Abstract
Our aim was to explore the burden of dementia in the Cretan Aging Cohort, comprised of 3140 persons aged ≥60 years (56.8% women, 5.8 ± 3.3 years formal education, 86.2% living in rural areas) who attended selected primary health-care facilities on the island of Crete, Greece. In the first study phase, a formal diagnosis of dementia had been reached in 4.0% of the participants. However, when selected 505 participants underwent thorough neuropsychiatric evaluation in the second phase of this study (344 with Mini-Mental State Examination [MMSE] <24 and 161 with MMSE ≥24), and results were extrapolated to the entire cohort, the prevalence of dementia and mild cognitive impairment was estimated at 10.8% (9.7%-11.9%) and 32.4% (30.8%-34.0%), respectively. Using both the field diagnostic data and the extrapolated data, the highest dementia prevalence (27.2%) was found in the 80- to 84-year-old group, who also showed the lowest educational level, apparently due to lack of schooling during World War II.
Keywords: dementia, education and dementia, mild cognitive impairment, dementia burden
Introduction
Prevalence of dementia increases with advancing age ranging from 2.6% in Africa to 6.2% in Europe and 6.5% in the Americas 1,2 for persons older than 60 years in 2010. Recent data show that the incidence of dementia is decreasing in the Western societies although, due to the aging population, the actual number of patients with dementia is increasing. 3,4 The reasons for the decreasing dementia incidence are not well understood, but there is evidence that this could relate to better control of vascular risk factors as well as the improving educational attainment of the currently aging population. 5
In Greece, there is paucity of data regarding the epidemiology of cognitive disorders. A study conducted 17 years ago in Northern Greece, 6 using a nonrandomized door-to-door approach, estimated a 5.5% dementia prevalence for those older than 70 years. The prevalence of dementia has been recently investigated for the population of a small rural area on the island of Crete (Mylopotamos) using again a door-to-door approach 7 and was found to range between 9.2% and 24.9% for those older than 60 years, depending on the method employed to achieve tentative diagnosis. Another study identified marital status, history of depression, and family history of dementia as risk factors for Alzheimer’s disease (AD) in the Greek population. 8 The ongoing Hellenic Longitudinal Investigation of Aging and Diet (HELIAD) study 9 was designed to estimate the prevalence of dementia in 2 mainly urban cohorts, one in Larissa, Thessaly (Central Greece), and one in Marousi, a suburb of Athens, Greece. This study also focused on the assessment of dietary habits, 10 since earlier studies have shown that adherence to Mediterranean diet was associated with lower incidence of dementia. 11 Accordingly, the Seven Countries study, which included Greece and Crete in particular, has shown the beneficial effect of Mediterranean diet on cardiovascular mortality and morbidity as well as on dementia-related mortality. 12 -15 Thus, it is of interest to examine the frequency of cognitive impairment, and associated social and financial burden, in rural areas of Crete, an island with rather homogeneous population and a unique type of Mediterranean diet in previous decades.
To answer these questions, we created the Cretan Aging Cohort (CAC), a large (n = 3140) community-dwelling, mainly rural, culturally homogeneous cohort of aged adults on the island of Crete, Greece. It was developed as part of a multidisciplinary study (“Thales: Multidisciplinary Network for the Study of Alzheimer’s Disease”) by a team of experts including primary health-care (PHC) physicians, neurologists, psychiatrists, neuropsychologists, geriatricians, geneticists, and other health-care professionals. The main objective of the overall project was to investigate the role of sociodemographic, medical, genetic, environmental, nutritional, lifestyle, and psychoemotional factors on the occurrence of neurocognitive decline. The present report focuses on the burden of dementia of any cause and mild cognitive impairment (MCI) in the CAC and aims to identify the basic clinical and demographic characteristics associated with cognitive disorders.
Methods
Study Phase I
The CAC is a cohort of 3140 community-dwelling adults, aged 60 to 100 years, who were recruited from PHC facilities (staffed by physicians participating in our PHC research network) in the district of Heraklion, Crete, Greece (Supplemental Figure 1), which houses about half (305490) of the population of Crete (623065; 2011 national census). This study was approved by the institutional review board of the University Hospital of Heraklion, Crete, Greece.
Eligible study participants were those aged ≥60 years who visited the selected PHC facilities in rural and urban areas of the Heraklion district for any reason (emergency or acute cases excluded). Consenting individuals (n = 3200) completed a structured face-to-face interview with a specially trained study nurse. A structured questionnaire was used to record sociodemographic information, anthropometric measurements, current physical and mental health problems based on history reported by patients and/or caregivers, and prescription of medications, as verified by attending PHC physicians. The main physical illnesses recorded were cardiometabolic (hypertension, diabetes mellitus, coronary heart disease, myocardial infraction, congestive heart failure, arrhythmias, peripheral vascular disease, and dyslipidemia), respiratory (bronchial asthma), hormonal (hyperthyroidism and hypothyroidism), gastrointestinal (peptic ulcer, liver disease, and irritable bowel syndrome), myoskeletal (arthritis and osteoporosis), renal (renal failure), neurological (stroke, Parkinson’s disease, multiple sclerosis, and epilepsy), urogenital (benign prostatic hyperplasia), hematological (anemia), and malignancy of any cause. The diagnosis of dementia by the attending PHC practitioner or another physician was also recorded. Global cognitive function was evaluated using the Greek version of the Mini-Mental State Examination test (MMSE 16,17 ), using a universal cutoff of 23/24 points (given that the majority of study participants had ≤6 years of formal education) for referral of participants for further evaluation.
After excluding participants with crucial missing data (MMSE and age), the final study sample consisted of 3140 persons (56.8% women), aged 73.7 ± 7.8 (60-100) years, who had completed an average of 5.8 ± 3.3 (0-18) years of formal education (82.7% and 23.9% with ≤6 years and ≤4 years of formal education, respectively) and lived mostly in rural areas (86.2%). To compare our community-dwelling sample of individuals older than 60 years to the Greek and the Cretan population of similar age, we used data from the 2011 national census (Supplemental Table 1). Results indicated that our sample included a significantly higher percentage of rural residents (86.2% vs 44.4% for the CAC and the Cretan population, respectively; P < .001), persons with low educational level (82.7% vs 71.5% with ≤6 of formal education, respectively, P < .001), females (56.8% vs 53.8%, respectively, P = .0002), and older persons (24.6% vs 21.3% more than 80 years of age, respectively, P < .0001). Results were similar when we compared the CAC cohort to the Greek population (Supplemental Table 1).
Study Phase II
Interview and neuropsychiatric assessment
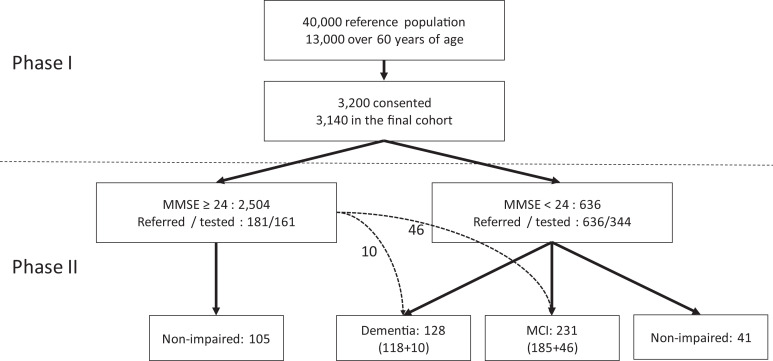
Participants showing probable cognitive impairment (MMSE <24; n = 636) were referred for a thorough neuropsychiatric and neuropsychological assessment in the second phase of the study. Among those with MMSE ≥24 (n = 2504), 181 (matched for place of residence with the low MMSE group) were also invited to undergo the same evaluation (Figure 1).
Figure 1.
Flowchart of the study (depicting diagnostic classification of phase II participants).
During phase II, participants were administered an extensive questionnaire in the context of a semi-structured interview, lasting approximately 2 hours and taking place, according to participants’ wishes, at the University Hospital of Heraklion, the facilities of the Medical School in Heraklion, the local Health Centers, or the participant’s home. Certified neurologists, psychiatrists, and internists completed these assessments with the aid of trained research assistants. The questionnaire was largely based on the questionnaire used in the HELIAD study, 9 rendering the 2 cohorts comparable for future analyses, despite different modes of participant selection. The questionnaire assessed, through adapted scales, determinants of health status including sociodemographic information, medical and family history, leisure activities, subjective functional assessment, daily activities and self-care habits, basic physical abilities, sleep patterns, memory/cognitive complaints, depression, anxiety, and neuropsychiatric (including psychotic and behavioral) symptoms. Dietary patterns were assessed using a semiqualitative food frequency scale. Questionnaires were supplemented by a semi-structured neurological evaluation. Clinicians involved in interviewing each participant wrote a 2- to 3-page report that included information on the medical and family history of the participant, his or her daily and other activities, the results of neuropsychiatric assessment, and the physician’s diagnostic impression.
Neuropsychological assessment
Neuropsychological assessment was performed by trained neuropsychologists. A full neuropsychological battery assessing a variety of cognitive domains, lasting about 2.5 hours, was administered. Verbal short-term and working memory and verbal episodic memory were assessed using corresponding tests from the Greek Memory Scale 18 supplemented by the Greek adaptation of the Rey Auditory Verbal Learning Test. 19,20 Visuoconstructive ability and visuospatial episodic memory were evaluated using the Taylor Complex Figure. 21 Language functions were examined using the short forms of the Greek adaptations of the Boston Naming and Peabody Picture Vocabulary tests 22 and the Semantic Verbal Fluency test. 23 Visuomotor processing speed and sustained attention were assessed through the Trail Making Test (TMT) Part A 24 and the Symbol Digit Modality Test. 25 Executive tests included TMT Part B, the Initiation/Perseveration subtests from the Mattis Dementia Rating Scale, and the General Ability Measure for Adults. 26,27 Daily functional capacity was assessed using the 13-item Greek Independent Activities of Daily Living (IADL) scale, 18 administered in the form of a structured interview to the participants’ closest relative or caregiver. Raw scores on all neuropsychological tests were converted to age- and education-adjusted standard scores using published norms developed for the Greek population. 18
Diagnosis assignment
All information obtained through history, neuropsychiatric and neuropsychological examination and corresponding reports, questionnaires, and any other additional data available (imaging studies and laboratory results) was reviewed by a certified neurologist (I.Z.) and a neuropsychologist (P.S.), and consensus diagnoses were reached. For the diagnosis of dementia and MCI (amnestic, nonamnestic single domain, and multidomain), the Diagnostic and Statistical Manual of Mental Disorders, Fourth ed criteria 28 and the International Working Group 29 criteria were used, respectively. Probable AD, vascular dementia (VaD), Lewy body dementia (LBD), behavioral variant frontotemporal dementia (bvFTD), and other types of FTD were diagnosed according to the National Institute of Neurological and Communicative Disorders and Stroke - Alzheimer's Disease and Related Disorders Association (NINCDS-ADRDA), 30 the National Institute of Neurological Disorders and Stroke - Association Internationale pour la Recherché et l' Enseignement en Neuroscience (NINDS-AIREN), 31 the Dementia with Lewy Bodies (DLB) Consortium, 32 the International Consortium on bvFTD, 33 and the Neary et al 34 criteria, respectively.
Statistical Analyses
Preliminary analyses established the demographic equivalence of participating and nonparticipating persons in phase II of the study as a function of MMSE score (Supplemental Table 2), allowing estimation of the frequency of dementia and MCI by extrapolation to the entire phase I cohort. Extrapolation, based on the assumptions described above, was performed by applying the frequency of dementia and MCI in MMSE-, age-, and sex-specific groups to the corresponding group of the entire cohort. Patients diagnosed with dementia and MCI were compared to cognitively nonimpaired elders using 1-way analysis of variance for continuous variables and Fisher’s exact test for categorical variables.
Results
Phase II Cohort Demographics
To further characterize in detail our cohort of 3140 participants undergoing initial evaluation in phase I, a subsample of n = 817 participants were invited to receive a thorough diagnostic evaluation. This invitation was addressed to all 636 participants with MMSE <24 and 181 of the 2504 participants with MMSE ≥24, with selection of the latter following a random procedure stratified by place of residence of participants in the low MMSE group. The response rate was 54.1% among participants with low MMSE score and 88.9% among participants with high MMSE score (Figure 1). Participants with low MMSE score who did not participate in phase II for any reason (n = 292; refused, could not be located or passed away) had similar clinical and demographic characteristics with those who were eventually evaluated (n = 344; Supplemental Table 2). This was also the case for participants with high MMSE who were eventually tested (n = 161) when compared to the participants with high MMSE who were not evaluated in phase II (n = 2,343; Supplemental Table 2).
Dementia and MCI Diagnoses in Phase II
Among the 344 individuals with low MMSE (<24), 185 (53.8%) were diagnosed with MCI and 118 (34.3%) with dementia of any type, whereas detailed neuropsychological assessment failed to detect significant cognitive deficits in 41 (11.9%) participants (Figure 1). Among the 161 tested participants of the MMSE >24 group, 10 (6.2%) and 46 (28.6%) were diagnosed with dementia and MCI, respectively (Figure 1). By extrapolation, the prevalence of dementia and MCI were estimated to be 10.8% (9.7-11.9) and 32.4% (30.8-34.0), respectively, in the entire cohort of 3140 participants.
The most frequent cause of dementia in phase II was probable AD (n = 107, 83.5%). Other disorders identified as causes of dementia were FTD (n = 6, 4.7%), LBD (n = 4, 3.1%), VaD (n = 5, 3.9%), and mixed dementia (n = 6, 4.7%; Supplemental Table 3). The distribution of MCI subtypes was 22.9% pure amnesic type, 51.9% amnesic multidomain, and 23.8% nonamnesic (Supplemental Table 3). The distribution of MCI subtypes did not vary significantly with gender, marital status, or geographic origin (rural/urban; data not shown).
Dementia Diagnosis by Age Group
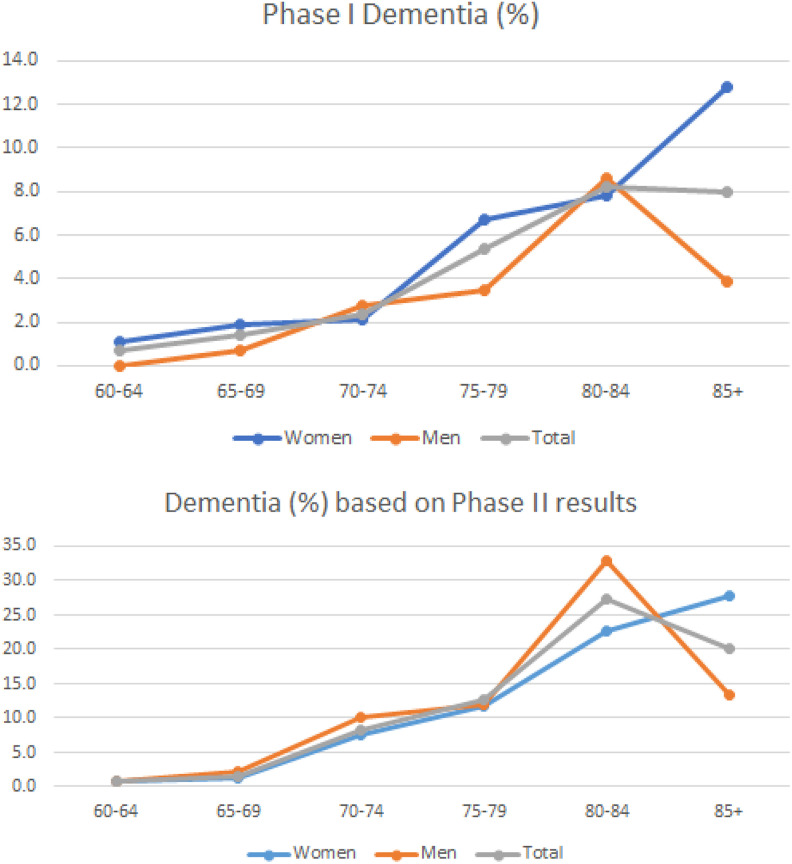
In the entire CAC cohort (n = 3140), a dementia diagnosis had been reached in phase I of the study in 4.0% of the participants (4.5% in women, n = 1785 and 3.3% in men, n = 1355; Table 1). As shown in Figure 2A, dementia rates increased throughout the age range of the study among women, whereas in men the maximum rate was noted in the 80- to 84-year-old group (8.55%), followed by a notable decline thereafter (3.87%). A similar trend was found in the rates of dementia as determined in phase II by specialists: There was a notable peak (27.2%) of extrapolated dementia frequency in the 80- to 84-year age-group, with decreasing frequency in ages older than 84 years (Figure 2B and Table 1). When analysis was performed by gender, this frequency peak was again found in men but not in women (Figure 2B). Of note, women had longer disease duration compared to men (3.6 ± 2.7 years and 2.2 ± 1.5 years, respectively; P = .003).
Table 1.
Demographic Characteristics and Clinical Diagnosis by Age-Group.
| Age Group, Years | n | Gender, Female, % | Years of Education, Mean | Dementia Diagnosed in Phase 1, % | Extrapolated Dementia Frequency, %, CIa | Extrapolated MCI Frequency, %, CIa |
|---|---|---|---|---|---|---|
| 60-64 | 418 | 65.3 | 7.5 | 0.72 | 0.9 (0.001-1.8) | 16.0 (12.5-19.5) |
| 65-69 | 643 | 57.5 | 6.8 | 1.40 | 1.5 (0.6-2.4) | 19.4 (16.3-22.5) |
| 70-74 | 626 | 58.8 | 6.1 | 2.40 | 8.2 (6.1-10.3) | 45.6 (41.7-49.5) |
| 75-79 | 676 | 57.1 | 5.2 | 5.33 | 12.6 (10.1-15.1) | 41.0 (37.3-44.7) |
| 80-84 | 489 | 52.1 | 4.1 | 8.18 | 27.2 (23.3-31.4) | 32.7 (28.5-36.9) |
| 85+ | 288 | 46.2 | 4.8 | 7.99 | 20.0 (15.4-24.6) | 38.7 (33.1-44.3) |
| Total | 3,140 | 56.8 | 5.8 | 4.01 | 10.8 (9.7-11.9) | 32.4 (30.8-34.0) |
Abbreviations: CI, 95% confidence interval; MCI, mild cognitive impairment.
a By extrapolation to the cohort of 3140 participants.
Figure 2.
A, Dementia frequency (as diagnosed by PHC physicians based on phase I data) in the entire cohort (n = 3140) by age-group and gender. B, Dementia frequency diagnosed in phase II by dementia specialists and extrapolated to the phase I cohort by age-group and gender. PHC indicates primary health care.
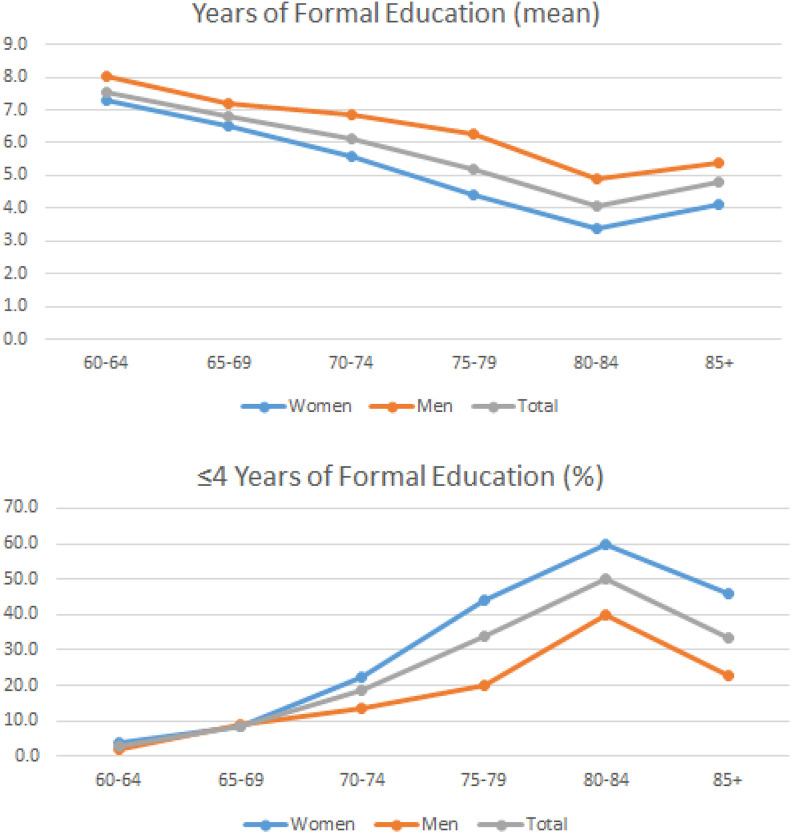
Next, we examined educational level as a function of age in the entire cohort, given that formal education was lower in patients with dementia compared to controls in our cohort (Table 2). Indeed, the observed dementia frequency distribution paralleled the educational status of the participants (as expressed by either the percentage of persons with ≤4 years of schooling or the mean years of formal education), since the age-group of 80- to 84-year-old had the lowest literacy of all age-groups (Figure 3 and Table 1).
Table 2.
Comparison of Patients With Dementia and MCI With Nonimpaired Participants on Sociodemographic, Anthropometric, Health, and Lifestyle Habits.
| A. Nonimpaired, n = 146 | B. MCI, n = 231 | C. Dementia, n = 128 | A Versus B | A Versus C | B Versus C | |
|---|---|---|---|---|---|---|
| Women, % | 63.7 | 72.7 | 61.7 | 0.09 | 0.9 | 0.06 |
| Age, years, mean (SD) | 73.4 (7.2) | 76.8 (7.0) | 79.9 (6.0) | 0.003 | 0.05 | 0.0001 |
| MMSE, mean (SD) | 26.1 (3.1) | 22.1 (2.7) | 17.8 (5.3) | 0.002 | 0.005 | 0.0001 |
| Education, years, mean (SD) | 5.2 (3.1) | 4.5 (3.0) | 4.3 (3.1) | 0.1 | 0.05 | 0.3 |
| Education, 0-4 years, % | 26.7 | 42.0 | 42.5 | 0.003 | 0.005 | 0.8 |
| Urban dwelling, % | 19.9 | 17.3 | 14.1 | 0.6 | 0.4 | 0.8 |
| Marital status, % | ||||||
| Married | 71.2 | 63.6 | 64.3 | 0.3 | 0.3 | 0.9 |
| Single | 2.7 | 4.8 | 3.9 | 0.5 | 0.5 | 0.8 |
| Divorced/widowed | 26.0 | 31.6 | 31.8 | 0.6 | 0.6 | 0.9 |
| Occupation, % | ||||||
| Household | 23.6 | 35.4 | 28.6 | 0.3 | 0.3 | 0.2 |
| Farmer | 48.2 | 43.5 | 48.0 | 0.1 | 0.9 | 0.3 |
| Laborer | 5.5 | 6.2 | 5.1 | 0.5 | 0.9 | 0.8 |
| Technician | 5.5 | 1.9 | 5.1 | 0.1 | 0.9 | 0.2 |
| Merchant | 5.5 | 6.2 | 8.2 | 0.7 | 0.4 | 0.5 |
| Clerical | 10.9 | 6.2 | 4.1 | 0.2 | 0.1 | 0.7 |
| Educator | 0.9 | 0.6 | 1.0 | 0.8 | 0.9 | 0.7 |
| Current smoking, % | 9.6 | 5.6 | 9.4 | 0.16 | 0.9 | 0.2 |
| Past smoking, % | 33.6 | 20.0 | 25.0 | 0.002 | 0.1 | 0.3 |
| Hypertension, % | 67.8 | 72.7 | 62.5 | 0.4 | 0.4 | 0.06 |
| Diabetes, % | 29.5 | 24.2 | 22.7 | 0.3 | 0.2 | 0.8 |
| Dyslipidemia, % | 47.9 | 43.7 | 39.8 | 0.4 | 0.2 | 0.5 |
| Hypothyroidism, % | 15.1 | 12.1 | 7.1 | 0.4 | 0.06 | 0.2 |
| BMI, mean (SD) | 30.5 (4.7) | 29.9 (4.6) | 29.3 (4.4) | 0.6 | 0.09 | 0.8 |
Abbreviations: BMI, body mass index; MCI, mild cognitive impairment; MMSE, Mini-Mental State Examination; SD, standard deviation.
Significant group differences (P < .05) are shown in bold.
Figure 3.
A, Average years of education per age group and gender. B, Percentage of persons with low education (4 years or less) per age group and gender. Data for both diagrams were derived from the entire cohort (phase I, n = 3140).
Other factors examined (place of residence, smoking) did not correlate with the dementia frequency distribution in terms of the frequency peak at 80 to 84 years of age. For example, the frequency of urban residence (Supplemental Figure 2) and past and current smoking (Supplemental Figure 3), both declining with age, did not show frequencies in the 80- to 84-year-old group who were divergent from the observed age-related trends.
Comparison of Dementia Diagnoses in Phases I and II
As presented earlier, 4.0% of participants carried a diagnosis of dementia, whereas the estimated prevalence after detailed neuropsychiatric evaluation by dementia specialists in phase II was 10.8% (Table 1), suggesting that in our cohort more than half of patients with dementia were undiagnosed as suffering from dementia until being examined by dementia specialists.
Indeed, when restricting our analysis only to patients examined at both phase I and phase II, we observed that 59.4% of our participants with dementia diagnosed in phase II by specialists were not diagnosed with dementia in phase I, and this rate of nondiagnosis was similar in both men and women (61.2% and 58.2%, respectively; Table 3).
Table 3.
Dementia Diagnosis by PHC Physicians in Phase I and Final Diagnosis by Dementia Specialists in Phase II of This Study.a
| Dementia Diagnosis in Phase I | |||||||
|---|---|---|---|---|---|---|---|
| Women, n = 340 | Men, n = 165 | Total, n = 505 | |||||
| No (%) | Yes (%) | No (%) | Yes (%) | No (%) | Yes (%) | ||
| Final diagnosis in phase II | Nonimpaired | 95.7 | 4.3 | 100.0 | 0.0 | 97.3 | 2.7 |
| MCI | 93.5 | 6.5 | 92.1 | 7.9 | 93.1 | 6.9 | |
| Dementia | 58.2 | 41.8 | 61.2 | 38.8 | 59.4 | 40.6 | |
Abbreviation: MCI, mild cognitive impairment.
a Patients were examined at both phases I and II.
Comparison of Patients with Dementia and MCI With the Nonimpaired Participants
A risk factor analysis comparing the 3 diagnostic groups (Table 2) in phase II showed that there were no gender differences between the 3 groups, although cognitive impairment (MCI and dementia) was associated with advancing age (Table 2). Also, as expected, patients with dementia scored worse on MMSE (17.8 ± 5.3 points) compared to those with MCI (22.1 ± 2.7 points, P = .0001), who in turn scored lower than nonimpaired individuals (26.1 ± 3.1; P = .0001). In respect to years of education, as noted above, patients with dementia had lower educational attainment (4.3 ± 3.1 years) than nonimpaired participants (5.2 ± 3.1 years, P = .05) with the difference between the former and the group of patients with MCI failing to reach significance (4.5 ± 3.0 years, P = 0.3). Furthermore, the proportion of persons with ≤4 years of education was significantly higher among patients with dementia (42.5%) and MCI (42.0%) than nonimpaired persons (26.7%, P = .005 and P = .003, respectively). Nonimpaired participants reported higher frequency of past smoking than patients with MCI (P = .002) but not than patients with dementia. Apart from that, the 3 groups were comparable on body mass index, occupation, marital status, rural dwelling, current smoking, hypertension, diabetes, dyslipidemia, and hypothyroidism (Table 2).
Discussion
Main Findings
The main finding of our study was the very high rate of undiagnosed cognitive disorders by PHC or other physicians caring for a rural-residing, mainly low-educated population on the island of Crete, Greece. Specifically, while a diagnosis of dementia in the entire cohort in phase I had been reached in only 4.0% of participants, the extrapolated frequency of dementia of any cause (based on diagnostic evaluation by specialists in phase II of the study) was estimated at 10.8%. Even when restricting our analysis to the 505 persons evaluated at both phase I and phase II of the study, about 60% of patients with dementia had remained undiagnosed until detailed neuropsychiatric evaluation. It was further estimated that an additional 32.4% of the persons visiting PHCs met formal criteria for MCI although they were in their overwhelming majority never diagnosed as suffering from a cognitive disorder. The second noteworthy finding of the present study was the pronounced increase in frequency of dementia in the 80 to 84 age-group, compared to both younger and older age-groups, particularly in men. This observation is at odds with previous epidemiological studies reporting a constant rise in dementia prevalence with increasing age. 2 This pattern is not specific to the region covered by the present study given that another door-to-door study conducted in a small rural area on the island of Crete reported a similar finding. 7
Discussing Low Diagnostic Rates for Dementia in Phase I of This Study
As noted earlier, only 40% of the patients actually suffering from dementia had received a formal diagnosis of dementia on the basis of the data collected in phase I of the study, in accordance with rates observed in several other studies. 35 There are many factors contributing to dementia underdiagnosis in rural-dwelling, low-educated populations such as the one targeted by the present study. 36 -39 Impaired self-awareness of cognitive deficits is one such potential factor, especially for persons living alone or with a nonrelative caregiver who is not a trained professional. Both patients and relatives may be reluctant to seek medical assistance for declining cognitive abilities adhering to the cultural belief that their symptoms are part of normal aging. Certain cases may have been actually missed by PHC physicians working in hectic rhythms due to time limitations, sparseness of suitable screening tools, 40 -42 and lack of support in dementia screening by trained nurses or other health-care professionals. 43 Lack of formal training and of continuing expert support to PHC physicians in the detection of cognitive problems may further contribute to underdiagnosis in their patients. 37,38,44 In addition, due to the remoteness of the villages included in this study from specialist services, it is probable that this underdiagnosis reflects limited health-care access. It should also be noted that MCI diagnosis can be especially challenging, even for dementia experts, and often is confounded by comorbidities, such as depression.
Regardless of cause, this decreased recognition of cognitive disorders (with MCI and dementia combined affecting more than 40% of individuals visiting PHC facilities in the areas where our cohort study took place) deprives patients from life-improving management and hinders appropriate care delivery for other, nondementia-related, health problems that often coexist in these patients.
Education as a Main Determinant of Dementia Occurrence
The 80- to 84-year-old age-group with the highest dementia prevalence in our study had also the lowest educational status as determined by average years of formal education and the proportion of individuals who had completed ≤4 years of formal education. In this subgroup of participants (born between 1929 and 1933), their primary schooling years coincided with the German occupation in Crete during World War II, a period during which formal education was abruptly discontinued and rapidly reintroduced thereafter. 45 In addition, many of the women in the oldest age-group (>85 years old) in our cohort were deprived of substantial education, probably due to social beliefs in the pre-war era. 46 This, together with the lengthier duration of cognitive symptoms in women in our cohort, could explain the fact that frequency of dementia in women does not strictly follow the educational patterns recorded in men.
Education is a well-known protective factor for dementia. 47 -52 Several recent studies have suggested that higher levels of education could have contributed to the recent decline in dementia incidence. 4,53,54 For example, data from the Framingham study showed that decreasing dementia incidence was observed only in high school graduates, with the limitation of this study being the small number of persons who had not completed high school. 4 In contrast, our study involves a cohort of mainly low-literacy elders, possibly enabling better dissection of the education effect.
Our Study in the Context of Epidemiological Studies on Dementia and MCI
The present study was not designed to provide estimates of population prevalence of dementia 7,9,55 -59 given that participant recruitment was restricted to elders visiting PHC facilities. This approach results in a representative sample of the target population which is by design biased against both very healthy individuals and nonambulatory elders. It should be noted, however, that the currently estimated frequency of dementia (10.8%) in our sample is well within the prevalence range reported for a different rural area of Crete, Greece, using a door-to-door approach. 7 Specifically, in the latter study, 7 which was based on a sample of 443 inhabitants (49.7% women, aged 75.3 ± 7.1 years, with 3.5 ± 2.7 years of formal education), the estimated prevalence of dementia, with or without significant self-reported depressive symptomatology, ranged between 9.2% and 24.9% depending on the method employed to achieve tentative diagnosis.
There have been numerous other epidemiological studies on dementia prevalence in rural populations worldwide. 56,60,61 These studies included random population samples and were less likely to sample populations where education was abruptly discontinued and rapidly reintroduced a few years later, as in our study population. For example, a study employing a door-to-door recruitment approach found an 8% prevalence of dementia in a rural area in Italy in persons aged older than 64 years. 61 Although a significant association between education and cognitive decline was demonstrated, there was a rather constant association between increasing rates of low education and increasing age. Furthermore, a very recent nationally representative population-based study from the United States showed an 11.6% prevalence of dementia in 2000 in those older than 65 years, a rate comparable to that in our cohort. 54 Dementia frequency decreased to 8.8% in 2012, and this was mainly attributed to the improvement in educational status. 54 A similar nationwide study in Poland showed an 12.1% prevalence of dementia in those older than 65 years. 62 In contrast, the prevalence of dementia was only 5.5% in a community-dwelling sample of individuals aged 65 to 85 years, visiting PHC practices in Amsterdam, the Netherlands (AMSTEL study 63 ). However, the AMSTEL study did not include participants older than 85 years, was focused on an urban population, and the percentage of participants with 6 or less years of formal education (42%) was nearly half of that observed in our study (82.7%). Although the prevalence of dementia in Europe has been estimated at 6.4% for those older than 65 years, 64 there is a considerable variation in dementia prevalence across European countries as well as significant regional variation within the same country. 65
In the present study, the estimated overall frequency of MCI based on comprehensive neuropsychological and neuropsychiatric assessment was 32.4% for those older than 60 years, with 22.9% of the pure amnesic type, 51.9% of the amnesic multidomain subtype, and 23.8% of the nonamnesic subtype. It is true that most epidemiological studies so far report lower rates of MCI 66,67 although with a similar subtype distribution. However, there has been great variation in the reported prevalence of MCI in those aged 60 years or more, with rates up to 42% reported, 68 a range that includes the prevalence estimated in our cohort. Also, our MCI prevalence is comparable to that found in a region in Northern Greece (35.1% 69 ) and well within the prevalence range recently reported for another rural area of Crete based on a sample of 443 inhabitants (12.2%-42.0% depending on the specific criteria used 7 ). This increased MCI prevalence in our cohort is probably related to the low-educated rural population included in our study, and underlines, taking also into account the increased dementia prevalence, the importance of screening for cognitive disorders patients presenting to PHC practitioners.
Strengths and Limitations of Our Study
The main strength of our study, compared to other similar studies, is the large number of participants. Furthermore, our mainly rural population is skewed toward advanced ages and is therefore suitable to address age-related conditions such as dementia. This is partly due to the urbanization process that takes place in Crete over the past few decades and largely involves younger individuals. Also, participants in our cohort share a common sociodemographic environment (including dietary habits), rendering this cohort suitable for assessing risk factors associated with cognitive impairment in a relatively homogeneous population. As a further strength of our study, the participants of our large cohort have been extensively characterized through detailed neuropsychiatric and neuropsychological assessments by skilled neuropsychologists and licensed neurologists, psychiatrists, and geriatricians, enabling accurate diagnostic evaluations.
Our study also has certain limitations. Although in phase I of the study, 3200 individuals agreed to participate (and 3140 were tested), participation rate in phase II was modest (61.8% across both MMSE groups). Sociodemographic characteristics (low education elders in remote rural areas who were minimally familiar with the concept of neuropsychiatric and neuropsychological testing) may have discouraged prospective participants to consent to lengthy interview/testing sessions. In comparison to several similar studies 70,71 and considering the cultural and sociodemographic characteristics of the target population, current participation rates may be considered as acceptable.
Another limitation of the present study relates to the cross-sectional nature of the reported data. Thus, in interpreting the current results, one should consider the possibility that a sizable percentage of participants who met formal criteria for MCI in our study may not develop dementia during their lifetime or even revert to normal cognition. According to a recent meta-analysis, 72 approximately 25% of patients with MCI revert to normal cognition, but this proportion varies considerably between studies. 73 This high percentage of nonprogression to dementia can be attributed to the fact that the life span of the participants in these studies does not permit them to reach the age at which they could develop dementia, to the well-documented intrasubject variability in cognitive performance over time and to the effect of additional neuropsychiatric symptoms. 74,75 There is, however, evidence that the risk for conversion to dementia is higher among persons who at some point in time met neuropsychiatric criteria for MCI but failed to do so on at least one subsequent visit, only temporarily reverting to normal or near normal cognition. 76 . It should further be noted that in our sample, nearly half of cases in the MCI group met criteria for the amnestic multidomain MCI type (51.9%) which is associated with the highest conversion rates to dementia. 77,78 Irrespective of this, only future follow-up studies could accurately assess the rate of MCI progression to dementia, stability over time, or reversion to normal in our population.
Concluding Remarks
The current results draw attention to a significant proportion of undiagnosed elders meeting formal criteria for dementia or MCI stressing the need for better diagnostic practices to ensure appropriate care delivery. The highest frequency of dementia in this large community-dwelling, predominantly rural, population was noted among the least educated age-group of 80 to 84 years olds. This finding links lack of childhood schooling (and its lifelong effect on overall learning and literacy 79 ) with the occurrence of dementia several decades later in a unique social setup (discontinuation of formal schooling for some years and its rapid reintroduction thereafter).
Supplemental Material
Supplemental Material, 20180718ZaganasetalCretanAgingCohortSupplementaryRevised for The Cretan Aging Cohort: Cohort Description and Burden of Dementia and Mild Cognitive Impairment by Ioannis V. Zaganas, Panagiotis Simos, Maria Basta, Stefania Kapetanaki, Symeon Panagiotakis, Irini Koutentaki, Nikolaos Fountoulakis, Antonios Bertsias, George Duijker, Chariklia Tziraki, Nikolaos Scarmeas, Andreas Plaitakis, Dimitrios Boumpas, Christos Lionis, and Alexandros N. Vgontzas in American Journal of Alzheimer's Disease & Other Dementias
Acknowledgments
The excellent assistance of Cynthia Manassaki in coordinating the overall project is cordially acknowledged. Also, this work would not be possible without the help from the nurses participating in the study (Albantaki Aikaterini, Fragkiadaki Georgia, Lironi Marina, Maniou Maria, Marinaki Sofia, Titaki Maria), the PHC physicians (Kalogridaki Irini, Klouva Eleni, Ladoukaki Eva, Makri Kornilia, Papadokostakis Polivios, Papamastorakis Emmanouil, Pateli Rodanthi, Prokopiadou Dimitroula, Symvoulakis Emmanouil, Stefanaki Ioanna, Tsakountakis Nikolaos, Tsiligianni Ioanna, Vasilaki Angeliki, Vasilopoulos Theodoros) involved in patient recruitment and data collection in phase I of the study and the medical students (Bellogianni Christina, Kardari Ioanna, Kounali Vassiliki, Selmanai Annieza) assisting with data collection and registration in phase II. Finally, we would like to thank all participants and, if applicable, their caregivers, for taking part in this cohort study.
Authors’ Note: Nikolaos Scarmeas is also affiliated with Department of Neurology, Taub Institute, Sergievsky Center, Columbia University, New York, NY, USA.
The authors declared no potential conflicts of interest with respect to the research, authorship, and/or publication of this article.
Funding: The authors disclosed receipt of the following financial support for the research, authorship, and/or publication of this article: This work has been supported by a grant from the European Union (European Social Fund, ESF) and Greek national funds through the Operational Program “Education and Lifelong Learning” of the National Strategic Reference Framework (NSRF)—Research Funding Program: THALES entitled “UOC-Multidisciplinary network for the study of Alzheimer’s Disease” (grant code: MIS 377299).
ORCID iD: Zaganas V. Ioannis  http://orcid.org/0000-0002-0774-5772
http://orcid.org/0000-0002-0774-5772
Supplemental Material: Supplemental material for this article is available online.
References
- 1. Sosa-Ortiz AL, Acosta-Castillo I, Prince MJ. Epidemiology of dementias and Alzheimer’s disease. Arch Med Res. 2012;43(8):600–608. [DOI] [PubMed] [Google Scholar]
- 2. Prince M, Bryce R, Albanese E, Wimo A, Ribeiro W, Ferri CP. The global prevalence of dementia: a systematic review and meta-analysis. Alzheimers Dement. 2013;9(1):63–75.e2. [DOI] [PubMed] [Google Scholar]
- 3. Larson EB, Yaffe K, Langa KM. New insights into the dementia epidemic. N Engl J Med. 2013;369(24):2275–2277. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 4. Satizabal CL, Beiser AS, Chouraki V, Chêne G, Dufouil C, Seshadri S. Incidence of dementia over three decades in the Framingham Heart Study. N Engl J Med. 2016;374(6):523–532. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 5. Jones DS, Greene JA. Is dementia in decline? Historical trends and future trajectories. N Engl J Med. 2016;374(6):507–509. [DOI] [PubMed] [Google Scholar]
- 6. Tsolaki M, Fountoulakis C, Pavlopoulos I, Chatzi E, Kazis A. Prevalence and incidence of Alzheimer’s disease and other dementing disorders in Pylea, Greece. Am J Alzheimers Dis Other Demen. 1999;14(3):138–148. [Google Scholar]
- 7. Tsolaki M, Gkioka M, Verykouki E, Galoutzi N, Kavalou E, Pattakou-Parasyri V. Prevalence of dementia, depression, and mild cognitive impairment in a rural area of the island of Crete, Greece. Am J Alzheimers Dis Other Demen. 2017;32(5):252–264. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 8. Tsolaki M, Fountoulakis K, Chantzi E, Kazis A. Risk factors for clinically diagnosed Alzheimer’s disease: a case–control study of a Greek population. Int Psychogeriatr. 1997;9(3):327–41. [DOI] [PubMed] [Google Scholar]
- 9. Dardiotis E, Kosmidis MH, Yannakoulia M, Hadjigeorgiou GM, Scarmeas N. The Hellenic Longitudinal Investigation of Aging and Diet (HELIAD): rationale, study design, and cohort description. Neuroepidemiology. 2014;43(1):9–14. [DOI] [PubMed] [Google Scholar]
- 10. Anastasiou CA, Yannakoulia M, Kosmidis MH, et al. Mediterranean diet and cognitive health: initial results from the Hellenic Longitudinal Investigation of Ageing and Diet. PLoS One. 2017;12(8):e0182048. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 11. Scarmeas N, Stern Y, Tang MX, Mayeux R, Luchsinger JA. Mediterranean diet and risk for Alzheimer’s disease. Ann Neurol. 2006;59(6):912–921. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 12. Keys A, Menotti A, Karvonen MJ, et al. The diet and 15-year death rate in the Seven Countries Study. Am J Epidemol. 1986;124(6):903–915. [DOI] [PubMed] [Google Scholar]
- 13. Alonso A, Jacobs DR, Jr, Menotti A, et al. Cardiovascular risk factors and dementia mortality: 40 years of follow-up in the seven countries study. J Neurol Sci. 2009;280(1):79–83. [DOI] [PubMed] [Google Scholar]
- 14. Hatzis C, Papandreou C, Patelarou E, et al. A 50-year follow-up of the Seven Countries Study: prevalence of cardiovascular risk factors, food and nutrient intakes among Cretans. Hormones (Athens). 2013;12(3):379–385. [DOI] [PubMed] [Google Scholar]
- 15. Hatzis CD, Sifaki-Pistolla D, Kafatos A. History of the Cretan cohort of the Seven Countries Study. Hormones (Athens). 2015;14(2):326–329. [DOI] [PubMed] [Google Scholar]
- 16. Folstein MF, Folstein SE, McHugh PR. . “Mini-Mental State”: a practical method for grading the cognitive state of patients for the clinician. J Psychol Res. 1975;12(3):189–198. [DOI] [PubMed] [Google Scholar]
- 17. Fountoulakis KN, Tsolaki M, Chantzi H, Kazis A. Mini Mental State Examination (MMSE): a validation study in Greece. Am J Alzheimers Dis. 2000;15(6):342–345. [Google Scholar]
- 18. Simos P, Papastefanakis E, Panou T, Kasselimis D. The Greek Memory Scale. Rethymno: Laboratory of Applied Psychology. Rethymno, Crete, Greece: University of Crete; 2011. [Google Scholar]
- 19. Constantinidou F, Zaganas I, Papastefanakis E, Kasselimis D, Nidos A, Simos PG. Age-related decline in verbal learning is moderated by demographic factors, working memory capacity, and presence of amnestic mild cognitive impairment. J Int Neuropsychol Soc. 2014;20(8):822–835. [DOI] [PubMed] [Google Scholar]
- 20. Geffen G, Geffen L. Auditory Verbal Learning Test (AVLT) Computerized Scoring Program and Population Norms. Australian Council for Educational Research. Melbourne: ACER Press; 2000. [Google Scholar]
- 21. Hubley A, Tremblay D. Comparability of total score performance on the Rey-Osterrieth Complex Figure and a modified Taylor Complex Figure. J Clin Exp Neuropsychol. 2002;24(3):370–382. [DOI] [PubMed] [Google Scholar]
- 22. Simos P, Kasselimis D, Mouzaki A. Age, gender, and education effects on vocabulary measures in Greek. Aphasiology. 2011;25:492–504. [Google Scholar]
- 23. Kosmidis MH, Vlahou CH, Panagiotaki P, Kiosseoglou G. The verbal fluency task in the Greek population: normative data, and clustering and switching strategies. J Int Neuropsychol Soc. 2004;10(2):164–172. [DOI] [PubMed] [Google Scholar]
- 24. Zalonis I, Kararizou E, Triantafyllou NI, et al. A normative study of the trail making test A and B in Greek adults. Clin Neuropsychol. 2008;22(5):842–850. [DOI] [PubMed] [Google Scholar]
- 25. Constantinidou F, Christodoulou M, Prokopiou J. . he effects of age and education on executive functioning and oral naming performance in Greek Cypriot adults: the neurocognitive study for the aging. Folia Phoniatr Logop. 2012;64(4):29–40. [DOI] [PubMed] [Google Scholar]
- 26. Coblentz J, Mattis S, Zingesser LH, Kasoff SS, Wiśniewski HM, Katzman R. Presenile dementia. Arch Neurol. 1973;29(5):299–308. [DOI] [PubMed] [Google Scholar]
- 27. Katsarou Z, Bostantjopoulou S, , Zikouli A, et al. Performance of Greek demented and nondemented subjects on the Greek version of the Mattis Dementia Rating Scale. A validation study. Int J Neurosci. 2010;120(11):724–730. [DOI] [PubMed] [Google Scholar]
- 28. American Psychiatric Association. DSM-IV Criteria. Diagnostic and Statistical Manual of Mental Disorders, 4th ed. Washington, DC, USA: American Psychiatric Association; 1994. [Google Scholar]
- 29. Winblad B, Palmer K, Kivipelto M, et al. Mild cognitive impairment, beyond controversies, towards a consensus: report of the International Working Group on Mild Cognitive Impairment. J Intern Med. 2004;256(3):240–246. [DOI] [PubMed] [Google Scholar]
- 30. McKhann G, Drachman D, Folstein M, Katzman R, Price D, Stadlan EM. Clinical diagnosis of Alzheimer’s disease: report of the NINCDS-ADRDA Work Group under the auspices of Department of Health and Human Services Task Force on Alzheimer’s Disease. Neurology. 1984;34(7):939–944. [DOI] [PubMed] [Google Scholar]
- 31. Roman GC, Tatemichi TK, Erkinjuntti T, et al. Vascular dementia: diagnostic criteria for research studies. Report of the NINDS-AIREN International Workshop. Neurology. 1993;43(2):250–260. [DOI] [PubMed] [Google Scholar]
- 32. McKeith I, Dickson DW, Lowe J, et al. ; Consortium on DLB. Diagnosis and management of dementia with Lewy bodies: third report of the DLB Consortium. Neurology. 2005;65(12):1863–1872. [DOI] [PubMed] [Google Scholar]
- 33. Rascovsky K, Hodges JR, Knopman D, et al. Sensitivity of revised diagnostic criteria for the behavioural variant of frontotemporal dementia. Brain. 2011;134(pt 9):2456–2477. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 34. Neary D, Snowden JS, Gustafson L, et al. Frontotemporal lobar degeneration: a consensus on clinical diagnostic criteria. Neurology. 1998;51(6):1546–1554. [DOI] [PubMed] [Google Scholar]
- 35. Eichler T, Thyrian JR, Hertel J, et al. Rates of formal diagnosis of dementia in primary care: the effect of screening. Alzheimers Dement (Amst). 2015;1(1):87–93. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 36. Russell P, Banerjee S, Watt J, et al. Improving the identification of people with dementia in primary care: evaluation of the impact of primary care dementia coding guidance on identified prevalence. BMJ Open. 2013;3(12):e004023. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 37. Lionis C, Tzagournissakis M, Iatraki E, Kozyraki M, Antonakis N, Plaitakis A. Are primary care physicians able to assess dementia? An estimation of their capacity after a short-term training program in rural Crete. Am J Geriatr Psychiatry. 2001;9(3):315. [PubMed] [Google Scholar]
- 38. Lionis C, Vlachonikolis J, Chatziarsenis M, et al. Managing Alzheimer’s disease in primary care in Crete, Greece: room for improvement. Qual Manag Health Care. 2001;9(2):16–21. [DOI] [PubMed] [Google Scholar]
- 39. De Lepeleire J, Wind AW, Iliffe S, et al. The primary care diagnosis of dementia in Europe: an analysis using multidisciplinary, multinational expert groups. Aging Ment Health. 2008;12(5):568–576. [DOI] [PubMed] [Google Scholar]
- 40. Prokopiadou D, Papadakaki M, Roumeliotaki T, et al. Translation and validation of a questionnaire to assess the diagnosis and management of dementia in Greek General Practice. Eval Health Prof. 2015;38(2):151–159. [DOI] [PubMed] [Google Scholar]
- 41. Iatraki E, Simos PG, Lionis CIatraki E, et al. Cultural adaptation, standardization and clinical validity of the test your memory dementia screening instrument in Greek. Dement Geriatr Cogn Disord. 2014;37(3-4):163–180. [DOI] [PubMed] [Google Scholar]
- 42. Prins A, Hemke F, Pols J, Moll van Charante EP. Diagnosing dementia in Dutch general practice: a qualitative study of GPs’ practices and views. Br J Gen Pract. 2016;66(647):e416–e422. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 43. Paterson NE, Pond D. The barriers to the early diagnosis of dementia and diagnostic disclosure in primary care. Alzheimer Dement J Alzheimer Assoc. 5(4):P185. [Google Scholar]
- 44. O’Connor DW, Pollitt PA, Hyde JB, et al. Do general practitioners miss dementia in elderly patients? BMJ. 1988;297(6656):1107–1110. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 45. Mazower M. Inside Hitler’s Greece: The Experience of Occupation, 1941-44. New Haven, CT, USA: Yale University Press; 2001. [Google Scholar]
- 46. Albisseti J, Goodman J, Girls RR. Secondary Education in the Western World: From the 18th to the 20th Century (Secondary Education in a Changing World). New York: Palgrave Macmillan; 2010. [Google Scholar]
- 47. Sharp ES, Gatz M. Relationship between education and dementia: an updated systematic review. Alzheimer Dis Assoc Disord. 2011;25(4):289–304. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 48. Meng X, D’Arcy C, Education and dementia in the context of the cognitive reserve hypothesis: a systematic review with meta-analyses and qualitative analyses. PLoS One. 2012;7:e38268. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 49. Shpanskaya KS, Choudhury KR, Hostage C, Jr, et al. Educational attainment and hippocampal atrophy in the Alzheimer’s disease neuroimaging initiative cohort. J Neuroradiol. 2014;41(5):350–357. [DOI] [PubMed] [Google Scholar]
- 50. Cook CJ, Fletcher JM, Can education rescue genetic liability for cognitive decline? Soc Sci Med. 2015;127:159–170. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 51. Xu W, Tan L, Wang HF, et al. Education and risk of dementia: dose–response meta-analysis of prospective cohort studies. Mol Neurobiol. 2016;53(5):3113–3123. [DOI] [PubMed] [Google Scholar]
- 52. Mirza SS, Portegies ML, Wolters FJ, et al. Higher education is associated with a lower risk of dementia after a stroke or TIA. The Rotterdam study. Neuroepidemiology. 2016;46(2):120–127. [DOI] [PubMed] [Google Scholar]
- 53. Kaup AR, Simonsick EM, Harris TB, et al. Older adults with limited literacy are at increased risk for likely dementia. J Gerontol Ser A. 2014;69(7):900–906. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 54. Langa KM, Larson EB, Crimmins EM, et al. A comparison of the prevalence of dementia in the united states in 2000 and 2012. JAMA Intern Med. 2017;177(1):51–58. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 55. Andersen K, Lolk A, Nielsen H, et al. Prevalence of very mild to severe dementia in Denmark. Acta Neurol Scand. 1997;96:82–87. [DOI] [PubMed] [Google Scholar]
- 56. Tognoni G, Ceravolo R, Nucciarone B, et al. From mild cognitive impairment to dementia: a prevalence study in a district of Tuscany, Italy. Acta Neurol Scand. 2005;112(2):65–71. [DOI] [PubMed] [Google Scholar]
- 57. Gostynski M, Ajdacic-Gross V, Gutzwiller F, Michel JP, Herrmann F. Prevalence of dementia in the City of Zurich. Soz Praventivmed. 2002;47:330–335. [DOI] [PubMed] [Google Scholar]
- 58. Gascon-Bayarri J, Reñé R, Del Barrio JL, et al. Prevalence of dementia subtypes in El Prat de Llobregat, Catalonia, Spain: the PRATICON study. Neuroepidemiology. 2007;28:224–234. [DOI] [PubMed] [Google Scholar]
- 59. Wu Y, Fratiglioni L, Matthews FE, et al. Dementia in Western Europe: epidemiological evidence and implications for policy making. Lancet Neurol. 2016;15:116–124. [DOI] [PubMed] [Google Scholar]
- 60. Fiest KM, Roberts JI, Maxwell CJ, et al. The prevalence and incidence of dementia: a systematic review and meta-analysis. Can J Neurol Sci. 2016;43(S1):S3–S50. [DOI] [PubMed] [Google Scholar]
- 61. Prencipe M, Casini AR, Ferretti C, Lattanzio MT, Fiorelli M, Culasso F. Prevalence of dementia in an elderly rural population: effects of age, sex, and education. J Neurol Neurosurg Psychiatry. 1996;60(6):628–633. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 62. Klich-Rączka A, Piotrowicz K, Mossakowska M, et al. The assessment of cognitive impairment suspected of dementia in Polish elderly people: results of the population-based PolSenior Study. Exp Gerontol. 2014;57(suppl C):233–242. [DOI] [PubMed] [Google Scholar]
- 63. Schmand B, Smit J, Lindeboom J, et al. Low education is a genuine risk factor for accelerated memory decline and dementia. J Clin Epidemiol. 1997;50(9):1025–1033. [DOI] [PubMed] [Google Scholar]
- 64. Lobo ALL, Fratiglioni L, Andersen K, et al. Prevalence of dementia and major subtypes in Europe: a collaborative study of population-based cohorts. Neurologic Diseases in the Elderly Research Group. Neurology. 2000;54(11 suppl 5):S4–S9. [PubMed] [Google Scholar]
- 65. Bruti G, Cavallucci E, Mancini M, et al. A systematic review of the quality of studies on dementia prevalence in Italy. BMC Health Serv Res. 2016;16(1):615. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 66. Petersen RC. Mild cognitive impairment. N Engl J Med. 2011;364(23):2227–2234. [DOI] [PubMed] [Google Scholar]
- 67. Di Carlo A, Lamassa M, Baldereschi M, et al. CIND and MCI in the Italian elderly: frequency, vascular risk factors, progression to dementia. Neurology. 2007;68(22):1909–1916. [DOI] [PubMed] [Google Scholar]
- 68. Hu C, Yu D, Sun X, Zhang M, Wang L, Qin H. The prevalence and progression of mild cognitive impairment among clinic and community populations: a systematic review and meta-analysis. Int Psychogeriatr. 2017;29(10):1595–1608. [DOI] [PubMed] [Google Scholar]
- 69. Tsolaki M, Kakoudaki T, Tsolaki A, Verykouki E, Pattakouet V. Prevalence of mild cognitive impairment in individuals aged over 65 in a rural area in North Greece. Adv Alzheimer’s Dis. 2014;3(1):11–19. [Google Scholar]
- 70. Heegaard KM, Holm-Pedersen P, Bardow A, Hvidtfeldt UA, Grønbaek M, Avlund K. The Copenhagen Oral Health Senior Cohort: design, population and dental health. Gerodontology. 2011;28(3):165–176. [DOI] [PubMed] [Google Scholar]
- 71. Matthews FE, Arthur A, Barnes LE, et al. A two-decade comparison of prevalence of dementia in individuals aged 65 years and older from three geographical areas of England: results of the Cognitive Function and Ageing Study I and II. Lancet. 2013;382(9902):1405–1412. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 72. Malek-Ahmadi M. reversion from mild cognitive impairment to normal cognition: a meta-analysis. Alzheimer Dis Assoc Disord. 2016;30(4):324–330. [DOI] [PubMed] [Google Scholar]
- 73. Shimada H, Makizako H, Doi T, Lee S, Lee S. Conversion and reversion rates in Japanese older people with mild cognitive impairment. J Am Med Directors Assoc. 2017;18(9):808.e1–808.e6. [DOI] [PubMed] [Google Scholar]
- 74. Kaduszkiewicz H, Eisele M, Wiese B, et al. Prognosis of mild cognitive impairment in general practice: results of the German AgeCoDe study. Ann Fam Med. 2014;12(2):158–165. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 75. Sugarman M, Alosco ML, Tripodis Y, Steinberg EG, Stern RA. Neuropsychiatric symptoms and the diagnostic stability of mild cognitive impairment. J Alzheimers Dis. 2018;62(4):1841–1855. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 76. Koepsell TD, Monsell SE. Reversion from mild cognitive impairment to normal or near-normal cognition: risk factors and prognosis. Neurology. 2012;79(15):1591–1598. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 77. Knopman DS, Beiser A, Machulda MM, et al. Spectrum of cognition short of dementia: Framingham Heart Study and Mayo Clinic Study Aging. Neurology. 2015;85(19):1712–1721. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 78. Tabert MH, Manly JJ, Liu X, et al. Neuropsychological prediction of conversion to Alzheimer disease in patients with mild cognitive impairment. Arch Gen Psychiatry. 2006;63(8):916–924. [DOI] [PubMed] [Google Scholar]
- 79. Kern ML, Friedman HS. Early educational milestones as predictors of lifelong academic achievement, midlife adjustment, and longevity. J Appl Dev Psychol. 2009;30(4):419–430. [DOI] [PMC free article] [PubMed] [Google Scholar]
Associated Data
This section collects any data citations, data availability statements, or supplementary materials included in this article.
Supplementary Materials
Supplemental Material, 20180718ZaganasetalCretanAgingCohortSupplementaryRevised for The Cretan Aging Cohort: Cohort Description and Burden of Dementia and Mild Cognitive Impairment by Ioannis V. Zaganas, Panagiotis Simos, Maria Basta, Stefania Kapetanaki, Symeon Panagiotakis, Irini Koutentaki, Nikolaos Fountoulakis, Antonios Bertsias, George Duijker, Chariklia Tziraki, Nikolaos Scarmeas, Andreas Plaitakis, Dimitrios Boumpas, Christos Lionis, and Alexandros N. Vgontzas in American Journal of Alzheimer's Disease & Other Dementias