit comparisons between older AD patients. In addition, in our sample it would have been relevant to evaluate and quantify the impact of vascular disease, as vascular factors may influence the presence of NPS in patients with AD. 34
Furthermore, there are methodological issues concerning the modest sample size, which may result in lack of statistical power, the retrospective nature of the study, and the restriction to a single center, which limit the generalization of results. Nonetheless, most studies analyzing NPS in early and late AD share small sample sizes, varying from 23 and 98 patients. 5,20,22,23 Also, our data being limited to cross-sectional observations, we can only infer the progression due to the influence of the nature of disease course through disease severity measures.
Other issues concerned the identification of NPS, which relied on caregiver report, and there was no information on caregiver distress or psychopathology. Although the literature 20,35 has consistently reported a strong correlation between the frequency and severity of NPS and caregiver distress, the role of caregiver psychopathology has not yet been studied. We can speculate that this variable may mediate the reports of caregivers, insofar as probably caregivers’ anxiety, depression, or sleep disturbances, overestimating the NPS reports. Moreover, our study supports the idea that the acknowledgment of any psychiatric symptom, even if subtle, can be a key to early diagnosis of AD, even in prodromal stages. 11
Further studies with larger samples and longitudinal data are needed to better understand whether the management of NPS based on the age of onset of disease might imply different clinical approaches.
Footnotes
Authors’ Note: The authors declare that the research was conducted at Hospital de Braga, Portugal, in the absence of any commercial or financial relationships that could be construed as a potential conflict of interest.
The author(s) declared no potential conflicts of interest with respect to the research, authorship, and/or publication of this article.
Funding: The author(s) received no financial support for the research, authorship, and/or publication of this article.
ORCID iD: Ana Sofia Costa http://orcid.org/0000-0002-1999-6616
References
- 1. Scheltens P, Blennow K, Breteler MMB, et al. Alzheimer’s disease. Lancet. 2016;6736(15):1–13. 10.1016/S0140-6736(15) 01124–1 [DOI] [Google Scholar]
- 2. Rossor MN, Fox NC, Mummery CJ, Schott JM, Warren JD. The diagnosis of young-onset dementia. Lancet Neurol. 2010;9(8):793–806. 10.1016/S1474-4422(10)70159-9 [DOI] [PMC free article] [PubMed] [Google Scholar]
- 3. Jack CR, Jr, Holtzman DM. Biomarker modeling of Alzheimer’s disease. Neuron. 2013;80(6):1347–1358. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 4. Koedam EL, Lauffer V, van der Vlies AE, van der Flier WM, Scheltens P, Pijnenburg YA. Early-versus late-onset Alzheimer’s disease: more than age alone. J Alzheimers Dis. 2010;19(4):1401–1408. 10.3233/JAD-2010-1337 [DOI] [PubMed] [Google Scholar]
- 5. Kaiser NC, Liang LJ, Melrose RJ, Wilkins SS, Sultzer DL, Mendez MF. Differences in anxiety among patients with early- versus late-onset Alzheimer’s disease. J Neuropsychiatry Clin Neurosci. 2014;26(1):73–80. [DOI] [PubMed] [Google Scholar]
- 6. Ossenkoppele R, Zwan MD, Tolboom N, et al. Amyloid burden and metabolic function in early-onset Alzheimer’s disease: parietal lobe involvement. Brain. 2012;135(Pt 7):2115–2125. [DOI] [PubMed] [Google Scholar]
- 7. Kaiser NC, Melrose RJ, Liu C, Sultzer DL, Jimenez E, Su M, Monserratt L, Mendez MF. Neuropsychological and neuroimaging markers in early versus late-onset Alzheimer’s disease. Am J Alzheimers Dis Other Demen. 2012;27(7):520–529. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 8. Licht EA, McMurtray AM, Saul RE, Mendez MF. Cognitive differences between early-and late-onset Alzheimer’s disease. Am J Alzheimers Dis Other Dement. 2007;22(3):218. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 9. Canevelli M, Cesari M, Lucchini F, et al. Need to recalibrate research outcomes in Alzheimer’s disease: focus on neuropsychiatric symptoms. J Am Geriatr Soc. 2017;65(9):2071–2073. [DOI] [PubMed] [Google Scholar]
- 10. Kiely KM, Mortby ME, Anstey KJ. Differential associations between sensory loss and neuropsychiatric symptoms in adults with and without a neurocognitive disorder. Int Psychogeriatr. 2017;20:1–12. [DOI] [PubMed] [Google Scholar]
- 11. Ismail Z, Smith EE, Geda Y, et al. Neuropsychiatric symptoms as early manifestations of emergent dementia: provisional diagnostic criteria for mild behavioral impairment. Alzheimers Dement. 2016;12(2):195–202. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 12. Mortby ME, Burns R, Eramudugolla R, Ismail Z, Anstey KJ. Neuropsychiatric symptoms and cognitive impairment: understanding the importance of co-morbid symptoms. J Alzheimers Dis. 2017;59(1):141–153. [DOI] [PubMed] [Google Scholar]
- 13. Zhao QF, Tan L, Wang HF, et al. The prevalence of neuropsychiatric symptoms in Alzheimer’s disease: systematic review and meta-analysis. J Affect Disord. 2016;190:264–271. 10.1016/j.jad.2015.09.069 [DOI] [PubMed] [Google Scholar]
- 14. Gatchel JR, Donovan NJ, Locascio JJ, et al. Regional 18F-fluorodeoxyglucose hypometabolism is associated with higher apathy scores over time in early Alzheimer disease. Am J Geriatr Psychiatry. 2017;25(7):683–693. 10.1016/j.jagp.2016.12.017 [DOI] [PMC free article] [PubMed] [Google Scholar]
- 15. Bature F, Guinn BA, Pang D, Pappas Y. Signs and symptoms preceding the diagnosis of Alzheimer’s disease: a systematic scoping review of literature from 1937 to 2016. BMJ Open. 2017;7(8):e015746. 10.1136/bmjopen-2016-015746 [DOI] [PMC free article] [PubMed] [Google Scholar]
- 16. McKhann GM, Knopman DS, Chertkow H, et al. The diagnosis of dementia due to Alzheimer’s disease: recommendations from the National Institute on Aging-Alzheimer’s Association workgroups on diagnostic guidelines for Alzheimer’s disease. Alzheimers Dement. 2011;7(3):263–269. doi:10.1016/j.jalz.2011.03.005 [DOI] [PMC free article] [PubMed] [Google Scholar]
- 17. Folstein MF, Folstein SE, McHugh PR. Mini- mental state: a practical method for grading the cognitive state of patients for the clinician. J Psychiatr Res. 1975;12(3):189–198. [DOI] [PubMed] [Google Scholar]
- 18. Reisberg B, Ferris SH, de Leon MJ, Crook T. The global deterioration scale for assessment of primary degenerative dementia. Am J Psychiatry. 1982;139(9):1136–1139. [DOI] [PubMed] [Google Scholar]
- 19. Cummings JL, Mega M, Gray K, Rosenberg-Thompson S, Carusi DA, Gornbein J. The Neuropsychiatric Inventory: comprehensive assessment of psychopathology in dementia. Neurology. 1994;44(12):2308–2314. [DOI] [PubMed] [Google Scholar]
- 20. van Vliet D, de Vugt ME, Aalten P, et al. Prevalence of neuropsychiatric symptoms in young-onset compared to late-onset Alzheimer’s disease - part 1: findings of the two-year longitudinal NeedYD-study. Dement Geriatr Cogn Disord. 2012;34(5-6):319–327. [DOI] [PubMed] [Google Scholar]
- 21. Nakaaki S, Sato J, Torii K, et al. Neuroanatomical abnormalities before onset of delusions in patients with Alzheimer’s disease: a voxel-based morphometry study. Neuropsychiatr Dis Treat. 2013;9:1–8. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 22. Toyota Y, Ikeda M, Shinagawa S, et al. Comparison of behavioral and psychological symptoms in early-onset and late-onset Alzheimer’s disease. Int J Geriatr Psychiatry. 2007;22(9):896–901. [DOI] [PubMed] [Google Scholar]
- 23. Hori K, Oda T, Asaoka T, et al. First episodes of behavioral symptoms in Alzheimer’s disease patients at age 90 and over, and early-onset Alzheimer’s disease: comparison with senile dementia of Alzheimer’s type. Psychiatry Clin Neurosci. 2005;59(6):730–735. [DOI] [PubMed] [Google Scholar]
- 24. Lyketsos CG, Lopez O, Jones B, Fitzpatrick AL, Breitner J, DeKosky S. Prevalence of neuropsychiatric symptoms in dementia and mild cognitive impairment: results from the cardiovascular health study. JAMA. 2002;288(12):1475–1483. [DOI] [PubMed] [Google Scholar]
- 25. Guercio BJ, Donovan NJ, Ward A, et al. Apathy is associated with lower inferior temporal cortical thickness in mild cognitive impairment and normal elderly individuals. J Neuropsychiatry Clin Neurosci. 2015. Winter;27(1):e22–e27. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 26. Cheng ST, Kwok T, Lam LC. Neuropsychiatric symptom clusters of Alzheimer’s disease in Hong Kong Chinese: prevalence and confirmatory factor analysis of the Neuropsychiatric Inventory. Int Psychogeriatr. 2012;24(9):1465–1473. [DOI] [PubMed] [Google Scholar]
- 27. Brodaty H, Connors MH, Xu J, Woodward M, Ames D; PRIME study group. The course of neuropsychiatric symptoms in dementia: a 3-year longitudinal study. J Am Med Dir Assoc. 2015;16(5):380–387. [DOI] [PubMed] [Google Scholar]
- 28. Tanaka H, Hashimoto M, Fukuhara R, et al. Relationship between dementia severity and behavioural and psychological symptoms in early-onset Alzheimer’s disease. Psychogeriatrics. 2015;15(4):242–247. [DOI] [PubMed] [Google Scholar]
- 29. Cho H, Seo SW, Kim JH, et al. Changes in subcortical structures in early- versus late-onset Alzheimer’s disease. Neurobiol Aging. 2013;34(7):1740–1747. [DOI] [PubMed] [Google Scholar]
- 30. Quaranta D, Vita MG, Bizzarro A, et al. Cognitive and behavioral determinants of psychotic symptoms in Alzheimer’s disease. Dement Geriatr Cogn Disord. 2015;39:194–206. [DOI] [PubMed] [Google Scholar]
- 31. Zahodne LB, Ornstein K, Cosentino S, Devanand DP, Stern Y. Longitudinal relationships between Alzheimer disease progression and psychosis, depressed mood, and agitation/aggression. Am J Geriatr Psychiatry. 2015;23(2):130–140. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 32. McLachlan E, Bousfield J, Howard R, Reeves S. Reduced parahippocampal volume and psychosis symptoms in Alzheimer’s disease [published online July 25, 2017]. Int J Geriatr Psychiatry. DOI: 10.1002/gps.4757. [DOI] [PubMed] [Google Scholar]
- 33. Tu MC, Huang WH, Hsu YH, Lo CP, Deng JF, Huang CF. Comparison of neuropsychiatric symptoms and diffusion tensor imaging correlates among patients with subcortical ischemic vascular disease and Alzheimer’s disease. BMC Neurol. 2017;17(1):144. doi:10.1186/s12883-017-0911-5. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 34. Steinberg M, Hess K, Corcoran C, et al. Vascular risk factors and neuropsychiatric symptoms in Alzheimer’s disease: the Cache County Study. Int J Geriatr Psychiatry. 2014;29(2):153–159. doi:10.1002/gps.3980 [DOI] [PMC free article] [PubMed] [Google Scholar]
- 35. Storti LB, Quintino DT, Silva NM, Kusumota L, Marques S. Neuropsychiatric symptoms of the elderly with Alzheimer’s disease and the family caregivers’ distress. Rev Lat Am Enfermagem. 2016;24:e2751. [DOI] [PMC free article] [PubMed] [Google Scholar]