Abstract
Diagnostic assessments for dementia include the evaluation of subjective memory impairment, dementia worries, or depressive symptoms. Data on the predictive value of these factors remain unclear, and varying help-seeking behavior may contribute to this finding. We investigate whether differentiating help-seeking motivation from other psychological factors associated with cognitive impairment would enhance the prediction of diagnostic outcomes in a memory clinic. We obtained information on help-seeking motivation from 171 patients who underwent routine diagnostic assessments. Utilizing a discriminant correspondence analysis, our results indicate that extrinsic motivation increases the likelihood of receiving a dementia diagnosis, whereas depression or the duration of deficits carries discriminatory information to further guide the differentiation of prodromal dementia. Recognizing motivational aspects of help-seeking behavior can complement the clinical evaluation of cognitive performance.
Keywords: help-seeking motivation, cognitive decline, subjective memory impairment, dementia worries, discriminant correspondence analysis
Introduction
Various healthy aging campaigns focus on detecting or preventing cognitive decline. Personal awareness and the recognition of new therapeutic developments in the media contribute to increasing numbers of patients being referred to memory clinics. Diagnostic procedures include behavioral and neuropsychological assessments, complemented by neuroimaging and laboratory (eg, cerebrospinal fluid) measures. Physicians also acknowledge subjective memory impairment, dementia worries, or depressive symptoms, and their association with cognitive decline has been studied previously. 1 –3
People with subjective memory impairment are at risk of developing dementia. 4 They show elevated Alzheimer’s disease cerebrospinal fluid biomarkers and structural changes in brain regions associated with early-stage neurodegeneration. 5 –7 Factors that contribute to worrying about dementia are the patient’s age, personal memory ratings, knowledge of Alzheimer’s disease, and the assumption that being a first-degree relative of someone with this disease increases one’s own risk. 8 However, investigating the predictive value of subjective memory impairment or dementia worries yielded variable findings across clinical and research settings. 2,9 –12 Differences in help-seeking behavior may contribute to this heterogeneity. Among individuals with subjective memory impairment, reduced memory self-efficacy, lower quality of life, and deterioration in daily functioning increase help-seeking motivation. 13 Perceiving subjective complaints as the result of biomedical changes in the brain and having no negative views about presenting to a primary care physician also increase help-seeking. 14 Whereas some people with subjective memory deficits may not worry about dementia, which could vary across specific settings (community, memory clinic) and socioeconomic networks, 12,14 others could even develop depressive symptoms. Therefore, both symptom anosognosia and mood changes could differentially affect help-seeking, but they could also be possible clinical features of prodromal dementia. 3,15
When we ask our patients whether they present self-motivated or extrinsically motivated (eg, by spouse or family physician), we intuitively think of differences in subjective memory impairment or dementia worries among these people, whereas the motivational aspect remains largely unstudied. We hypothesized that differentiating help-seeking motivation from other psychological factors associated with cognitive impairment would enhance the prediction of diagnostic outcomes in a memory clinic.
Methods
Participants and Procedures
One hundred seventy-one people (85 women and 86 men) participated in this study. We aimed at recruiting patients who had been referred to our memory clinic (university hospital, outpatient service) for the first time from January to December 2016. Patients with previously established diagnoses presenting for follow-up assessments and participants without the cognitive capacity to consent were not invited to participate. After providing complete description of the study to the patients, written informed consent was obtained in accordance with our university’s ethics committee. Irrespective of study procedures, all participants underwent routine diagnostic assessments including detailed medical and neuropsychological examinations, laboratory testing, and brain imaging. Diagnoses were established according to standard clinical criteria, for example National Institute of Neurological and Communicative Diseases and Stroke/Alzheimer’s Disease and Related Disorders Association criteria, for Alzheimer’s disease 16 or Petersen criteria for mild cognitive impairment. 17 For this study, in addition to basic demographic and clinical information (such as the years of education, a personal or family history of dementia and/or depression, subjective cognitive impairment, and dementia worries), we specifically obtained information on help-seeking motivation (self-motivated/extrinsically motivated). Self-motivation was defined as an individual’s own decision to make an appointment in our memory clinic either directly or after asking family members, friends, or a primary care provider for advice. Extrinsic motivation is reflected by a situation, where others recommend the appointment in the memory clinic without prior indication that the person would find this necessary. In order to obtain a standardized investigation of all demographic and clinical variables, we used a questionnaire that was completed by the physician during the initial medical examination and patient/caregiver interview.
Statistical Analyses
We examined all variables (as shown in Table 1) with a discriminant correspondence analysis (DICA) 18,19 in order to reveal the variables important to discriminate between major diagnostic groups: “normal objective cognition (NC),” “mild cognitive impairment (MCI),” and “dementia (DEM).” The multivariate technique has been used in dementia research. 20,21 We normalized continuous variables (eg, age) with percentile ranges. We performed additional multinomial regression analyses to examine possible adjusted effects, with diagnostic group regressed on all other variables. We used the NC group as a reference level. Statistical computations were conducted with the R (3.40) statistical programming environments. 22 For DICA, we used the “Inposition” package, 23 accounting for missing values (1%) with “missMDA.” 24
Table 1.
Demographic Characteristics and Clinical Variables Across Diagnostic Groups.
| Variable | Value | NC | MCI | DEM |
|---|---|---|---|---|
| Age | <67 years | 26 | 25 | 9 |
| 67-76 years | 11 | 23 | 23 | |
| >76 years | 8 | 13 | 33 | |
| Depression | Current: no | 23 | 36 | 58 |
| Past: yes | 18 | 13 | 4 | |
| Current or past: yes | 4 | 12 | 3 | |
| Education | University: yes | 22 | 18 | 22 |
| University: no | 23 | 43 | 43 | |
| >13 years | 21 | 22 | 26 | |
| <13 years | 24 | 39 | 39 | |
| Dementia worries | No | 5 | 13 | 27 |
| Few | 8 | 21 | 24 | |
| Some | 21 | 16 | 10 | |
| Significant | 11 | 11 | 4 | |
| Motivation | Extrinsic | 8 | 20 | 50 |
| Intrinsic | 37 | 41 | 15 | |
| Symptom onset | <22 months | 15 | 27 | 14 |
| 22-40 months | 12 | 20 | 25 | |
| >40 months | 18 | 14 | 26 | |
| Sex | Female | 20 | 30 | 35 |
| Male | 25 | 31 | 30 | |
| Dementia family history | No | 30 | 42 | 39 |
| Yes | 15 | 19 | 26 | |
| First symptom | Attention deficit | 7 | 4 | 2 |
| Memory impairment | 29 | 32 | 43 | |
| Orientation deficit | 4 | 2 | ||
| Word finding impairment | 7 | 7 | 3 | |
| Unspecified | 2 | 14 | 15 | |
| Subjective memory impairment | No | 5 | 19 | 40 |
| Yes | 40 | 42 | 25 |
Abbreviations: DEM, dementia; MCI, mild cognitive impairment; NC, normal objective cognition.
Results
Among our study participants were 45 participants with normal cognition (Mini-Mental State Examination, 25 [MMSE] score, mean [standard deviation]: 29.2 [1.2]), 61 patients with mild cognitive impairment (MMSE 27.5 [2.1], and 65 patients with dementia (MMSE: 21.1 [6.5]). Further demographic characteristics and clinical measures are highlighted in Table 1.
The DICA resulted in a significant omnibus test (P perm = .001). In the 2-dimensional maps, the proximity between the groups (Figure 1) or between individual observations (ie, participants) within a group (Figure 2) represents their similarity. 18,20 The quality of group assignment was r 2 = .3, P perm = .001, indicating that the model yields a significant (and not random) assignment of observations to a group. We rather show that the diagnostic groups could be separated from each other using 2 components. A component reflects a set of variables (ie, the items we investigated) important to discriminate between diagnostic groups. The accuracy (prediction × observed group-assignment) as indicated by a confusion matrix was acc = 61.3%. A leave-one-out cross validation led to an estimate of accloo = 60%.
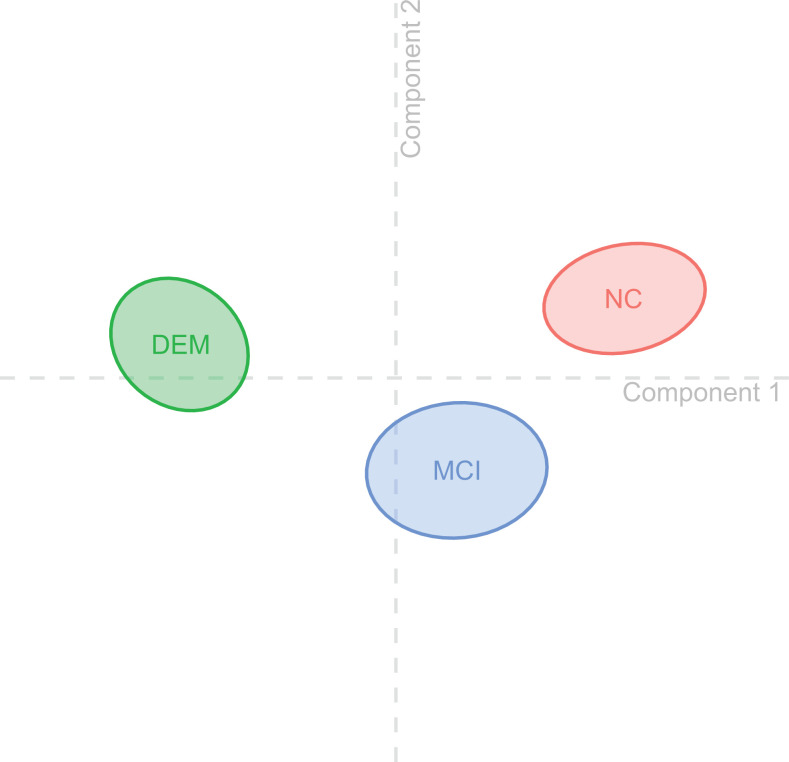
Figure 1.
Group centroids. The figure visualizes the diagnostic groups with 95% bootstrap confidence intervals in 2-dimensional space resulting from the discriminant correspondence analysis (DICA) components. As none of the confidence intervals overlap, all groups significantly differ from each other. The horizontal axis (component 1) represents discriminatory factors that separate the groups “normal objective cognition (NC)” and “dementia (DEM),” whereas the vertical axis (component 2) represents factors that separate the groups “normal objective cognition (NC)” and “mild cognitive impairment (MCI).”
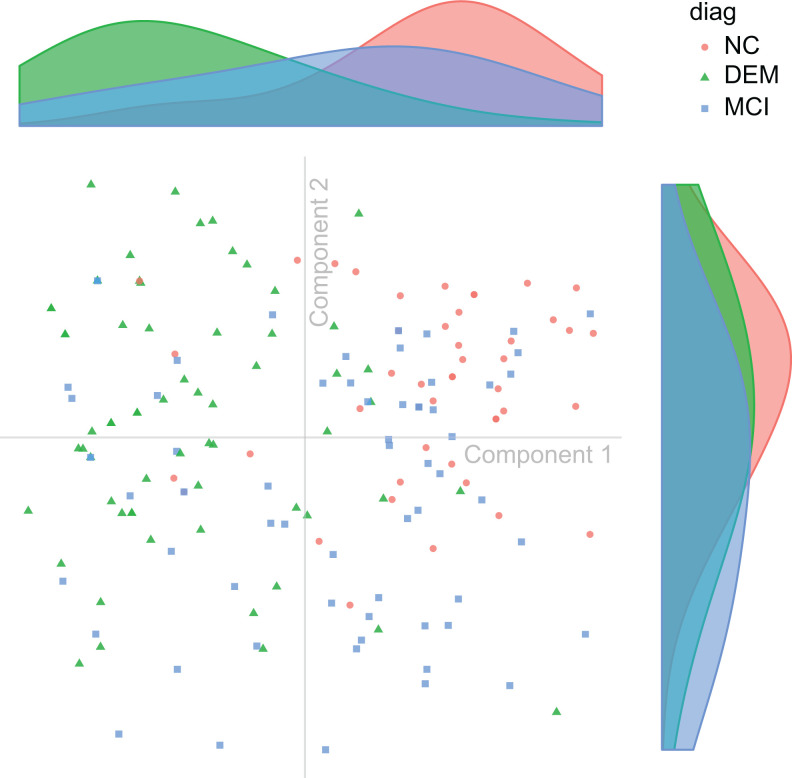
Figure 2.
Subject distribution. Discriminant correspondence analysis (DICA) map of observations (ie, patients), color coded by diagnostic group. Each point shows the 2-dimensional DICA representation of the response profile from all variables given by a patient. On the top and right aligned are the respective marginal densities of component 1 (top) and component 2 (right).
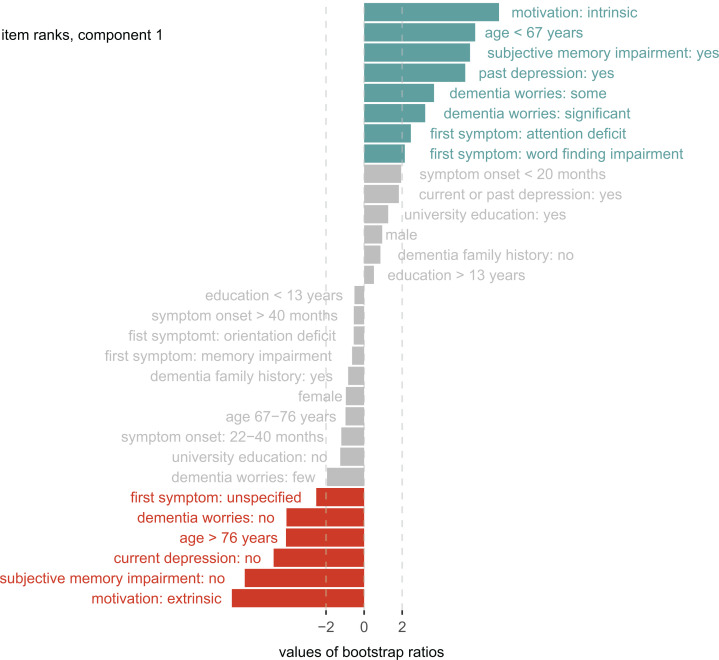
Component 1 explained 86% of the variance (pperm = .001), and discriminated between the groups NC and DEM, there was a trend for the discrimination of NC and MCI (t = 1.3, P = .09). Help-seeking motivation was the variable with the highest coefficients (intrinsic motivation, t = 7.1, P = .001, extrinsic motivation = −6.9, P = .001) for group discrimination. It means that help-seeking motivation contributes more than other variables, such as subjective memory impairment, to the discrimination of NC and DEM. All the variables within component 1 that contributed (or did not significantly contribute) to the NC/DEM group discrimination are highlighted in Figure 3.
Figure 3.
Group discrimination (component 1). The bar lengths represent bootstrap ratios for the discriminant correspondence analysis (DICA) component 1 factor variables ordered by their magnitude. The dotted line represents the critical value for a single null hypothesis test. Colored variables significantly contribute to group discrimination (blue: predictive toward normal objective cognition, red: predictive toward dementia).
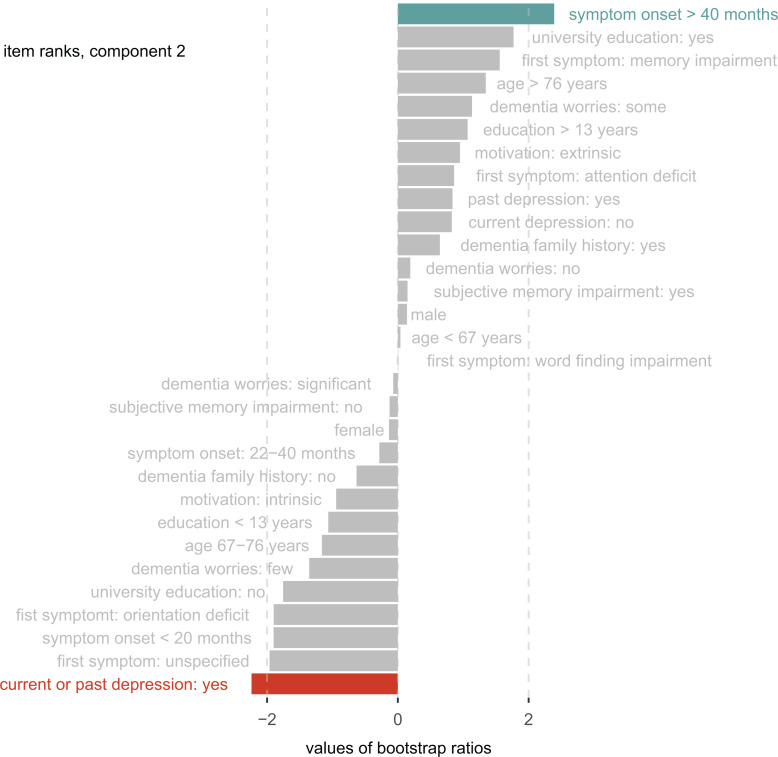
Component 2 explained 14% of the variance (P perm = .006) and discriminated between the NC and MCI groups. The variables “depression” and “onset of symptoms” resulted in significant coefficients for group discrimination (Figure 4). Patients with current or past depression were more likely to receive a diagnosis of mild cognitive impairment, whereas people reporting a long symptom duration were more likely to receive no diagnosis. See Supplementary Table 1 for additional information on bootstrap ratios, factor scores, and contributions for all variables within the DICA components.
Figure 4.
Group discrimination (component 2). The bar lengths represent bootstrap ratios for the discriminant correspondence analysis (DICA) component 2 factor variables ordered by their magnitude. The dotted line represents the critical value for a single null hypothesis test. Colored variables significantly contribute to group discrimination (blue: predictive toward normal objective cognition, red: predictive toward mild cognitive impairment).
Within multinomial regression, the analysis of deviance showed significant effects for help-seeking motivation (χ2(2) = 9.39, P = .01) and depression (χ2(4) = 15.53, P = .004), whereas other factors were not significant. This suggests that even after adjusting for all other variables, these items carry discriminatory information. Extrinsically motivated people were 6.4 times more likely to receive a dementia diagnosis than receiving no diagnosis (b = 1.85, SE = .87, z = 2.14, P = .0327). The likelihood of mild cognitive impairment increased 7 times (b = 1.93, SE = .83, z = 2.35, P = .02) if the patient reported current or past depression.
Discussion
This study demonstrates the predictive value of help-seeking motivation differences among people presenting to a memory clinic. Extrinsic motivation increases the likelihood of receiving a dementia diagnosis, whereas depressive symptoms or the duration of subjective deficits further guide the differentiation of prodromal dementia.
Although subjective memory impairment is associated with a risk of developing dementia, 4 people may still choose to not seek help because of several factors. They may not perceive significant changes in daily functioning or in the quality of life, 13 attribute their deficits to normal aging and not pathological brain dysfunction, 26,27 and are less likely to compare their cognitive performance to others’. 27 Irrespective of whether it indicates impairment anosognosia or unawareness for the possibilities to treat cognitive decline, extrinsic help-seeking motivation signals a symptom appraisal discrepancy between an individual and its social environment. Hurt and colleagues 27 show that help-seeking behavior is unrelated of cognitive impairment severity. Therefore, an extrinsically motivated presentation highlights better than symptom awareness or symptom severity that caregivers recognize a deviation of how help-seeking is guided by a common-sense model of illness perception 28 in this subject and within a given socioeconomic context.
Data presented by Perrotin and colleagues 29 encourage the investigation of mood changes in people with subjective cognitive decline. The authors highlight that subclinical depression and hippocampal atrophy were associated with medical help-seeking in people with subjective memory impairment. Our data also show that mood changes, and not the severity of memory complaints, are helpful indicators for the differentiation of prodromal dementia stages. In line with this, La Joie and colleagues 30 found that people with subjective cognitive impairment and mild objective deficits differ in measures, such as temporal orientation, that are not primarily memory related but may also indicate hippocampal network dysfunction.
Increasing knowledge of neurodegenerative diseases, and the awareness that preventing cognitive decline contributes to healthy aging, will result in more and younger people being referred to memory clinics. Among these people, a family history of dementia, a positive view about presenting to a physician, subjective symptom progression, and concern about possible brain pathology increase help-seeking motivation. 13,14,27 However, people experiencing subjective deficits may show different help-seeking behavior within specialized hospital services and the community. Archer and colleagues 12 show that the above-mentioned factors associated with increased help-seeking are less frequent in community participants with subjective memory deficits. The desire to avoid medicalization (and stigma) of normal aging-related changes in cognition could modulate help-seeking behavior. In contrast, a lack of concern associated with subjective impairment could reflect shared but dysfunctional beliefs within a social network about which changes normal cognitive aging might be associated with. 14 Furthermore, differences in health-care access as well as physical comorbidities could influence help-seeking. Begum and colleagues 14 show that people who are already present in the medical system, for example, due to physical illnesses, may have more opportunity to seek formal help or to be under medical surveillance. Open-access services and general practitioner training on the early diagnosis of dementia could enhance help-seeking behavior. 14 The authors and others 31 demonstrate how Open House initiatives and public campaigns focusing on the importance of memory complaints and self-evaluation can provide guidance on how to access the medical system and what support or treatment to expect. 14 Despite these limitations, we show that subjective memory impairment or dementia worries do not simply induce help-seeking. Recognizing its motivational aspects can complement the standard clinical evaluation of cognitive performance.
Supplemental Material
Supplemental Material, Suppl_Table1 for Extrinsic and Intrinsic Help-Seeking Motivation in the Assessment of Cognitive Decline by Robert Haussmann, René Mayer-Pelinski, Maike Borchardt, Fabrice Beier, Franziska Helling, Maria Buthut, Gisa Meissner, Jan Lange, Anne Zweiniger, and Markus Donix in American Journal of Alzheimer's Disease & Other Dementias
Footnotes
The authors declared no potential conflicts of interest with respect to the research, authorship, and/or publication of this article.
Funding: The authors disclosed receipt of the following financial support for the research, authorship, and/or publication of this article: Dr. Donix has received research support from the Roland Ernst Stiftung and a lecture fee from Mundipharma.
Supplemental Material: Supplementary material for this article is available online.
References
- 1. Mol ME, van Boxtel MP, Willems D, Jolles J. Do subjective memory complaints predict cognitive dysfunction over time? A six-year follow-up of the maastricht aging study. Int J Geriatr Psychiatry. 2006;21(5):432–441. [DOI] [PubMed] [Google Scholar]
- 2. Pietrzak RH, Maruff P, Woodward M, et al. Mild worry symptoms predict decline in learning and memory in healthy older adults: a 2-year prospective cohort study. Am J Geriatr Psychiatry. 2012;20(3):266–275. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 3. Singh-Manoux A, Dugravot A, Fournier A, et al. Trajectories of depressive symptoms before diagnosis of dementia: a 28-year follow-up study. JAMA Psychiatry. 2017;74(7):712–718. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 4. Jessen F, Wiese B, Bachmann C, et al. ; German Study on Aging, Cognition and Dementia in Primary Care Patients Study Group. Prediction of dementia by subjective memory impairment: effects of severity and temporal association with cognitive impairment. Arch Gen Psychiatry. 2010;67(4):414–422. [DOI] [PubMed] [Google Scholar]
- 5. Peter J, Scheef L, Abdulkadir A, et al. ; Alzheimer’s Disease Neuroimaging Initiative. Gray matter atrophy pattern in elderly with subjective memory impairment. Alzheimers Dement. 2014;10(1):99–108. [DOI] [PubMed] [Google Scholar]
- 6. Ryu SY, Lim EY, Na S, et al. Hippocampal and entorhinal structures in subjective memory impairment: a combined MRI volumetric and DTI study. Int Psychogeriatr. 2017;29(5):785–792. [DOI] [PubMed] [Google Scholar]
- 7. Visser PJ, Verhey F, Knol DL, et al. Prevalence and prognostic value of CSF markers of Alzheimer’s disease pathology in patients with subjective cognitive impairment or mild cognitive impairment in the DESCRIPA study: a prospective cohort study. Lancet Neurol. 2009;8(7):619–627. [DOI] [PubMed] [Google Scholar]
- 8. Cutler SJ. Worries about getting Alzheimer’s: who’s concerned? Am J Alzheimers Dis Other Demen. 2015;30(6):591–598. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 9. Kryscio RJ, Abner EL, Cooper GE, et al. Self-reported memory complaints: implications from a longitudinal cohort with autopsies. Neurology. 2014;83(15):1359–1365. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 10. Eichler T, Thyrian JR, Hertel J, et al. Subjective memory impairment: no suitable criteria for case-finding of dementia in primary care. Alzheimers Dement (Amst). 2015;1(2):179–186. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 11. Mitchell AJ. The clinical significance of subjective memory complaints in the diagnosis of mild cognitive impairment and dementia: a meta-analysis. Int J Geriatr Psychiatry. 2008;23(11):1191–1202. [DOI] [PubMed] [Google Scholar]
- 12. Archer HA, Newson MA, Coulthard EJ. Subjective memory complaints: symptoms and outcome in different research settings. J Alzheimers Dis. 2015;48(suppl 1):S109–S114. [DOI] [PubMed] [Google Scholar]
- 13. Ramakers IH, Visser PJ, Bittermann AJ, Ponds RW, van Boxtel MP, Verhey FR. Characteristics of help-seeking behaviour in subjects with subjective memory complaints at a memory clinic: a case-control study. Int J Geriatr Psychiatry. 2009;24(2):190–196. [DOI] [PubMed] [Google Scholar]
- 14. Begum A, Whitley R, Banerjee S, Matthews D, Stewart R, Morgan C. Help-seeking response to subjective memory complaints in older adults: toward a conceptual model. Gerontologist. 2013;53(3):462–473. [DOI] [PubMed] [Google Scholar]
- 15. Lin F, Wharton W, Dowling NM, et al. Awareness of memory abilities in community-dwelling older adults with suspected dementia and mild cognitive impairment. Dement Geriatr Cogn Disord. 2010;30(1):83–92. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 16. McKhann G, Drachman D, Folstein M, Katzman R, Price D, Stadlan EM. Clinical diagnosis of Alzheimer’s disease: report of the NINCDS-ADRDA Work group under the auspices of Department of Health and Human Services Task Force on Alzheimer’s Disease. Neurology. 1984;34(7):939–944. [DOI] [PubMed] [Google Scholar]
- 17. Petersen R. Mild cognitive impairment as a diagnostic entity. J Intern Med. 2004;256(3):183–194. [DOI] [PubMed] [Google Scholar]
- 18. Abdi H. Discriminant correspondence analysis. In: Salkind N, ed. Encyclopedia of Measurement and Statistics. Thousand Oaks, CA: Sage; 2007. [Google Scholar]
- 19. Greenacre MJ. Correspondence Analysis in Practice. 2nd ed. Boca Raton, FL: Chapman & Hall/CRC; 2007. [Google Scholar]
- 20. Williams LJ, Abdi H, French R, Orange JB. A tutorial on multiblock discriminant correspondence analysis (MUDICA): a new method for analyzing discourse data from clinical populations. J Speech Lang Hear Res. 2010;53(5):1372–1393. [DOI] [PubMed] [Google Scholar]
- 21. Beaton D, Dunlop J, Abdi H; Alzheimer’s Disease Neuroimaging I. Partial least squares correspondence analysis: a framework to simultaneously analyze behavioral and genetic data. Psychol Methods. 2016;21(4):621–651. [DOI] [PubMed] [Google Scholar]
- 22. R Core Team. R. A Language and Environment for Statistical Computing. Vienna, Austria: R Foundation for Statistical Computing; 2016. [Google Scholar]
- 23. Beaton D, Chin Fatt CR, Abdi H. An ExPosition of multivariate analysis with the singular value decomposition in R. Comput Stat Data Anal. 2014;72:176–189. [Google Scholar]
- 24. Josse J, Husson F. missMDA: a package for handling missing values in multivariate data analysis. J Stat Softw. 2016;70(1):1–31. [Google Scholar]
- 25. Folstein MF, Folstein SE, McHugh PR. Mini-Mental-State: a practical method for grading the cognitive state of patients for the clinician. J Psychiatr Res. 1975;12(3):189–198. [DOI] [PubMed] [Google Scholar]
- 26. Werner P. Beliefs about memory problems and help seeking in elderly persons. Clin Gerontol. 2004;27(4):19–30. [Google Scholar]
- 27. Hurt CS, Burns A, Brown RG, Barrowclough C. Why don’t older adults with subjective memory complaints seek help? Int J Geriatr Psychiatry. 2012;27(4):394–400. [DOI] [PubMed] [Google Scholar]
- 28. Hamilton-West KE, Milne AJ, Chenery A, Tilbrook C. Help-seeking in relation to signs of dementia: a pilot study to evaluate the utility of the common-sense model of illness representations. Psychol Health Med. 2010;15(5):540–549. [DOI] [PubMed] [Google Scholar]
- 29. Perrotin A, La Joie R, de La Sayette V, et al. Subjective cognitive decline in cognitively normal elders from the community or from a memory clinic: differential affective and imaging correlates. Alzheimers Dement. 2017;13(5):550–560. [DOI] [PubMed] [Google Scholar]
- 30. La Joie R, Perrotin A, Egret S, et al. Qualitative and quantitative assessment of self-reported cognitive difficulties in nondemented elders: association with medical help seeking, cognitive deficits, and beta-amyloid imaging. Alzheimers Dement (Amst). 2016;5:23–34. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 31. Rodriguez-Gomez O, Abdelnour C, Jessen F, Valero S, Boada M. Influence of sampling and recruitment methods in studies of subjective cognitive decline. J Alzheimers Dis. 2015;48(suppl 1):S99–S107. [DOI] [PubMed] [Google Scholar]
Associated Data
This section collects any data citations, data availability statements, or supplementary materials included in this article.
Supplementary Materials
Supplemental Material, Suppl_Table1 for Extrinsic and Intrinsic Help-Seeking Motivation in the Assessment of Cognitive Decline by Robert Haussmann, René Mayer-Pelinski, Maike Borchardt, Fabrice Beier, Franziska Helling, Maria Buthut, Gisa Meissner, Jan Lange, Anne Zweiniger, and Markus Donix in American Journal of Alzheimer's Disease & Other Dementias