Abstract
Objective:
This study aimed to evaluate the effect induced by musk on Alzheimer’s disease—such as neurodegenerative changes in mice exposed to chronic unpredictable mild stress (CUMS).
Material and Methods:
Forty male Swiss albino mice were divided into 4 groups (n = 10); control, CUMS, CUMS + fluoxetine, CUMS + musk. At the end of the experiment, behavior of the mice was assessed. Serum corticosterone level, hippocampal protein level of the glucocorticoid receptors, and brain-derived neurotropic factor were also assessed. Hippocampus was histopathologically examined.
Results:
Musk improved depressive status induced after exposure to CUMS as evidenced by the forced swimming and open field tests and improved the short-term memory as evidenced by the elevated plus maze test. Musk reduced both corticosterone levels and the hippocampal neurodegenerative changes observed after exposure to CUMS. These improvements were comparable to those induced by fluoxetine.
Conclusion:
Musk alleviated the memory impairment and neurodegenerative changes induced after exposure to the chronic stress.
Keywords: musk, neurodegenerative, depression, chronic stress, Alzheimer, corticosterone, GR-BDNF
Introduction
About 44 million people, worldwide, have Alzheimer or a related dementia. 1 In North Africa and the Middle East, there were 2.34 million people with dementia in 2015 which is expected to be raised to 4.35 million in 2030 and to 10.04 million in 2050. 2 Alzheimer’s disease (AD) accounting for nearly half the number of cases of dementia; so this has become a big social problem. 3 Alzheimer’s disease is a state of progressive neurodegenerative brain disorder where the person exhibits dementia associated with various degrees of memory disorder and cognitive deterioration. 4 This disorder could be considered as a squeal of altered living conditions associated with the industrialization of society. 5
Epidemiologically, stress was reported to be a risk factor for AD. 6,7 Chronic stress, specifically, may play a critical role in the etiology of sporadic AD. Chronic stress had been reported to induce atrophy and functional impairments in several brain areas such as the frontal cortex and hippocampus that play an important role in the generation and progression of depression and AD. 8,9 Stress induces an increase in steroids that was reported to alter neuronal structure and function as well as inhibit general brain activity. 10 Stress also increases hippocampal vulnerability to neuronal atrophy that reversibly impairs cognitive function. 11
The preventive medicine for dementia has become more important. 12 Recently, complementary alternative medicine, which, in addition to using medications, also makes use of various “nonpharmacological” approaches, has become an attractive alternative in the treatment of senile dementia. 13
Essential volatile oils from plant or animal sources were used in preventing and treating many clinical problems, and this is known as aromatherapy. 14 In psychiatry, aromatherapy was used for improving emotional changes in different neurodegenerative diseases specifically in treating dementia in both experimental animal model and human. 15 –17 Musk is a powerful odoriferous substance obtained from a special gland of male musk deer. Khan and Abourashed reported that musk was utilized by Chinese long time ago for patient with stroke, coma, neurasthenia, and convulsions. 18 Musk was described to reduce stress and induced an antidepressant-like effect. 19,20 Therefore, this study aimed to assess the effect of chronic unpredictable mild stress (CUMS) on the hippocampus of the mice and to investigate the possibility to alleviate this effect by musk inhalation. We hypothesized that musk can alleviate the chronic stress-induced memory impairment as well as the AD-like neurodegenerative changes in mice.
Material and Methods
This study design is compliant with the guidelines of dealing with experimental animals that were followed in the King Fahed Medical Research Center (KFMRC) and were compliant with Declaration of Helsinki. 21 An ethical approval for this study was obtained from the research ethics, King Abdulaziz University, Jeddah, Saudi Arabia with a reference number (48-16).
Musk (Moschus moschiferus) used in this study was obtained from 1 of Jeddah markets. The constituents of the musk were identified using gas chromatography ( GC-MS; Agilent, Columbia, South Carolina) and were shown in the Supplemental table. Methods of administration of musk were previously described. 20 Fluoxetine (FLU; Dar Al Dawa Pharmaceuticals Co, Ltd, Amman, Jordan) used in this study was dissolved in 0.03% sodium carboxymethyl cellulose (CMC-Na). It was given through the intragastric gavage with a dose of 20 mg/kg. 22 Five percent amyl acetate, an odorous substance with no effect on anxiety, was obtained from Sigma (St Louis, Missouri) and was given to the positive control group. 23
Forty male Swiss albino mice (5 weeks old) were purchased from animal unit at the KFMRC, Jeddah, Saudi Arabia, and left to acclimatize for 2 week to the laboratory conditions (22°C ± 3°C and relative humidity of 44%-55% with a 12-hour dark/light cycle). The animals were divided into 2 groups; the control group (n = 10) left without exposure to stress and an experimental group (n = 30) that was exposed to CUMS for 4 weeks. This group was further divided into 3 groups (n = 10 each); a positive control group (CUMS) that was treated with amyl acetate, CUMS + FLU that was treated with FLU, and CUMS + M that was treated with musk inhalation. The CUMS procedure included subjecting the mice at different time points during the day to different stressors for 4 weeks. 24 All treatments were started after stopping the exposure to CUMS and were administrated for 2 weeks (see Figure 1). Fluoxetine was given through the intragastric gavage. Amyl acetate and musk were inhaled by mice for 15 minutes once per day in an odor-isolated chamber according to Chioca et al 25 Two cotton balls embedded with 2.5 mL per unit of musk were utilized to be the source of the musk odor in the chamber. They were placed in the top wall holes of the apparatus. These cotton balls were replaced with another fully impregnated ones every session to maintain the concentration of musk odor in the apparatus.
Figure 1.
Procedure of the experiment. Mice were exposed to chronic unpredictable mild stress (CUMS) from day 1 to day 28. On day 29 to day 42, the mice were exposed daily to the control treatment (amyl acetate), fluoxetine, or muck. On the days 43 to 45, the behavioral tests were sequentially done for all groups. On the day 46, blood samples were obtained then the mice were sacrificed.
Procedures
At the end of the experiment, the behavior of the mice was assessed through 3 behavioral tests that were previously described by Ayuob et al in order to confirm the development of the depression. 20 The behavior tests were performed between 08.00 and 11.30 hours in a dimly lit room starting with the elevated plus maze (EPM) test, open field test (OFT), and finally the forced swimming test (FST).
Elevated plus maze test was conducted according to Carobrez and Bertoglio. 24 The numbers of closed arms entries in 6 minutes and time spent by each mouse inside the open and closed arms were observed and recorded expressed in seconds. Transfer latency (TL), the time it took for the mouse to move from the open arm to either of the closed arms when it placed in the open arm, was also recorded in 3 successive days in order to examine whether the mice learn this scape behavior as described by Itoh et al. 26 The TL of the third day was included in the analysis.
Open field test was carried out according to Mineur et al. 27 The number of mouse rearing in 25 minutes was registered manually, and the distance traveled by the mouse during these 25 minutes was also measured through video tracking system (Columbus Instruments, Ohio, Washington).
Forced swimming test was done according to Doro et al. 28 In this test, the total time spent immobile by the mouse during the 6 minutes was assessed and presented in seconds.
The day after finishing the behavior tests, the mice were anesthetized using light ether, and blood samples were collected from retro-orbital venous plexus, centrifuged for 10 minutes (2200g, 4uC), and kept at the refrigerator till the estimation of serum corticosterone levels using the Radioimmunoassay technique (ALPCO Diagnostics, Orangeburg, New York).
After finishing blood sampling, the animals were scarified by cervical dislocation. The brain was immediately extracted from the skull and cut on an ice plate in the sagittal plane into 2 halves. The hippocampus of the left half of the brain was dissected out as described by Paxinos and Watson. 29 Tissue punches from the hippocampus were obtained and homogenized in cold extraction buffer (Tris-buffered saline, pH 8.0, with 1% NP-40, 10% glycerol, 5 mM sodium metavanadate, 10 mM Phenylmethanesulfonyl Fluoride (PMSF), 100 µg/mL aprotinin, and 10 µg/mL leupeptin) and processed by sandwich enzyme-linked immunosorbent assay (ELISA) described by Baker-Herman et al to assess the brain-derived neurotropic factor (BDNF) and the glucocorticoid receptors (GRs) protein levels. 30
The right half of the brain was fixed overnight in 10% neutral-buffered formalin and processed for the routine histopathological examination according to Bancroft and Gamble. 31 Congo red stain, an accepted histochemical marker for the β-pleated sheet structure of amyloid, was also used as described by Nobakht et al. 32
Immunohistochemical staining was performed using the peroxidase-labeled streptavidin–biotin technique. The paraffin sections were processed according to Makhlouf et al. 33 Anti-caspase-3, the primary antibody used for the demonstration of apoptosis, was obtained from Santa Cruz Biotechnology, Dallas, USA. and utilized with the dilution of 1:1000. For demonstration of astrocytes, anti-GFAP (Dako Cytomation, with the dilution of 1:1000) was utilized for 1 hour. Anti-Ki-67 (Abcam, Cambridge, United Kingdom, at dilution 1:100) was utilized to determine proliferating cells. In order to examine and photograph the sections, a digital camera connected to a light microscope (Olympus; Los Angeles, California) was utilized. Morphometric measurements of the thickness and the surface area of cornu ammonis (CA1) pyramidal cell layer and dentate gyrus (DG) granular cell layer were performed using the Image ProPlus (Cybernetics, Rockville, USA). Counting the number of GFAP- and Caspase-3-positive cells in both CA1 and DG as well as the number of Ki67-positive cells in DG only was also done. The counting was performed in 5 high power field (×400) in each mouse as described by Makhlouf et al. 33
The SPSS (version 16) software was used to analyze the data, and the results were presented as mean and standard deviation. Analysis of variance (F test) was used to compare between the studied groups followed by Bonferroni post hoc test. A P value less than .05 was considered significant.
Results
Effect on Behavioral Tests
The immobility time recorded during the FST, an indicator of the depressive status, was significantly (P < .001) prolonged in mice exposed to CUMS when compared to the unexposed group. Treatment with either FLU or musk significantly (P = .008, P = .006, respectively) reduced this time compared to the untreated group (Figure 2).
Figure 2.
Forced swimming test (A) and open field test (B, C) of the control, chronic unpredictable mild stress (CUMS), fluoxetine-treated (CUMS + FLU), and musk-treated (CUMS + M) groups (n = 10 each). Data were shown as mean (SD). # indicates significance compared to the control group; *indicates significance compared to the CUMS group.
During the EPM, the mice exposed to CUMS spent a significantly (P < .001) shorter time in the open arms in comparison with the unexposed mice indicating anxiety. In addition, both TL on the third and the time spent by the mice in the closed arm were also prolonged. After treatment with FLU or musk, the stressed mice spent longer times in the open arms in comparison with the CUMS mice (P = .001, P < .001) indicating relief of anxiety. Adding to that the noticed improvement in mice memory evidenced by shorting in the TL on the third day and the time spent in the closed arm (Table 1). A significant increase in the number of closed arm entries in the EPM of the CUMS group compared to the control mice was also observed (P < .001) while administration of either FLU or musk significantly reduced it in comparison with the CUMS group (P < .001, P = .01; respectively; Table 1).
Table 1.
Effect of Musk on The Behavior Observed During The Elevated Plus Maze Test.a,b,c,d
| Parameter | Control (n = 10) | CUMS (n = 10) | CUMS + FLU (n = 10) | CUMS + M (n = 10) |
|---|---|---|---|---|
| Time spent in the open arm (seconds) | 27.4 (3.5) | 11.8 (0.93); P < .001 | 14.1 (2.5); P1 = .01 | 15.5 (3.5); P1 = .004 |
| Time spent in the closed arm (seconds) | 126.4 (13.9) | 213.6 (17.4); P < .001 | 202.1 (34); P1 = .97 | 163.9 (26.6); P1 = .001 |
| Transfer latency of the third day (seconds) | 13.4 (2.3) | 24.9 (4.1); P < .001 | 23.5 (5.8); P1 = .98 | 18.1 (2.3); P1 = .002 |
| Number of closed arm entries | 17.3 (2.6) | 25.06 (1.4); P < .001 | 18.3 (5.6); P1 = .002 | 19.9 (5.4); P1 = .01 |
Abbreviations: CUMS, chronic unpredictable mild stress; FLU, fluoxetine; M, musk.
aData are expressed as mean (SD).
b P indicates significance versus group control.
c P1 indicates significance versus group CUMS.
dSignificance is considered at P < .05.
During the OFT, the mice exposed to the CUMS traveled significantly (P < .001) more than the unexposed mice indicating an exaggeration in their spontaneous locomotor activity. Administration of either FLU or musk significantly (P < .001) minimized this activity when compared to the untreated group. The number of rearing was significantly (P = .02) increased after exposure to CUMS, while administration of either FLU (P < .001) or musk (P = .007) significantly reduced it (Figure 2).
Effect on the Biochemical Tests
Mice exposed to CUMS showed a significant (P < .001) increase in the basal serum corticosterone when compared to the unexposed mice. Treating stressed mice with FLU or musk significantly (P < .001) decreased it (Figure 3).
Figure 3.
A, Serum level of corticosterone shown as mean (SD). Glucocorticoid receptor protein (B) and BDNF protein (C) expression levels in the hippocampus assessed by ELISA and expressed as percentage of control value (SD; n = 10 each). #indicates significance compared to the control group; *indicates significance compared to the CUMS group. BDNF indicates brain-derived neurotropic factor; CUMS, chronic unpredictable mild stress, ELISA, enzyme-linked immunosorbent assay; FLU, fluoxetine; M, musk; SD, standard deviation.
Protein expression levels of GR and BDNF, assessed by ELISA, were significantly (P < .001) downregulated in stressed mice when compared to the unstressed mice while administrating FLU (P < .001, P < .001), or musk significantly (P < .001, P = .02) upregulated it compared to the unexposed group (Figure 3).
Effect on the Histological Structure
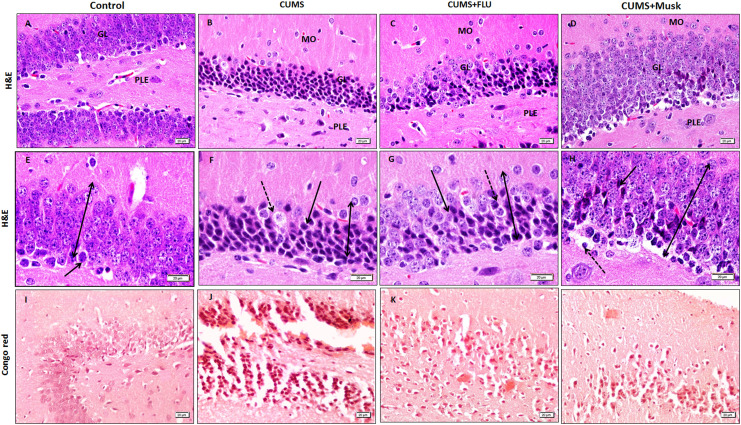
The hippocampus includes the DG and “CA” that has a characteristic C shape and included 3 regions; the CA1, the CA2, and the CA3. Both the DG and the CA1, the affected sites in AD, were critically investigated. The CA1 included the polymorphic, molecular, and pyramidal layers. The latter was formed of multilayer pyramidal neurons that have large vesicular nuclei and slightly basophilic cytoplasm, while the glia cells, on the other hand, have dark small nuclei. In stressed mice, many of these pyramidal cells had dark nuclei and cytoplasm. The size of this layer was decreased as evidenced by the significant (P < .001, P = .002, respectively) reduction in its thickness and surface area. Eosinophilic intraneural structures; Hirano bodies (HBs) were frequently observed in the pyramidal cells of CA1. Treatment with FLU or musk reduced these changes and significantly restored the pyramidal cell layer thickness and surface area compared to the CUMS group. The HBs were less frequently observed (Figures 4 and 5A).
Figure 4.
The hippocampal CA1 is formed of the molecular layer (MO), the pyramidal layer (PY), and the polymorphic layer (PO). The thickness of the PY layer appears smaller in the chronic unpredictable mild stress (CUMS) compared to the control (bi-head arrow). Some darkly stained cells (interrupted arrow) are observed. An eosinophilic structure (black arrow) is seen related to the pyramidal cell. Amyloid deposition with salmon red coloration observed near CA1 (insert show blood vessel with amyloid deposition in its wall). Immunoexpression of Caspase, GFAP, in the hippocampal CA1 are shown (A-D, I-T x 400, E-Hx1000). CUMS indicates chronic unpredictable mild stress, FLU, fluoxetine; M, musk.
Figure 5.
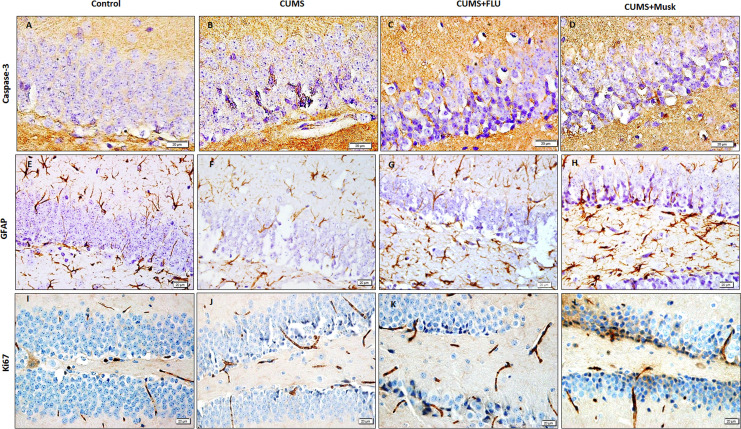
Thickness and surface area of CA1 (A) and dentate gyrus (B). Immunoexpression of GFAP (C), Caspase-3 (D), Ki67 (E), in the studied groups. Data are expressed as mean. # indicates significance versus control; * indicates significance versus CUMS. CA indicates cornu ammonis; CUMS, chronic unpredictable mild stress; FLU, fluoxetine; M, musk.
The granular cell layer of the DG was formed of polygonal cells with open face nuclei and lightly basophilic cytoplasm. In stressed mice, many of these granular cells had deeply stained cytoplasm and dark nuclei, while others appeared vacuolated and the number of the immature cell that had darkly stained nuclei was increased. These changes were less evidenced in groups treated with FLU or musk (Figure 6). The granular cell layer in mice treated with FLU (P = .04, P = .02) or musk (P = .04, P = .01) appeared significantly thicker and with larger surface area compared to the untreated mice, respectively (Figure 5B). On examining Congo red-stained hippocampus of mice exposed to CUMS, some scattered amyloid cores or plaques were observed in both the CA1 and DG near the pyramidal and granular cell layers, respectively. These amyloid cores were reduced in both size and number in FLU or musk-treated mice (Figures 4 and 6).
Figure 6.
The hippocampal DG is formed of the PLE, the GL, and the MO. The thickness of the GL layer appears smaller in the CUMS compared to the control (bi-head arrow). Some cells appear vacuolated cells (interrupted arrow) are observed. Note the increased number of the immature cell that have darkly stained nuclei (arrow). Amyloid deposition with salmon red coloration observed as patches as well as aggregation around the granular cell of the DG (A-D, I-L x400, E-H x1000). CUMS indicates chronic unpredictable mild stress, DG, dentate gyrus; FLU, fluoxetine; GL, granular cell layer; M; musk; MO, molecular; PLE, pleomorphic layer.
When it came to the effect of exposure to CUMS on the glial cells, immunohistochemical staining with GFAP antibody was used to assess the integrity of these cells (Figures 4 and 6). It was found that the GFAP-positive cells significantly decreased in number in both CA1 (P = .004) and DG (P = .02) of mice exposure to CUMS compared to the control, respectively. On the other hand, a significant increase in the number of these cells was observed in both groups treated with FLU (P = .03, P = .04) or musk (P = .01, P = .04; Figure 5C). Immunohistochemical stain using Capsase-3 antibody was used to detect apoptotic changes in the hippocampus (Figures 4 and 7). The number of Caspase-3 positive cells were significantly (P < .001) increased in both CA1 and DG of the CUMS group compared to the control, while their number showed a significant decrease after administration of FLU (P < .001) and musk (P < .001), respectively (Figure 5). Exposure to CUMS also resulted in a significant (P < .001) reduction in the DG neurogenesis indicated by immunohistochemical staining with Ki67 (Figure 7). Administration of FLU or musk resulted in a significant increase in Ki67-positive cells (P = .003, P = .001) in the subgranular zone (SGZ) of DG in comparison with the unexposed group respectively indicating proliferation of these cells (Figure 5C).
Figure 7.
Immunoexpression of GFAP, Caspase-3, Ki67, in the hippocampal dentate gyrus (A-D x600 and E-L x400). CUMS indicates chronic unpredictable mild stress; FLU, fluoxetine; M, musk.
Discussion
Anxiety, depression, and cognitive impairment are comorbidities triggered by psychological stress. 34 In more recent studies, the association between exposure to chronic stress and neurodegenerative disease was described. 35,36 The underlying mechanism of AD, one of the neurodegenerative diseases, in case of exposure to chronic stress, was a field of controversy. Anyway, studies on the discovery of drugs that can improve chronic stress-induced learning and memory impairments and neurodegeneration are of great importance for the treatment of AD. 37
Mice exposed to stress, in this study, developed depression evidenced by the observed elongation in the immobility time during the FST. The reduction in the time spent by mice in the open arm during the EPM test and exaggerated spontaneous locomotor activity during the OFT was an evidence in developing anxiety. These findings were supported by previous studies of Liu et al. 38 The EPM test was employed also to evaluate the short-term memory, and this was reported by many researchers. 27,39 In this study, the TL and the time spent by the mice in the closed arm were measured on the third day to assess the mice learning of the escape behavior in the first and second days. It was observed that the TL on third was prolonged after exposure to CUMS indicating an impairment in mice memory as was previously reported by Itoh et al. 26 One of the explanation of these behavior changes induced by stress was postulated by Sotiropoulos et al as he reported that chronic stress induces abnormal hyperphosphorylation of TAU in the hippocampus and prefrontal cortex (PFC), with contemporaneous impairments of hippocampus- and PFC-dependent behaviors. 8 The contributions of the PFC and the hippocampus to learning and memory have been known for a long time. 37
Previous studies on both human and animal showed that hippocampus was the part of the brain responsible for cognitive and memory functions. It was impaired very early in AD, and its impairment, especially CA1 region, resulted in the development of dementia and early signs of AD. 33,34,40 In the present study, exposure to CUMS for 4 weeks resulted in pathological changes included many apoptotic neurons in both CA1 and DG in addition to many vacuolated granular cells in DG. Adding to reduced thickness of both CA1 and DG and reduced number of the GFAP-positive astrocytes. These changes were in accordance with those reported by previous researchers. 9,41 These pathological changes were similar to what were described by Okasha as a neurodegenerative changes observed in an Alzheimer-induced model in adult male albino rats. 42 Hirano’s bodies (, eosinophilic intraneural structures in the hippocampal pyramidal cells, and the amyloid plaques, observed in Congo red-stained hippocampus in this study, were among the neuropathological hallmarks of Alzheimer described by Cvetković-Dožić et al. 43 The DG is one of the few regions of the adult brain where neurogenesis takes place. Neurogenesis is thought to play a role in the formation of new memories. 44 Gould et al and Li et al reported that the differentiation, not only the birth, of the newborn cells into functional neurons is required for the maintenance of cognitive abilities and normal learning and memory. 22,45 This differentiation is compromised in AD although neuroproliferation is increased. 22 This explained the presence of increased number of the new born cells, with dark nuclei, in the SGZ of the DG, after exposure to CUMS in this study. The presence of large number of these cells meant that they remained immature and did not differentiate into mature neurons. These findings indicated that CUMS induced AD-like neurodegenerative changes in mice.
In the present study, an increase in corticosterone level was observed following the exposure of mice to CUMS, and this was in concordance with Gong et al. 46 Increased cerebrospinal fluid cortisol level in dementia of Alzheimer’s type was also reported by Popp et al. 47 Gądek-Michalska et al added that alteration in cortisol level either systematically or in the vicinity of neuronal element may play a role in stress and its consequences. 48 It was reported that stress activated the hypothalamic–pituitary–adrenal axis with subsequent increase in glucocorticoids blood levels and activation of type II GRs which then trigger modifications in gene expression and negative consequences for hippocampal function. 49 Hippocampus possessed plenty of GRs that are involved in cognition, so chronic stress is proposed to induce a harmful impact on the hippocampal. 41 This could explain the CUMS-induced neurodegenerative changes described above. Another explanation of these changes is the reduction in the BDNF level which is confirmed biochemically in this study by assessing BDNF hippocampal protein level. Aleisa et al reported that exposure to chronic stress resulted in marked reduction in BDNF levels in hippocampal CA1 with subsequent hinder of the repair process and hence aggravating the impact of amyloid β (Aβ). 50 Stress may also modify the assembly of various AD-related proteins, for example, Amyloid Precursor Protein (APP) and drive its processing toward the amyloid production path that resulted in elevated Aβ levels reported with stress. 51
Fluoxetine, the antidepressant utilized to treat stress-induced depression in mice of the positive control group was reported to attenuate the behavioral changes induced by unpredictable stress and improve depression-associated changes in hippocampal C1 region and corticosterone level. 52 These results were supportive to the findings of this study.
Chemical therapeutic for AD are often of no vast benefit. Aicardi reported that current pharmacological therapy of AD partially masks the symptoms, while degenerative changes still progresses within the brain. 53 Aicardi reported that using essential oil from natural sources, what is called aromatherapy, was reported to be safe compared to the chemical drugs. 54 Those applied it for alleviation of mental and psychological disorders claimed that it is effective in both human and experimental animal models. 55,56 In the present study, inhalation of musk alleviate the depressive status induced by exposure to CUMS. It also shorten the TL and the time the mice spent in the closed arm indicating that it has improved the short memory and learning of the mice. Musk also reduced the corticosterone level and the pathological changes induced by stress on the hippocampal neuron in a pattern comparable to that induced by FLU. Neurogenesis in the hippocampal is an essential part of the FLU antidepressant action induced by augmenting proliferation of neural progenitor cells in the DG. 57 In this study, musk increased hippocampal neurogenesis as evident by increased Ki67 positive cell in the SGZ. Musk was reported to possess anti-inflammatory activities and was used as CNS stimulant by the old Chinese. 58 Thus, the anti-inflammatory action of musk could explain the neuroprotective effect observed on the hippocampus in this study. The direct action of musk through GRs could also explain for the antidepressant-like effect of the musk. 59 Natural musk was reported to contain steroids and androgen sex hormone derivatives. 60 This was consistent with what was recorded in this study as steroids accounted for up to 14% of musk’s constituents. Adding to that muscone, the active ingredient of musk was proved to have antiapoptotic effects. 9
In conclusion, musk of animal origin was found to alleviate the memory impairment and neurodegenerative changes induced in the hippocampus after exposure to the chronic unpredictable stress indicating that it is the time to test its effectiveness on experimental models of AD.
Supplemental Material
Supplemental Material, supplementary_table for The Role of Musk in Relieving the Neurodegenerative Changes Induced After Exposure to Chronic Stress by Manal Galal Abd El Wahab, Soad Shaker Ali, and Nasra Naeim Ayuob in American Journal of Alzheimer's Disease & Other Dementias
Footnotes
The authors declared no potential conflicts of interest with respect to the research, authorship, and/or publication of this article.
Funding: The author(s) disclosed receipt of the following financial support for the research, authorship, and/or publication of this article: the authors received funding from Yousef Abdullatif Jameel, Chair of Prophetic Medical Applications (YAJCPMA), Faculty of Medicine, King Abdulaziz University, Jeddah, Saudi Arabia to conduct the experiment of this research.
Supplemental Material: Supplementary material for this article is available online.
References
- 1. World Alzheimer Report 2016. Improving healthcare for people living with dementia coverage, Quality and costs now and in the future. Alzheimer’s Disease International (ADI), London. https://www.alz.co.uk/research/WorldAlzheimerReport2016.pdf Accessed January 19, 2018. [Google Scholar]
- 2. World Alzheimer Report 2015. The Global Impact of Dementia An analysis of revalence, incidence, cost and trends. Executive summary. Alzheimer’s Disease International (ADI), London. https://www.alz.co.uk/research/worldalzheimerreport2015summary.pdf Accessed January 19, 2018.
- 3. Yamada T, Hattori H, Miura A, Tanabe M, Yamori Y. Prevalence of Alzheimer’s disease, vascular dementia and dementia with Lewy bodies in a Japanese population. Psychiatry Clin Neurosci. 2001;55(1):21–25. [DOI] [PubMed] [Google Scholar]
- 4. Chung JK, Plitman E, Nakajima S, et al. ; Alzheimer’s Disease Neuroimaging Initiative. Cortical amyloid β deposition and current depressive symptoms in Alzheimer disease and mild cognitive impairment. J Geriatr Psychiatry Neurol. 2016;29(3):149–159. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 5. Choudhury B, Saytode P, Shah V. Neurodegenrative disorders: past, present and future. Int J Appl Biol Pharma Technol. 2014;5(2):1–14. [Google Scholar]
- 6. Wilson RS, Barnes LL, Bennett DA, et al. Proneness to psychological distress and risk of Alzheimer disease in a biracial community. Neurol. 2005;64(2):380–382. [DOI] [PubMed] [Google Scholar]
- 7. Johansson L, Guo X, Waern M, et al. Midlife psychological stress and risk of dementia: a 35-year longitudinal population study. Brain. 2010;133(pt 8):2217–2224. [DOI] [PubMed] [Google Scholar]
- 8. Sotiropoulos I, Catania C, Pinto LG, et al. Stress acts cumulatively to precipitate Alzheimer’s disease-like tau pathology and cognitive deficits. J Neurosci. 2011;31(21):7840–7847. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 9. Wang Y, Kan H, Yin Y, et al. Protective effects of ginsenoside Rg1 on chronic restraint stress induced learning and memory impairments in male mice. Pharmacol Biochem Behav. 2014;120;73–81. [DOI] [PubMed] [Google Scholar]
- 10. Sandi C, Pinelo-Nava MT. Stress and memory: behavioral effects and neurobiological mechanisms. Neural Plast. 2007;2007:78970. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 11. Wingenfeld K, Wolf OT. Stress, memory, and the hippocampus. Front Neurol Neurosci. 2014;34:109–120. [DOI] [PubMed] [Google Scholar]
- 12. Urakami K. Prevention of dementia. Psychogeriatrics 2007;7(3):93–97. [Google Scholar]
- 13. Jimbo D, Kimura Y, Taniguchi M, Inoue M, Urakami K. Effect of aromatherapy on patients with Alzheimer’s disease. Psychogeriatrics 2009;9(4):173–179. [DOI] [PubMed] [Google Scholar]
- 14. Irmak Sapmaz H, Uysal M, Taş U, et al. The effect of lavender oil in patients with renal colic: a prospective controlled study using objective and subjective outcome measurements. J Altern Complement Med. 2015;21(10):617–622. [DOI] [PubMed] [Google Scholar]
- 15. Yoshiyama K, Arita H, Suzuki J. The effect of aroma hand massage therapy for people with dementia. J Altern Complement Med. 2015;21(12):759–765. [DOI] [PubMed] [Google Scholar]
- 16. Hritcu L, Cioanca O, Hancianu M. Effects of lavender oil inhalation on improving scopolamine-induced spatial memory impairment in laboratory rats. Phytomed. 2012;19(6):529–534. [DOI] [PubMed] [Google Scholar]
- 17. Bae D, Seol H, Yoon HG, et al. Inhaled essential oil from chamaecyparis obtuse ameliorates the impairments of cognitive function induced by injection of β-amyloid in rats. Pharm Biol. 2012;50(7):900–910. [DOI] [PubMed] [Google Scholar]
- 18. Khan IA, Abourashed EA. Leung’s Encyclopedia of Common Natural Ingredients. Hoboken, New Jersey: John Wiley & Sons, Inc. 2010:455–465. [Google Scholar]
- 19. Fukui H, Komaki R, Okui M, Toyoshima K, Kuda K. The effects of odor on cortisol and testosterone in healthy adults. Neuro Endocrinol Lett. 2007;28(4):433–437. [PubMed] [Google Scholar]
- 20. Ayuob NN, Ali SS, Suliaman M, El Wahab MG, Ahmed SM. The antidepressant effect of musk in an animal model of depression: a histopathological study. Cell Tissue Res. 2016;366(2):271–284. [DOI] [PubMed] [Google Scholar]
- 21. World Medical Association Declaration of Helsinki. Ethical principles for medical research involving human subjects. JAMA. 2013;310(20):2191–2194. [DOI] [PubMed] [Google Scholar]
- 22. Li M, Fu Q, Li Y, Li S, Xue J, Ma S. Emodin opposes chronic unpredictable mild stress induced depressive-like behavior in mice by upregulating the levels of hippocampal glucocorticoid receptor and brain-derived neurotrophic factor. Fitoterapia. 2014;98;1–10. [DOI] [PubMed] [Google Scholar]
- 23. Kilpatrick L, Cahill L. Amygdala modulation of parahippocampal and frontal regions during emotionally influenced memory storage. NeuroImage. 2003;20(4):2091–2099. [DOI] [PubMed] [Google Scholar]
- 24. Carobrez AP, Bertoglio LJ. Ethological and temporal analyses of anxiety-like behavior: the elevated plus-maze model 20 years on. Neurosci Biobehav Rev. 2005;29(8):1193–1205. [DOI] [PubMed] [Google Scholar]
- 25. Chioca LR, Ferro MM, Baretta IP, et al. Anxiolytic-like effect of lavender essential oil inhalation in mice. Participation of serotonergic but not GABA neurotransmission. J Ethnopharmacol. 2013;147(2);412–418. [DOI] [PubMed] [Google Scholar]
- 26. Itoh J, Nabeshima T, Kameyama T. Utility of an elevated plus-maze for the evaluation of memory in mice: effects of nootropics, scopolamine and electroconvulsive shock. Psychopharmacol (Berl). 1990;101(1):27–33. [DOI] [PubMed] [Google Scholar]
- 27. Mineur YS, Belzung C, Crusio WE. Effects of unpredictable chronic mild stress on anxiety and depression-like behavior in mice. Behav Brain Res. 2006;175(1):43–50. [DOI] [PubMed] [Google Scholar]
- 28. Doro R, Lotan D, Versano Z, et al. Escitalopram or novel herbal mixture treatments duringor following exposure to stress reduce anxiety like behavior through corticosterone and BDNF modifications. PLoS One. 2014;9(4):e91455. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 29. Paxinos G, Watson C. The Rat Hippocampus in Stereotaxic Coordinates. San Diego, CA: Academic Press; 1998. [Google Scholar]
- 30. Baker-Herman TL, Fuller DD, Bavis RW, et al. BDNF is necessary and sufficient for spinal respiratory plasticity following intermittent hypoxia. Nat Neurosci. 2014;7(1):48–55 [DOI] [PubMed] [Google Scholar]
- 31. Bancroft JD, Gamble M. Theory and practice of histological techniques. 6th ed. Philadelphia, PA: Churchill Livingstone; 2008. [Google Scholar]
- 32. Nobakht M, Hoseini SM, Mortazavi P, et al. Neuropathological changes in brain cortex and hippocampus in a rat model of Alzheimer’s Disease. Iran Biomed J. 2011;15(1-2):51–58. [PMC free article] [PubMed] [Google Scholar]
- 33. Makhlouf NA, El-Beshbishy RA, Abousetta A. Ginkgo modulates noise-induced hippocampal damage in male albino rats: a light and electron microscopic study. The Egypt J Histol. 2014;37(1):159–174. [Google Scholar]
- 34. McEwen BS. Physiology and neurobiology of stress and adaptation: central role of the brain. Physiol Rev. 2007;87(3):873–904. [DOI] [PubMed] [Google Scholar]
- 35. Chaudhry M, Hasnain S, Snitz BE, et al. Association of APOE polymorphisms and stressful life events with dementia in a Pakistani population. Neurosci Lett. 2014;570:42–46. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 36. Huang Y, Skwarek-Maruszewska A, Horré K, et al. Loss of GPR3 reduces the amyloid plaque burden and improves memory in Alzheimer’s disease mouse models. Sci Transl Med. 2015;7(309):309ra164. [DOI] [PubMed] [Google Scholar]
- 37. Laroche S, Davis S, Jay TM. Plasticity at hippocampal to prefrontal cortex synapses: dual roles in working memory and consolidation. Hippocampus. 2000;10(4):438–446. [DOI] [PubMed] [Google Scholar]
- 38. Liu D, Xie K, Yang X, et al. Resveratrol reverses the effects of chronic unpredictable mild stress on behavior, serum corticosterone levels and BDNF expression in rats. Behav Brain Res. 2014;264:9–16. [DOI] [PubMed] [Google Scholar]
- 39. Kulkarni SK. Hand book of Experimental Pharmacology, 3rd ed. Vallabh Prakashan, Delhi: 2007:36–38. [Google Scholar]
- 40. Tamagnini F, Scullion S, Brown JT, Randall AD. Intrinsic excitability changes induced by acute treatment of hippocampal CA1 pyramidal neurons with exogenous amyloid β peptide. Hippocampus. 2015;25(7):786–797. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 41. McEwen BS. Central effects of stress hormones in health and disease: understanding the protective and damaging effects of stress and stress mediators. Eur J Pharmacol. 2008;583(2-3):174–185. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 42. Okasha EF. Dentate gyrus changes in an Alzheimer-induced model in adult male albino rats and the possible protection by ginger: histological and immunohistochemical study. Egypt J Histol. 2012;35:711–720. [Google Scholar]
- 43. Cvetković-Dožić D, Skender-Gazibara M, Dožić S. Neuropathological hallmarks of Alzheimer’s disease. Arch Oncol. 2001;9(3):195–199. [Google Scholar]
- 44. Saab BJ, Georgiou J, Nath A, et al. NCS-1 in the dentate gyrus promotes exploration, synaptic plasticity, and rapid acquisition of spatial memory. Neuron. 2009;63(5):643–656. [DOI] [PubMed] [Google Scholar]
- 45. Gould E, Beylin A, Tanapat P, et al. Learning enhances adult neurogenesis in the hippocampal formation. Nat Neurosci. 1999;2(3):260–265 [DOI] [PubMed] [Google Scholar]
- 46. Gong S, Miao YL, Jiao GZ, et al. Dynamics and correlation of serum cortisol and corticosterone under different physiological or stressful conditions in mice. PLoS One. 2015;10(2):e0117503. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 47. Popp J, Wolfsgruber S, Heuser I, et al. Cerebrospinal fluid cortisol and clinical disease progression in MCI and dementia of Alzheimer’s type. Neurobiol Aging. 2015;36(2):601–607. [DOI] [PubMed] [Google Scholar]
- 48. Gądek-Michalska A, Spyrka J, Rachwalska P, Tadeusz J, Bugajski J. Influence of chronic stress on brain corticosteroid receptors and HPA axis activity. Pharmacol Rep. 2013;65(5):1163–1175. [DOI] [PubMed] [Google Scholar]
- 49. Duman RS, Monteggia LM. A neurotrophic model for stress-related mood disorders. Biol Psychiatry. 2006;59(12):1116–1127. [DOI] [PubMed] [Google Scholar]
- 50. Aleisa AM, Alzoubi KH, Gerges NZ, Alkadhi KA. Chronic psychosocial stress-induced impairment of hippocampal LTP: possible role of BDNF. Neurobiol Dis. 2006;22(3):453–462. [DOI] [PubMed] [Google Scholar]
- 51. Srivareerat M, Tran TT, Salim S, Aleisa AM, Alkadhi KA. Chronic nicotine restores normal Aβ levels and prevents short-term memory and E-LTP impairment in Aβ rat model of Alzheimer’s disease. Neurobiol Aging. 2011;32(5):834–844. [DOI] [PubMed] [Google Scholar]
- 52. Dwivedi Y, Rizavi HS, Pandey GN. Antidepressants reverse corticosterone-mediated decrease in brain-derived neurotrophic factor expression: differential regulation of specific exons by antidepressants and corticosterone. Neuroscience. 2006;139(3):1017–1029. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 53. Aicardi G. New hope from an old drug: fighting Alzheimer’s disease with the cancer drug bexarotene (targretin)? Rejuvenation Res. 2013;16(6):524–528. [DOI] [PubMed] [Google Scholar]
- 54. Schmidt NB, Keough ME, Hunter LR, Funk AP. Physical illness and treatment of anxiety disorders: A review In: Zvolensky MJ, Smits J, eds. Series in Anxiety and Related Disorders: Anxiety in Health Behaviors and Physical Illness. New York: Springer; 2008:341–366. [Google Scholar]
- 55. Goes TC, Antunes FD, Alves PB, Teixeira-Silva F. Effect of sweet orange aroma on experimental anxiety in humans. J Altern Complement Med. 2012;18(8):798–804. [DOI] [PubMed] [Google Scholar]
- 56. Oyemitan IA, Olayera OA, Alabi A, et al. Psychoneuropharmacological activities and chemical composition of essential oil of fresh fruits of Piper guineense (Piperaceae) in mice. J Ethnopharmacol. 2015;166:240–249. [DOI] [PubMed] [Google Scholar]
- 57. David DJ, Samuels BA, Rainer Q, et al. Neurogenesis-dependent and -independent effects of fluoxetine in an animal model of anxiety/depression. Neuron. 2009;62(4):479–493. doi:10.1016/j.neuron.2009.04.017 [DOI] [PMC free article] [PubMed] [Google Scholar]
- 58. Kimura K, Kimura Y, Ohata K, Takagi H. Effects of several monoamine-related compounds on the reserpine-induced spikes recorded from the medial nucleus trapezoides in rabbits. Jpn J Pharmacol. 1978;28(2):317–327. [DOI] [PubMed] [Google Scholar]
- 59. McEwen BS, Magarinos AM. Stress and hippocampal plasticity: implications for the pathophysiology of affective disorders. Hum Psychopharmacol. 2001;16(S1):S7–S19 [DOI] [PubMed] [Google Scholar]
- 60. Oh SR, Lee JP, Chang SY, et al. Androstane alkaloids from musk of Moschus moschiferus. Chem Pharm Bull. 2002;50(5):663–664. [DOI] [PubMed] [Google Scholar]
Associated Data
This section collects any data citations, data availability statements, or supplementary materials included in this article.
Supplementary Materials
Supplemental Material, supplementary_table for The Role of Musk in Relieving the Neurodegenerative Changes Induced After Exposure to Chronic Stress by Manal Galal Abd El Wahab, Soad Shaker Ali, and Nasra Naeim Ayuob in American Journal of Alzheimer's Disease & Other Dementias